Abstract
An investigation into the optical and mechanical properties of a novel borate glasses with the chemical composition of 70 B2O3-10 Li2O-10ZnO-5Bi2O3-5CaO-xEu2O3 was conducted. The glassy specimens of Eu3+-doped borate were prepared by the melting-quenching technique. An enhanced density from 3.0860 to 3.2176 g cm−3 and reduced molar volume from 29.27819 to 29.17447 (cm3 mol−1) are the outcome of increasing the concentration of Eu3+ in glasses. Plotting the extinction coefficient, dielectric constant (ε1, ε2), and refractive index (n) against wavelength reveals that they all rise as level of Eu3+ elements in the glass lattice increases. An increase in Eu3+ concentration results in a decrease in both the volume (VELF) and surface (SELF) energy loss functions. Also, all elastic-mechanical moduli (such as Young’s, Bulk, Shear, and Elongation) increase with increasing the quantity of Eu3+ ions in the glass lattice. The Young’s modulus (Y, GPa) of the glassy specimens was 34.512, 36.089, 36.504, 36.730 and 37.114 GPa for x equal 0, 0.25, 0.5, 0.75 and 1 mol ratio in the glass system, and coded by Eu-0.0, Eu-0.25, Eu-0.5, Eu-0.75 and Eu-1.0, respectively. Growing Eu2O3 levels resulted in an increase in Micro-Hardness from 2.050 to 2.146 GPa. Poisson’s ratio values for Eu-0.0, Eu-0.250, Eu-0.5, Eu-0.75 and Eu-1.0 were 0.273, 0.275, 0.277, 0.277 and 0.278, respectively.
Keywords: Micro-hardness, Young’s modulus, Volume energy loss function (VELF), Eu3+, Molar volume
Subject terms: Optics and photonics, Physics
Introduction
Since long ago, glass, a non-crystalline substance, has been employed in a variety of applications. Furthermore, glasses are significant optical materials that are used extensively in both traditional and contemporary technological advancements due to their linear and nonlinear optical properties. It’s important to note that glass’s capabilities mostly depend on its ingredients. Glass composition and its fundamental structural component are key factors in determining its characteristics. Additionally, glass composition can be changed to satisfy glass technology requirements. In an attempt to link compositional changes with the evolution of characteristics, many glass systems were developed and investigated1–4. Borate glass is regarded as notable among the many oxide glasses because of its unique properties, which include low point of melting, significant RE solubility, good thermal stability, transparency, large refractive index, and affordability5–8. Furthermore, because it does not crystallize on its own, it has the highest inclination to create glasses. One of the unique qualities of borate-based compositions is their ability to use the BO3 and BO4 structural elements to create a variety of structures9,10. Then, new structures can be created by modifying these ones11,12. Zinc oxide (ZnO) is added to borate glass to improve the chemical durability of the glass matrix, reduce crystallization, and boost thermal stability13. The presence of lithium oxide lowers the glass transition temperature while increasing optical transmission and glass thermal stability14. Lithium-based glasses are utilized in modulators, electro-optic switches, and optical parametric devices due to their high conductivity14. Bismuth and other heavy metal oxides increase the glass creation range and fluorescence of oxide glasses15. Glasses made of bismuth borate have amazing uses in optical devices and non-linear optics. The substantial applications of Bi2O3 and ZnO in glasses, such as thermal and mechanical sensors, optical and electrical device layers, infrared transmitting windows, and glass ceramics, have attracted the interest of the scientific community16.
Rare-Earth (RE) ions have the benefit of having f-f transitions that are less affected by ligand field fluctuation. Glasses loaded with RE ions exhibit exceptional emission effectiveness resulting from both 4f-5d and 4f-4f electronic transitions, making them highly efficient luminous materials. Due to its broad emission spectral span, which extends from the blue towards the near-infrared (NIR) location, narrowed emission band, potent emission intensity, beneficial red emission effectiveness, and lengthy excited-state lifespan, europium (Eu3+) is considered to be one of the most suitable rare earth ions for injection17–22.
Glasses doped with Eu3+ ions, in particular, have a lot of potential as effective red phosphors and are used in a variety of photonic applications. This is because they have a long radiative lifetime and can emit monochromatic light23. The phonon energy of the host material influences the quantum efficiency of rare earth ion emissions. Borate glasses generally have high phonon energy levels because of the network-forming oxides’ stretching vibrations. Moreover, in their pure form, it consists of boroxyl BO3 subunits forming random networks24. It is commonly known that the spectroscopic properties of generated (RE) glasses can be improved by the addition of heavier metal oxides, like Bi2O3, PbO, CdO, and Sb2O3, by lowering the phonon energy, increasing the glass-forming span, and improving the polarizable character of the resulting glass matrix25–28.
In this work, we have effectively synthesized a variety of new glasses doped with europium oxide that contain lithium, zinc, borate, and heavy metal oxide (bismuth). 70 B2O3-10 Li2O-10ZnO-5Bi2O3-5CaO-xEu2O3, where x has values of 0.0, 0.25, 0.5, 0.75, and 1.0 (mole ratio), is the formula for the glass compositions. The recently created glasses were then thoroughly characterized to investigate the effects of Eu3+ ions on the host glass network structural. This examination included a review of the glasses’ mechanical, structural, optical, and physical characteristics.
Experimental
The new glasses used in this study have the following composition: 70 B2O3-10 Li2O-10ZnO-5Bi2O3-5CaO-xEu2O3, where x (mole ratio) has values of 0.0, 0.25, 0.5, 0.75, and 1.0. Bi2O3, B2O3, CaO, ZnO, Li2O, and Eu2O3 were all used in extremely pure (99.9%) forms (Aldrich). Twelve grams are produced overall for each composite. The calculated powders are combined in a mortar in a molar ratio for thirty minutes to produce an excellent, homogenous blend. The mixture was melted at a temperature of roughly 1100 °C for 40 min in a porcelain crucible. A homogeneous solution was achieved by physically rotating the porcelain crucible containing the glass sample during the melting process. A brass mold was then used to quench it. Next, in order to remove bubbles and thermal stress from the samples that were created, glasses in a brass mold were placed in a furnace set to 350 °C for 12 h, and the furnace was then allowed to cool to ambient temperature during the annealing process. For the glass samples, the following names are applicable:
Based on the Archimedes Principle, the density of the generated glass samples was computed using the following formula:
| 1 |
where WAir and WLiquid represent the sample weight in air and liquid, respectively (the liquid employed was xylene, which has a density of ρLiquid = 0.863 g cm−3).
