Abstract
Background
The wearing-off phenomenon is a key driver of medication change for patients with Parkinson’s disease (PD) treated with levodopa. Common first-line options include increasing the levodopa dose or adding a catechol-O-methyltransferase (COMT) inhibitor, but there are no trials comparing the efficacy of these approaches. We evaluated the effectiveness of adjunct opicapone versus an additional 100 mg levodopa dose in PD patients with early wearing-off using pooled data from 2 randomized studies.
Methods
The ADOPTION study program included two similarly designed 4-week, open-label studies conducted in South Korea (NCT04821687) and Europe (NCT04990284). Patients with PD, treated with 3–4 daily doses of levodopa therapy and with signs of early wearing-off were randomized (1:1) to adjunct opicapone 50 mg or an additional dose of levodopa 100 mg. Patient-level data from the two studies were pooled.
Results
The adjusted mean [SE] change from baseline to week 4 in absolute OFF time (key endpoint) was − 62.8 min [8.8] in the opicapone group and − 33.8 min [9.0] in the levodopa 100 mg group, the difference significantly favoring opicapone (− 29.0 [− 53.8, − 4.2] min, p = 0.02). Significant differences in the Movement Disorder Society—Unified Parkinson’s Disease Rating Scale Part III subscore (− 4.1 with opicapone vs − 2.5 with levodopa 100 mg), also favored opicapone (− 1.7 [− 3.3, − 0.04], p < 0.05). Dyskinesia was the most frequently reported adverse event (opicapone 7.2% vs. levodopa 100 mg 4.2%).
Conclusions
In these short-term trials, introducing adjunct opicapone was more effective at reducing OFF time than adding another 100 mg levodopa dose in PD patients with early signs of wearing-off.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00415-024-12614-8.
Keywords: COMT, Levodopa, Motor fluctuations, Opicapone, Parkinson’s disease, Wearing-off
Introduction
In the last decade, there has been a general return to the use of levodopa as initial monotherapy for most patients with Parkinson’s disease (PD) [1], but with the aim of keeping the dose as low as possible for as long as possible to avoid the development of levodopa motor complications which are a significant source of disability. Often, one of the first motor complications to emerge are ‘wearing-off’ motor fluctuations reflecting a decrease in the duration of effect of each individual dose of levodopa with increasing disease progression and duration of drug treatment [2]. Wearing-off fluctuations can already emerge within the first few years after levodopa initiation [3], but early wearing-off may go unrecognized at office visits, particularly in patients with a shorter disease duration [4]. Predictors of wearing-off (in addition to duration of treatment and disease severity) include a younger age, weight, and female sex [4, 5], with women showing an 80% increased risk for wearing-off [6].
The development of wearing-off fluctuations is a key driver of medication changes [7]. One of the most common approaches to treating early wearing-off is to alter the levodopa dosing regimen, for example, by shortening interdose intervals and/or adding another dose. However, the introduction of a 4th or 5th levodopa dose poses challenges for adherence [8, 9] and is often only effective for a limited period after which further increases in dosing frequency usually are necessary (in turn increasing the risk of inducing dyskinesias). COMT inhibitors, such as opicapone, are recommended by evidence-based guidelines as effective treatment for patient with motor complications [10]. Such recommendations are based on a wealth of data from clinical trials such as the BIPARK pivotal studies [11, 12]. However, these studies enrolled patients who had well established motor fluctuations (average disease duration of 8 years and time with motor fluctuations of 3 years) and a mean daily OFF time of over 6 h [13] indicative of a population with chronic motor complications. However, post-hoc analyses of the pivotal studies suggested the efficacy of opicapone 50 mg in those patients who had developed motor fluctuations within the prior 2 years (placebo-adjusted reduction of OFF time of − 68.5 min, p = 0.0003 vs. placebo) [13, 14].
The eArly levoDopa with Opicapone in Parkinson’s paTients wIth motOr fluctuatioNs (ADOPTION) clinical program aimed to evaluate the efficacy and safety of opicapone 50 mg in PD patients with early wearing-off. To improve study feasibility, the overall Phase 4 program has been designed such that all component studies (at national or regional levels) can be kept relatively small but with the intention of pooling for additional power and to support international generalizability. Thus far, two similarly designed trials have been conducted in Europe and South Korea. The results of the South Korean study have recently been published [15]. Here, we report findings from the pooled analysis of both studies.
Methods
Study conduct
The ADOPTION studies were two randomized, parallel-group, multicenter, prospective, open-label, exploratory, Phase 4 studies conducted from June 2021 to August 2022 in Korea and from November 2021 to April 2023 in Europe (Germany, Italy, Portugal, Spain, and the UK). Both studies were conducted in accordance with the Declaration of Helsinki; each study protocol was approved by the ethics committee at each site and all patients provided informed consent.
Study population
Full protocol details have been previously described [15]. Briefly, male or female patients aged ≥ 30 years were eligible if they had a diagnosis of PD, a Hoehn and Yahr stage of 1–3 (during ON) and were being treated with 3–4 daily doses of levodopa/dopa-decarboxylase inhibitor therapy (maximum 600 mg) for ≥ 4 weeks prior to study entry. Patients had early signs of wearing-off, defined as having at least 1 h of daily OFF time for at least 4 weeks, but for less than 2 years. Key exclusion criteria included atypical parkinsonism, severe and/or unpredictable OFF periods, or awake daily OFF time of more than 5 h. Previous or planned surgery for deep brain stimulation and treatment with monoamine oxidase inhibitors (except for selegiline ≤ 10 mg/day oral, ≤ 1.25 mg/day buccal absorption formulations, rasagiline ≤ 1 mg/day, and safinamide ≤ 100 mg/day), opicapone, entacapone, tolcapone and anti-emetics with anti-dopaminergic action (except domperidone) were prohibited during the study (withdrawn ≥ 4 weeks before screening).
