Abstract
Calciprotein particles (CPPs) are an endogenous buffering system, clearing excessive amounts of Ca2+ and PO43- from the circulation and thereby preventing ectopic mineralization. CPPs circulate as primary CPPs (CPP1), which are small spherical colloidal particles, and can aggregate to form large, crystalline, secondary CPPs (CPP2). Even though it has been reported that CPPs are toxic to vascular smooth muscle cells (VSMC) in vitro, their effect(s) on the vasculature remain unclear. Here we have shown that CPP1, but not CPP2, increased arterial stiffness ex vivo. Interestingly, the effects were more pronounced in the abdominal infrarenal aorta compared to the thoracic descending aorta. Further, we demonstrated that CPP1 affected both endothelial and VSMC function, impairing vasorelaxation and contraction respectively. Concomitantly, arterial glycosaminoglycan accumulation was observed as well, which is indicative of an increased extracellular matrix stiffness. However, these effects were not observed in vivo. Hence, we concluded that CPP1 can induce vascular dysfunction.
Subject terms: Calcification, Calcium and vitamin D, Computational models, Mechanisms of disease
Calciprotein particles (CPPs) help clear excess Ca2+ and PO43- to prevent mineralization. However, this study unveils the pathophysiological role of CPPs in arterial stiffness and impaired smooth muscle cell function ex vivo.
Introduction
Physiological mineralization in the human body is restricted to bones, teeth and cartilage. It highly depends upon the metabolism of calcium and phosphate, which is facilitated by many organs (e.g., absorption by the intestines, excretion and reabsorption by the kidneys and (de)mineralization of bone tissue) as extensively reviewed elsewhere1–3. Pathological mineralization can occur spontaneously if calcium and phosphate serum levels are not tightly regulated4. Different inhibitory mechanisms, such as the presence of endogenous inhibitors, continuously prevent the manifestation of ectopic mineralization. Fetuin-A, a liver derived protein, scavenges serum Ca2+ and PO43− and leads to the formation of calciprotein particles (CPPs), thereby preventing mineral precipitation. CPPs are nano-sized protein-mineral complexes that can form spontaneously in fluids containing supersaturated amounts of calcium and phosphate as well as protein. First, a calciprotein monomer (CPM) is formed which consists of small calcium phosphate ion clusters, bound by monomeric fetuin-A. Afterwards, CPMs will combine to form amorphous spherically shaped particles termed primary CPPs (CPP1). In supersaturated conditions, the mineral phase will undergo amorphous-to-crystalline phase transitioning and form secondary CPPs (CPP2), which are large needle-shaped particles (due to the presence of hydroxyapatite crystal). CPMs are cleared from the circulation via the kidneys whereas CPP1 and CPP2 are endocytosed by liver endothelial cells (ECs) and resident liver macrophages, respectively. Both CPMs and CPPs mediate clearance of excessive minerals, preventing their precipitation. The total amount of CPM, CPP1 and CPP2 present in the circulation depends on the health of the organs responsible for their systemic clearance5. The necessity of these anti-calcifying mechanisms becomes clear when observing the dramatic calcification phenotype of DBA/2 mice with a defect in the gene encoding Fetuin-A (Ashg−/−). DBA/2 mice have a deficiency in two important regulators of extracellular matrix mineralization (pyrophosphate and magnesium) and, combined with the Ashg−/− genotype, they exhibit severe and spontaneous soft tissue calcification6. Another example of ectopic calcification due to a lack of endogenous inhibitors are mice lacking matrix GLA protein and/or osteopontin of which the former even displays a lethal phenotype7,8. Hence, there are mechanisms in place that prevent pathological mineralization and a deficiency in this regulation has harmful effects.
In recent years, a clinical role for CPPs has emerged. Indeed, CPPs are important regulators of calcium and phosphate serum concentrations, facilitating rapid clearance of excess minerals from the circulation. However, CPP levels are elevated in patients with chronic kidney disease (CKD) and have been associated with the development of arterial stiffness, atherosclerosis, vascular calcification and rheumatoid arthritis9–13. CPPs are also a strong, independent predictor of mortality in kidney transplant recipients14. In vitro and in vivo studies further demonstrated cytotoxic and inflammatory properties of CPPs. Indeed, CPPs have been shown to induce both lysosomal dysfunction, thereby inhibiting autophagic activity, and NLRP3 inflammasome activation15,16. Regarding the vasculature, CPPs have been found to activate pro-inflammatory pathways in ECs in vitro, often referred to as endothelial dysfunction. Moreover, CPPs stimulate overexpression of cell adhesion molecules VCAM1 and ICAM1 and enhance the secretion of IL-6 and IL-817,18. CPPs also induce VSMC calcification and activate TNFα expression19. All these studies demonstrate that while CPPs are necessary for the clearance of excessive mineral ions, they possess intrinsic cytotoxic properties, albeit weaker compared to inorganic calcium-phosphate crystals17.
Although several studies highlight the pro-inflammatory and cytotoxic nature of CPPs in an in vitro setting, the effect of CPPs on arterial stiffness is poorly studied. Indeed, even though vascular medial calcification is a well-known inducer of arterial stiffening, the involvement of CPPs in aortic stiffness is less clear20,21. One study in patients with CKD reported an association between fetuin-A containing CPPs and aortic pulse wave velocity (PWV), which is a measure of arterial stiffness10. Another study investigated whether CPP2 size affects arterial stiffness and/or arterial medial calcification in patients undergoing haemodialyses, but found no association22. Therefore, more studies are warranted to investigate how CPPs are involved in the pathogenesis of vascular calcification and arterial stiffness and how they can be used as novel therapeutic targets.
The current study aimed to unveil whether CPPs induce arterial stiffening and/or vascular medial calcification when taken out of the pro-calcific milieu in which CPPs are formed (i.e.; mineral imbalance). In this way, effects of the toxic, pro-calcific environment on arterial stiffness are excluded. Because the detrimental effects of CPPs on the vasculature are elusive, the present study additionally aimed to further address this knowledge gap. In vitro and ex vivo (i.e. tissue culture) studies were carried out to assess the pathophysiology of CPP-induced vasculopathy on a molecular, functional and biomechanical level. Lastly, an acute in vivo study was performed to determine whether short-term CPP1/2 exposure induces arterial stiffening.
Results
Calciprotein particles are taken up by vascular cells in vitro
Synthesized CPP1s differed in size and structure compared to CPP2 (Supplementary Fig. 1). HAoECs and HAoSMCs were treated in parallel (i.e. on the same day) with the same batch of freshly prepared CPP1 and CPP2 to evaluate differential CPP uptake. Both HAoECs and HAoSMCs showed a significant increase in fluorescent signal over time, indicative of CPP uptake (Supplementary Fig. 2A). CPP1 and CPP2 were taken up by both cell types. Interestingly, CPP1 uptake was faster in HAoECs than in HAoSMCs (Supplementary Fig. 2B). Whereas both cell types showed affinity for CPP2, there was no time-dependent difference in CPP2 uptake (Supplementary Fig. 2C).
Calciprotein particles induce arterial stiffening ex vivo
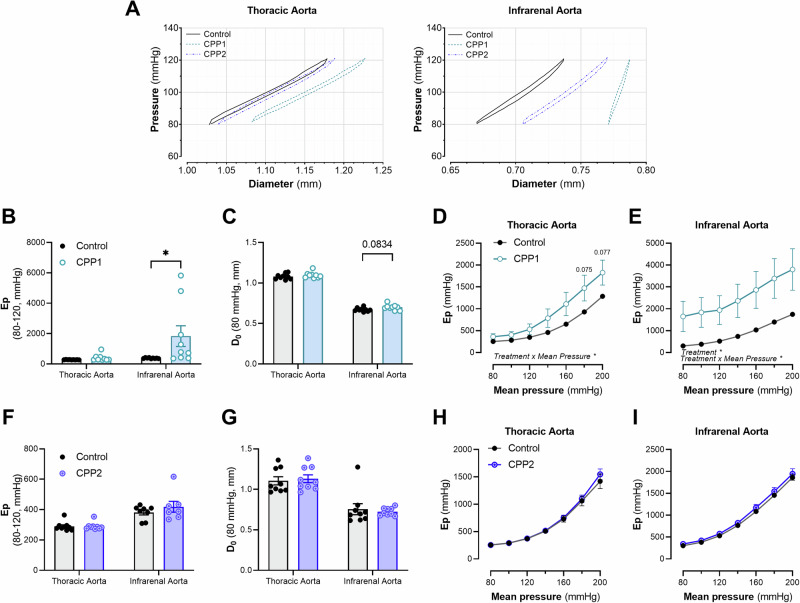
TDA and AIA segments were incubated ex vivo (in the ROTSAC set-up) with CPP1 or CPP2 (100 µg/mL [Ca2+]) for 24 h. Afterwards, the segments were subjected to biomechanical testing. CPP1 increased the Peterson’s modulus (Ep) (i.e., aortic stiffness) at normal, physiological pressures in the AIA, but not in the TDA (Fig. 1A, B). Furthermore, an increase in diameter (at 80 mmHg) after CPP1 treatment was observed in the AIA, but not in the TDA (Fig. 1C), however, this increase in diameter was not significant after statistical analysis (p = 0.084). Ex vivo stiffness was further assessed in isobaric conditions (Ep over a pressure range). Both the TDA and AIA showed a significantly (p < 0.05) increased stiffness over the applied pressure range (60–100 mmHg to 180–220 mmHg) after CPP1 treatment (Fig. 1D, E). There were no changes in biomechanical properties in basal conditions after 24 hours ex vivo CPP2 treatment (Fig. 1F–I). A viscoelastic analysis was performed on pressure-diameter loops at physiological pressure (80–120 mmHg) to further evaluate changes in intrinsic vessel wall properties (Table 1). 24 h of ex vivo incubation with CPP1, but not CPP2, significantly increased (p < 0.001) the viscous modulus of the AIA but not the TDA. Additionally, CPP1 significantly increased (p < 0.01) the elastic modulus of the AIA. CPP1, but not CPP2, changed the intrinsic viscoelastic properties of the AIA at physiological pressures.
