Abstract
Effective antihypertensive therapy is essential for achieving optimal blood pressure (BP) control and reducing cardiovascular events. This double-blind, multicenter, randomized trial aimed to compare the antihypertensive efficacy and safety of a combination of amlodipine (AML) and candesartan cilexetil (CC) versus AML monotherapy in patients with essential hypertension (HTN). After a 4-week run-in period with AML 5 mg, patients whose HTN remained uncontrolled (diastolic BP [DBP]) ≥ 90 mmHg and < 120 mmHg) were randomized to receive either AML + CC or AML alone for 8 weeks. Efficacy was assessed by measuring changes in DBP and systolic BP (SBP). The primary safety measure was the incidence of adverse events (AEs). A total of 174 participants were included in the efficacy analysis. After 8 weeks, DBP decreased by -9.92 ± 0.86 mmHg in the AML + CC arm and - 2.08 ± 0.86 mmHg in the AML arm (p < 0.0001). SBP decreased by -14.27 ± 1.39 mmHg in the AML + CC arm versus - 2.77 ± 1.39 mmHg in the AML arm (p < 0.0001). AEs occurred in 11.24% of the AML + CC group and 5.62% of the AML group (p = 0.1773). AML + CC combination therapy demonstrated superior efficacy with good tolerance, making it a promising option for patients with inadequately controlled hypertension on amlodipine alone.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-74003-5.
Keywords: HTN, Amlodipine, Candesartan Cilexetil, Blood pressure, Angiotensin receptor blocker, Antihypertensive
Subject terms: Cardiology, Hypertension, Drug development
Introduction
Hypertension (HTN) represents a paramount global health challenge, particularly because its prevalence continues to increase, affecting approximately one-third of the adult population worldwide. This silent but insidious condition is the principal risk factor for several devastating cardiovascular events, including stroke, myocardial infarction, heart failure, and renal dysfunction1. Given its widespread impact on public health, the control of blood pressure (BP) is crucial. The cornerstone of HTN management is BP control, which has consistently been shown to reduce the incidence of cardiovascular events and overall mortality2. Based on the recent Systolic Blood Pressure Intervention Trial, guidelines recommend more intensive BP control in addition to lifestyle modification1–5. Further, combination antihypertensive therapy has emerged as a compelling strategy to address the multifactorial nature of HTN, enhancing treatment outcomes1–3.
More than two-thirds of patients with HTN have a BP uncontrolled by a single agent, and a combination of antihypertensive agents with different mechanisms of action is needed. As the BP-lowering effect slightly differs among agents, it is difficult to predict outcomes; hence, the combination of agents with demonstrated clinical efficacy is preferred1–3. The combination of angiotensin II receptor blockers (ARBs) and calcium channel blockers (CCBs) is recognized as an effective combination therapy6–8. Further, antihypertensive combination therapy is increasing treatment compliance9,10.
Candesartan cilexetil (CC) is an ARB that blocks the binding of angiotensin II by selectively binding to the angiotensin type 1 (AT1) receptor, which mediates vasoconstriction and aldosterone secretion. CC is widely used to treat HTN11. Amlodipine besylate (AML) is a dihydropyridine-type CCB that exerts its tonic action primarily by blocking the influx of extracellular calcium ions into the vascular smooth muscle. It similarly is a major antihypertensive12,13. Although there are only a few studies on the combination of AML and CC as a type of CCB and ARB combination, these studies have demonstrated efficacy and safety, mainly in Japan12,13.
Combination therapies with different mechanisms of action may benefit patients with uncontrolled BP by enhancing the BP-lowering effects, counteracting side effects, and increasing adherence. Therefore, this study aimed to investigate the effectiveness and safety of combination therapy with AML and CC in patients with uncontrolled HTN.
Methods
Study design
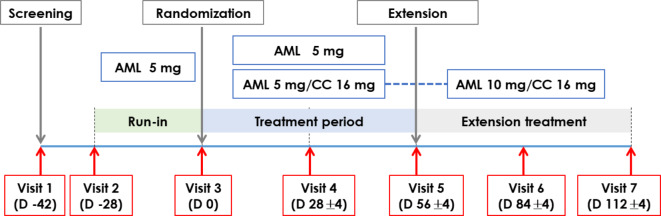
This study is a multicenter, randomized, double-blind study designed to evaluate the antihypertensive efficacy and safety of AML plus CC compared to AML monotherapy in Korean patients with essential hypertension uncontrolled by AML monotherapy, with the additional goal of obtaining approval from the Korean Ministry of Food and Drug Safety (KMFDS) for the use of the newly developed AML/CC fixed-dose combination. Patients with essential HTN who met the eligibility criteria based on screening tests received a 4-week treatment with AML (5-mg tablet) prescribed at visit 2. Participants who had already been on antihypertensive therapy were asked to stop the administration of the previous antihypertensive agent to prevent any potential effects on the study results and to precisely determine the antihypertensive efficacy of the investigational products. After the 4-week single-agent run-in period, participants whose HTN was not adequately controlled (diastolic BP (DBP) ≥ 90 mmHg and < 120 mmHg) were randomized to one of two treatment arms (combination therapy with CC or AML monotherapy) with in 1:1 ratio. The study treatment was taken at the same dose throughout the 8-week treatment period without dose modification (Fig. 1).
