Abstract
We aimed to compare the associations of metabolic dysfunction-associated steatotic liver disease (MASLD) and metabolic dysfunction-associated fatty liver disease (MAFLD) with coronary artery calcification (CAC). Patients who simultaneously underwent ultrasonography to diagnose hepatic steatosis and cardiac computed tomography to detect CAC were included. The presence and severity of CAC were defined with CAC-score thresholds of > 0 and > 300, respectively, and patients were divided into the following groups: no MASLD or MAFLD (reference), MASLD-only, MAFLD-only, and overlapping groups. Overall, 1,060/2,773 (38.2%) patients had CAC, of which 196 (18.5%) had severe CAC. The MASLD and MAFLD prevalence rates were 32.6% and 45.2%, respectively, with an overlap of 30.7%. In an ASCVD risk score-adjusted model, both MASLD (adjusted odd ratios [aOR], 1.21; 95% confidence interval [CI], 1.02–1.44; p = 0.033) and MAFLD (aOR 1.20; 95% CI 1.01–1.42, p = 0.034) were associated with CAC, whereas only MASLD (aOR 1.38; 95% CI 1.01–1.89, p = 0.041) was associated with severe CAC. Compared to the reference group, the overlapping group showed an association with CAC (aOR 1.22; 95% CI 1.01–1.47; p = 0.038); however, the MASLD and MAFLD subgroups did not differ in their association with CAC. MASLD may predict a higher risk of ASCVD more effectively than MAFLD.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-74287-7.
Keywords: Metabolic dysfunction-associated steatotic liver disease, Metabolic dysfunction-associated fatty liver disease, Coronary artery calcification, Cardiovascular disease
Subject terms: Hepatology, Non-alcoholic fatty liver disease
Introduction
Non-alcoholic fatty liver disease (NAFLD) is defined as hepatic steatosis of ≥ 5% without the evidence of excessive alcohol consumption and other chronic liver diseases1,2. It has a global prevalence of approximately 30–35% and coexists with obesity, insulin resistance, and diabetes, which accounts for a large socioeconomic burden1,3. The prognosis of NAFLD varies depending on the degree of fibrosis, with cardiovascular outcomes being the primary cause of mortality1,4. However, due to the heterogenous pathogenesis of NAFLD and uncertainty regarding its classification, NAFLD nomenclature underestimates the significance of metabolic comorbidities and extrahepatic manifestations5.
The nomenclature for metabolic dysfunction-associated fatty liver disease (MAFLD) was introduced in 20196. Compared with NAFLD, MAFLD is a positive diagnosis that includes other chronic liver diseases and is categorised into three stratified subtypes based on diabetes, obesity, and other metabolic components. Several studies have indicated that the MAFLD criteria may identify a higher risk of cardiovascular disease (CVD) than NAFLD7–9. However, different subtypes of MAFLD—such as diabetic and non-diabetic MAFLD—have different CVD morbidity and mortality rates10,11. Further drawbacks of the MAFLD nomenclature include heterogeneous pathogeneses resulting from mixed aetiologies and a restricted comprehension of the disease’s natural history due to more lenient alcohol consumption12,13.
In 2023, metabolic dysfunction-associated steatotic liver disease (MASLD) emerged as the new nomenclature for steatotic liver disease (SLD), replacing both types of fatty liver disease, which have potentially stigmatising descriptions12,13. MASLD is defined as the presence of at least one of five cardiometabolic criteria of hepatic steatosis without any other apparent causes12,13. This umbrella term provides a comprehensive definition of SLD, with a focus on metabolic dysfunction12,13. Due to the recent change in the nomenclature, the relationship between MASLD and the risk of CVD remains uncertain. Given the high predictive value of MAFLD for predicting the risk of CVD, it is important to elucidate the association between MASLD and CVD risk and compare it to that of MAFLD.
Quantitatively scoring coronary artery calcification (CAC) is a reliable, non-invasive tool for detecting the presence of coronary artery disease and assessing the severity of CVD in asymptomatic individuals. This score is closely correlated with the degree of atherosclerosis and cardiovascular mortality, regardless of traditional risk factors14,15.
This study aimed to investigate the potential of MASLD and MAFLD as predictors of CVD risk based on the presence and severity of CAC. Additionally, we compared the associations between the MASLD and MAFLD subgroups with respect to the risk of CVD.
Results
Baseline characteristics
Table 1 summarises the baseline characteristics of the patients with CAC (CAC group) and those without CAC (no-CAC group). Of the 2773 participants, 1060 (38.2%) were categorised into the CAC group. Notably, compared to patients without CAC, those with CAC were older (55.0 [49.0–61.0] vs. 60.0 [55.0–66.0] years, p < 0.001), were more likely to be male (61.5% vs. 79.6%, p < 0.001), had a higher body mass index (BMI) (23.7 ± 2.9 vs. 24.0 ± 2.8 kg/m2, p = 0.001), were more likely to have diabetes (13.0% vs. 27.5%, p = 0.002), were more likely to be current smokers (50.1% vs. 67.3%, p < 0.001), had higher aspartate aminotransferase (AST) (27.6 ± 15.5 vs. 29.4 ± 16.2, p = 0.003), but not alanine aminotransferase (ALT) (27.8 ± 19.5 vs. 29.3 ± 19.9, p = 0.055), had lower platelet counts (243.5 ± 59.4 vs. 233.2 ± 54.1, p < 0.001), had a higher prevalence of statin usage (14.1% vs. 27.9%, p < 0.001), had no significant difference in high-sensitivity C-reactive protein (hsCRP) levels (0.4 [0.1–0.8] vs. 0.4 [0.1–0.8], p = 0.011), had high proportions of advanced fibrosis (3.2% vs. 7.3%, p < 0.001), and had higher atherosclerotic cardiovascular disease (ASCVD) risk scores (5.6 [2.4–10.8] vs. 13.1 [7.4–20.1], p < 0.001). In the CAC group, the median CAC score was 63.9 and the prevalence rates of mild, moderate, and severe CAC were 60.1%, 21.4%, and 18.5%, respectively.
Table 1.