Fig. 1. CPP1, but not CPP2, induces arterial stiffness ex vivo.
Aortic segments (from the thoracic descending aorta and the abdominal infrarenal aorta) were incubated with 150 µg/mL [Ca2+]CPP for 24 h in the ROTSAC. A Pressure-diameter tracings, recorded 24 h after CPP exposure in the organ baths. B After 24 h, the stiffness of the infrarenal aorta at physiological pressures (80–120 mmHg, diastolic and systolic pressure) was significantly (p < 0.05) increased after CPP1, but not CPP2 exposure. Whereas there was no change in C diameter after CPP1 exposure, the pressure-stiffness relationship was significantly (p < 0.05) increased in aortic tissue from both the D thoracic descending and E abdominal infrarenal aorta. F–I There were no changes in basal biomechanical properties of aortic tissue after CPP2 incubation. n = 9 (except for F; CPP2-infrarenal aorta; n = 7). Statistical analysis was performed using a two-way mixed ANOVA with a Sidak post hoc test for multiple comparisons. *p < 0.05. Error bars represent standard error of the mean (SEM). CPP1 Primary calciprotein particle, CPP2 Secondary calciprotein particle, D0 Diastolic Diameter, Ep Peterson’s Modulus.
Table 1.
Viscoelastic properties of aortic tissue after incubation (for 24 h) with calciprotein particles
| Viscous modulus (mmHg∙s/mm) | Elastic modulus (mmHg/mm) | |
|---|---|---|
| Thoracic Aorta | ||
| Control | 0.217 ± 0.013 | 276 ± 4 |
| CPP1 | 0.372 ± 0.101 | 398 ± 73 |
| CPP2 | 0.210 ± 0.007 | 274 ± 9 |
| Infrarenal Aorta | ||
| Control | 0.563 ± 0.023 | 612 ± 16 |
| CPP1 | 3.434 ± 1.207*** | 2975 ± 1140** |
| CPP2 | 0.625 ± 0.095 | 620 ± 61 |
The viscous and elastic moduli were calculated from pressure-diameter tracings, recorded between 80 and 120 mmHg diastolic and systolic pressure, respectively. Data are expressed as mean ± standard error of the mean (SEM). n = 9. Statistical analysis was performed using a Two-Way ANOVA with a Tukey post-hoc test for multiple comparisons. Results of multiple comparison (versus “control”) analysis are shown in the table.
CPP1 Primary Calciprotein Particle(s), CPP2 Secondary Calciprotein Particle(s).
**p < 0.01; ***p < 0.001.
Ex vivo incubation with calciprotein particles does not induce aortic mineralization
Calciprotein particles have mostly been investigated in the context of vascular calcification. Surprisingly, no medial mineralization (i.e., Von Kossa positivity on histological cross sections) was observed in either CPP1 or CPP2 treated segments (Supplementary Fig. 3A, B). Therefore, the increased stiffening after CPP1 treatment is not caused by calcification of the medial layer.
Calciprotein particles impair VSMC-mediated active modulation of aortic biomechanics and induce endothelial dysfunction
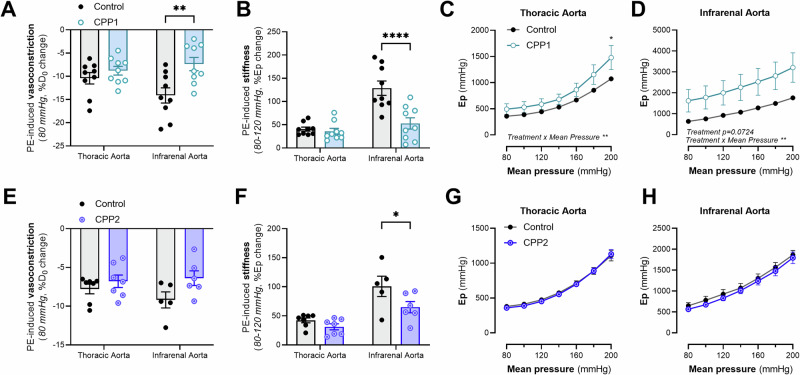
VSMCs play a critical role in regulating the biomechanical properties of the aorta. Hence, we studied the effect of CPPs on VSMCs in aortic tissue ex vivo and on human aortic cells in vitro. VSMC contraction-induced stiffening was studied in aortic rings by stimulating α1-adrenergic VSMC contraction with 2 µM PE. Vasoconstriction was impaired after CPP1 treatment, as indicated by a smaller decrease in diastolic diameter after stimulation with PE (Fig. 2A). Additionally, contraction-induced stiffening was significantly reduced after CPP1 treatment (Fig. 2B). When evaluating the pressure-stiffness relationship, there was a significant (p = 0.043) difference in stiffness of CPP1-treated TDA segments at 200 mmHg and a significant interaction between “Pressure” and “Treatment” on Ep (two-way ANOVA interaction effect, p = 0.0091) (Fig. 2C). Contracted CPP1-treated AIA segments were stiffer over the whole pressure range than their respective controls (p = 0.0724) (Fig. 2D). Interestingly, CPP2 treatment significantly (p = 0.0326) attenuated PE-induced stiffening without decreasing PE-induced vasoconstriction in the AIA (Fig. 2E, F). No changes were observed in the pressure-stiffness relationship in contractile state, after CPP2 incubation in either the TDA or AIA segments (Fig. 2G, H).
Fig. 2. Calciprotein particles impair VSMC-mediated regulation of aortic mechanics.
Aortic segments (from the thoracic descending aorta and the abdominal infrarenal aorta) were incubated with 150 µg/mL [Ca2+]CPP for 24 h in the ROTSAC. Afterwards, aortic segments were stimulated with 2 µM phenylephrine (PE) to induce vascular smooth muscle cell (VSMC) contraction. A–H Biomechanical analysis of aortic segments, incubated with CPPs, after inducing VSMC contraction. Statistical analyses: Two-Way ANOVA with a Sidak post hoc test for multiple comparisons. Non-responders to PE were excluded from the analysis Control/CPP1; n = 9. E–G Control/CPP2 – thoracic aorta; n = 7. E, F, H Control-infrarenal aorta; n = 5. E, F, H CPP2-infrarenal aorta; n = 6. *p < 0.05, **p < 0.01, ****p < 0.0001. Error bars represent standard error of the mean (SEM). PE Phenylephrine, CPP1 Primary calciprotein particle, CPP2 Secondary calciprotein particle.
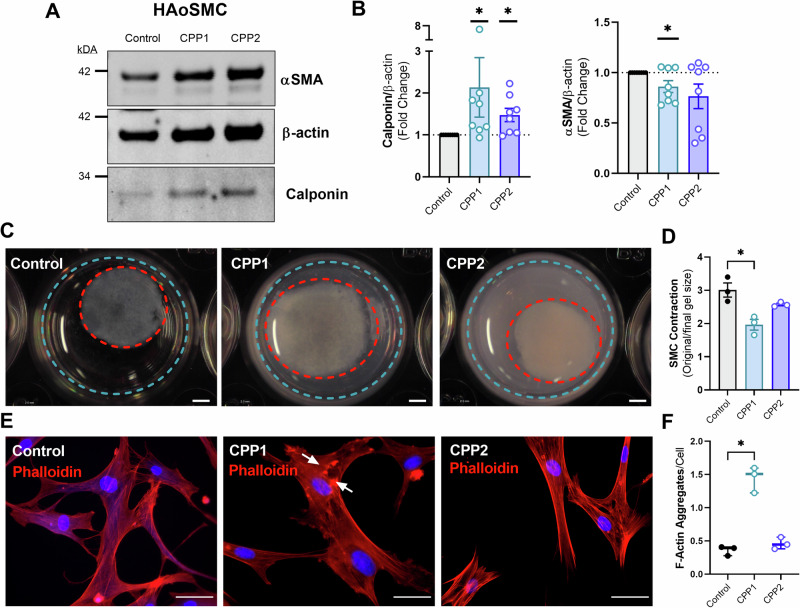
To shed more light on the mechanism of impaired VSMC contraction, western blots were performed. HAoSMCs showed a decrease in the contractile element α-SMA after treatment with CPP1 and an increase in the contraction inhibiting protein calponin after treatment with either CPP1 or CPP2 (Fig. 3A, B). An in vitro collagen contraction assay was employed to further study contractility of HAoSMCs after CPP treatment. The ability of the HAoSMC to decrease the circumference of the gel (i.e. a measure of VSMC contractility) was significantly (p = 0.0146) decreased after 24 h by CPP1, but not by CPP2 treatment (Fig. 3C, D). A phalloidin staining revealed an increased number of F-actin aggregates (p = 0.025) in HAoSMCs treated with CPP1, indicating a dysregulated actin network (Fig. 3E, F).