Fig. 1.
Study design. AML, amlodipine; CC, candesartan cilexetil.
During the 8-week treatment period, participants visited their site at week 4 (visit 4, day 28 ± 4 days) and week 8 (visit 5, day 56 ± 4 days). After a review of the inclusion/exclusion criteria for extension at visit 5, eligible participants who had been allocated to the combination therapy arm and received the 8-week study treatment visited the site at week 12 (visit 6, day 84 ± 4 days) and week 16 (visit 7, day 112 ± 4 days) and underwent scheduled study procedures during the additional 8-week extension period.
All procedures performed involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. All experimental protocols were approved by the ethics committees of Seoul St. Mary’s Hospital, Kangbuk Samsung Hospital, Korea University Guro Hospital, Korea University Anam Hospital, Seoul National University Bundang Hospital, Asan Medical Center, Seoul Medical Center, Soonchunhyang University Hospital, Ajou University Hospital, Wonju Severance Christian Hospital, Yeungnam University Hospital, Ewha Womans University Hospital, Inje University Busan Paik Hospital, Inje University Ilsan Paik, Inje University Haeundae Paik, Inha University Hospital, Chonnam National University Hospital, Hallym University Sacred Heart Hospital, Severance Hospital, and Daedong Hospital. Written informed consent was obtained from all patients. The trial was registered in the US Clinical Trials Registry (NCT02368665, 23/02/2015).
Study population and randomization
Binary adult men and women aged 19–75 years with essential HTN were recruited from 20 institutions in Korea between December 2014 and July 2015. Patients were randomized if their mean DBP was ≥ 90 mmHg and < 120 mmHg, as measured twice after 4 weeks of single-agent treatment. Patients with a mean DBP ≥ 90 mmHg and < 120 mmHg at visit 5, the end of the treatment period, were further studied as the extension treatment arm. Patients with a mean DBP ≥ 120 mmHg or a mean systolic BP (SBP) ≥ 200 mmHg at screening, a bilateral BP difference of ≥ 10 mmHg in DBP or ≥ 20 mmHg in systolic BP were excluded. Patients with possible secondary HTN, severe heart disease, cerebrovascular disorders, cancer, and dialysis were also excluded. Severe heart disease was defined as heart failure (NYHA class 3 or 4), ischemic heart disease (unstable angina, myocardial infarction), percutaneous coronary intervention, or coronary artery bypass grafting within the last 6 months; and severe cerebrovascular disorder was defined as a diagnosis of stroke, cerebral infarction, or cerebral hemorrhage within the last 6 months. Patients with a mean SBP ≥ 180 mmHg measured at Visit 3 after 4 weeks of AML monotherapy were also excluded.
Due to the absence of similar existing studies, the change in sitting DBP and the standard deviation (SD) of the pooled variance for AML 5 mg/CC 16 mg were estimated using data from a factorial design study by Punzi HA, et al.14, which compared the BP lowering effects of various dose combinations of telmisartan and AML. Based on these estimates, the sample size was calculated with a significance level of 5% and a power of 90% using the following formula, resulting in a requirement of 63 participants per arm.
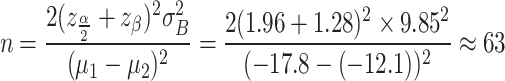 |
Considering a 20% dropout rate, the minimum final enrollment for adequate analysis was targeted to be 79 patients per arm across the combination and monotherapy groups, for a total of 158 patients.
Study outcomes
The primary efficacy outcome was the changes in DBP at week 8 from baseline. The secondary efficacy outcome was the changes in DBP at week 4 and changes in SBP at weeks 4 and 8 from baseline. For patients assigned to the extension treatment arm, changes in DBP and SBP at weeks 12 and 16 compared with those at week 8 of treatment were additionally evaluated. The occurrence of adverse events (AEs) in each system was measured as the safety outcome. The participants were instructed to return all remaining study drugs at each visit during the treatment period, and treatment compliance was assessed by counting the remaining pills.