To calculate the molar volume (Vm) of the glass block, the following expression was used:
| 2 |
The photograph, density, and estimated molar volume are tabulated in Table 1.
Table 1.
Density, photo, molar volume and mechanical characteristics of the present glass systems.
| Glass code | Sample photo | Density, ρ (g cm−3) |
Molar volume (cm3 mol−1) |
Mechanical characteristics | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gt (KJ cm−3) |
Vi (cm3 mol−1) |
Vt (cm3 mol−1) |
Y (GPa) | B (GPa) | S (GPa) | L (GPa) | H (GPa) | |||||
| Eu-0.0 |  |
3.0860 | 29.27819 | 28.185 | 17.925 | 0.612 | 34.512 | 25.355 | 13.554 | 35.520 | 0.273 | 2.050 |
| Eu-0.25 |  |
3.1180 | 29.25988 | 29.190 | 18.088 | 0.618 | 36.089 | 26.771 | 14.149 | 37.383 | 0.275 | 2.119 |
| Eu-0.5 |  |
3.1490 | 29.25123 | 29.334 | 18.201 | 0.622 | 36.504 | 27.256 | 14.295 | 37.977 | 0.277 | 2.127 |
| Eu-0.75 |  |
3.1808 | 29.23538 | 29.479 | 18.213 | 0.623 | 36.730 | 27.459 | 14.381 | 38.245 | 0.277 | 2.137 |
| Eu-1.0 |  |
3.2176 | 29.17447 | 29.623 | 18.276 | 0.626 | 37.114 | 27.900 | 14.517 | 38.787 | 0.278 | 2.146 |
A JASCO UV–Vis-NIR double -beam spectrophotometer model V-570 was used to measure the glasses’ optical characteristics.
The Makishima–Mackenzie theory was used in this investigation to compute the elastic mechanical properties of currently produced glass composites29,30. The mechanical results depends on the dissociation energy per unit volume (Gt, KJ cm−3) and the packing density (Vt, cm3 mol−1) of each composite, which given by and , where , and represents the dissociation energy per unit volume, packing factor and molar fraction of ith oxide. Through the dissociation energy (Gt) and packing density (Vt) of the studied glass composites estimated by Makishima–Mackenzie’s29,30, the elastic moduli (such as Young (Y), Bulk (B), Shear (S) and Longitudinal (L) modulus) in addition to the Poisson's ratio () as well as the Micro-hardness (Hm) values can be determined from the following relations.
| 3 |
| 4 |
| 5 |
| 6 |
| 7 |
| 8 |
Compliance with ethical standards
The authors affirm that this manuscript is original, has not been published before, and is not currently being considered for publication elsewhere.
Result and discussion
Density
Table 1 shows that the average density of glasses under assessment increases steadily from 3.0860 towards 3.2176 g cm−3 as the Eu level rises from 0.0 to 1.0 mol ratio. Compared to B2O3, which is less heavy at 2.46 g cm−3, Eu2O3 density (7.4 g cm−3) is responsible for this increase in density and is essential for the growth of the glass network. An further reason for the greater density of the glasses under investigation is the larger molecular weight of Eu additives (351.926 g mol−1) in comparison to B2O3 (69.617 g mol−1). The observed decrease in the molar volume shown in Table 1 could be the result of a reduction in the link length or the interatomic spacing within the glass network, which would create a compact structure within the glass.
Optical properties
The variation of reflectance (R) and transmittance (T) over wavelength (λ) are illustrated in Figs. 1 and 2. Reduced transmittance and increased reflectance are seen. Once the band edge was reached, all of the samples showed high transparency, with the significant transmission range being within 400 and 700 nm throughout the visible region. Transmission measurements (see Fig. 1) show a red shift in the cutoff wavelength, the red shift is mainly attributed to a conversion of three coordination boron atoms BO3/2 into BO4/231. Eu-0.25 is the sample that yields the greatest transmittance at a rate of almost 70% and smallest reflectance, Eu-0.25 obeys a different trend in the visible region compared to the other samples, higher transmittance of Eu-0.25 indicates higher concentration of BO432, or less absorption by the sample, may due to decrease in the concentration of absorbing molecules. More specifically, Figs. 1 and 2 also show that the reflectance and transmittance spectra for every specimen can be categorized into three spectral regions, (I) a strong-absorption locale, where λ is usually shorter than 250 nm, (II) transparent with low reflectance areas where λ usually lies between 250 and 650 nm, (III) no absorption location, which is found at wavelengths beyond 650 nm. In ref.33, identical behavior was noted.
Figure 1.
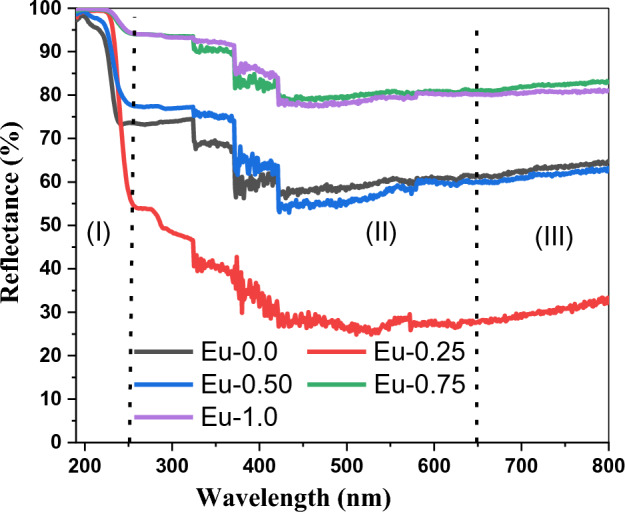
Variation of the reflectance (R) of Eu2O3 doped borate glass.
Figure 2.
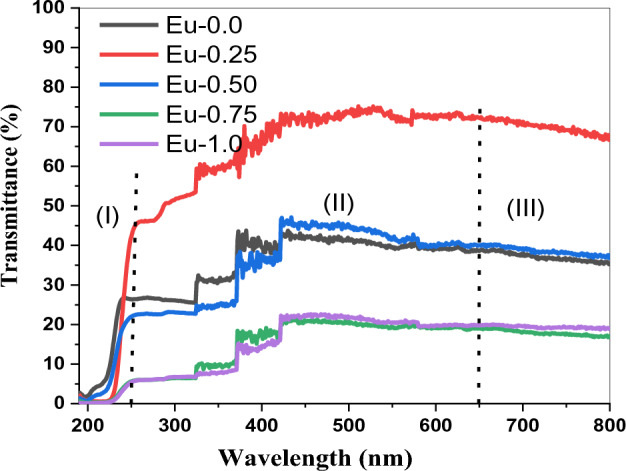
Variation of the Transmittance (T) of Eu2O3 doped borate glass.