Fig. 3. Calciprotein particles decrease VSMC contractility and disorganize F-actin network.
Human aortic vascular smooth muscle cells were incubated with CPP1 or CPP2 for 24 h to study the effect of CPPs on VSMC function/contractility. A, B Representative image of western blot of αSMA, β-actin and calponin in HAoSMCs treated for 24 h with CPP1 or CPP2, including analysis graphs. C, D Representative images and analysis of collagen contraction assay after CPP incubation. Scale bar = 2 mm. E, F Representative images and analysis of phalloidin-stained HAoSMCs after 24 h incubation with CPPs to visualize F-actin. White arrows indicate F-actin aggregates. Scale bar = 40 µm. Statistical analyses: B One-sample T test on Log2 transformed data (hypothetical value = 0). n = 8. D, F Kruskal–Wallis test with Dunn’s post hoc test for multiple comparisons. n = 3. *p < 0.05. Error bars represent standard error of the mean (SEM). PE Phenylephrine, (HAo)SMC (Human aortic) smooth muscle cell, CPP1 Primary calciprotein particle, CPP2 Secondary calciprotein particle.
Immunohistochemical staining of infrarenal aortic segments treated with or without CPP1 for 24 h, revealed a significant decrease in luminal CD31 coverage in CPP1 treated segments (Supplementary Fig. 4A, B). We further explored the effect of CPP1 on aortic endothelial cell function by incubating aortic segments for 24 h with CPP1 (150 µg/mL [Ca2+]) in a ROTSAC setup. Afterwards the isobaric conditions were adjusted to isometric conditions to measure isometric force. Maximal VSMC contraction was performed with PE (2 µM) after which acetylcholine (ACh) concentration-response curves (Supplementary Fig. 4C) revealed endothelial dysfunction in CPP1 treated infrarenal aorta segments. Indeed, aortic segments treated with CPP1 did not respond to ACh. Endothelium-independent relaxations were elicited by DEANO concentration-response stimulation after pre-contraction with 2 µM PE and in the presence of 300 µM L-NAME to exclude effects of endogenously produced nitric oxide. There was no difference in DEANO induced relaxation between control and CPP1-treated aortic segments (Supplementary Fig. 4D).
Primary calciprotein particles increase aortic extracellular matrix stiffness
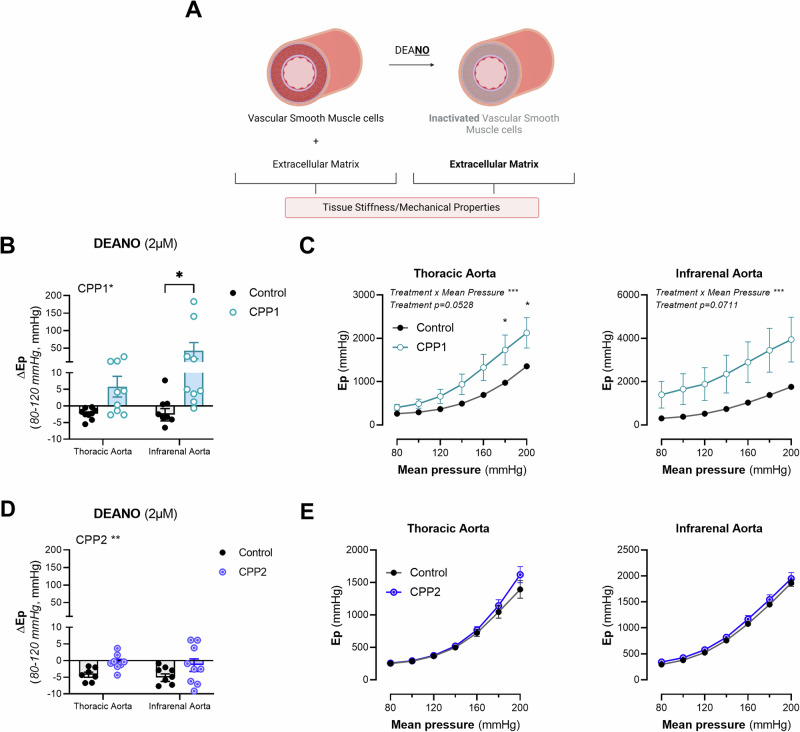
To study passive aortic wall behaviour ex vivo, vasodilators such as DEANO are often used to induce VSMC relaxation (Fig. 4A). 2 µM of DEANO was administered to aortic segments, incubated with either CPP1 or CPP2, in basal, uncontracted state to induce vasodilation and to remove basal tone. Interestingly, CPP1 treatment significantly (p = 0.0291) altered the response of Ep after DEANO administration at 80–120 mmHg (diastolic and systolic pressure respectively). Whereas the stiffness of untreated aortic segments slightly decreased after stimulation with DEANO, segments treated with CPP1 significantly (p = 0.02), and paradoxically, stiffened after vasorelaxation (Fig. 4B). Furthermore, CPP1 treatment altered the pressure-stiffness relationship of the thoracic descending aorta, but this effect was not statistically significant (p = 0.0528) (Fig. 4C). However, a significant (p = 0.0008) interaction effect (between “pressure” and “treatment”) was observed in the TDA. After multiple comparisons, a significantly greater stiffness was observed at high pressure in the CPP1-treated TDA segments (p = 0.0221 at 180 mmHg mean pressure, p = 0.0185 at 200 mmHg mean pressure). Furthermore, whereas CPP2 treatment significantly (p = 0.0096) altered the response of Ep on DEANO at 80–120 mmHg (diastolic and systolic pressure respectively), there were no significant differences after multiple comparisons analysis (Fig. 4D). There were also no significant changes in the pressure x stiffness relationship of either the TDA or AIA after CPP2 treatment (Fig. 4E).
Fig. 4. Vascular smooth muscle cell relaxation/vasodilation with DEANO increases aortic stiffness of CPP1-treated infrarenal aortic segments.
A In basal unstimulated conditions, both the vascular (smooth muscle) cells and the extracellular matrix proteins together define the overall stiffness/biomechanical properties of arterial tissue. However, when a maximum concentration of a vasodilator such as DEANO is administered, the VSMCs relax completely. Consequentially, the ECM becomes the main determinant of the biomechanical properties of the aorta. B Aortic segments, treated for 24 h with CPP1, were challenged with 2 µM DEANO to induce vasodilatation in otherwise unstimulated, ex vivo, conditions. Interestingly, the stiffness of CPP1-treated aortic segments significantly increased after vasorelaxation. C Pressure-stiffness relationship between Ep and pressure for CPP1-treated thoracic descending aorta (TDA) and abdominal infrarenal aorta (AIA) tissue segments. D Additionally, whereas ΔEp in untreated samples was negative after DEANO administration, CPP2-treated segments showed a significantly attenuated response. E Pressure-stiffness relationship between Ep and pressure for CPP2-treated TDA and AIA tissue segments. n = 9, except: [(D, E) control-thoracic aorta; n = 7, (D, E) CPP2-infrarenal aorta; n = 8]. Statistical analysis was performed using a Two-Way ANOVA with a Sidak post hoc test for multiple comparisons. *p < 0.05, ***p < 0.001. Error bars represent standard error of the mean (SEM). ΔEp = Ep after DEANO – Ep before DEANO.
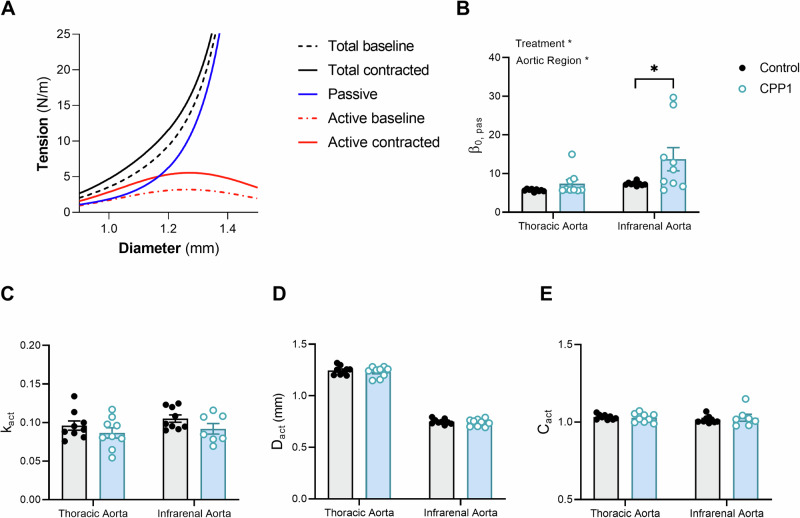
Next, we modelled the effect of CPP1 on arterial biomechanics with a mathematical model that distinguishes passive ECM behaviour from the effect of active VSMC contraction (Fig. 5A). Computational modelling pointed towards a significant increase in the ECM stiffness parameter () after CPP1 treatment in the IRA, but not in the TDA (Fig. 5B), although the treatment led to a larger spread in this parameter for both arteries. Moreover, the contraction parameter () showed a decreasing trend with CPP1 treatment in both arteries, although this was not statistically significant (Fig. 5C). CPP1 treatment did not affect the reference diameter (, Fig. 5D) and the middle of the active curve (, Fig. 5E).
Fig. 5. Computational modelling of the arterial wall revealed increased extracellular matrix stiffening after CPP1 incubation.