Baseline characteristics of the patients.
| Absence of CAC n = 1713 (61.8%) |
Presence of CAC n = 1060 (38.2%) |
p-value | |
|---|---|---|---|
| Demographic profile | |||
| Age (year) | 55.0 [49.0–61.0] | 60.0 [55.0–66.0] | < 0.001 |
| Male | 1054 (61.5) | 844 (79.6) | < 0.001 |
| BMI (kg/m2) | 23.7 ± 2.9 | 24.0 ± 2.8 | 0.001 |
| Waist circumference (cm) | 84.8 ± 9.1 | 87.1 ± 8.5 | < 0.001 |
| Diabetes | 223 (13.0) | 291 (27.5) | 0.002 |
| Current smoker | 858 (50.1) | 713 (67.3) | < 0.001 |
| Liver function tests | |||
| AST (U/L) | 27.6 ± 15.5 | 29.4 ± 16.2 | 0.003 |
| ALT (U/L) | 27.8 ± 19.5 | 29.3 ± 19.9 | 0.055 |
| Platelet counts (×109/L) | 243.5 ± 59.4 | 233.2 ± 54.1 | < 0.001 |
| Albumin (g/dL) | 4.5 ± 0.4 | 4.7 ± 0.6 | 0.197 |
| Total bilirubin (mg/dL) | 0.8 ± 1.7 | 0.8 ± 0.4 | 0.539 |
| Metabolic profile (mg/dL) | |||
| Fasting glucose | 98.5 ± 22.2 | 105.4 ± 27.8 | < 0.001 |
| Total cholesterol | 191.9 ± 38.3 | 182.5 ± 40.5 | < 0.001 |
| TG | 124.4 ± 89.6 | 130.1 ± 83.3 | 0.091 |
| HDL-C | 56.5 ± 15.4 | 53.6 ± 14.4 | < 0.001 |
| LDL-C | 128.8 ± 46.0 | 120.5 ± 38.8 | < 0.001 |
| Use of statin | 241 (14.1) | 296 (27.9) | < 0.001 |
| hsCRP | 0.4 [0.1–0.8] | 0.4 [0.1–0.8] | 0.011 |
| Fib-4 ≥ 2.67 | 54 (3.2) | 77 (7.3) | < 0.001 |
| Cardiovascular profile | |||
| ASCVD risk score | 5.6 [2.4–10.8] | 13.1 [7.4–20.1] | < 0.001 |
| CAC score | 63.9 [14.8–202.5] | ||
| CAC grade | |||
| Mild (CAC score, 1–99) | 637 (60.1) | ||
| Moderate (CAC score, 100–299) | 227 (21.4) | ||
| Severe (CAC score, ≥ 300) | 196 (18.5) | ||
Data are presented as mean ± standard deviation, median [interquartile range], or number (%). Presence of CAC is defined as a CAC score > 0. CAC coronary artery calcification, BMI body mass index, AST aspartate aminotransferase, ALT alanine aminotransferase, hsCRP high-sensitivity C-reactive protein, TG triglycerides, HDL-C high-density lipoprotein cholesterol, LDL low-density lipoprotein cholesterol, Fib-4 fibrosis-4 index, ASCVD atherosclerotic cardiovascular disease.
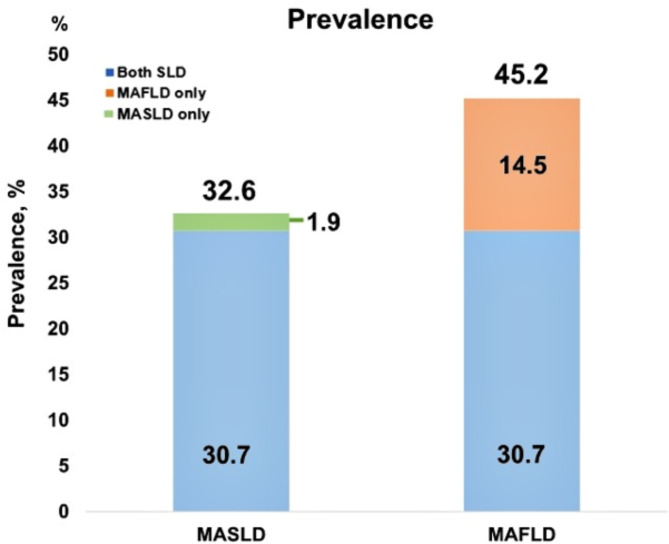
In contrast, the prevalence rates of MASLD and MAFLD were 32.6% and 45.2%, respectively. Furthermore, the prevalence of satisfying both MASLD and MAFLD criteria was 30.7%, compared with 1.9% for MASLD alone and 14.5% for MAFLD alone (Fig. 1).
Fig. 1.
The prevalence of MASLD and MAFLD.
Adjusted associations of CAC based on the presence or absence of MASLD or MAFLD
Table 2 shows the adjusted risk of CAC according to the presence or absence of MASLD or MAFLD. Compared with the no-MASLD group, the patients with MASLD remained at higher risk of CAC after adjustment for age and sex (Model 1: adjusted odds ratio (aOR), 1.36; 95% confidence interval (CI), 1.13–1.63; p = 0.001); smoking status, use of statins, diabetes, and advanced fibrosis (Model 2: aOR 1.25; 95% CI 1.04–1.51; p = 0.018); and ASCVD risk score (Model 3: aOR 1.21; 95% CI 1.02–1.44; p = 0.033).
Table 2.