Study design
Following screening, eligible patients were randomized (1:1) to opicapone 50 mg or levodopa 100 mg for 4 weeks in addition to their current levodopa therapy. Opicapone was administered once daily at bedtime 1 h after levodopa intake, and levodopa 100 mg was either taken as one full additional administration or by increasing one or more doses of the established levodopa regimen without altering dose frequency. During the first 2 weeks of study treatment, the total daily dose of levodopa (excluding the additional 100 mg levodopa dose) or the intake intervals could be adjusted in case of dopaminergic adverse events (AEs). However, daily doses were kept stable for the last 2 weeks of the study.
Study endpoints
The key efficacy endpoint was the change from baseline to the end of study treatment in absolute OFF time, as assessed by daily paper patient diaries (mean of the 3 preceding diary days before each visit) [16]. We also assessed the change from baseline to the end of study treatment in the proportion of patients (i.e., responders) achieving (i) ≥ 1 h reduction in absolute OFF time and (ii) ≥ 1 h increase in absolute total ON time. Other diary-based efficacy variables were changes from baseline in the percentage of OFF time, in absolute ON time, in the percentage of ON time, and in ON time with and without dyskinesia. Percentages of OFF and ON time were calculated as the sum in minutes from 30-min periods classified as OFF or ON state divided by the total time awake.
Scale-based efficacy variables were the change from baseline in the Movement Disorder Society-Sponsored Revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS) [17, 18] assessed in the ON state, 8-item Parkinson’s Disease Questionnaire (PDQ-8) [19], and the Clinical Global Impression of Improvement (CGI-I) and Patient Global Impression of Improvement (PGI-I). Levodopa equivalent daily dose (LEDD) was calculated for monoamine oxidase B (MAO-B) inhibitors and dopamine agonists using the existing conversion factors [20].
Safety was assessed for the duration of the study and up to 2 weeks after the end of study by evaluating AEs, serious AEs, drug-related AEs, and AEs leading to discontinuation.
Statistical analyses
Similarities in the design of the two clinical trials permitted a pooled analysis. This pooled analysis was based on integration of individual participant data. Like the two component studies, this preplanned integrated analysis was designed to be exploratory—without hierarchical hypothesis-testing (due to lack of previous trial data on the expected magnitude of effect for levodopa 100 mg) [15]. Patient-level data were combined and analyzed. ANCOVA models were used to evaluate the applicable endpoints as independent variables, treatment arm as fixed effect and baseline values as covariate. Only patients with available (non-missing) data for a particular variable were included in the calculation of a percentage. A mixed-effect linear model with repeated measures (MMRM) were used to evaluate the applicable endpoints as independent variables, with fixed effect for treatment group, visit, interaction between treatment group and visit, and a covariance for the baseline value (visit and corresponding interaction was not included for secondary efficacy endpoints). Responder rates of change in OFF/ON time were compared between the treatment groups using the Chi-square test.
Population sets were defined as the safety set, which included all randomly assigned patients who received at least one dose of study drug and the full analysis set (FAS), which included all randomly assigned patients who took at least one dose of study drug and had at least one efficacy assessment after baseline. Only patients with available (non-missing) data for a particular variable were included in the calculation of a percentage.
Results
Study population
Of the 244 patients enrolled across both studies, 243 patients were randomized and treated with either opicapone 50 mg (N = 125) or levodopa 100 mg (N = 118). Overall, 225 (92%) patients completed the studies; 18 patients (opicapone n = 10, levodopa n = 8) early discontinued (Fig. e1).
Baseline demographics and disease characteristics were similar between the two treatment arms (Table 1) and were consistent with an early fluctuating population. Data for the 2 separate studies are given in Table e1. Overall, the average duration of PD was 5.2 years, and the mean baseline levodopa daily dose was ~ 405 mg/day, distributed mostly over three (80%) daily intakes. Patients had a mean (SD) daily OFF time of 3.4 (1.0) h (~ 22% of their awake time) and most ON time was without dyskinesia (~ 89% of total ON time). Concomitant PD medication was common, with 62% of patients already receiving a dopamine agonist and 54% receiving an MAO-B inhibitor; the mean LEDD being 566 mg/day.
Table 1.