A Representative graph of the mathematical model fit for a thoracic descending aortic sample, treated with CPP1. B Aortic segments, treated with CPP1 for 24 h, increased (passive) wall stiffness as seen by an increase in β0, pas. C–E No differences were observed in the other parameters. Statistical analysis was performed using a Two-Way ANOVA with a Sidak post hoc test for multiple comparisons. n = 9, except: E CPP1-infrarenal aorta; n = 7. *p < 0.05. Error bars represent standard error of the mean (SEM).
Primary calciprotein particles increase glycosaminoglycan deposition in the aortic wall of the infrarenal aorta
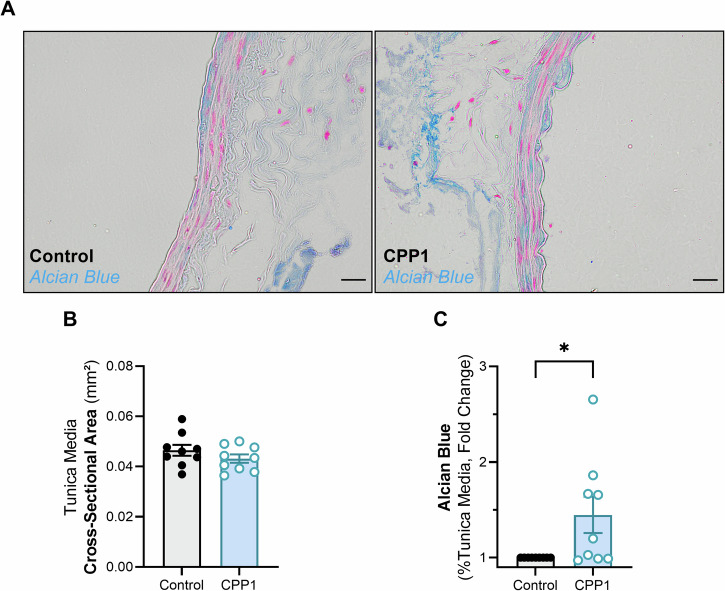
Glycosaminoglycans have recently been shown to be key regulators of aortic biomechanics, especially in the context of aortic swelling and delamination23. Because CPP1 increased the passive wall stiffness of the infrarenal aorta, we examined glycosaminoglycan deposition, through an Alcian Blue staining, in the medial arterial wall (Fig. 6A). The cross-sectional area of the aortic wall between untreated and CPP1 treated aortic segments did not differ (Fig. 6B). However, CPP1 treated segments had a significantly higher amount of Alcian Blue positivity in the medial wall (Fig. 6C).
Fig. 6. CPP1 induces glycosaminoglycan accumulation in the abdominal infrarenal aorta.
A Abdominal infrarenal aortic samples were stained with Alcian Blue to visualize glycosaminoglycans. B There was no significant difference in cross-sectional area of the tunica media between CPP1-treated samples and control. C Interestingly, there was a significantly increased amount of Alcian blue positivity in the tunica media of CPP1-treated infrarenal aortic samples. Scale bar = 20 µm. n = 9. C Statistical analysis was performed using a one sample t-test (hypothetical value = 0) on log2 transformed values. *p < 0.05. Error bars represent standard error of the mean (SEM). CPP1 = Primary calciprotein particle.
Acute in vivo CPP1 treatment only slightly affects passive stiffness of the abdominal infrarenal aorta
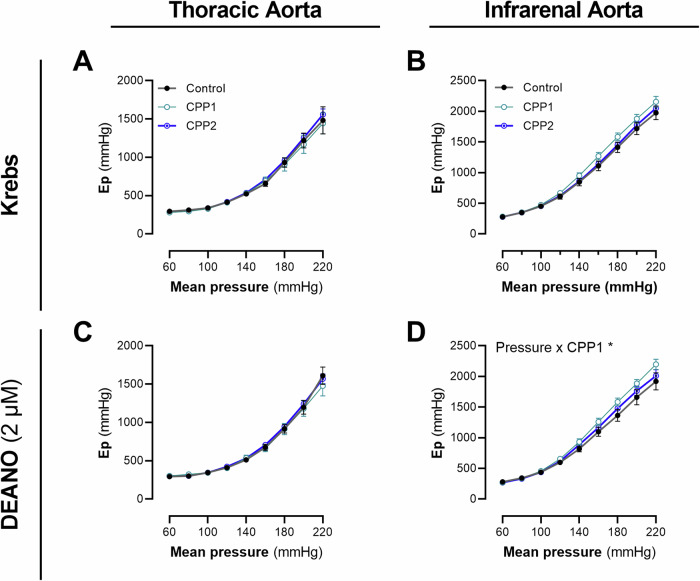
An in vivo study was set up to evaluate the effect of an acute increase in circulating CPPs on the biomechanical properties of the central aorta. Healthy C57Bl6/J mice (n = 6) were injected with CPPs (100 µg Ca2+) i.v. three times per week for two weeks. Afterwards, the thoracic descending aorta and the abdominal infrarenal aorta were subjected to biomechanical testing in a ROTSAC. No differences in stiffness of both the TDA and AIA aorta were observed between CPP1-treated, CPP2-treated and control littermates in basal, unstimulated conditions (Fig. 7A, B). After stimulation with DEANO, to measure passive biomechanics, a significant interaction between Pressure and Treatment was observed on the Ep (ANOVA interaction: p = 0.0452) in the AIA but not in the TDA (Fig. 7C, D).
Fig. 7. Acute in vivo exposure (2 weeks) to CPP1 induces slight changes in passive wall mechanics of the abdominal infrarenal aorta.
Healthy adult male C57Bl6/J mice were injected i.v. with calciprotein particles (100 µg/mL [Ca2+]CPP) three times per week for two weeks. Afterwards, the mice were sacrificed and the aorta was collected for biomechanical testing. No differences were observed in ex vivo stiffness of the thoracic aorta between the different groups in basal conditions (A, C). Also for the infrarenal aorta, no differences were observed in ex vivo stiffness between the different groups (B, D). However, there was a significant pressure x stiffness interaction effect (after DEANO stimulation) in the infrarenal aorta for CPP1 treated mice. n = 6, [except: (A, C) CPP1; n = 4, (D) Control/CPP2, n = 4]. Statistical analyses were performed using Two-Way ANOVA(s) with a Dunett’s post hoc test for multiple comparisons. *p < 0.05. Error bars represent standard error of the mean (SEM). CPP1 Primary Calciprotein Particle, CPP2 Secondary calciprotein particle.
Discussion
In the present study, we have shown that calciprotein particles (CPPs) are taken up by vascular cells (VSMCs and ECs) causing overall cell damage (i.e., reduced VSMC contractility and EC dysfunction). Moreover, CPPs induce ex vivo stiffening of the abdominal infrarenal aorta, but not the thoracic descending aorta. Further experiments and computational modelling of the vessel wall revealed an increase in passive wall stiffness of the infrarenal aorta. Although CPPs did not induce mineralization of the vessel wall, an increase in passive wall components (i.e., glycosaminoglycans) was observed. Despite ex vivo stiffening, an acute increase in CPPs did not result in arterial stiffening in vivo.
In the next section we will discuss the regional differences in CPP-induced arterial stiffening, the effect of CPPs on (vascular) cell function, how CPPs affect passive wall mechanics and finally, the potential (clinical) impact of our results and future directions.
We have demonstrated that 24 h of ex vivo exposure to CPP1, albeit not to CPP2, induced vascular dysfunction. Moreover, biomechanical changes were observed in infrarenal aortic tissue, whereas this was less pronounced in thoracic descending aortic tissue. Thus, there is not only a difference between CPP1 and CPP2 in their ability to induce vascular stiffness, but also an aortic region-specific susceptibility to such stiffening. Regional differences have been described in earlier studies, showing that the abdominal aorta is more sensitive to undergo calcification opposed to the thoracic descending aorta24–27. Both the matrix structure and the molecular signature of the abdominal aorta differs from that of the thoracic aorta, which could play a potential role in these regional differences28–30. Moreover, the abdominal infrarenal aorta has a lower number of elastin fibres, but a higher amount of collagen in its matrix compared to the thoracic descending aorta. Matrix proteins such as elastin and collagen act as solid-phase catalysts and bind calcium31. Therefore, differences in aortic wall composition result in different mineral binding properties. Hence, we speculate that the matrix of the abdominal infrarenal aorta has as a higher affinity for CPPs than that of the thoracic descending aorta.
Alternatively, differences in blood flow patterns between the thoracic and infrarenal aorta are important to consider. More specifically, it has been shown that blood flow velocity and shear stress, are both lower and more oscillatory in the infrarenal aorta compared to the descending aorta32. This is important, as it is known that (oscillatory) wall shear stress has been correlated with inflammatory markers in vascular ECs and is therefore considered a predictor of cardiovascular diseases such as atherosclerotic plaque build-up and aortic aneurysm formation33–35. Therefore, one needs to consider these hemodynamic differences in future studies which focus on unravelling regional differences in CPP-induced vascular damage.