Statistical analysis
Clinicodemographic patient characteristics were presented using descriptive statistics. Continuous variables were presented as the mean and standard deviation, while categorical variables were presented as the frequency and percentage. The least square (LS) mean change in baseline-adjusted DBP from baseline to after 8 weeks of study treatment and its standard error (SE) were presented. The inter-arm superiority test was conducted using the analysis of covariance (ANCOVA) with change in the DBP from baseline after 8 weeks of treatment as the response variable and the baseline DBP and treatment arm as independent variables. The same statistical approach was applied to the change in DBP at week 8 and in SBP at weeks 4 and 8.
To evaluate the antihypertensive efficacy of the combination of AML 10 mg and CC 16 mg, the mean and standard deviation of the changes in DBP and SBP from week 8 to weeks 12 and 16 of the study treatment were calculated, and statistical tests with the paired t-test or Wilcoxon’s signed rank test were conducted depending on the normality of the change in DBP. Adverse events were counted, and the number of events/reactions and their incidence rates were recorded. The difference in the change from baseline to post-treatment measurements between the combination therapy and monotherapy arms was tested using the unpaired t-test or Wilcoxon’s rank sum test. AEs were compared between the AML 5 mg and AML 5 mg + CC 16 mg groups using the chi-square or Fisher exact test. All statistical analyses were performed using SAS (version 9.3). A p-value of < 0.05 was considered significant.
Results
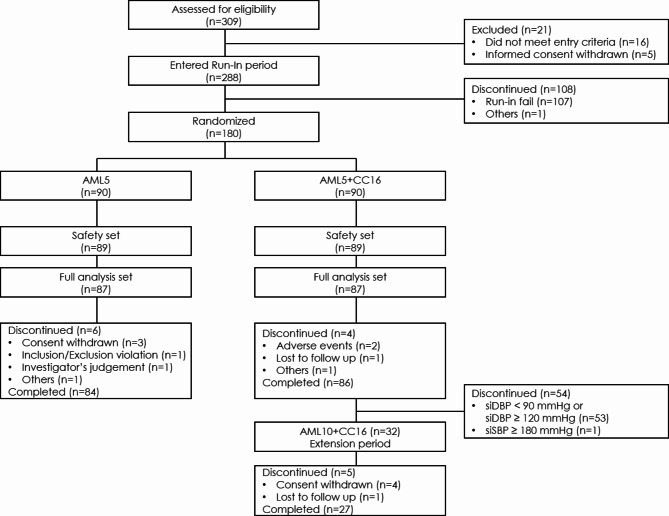
In total, 288 participants were recruited during the run-in period; among them, 180 participants were randomized, and 174 and 178 participants were included in the full analysis set (FAS) and safety set, respectively (Fig. 2). There was no significant difference in mean age (55 years vs. 56 years) and proportion of male patients (84% vs. 89%) at baseline between the AML 5 mg monotherapy and AML 5 mg + CC 16 mg arms. The mean baseline DBP and SBP was 96 mmHg and 148 mmHg, respectively, in the AML arm and was 97 mmHg and 150 mmHg, respectively, in the AML + CC arm (Table 1). In addition, there were no significant differences in the past medical history such as hyperlipidemia and diabetes between the two arms. The concurrent medication history was not different between the two arms, with renin-angiotensin system (RAS) inhibitors being 67.82% in the AML 5 mg arm and 60.92% in the AML 5 mg + CC 16 mg arm, and CCB being 25.29% in the AML 5 mg arm and 33.33% in the AML 5 mg + CC 16 mg arm.
Fig. 2.
Disposition of the participants. AML, amlodipine; CC, candesartan cilexetil.
Table 1.
Comparison of baseline participant characteristics between the study arms.
| AML5 (n = 87) | AML5 + CC16 (n = 87) | p-value | |
|---|---|---|---|
| Age (years) | 55.09 ± 9.11 | 56.21 ± 10.18 | 0.4476 |
| Sex (male (%)) | 73 (83.91) | 77 (88.51) | 0.3792 |
| Weight (kg) | 74.22 ± 12.16 | 75.46 ± 13.07 | 0.5167 |
| Height (cm) | 167.88 ± 6.70 | 168.20 ± 6.71 | 0.7544 |
| SBP (mmHg) | 148.27 ± 11.27 | 150.18 ± 12.68 | 0.2930 |
| DBP (mmHg) | 95.77 ± 4.65 | 96.93 ± 5.86 | 0.1519 |
| Heart rate (beats/min) | 73.86 ± 8.00 | 73.99 ± 10.03 | 0.9209 |
| Past health history | |||
| Hyperlipidemia (N (%)) | 19 (21.84) | 20 (22.99) | 0.8558 |
| Diabetes mellitus (N (%)) | 8 (9.20) | 10 (11.49) | 0.6186 |
| Gout (N (%)) | 3 (3.45) | 1 (1.15) | 0.6206 |
| Cerebral infarction | 0 (0.00) | 1 (1.15) | 1.0000 |
| Angina pectoris | 2 (2.30) | 0 (0.00) | 0.4971 |
| Myocardial infarction | 1 (1.15) | 0 (0.00) | 1.0000 |
| Pre-medication | |||
| RAS inhibitor | 59 (67.82) | 53 (60.92) | 0.3422 |
| Calcium channel blockers | 22 (25.29) | 29 (33.33) | 0.2437 |
| Diuretics | 5 (5.75) | 5 (5.75) | 1.0000 |
| Beta blockers | 6 (6.90) | 4 (4.60) | 0.5148 |
| Lipid modifying agents | 23 (26.44) | 22 (25.29) | 0.8626 |
AML, amlodipine; CC, candesartan cilexetil; SBP, systolic blood pressure; DBP, diastolic blood pressure; RAS, renin–angiotensin system.