The fraction of incident light dissipated throughout transmission across the medium can be identified via the extinction coefficient (), which is calculated as = (αλ/4π)34,35. When electromagnetic waves pass through a substance, the quantity of energy that is absorbed through the material is indicated by the extinction coefficient (), also known as the absorption index. This indicates that the intensity of the electromagnetic waves will attenuate as they pass along the medium. The proportion of free electrons within the substance and the presence of structural imperfections affect the values of the extinction coefficient (). Figure 3 displays the variation of () with wavelength (λ). We can see from the figure the increasing of () levels with increasing Eu2O3 concentration. The enhancement in the values of () may be assigned to creation the structure defect in the system under consideration by Eu3+ content. Absorption intensity of the absorption peaks are increased with Eu3O2 content. It can be seen that, most of characteristic absorption bands of Eu3O2 ion in the UV and visible region are masked due to strong absorption of the host glass36,37. The presence of heavy metal cations such as Bi3+ might be responsible for the strong absorption of the glasses. Appearance of absorption peaks in the UV region (200–350 nm) associated with amorphous glass matrix convoyed to chemicals used in preparing glasses. Dufy38 attributed strong UV absorbance in glasses to the presence of some transition metal ions, e.g. Cr6+ and Fe3+, because of the possible charge electron transfer mechanism, even the traces of impurities are in ppm level. It is suggested that the production of superior optical glasses requires the use of exceptionally pure chemicals since they have the potential to impart a strong absorption and the presence of iron ion traces as impurities lower the optical glasses’ transmittance39.
Figure 3.
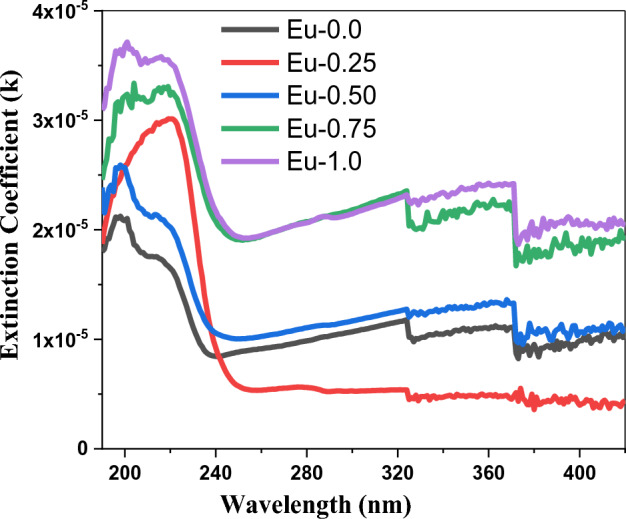
The fluctuation of (k) with wavelength (λ) of Eu2O3 doped borate glass.
The following equation can be used to estimate the variation of the refractive index (n) of the present Eu3+ ion-doped glass specimens based on absolute value of reflectance (R) as well as extinction coefficient ()35,40–42:
| 9 |
Figure 4 shows the numerical values of (n) with wavelength λ (nm). It was found that the inclusion of Eu3+ ions into the glass structures increased the n levels. The refractive index peak in Fig. 4 increases from sample Eu-0.0 until Eu-1.0 sample, the rise in refractive index indicates a higher optical density for the material.
Figure 4.
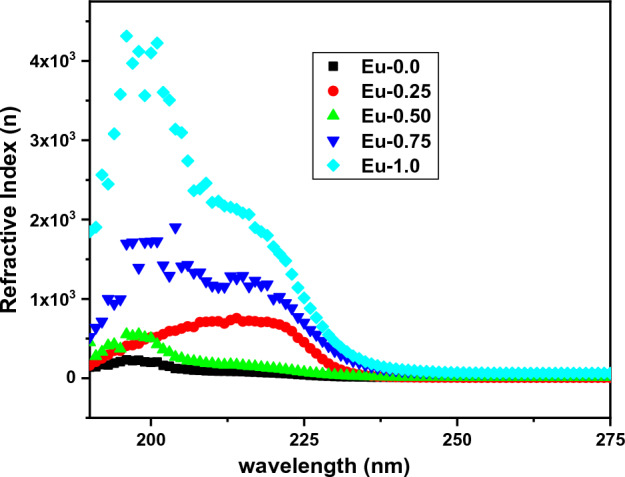
The values of (n) as a function of with wavelength (λ) of Eu2O3 doped borate glass.
The dependence of real part of dielectric constant ε1 on wavelength is shown in Fig. 5, which can be calculated using the following relation35,40:
| 10 |
we notice from Fig. 5 that, the effect of the increasing of Eu3+ content appears only at lower wavelength or higher energy, also the increase of real part of dielectric constant ε1 with the variations of the mole ratio of Eu3+ ions agreement with the increase of the refractive index level (n).
Figure 5.
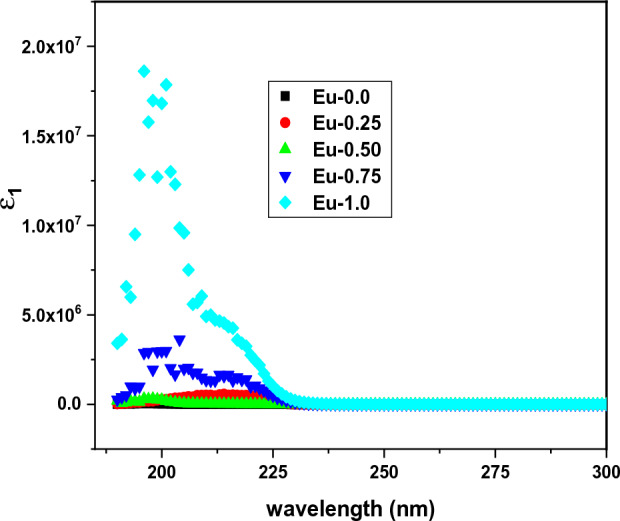
The variations of (ε1) as a function of wavelength (λ) of Eu2O3 doped borate glass system.
The imaginary part (ε2) of dielectric constant was calculated using the equation that follows35,40:
| 11 |
Figure 6 shows the spectrum dependence of ε2 on wavelength λ (nm) across all doped glass borate samples. The value of both ε1 and ε2 were boosted with increase of Eu3+ concentration.