Further, CPP1 is the precursor of CPP2 and both CPP subtypes are simultaneously present in the circulation. However, CPP1 is more abundantly present in vivo than CPP2. In the present study, we have demonstrated that CPP1 is more harmful to the vasculature than CPP2. The discrepancy between CPP subtypes has been reported in another study, where CPP1, but not CPP2, was able to induce systemic inflammation17. Moreover, CPP1 and CPP2 activate distinct signalling cascades, partially due to their different solid states36. CPP1, an amorphous solid, is more dissolvable than the crystalline CPP2, resulting in a higher availability of Ca2+ ions. Therefore, the differential biochemical properties between CPPs could play a role in whether they induce vascular toxicity.
VSMCs are important regulators of the biomechanical properties of the aorta37–39. Because CPP1 was able to induce aortic stiffness, we investigated if (and how) CPPs affect aortic VSMC function. In the present study, we have demonstrated that CPPs affect contractility of both VSMCs in vitro and aortic rings ex vivo. Decreased vasoconstriction after challenging aortic segments with a vasoconstrictor, decreased in vitro contractility of primary isolated aortic VSMCs, and a molecular signature that indicates an abolished contractile apparatus was observed after treatment with both CPP1 and CPP2. A decrease of VSMC contractility in conditions of vascular calcification has been reported in other studies as well, often attributed to a change from a “contractile” VSMC phenotype towards a “osteochondrogenic” phenotype with consequent loss of contractile proteins40,41. However, a change in VSMC phenotype as an explanation for reduced contractility seems unlikely as we observed an increase in calponin levels after CPP treatment, which contradicts phenotype switching. Indeed, contractile proteins should decrease in such an event42. Hence, the decreased contractility of VSMCs is rather a reflection of the toxicity of CPPs, presumably by increasing cytosolic [Ca2+], as seen in studies with hydroxyapatite/CaPi crystals43.
Furthermore, we have demonstrated that CPP1 perturbs the aortic endothelial layer and abolishes acetylcholine-mediated vasorelaxation indicating that CPPs, besides VSMC dysfunction, induce EC dysfunction as well. These results are in line with earlier studies demonstrating that CPPs impair eNOS function and induce a pro-inflammatory response in ECs17,44. Clearly, CPPs are harmful to vascular cells raising the question whether CPPs affect cellular metabolism, such as autophagy, a crucial cellular homoeostatic mechanism45.
Counterintuitively, nitroglycerin-induced vasorelaxation in elderly patients has been found to acutely increase central aortic stiffness46. The authors demonstrated that an increase in ECM stiffness upon ageing is responsible for such a contradictory effect. Indeed, as vasodilation shifts the load from the VSMCs towards the ECM, a stiffer ECM will result in an increased stiffness after vasodilation. In the present study, a similar effect occurred, as ex vivo vasodilation with DEANO resulted in an increase in aortic stiffness. This suggests an increase in ECM stiffness after CPP1 treatment. Surprisingly, no medial/VSMC mineralization was observed in the aortic segments after CPP treatment and is therefore not the cause of the observed increase in tissue stiffness. This is in strong contrast with previously published in vitro studies where CPPs have been shown to mineralize cell monolayers after 24 h19,47,48. This could be due to differential cellular uptake of CPPs between cell monolayers and aortic tissue. Furthermore, constant aeration of the organ baths creates turbulence, thereby preventing passive mineralisation through gravity, as it might occur in conventional cell culture experiments. Since mathematical modelling revealed increased passive wall stiffness in the infrarenal aorta after CPP1 incubation (increased parameter) in the absence of medial mineralization, another mechanism is activated by CPP1 resulting in increased ECM stiffness. Interestingly, a higher amount of glycosaminoglycans (GAGs) was observed in the medial layer of the infrarenal aorta after CPP1 incubation. Indeed, GAG accumulation in the ECM has been associated with increased aortic stiffness and local inflammation, as well as with elastic lamellae delamination49–51. Furthermore, the overproduction of GAGs has been shown to induce vascular calcification as well52,53. Oxidative stress is a known trigger for TGF-β expression which, in turn, induces GAG synthesis54,55. Thus, if CPPs indeed increase oxidative stress in vascular cells, ECM remodelling (e.g., GAG deposition due to either increased production or reduced breakdown) can be expected. Therefore, the toxic effects of CPPs predispose mineralization and could therefore play a role in creating a “pro-calcific” environment that would eventually lead to aortic wall calcification. However, the exact mechanisms causing and/or leading to such events, remain elusive and warrant further research to uncover novel targets in treating vascular calcification.
Arterial stiffening and vascular calcification increase the risk for cardiovascular events in patients56,57. However, in the present study, a two-week exposure to CPPs in vivo did not alter the biomechanical properties of the central aorta. Even though we have demonstrated that short term CPP exposure ex vivo and in vitro causes vascular dysfunction, this did not occur to the same extent in vivo. There are several possibilities that could explain these dissonant results such as a different local residence time of CPPs (i.e., continuous presence ex vivo and in vitro vs. rapid systemic clearance in vivo). Nevertheless, it is not surprising that an acute increase in CPPs did not result in arterial stiffening in our in vivo study. CPP formation is a physiological process that occurs due to the absorption of minerals (for example via the diet). Because CPPs are efficiently cleared in healthy individuals (and mice), their residence time in the circulation is probably too low to induce vascular toxicity58. Therefore, future studies should investigate whether a longer exposure to high circulating amounts of CPPs, as seen as in patients with reduced renal function, leads to vascular injury. Alternatively, one could investigate synergistic effects of CPP administration with an experimental model of reduced clearance (e.g., kidney dysfunction)58,59. This would shed light on whether chronic CPP exposure, either through the diet or due to increased endogenous production, in combination with reduced systemic clearance would result in the toxic effects that we observed ex vivo.
Lastly, it needs to be mentioned that data on circulating CPP concentrations are lacking, but very much needed. Measuring T50 (i.e., the transition time from CPP1 to CPP2) is relatively fast and effective in estimating in vivo CPP burden in patients, yet its translation to CPP composition and concentration remains ambiguous60. A recently established flow cytometry-based technique makes quantification of circulating CPPs now a possible, even in animal experiment studies61. This technique offers an important future perspective in order to further elucidate the role of CPPs in cardiovascular disease.
In conclusion, calciprotein particles (and more specifically; CPP1) induce arterial stiffness ex vivo, which is more pronounced in the abdominal infrarenal aorta as compared to the thoracic descending aorta. However, an acute in vivo treatment was not associated with arterial stiffening. Lastly, CPPs induce vascular cell toxicity as demonstrated by EC dysfunction and decreased VSMC contractility. These results suggest that prolonged exposure to CPPs can be toxic to the vasculature and can induce arterial stiffening. Hence, the monitoring of CPP levels (or CPP residence time) could be of clinical value as a predictor of cardiovascular risk and should be further explored as a potential biomarker for vascular toxicity and local arterial stiffening.
While this study provides valuable insights into the effects of calciprotein particles (CPPs) on vascular function, several limitations should be acknowledged. Because of the extensive testing protocol that was used to assess aortic biomechanics, we were not able to evaluate dose-dependent effects of CPPs on arterial function. Concentration-dependent effects of CPPs on endothelial function have been described44. The concentration that was used in our experiments is widely used across different research groups, which allows comparison of our data to other, scarcely available, CPP studies15,44,47,62. Secondly, aortic segments were incubated with CPPs in the ROTSAC setup for 24 h using regular Krebs-Ringer buffer. Damage to the tissue by the wires of the setup cannot be excluded and explains why luminal CD31 coverage in control samples was not 100% (but rather 70%). Nonetheless, all aortic segments were exposed to the same experimental conditions. Thirdly, we only used male C57Bl6/J mice in our acute in vivo study. Therefore, not only the use of C57Bl6/J mice (which are naturally more resistant to the development of calcifications) but also possible sex-related differences should be taken into account. Indeed, it has been shown that female mice have a different susceptibility for developing calcification compared to male mice63. Moreso, differences in T50 values distributions between males and females have been reported, but its significance is not fully understood64. Hence, future studies should include “sex” as a parameter in unravelling the pathophysiology of CPP-induced arterial stiffening.
Methods
Synthesis and characterization of calciprotein particles
Calciprotein particles were generated in vitro in pre-warmed DMEM (w/o phenol red), supplemented with 10% heat-inactivated FBS. Phosphate (NaH2PO4/Na2HPO4,19%/81% mixture, 1 M) and calcium (CaCl2,1 M) stock solutions were used to increase [PO43−] and [Ca2+] in the DMEM/FBS solution to 3.5 mM and 1 mM, respectively. Phosphate was added first, followed by vortexing, after which calcium was added, followed again by vortexing. The solution was incubated at 37 °C for either 1 day or 7 days to generate primary (CPP1) or secondary (CPP2) CPPs, respectively. The CPP-containing solution was centrifuged at 20,000 × g for 90 min. The CPP-containing pellet was resuspended in sterile phosphate-buffered saline (PBS) to generate a “CPP batch”. CPP batches were freshly prepared for every individual experiment, since freeze-thaw cycles would affect CPP stability and function. Calcium content of the CPPs was measured by flame atomic absorption spectrometry (FAAS), as described elsewhere65. In addition, CPPs were visualized with transmission electron microscopy (TEM) as described by Köppert et al.62. Briefly, CPPs were diluted 1:10 in MilliQ water after which a sample (1 µL) was applied on Formvar-coated nickel grids. The samples were dried at room temperature after which they were imaged via a Tecnai G2 Spirit Bio TWIN microscope (Thermo Fisher Scientific, Eindhoven, The Netherlands) at 120 kV, without staining.