Baseline characteristics (randomized set)
| Opicapone 50 mg N = 126 |
Levodopa 100 mg N = 118 |
Total N = 244 |
|
|---|---|---|---|
| Age, year | 64.1 (8.3) | 64.6 (9.1) | 64.3 (8.7) |
| Male, n (%) | 65 (51.6) | 63 (52.9) | 128 (52.8) |
| Hoehn and Yahr, stage | 2.0 (0.5) | 2.1 (0.5) | 2.0 (0.5) |
| PD duration, year | 5.1 (3.6) | 5.3 (3.6) | 5.2 (3.6) |
| MDS-UPDRS motor score | 23.7 (10.8) | 24.7 (11.5) | 24.2 (11.1) |
| PDQ-8 summary index | 17.4 (13.2) | 18.4 (14.6) | 17.9 (13.9) |
| Daily OFF time, h | 3.4 (1.0) | 3.4 (1.0) | 3.4 (1.0) |
| Total ON time, h | 12.8 (1.6) | 12.8 (1.6) | 12.8 (1.6) |
| ON time without dyskinesia, h | 11.6 (2.6) | 11.2 (3.3) | 11.4 (3.0) |
| Levodopa dose at baseline, mg | 398.3 (117.4) | 412.4 (119.5) | 405.2 (118.7) |
| Patients receiving 3 or 4 levodopa intakes per day, n (%)a | |||
| 3 intakes | 101 (80.2) | 93 (78.2) | 194 (79.5) |
| 4 intakes | 24 (19.0) | 25 (21.0) | 49 (20.1) |
| Concomitant therapy, n (%)a | 106 (84.1) | 99 (83.9) | 205 (84.0) |
| Dopamine agonist | 77 (61.1) | 75 (63.6) | 152 (62.3) |
| Pramipexole | 56 (44.4) | 53 (44.9) | 109 (44.7) |
| Rotigotine | 5 (4.0) | 5 (4.2) | 10 (4.1) |
| Ropinirole | 22 (17.5) | 19 (16.1) | 41 (16.8) |
| MAO-B inhibitor | 67 (53.2) | 67 (56.8) | 134 (54.9) |
| Rasagiline | 59 (46.8) | 51 (43.2) | 110 (45.1) |
| Safinamide | 7 (5.6) | 13 (11.0) | 20 (8.2) |
| Selegiline | 2 (1.6) | 3 (2.5) | 5 (2.0) |
| LEDDb, mg | 556.8 (166.5) | 575.6 (170.7) | 565.9 (168.5) |
Data are mean (SD) unless otherwise specified
aOne patient took 5 intakes in the opicapone 50 mg group; bcalculated for Levodopa, MAO-Bi and dopamine agonists only
LEDD levodopa equivalent daily dose, MAO-Bi Monoamine oxidase B inhibitor; MDS-UPDRS Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale, PD Parkinson’s disease, PDQ Parkinson’s disease questionnaire
Efficacy
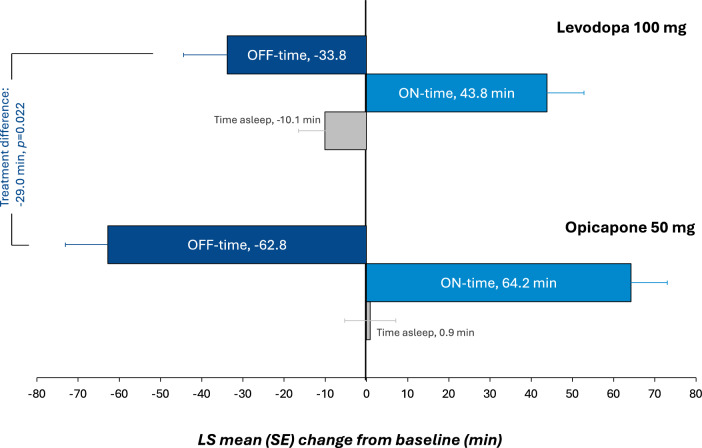
Results for the integrated efficacy analysis are summarized in Table 2. For the key efficacy endpoint, the adjusted (least squares [LS]) mean (SE) change from baseline to end of study treatment in absolute OFF time was − 62.8 min [8.8] in the opicapone 50 mg and − 33.8 min [9.0] in the levodopa 100 mg group, resulting in a significant difference of − 29.0 [− 53.8, − 4.2] min favoring opicapone 50 mg (p = 0.022) (Fig. 1). Results were similar in males and females (Table e2). The percentage of OFF time reduction was also significantly higher with opicapone vs levodopa (p = 0.03), and the proportion of patients with a reduction in OFF time of at least 1 h was numerically higher in the opicapone 50 mg group than in the levodopa 100 mg group (56.7% vs. 45.6%, respectively).
Table 2.
Change from baseline to week 4 in outcome measures (full analysis set)
| Opicapone 50 mg N = 122 |
Levodopa 100 mg N = 118 | |
|---|---|---|
| OFF time (min) | ||
| LS mean (SE) change from baseline | − 62.8 (8.8) | − 33.8 (9.0) |
| LS mean difference versus levodopa 100 mg (95% CI) | − 29.0 (− 53.8, − 4.2) | |
| p value for opicapone 50 mg versus levodopa 100 mg | 0.0222 | |
| Percent OFF time (%) | ||
| LS mean (SE) change from baseline | − 6.5 (0.9) | − 3.7 (0.9) |
| LS mean difference versus levodopa 100 mg (95% CI) | − 2.8 (− 5.4, − 0.3) | |
| p-value for opicapone 50 mg versus levodopa 100 mg | 0.0309 | |
| OFF-time responder rate (reduction of ≥ 1 h); n/N (%) | 69/122 (56.7%) | 54/116 (45.6%) |
| Total ON time (min) | ||
| LS mean (SE) change from baseline | 64.2 (10.3) | 43.8 (10.6) |
| LS mean difference versus levodopa 100 mg (95% CI) | 20.4 (− 8.8, 49.6) | |
| p value for opicapone 50 mg versus levodopa 100 mg | 0.1693 | |
| ON time without dyskinesia (min) | ||
| LS mean (SE) change from baseline | 54.9 (12.1) | 59.3 (12.4) |
| LS mean difference versus levodopa 100 mg (95% CI) | − 4.4 (− 38.5, 29.7) | |
| p value for opicapone 50 mg versus levodopa 100 mg | 0.8008 | |
| ON time with troublesome dyskinesia (min) | ||