Adjusted risk for CAC according to the presence or absence of MASLD or MAFLD.
| Number | Presence of CAC | Model 1 aOR (95% CI) |
Model 2 aOR (95% CI) |
Model 3† aOR (95% CI) |
|
|---|---|---|---|---|---|
| MASLD | |||||
| No-MASLD | 1868 | 664 | 1 (ref.) | 1 (ref.) | 1 (ref.) |
| MASLD | 905 | 396 | 1.36 (1.13–1.63)* | 1.25 (1.04–1.51)* | 1.21 (1.02–1.44)* |
| MAFLD | |||||
| No-MAFLD | 1520 | 503 | 1 (ref.) | 1 (ref.) | 1 (ref.) |
| MAFLD | 1253 | 557 | 1.48 (1.25–1.75)* | 1.33 (1.12–1.58)* | 1.20 (1.01–1.42)* |
*A p-value < 0.05. Model 1, adjusted for age and sex; Model 2, Model 1 + smoking, use of statins, diabetes, and advanced fibrosis; Model 3: †ASCVD risk score. Presence of CAC is defined as a CAC score > 0 and advanced fibrosis is defined as a fibrosis-4 index ≥ 2.67. †The ASCVD risk score includes age, sex, race, smoking status, blood pressure, medication use for hypertension, diabetes status, total cholesterol, and high-density lipoprotein cholesterol. CAC coronary artery calcification, MASLD metabolic dysfunction-associated steatotic liver disease, MAFLD metabolic dysfunction-associated fatty liver disease, aOR adjusted odds ratio, CI confidence interval, ref. reference, ASCVD atherosclerotic cardiovascular disease.
Similarly, compared with the no-MAFLD group, the patients with MAFLD had a higher risk of CAC after adjustment for age and sex (Model 1: aOR 1.48; 95% CI 1.25–1.75; p < 0.001); smoking status, use of statins, diabetes, and advanced fibrosis (Model 2: aOR 1.33; 95% CI 1.12–1.58; p = 0.001); and ASCVD risk score (Model 3: aOR 1.20; 95% CI 1.01–1.42; p = 0.034).
Adjusted associations of severe CAC based on the presence or absence of MASLD or metabolic dysfunction-associated fatty liver disease
Table 3 shows the adjusted associations of CAC severity in patients with MASLD and MAFLD compared to those without. Compared with the no-MASLD group, the patients with MASLD maintained a higher risk of severe CAC after adjustment for age and sex (Model 1: aOR 1.60; 95% CI 1.16–2.22; p = 0.005); smoking status, use of statins, diabetes, and advanced fibrosis (Model 2: aOR 1.49; 95% CI 1.07–2.08; p = 0.017); and ASCVD risk score (Model 3: aOR 1.38; 95% CI 1.01–1.89; p = 0.041).
Table 3.
Adjusted risk of severe CAC according to the presence or absence of MASLD or MAFLD.
| Number | Severe CAC | Model 1 aOR (95% CI) |
Model 2 aOR (95% CI) |
Model 3† aOR (95% CI) |
|
|---|---|---|---|---|---|
| MASLD | |||||
| No-MASLD | 1868 | 115 | 1 (ref.) | 1 (ref.) | 1 (ref.) |
| MASLD | 905 | 81 | 1.60 (1.16–2.22)* | 1.49 (1.07–2.08)* | 1.38 (1.01–1.89)* |
| MAFLD | |||||
| No-MAFLD | 1520 | 86 | 1 (ref.) | 1 (ref.) | 1 (ref.) |
| MAFLD | 1253 | 110 | 1.49 (1.10–2.03)* | 1.33 (0.97–1.82) | 1.15 (0.84–1.57) |
*A p-value < 0.05. Model 1, adjusted for age and sex; Model 2, Model 1 + smoking, use of statins, diabetes, and advanced fibrosis; Model 3: †ASCVD risk score. Presence of severe CAC is defined as a CAC score > 300 and advanced fibrosis is defined as a fibrosis-4 index ≥ 2.67. †The ASCVD risk score includes age, sex, race, smoking status, blood pressure, medication use for hypertension, diabetes status, total cholesterol, and high-density lipoprotein cholesterol. CAC coronary artery calcification, MASLD metabolic dysfunction-associated steatotic liver disease, MAFLD metabolic dysfunction-associated fatty liver disease, aOR adjusted odds ratio, CI confidence interval, ref. reference, ASCVD atherosclerotic cardiovascular disease.
Unlike the no-MAFLD group, the patients with MAFLD had an increased risk of severe CAC only after adjustment for age and sex (Model 1: aOR 1.49; 95% CI 1.10–2.03; p = 0.011), whereas no association with severe CAC was observed upon further adjustment (Table 3).
Adjusted associations of CAC according to subgroups of different steatotic liver statuses
We assessed the effect of CAC on four subgroups of patients categorised according to their steatotic liver status (Table 4). Compared with the group with neither MASLD nor MAFLD, both the MASLD and MAFLD groups were associated with an increased risk of CAC after adjustment for age and sex (Model 1: aOR 1.48; 95% CI 1.22–1.81; p < 0.001); smoking status, use of statins, diabetes, and advanced fibrosis (Model 2: aOR 1.34; 95% CI 1.09–1.64; p = 0.005); and ASCVD risk score (Model 3: aOR 1.22; 95% CI 1.01–1.47; p = 0.038), whereas the MASLD-only and MAFLD-only groups were not.
Table 4.
Adjusted risk of CAC according to the subgroups of different steatotic liver statuses.
| Number | Presence of CAC | Model 1 aOR (95% CI) |
Model 2 aOR (95% CI) |
Model 3† aOR (95% CI) |
|
|---|---|---|---|---|---|
| Neither MASLD nor MAFLD | 1466 | 482 | 1 (ref.) | 1 (ref.) | 1 (ref.) |
| MASLD only | 54 | 21 | 1.30 (0.73–2.25) | 1.30 (0.73–2.25) | 1.30 (0.73–2.25) |
| MAFLD only | 402 | 182 | 1.45 (1.13–1.86)* | 1.29 (0.99–1.67) | 1.11 (0.86–1.43) |
| Both MASLD and MAFLD | 851 | 375 | 1.48 (1.22–1.81)* | 1.34 (1.09–1.64)* | 1.22 (1.01–1.47)* |
*A p-value < 0.05. Model 1, adjusted for age and sex; Model 2, Model 1 + smoking, use of statins, diabetes, and advanced fibrosis; Model 3: †ASCVD risk score. Presence of CAC is defined as a CAC score > 0 and advanced fibrosis is defined as a fibrosis-4 index ≥ 2.67. †The ASCVD risk score includes age, sex, race, smoking status, blood pressure, medication use for hypertension, diabetes status, total cholesterol, and high-density lipoprotein cholesterol. CAC coronary artery calcification, aOR adjusted odds ratio, CI confidence interval, MASLD metabolic dysfunction-associated steatotic liver disease, MAFLD metabolic dysfunction-associated fatty liver disease, ref. reference.