Animals
For ex vivo treatment with CPPs, aortas were collected from 6-month-old male C57Bl6/J mice (n = 9). Additionally, 6-month-old male C57Bl6/J mice were treated with 100 µg/mL [Ca2+] CPP1, CPP2 or saline through i.v. injections in the tail vein (n = 6 per group; 100 µl/mouse). Mice were injected 3×/week for 2 weeks to study the acute effect of CPPs on the vasculature. The mice were sacrificed after 2 weeks by an intraperitoneal injection of sodium pentobarbital (200 mg/kg; Sanofi, Machelen, Belgium), followed by perforation of the diaphragm whilst under deep anaesthesia (confirmed by a complete loss of reflexes). Aortic tissue from the thoracic descending aorta and the abdominal infrarenal aorta were harvested from the animal, stripped from adipose tissue and subjected to biomechanical testing. All animals were housed in the animal facility of the University of Antwerp in standard cages with 12 h–12 h light-dark cycles and had free access to regular chow and tap water. The study was conducted in accordance with the Declaration of Helsinki and was approved by the ethics committee of the University of Antwerp (file number: 2022-44). Hence, we have complied with all relevant ethical regulations for animal use.
Ex vivo aortic biomechanics and vascular function
The thoracic descending aorta (TDA) and the abdominal infrarenal aorta (AIA) were removed from the mice and cleaned from adipose tissue and cut each in a set of 2 mm segments. The segments were immersed in Krebs Ringer solution (37 °C, 95% O2/5% CO2, pH 7.4) containing (in mM): NaCl 118, KCl 4.7, CaCl2 2.5, KH2PO4 1.2, MgSO4 1.2, NaHCO3 25, CaEDTA 0.025 and glucose 11.1. Two TDA and two AIA segments were analysed simultaneously using an in-house developed rodent oscillatory tension set-up for measuring arterial compliance (ROTSAC)66. In brief, aortic segments were mounted between two parallel wire hooks in organ baths of 10 mL. Displacement of the upper hook was measured with a force-length transducer. The segments were continuously stretched, oscillating between alternating preloads, corresponding to a “diastolic” and “systolic” transmural pressure at a frequency of 10 Hz. Calibration of displacement of the upper hook allowed the calculation of the vessel diameter. The Laplace relationship was used to calculate the transmural pressure from the distension force and vessel dimensions:
With F the force, l the length and D the diameter of the vessel segment. While pressure is not generated in the ROTSAC setup, the calculated pressure has been successfully used in previous research, with good translation to in vivo PWV measurements in mice. At any given pressure, calibration of the upper hook allowed calculation of the vessel diameter (both systolic and diastolic diameter) and stiffness of the aortic segments was quantified through the Peterson’s pressure-strain modulus of elasticity (Ep). The Ep was calculated as follows:
With D0 as the diastolic diameter, ∆D the distension and ∆P the pulse pressure.
One TDA and one AIA segment, originating from the same animal, were incubated with CPPs (CPP1 or CPP2, 150 µg/mL [Ca2+]) at 37 °C in the ROTSAC for 24 hours under continuous cyclic loading, whereas the other TDA and AIA segment served as controls and were exposed to the same conditions (with exception of the presence of CPPs). After the incubation period, CPPs were washed out of the organ baths after which the aortic segments were subjected to biomechanical testing. Furthermore, segments were challenged with phenylephrine (PE, 2 µM), an α1-adrenergic agonist, to induce vascular smooth muscle cell (VSMC) contraction. Contractions were performed under dynamic conditions to measure vasoconstriction and contraction-induced stiffness. VSMC relaxation experiments were conducted under isometric conditions. Endothelium-dependent relaxations were evaluated by adding cumulative concentrations of acetylcholine (ACh, 3 nM–10 μM), a muscarinic receptor agonist, on pre-contracted aortic rings (PE 2 µM). Endothelium-independent relaxations were measured using the nitric oxide donor Diethylamine Nitric Oxide (DEANO, 2 µM) on pre-contracted segments.
Pressure-diameter relationships, generated by the ROTSAC, were used to analyse viscoelasticity, by using an adapted Kelvin-Voigt model as previously described30,67. This model separates the total pressure-diameter relationship in an elastic and a viscous component. These components can be calculated using the following formula:
Where and are the viscous and inertial modulus, respectively. For each “raw” pressure-diameter tracing obtained from the ROTSAC, the viscous and inertial modulus were manually iterated to obtain the minimum area of hysteresis. The corresponding value was considered to be a measure of the amount of hysteresis in the sample. Afterwards, the slope of the resulting, elastic, pressure-diameter relationship was considered as the elastic modulus ().
The pressure-diameter data from the ROTSAC experiments were used as input to investigate the distinct effect of CPP1 on the extracellular matrix (ECM) and VSMCs by in silico modelling. A physiologically relevant mathematical model of the arterial wall which accounts for the summed contribution of the ECM and VSMCs was fitted to the experimental data at baseline and after PE-induced contraction68. The ECM pressure-diameter relationship is mathematically described by an exponential curve. VSMC force generation is length-dependent, due to the design of the contractile apparatus. In the model, this length-dependency is captured by a Gaussian-shaped curve, resulting in the following model equation:
where is the wall tension, calculated from the diameter () and pressure () data (); is the arterial outer diameter; and are the reference pressure (fixed at 80 mmHg) and corresponding diameter, respectively; is a passive ECM stiffness parameter; and govern the contractile state of VSMCs (); is a normalized VSMC optimal working length; and governs the width of the Gaussian function. In the final fitting procedure, was kept constant because of its large confidence intervals when included in the fitting routine. We chose to set = 0.26, i.e., the average value obtained in this preliminary analysis. The model parameters were fitted to the experimental data with the MATLAB (The MathWorks Inc. R2021b) nonlinear least-squares solver lsqnonlin. Furthermore, because of the potential existence of different local minima in the optimization function, we used the MATLAB function MultiStart to repeat the nonlinear least-square optimization starting from n = 100 different initial parameter guesses.
In vitro uptake of calcein-labelled calciprotein particles
Human aortic endothelial cells (HAoECs, Merck Life Sciences, 304-05A) and human aortic vascular smooth muscle cells (HAoSMCs, Merck Life Sciences, 354-05A) were used to study CPP uptake in vascular cells. CPPs were labelled with non-membrane permeable calcein (Merck Life Sciences, C0875) to allow for fluorescent detection of CPPs. Labelling was performed by supplementing the CPP solution with 15 µM calcein. Calcein fluorescence (i.e., CPP uptake) was measured at different timepoints after incubation with calcein-labelled CPPs through fluorescent imaging using a CELENA S fluorescence microscope (Logos Biosystems).
Collagen gel contraction assay
VSMC contraction was measured in vitro after incubation with CPPs. In this way, there was no interference from interactions with either ECs or with the complex aortic extracellular matrix. HAoSMCs (Merck Life Sciences, 354-05A) were used for this experiment (passage 5–7). The protocol was adapted from Pincha et al.69. In brief, 1.2 mL of cell suspension (75,000 cells) in cell culture medium (smooth muscle cell growth medium; Sigma-Aldrich, 311–500) was mixed with 600 µL rat tail collagen 1 (3 mg/mL, Thermo Fisher Scientific, A1048301) on ice. 15 µL 1 M NaOH was added to the suspension to induce gel solidification. This solution was divided over three wells of a 24 well plate. The plate was left for 30 minutes at room temperature to induce gel formation. After the gels solidified, 500 µL of cell culture medium was added on top of the gels. After 24 h incubation with CPPs (CPP1 and CPP2, 100 µg/mL [Ca2+]) the gels were dissociated from the well plate walls by gentle scraping at the borders with a 10 µL pipette tip. Afterwards, 2 µM PE was added to induce VSMC contraction. After a 24 h incubation with PE at 37 °C in 95% air/5% CO2, pictures of the gels were taken with a Dino-Lite edge Digital Microscope. Both the circumference of the well plate (i.e., the original circumference of the gel, before contraction) and the circumference of the contracted gel were measured using a Dino-Lite digital microscope and DinoCapture 2.0 software. The change in circumference of the gel was considered a measure of VSMC contractility.
Western blotting
HAoECs and HAoSMCs were collected in Laemmli sample buffer (Bio-Rad), supplemented with β-mercaptoethanol (5%), to facilitate cell lysis. Samples were heat-denatured for 5 min at 100 °C. Afterwards, samples were loaded on Bolt 4–12% bis-tris gels (Invitrogen) for gel electrophoresis, followed by wet transfer on polyvinylidene fluoride membranes. Membranes were blocked in Odyssey® Blocking Buffer (Li-Cor Bioscience) and probed with primary antibody (overnight, 4 °C). The following primary antibodies and dilutions were used: anti-α-smooth muscle cell actin (1/1000 dilution, Abcam; ab5694), anti-β-actin (1/2000 dilution, Abcam; 8226), anti-calponin (1/1000 dilution, DAKO; M3556) and anti-CD31 (1/100 dilution, Cell Signalling; #77699). Subsequentially, (IR)-conjugated secondary antibodies (anti-rabbit: IgG926-32211 and anti-mouse: IgG926-68070; Li-Cor Biosciences) were used for IR fluorescence detection using an Odyssey SA infrared imaging system (LI-COR Biosciences). Western blot signal was analysed using Image Studio Lite (LI-COR).