Figure 6.
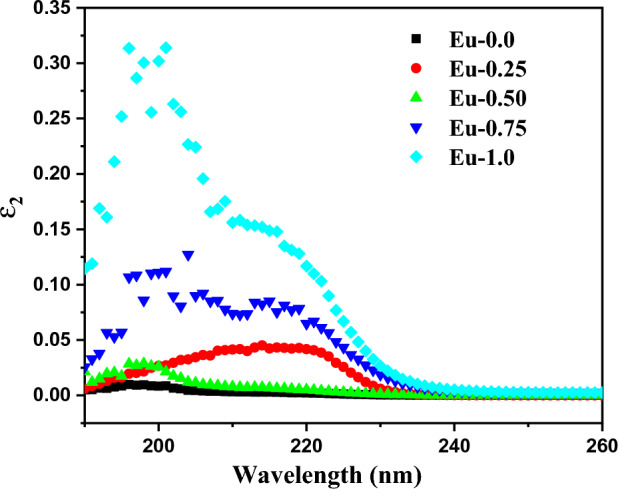
The variations of (ε2) as a function of wavelength (λ) of Eu2O3 doped borate glass system.
Two other important features that can be employed to identify the transitions in optical properties for electrons in this studied are the surface energy loss function (SELF) and the volume energy loss function (VELF). Both factors were utilized to calculate the energy loss rate of electrons during travelling through glass. Via the dielectric function, the energy dissipation is connected to the material's optical characteristics. The probability that the fast electrons will lose power as they go through the bulk and surface of the material is represented by the volume and surface energy loss functions, respectively. The electron transitions within this substance at low and high energies are described by the value of SELF/VELF. VELF and SELF, the two parameters, are provided by equations40–45:
| 12 |
| 13 |
The volume energy loss function (VELF) of the doped borate glass samples with different quantities of Eu2O3 was illustrated as a function of wavelength as shown in Fig. 7. As can be observed from that figure, a change in the quantity of Eu2O3 causes a change in the electron transition energy, which in turn causes variation in the volume energy loss. Also, the Surface energy loss function is varied due to the variation in Eu3+ content as shown in Fig. 8. The values of SELF/VELF as functions of wavelength are plotted in Fig. 9. It is evident that, at the incident wavelength, the volume energy loss was larger than the surface energy loss.
Figure 7.
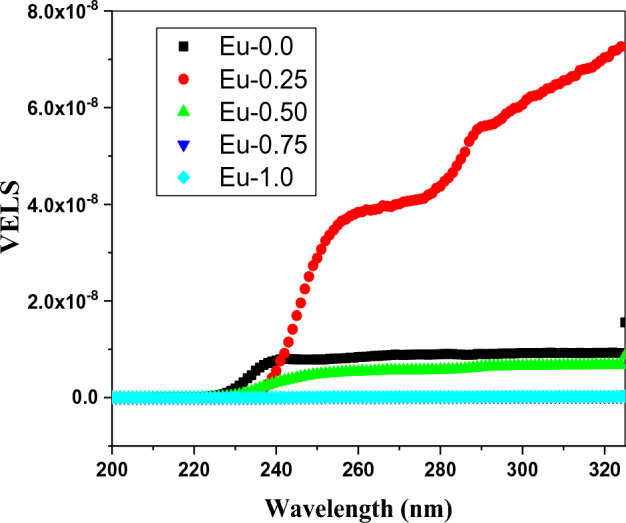
The variations of (VELF) as a function of wavelength (λ) of Eu2O3 doped borate glass system.
Figure 8.
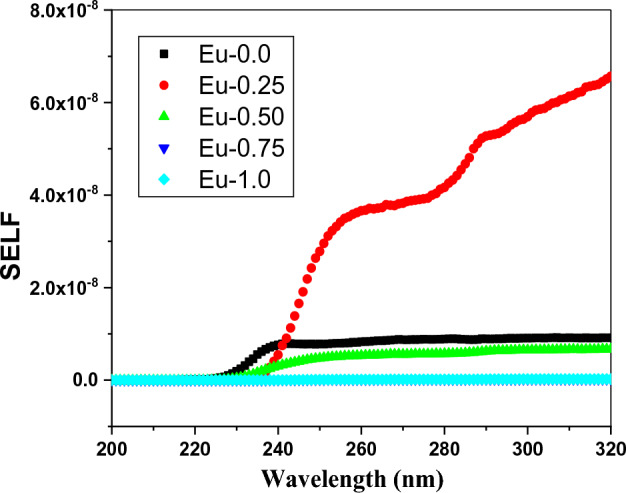
The variations of (SELF) as a function of wavelength (λ) of Eu2O3 doped borate glass system.
Figure 9.
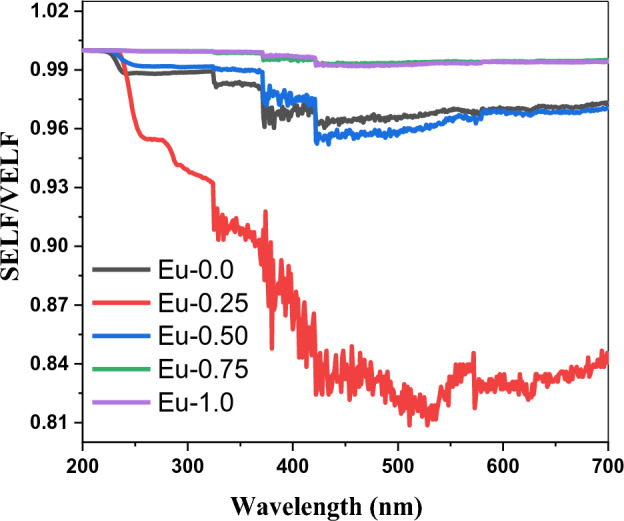
The variations of (VELF/SELF) as a function of wavelength (λ) of Eu2O3 doped borate glass system.