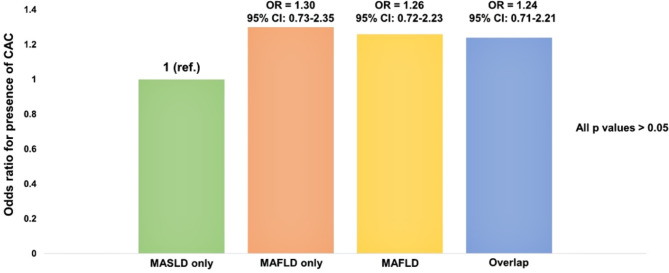
To directly compare the impact of CAC on each subgroup of patients with MASLD and/or MAFLD, we established the MASLD-only group as a reference (Fig. 2). Compared with the MASLD-only group, the MAFLD-only, MAFLD, and both MASLD and MAFLD groups were not significantly associated with CAC (all p > 0.05).
Fig. 2.
Odds ratios for the presence of CAC between the subgroups of MASLD and MAFLD.
Discussion
In this study, we identified an association between MASLD and the presence and severity of CAC. In addition, we found that MASLD has a cardiovascular risk comparable to that of MAFLD but tends to outperform MAFLD in the assessment of CVD severity using the CAC score. This suggests that MASLD, as a novel nomenclature, has the potential to replace MAFLD when predicting the CVD risk.
This study has some clinical implications. First, the prevalence of MASLD in the two health-promotion centre populations was 32.6%. In two recent studies, the prevalence of MASLD was 33.4% in a Brazilian cohort and 14.87% in a Chinese cohort16,17. These discrepancies are possibly due to differences in the enrolled populations, including the timing of cohort recruitment and ethnic differences. Moreover, compared to a previous meta-analysis that reported the prevalence of MAFLD to be 38.77% (95% CI 32.94–44.95%)18, the prevalence of MAFLD in our study was slightly higher at 45.2%. Additionally, the prevalence of overlap between MASLD and MAFLD in our study was 30.7%, which was comparable to that between traditional NAFLD and MAFLD (27.4%) in a previous study7. Further research is required to investigate the prevalence of MASLD in a large population cohort that reflects sex- and ethnicity-specific characteristics.
When compared with the non-MASLD or non-MAFLD groups, both the MASLD (odds ratio [OR] 1.21; 95% CI 1.02–1.44) and MAFLD (OR 1.20; 95% CI 1.01–1.42) groups showed an ASCVD risk score-adjusted association with the presence of CAC. Similarly, both MAFLD and NAFLD have been associated with an approximately 1.2 to 2–fold increased risk of CVD in previous studies9,19,20. A previous meta-analysis revealed an association between the presence of CAC (OR 1.272; 95% CI 1.114–1.452), severe CAC defined as CAC > 100 (OR 1.242; 95% CI 1.017–1.516) and NAFLD, irrespective of traditional risk factors, which is similar to our results21.
However, in the present study, MASLD showed an ASCVD risk score-adjusted association with severe CAC (OR 1.38; 95% CI 1.01–1.89), whereas MAFLD did not (OR 1.15; 95% CI 0.84–1.57). These results may be related to the heterogeneous subtypes of MAFLD. Among the three MAFLD subtypes, diabetes is the most well-established risk factor for CAC, and the MAFLD-diabetic subtype is associated with an increased risk of all-cause mortality and CVD11,22. However, recent research indicates that the MAFLD-overweight/obese subtype does not increase the risk of all-cause mortality or cardiovascular morbidity10,11. Additionally, the MAFLD-lean subtype with less than two metabolic factors is not associated with CAC11. To investigate the association between the presence of CAC and MAFLD subtypes, we divided the MAFLD group into diabetic and non-diabetic subgroups. We found that the presence of CAC was significantly associated with the MAFLD-diabetic subtype when adjusted for age and sex, but not with the MAFLD-non-diabetic subtype when unadjusted (Supplementary Table 1). This implies that the associations of overall MAFLD with severe CAC may differ due to its heterogeneous subtypes, indicating that MASLD may be more effective than MAFLD when evaluating the severity of CVD. Moreover, a previous study suggested that light and moderate alcohol consumption may have a protective effect on CVD mortality23. Therefore, MAFLD may be associated with a reduced risk of CAC due to the higher proportion of light-to-moderate drinkers than in patients with MASLD; however, further research is required to reflect the exact impact of alcohol intake on CAC.
Furthermore, our subgroup analysis revealed that only overlap between the MASLD and MAFLD groups was associated with the presence of CAC when neither group was used as a reference. However, the MASLD-only and MAFLD-only groups showed no association with CAC, which is a distinctive finding. Additionally, there was no evidence to suggest the superiority of CAC in the definition of either type of SLD through comparisons between the MASLD-only and MAFLD subgroups. Contrary to our findings, previous studies comparing the efficacies of NAFLD and MAFLD in predicting the risk of CVD showed that the MAFLD group and the overlap between the NAFLD and MAFLD groups were associated with an increased risk of CVD7,9. In one study, the aOR for a high ASCVD risk in the MAFLD-only and MAFLD groups were 3.26 and 3.14, respectively, using the NAFLD-only group as a reference group24. In addition, previous studies have shown that the MAFLD definition is more effective than the NAFLD definition when predicting the prognosis of other diseases25,26.
The subgroups in this study were characterised as follows: the MAFLD-only group included patients with metabolic risk factors and other aetiologies such as alcohol consumption and chronic hepatitis C, whereas the MASLD-only group had a comparatively favourable metabolic status, with all metabolic risk factors scoring 1. Compared to the definition of NAFLD, the MASLD definition is believed to more directly and comprehensively include the metabolic components of MAFLD, thereby homogenising the overlap of the metabolically unfavourable portions. In other words, groups other than the homogeneous groups (NAFLD-only and MAFLD-only) showed a favourable metabolic status when compared with the overlap group. Future studies should use large longitudinal data to determine the comparative associations between MASLD, MAFLD, and CVD risk.