| LS mean (SE) change from baseline | − 3.0 (6.2) | − 2.1 (6.4) |
| LS mean difference versus levodopa 100 mg (95% CI) | − 0.9 (− 18.3, 16.6) | |
| p value for opicapone 50 mg versus levodopa 100 mg | 0.9231 | |
| Asleep (min) | ||
| LS mean (SE) change from baseline | 0.9 (6.2) | − 10.1 (6.4) |
| LS mean difference versus levodopa 100 mg (95% CI) | 10.9 (− 6.6, 28.4) | |
| p value for opicapone 50 mg versus levodopa 100 mg | 0.2217 | |
| MDS-UPDRS scores | ||
| Part III | ||
| LS mean (SE) change from baseline | − 4.1 (0.6) | − 2.5 (0.6) |
| LS mean difference versus levodopa 100 mg (95% CI) | − 1.7 (− 3.3, − 0.04) | |
| p value for opicapone 50 mg versus levodopa 100 mg | 0.0445 | |
| Part IV | ||
| LS mean (SE) change from baseline | − 1.1 (0.2) | − 0.8 (0.2) |
| LS mean difference versus levodopa 100 mg (95% CI) | − 0.3 (− 0.7, 0.1) | |
| p value for opicapone 50 mg versus levodopa 100 mg | 0.1734 | |
| PDQ-8 summary index | ||
| LS mean (SE) change from baseline | − 2.7 (1.0) | − 1.9 (1.0) |
| LS mean difference versus levodopa 100 mg (95% CI) | − 0.9 (− 3.6, 1.9) | |
| p value for opicapone 50 mg versus levodopa 100 mg | 0.5447 | |
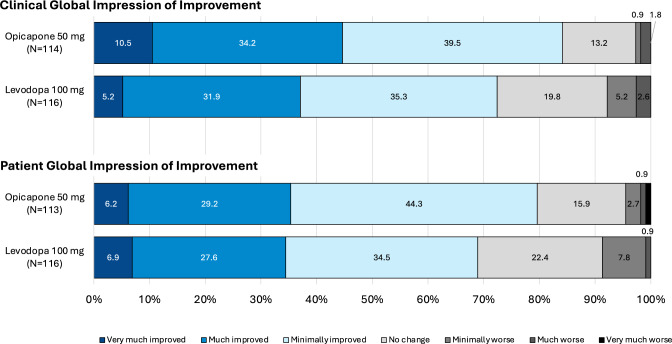
| Improvement on CGI-Ia, n/N (%) | 96/114 (84.2%) | 84/116 (72.4%) |
| Improvement on PGI-Ia, n/N (%) | 90/113 (79.7%) | 80/116 (69.0%) |
| Mean (SD) LEDD at end of study | 744.4b (218.5) | 664.9 (181.7) |
Significant p values are in bold. aincludes any improvement (minimal, much, and very much); bapplying 0.5 conversion factor for opicapone (same as tolcapone). Abbreviations: CI, confidence interval; CGI-I, Clinical Global Impressions of Improvement; LS, least square; LEDD, levodopa equivalent daily dose; MDS-UPDRS, Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale; PDQ, Parkinson’s disease questionnaire; SD, standard deviation; PGI-I, Patient’s Global Impression of Improvement; SE, standard error
Fig. 1 .
LS mean (SE) change from baseline to end of study treatment in absolute OFF, ON and asleep time. The treatment difference for change in ON time was non-significant. LS least square, SE standard error
Findings from the other diary-reported efficacy endpoints showed non-significant trends in line with the reduction in OFF time. Treatment with opicapone 50 mg resulted in a correspondingly greater increase than treatment with levodopa 100 mg in total ON time (64.2 min vs. 43.8 min; p = 0.17); most of which was without any dyskinesia. Adjusted mean ON time with troublesome dyskinesia remained unchanged with no difference between groups (–3.0 min with opicapone vs − 2.1 min with levodopa 100 mg; p = 0.9231).
A higher proportion of patients in the opicapone 50 mg group than those in the levodopa 100 mg group showed improvements (minimally, much, or very much) from baseline to end of study treatment as assessed by CGI-I and PGI-I (Fig. 2). Overall, both groups showed improvements from baseline to end of study in MDS-UPDRS and PDQ-8 scores. The change from baseline to end of study treatment in MDS-UPDRS III (motor) scores (during ON) was significantly greater for the opicapone 50 mg group than the levodopa 100 mg group (− 4.1 vs − 2.5, respectively; p = 0.04). While levodopa dose adjustments were minimal during the study, the mean LEDD was higher for the opicapone 50 mg group than for the levodopa 100 mg group (Table 2).
Fig. 2.
Clinical and patient global impressions of improvement
Safety
Overall, 38% of patients reported at least one AE during treatment with opicapone compared with 25% in the levodopa 100 mg group (Table 3). Dyskinesia was the most frequently reported AE possibly related to the study drug, with the highest incidence observed in the opicapone 50 mg group (9 cases, 7%). The incidence of serious AEs was low (< 3%) and similar between the treatment groups; only one case was judged to be related to the study drug and no deaths occurred during the study.
Table 3.