Mechanical characteristics
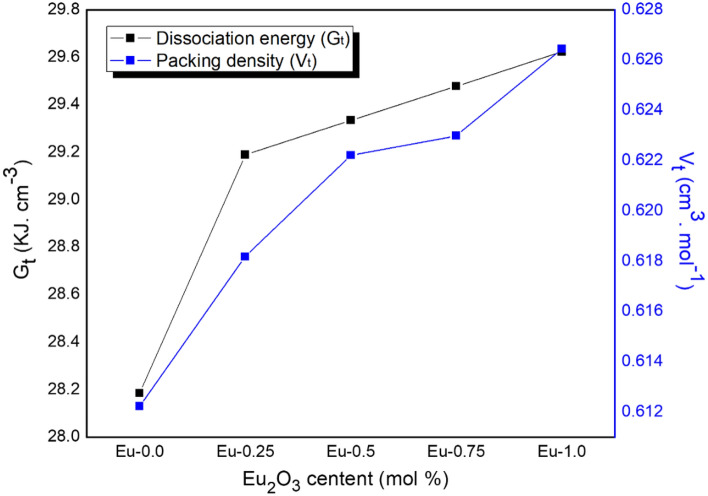
The dissociation energy and packing density were calculated and plotted in Fig. 10. The figure shows an increase in the two factors with the addition of Eu2O3 concentration from 0 to 1 mol ratio. The dissociation energy increased from 28.185 to 29.623 kJ cm−3, while the packing density increased from 0.612 to 0.626 cm3 mol−1 as shown in Table 1.
Figure 10.
The variation of dissociation energy and packing density of Eu-glass composites.
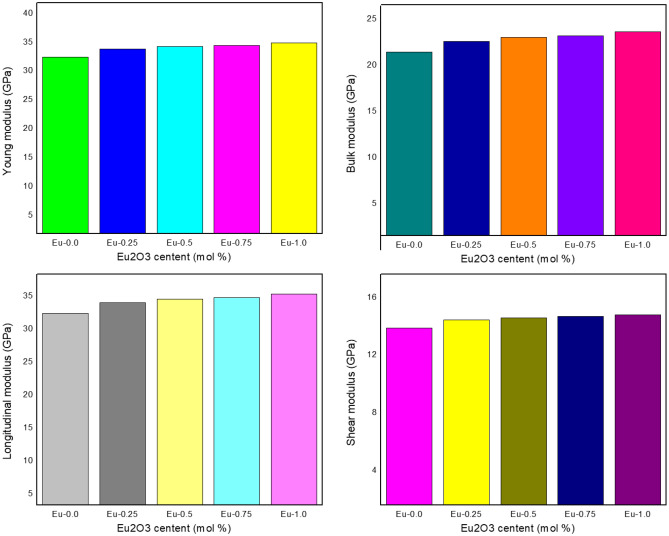
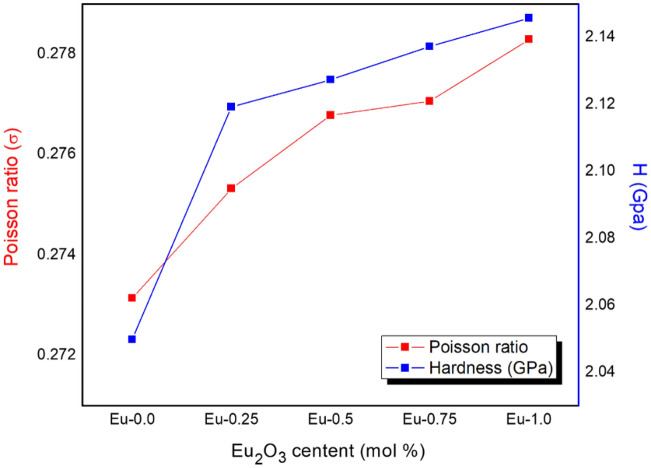
The Young’s modulus (Y, GPs) was estimated by the formula in section “Experimental” which measures the rigidity of the fabricated glass. The moduli elasticity results were shown in Fig. 11. The Young’s modulus values increased from 34.512 to 37.114 GPs with raising the Eu2O3 additives from 0 to 1 mol ratio in the glass system. Due to the addition Eu2O3 rare earth to the glass composites, the dissociation energy, or Gibbs-free energy increased which lead to increasing the young’s modulus. The other elastic moduli such as Bulk modulus, Shear modulus and longitudinal modulus related to the young modulus are estimated and tabulated in Table 1, and Fig. 11. The longitudinal modulus was greater than the shear and bulk modulus. Poisson’s ratio (σ) as well as the microhardness (H, GPa) of the prepared glass composites were calculated based on the results of the elastic modulus, and these parameters measure the extent of expansion/contraction and hardness of the manufactured glass, respectively. The values of Poisson's ratio were 0.273, 0.275, 0.277, 0.277 and 0.278 for Eu-0.0, Eu-0.250, Eu-0.5, Eu-0.75 and Eu-1.0, respectively as shown in Fig. 12, which indicated that Poisson’s ratio is approximately relatively constant with Eu2O3 additions. Also, the Micro-Hardness was estimated and plotted in Fig. 12, the results increased from 2.050 to 2.146 GPa which showed that the hardness was raised with increasing Eu2O3 contents.
Figure 11.
The variation of Young, shear, Longitudinal and Bulk modulus of Eu-glass composites.
Figure 12.
The variation of Poisson ratio and micro-hardness of Eu-glass composites.
The mechanical results depend on the structure of glass composites with changing the doping Eu2O3. The FTIR spectra of the borate glasses without Eu2O3 and doped with 0.75, and 1.0 mol ratio of Eu2O3 is illustrated in Fig. 13 over the spectral range 400–2000 cm−1. From these peaks, three zones can be identified. The first region, spanning 600–700 cm−1, is caused by a B—O—B bending vibration bond in the borate network. The tetrahedral BO4 units’ B—O linkage stretching vibration is responsible for the second region, which spans from 800 to 1200 cm−1, and the triangles-BO3 units’ B—O stretching vibration is responsible for the third zone, which spans from 1200 to 1600 cm−146–48. More information regarding structural alterations was found by using FTIR deconvolution techniques shown in Fig. 14. Several peaks were seen in the infrared spectra of the glasses at 673, 882, 1048, 1239, 1405, and 1754 cm−1. The bending vibrations of bridging oxygen (B—O) over trigonal BO3 groups are suggested to be the origin of a band at 673 cm−149. The band at 882 cm−1 further confirms connectivity between the diporate and B-O-B networks. Furthermore, the bands at 1239 cm−1 were shown to be caused by the asymmetric stretching of the B–O connection in BO3 units made from boroxels. The symmetric stretching relaxation of the B—O band, caused by the trigonal BO3 units, is responsible for the peak at around 1405 cm−150. The two other peaks, measured at 1572 and 1969 cm−1, are the result of B—O stretching vibrations, or BO3 units, in triangles46–48.