In this study, we also compared the CAC risk of each MASLD and MAFLD adjusted for the presence of advanced fibrosis, defined as Fib-4 ≥ 2.67, in addition to traditional risk factors. NAFLD is known to be associated with both fatal and nonfatal CVD events, depending on the stage of liver fibrosis27. We have previously shown that significant fibrosis, as defined by MRE, is associated with the presence of CAC in patients with NAFLD28. Furthermore, considering that MAFLD may better identify significant liver fibrosis than NAFLD, the assessment of the fibrosis stage may be an important consideration when comparing CVD risk between MAFLD and MASLD29.
Our study had several limitations. First, this was a cross-sectional retrospective study that does not establish causality with CVD in patients with SLD. Given that MASLD is significantly associated with clinical CVD including atrial fibrillation, valvular heart disease, and heart failure with or without reduced ejection fraction, future studies to elucidate the association between each CVD would clarify the pathophysiology of MASLD30. Second, we only included individuals who voluntarily visited the health-promotion centre and could afford the full test, including non-contrast cardiac CT, which may have resulted in a selection bias. Additionally, non-contrast imaging has limitations in quantifying calcified plaque burden without non-calcified plaque, which limits the ability to accurately predict CVD risk. Non-calcified plaques, which are mainly composed of lipids, cholesterol, cell debris, and inflammatory cells, are known to be more unstable than calcified plaques and easily rupture due to plaque vulnerability, which can cause serious CVD events such as myocardial or cerebral infarction31. It is important to clearly evaluate non-calcified plaque burden through contrast-based imaging to predict prognosis, but this study has the disadvantage of measuring only calcified plaque burden using non-contrast cardiac CT. However, considering the cost effectiveness and contrast-related adverse effects (contrast-induced nephrotoxicity and allergic reaction) of performing contrast heart CT for all participants without heart-related symptoms, we think that the implementation of contrast CT in all participants of health promotion center is realistically limited. Furthermore, we did not measure epicardial adipose distribution, which is independently associated with MASLD and an increased risk for CAC. Future expanded research is needed to supplement these points. Third, this study was conducted in South Korea, potentially restricting the generalisability of the findings to the entire population. Therefore, prospective, well-designed longitudinal studies are warranted to validate the causal relationship between MASLD and the incident and prevalent CVD risks. Fourth, in our study, steatotic liver was diagnosed using abdominal ultrasound (US), which is subject to inter-observer and intra-observer variability, as well as inaccurate detection of the presence of mild steatosis (10–15%)32,33. The US fatty liver index (US-FLI) and or transient elastography-based controlled attenuation parameters (CAP) demonstrates excellent reproducibility and a satisfactory discriminative capacity for the various grades of steatosis34. While the recently published guideline does not provide a definitive definition of imaging-based steatosis diagnosis, the use of US-FLI or CAP may help to address the limitations of US12. A drawback is that transient elastography was not available in the health promotion centers included in our study. It is imperative that clear criteria for the definition of hepatic steatosis be established through the implementation of multiple studies. Fifth, the subjects enrolled in this study did not meet the criteria for MAFLD owing to the lack of homeostasis model assessment of insulin resistance (HOMA-IR) measurements. However, owing to the high cost and complexity of this procedure, the use of IR markers as the primary test for identifying MAFLD in the general population is challenging. In contrast, the MASLD can identify and assess risk factors through simple blood tests and demographic measurements. Recently, given that triglyceride glucose index (TyG) has emerged as a novel predictive biomarker of IR and ASCVD events, the measurement of TyG demonstrates the potential to replace measurement of HOMA-IR35. Our study has a limitation in that it did not measure TyG but considering that the CAC group had higher glucose levels compared to the non-CAC group, it can be indirectly estimated that the TyG level was higher. However, this study had limitations in collecting data on factors affecting high-TG medications (fibrates, omega-3, etc.).
In conclusion, beyond being associated with the presence of CAC, independent of traditional risk factors, MASLD was more effective than MAFLD in identifying severe CAC. Moreover, compared with the definition of MAFLD, the new MASLD nomenclature might not show any gap in the prediction of CVD risk using the CAC score. Therefore, the assessment and stratification of CAC may be useful in predicting a high risk of CVD events in patients with MASLD.
Methods
Patients
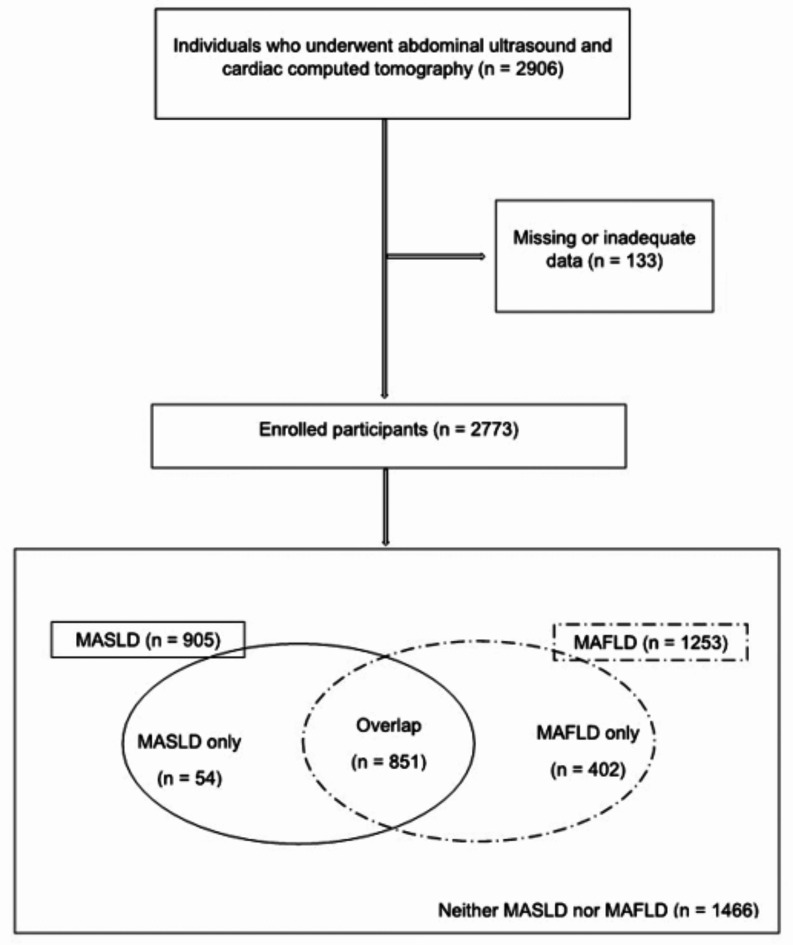
This cross-sectional, multicentre, retrospective study enrolled individuals who underwent comprehensive health screening using abdominal ultrasonography and cardiac computed tomography (CT) between January 2017 and December 2021 at two health-promotion centres in Daegu, South Korea. In total, 2906 individuals who underwent abdominal ultrasonography and cardiac CT were examined. The exclusion criteria were a documented history of significant CVD or cardiac intervention and missing or inadequate data. Of the 2906 patients, none experienced CVD events; however, 133 individuals had missing or inadequate data. Therefore, a total of 2773 participants were enrolled in this study.