Summary of safety (safety analysis set)
| Opicapone 50 mg N = 125 |
Levodopa 100 mg N = 118 |
|
|---|---|---|
| Any AE, n (%) * | 47 (37.6) | 29 (24.6) |
| Dyskinesia | 9 (7.2) | 5 (4.2) |
| Dizziness | 8 (6.4) | 3 (2.5) |
| Nausea | 3 (2.4) | 4 (3.4) |
| Insomnia | 4 (3.2) | – |
| Constipation | 4 (3.2) | 2 (1.7) |
| Severity, n (%) | ||
| Mild | 42 (33.6) | 24 (20.3) |
| Moderate | 9 (7.2) | 6 (5.1) |
| Severe | 1 (0.8) | 1 (0.8) |
| Serious AEs, n (%) | 3 (2.4) | 2 (1.7) |
| Spinal compression fracture | 2 (1.6) | – |
| Subdural hematoma | 1 (0.8) | – |
| Upper limb fracture | – | 1 (0.8) |
| Retinal detachment | – | 1 (0.8) |
| AEs leading to discontinuation, n (%) | 4 (3.2) | 2 (1.7) |
| Vertigo | 1 (0.8) | – |
| Somnolence | 1 (0.8) | – |
| Tremor | 1 (0.8) | – |
| Spinal compression fracture | 1 (0.8) | – |
| Upper limb fracture | – | 1 (0.8) |
| Dizziness | 1 (0.8) | – |
| Insomnia | – | 1 (0.8) |
| Rash | 1 (0.8) | – |
| Any drug-related AE, n (%)a | 32 (25.6) | NAb |
| Dyskinesia | 6 (4.8) | – |
| Dizziness | 5 (4.0) | – |
| Constipation | 4 (3.2) | – |
| Insomnia | 4 (3.2) | – |
| Headache | 3 (2.4) | – |
| Nausea | 3 (2.4) | – |
| Somnolence | 2 (1.6) | – |
| Serious drug-related AE, n (%) | 1 (0.8) | – |
| Subdural hematoma | 1 (0.8) | – |
n represents number of patients
a ≥ 3% of patients; brelationship was only considered for the opicapone group
AE adverse event
The percentage of patients who discontinued due to AEs was low (< 5%). There was no common AE leading to discontinuation; all six patients who discontinued due to AEs reported different reasons (Table 3).
Discussion
Results of this preplanned pooled analysis of two randomized 4-week studies demonstrate that the introduction of adjunct opicapone 50 mg was significantly more efficacious in reducing OFF time compared with adding another levodopa dose of 100 mg in patients with early wearing-off fluctuations. Treatment with opicapone 50 mg was generally safe and well tolerated.
The ADOPTION clinical program was designed to address the common practical question of which strategies should be used to treat early wearing-off symptoms. In this analysis, the mean treatment difference in OFF time between the introduction of adjunct opicapone and the addition of an extra 100 mg/day of levodopa was 29 min. While this is below the minimal clinically important difference (MCID) of 1 h previously reported for OFF time versus placebo [21], it is pertinent that the difference is versus an active intervention (an additional 100 mg of levodopa) and not placebo. Since all patients were already receiving levodopa therapy—and the comparator treatment was an additional levodopa dose—we did not expect any between group differences in motor symptom severity. However, in line with recent data showing that opicapone provides additional symptomatic efficacy in levodopa-treated patients [22], MDS-UPDRS III scores (during ON) showed significantly greater improvements with the introduction of opicapone 50 mg versus the addition of levodopa 100 mg. One of the key findings in the pivotal studies was the maintenance of levodopa dose over an extended period of time (in contrast to the frequent changes often required when trying levodopa modification strategies). Unfortunately, this study was too short to examine this important aspect of therapy, which could also impact the development of dyskinesia.
This pooled analysis was preplanned to reduce recruitment time pressures for each single study, although recruitment was easier in South Korea than Europe (where it was harder to find eligible patients not already on opicapone). When analyzed separately, only the South Korean study showed a significant difference favoring opicapone [15], while OFF time reductions were similar for both treatment arms in the European study (Table e2). However, the European study was smaller and included a subset of ‘outlier’ patients who, in post hoc analyses, showed signs of atypical disease progression. Other potential reasons for the difference between the two studies could include novelty bias (opicapone had only been launched 6 months earlier in South Korea), and in how the additional levodopa doses were given (the method of administering the additional 100 mg levodopa was not specified in the protocol). For example, in the South Korean study, > 90% of patients came into the study on 3 daily intakes of levodopa, and the additional 100 mg was often distributed across those three intakes and not as a 4th full intake. By contrast, about half of the patients in the European study were on 4 daily levodopa doses, and the additional 100 mg of levodopa was frequently added as a full 4th or 5th intake (i.e., shortening of the interdose interval). The relative efficacy of increasing levodopa dose frequency (fractionation) vs increasing individual doses has not been well studied.
Opicapone was safe and well tolerated, and most AEs, in both groups, were as expected for a dopaminergic replacement therapy. Consistent with the higher LEDD in the opicapone group, the incidence of treatment-emergent dyskinesia reported as an AE was slightly higher in the opicapone than the levodopa 100 mg group; however, this was mainly reported as mild. On the other hand, patients showed an increase in ON time without any dyskinesia. Recent pharmacokinetic data suggest that this may be because opicapone acts to improve the levodopa AUC without significant impact the levodopa Cmax [23].
Strengths of the ADOPTION clinical program lie in the similar designs of the study which enabled patient level integration of data. Similar studies in other countries are currently under consideration and could be added to the dataset. Although open-label, the program was designed to include two randomized studies that evaluated the introduction of adjunct opicapone against one of the most commonly used and potent strategies—namely levodopa dose increase. The study also specifically recruited patients who were earlier in their disease course than the pivotal studies (patients in the BIPARK studies had more advanced PD as evidence by a longer disease duration of ~ 8 years; baseline daily OFF time of ~ 6 h; and a daily levodopa dose of ~ 700 mg/day); as such, the results of the current study should not be compared to the pivotal studies. Limitations of the program lie in its open-label design, variability in the ways that the additional 100 mg levodopa was given (the manner was not prespecified nor recorded, although the European preference for giving the 100 mg levodopa as a 4th or 5th intake vs the South Korean preference for remaining at 3 intakes is evidenced by diary data) and short study duration. Further prospective studies powered to evaluate direct comparisons between opicapone and different levodopa regimens are warranted. Previous post hoc analyses of the pivotal studies have suggested added benefit of opicapone as a first-line adjunctive therapy to levodopa [14]. While patient numbers were too low in the present analysis, it would also be of interest to compare outcomes in patients previously on levodopa monotherapy (i.e., opicapone as first-line adjunct therapy).