Histology
After biomechanical testing in the ROTSAC, aortic tissues were carefully removed from the organ baths and fixated in 4% formol (PBS buffered). After 24 h of fixation, samples were transferred to well plates with 60% isopropanol followed by paraffin-embedding. Transversal sections were stained with a Von Kossa (VK) staining to visualize mineralization and Alcian Blue (AB) to detect glycosaminoglycans (%area positivity of VK or AB in medial aortic layer). As both control and CPP-treated samples originated from the same animal, comparisons were made between corresponding samples (i.e., fold change). Additionally, an immunohistochemical staining for CD31 was performed on abdominal infrarenal aortic tissue to evaluate luminal endothelial cell coverage (coverage = the length of CD31 positive cells along the luminal border as percentage of the total perimeter). Rabbit anti-mouse CD31/PECAM-1 (1/100 dilution) was purchased from Cell Signalling (#77699). All images were acquired with Universal Grab 6.1 software using an Olympus BX40 microscope. Image analysis was conducted in Fiji/ImageJ.
Statistics and reproducibility
All results are expressed as the mean ± SEM with n representing the number of mice. Analyses were performed using GraphPad Prism 10.0 (GraphPad Software, La Jolla, CA, USA). The statistical tests used in the experiments are mentioned in the corresponding figure legends. A 5% level of significance was selected. All attempts at replication were successful.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Supplementary information
Description of additional supplementary files
Acknowledgements
C.H.G.N. was funded by the Fund for Scientific Research (FWO)-Flanders (grant number: 1S24720N) and by the Research Council of the University of Antwerp (BOF UAntwerp ID: 48316). C.D.W. is an Early-Stage Researcher (ESR) in the INSPIRE project, which has received funding from the European Union’s Horizon 2020 Research and Innovation Program (H2020-MSCA-ITN program, Grant Agreement: No858070). C.v.L. is funded by a CARIM School for Cardiovascular Diseases 2021 PhD fellowship. L.R. is funded by the Research Council of the University of Antwerp (BOF UAntwerp ID: 45846) and the FWO-Flanders (G060723N). W.M. and G.R.Y.D.M received funding from the iBOF (interuniversitair Bijzonder Onderzoeksfonds) ATLANTIS (grant iBOF-21-053). W.H.D.V. received funding from the FWO-Flanders (IRI: I000321N).
Author contributions
Conceptualization: Cédric H.G. Neutel, Pieter-Jan Guns, Wim Martinet. Methodology: Cédric H.G. Neutel, Callan D. Wesley, Cindy van Loo, Winnok De Vos. Software: Winnok De Vos. Investigation: Cédric H.G. Neutel, Callan D. Wesley, Cindy van Loo, Celine Civati, Michelle Zurek. Formal analysis: Cédric H.G. Neutel, Cindy van Loo, Freke Mertens, Isabel Pintelon. Visualisation: Cédric H.G. Neutel. Writing - original draft preparation: Cédric H.G. Neutel. Writing - review and editing: Callan D. Wesley, Anja Verhulst, Bart Spronck, Winnok De Vos, Lynn Roth, Guido R.Y. De Meyer, Wim Martinet, Pieter-Jan Guns.
Peer review
Peer review information
Communications biology thanks the anonymous reviewers for their contribution to the peer review of this work. Primary Handling Editors: Jesmond Dalli and Dario Ummarino. A peer review file is available.
Data availability
The numerical source data that make up the graphs and charts as well as the uncropped/unedited western blot images are available in Supplementary Data 1. Other datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s42003-024-06895-y.
References
- 1.Peacock, M. Calcium metabolism in health and disease. Clin. J. Am. Soc. Nephrol.5, S23–S30 (2010). [DOI] [PubMed] [Google Scholar]
- 2.Peacock, M. Phosphate Metabolism in Health and Disease. Calcif. Tissue Int.108, 3–15 (2021). [DOI] [PubMed] [Google Scholar]
- 3.Renkema, K. Y., Alexander, R. T., Bindels, R. J. & Hoenderop, J. G. Calcium and phosphate homeostasis: concerted interplay of new regulators. Ann. Med.40, 82–91 (2008). [DOI] [PubMed] [Google Scholar]
- 4.Smith, E. R., Hewitson, T. D. & Jahnen-Dechent, W. Calciprotein particles: mineral behaving badly? Curr. Opin. Nephrol. Hypertens.29, 378–386 (2020). [DOI] [PubMed] [Google Scholar]
- 5.Jahnen-Dechent, W. & Smith, E. R. Nature’s remedy to phosphate woes: calciprotein particles regulate systemic mineral metabolism. Kidney Int.97, 648–651 (2020). [DOI] [PubMed] [Google Scholar]
- 6.Babler, A. et al. Microvasculopathy and soft tissue calcification in mice are governed by fetuin-A, magnesium and pyrophosphate. PLoS One15, e0228938 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Luo, G. et al. Spontaneous calcification of arteries and cartilage in mice lacking matrix GLA protein. Nature386, 78–81 (1997). [DOI] [PubMed] [Google Scholar]
- 8.Speer, M. Y. et al. Inactivation of the osteopontin gene enhances vascular calcification of matrix Gla protein-deficient mice: evidence for osteopontin as an inducible inhibitor of vascular calcification in vivo. J. Exp. Med.196, 1047–1055 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smith, E. R. et al. Serum fetuin-A concentration and fetuin-A-containing calciprotein particles in patients with chronic inflammatory disease and renal failure. Nephrology18, 215–221 (2013). [DOI] [PubMed] [Google Scholar]
- 10.Smith, E. R. et al. Phosphorylated fetuin-A-containing calciprotein particles are associated with aortic stiffness and a procalcific milieu in patients with pre-dialysis CKD. Nephrol. Dial. Transpl.27, 1957–1966 (2012). [DOI] [PubMed] [Google Scholar]
- 11.Nakazato, J. et al. Association of calciprotein particles measured by a new method with coronary artery plaque in patients with coronary artery disease: A cross-sectional study. J. Cardiol.74, 428–435 (2019). [DOI] [PubMed] [Google Scholar]
- 12.Chen, W. et al. Patients with advanced chronic kidney disease and vascular calcification have a large hydrodynamic radius of secondary calciprotein particles. Nephrol. Dial. Transpl.34, 992–1000 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jäger, E. et al. Calcium-sensing receptor-mediated NLRP3 inflammasome response to calciprotein particles drives inflammation in rheumatoid arthritis. Nat. Commun.11, 4243 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dahle, D. O. et al. Serum Calcification Propensity Is a Strong and Independent Determinant of Cardiac and All-Cause Mortality in Kidney Transplant Recipients. Am. J. Transpl.16, 204–212 (2016). [DOI] [PubMed] [Google Scholar]
- 15.Kunishige, R. et al. Calciprotein particle-induced cytotoxicity via lysosomal dysfunction and altered cholesterol distribution in renal epithelial HK-2 cells. Sci. Rep.10, 20125 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Anzai, F. et al. Calciprotein Particles Induce IL-1β/α-Mediated Inflammation through NLRP3 Inflammasome-Dependent and -Independent Mechanisms. Immunohorizons5, 602–614 (2021). [DOI] [PubMed] [Google Scholar]
- 17.Shishkova, D. et al. Calciprotein Particles Cause Physiologically Significant Pro-Inflammatory Response in Endothelial Cells and Systemic Circulation. Int. J. Mol. Sci.23, 14941 (2022). [DOI] [PMC free article] [PubMed]
- 18.Shishkova, D. K. et al. Calciprotein Particles Link Disturbed Mineral Homeostasis with Cardiovascular Disease by Causing Endothelial Dysfunction and Vascular Inflammation. Int J. Mol. Sci.22, 12458 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aghagolzadeh, P. et al. Calcification of vascular smooth muscle cells is induced by secondary calciprotein particles and enhanced by tumor necrosis factor-α. Atherosclerosis251, 404–414 (2016). [DOI] [PubMed] [Google Scholar]
- 20.Van den Bergh, G., Opdebeeck, B., D’Haese, P. C. & Verhulst, A. The Vicious Cycle of Arterial Stiffness and Arterial Media Calcification. Trends Mol. Med.25, 1133–1146 (2019). [DOI] [PubMed] [Google Scholar]
- 21.Chen, Y., Zhao, X. & Wu, H. Arterial Stiffness: A Focus on Vascular Calcification and Its Link to Bone Mineralization. Arterioscler Thromb. Vasc. Biol.40, 1078–1093 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen, W. et al. Associations of Serum Calciprotein Particle Size and Transformation Time With Arterial Calcification, Arterial Stiffness, and Mortality in Incident Hemodialysis Patients. Am. J. Kidney Dis.77, 346–354 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ghadie, N. M., St-Pierre, J. P. & Labrosse, M. R. The Contribution of Glycosaminoglycans/Proteoglycans to Aortic Mechanics in Health and Disease: A Critical Review. IEEE Trans. Biomed. Eng.68, 3491–3500 (2021). [DOI] [PubMed] [Google Scholar]
- 24.Muyor, K. et al. Vascular calcification in different arterial beds in ex vivo ring culture and in vivo rat model. Sci. Rep.12, 11861 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ameer, O. Z., Salman, I. M., Avolio, A. P., Phillips, J. K. & Butlin, M. Opposing changes in thoracic and abdominal aortic biomechanical properties in rodent models of vascular calcification and hypertension. Am. J. Physiol. Heart Circ. Physiol.307, H143–H151 (2014). [DOI] [PubMed] [Google Scholar]