Figure 13.
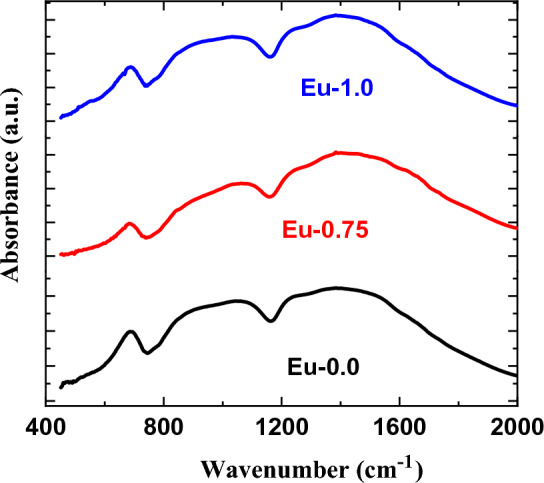
FTIR spectra of the borate glasses without Eu2O3 and doped with 0.75, and 1.0 mol ratio of Eu2O3.
Figure 14.
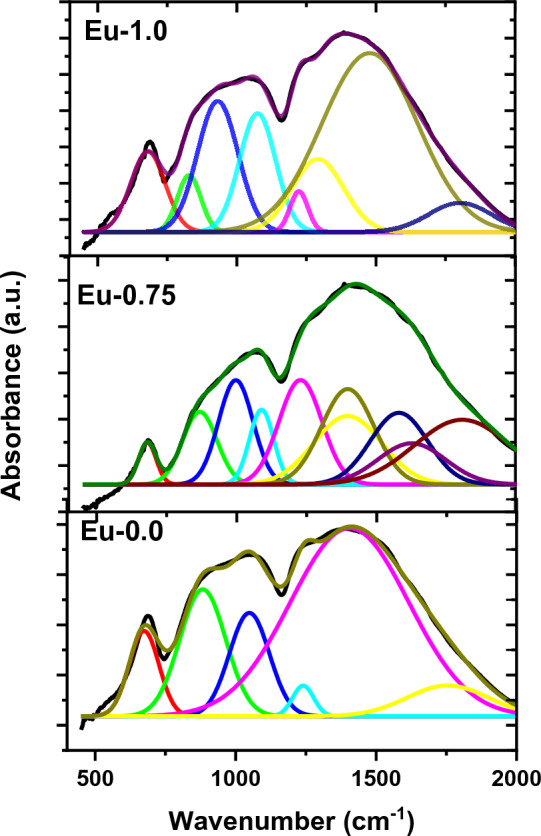
FTIR deconvolution of the borate glasses without Eu2O3 and doped with 0.75, and 1.0 mol ratio of Eu2O3.
The change in the relative areas of peak absorption linked to BO4 and BO3 groups, N4 and N3, respectively, is one of the most significant finds. N4 and N3 can be calculated using the relations listed below.
| 14 |
In the present case, the regions under the BO3 and BO4 group's absorption peaks of Eu-0.0, Eu-0.75, and Eu-1.0 are equal 0.653, 0.634, 0.632 and 0.347, 0.366, 0.368, respectively. As clear, the relative value of BO4 rises with lowering BO3 values that cause improving the compatibility and stiffness for the doped samples. Increments in BO4 result in increasing not only the density but also the connectivity and the stiffness and elasticity of the glass system. It is clear that the structure with doping Eu2O3 increase the mechanical properties of present glass.
Finally, the mechanical results of this study were compared with related published values for BaO-PbO-P2O5 glass system51. The comparison showed a superiority in the mechanical properties of the manufactured glass samples compared to BaO-PbO-P2O5 system, as the maximum young modulus in the published sample BPP7 (50 BaO + 50 P2O5) was 36.655 GPa, while the maximum value in the present results was 37.114 GPs. The current young modulus results also compared with the Bi2O3–PbO–CdO–B2O352, the sample with the highest density consists of 25Bi2O3–30PbO–10CdO–35B2O3, and its young’s modulus was 35.07 GPa, while the bulk modulus was 19.97 GPa. These values are lower than the values for the present glass composites studied in the current work, which indicates that they provide good mechanical results and can be used in different applications.
Conclusions
A study was carried out to examine the optical and mechanical characteristics of a new borate glass system that contains 70 B2O3-10 Li2O-10ZnO-5Bi2O3-5CaO-xEu2O3 that were made using the melting-quenching method. The result of raising the Eu3+ concentration in glasses is an improved density. The glass structure exhibits an increase in dielectric constant (ε1, ε2), extinction coefficient, reflectance, and refractive index as the quantity of Eu3+ ions increases. The spectrum transmittance and both surface and volume energy loss function (SELF and VELF) are decrease with increasing Eu3+ ions. The result of raising the Eu3+ concentration in fabricated glass led to improve the mechanical properties. The young’s modulus, shear modulus, bulk modulus and micro- Hardness are increases with increasing Eu3+ ions in the glass structure, this is due to the raising the dissociation energy in the glass composite increased with increasing the Eu3+ concentration.
Acknowledgements
The authors express their gratitude to Princess Nourah bint Abdulrahman University Researchers Supporting Project Number (PNURSP2024R173), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia. The authors extend their appreciation to the Deanship of Scientific Research at Northern Border University, Arar, Kingdom of Saudi Arabia for funding this research work through the project number “NBU-FFR-2024-289-09”.
Author contributions
G AA, AMM, and ME wrote the main body of the manuscript; RAE, SAS, SMS, and NME created and interpreted all of the figures; all authors revised the work.