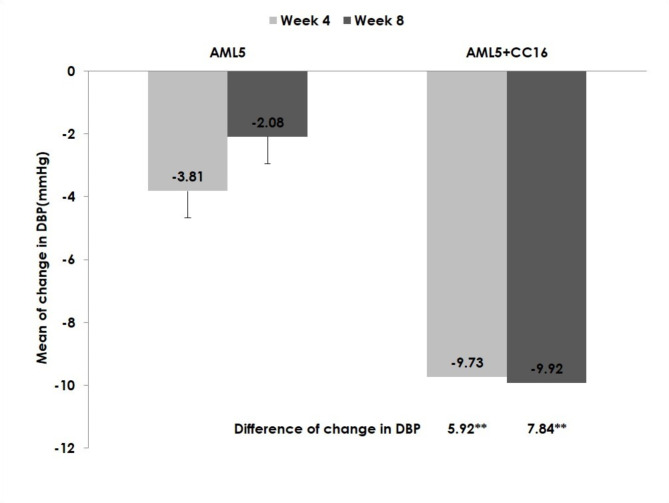
Based on the data from 174 participants in the efficacy set, the LS-mean (± SE) change in DBP from baseline to after 8 weeks of study treatment was - 9.92 ± 0.86 mmHg in the AML 5 mg + CC 16 mg arm and - 2.08 ± 0.86 mmHg in the AML 5 mg arm, with the difference being significant (-7.84 ± 1.22 mmHg, p < 0.0001) (Fig. 3; Table 2). In addition, the LS-mean (± SE) change in DBP from baseline to after 4 weeks of study treatment was - 9.73 ± 0.85 mmHg in the AML 5 mg + CC 16 mg arm and - 3.81 ± 0.85 mmHg in the AML 5 mg arm, and the difference was also significant (-5.92 ± 1.20, p < 0.0001).
Fig. 3.
Change in DBP from baseline. ** p -value less than 0.001. DBP, diastolic blood pressure; AML, amlodipine; CC, candesartan cilexetil.
Table 2.
Mean change in DBP from baseline to after treatment.
| DBP (mmHg) | |||
|---|---|---|---|
| AML5 (n = 87) | AML5 + CC16 (n = 87) | ||
| Baseline | Mean ± SD | 95.77 ± 4.65 | 96.93 ± 5.86 |
| Week 4 | Mean ± SD | 92.16 ± 7.77 | 87.00 ± 9.33 |
| Week 8 | Mean ± SD | 93.78 ± 8.40 | 86.91 ± 9.74 |
| Change from baseline to week 4 |
LS mean ± SE 95% CI |
-3.81 ± 0.85 [-5.48, -2.14] |
-9.73 ± 0.85 [-11.40, -8.06] |
| p-value (within group) 1 | < 0.0001 | < 0.0001 | |
| Between-arm difference in change |
LS mean ± SE 95% CI |
5.92 ± 1.20 [3.55, 8.29] |
|
| p-value (between groups) 2 | < 0.0001 | ||
|
Change from baseline to week 8 |
LS Mean ± SE 95% CI |
-2.08 ± 0.86 [-3.77, -0.39] |
-9.92 ± 0.86 [-11.62, -8.23] |
| p-value (within arm) 1 | 0.0161 | < 0.0001 | |
| Between-arm difference in change |
LS Mean ± SE 95% CI |
7.84 ± 1.22 [5.44, 10.24] |
|
| p-value (between arms) 2 | < 0.0001 | ||
1Unpaired t-test value calculated using ANCOVA with baseline DBP and treatment as independent variables.
2ANCOVA with baseline DBP and treatment as independent variables.
DBP, diastolic blood pressure; AML, amlodipine; CC, candesartan cilexetil; SD, standard deviation; LS, least square; SE, standard error; CI, confidence interval.