Definition and classification of MASLD and MAFLD
Hepatic steatosis was assessed based on the following criteria: increased echogenicity of the liver compared with that of the renal cortex, deep attenuated beam, and blurred intrahepatic vessels on abdominal US36. MASLD is defined as hepatic steatosis in addition to at least one of the five cardiometabolic criteria12,13, whereas MAFLD is defined as hepatic steatosis in addition to the presence of one of the following metabolic conditions: diabetes, overweight/obesity, or evidence of at least two out of seven metabolic abnormalities6. Due to all the enrolled patients being Korean, waist circumference and BMI were determined using cut-off values for Asians. HOMA-IR values was excluded due to the absence of fasting insulin levels. Individuals who did not fulfil the MASLD or MAFLD criteria were classified into the no-MASLD or no-MAFLD groups, respectively. Moreover, individuals who met neither the MASLD nor the MAFLD criteria were classified into the neither MASLD nor MAFLD group.
Of the 2773 participants, 905 met the criteria for MASLD, 1253 met the criteria for MAFLD, 1466 had neither MASLD nor MAFLD, and 851 met the criteria for overlap between MAFLD and MASLD. Moreover, 54 patients did not fulfil the MAFLD criteria and were diagnosed with MASLD only, while 402 did not meet the MASLD criteria and were diagnosed with MAFLD only (Fig. 3).
Fig. 3.
Flow charts and Venn diagram of the enrolled participants.
Quantification of CAC using cardiac CT
Two non-contrast cardiac prospective electrocardiogram-gated volumetric CT scans were performed at each institution using a 256-slice CT scanner (Revolution; GE Healthcare) and a 320-slice CT scanner (SOMATOM Force, Siemens Healthineers). At the end of inspiration, individuals were instructed to hold their breath while undergoing a scan ranging from the base of the heart to the carina. The tube voltage ranged from 100 to 120 kVp. Five filter revolutions were used for reconstruction, resulting in 2.5 mm-thick reconstruction slices. Subsequently, the CAC was calculated for each cardiac CT protocol. The CAC scores were calculated using the Agatston scoring method through independent postprocessing software (TeraRecon and Syngo.via; Siemens Healthineers). The presence of CAC was determined by a CAC score of > 0, whereas severe CAC was defined as a score of > 30014,37.
Statistical analysis
Data are expressed as means with standard deviations, medians with interquartile ranges, or numbers and percentages, as appropriate. Continuous data were analysed using the Student t-test or Mann–Whitney U test, and categorical data were compared using the chi-square test or Fisher exact test after testing for normality. To evaluate the associations of MASLD and MAFLD with the presence or severity of CAC, adjusted logistic regression models with stepwise backward elimination of OR were assessed. Model 1 was adjusted for age and sex, whereas Model 2 was adjusted for smoking, statin use, diabetes, and advanced fibrosis, defined as a fibrosis-4 index ≥ 2.67 after adjustment for Model 1. To avoid multicollinearity, Model 3 was adjusted for the ASCVD risk score only, which included age, sex, race, smoking status, blood pressure, use of antihypertensive medication, diabetes status, and levels of total and high-density lipoprotein cholesterol.
All statistical analyses were performed using the R software (version 4.1.0; R Core Team, 2021; R Foundation for Statistical Computing, Vienna, Austria), and statistical significance was set at p < 0.05.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Abbreviations
- ALT
Alanine aminotransferase
- aOR
Adjusted odds ratio
- ASCVD
Atherosclerotic cardiovascular disease
- AST
Aspartate aminotransferase
- BMI
Body mass index
- CAC
Coronary artery calcification
- CAP
Controlled attenuation parameters
- CI
Confidence interval
- CT
Computed tomography
- CVD
Cardiovascular disease
- Fib-4
Fibrosis-4 index
- HDL-C
High-density lipoprotein cholesterol
- HOMA-IR
Homeostasis model assessment of insulin resistance
- HsCRP
High-sensitivity C-reactive protein
- LDL
Low-density lipoprotein cholesterol
- MAFLD
Metabolic dysfunction-associated fatty liver disease
- MASLD
Metabolic dysfunction-associated steatotic liver disease
- SLD
Steatotic liver disease
- TG
Triglycerides
- TyG
Triglyceride glucose index
- US
Ultrasound
- US-FLI
Ultrasound fatty liver index
Author contributions
MKK and JGP are guarantor of integrity of the entire study and designated the study and partially supervised by RL. All authors except RL collected data, which were reviewed and analysed based on the statistical analysis plan by MKK, JES, YRL. MKK and YRL drafted the manuscript, which was critically revised by all authors. All authors were responsible for collecting and interpretation of the data and approved final version of manuscript.