In summary, the results of this pooled analysis indicate that opicapone is a well-tolerated and effective option for patients who have developed the early signs of wearing-off.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The ADOPTION clinical program was funded by BIAL—Portela & Ca, S.A. and SK Chemicals (Korean study). Medical writing assistance was provided by Anita Chadha-Patel PhD (ACP Clinical Communications Ltd, funded by BIAL—Portela & Ca, S.A) in accordance with good publications practice.
Author contributions
All the authors contributed to the study design. Joaquim J. Ferreira, Jee-Young Lee, Hyeo-il Ma, Beomseok Jeon, Werner Poewe, Angelo Antonini, Fabrizio Stocchi, and Olivier Rascol were study investigators. The first draft of the manuscript was written by Joaquim J. Ferreira and José-Francisco Rocha, and all the authors commented on previous versions of the manuscript. All the authors read and approved the final manuscript.
Funding
Open access funding provided by FCT|FCCN (b-on). Joaquim J. Ferreira has provided consultancy and received speaker fees from Lundbeck, BIAL, Biogen, Acadia, Abbvie, Sunovion Pharmaceuticals, Zambon, Affiris, Roche, ONO and SK Chemicals and has received grants from Abbvie, Bial, Medtronic and Angelini. Jee-Young Lee received a research grant of NRF funded by the MSIT of Korea and a multidisciplinary research grant-in-aid and a focused clinical research grant-in-aid from the Seoul Metropolitan Government-Seoul National University (SMG-SNU) Boramae Medical Center, and speaker honorarium from Esai Korea, Bial, SK chemicals and Scientific Advisory Board of Regeners Inc. Beomseok Jeon has received research grant from Peptron and Abbvie Korea and has participated in an expert panel for Aspen Neuroscience. Werner Poewe has received lecture fees and honoraria for consultancy in relation to clinical drug development programs from Alterity, AbbVie, Affiris, AstraZeneca, Axovant, BIAL, Biogen, Britannia, Lilly, Lundbeck, NeuroDerm, Neurocrine, Denali Pharmaceuticals, Orion Pharma, Roche, Stada, Sunovion, Takeda, UCB and Zambon, as well as grant support from the MJFF and the EU FP7 & Horizon 2020 programs. Angelo Antonini has received compensation for consultancy and speaker-related activities from UCB, Boehringer Ingelheim, Britannia, AbbVie, Zambon, Bial, NeuroDerm, Theravance Biopharma, Roche; he receives research support from Chiesi Pharmaceuticals, Lundbeck, Horizon 2020—Grant 825785, Horizon2020 Grant 101016902, Ministry of Education University and Research (MIUR) Grant ARS01_01081, Cariparo Foundation. He serves as consultant for Boehringer Ingelheim for legal cases on pathological gambling; owns Patent WO2015110261-A1; and owns shares in PD Neurotechnology Limited. Fabrizio Stocchi has received compensation for consultancy and speaker-related activities from Lundbeck, UCB, Chiesi, Zambon, Britannia, Cynapsus, Sunovion, Kyowa, Abbvie, NeuroDerm, Biogen, Bial. Daniela M. Rodrigues, Miguel M. Fonseca, Guillermo Castilla-Fernández, Joerg Holenz and José-Francisco Rocha are employed by BIAL. Olivier Rascol has participated in advisory boards and/or provided consultancy for AbbVie, Adamas, Acorda, Addex, AlzProtect, ApoPharma, AstraZeneca, Axovant, Bial, Biogen, Britannia, Buckwang, CereSpir, Clevexel, Denali, INC Research, IPMDS, Lundbeck, Lupin, Merck, MundiPharma, NeurATRIS, NeuroDerm, Novartis, ONO Pharma, Osmotica, Parexel, Pfizer, Prexton Therapeutics, Quintiles, Roche, Sanofi, Servier, Sunovion, Theranexus, Takeda, Teva, UCB, Vectura, Watermark Research, XenoPort, XO, Zambon; received grants from Agence Nationale de la Recherche (ANR), CHU de Toulouse, France-Parkinson, INSERM-DHOS Recherche Clinique Translationnelle, MJ Fox Foundation, Programme Hospitalier de Recherche Clinique, European Commission (FP7, H2020), Cure Parkinson UK; and received a grant to participate in a symposium and contribute to the review of an article by the International Parkinson and Movement Disorder Society.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Declarations
Conflict of interest