The LS-mean (± SE) change in SBP from baseline to after 4 weeks of study treatment was - 13.20 ± 1.39 mmHg in the AML 5 mg + CC 16 mg arm and - 5.28 ± 1.39 mmHg in the AML 5 mg arm, and the difference was significant (-7.92 ± 1.96 mmHg, p < 0.0001) (Fig. 4; Table 3). The LS-mean (± SE) change in SBP from baseline to after 8 weeks of study treatment was - 14.27 ± 1.39 mmHg in the AML 5 mg + CC 16 mg arm and - 2.77 ± 1.39 mmHg in the AML 5 mg arm, and the difference was significant (-11.50 ± 1.97 mmHg, p < 0.0001).
Fig. 4.
Change in SBP from baseline. **p-value less than 0.001. SBP, systolic blood pressure; AML, amlodipine; CC, candesartan cilexetil.
Table 3.
Mean change in SBP from baseline to after treatment.
| SBP (mmHg) | |||
|---|---|---|---|
| AML5 (n = 87) | AML5 + CC16 (n = 87) | ||
| Baseline | Mean ± SD | 148.27 ± 11.27 | 150.18 ± 12.68 |
| Week 4 | Mean ± SD | 143.42 ± 12.92 | 136.56 ± 15.90 |
| Week 8 | Mean ± SD | 145.85 ± 13.78 | 135.56 ± 16.03 |
| Change from baseline to week 4 |
LS Mean ± SE 95% CI |
-5.28 ± 1.39 [-8.01, -2.54] |
-13.20 ± 1.39 [-15.93, -10.46] |
| p-value (within arm) 1 | 0.0002 | < 0.0001 | |
| Between-arm difference in change |
LS Mean ± SE 95% CI |
7.92 ± 1.96 [4.04, 11.80] |
|
| p-value (between arms) 2 | < 0.0001 | ||
| Change from baseline to week 8 |
LS Mean ± SE 95% CI |
-2.77 ± 1.39 [-5.52, -0.03] |
-14.27 ± 1.39 [-17.01, -11.53] |
| p-value (within arm) 1 | 0.0477 | < 0.0001 | |
| Between-arm difference in change |
LS Mean ± SE 95% CI |
11.50 ± 1.97 [7.61, 15.39] |
|
| p-value (between arms) 2 | < 0.0001 | ||
1 Unpaired t-test value calculated using ANCOVA with baseline DBP and treatment as independent variables.
2 ANCOVA with baseline DBP and treatment as independent variables.
SBP, systolic blood pressure; AML, amlodipine; CC, candesartan cilexetil; SD, standard deviation; LS, least square; SE, standard error; CI, confidence interval.
Participants whose mean DBP was ≥ 90 mmHg and < 120 mmHg despite an 8-week study treatment with AML 5 mg + CC 16 mg received combination therapy with an increase in the dose of amlodipine, that is, AML 10 mg + CC 16 mg, for an additional 8 weeks. For these 28 participants, the mean (± SD) change in DBP from week 8 to weeks 12 and 16 of the study treatment were - 8.48 ± 7.34 mmHg and - 9.39 ± 8.39 mmHg, respectively (TableS1). The mean (± SD) changes in SBP from week 8 to weeks 12 and 16 of the study treatment were - 12.39 ± 12.69 mmHg and - 14.50 ± 10.20 mmHg, respectively (Table S2). At week 8, response rates were also 4.60% in the AML 5 mg arm and 25.29% in the AML 10 mg + CC 16 mg arm, respectively (Table S3).
Among the 178 patients included in the safety set, 15 participants reported 19 AEs (Table 4). A total of 11% of the patients (10/89, 12 events) in the AML 5 mg + CC 16 mg arm and 6% of the patients (5/89, 7 events) in the AML 5 mg arm reported AEs. The incidence rate of AEs was not significantly different between the treatment arms (p = 0.1773). The reported AEs included chest discomfort, edema, and dizziness. There was no significant difference in medication compliance or duration between the AML 5 mg + CC 16 mg and AML5 mg arms (Table S4).
Table 4.
Incidence of adverse events.