- 26.Sørensen, I. M. H. et al. Regional distribution and severity of arterial calcification in patients with chronic kidney disease stages 1-5: a cross-sectional study of the Copenhagen chronic kidney disease cohort. BMC Nephrol.21, 534 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jadidi, M. et al. Calcification prevalence in different vascular zones and its association with demographics, risk factors, and morphometry. Am. J. Physiol. Heart Circ. Physiol.320, H2313–h23 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Concannon, J. et al. Quantification of the regional bioarchitecture in the human aorta. J. Anat.236, 142–155 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Suslov, A. V., et al. Molecular Pathogenesis and the Possible Role of Mitochondrial Heteroplasmy in Thoracic Aortic Aneurysm. Life. 11, 14941 (2021). [DOI] [PMC free article] [PubMed]
- 30.Neutel, C. H. G., Wesley, C. D., De Meyer, G. R. Y., Martinet, W. & Guns, P.-J. The effect of cyclic stretch on aortic viscoelasticity and the putative role of smooth muscle focal adhesion. Front. Physiol.14, 1218924 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Villa-Bellosta, R. & O’Neill, W. C. Pyrophosphate deficiency in vascular calcification. Kidney Int.93, 1293–1297 (2018). [DOI] [PubMed] [Google Scholar]
- 32.Trenti, C. et al. Wall shear stress and relative residence time as potential risk factors for abdominal aortic aneurysms in males: a 4D flow cardiovascular magnetic resonance case–control study. J. Cardiovasc. Magn. Reson.24, 18 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pedersen, E. M. et al. Distribution of early atherosclerotic lesions in the human abdominal aorta correlates with wall shear stresses measured in vivo. Eur. J. Vasc. Endovasc. Surg.18, 328–333 (1999). [DOI] [PubMed] [Google Scholar]
- 34.Katoh, K. Effects of Mechanical Stress on Endothelial Cells In Situ and In Vitro. Int. J. Mol. Sci.24, 16518 (2023). [DOI] [PMC free article] [PubMed]
- 35.Zhou, G., Zhu, Y., Yin, Y., Su, M. & Li, M. Association of wall shear stress with intracranial aneurysm rupture: systematic review and meta-analysis. Sci. Rep.7, 5331 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kutikhin, A. G. et al. Calciprotein Particles: Balancing Mineral Homeostasis and Vascular Pathology. Arterioscler Thromb. Vasc. Biol.41, 1607–1624 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Leloup, A. J. A. et al. Vascular smooth muscle cell contraction and relaxation in the isolated aorta: a critical regulator of large artery compliance. Physiol. Rep.7, e13934 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Neutel, C. H. G. et al. High Pulsatile Load Decreases Arterial Stiffness: An ex vivo Study. Front. Physiol.12, 741346 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kochová, P. et al. The contribution of vascular smooth muscle, elastin and collagen on the passive mechanics of porcine carotid arteries. Physiol. Meas.33, 1335–1351 (2012). [DOI] [PubMed] [Google Scholar]
- 40.Van den Bergh, G. et al. Endothelial dysfunction aggravates arterial media calcification in warfarin administered rats. FASEB J.36, e22315 (2022). [DOI] [PubMed] [Google Scholar]
- 41.Shi, J., Yang, Y., Cheng, A., Xu, G. & He, F. Metabolism of vascular smooth muscle cells in vascular diseases. Am. J. Physiol. Heart Circ. Physiol.319, H613–h31 (2020). [DOI] [PubMed] [Google Scholar]
- 42.Rensen, S. S., Doevendans, P. A. & van Eys, G. J. Regulation and characteristics of vascular smooth muscle cell phenotypic diversity. Neth. Heart J.15, 100–108 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ewence, A. E. et al. Calcium phosphate crystals induce cell death in human vascular smooth muscle cells: a potential mechanism in atherosclerotic plaque destabilization. Circ. Res.103, e28–e34 (2008). [DOI] [PubMed] [Google Scholar]
- 44.Feenstra, L. et al. Calciprotein Particles Induce Endothelial Dysfunction by Impairing Endothelial Nitric Oxide Metabolism. Arterioscler. Thromb. Vasc. Biol.43, 443–455 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Neutel, C. H. G., Hendrickx, J. O., Martinet, W., De Meyer, G. R. Y. & Guns, P. J. The Protective Effects of the Autophagic and Lysosomal Machinery in Vascular and Valvular Calcification: A Systematic Review. Int. J. Mol. Sci.21, 8933 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pewowaruk, R. J., Hein, A. J., Carlsson, C. M., Korcarz, C. E. & Gepner, A. D. Effects of nitroglycerin-induced vasodilation on elastic and muscular artery stiffness in older Veterans. Hypertens. Res.45, 1997–2007 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ter Braake, A. D. et al. Calciprotein particle inhibition explains magnesium-mediated protection against vascular calcification. Nephrol. Dial. Transpl.35, 765–773 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zeper, L. W. et al. Calciprotein Particle Synthesis Strategy Determines In Vitro Calcification Potential. Calcif. Tissue Int.112, 103–117 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Humphrey, J. D. Possible mechanical roles of glycosaminoglycans in thoracic aortic dissection and associations with dysregulated transforming growth factor-β. J. Vasc. Res.50, 1–10 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chai, S. et al. Overexpression of Hyaluronan in the Tunica Media Promotes the Development of Atherosclerosis. Circ. Res.96, 583–591 (2005). [DOI] [PubMed] [Google Scholar]
- 51.Ahmadzadeh, H., Rausch, M. K. & Humphrey, J. D. Particle-based computational modelling of arterial disease. J. R. Soc. Interface15, 20180616 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Purnomo, E. et al. Glycosaminoglycan overproduction in the aorta increases aortic calcification in murine chronic kidney disease. J. Am. Heart Assoc.2, e000405 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bramsen, J. A. et al. Glycosaminoglycans affect endothelial to mesenchymal transformation, proliferation, and calcification in a 3D model of aortic valve disease. Front. Cardiovasc. Med.9, 975732 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liu, R. M. & Gaston Pravia, K. A. Oxidative stress and glutathione in TGF-beta-mediated fibrogenesis. Free Radic. Biol. Med.48, 1–15 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.D’Angelo, M. & Greene, R. M. Transforming growth factor-beta modulation of glycosaminoglycan production by mesenchymal cells of the developing murine secondary palate. Dev. Biol.145, 374–378 (1991). [DOI] [PubMed] [Google Scholar]
- 56.Mitchell, G. F. et al. Arterial stiffness and cardiovascular events: the Framingham Heart Study. Circulation121, 505–511 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Demer, L. L. & Tintut, Y. Vascular Calcification. Circulation117, 2938–2948 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tiong, M. K. et al. Effect of nutritional calcium and phosphate loading on calciprotein particle kinetics in adults with normal and impaired kidney function. Sci. Rep.12, 7358 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Thiem, U. et al. Effect of the phosphate binder sucroferric oxyhydroxide in dialysis patients on endogenous calciprotein particles, inflammation, and vascular cells. Nephrol. Dial. Transpl.38, 1282–1296 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pasch, A. Novel assessments of systemic calcification propensity. Curr. Opin. Nephrol. Hypertens.25, 278–284 (2016). [DOI] [PubMed] [Google Scholar]
- 61.Smith, E. R. et al. A novel fluorescent probe-based flow cytometric assay for mineral-containing nanoparticles in serum. Sci. Rep.7, 5686 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Köppert, S. et al. Cellular Clearance and Biological Activity of Calciprotein Particles Depend on Their Maturation State and Crystallinity. Front. Immunol.9, 1991 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Woodward, H. J., Zhu, D., Hadoke, P. W. F. & MacRae, V. E. Regulatory Role of Sex Hormones in Cardiovascular Calcification. Int. J. Mol. Sci.22, 4620 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Eelderink, C. et al. Serum Calcification Propensity and the Risk of Cardiovascular and All-Cause Mortality in the General Population. Arteriosclerosis Thrombosis Vasc. Biol.40, 1942–1951 (2020). [DOI] [PubMed] [Google Scholar]
- 65.Van den Bergh, G. et al. Endothelial Contribution to Warfarin-Induced Arterial Media Calcification in Mice. Int J. Mol. Sci.22, 11615 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Leloup, A. J. et al. A novel set-up for the ex vivo analysis of mechanical properties of mouse aortic segments stretched at physiological pressure and frequency. J. Physiol.594, 6105–6115 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bia, D. et al. Regional Differences in Viscosity, Elasticity and Wall Buffering Function in Systemic Arteries: Pulse Wave Analysis of the Arterial Pressure-Diameter Relationship. Rev. Española de. Cardiol.ía58, 167–174 (2005). [PubMed] [Google Scholar]
- 68.van Loo, C., Giudici, A. & Spronck, B. Potential adverse effects of vasodilatory antihypertensive medication on vascular stiffness in elderly individuals. Hypertens. Res.45, 2024–2027 (2022). [DOI] [PubMed] [Google Scholar]
- 69.Pincha, N., Saha, D., Bhatt, T., Zirmire, R. K. & Jamora, C. Activation of Fibroblast Contractility via Cell-Cell Interactions and Soluble Signals. Bio Protoc.8, e3021 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Description of additional supplementary files
Data Availability Statement
The numerical source data that make up the graphs and charts as well as the uncropped/unedited western blot images are available in Supplementary Data 1. Other datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.