Data availability
All data generated or analyzed during this study are included in this published article.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ahmmad, S. K. et al. Machine learning density prediction and optical properties of calcium boro-zinc glasses. Opt. Mater.134, 113145 (2022). [Google Scholar]
- 2.Alsaif, N. A. et al. Antimony (III) oxide-reinforced lithium-calcium borate glasses: Preparation and characterization of physical, optical, and γ-ray shielding behavior through experimental and theoretical methods. J. Mater. Sci.: Mater. Electron.51, 5869–5879 (2022). [Google Scholar]
- 3.Alsaif, N. A. M., Khattari, Z. Y., Rammah, Y. S., Shams, M. S. & Elsad, R. A. Bismo-borate glasses doped with La3+ and Eu3+ ions: Synthesis, physical, optical and electrical characteristics. J. Mater. Sci.: Mater. Electron.33, 19667–19677 (2022). [Google Scholar]
- 4.Rammah, Y. S. et al. Synthesis, physical, FTIR, and optical characteristics of B2O3·CaO·ZnO glasses doped with Nb2O5 oxide: Experimental investigation. J. Mater. Sci. Mater.33, 23749–23760 (2022). [Google Scholar]
- 5.Kavaz, E. An experimental study on gamma-ray shielding features of lithium borate glasses doped with dolomite, hematite and goethite minerals. Radiat. Phys. Chem.160, 112–123. 10.1016/j.radphyschem.2019.03.032 (2019). [Google Scholar]
- 6.Alsaif, N. A. M. et al. Synthesis, structure, radiation attenuation efficacy as well as prediction of density using artificial intelligence techniques of lead borate lithium zinc strontium glasses. Opt. Mater.137, 113599 (2023). [Google Scholar]
- 7.Shaaban, S. M. et al. Influence of copper ions on the structural, mechanical, radiation shielding and dielectric properties of borate zinc-fluoride glasses. J. Electron. Mater.52, 6269–6276 (2023). [Google Scholar]
- 8.Abdel-Aziz, A. M. et al. Synthesis, physical, ultrasonic waves, mechanical, FTIR, and dielectric characteristics of B2O3/Li2O/ZnO glasses doped with Y3+ ions. J. Mater. Sci. Mater. Electron.33, 6603–6615 (2022). [Google Scholar]
- 9.Deopa, N. & Rao, A. S. Spectroscopic studies of Sm3+ ions activated lithium lead alumino-borate glasses for visible luminescent device applications. Opt. Mater.72, 31–39 (2017). [Google Scholar]
- 10.Maheshvaran, K., Veeran, P. K. & Marimuthu, K. Structural and optical studies on Eu3+ doped boro-tellurite glasses. Solid State Sci.17, 54–62 (2013). [Google Scholar]
- 11.Khalil, E. M. A. et al. UV-visible and IR spectroscopic studies of gamma irradiated transition metal doped lead silicate glasses. Silicon2(1), 49 (2010). [Google Scholar]
- 12.Abdelghany, A. M., Abdelrazek, E. M., Badr, S. I., Abdel-Aziz, M. S. & Morsi, M. A. Efect of Gamma-irradiation on biosynthesized gold nanoparticles using Chenopodiummurale leaf extract. J. Saudi Chem. Soc.21(5), 528 (2017). [Google Scholar]
- 13.Ahlawat, J. et al. Structural and optical characterization of IR transparent sodium-modified zinc borate oxide glasses. Appl. Phys. A128, 923 (2022). [Google Scholar]
- 14.Pawaria, S. et al. Structural and optical characterization of semiconducting lithium modified zinc borate glassy system for UV band reject filter. J. Mol. Struct.1270, 133836 (2022). [Google Scholar]
- 15.Narwal, P. et al. Improved white light emission in Dy3+ doped LiF – CaO – Bi2O3 – B2O3 glasses. J. Non Cryst. Solids498, 470–479. 10.1016/j.jnoncrysol.2018.01.042 (2018). [Google Scholar]
- 16.Bala, M. et al. Effect of replacement of Bi2O3 by Li2O on structural, thermal, optical and other physical properties of zinc borate glasses. J. Mol. Struct.1219, 128589 (2020). [Google Scholar]
- 17.Bouabdalli, E. M., El Jouad, M., Hajjaji, A., Garmim, T. & Touhtouh, S. Synthesis and characterization of silicophosphate glasses doped with europium. Mater. Today: Proc.10.1016/J.MATPR.2022.04.299 (2022). [Google Scholar]
- 18.Anjaiah, J., Laxmikanth, C. & Veeraiah, N. Spectroscopic properties and luminescence behavior of europium doped lithium borate glasses. Phys. B Condens. Matter454, 148–156. 10.1016/j.physb.2014.07.070 (2014). [Google Scholar]
- 19.Selvi, S., Marimuthu, K. & Muralidharan, G. Structural and luminescence studies of Eu3+: TeO2[sbnd]B2O3[sbnd]AO[sbnd]AF2 (A = Pb, Ba, Zn, Cd, Sr) glasses. J. Mol. Struct.1144, 290–299. 10.1016/j.molstruc.2017.05.031 (2017). [Google Scholar]
- 20.Selvi, S., Marimuthu, K., Suriya-Murthy, N. & Muralidharan, G. Red light generation through the lead boro-telluro-phosphate glasses activated by Eu3+ ions. J. Mol. Struct.1119, 276–285. 10.1016/j.molstruc.2016.04.073 (2016). [Google Scholar]
- 21.Sajna, M. S. et al. Spectroscopic investigations and phonon side band analysis of Eu3+-doped multicomponent tellurite glasses. Opt. Mater. Amst.70, 31–40. 10.1016/j.optmat.2017.04.064 (2017). [Google Scholar]
- 22.El-Jouad, M. et al. Red luminescence and UV light generation of europium doped zinc oxide thin films for optoelectronic applications. EPJ Appl. Phys.2020, 91. 10.1051/epjap/2020200133 (2020). [Google Scholar]
- 23.Malchukova, E., Boizot, B., Ghaleb, D. & Petite, G. Optical properties of pristine and γ-irradiated Sm doped borosilicate glasses. Nucl. Instrum. Methods Phys. Res.537, 411–414. 10.1016/j.nima.2004.08.054 (2005). [Google Scholar]
- 24.Hatefi, Y., Shahtahmasebi, N., Moghimi, A. & Attaran, E. Frequency-conversion properties of Eu3+ doped chlorophosphate glass ceramics containing CaCl2nanocrystals. J. Lumin.131, 114–118. 10.1016/j.jlumin.2010.09.029 (2010). [Google Scholar]
- 25.Doerenkamp, C. et al. Composition–structure–property correlations in rare-earth-doped heavy metal oxyfluoride glasses. J. Phys. Chem. C123(36), 22478–22490. 10.1021/acs.jpcc.9b05531 (2019). [Google Scholar]