| AML5 (n = 89) |
AML5 + CC16 (n = 89) |
p-value | |
|---|---|---|---|
| General disorders | 1 (1.12) | 3 (3.37) | 0.6207 |
| Chest discomfort | 0 (0.00) | 2 (2.25) | 0.4972 |
| Chest pain | 0 (0.00) | 1 (1.12) | 1.0000 |
| Edema | 1 (1.12) | 0 (0.00) | 1.0000 |
| Nervous system disorders | 2 (2.25) | 2 (2.25) | 1.0000 |
| Head discomfort | 0 (0.00) | 1 (1.12) | 1.0000 |
| Headache | 0 (0.00) | 1 (1.12) | 1.0000 |
| Dizziness | 2 (2.25) | 0 (0.00) | 0.4972 |
| Gastrointestinal disorders | 0 (0.00) | 1 (1.12) | 1.0000 |
| Pancreatitis | 0 (0.00) | 1 (1.12) | 1.0000 |
| Infections and infestations | 1 (1.12) | 1 (1.12) | 1.0000 |
| Acute sinusitis | 0 (0.00) | 1 (1.12) | 1.0000 |
| Pharyngitis | 0 (0.00) | 1 (1.12) | 1.0000 |
| Nasopharyngitis | 1 (1.12) | 0 (0.00) | 1.0000 |
| Investigations | 1 (1.12) | 1 (1.12) | 1.0000 |
| Liver function test abnormal | 0 (0.00) | 1 (1.12) | 1.0000 |
| Creatine phosphokinase increased | 1 (1.12) | 0 (0.00) | 1.0000 |
| Lactate dehydrogenase increased | 1 (1.12) | 0 (0.00) | 1.0000 |
| Injury and procedural complications | 0 (0.00) | 1 (1.12) | 1.0000 |
| Ligament sprain | 0 (0.00) | 1 (1.12) | 1.0000 |
| Metabolism and nutrition disorders | 0 (0.00) | 1 (1.12) | 1.0000 |
| Hypertriglyceridemia | 0 (0.00) | 1 (1.12) | 1.0000 |
| Musculoskeletal disorders | 0 (0.00) | 1 (1.12) | 1.0000 |
| Myalgia | 0 (0.00) | 1 (1.12) | 1.0000 |
| Reproductive system disorders | 1 (1.12) | 0 (0.00) | 1.0000 |
| Menopausal disorder | 1 (1.12) | 0 (0.00) | 1.0000 |
| Total | 5 (5.62) | 10 (11.24) | 0.1773 |
Data are presented as the n (%).
AML, amlodipine; CC, candesartan cilexetil.
Discussion
This study found that 4 and 8 weeks of treatment with the combination of AML and CC provided superior antihypertensive efficacy, for both DBP and SBP, to AML monotherapy at the corresponding dose without increasing AEs.
A same-dose AML non-responder study demonstrated that the combination of AML and an ARB was well tolerated and effective in reducing BP in patients with uncontrolled BP than AML alone7,15. The current study replicated and extended these findings by demonstrating the superior efficacy and tolerability of AML 5 mg + CC 16 mg over AML 5 mg alone. Additionally, in participants who received further treatment with increased doses of AML and CC for an additional 8 weeks owing to inadequate HTN control, treatment with an increased dose of AML 10 mg and of CC 16 mg was effective for the control of both DBP and SBP, with significant improvements. These results support the usefulness of a high-dose combination of AML and CC in patients with uncontrolled HTN.
BP reduction is known to reduce various cardiovascular risks, with a 10-mmHg reduction in SBP being associated with a 20% reduction in major cardiovascular events, 27% reduction in stroke, and 28% reduction in heart failure (HF)16,17. This is especially true given the recent increase in BP treatment targets and prevalence of HTN in Asians1,2,18. In addition, the recent increase in the incidence of HF, especially in aging populations, underscores the importance of combinations containing ARBs or RAS inhibitors. Candesartan has its own cardioprotective effects in HF, in addition to its BP-lowering effects. Further, it is effective in chronic HF patients with a low-to-preserved ejection fraction, the classification recently emphasized in clinical field19–21. In addition to its cardiovascular effects, candesartan has also been shown to be useful in the treatment of stroke and cognitive impairment22,23.
Previous studies have demonstrated the effectiveness of a fixed-dose combination treatment with AML and CC for BP reduction24,25. Yamaguchi J, et al. demonstrated that AML/CC combination was effective in reducing cardiovascular events compared to AML monotherapy in hypertensive patients with coronary artery disease, and Yasuno S, et al. demonstrated the effectiveness of AML/CC fixed-dose combination in hypertensive patients in Japan. In addition to BP reduction and other clinical benefits, medication adherence is another aspect that must be addressed. In this study, adequate compliance and treatment duration were maintained in both the monotherapy and combination arms, with no significant differences. These findings have important implications in older, multi-medicated patients9,10. In the safety set, 15 participants reported a total of 19 AEs, and 4 participants reported a total of 4 adverse drug reactions (ADRs), including chest discomfort, edema, and dizziness. All four ADR events were consistent with those previously reported for each commercially available single agent, and no other unexpected ADR were observed. This shows that there are no additional risks associated with combination therapy.
CCBs are effective hypertension medications, but they can have side effects such as ankle edema, for which ARBs are helpful26. Candesartan is also favorable in this aspect, and its benefits with respect to renal protection will be even more important for volume issues in HTN patients27. Furthermore, it has good bioavailability and even greater synergistic effects11,28. The efficient bioavailability and long-lasting binding of candesartan to the AT1 receptor make it a highly effective blocker of negative cardiovascular effects, reducing issues for incorrect drug doses.