Funding
This study was supported by the 2023 Yeungnam University Research Grant (number: 223A061030, by MKK). RL receives funding support from NCATS (5UL1TR001442), NIDDK (U01DK061734, U01DK130190, R01DK106419, R01DK121378, R01DK124318, P30DK120515), NHLBI (P01HL147835), and John C Martin Foundation (RP124).
Data availability
The data supporting findings of this study are available from the corresponding authors upon reasonable request.
Declarations
Competing interests
RL serves as a consultant to Aardvark Therapeutics, Altimmune, Arrowhead Pharmaceuticals, AstraZeneca, Cascade Pharmaceuticals, Eli Lilly, Gilead, Glympse bio, Inipharma, Intercept, Inventiva, Ionis, Janssen Inc., Lipidio, Madrigal, Neurobo, Novo Nordisk, Merck, Pfizer, Sagimet, 89 bio, Takeda, Terns Pharmaceuticals and Viking Therapeutics. In addition, his institution received research grants from Arrowhead Pharmaceuticals, Astrazeneca, Boehringer-Ingelheim, Bristol-Myers Squibb, Eli Lilly, Galectin Therapeutics, Gilead, Intercept, Hanmi, Intercept, Inventiva, Ionis, Janssen, Madrigal Pharmaceuticals, Merck, Novo Nordisk, Pfizer, Sonic Incytes and Terns Pharmaceuticals. Co-founder of LipoNexus Inc.
Ethics approval
This study was approved by the Institutional Review Board (IRB) of the Yeungnam University Hospital (IRB No. 2023-11-026) and performed in accordance with the ethical guidelines of the 1975 Declaration of Helsinki. Owing to the retrospective nature of this study, the requirement for written informed consent was waived.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Yu Rim Lee, Email: deblue00@naver.com.
Jung Gil Park, Email: gsnrs@naver.com.
References
- 1.Kang, S. H. et al. KASL clinical practice guidelines: management of nonalcoholic fatty liver disease. Clin. Mol. Hepatol.27, 363–401. 10.3350/cmh.2021.0178 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Loomba, R. & Sanyal, A. J. The global NAFLD epidemic. Nat. Rev. Gastroenterol. Hepatol.10, 686–690. 10.1038/nrgastro.2013.171 (2013). [DOI] [PubMed] [Google Scholar]
- 3.Teng, M. L. et al. Global incidence and prevalence of nonalcoholic fatty liver disease. Clin. Mol. Hepatol.2910.3350/cmh.2022.0365 (2023). S32-S42. [DOI] [PMC free article] [PubMed]
- 4.Loomba, R., Friedman, S. L. & Shulman, G. I. Mechanisms and disease consequences of nonalcoholic fatty liver disease. Cell. 184, 2537–2564. 10.1016/j.cell.2021.04.015 (2021). [DOI] [PubMed] [Google Scholar]
- 5.Kang, S. H. et al. From nonalcoholic fatty liver disease to metabolic-associated fatty liver disease: big wave or ripple? Clin. Mol. Hepatol.27, 257–269. 10.3350/cmh.2021.0067 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eslam, M. et al. A new definition for metabolic dysfunction-associated fatty liver disease: an international expert consensus statement. J. Hepatol.73, 202–209. 10.1016/j.jhep.2020.03.039 (2020). [DOI] [PubMed] [Google Scholar]
- 7.Lee, H., Lee, Y. H., Kim, S. U. & Kim, H. C. Metabolic dysfunction-associated fatty liver disease and incident cardiovascular disease risk: a nationwide cohort study. Clin. Gastroenterol. Hepatol.19, 2138–2147e2110. 10.1016/j.cgh.2020.12.022 (2021). [DOI] [PubMed] [Google Scholar]
- 8.Mantovani, A., Csermely, A., Tilg, H., Byrne, C. D. & Targher, G. Comparative effects of non-alcoholic fatty liver disease and metabolic dysfunction-associated fatty liver disease on risk of incident cardiovascular events: a meta-analysis of about 13 million individuals. Gut. 72, 1433–1436 (2023). [DOI] [PubMed] [Google Scholar]
- 9.Sung, K. C. et al. Comparative associations of nonalcoholic fatty liver disease and metabolic dysfunction-associated fatty liver disease with coronary artery calcification: a cross-sectional and longitudinal cohort study. Arterioscler. Thromb. Vasc. Biol.43, 482–491. 10.1161/ATVBAHA.122.318661 (2023). [DOI] [PubMed] [Google Scholar]
- 10.Chen, X., Chen, S., Pang, J., Tang, Y. & Ling, W. Are the different MAFLD subtypes based on the inclusion criteria correlated with all-cause mortality? J. Hepatol.75, 987–989. 10.1016/j.jhep.2021.06.013 (2021). [DOI] [PubMed] [Google Scholar]
- 11.Kang, M. K. et al. Impact of metabolic factors on risk of cardiovascular disease in nondiabetic metabolic dysfunction-associated fatty liver disease. Hepatol. Int.17, 626–635. 10.1007/s12072-023-10517-w (2023). [DOI] [PubMed] [Google Scholar]
- 12.Rinella, M. E. et al. A multisociety Delphi consensus statement on new fatty liver disease nomenclature. J. Hepatol.79, 1542–1556. 10.1016/j.jhep.2023.06.003 (2023). [DOI] [PubMed] [Google Scholar]
- 13.Rinella, M. E. et al. A multisociety Delphi consensus statement on new fatty liver disease nomenclature. Hepatology. 10.1097/HEP.0000000000000520 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Obisesan, O. H., Osei, A. D., Uddin, S. M. I., Dzaye, O. & Blaha, M. J. An update on coronary artery calcium interpretation at chest and cardiac CT. Radiol. Cardiothorac. Imaging. 3, e200484. 10.1148/ryct.2021200484 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Uddin, S. M. I. et al. Coronary artery calcium scoring for adults at borderline 10-year ASCVD risk: the CAC consortium. J. Am. Coll. Cardiol.78, 537–538. 10.1016/j.jacc.2021.05.036 (2021). [DOI] [PubMed] [Google Scholar]
- 16.Perazzo, H., Pacheco, A. G. & Griep, R. H. Collaborators. Changing from NAFLD through MAFLD to MASLD: similar prevalence and risk factors in a large Brazilian cohort. J. Hepatol.80, e72–e74. 10.1016/j.jhep.2023.08.025 (2024). [DOI] [PubMed] [Google Scholar]