- 26.Ouannes, K. et al. New Er3+ doped antimony oxide based glasses: Thermal analysis, structural and spectral properties. J. Alloys Compd.649, 564–572. 10.1016/j.jallcom.2015.07.113 (2015). [Google Scholar]
- 27.Marzouk, M. A., Ouis, M. A. & Hamdy, Y. M. Spectroscopic studies and luminescence spectra of Dy2O3 doped lead phosphate glasses. Silicon4(3), 221–227. 10.1007/s12633-012-9125-z (2012). [Google Scholar]
- 28.ElBatal, F. H., Marzouk, M. A. & Abdel-Ghany, A. M. Gamma rays interaction with bismuth borate glasses doped by transition metal ions. J. Mater. Sci.46, 5140–5152. 10.1007/s10853-011-5445-4 (2011). [Google Scholar]
- 29.Makishima, A. & Mackenzie, J. D. Direct calculation of Young’s modulus of glass. J. Non-Cryst. Solids12, 35–45 (1973). [Google Scholar]
- 30.Makishima, A. & Mackenzie, J. D. Calculation of Bulks modulus, Shear modulus and Piosson’s ratio of glass. J. Non-Cryst. Solids17, 147–157 (1975). [Google Scholar]
- 31.El-Diasty, F., Moustafa, F. A., Abdel-Wahab, F. A., Abdel-Baki, M. & Fayad, A. M. Role of 4p–3d orbital hybridization on band gap engineering of heavy metal glass for optoelectronic applications. J. Alloys Compd.605, 157–136 (2014). [Google Scholar]
- 32.Colak, S. C. & Kilic, G. Structural, physical, and optical characterization of (Nd3+/Eu3+)-doped zinc-rich silica–borate glasses. J. Mater. Sci.: Mater. Electron.33, 21852–21863 (2022). [Google Scholar]
- 33.Hassanien, A. S. & Akl, A. A. Optical characteristics of iron oxide thin films prepared by spray pyrolysis technique at different substrate temperatures. Appl. Phys. A124, 752. 10.1007/s00339-018-2180-6 (2018). [Google Scholar]
- 34.Hodgson, J. N. Optical Absorption and Dispersion in Solids (Springer, New York, 2012). 10.1007/978-1-4613-3321-0. [Google Scholar]
- 35.Mahmoud, W. E., Shirbeeny, W., Al-Ghamdi, A. A. & Al-Heniti, S. Synthesis and characterization of CdxZn1−xO nanoparticles-doped aryl poly ether ketone for novel application potentials. J. Appl. Poly. Sci.125, 339. 10.1002/app.35570 (2012). [Google Scholar]
- 36.Jamalaiah, B. C., Moorthy, L. R., Seo, H. J. & Non-Cryst, J. Effect of lead oxide on optical properties of Dy3+ ions in PbO–H3BO3–TiO2–AlF3 glasses. Solids358, 204–209 (2012). [Google Scholar]
- 37.Ramesh, P., Hegde, V., Pramod, A. G., Eraiah, B. & Agarkov, D. A. Compositional dependence of red photoluminescence of Eu3O2 ions in lead and bismuth containing borate glasses. Solid State Sci.107, 106360 (2020). [Google Scholar]
- 38.Dufy, J. A. Charge transfer spectra of metal ions in glass. Phys. Chem. Glasses38, 289–292 (1997). [Google Scholar]
- 39.Hussein, E. M. A. & Rammah, Y. S. Optical UV–visible, Raman spectroscopy, and gamma radiation shielding properties of borate glass systems; B2O3+Na2O+Al2O3/MgO/Li2O. Opt. Quant. Electron.56, 387 (2024). [Google Scholar]
- 40.Taha, T. A. & Rammah, Y. S. Optical characterization of new borate glass doped with titanium oxide. J. Mater. Sci. Mater. Electron.27(2), 1384–1390. 10.1007/s10854-015-3901-7 (2016). [Google Scholar]
- 41.Taha, T. A. & Abouhaswa, A. S. Preparation and optical properties of borate glass doped with MnO2. J. Mater. Sci.: Mater. Electron.29, 8100–8106 (2018). [Google Scholar]
- 42.Hodgson, J. N. Optical Absorption and Dispersion in Solids (Springer, 2012). [Google Scholar]
- 43.Ebnalwaled, A. A., Sadek, A. H., Ismail, S. H. & Mohamed, G. G. Structural, optical, dielectric and surface properties of polyimide hybrid nanocomposites films embedded mesoporous silica nanoparticles synthesized from rice husk ash for optoelectronic applications. Opt. Quant. Electron.54, 690. 10.1007/s11082-022-03976-2 (2022). [Google Scholar]
- 44.Sarkar, S., Das, N. S. & Chattopadhyay, K. K. Optical constants, dispersion energy parameters and dielectric properties of ultra-smooth nanocrystalline BiVO4 thin films prepared by rf-magnetron sputtering. Solid State Sci.33, 58–66. 10.1016/j.solidstatesciences.2014.04.008 (2014). [Google Scholar]
- 45.Ali, A. I., Son, J. Y., Ammar, A. H., Moez, A. A. & Kim, Y. S. Optical and dielectric results of Y0.225Sr0.775CoO3±δ thin films studied by spectroscopic ellipsometry technique. Results Phys.3, 167–172. 10.1016/j.rinp.2013.08.004 (2013). [Google Scholar]
- 46.Gupta, N. et al. Structure-property correlations in TiO2-Bi2O3-B2O3-TeO2 glasses. J. Non-Cryst. Solids470, 168–177 (2017). [Google Scholar]
- 47.Kaky, K. M. et al. Stuctural, optical and radiation shielding properties of zinc boro-tellurite alumina glasses. Appl. Phys. A.125, 32 (2019). [Google Scholar]
- 48.Malge, A. et al. Structural and DC conductivity studies of borotellurite glasses doped with ZnO, Li2O and Dy2O3. Mater. Today: Proc.26, 1960–1963 (2020). [Google Scholar]
- 49.Es-soufi, H., Bih, H., Sayyed, M. I. & Bih, L. Impact of TiO2 on physical, optical, and radiation shielding properties of tungsten-based glasses. Optik272, 170400 (2023). [Google Scholar]
- 50.Kaur, R. et al. Physical, optical, structural and thermoluminescence behaviour of borosilicate glasses doped with trivalent neodymium ions. Opt. Mater.117, 111109 (2021). [Google Scholar]
- 51.Mahmoud, K. A., El-Agawany, F. I., Tashlykov, O. L., Ahmed, E. M. & Rammah, Y. S. The influence of BaO on the mechanical and gamma/fast neutron shielding properties of lead phosphate glasses. Nucl. Eng. Technol.53, 3816–3823 (2021). [Google Scholar]
- 52.Almuqrin, A. H. et al. Mechanical and gamma-ray shielding examinations of Bi2O3–PbO–CdO–B2O3 glass system. Open Chem.20, 808–815. 10.1515/chem-2022-0195 (2022). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.