Combination therapy of AML and CC demonstrated superior antihypertensive effects and safety compared to AML monotherapy in this study. This result supports the need for the development and use of a fixed-dose combination of the two components. Considering HF to a major complication of HTN, a fixed-dose combination based on candesartan, which has proven to be highly effective in the prevention and treatment of HF, is expected to be an excellent treatment option enhancing medication adherence to improve clinical benefit in terms of not only effective BP control but also HF management18–20.
The limitations of this study include the relatively small number of patients and the inability to apply the recently strengthened criteria for HTN1–3. In addition, since one of the objectives of the study was to obtain approval for the use of the newly developed drug from the KMFDS, the requirements of the KMFDS were reflected in the study design. To meet the KMFDS requirements, the trial design mandated that AML 5 mg be continued in one of the intervention arms throughout the study period, even in cases where the BP was not adequately controlled with AML 5 mg monotherapy. This study design, while necessary for regulatory approval, may not fully reflect standard clinical practice.
In conclusion, in patients with essential HTN whose BP was not adequately controlled by AML monotherapy, the combination therapy of AML + CC demonstrated superior antihypertensive efficacy to AML monotherapy, with good tolerability. The combination of these two agents is effective and safe option for treating patients whose BP is not adequately controlled by AML monotherapy.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors thank all the investigators who contributed to this study.
Author contributions
All authors participated in acquisition and interpretation of data, reviewing and revising the manuscript for important intellectual content, and approved the final version for publication.
Funding
This study was funded in full by HK inno.N Corp.(formerly CJ healthcare), Seoul, Korea.
Data availability
Data supporting the findings of this study are available from the corresponding author upon reasonable request.
Declarations
Competing interests
This study was funded by HK inno.N Corp. (formerly CJ Healthcare), Seoul, Korea. The sponsor was responsible for data collation and statistical analysis, which was supported by Chung Hyun Choi, an employee of HK inno.N Corp. Soohong Kim, an employee of HK inno.N Corp., assisted with the preparation of the manuscript, but did not meet the criteria for authorship. The authors declare no other competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Hong-Seok Lim, Email: camdhslim@ajou.ac.kr.
Seung-Jea Tahk, Email: sjtahk@gmail.com.
References
- 1.Mancia, G. et al. 2023 ESH guidelines for the management of arterial hypertension the Task Force for the management of arterial hypertension of the European Society of Hypertension: endorsed by the International Society of Hypertension (ISH) and the European Renal Association (ERA). J. Hypertens.41, 1874–2071 (2023). [DOI] [PubMed] [Google Scholar]
- 2.Whelton, P. K. et al. ACC/AHA/AAPA/ABC/ACPM/AGS. A/ASH/ASPC/NMA/PCNA Guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension 324, ;71:1269 (2017) APh. (2018). [DOI] [PubMed]
- 3.Unger, T. et al. International Society of Hypertension global hypertension practice guidelines. J. Hypertens. 38, 982–1004 (2020). (2020). [DOI] [PubMed]
- 4.SPRINT Research Group. A randomized trial of intensive versus standard blood-pressure control. N Engl. J. Med.373, 2103–2116 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang, W. et al. Trial of intensive blood-pressure control in older patients with hypertension. N Engl. J. Med.385, 1268–1279 (2021). [DOI] [PubMed] [Google Scholar]
- 6.Schunkert, H. et al. Efficacy and tolerability of amlodipine/valsartan combination therapy in hypertensive patients not adequately controlled on amlodipine monotherapy. Curr. Med. Res. Opin.25, 2655–2662 (2009). [DOI] [PubMed] [Google Scholar]
- 7.Kang, S. M. et al. Comparative efficacy and safety profile of amlodipine 5 mg/losartan 50-mg fixed-dose combination and amlodipine 10 mg monotherapy in hypertensive patients who respond poorly to amlodipine 5 mg monotherapy: an 8-week, multicenter, randomized, double-blind phase III noninferiority study. Clin. Ther.33, 1953–1963 (2011). [DOI] [PubMed] [Google Scholar]
- 8.Barrios, V., Brommer, P., Haag, U., Calderón, A. & Escobar, C. Olmesartan medoxomil plus amlodipine increases efficacy in patients with moderate-to-severe hypertension after monotherapy: a randomized, double-blind, parallel-group, multicentre study. Clin. Drug Investig. 29, 427–439 (2009). [DOI] [PubMed] [Google Scholar]