- 17.Zhao, Q. & Deng, Y. Comparison of mortality outcomes in individuals with MASLD and/or MAFLD. J. Hepatol.80, e62–e64. 10.1016/j.jhep.2023.08.003 (2024). [DOI] [PubMed] [Google Scholar]
- 18.Chan, K. E. et al. Global prevalence and clinical characteristics of metabolic-associated fatty liver disease: a meta-analysis and systematic review of 10,739,607 individuals. J. Clin. Endocrinol. Metab.107, 2691–2700. 10.1210/clinem/dgac321 (2022). [DOI] [PubMed] [Google Scholar]
- 19.Targher, G., Byrne, C. D., Lonardo, A., Zoppini, G. & Barbui, C. Non-alcoholic fatty liver disease and risk of incident cardiovascular disease: a meta-analysis. J. Hepatol.65, 589–600. 10.1016/j.jhep.2016.05.013 (2016). [DOI] [PubMed] [Google Scholar]
- 20.Wijarnpreecha, K., Aby, E. S., Ahmed, A. & Kim, D. Evaluation and management of extrahepatic manifestations of nonalcoholic fatty liver disease. Clin. Mol. Hepatol.27, 221–235. 10.3350/cmh.2020.0239 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jaruvongvanich, V., Wirunsawanya, K., Sanguankeo, A. & Upala, S. Nonalcoholic fatty liver disease is associated with coronary artery calcification: a systematic review and meta-analysis. Dig. Liver Dis.48, 1410–1417. 10.1016/j.dld.2016.09.002 (2016). [DOI] [PubMed] [Google Scholar]
- 22.Kronmal, R. A. et al. Risk factors for the progression of coronary artery calcification in asymptomatic subjects: results from the multi-ethnic study of atherosclerosis (MESA). Circulation. 115, 2722–2730. 10.1161/CIRCULATIONAHA.106.674143 (2007). [DOI] [PubMed] [Google Scholar]
- 23.Xi, B. et al. Relationship of alcohol consumption to all-cause, cardiovascular, and cancer-related mortality in US adults. J. Am. Coll. Cardiol.70, 913–922. 10.1016/j.jacc.2017.06.054 (2017). [DOI] [PubMed] [Google Scholar]
- 24.Chun, H. S. et al. Metabolic dysfunction associated fatty liver disease identifies subjects with cardiovascular risk better than non-alcoholic fatty liver disease. Liver Int.43, 608–625. 10.1111/liv.15508 (2023). [DOI] [PubMed] [Google Scholar]
- 25.Miao, L. et al. Metabolic dysfunction-associated fatty liver disease is associated with greater impairment of lung function than nonalcoholic fatty liver disease. J. Clin. Transl. Hepatol.10, 230–237. 10.14218/JCTH.2021.00306 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sun, D. Q. et al. MAFLD and risk of CKD. Metabolism115, 154433. 10.1016/j.metabol.2020.154433 (2021). [DOI] [PubMed]
- 27.Mantovani, A. et al. Non-alcoholic fatty liver disease and risk of fatal and non-fatal cardiovascular events: an updated systematic review and meta-analysis. Lancet Gastroenterol. Hepatol.6, 903–913. 10.1016/S2468-1253(21)00308-3 (2021). [DOI] [PubMed] [Google Scholar]
- 28.Park, J. G. et al. Liver stiffness by magnetic resonance elastography is associated with increased risk of cardiovascular disease in patients with non-alcoholic fatty liver disease. Aliment. Pharmacol. Ther.53, 1030–1037. 10.1111/apt.16324 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yamamura, S. et al. MAFLD identifies patients with significant hepatic fibrosis better than NAFLD. Liver Int.40, 3018–3030. 10.1111/liv.14675 (2020). [DOI] [PubMed] [Google Scholar]
- 30.Stahl, E. P. et al. Nonalcoholic fatty liver disease and the heart: JACC state-of-the-art review. J. Am. Coll. Cardiol.73, 948–963. 10.1016/j.jacc.2018.11.050 (2019). [DOI] [PubMed] [Google Scholar]
- 31.Yamaura, H. et al. Determinants of non-calcified low-attenuation coronary plaque burden in patients without known coronary artery disease: a coronary CT angiography study. Front. Cardiovasc. Med.9, 824470. 10.3389/fcvm.2022.824470 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mathiesen, U. et al. Increased liver echogenicity at ultrasound examination reflects degree of steatosis but not of fibrosis in asymptomatic patients with mild/moderate abnormalities of liver transaminases. Dig. Liver Disease. 34, 516–522 (2002). [DOI] [PubMed] [Google Scholar]
- 33.Saverymuttu, S., Joseph, A. & Maxwell, J. Ultrasound scanning in the detection of hepatic fibrosis and steatosis. Br. Med. J. (Clin Res. Ed). 292, 13–15 (1986). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xavier, S. A. et al. US-FLI score—is it possible to predict the steatosis grade with an ultrasonographic score? Mol. Genet. Metab.132, 204–209. 10.1016/j.ymgme.2021.01.007 (2021). [DOI] [PubMed] [Google Scholar]
- 35.Otsuka, K. et al. Impact of diabetes mellitus and triglyceride glucose index on mortality and cardiovascular outcomes in patients with chronic coronary syndrome undergoing coronary computed tomography angiography. Int. J. Cardiol. Cardiovasc. Risk Prev.20, 200250. 10.1016/j.ijcrp.2024.200250 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dasarathy, S. et al. Validity of real time ultrasound in the diagnosis of hepatic steatosis: a prospective study. J. Hepatol.51, 1061–1067. 10.1016/j.jhep.2009.09.001 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Agatston, A. S. et al. Quantification of coronary artery calcium using ultrafast computed tomography. J. Am. Coll. Cardiol.15, 827–832. 10.1016/0735-1097(90)90282-t (1990). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data supporting findings of this study are available from the corresponding authors upon reasonable request.