Joaquim J. Ferreira has provided consultancy and received speaker fees from Lundbeck, BIAL, Biogen, Acadia, Abbvie, Sunovion Pharmaceuticals, Zambon, Affiris, Roche, ONO and SK Chemicals and has received grants from Abbvie, Bial, Medtronic and Angelini. Jee-Young Lee received a research grant of NRF funded by the MSIT of Korea and a multidisciplinary research grant-in-aid and a focused clinical research grant-in-aid from the Seoul Metropolitan Government-Seoul National University (SMG-SNU) Boramae Medical Center, and speaker honorarium from Esai Korea, Bial, SK chemicals and Scientific Advisory Board of Regeners Inc. Beomseok Jeon has received research grant from Peptron and Abbvie Korea and has participated in an expert panel for Aspen Neuroscience. Werner Poewe has received lecture fees and honoraria for consultancy in relation to clinical drug development programs from Alterity, AbbVie, Affiris, AstraZeneca, Axovant, BIAL, Biogen, Britannia, Lilly, Lundbeck, NeuroDerm, Neurocrine, Denali Pharmaceuticals, Orion Pharma, Roche, Stada, Sunovion, Takeda, UCB and Zambon, as well as grant support from the MJFF and the EU FP7 & Horizon 2020 programs. Angelo Antonini has received compensation for consultancy and speaker-related activities from UCB, Boehringer Ingelheim, Britannia, AbbVie, Zambon, Bial, NeuroDerm, Theravance Biopharma, Roche; he receives research support from Chiesi Pharmaceuticals, Lundbeck, Horizon 2020 - Grant 825785, Horizon2020 Grant 101016902, Ministry of Education University and Research (MIUR) Grant ARS01_01081, Cariparo Foundation. He serves as consultant for Boehringer Ingelheim for legal cases on pathological gambling; owns Patent WO2015110261-A1; and owns shares in PD Neurotechnology Limited. Fabrizio Stocchi has received compensation for consultancy and speaker related activities from Lundbeck, UCB, Chiesi, Zambon, Britannia, Cynapsus, Sunovion, Kyowa, Abbvie, NeuroDerm, Biogen, Bial. Daniela M. Rodrigues, Miguel M. Fonseca, Guillermo Castilla-Fernández, Joerg Holenz and José-Francisco Rocha are employed by BIAL. Olivier Rascol has participated in advisory boards and/or provided consultancy for AbbVie, Adamas, Acorda, Addex, AlzProtect, ApoPharma, AstraZeneca, Axovant, Bial, Biogen, Britannia, Buckwang, CereSpir, Clevexel, Denali, INC Research, IPMDS, Lundbeck, Lupin, Merck, MundiPharma, NeurATRIS, NeuroDerm, Novartis, ONO Pharma, Osmotica, Parexel, Pfizer, Prexton Therapeutics, Quintiles, Roche, Sanofi, Servier, Sunovion, Theranexus, Takeda, Teva, UCB, Vectura, Watermark Research, XenoPort, XO, Zambon; received grants from Agence Nationale de la Recherche (ANR), CHU de Toulouse, France-Parkinson, INSERM-DHOS Recherche Clinique Translationnelle, MJ Fox Foundation, Programme Hospitalier de Recherche Clinique, European Commission (FP7, H2020), Cure Parkinson UK; and received a grant to participate in a symposium and contribute to the review of an article by the International Parkinson and Movement Disorder Society.
References
- 1.Pringsheim T, Day GS, Smith DB, Rae-Grant A, Licking N, Armstrong MJ, de Bie RMA, Roze E, Miyasaki JM, Hauser RA, Espay AJ, Martello JP, Gurwell JA, Billinghurst L, Sullivan K, Fitts MS, Cothros N, Hall DA, Rafferty M, Hagerbrant L, Hastings T, O’Brien MD, Silsbee H, Gronseth G, Lang AE (2021) Dopaminergic therapy for motor symptoms in early Parkinson disease practice guideline summary: a report of the AAN guideline subcommittee. Neurology 97:942–957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jenner P, Rocha JF, Ferreira JJ, Rascol O, Soares-da-Silva P (2021) Redefining the strategy for the use of COMT inhibitors in Parkinson’s disease: the role of opicapone. Expert Rev Neurother 21:1019–1033 [DOI] [PubMed] [Google Scholar]
- 3.Stocchi F, Jenner P, Obeso JA (2010) When do levodopa motor fluctuations first appear in Parkinson’s disease? Eur Neurol 63:257–266 [DOI] [PubMed] [Google Scholar]
- 4.Stocchi F, Antonini A, Barone P, Tinazzi M, Zappia M, Onofrj M, Ruggieri S, Morgante L, Bonuccelli U, Lopiano L, Pramstaller P, Albanese A, Attar M, Posocco V, Colombo D, Abbruzzese G, group Ds (2014) Early DEtection of wEaring off in Parkinson disease: the DEEP study. Parkinsonism Relat Disord 20:204–211 [DOI] [PubMed] [Google Scholar]
- 5.Olanow CW, Kieburtz K, Rascol O, Poewe W, Schapira AH, Emre M, Nissinen H, Leinonen M, Stocchi F, Stalevo Reduction in Dyskinesia Evaluation in Parkinson’s Disease I (2013) Factors predictive of the development of Levodopa-induced dyskinesia and wearing-off in Parkinson’s disease. Mov Disord 28:1064–1071 [DOI] [PubMed] [Google Scholar]
- 6.Colombo D, Abbruzzese G, Antonini A, Barone P, Bellia G, Franconi F, Simoni L, Attar M, Zagni E, Haggiag S, Stocchi F (2015) The “gender factor” in wearing-off among patients with Parkinson’s disease: a post hoc analysis of DEEP study. ScientificWorldJournal 2015:787451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Armstrong MJ, Okun MS (2020) Diagnosis and treatment of Parkinson disease: a review. JAMA 323:548–560 [DOI] [PubMed] [Google Scholar]
- 8.Grosset KA, Bone I, Grosset DG (2005) Suboptimal medication adherence in Parkinson’s disease. Mov Disord 20:1502–1507 [DOI] [PubMed] [Google Scholar]
- 9.Grosset D, Antonini A, Canesi M, Pezzoli G, Lees A, Shaw K, Cubo E, Martinez-Martin P, Rascol O, Negre-Pages L, Senard A, Schwarz J, Strecker K, Reichmann H, Storch A, Löhle M, Stocchi F, Grosset K (2009) Adherence to antiparkinson medication in a multicenter European study. Mov Disord Off J Mov Disord Soc 24:826–832 [DOI] [PubMed] [Google Scholar]