- 9.Mazzaglia, G. et al. Adherence to antihypertensive medications and cardiovascular morbidity among newly diagnosed hypertensive patients. Circulation. 120, 1598–1605 (2009). [DOI] [PubMed] [Google Scholar]
- 10.Gupta, P. et al. Risk factors for nonadherence to antihypertensive treatment. Hypertension. 69, 1113–1120 (2017). [DOI] [PubMed] [Google Scholar]
- 11.Khawaja, Z. & Wilcox, C. S. An overview of candesartan in clinical practice. Expert Rev. Cardiovasc. Ther.9, 975–982 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abernethy, D. R. Pharmacokinetics and pharmacodynamics of amlodipine. Cardiology. 80 (Suppl 1), 31–36 (1992). [DOI] [PubMed] [Google Scholar]
- 13.Ferrari, R. et al. Anti-anginal drugs-beliefs and evidence: systematic review covering 50 years of medical treatment. Eur. Heart J.40, 190–194 (2019). [DOI] [PubMed] [Google Scholar]
- 14.Punzi, H. A. et al. The effects of telmisartan and amlodipine in Treatment-Naïve and Previously Treated Hypertensive Patients: a subanalysis from 4X4 Factorial Design Study. Clin. Exp. Hypertens.35, 330–340 (2013). [DOI] [PubMed] [Google Scholar]
- 15.Ke, Y. N. et al. Efficacy and safety of a single-pill combination of amlodipine/valsartan in Asian hypertensive patients inadequately controlled with amlodipine monotherapy. Curr. Med. Res. Opin.26, 1705–1713 (2010). [DOI] [PubMed] [Google Scholar]
- 16.Lewington, S. et al. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 360, 1903–1913 (2002). [DOI] [PubMed] [Google Scholar]
- 17.Ettehad, D. et al. Blood pressure lowering for prevention of cardiovascular disease and death: a systematic review and meta-analysis. Lancet. 387, 957–967 (2016). [DOI] [PubMed] [Google Scholar]
- 18.Kim, H. C. et al. Korea hypertension fact sheet 2022: analysis of nationwide population-based data with a special focus on hypertension in the elderly. Clin. Hypertens.29, 22 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pfeffer, M. A. et al. Effects of Candesartan on mortality and morbidity in patients with chronic heart failure: the CHARM-Overall programme. Lancet. 362, 759–766 (2003). [DOI] [PubMed] [Google Scholar]
- 20.Young, J. B. et al. Mortality and morbidity reduction with candesartan in patients with chronic heart failure and left ventricular systolic dysfunction: results of the CHARM low-left ventricular ejection fraction trials. Circulation. 110, 2618–2626 (2004). [DOI] [PubMed] [Google Scholar]
- 21.Lund, L. H. et al. Heart failure with mid-range ejection fraction in CHARM: characteristics, outcomes and effect of candesartan across the entire ejection fraction spectrum. Eur. J. Heart Fail.20, 1230–1239 (2018). [DOI] [PubMed] [Google Scholar]
- 22.Li, N. C. et al. Use of angiotensin receptor blockers and risk of dementia in a predominantly male population: prospective cohort analysis. BMJ. 340, b5465 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schrader, J. et al. The ACCESS study. Evaluation of acute candesartan cilexetil therapy in stroke survivors. Stroke. 34, 1699–1703 (2003). [DOI] [PubMed] [Google Scholar]
- 24.Yamaguchi, J. et al. Effect of amlodipine + candesartan on cardiovascular events in hypertensive patients with coronary artery disease (from the heart institute of Japan Candesartan randomized trial for evaluation in coronary artery disease [HIJ-CREATE] study). Am. J. Cardiol.106, 819–824 (2010). [DOI] [PubMed] [Google Scholar]
- 25.Yasuno, S., Fujimoto, A., Nakagawa, Y., Kuwahara, K. & Ueshima, K. Fixed-dose combination therapy of candesartan cilexetil and amlodipine besilate for the treatment of hypertension in Japan. Expert Rev. Cardiovasc. Ther.10, 577–583 (2012). [DOI] [PubMed] [Google Scholar]
- 26.Fogari, R. et al. Effect of valsartan or olmesartan addition to amlodipine on ankle edema in hypertensive patients. Adv. Ther.27, 48–55 (2010). [DOI] [PubMed] [Google Scholar]
- 27.Mogensen, C. E. et al. Randomised controlled trial of dual blockade of renin-angiotensin system in patients with hypertension, microalbuminuria, and non-insulin dependent diabetes: the candesartan and lisinopril microalbuminuria (CALM) study. BMJ. 321, 1440–1444 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vanderheyden, P. M. L., Fierens, F. L. P. & Vauquelin, G. Angiotensin II type 1receptor antagonists. Why some Them Produce Insurmountable Inhib. ? Biochem. Pharmacol.60, 1557 (2000). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data supporting the findings of this study are available from the corresponding author upon reasonable request.