- 10.Fox SH, Katzenschlager R, Lim SY, Barton B, de Bie RMA, Seppi K, Coelho M, Sampaio C, Movement Disorder Society Evidence-Based Medicine C (2018) International Parkinson and movement disorder society evidence-based medicine review: Update on treatments for the motor symptoms of Parkinson’s disease. Mov Disord 33:1248–1266 [DOI] [PubMed] [Google Scholar]
- 11.Ferreira JJ, Lees A, Rocha JF, Poewe W, Rascol O, Soares-da-Silva P, Bi-Park i, (2016) Opicapone as an adjunct to levodopa in patients with Parkinson’s disease and end-of-dose motor fluctuations: a randomised, double-blind, controlled trial. Lancet Neurol 15:154–165 [DOI] [PubMed] [Google Scholar]
- 12.Lees AJ, Ferreira J, Rascol O, Poewe W, Rocha JF, McCrory M, Soares-da-Silva P, Investigators B-S (2017) Opicapone as Adjunct to levodopa therapy in patients with Parkinson disease and motor fluctuations: a randomized clinical trial. JAMA Neurol 74:197–206 [DOI] [PubMed] [Google Scholar]
- 13.Ferreira JJ, Lees A, Rocha JF, Poewe W, Rascol O, Soares-da-Silva P (2019) Long-term efficacy of opicapone in fluctuating Parkinson’s disease patients: a pooled analysis of data from two phase 3 clinical trials and their open-label extensions. Eur J Neurol 26:953–960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rocha JF, Ebersbach G, Lees A, Tolosa E, Ferreira JJ, Poewe W, Rascol O, Stocchi F, Antonini A, Magalhaes D, Gama H, Soares-da-Silva P (2021) The added benefit of opicapone when used early in Parkinson’s disease patients with levodopa-induced motor fluctuations: a post-hoc analysis of BIPARK-I and -II. Front Neurol 12:754016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee JY, Ma HI, Ferreira JJ, Rocha JF, Sung YH, Song IU, Ahn TB, Kwon DY, Cheon SM, Kim JM, Lee CS, Lee PH, Park JH, Lee JH, Park MY, Kim SJ, Baik JS, Choi SM, Shin HW, Lee HW, Kang SY, Jeon B (2024) Opicapone to treat early wearing-off in Parkinson’s disease patients: the Korean ADOPTION trial. Mov Disord Clin Pract 11:655–665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hauser RA, Friedlander J, Zesiewicz TA, Adler CH, Seeberger LC, O’Brien CF, Molho ES, Factor SA (2000) A home diary to assess functional status in patients with Parkinson’s disease with motor fluctuations and dyskinesia. Clin Neuropharmacol 23:75–81 [DOI] [PubMed] [Google Scholar]
- 17.Park J, Koh SB, Kwon KY, Kim SJ, Kim JW, Kim JS, Park KW, Paik JS, Sohn YH, Ahn JY, Oh E, Youn J, Lee JY, Lee PH, Jang W, Kim HJ, Jeon BS, Chung SJ, Cho JW, Cheon SM, Kang SY, Park MY, Park S, Huh YE, Kang SJ, Kim HT (2020) Validation study of the official korean version of the movement disorder society-unified Parkinson’s disease rating scale. J Clin Neurol 16:633–645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goetz CG, Tilley BC, Shaftman SR, Stebbins GT, Fahn S, Martinez-Martin P, Poewe W, Sampaio C, Stern MB, Dodel R, Dubois B, Holloway R, Jankovic J, Kulisevsky J, Lang AE, Lees A, Leurgans S, LeWitt PA, Nyenhuis D, Olanow CW, Rascol O, Schrag A, Teresi JA, van Hilten JJ, LaPelle N, Movement Disorder Society URTF (2008) Movement disorder society-sponsored revision of the unified parkinson’s disease rating scale (MDS-UPDRS): scale presentation and clinimetric testing results. Mov Disord 23:2129–2170 [DOI] [PubMed] [Google Scholar]
- 19.Jenkinson C, Fitzpatrick R, Peto V, Greenhall R, Hyman N (1997) The PDQ-8: development and validation of a short-form parkinson’s disease questionnaire. Psychol Health 12:805–814 [Google Scholar]
- 20.Schade S, Mollenhauer B, Trenkwalder C (2020) Levodopa equivalent dose conversion Factors: An Updated Proposal Including Opicapone and Safinamide. Mov Disord Clin Pract 7:343–345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hauser RA, Gordon MF, Mizuno Y, Poewe W, Barone P, Schapira AH, Rascol O, Debieuvre C, Frassdorf M (2014) Minimal clinically important difference in Parkinson’s disease as assessed in pivotal trials of pramipexole extended release. Parkinsons Dis 2014:467131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ferreira JJ, Rascol O, Stocchi F, Antonini A, Moreira J, Castilla-Fernandez G, Rocha JF, Holenz J, Poewe W (2023) Opicapone as adjunctive to levodopa-treated Parkinson's disease patients without motor complications: preliminary data from the EPSILON Study [Abstract]. https://www.mdscongress.org/IC23-Late-BreakingAbstractBooklet.pdf.
- 23.Ferreira JJ, Poewe W, Rascol O, Stocchi F, Antonini A, Moreira J, Guimaraes B, Rocha JF, Soares-da-Silva P (2022) Effect of opicapone on levodopa pharmacokinetics in patients with fluctuating Parkinson’s disease. Mov Disord 37:2272–2283 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.