Abstract
Although immune checkpoint blockade (ICB) has become the mainstay of treatment for advanced solid organ malignancies, success in revitalizing the host anticancer immune response remains limited. G-protein coupled receptors (GPCRs) are a broad family of cell-surface proteins that have been regarded as main players in regulating the immune system, namely by mediating the activity of T lymphocytes. Among the most novel immunoregulatory GPCRs include GPR171, lysophosphatidic acid receptors (LPARs), GPR68, cannabinoid receptor 2 (CB2), and prostaglandin E receptors, many of which have shown promise in mediating antitumor response via activation of cytotoxic T cells, inhibiting immunosuppressive lymphocytes, and facilitating immune cell infiltration within the tumor microenvironment across multiple types of cancers. This paper reviews our current understanding of some of the most novel GPCRs—their expression patterns, evolving roles within the immune system and cancer, potential therapeutic applications, and perspective for future investigation.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00262-024-03801-7.
Keywords: GPCRs, Immune checkpoint inhibitor, T cell, Tumor immunology
Introduction
Immunotherapy in the form of immune checkpoint blockade (ICB) has revolutionized the treatment of various solid organ cancers; however, the widespread success of these therapies remains limited [1]. Currently, the most prominent ICB agents are monoclonal antibodies (mAbs) that block the co-inhibitory interactions of the B7-CD28 superfamily such as cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) and programmed death-(ligand) 1 (PD-1/-L1) on T cells with their ligands, CD80/86 and PD-L1, respectively, on antigen-presenting cells (APCs) and/or cancer cells [2]. These ICBs can reinvigorate the host adaptive immune system to target various tumors generating durable and specific antitumor effect. As such, they have become standard of care for advanced stage melanoma, renal cell carcinoma, esophageal, and non-small cell lung carcinoma following randomized trials demonstrating significant survival benefit with ICB therapy [3–5]. Despite marked improvement in outcomes for these select cancers, ICBs continue to be limited by tumor immunogenicity and the presence of alternative immune escape pathways, resulting in overall tumor response rates less than 30% [6].
G-protein coupled receptors (GPCRs) comprise a ubiquitous family of cell-surface receptors that are known to be important for immune response, with a primary role as chemokine receptors in mediating immune cell migration [7, 8]. Recent investigation into GPCRs as T cell immune checkpoints has given new insight into the mechanisms of tumor immune evasion as well as potential antitumor therapeutic targets [9, 10]. In this review, we provide a summary of select novel GPCRs with immunoregulatory functions within the broader context of immune checkpoints and separated by predominant effect on CD8 + or CD4 + T cells, with emphasis on GPCRs’ roles in tumor immune escape and how we can leverage knowledge of these mechanisms to generate meaningful cancer therapies.
Emerging GPCR members with T cell modulatory functions
Activated T cells, namely CD4 + and CD8 + lymphocytes, are critical to an effective adaptive immune response to cancer. Activation and regulation of T cells are balanced by a two-signal activation/suppression system largely comprised of ligands from the B7-CD28 cell-surface immunoglobulin family. Manipulation of B7-CD28 members such as PD-1 and CTLA-4 that play essential roles in driving the stimulation or inhibition of T cell activity have ushered in a new era of cancer treatment and demonstrated the promising efficacy and durability of immunotherapy.
While blocking the interactions of the co-inhibitory B7-CD28 immunoglobulin family has demonstrated tremendous success in activating cytotoxic T cell function in the clinic, it has also exposed the complexity of the numerous mechanisms by which tumors continue to evade and/or attenuate the host immune response despite ICB [11]. Apart from the B7-CD28 co-inhibitory effects on T cells, the activity of cell-surface GPCRs have emerged as important players in regulating T cell-based immunity that may offer novel avenues to facilitate the host antitumor response [9, 12]. GPCRs are among the most ubiquitous cell-surface proteins known in humans, comprising 3% of the human genome translating to over 800 individual proteins [7, 8]. While GPCRs can be classified into six subfamilies based on amino acid sequence, the vast majority are part of the class A family of rhodopsin-like proteins [7]. GPCRs share a conserved architecture: a three-loop extracellular N-terminus followed by seven transmembrane alpha helical segments followed by a three-loop intracellular C-terminus [7, 13]. The predominant function of GPCRs is to transmit extracellular stimuli into intracellular signals, a role that is universally involved in normal, physiologic functions of the human body spanning neurotransmission to cardiac myocyte contraction to hormone signaling [7, 8]. In cancer, GPCRs can promote tumorigenesis by facilitating tumor cell proliferation, migration, and angiogenesis via mutations or aberrant expression of GPCRs [14]. A growing body of literature also suggests that malignancies can manipulate GPCR activity and expression to downregulate the T cell host immune response [9, 15]. Chemokine receptors, such as CXCR3 and 4, are prime examples of GPCRs expressed on the surface of T cells that mediate T cell function and migration within the TME via binding of soluble chemokines that control T cell activation and into the TME [16, 17]. Beyond chemokine receptors, additional novel GPCRs have been characterized on the cell surface of T cells as well as cells of the TME that execute regulatory roles on T cell proliferation, migration, and activation. In the subsequent sections, we review the mechanisms and evidence supporting the regulatory role of select novel non-chemokine receptor GPCRs on T cell function stratified by major phenotype (CD8 + and CD4 + T cells) within the context of the TME.
Modulators of CD8 + T cells
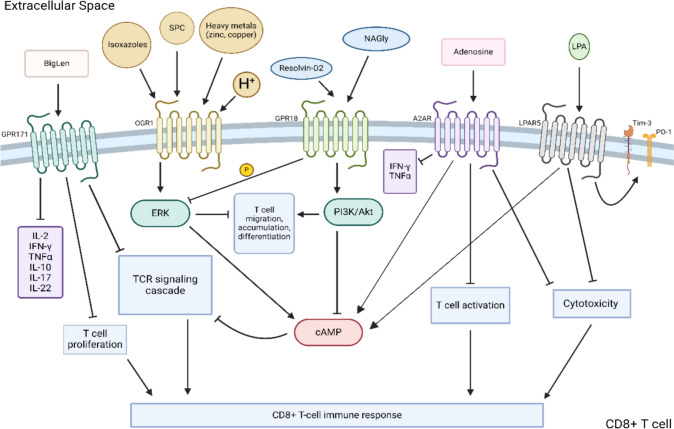
CD8 + T cells are the main effector cells of the adaptive immune system [18]. Activation of CD8 + T cells leads to direct target-cell death via a variety of mechanisms, including release of cytotoxic granules (e.g., granzyme, perforin, and granulysin), direct endocytosis, or activation of death domains via Fas ligand interactions [19, 20]. Several GPCR molecules, including GPR171, A2AR, LPARs, ovarian cancer GPCR 1 (OGR1), and GPR28, have been described to modulate CD8 + T cell functions within the TME. Figure 1 displays a schematic summary of intracellular signaling pathways and downstream function of CD8 + T cell immunomodulatory GPCRs.
Fig. 1.
Summary of GPCR signaling modulating CD8 + T cell activity. SPC, sphingosylphosphorylcholine; OGR1, ovarian cancer GPCR 1; NAGly, N-arachidonyl glycine; A2AR, adenosine A2A receptor; LPAR, lysophosphatidic acid receptor; LPA, lysophosphatidic acid; Tim-3, T cell immunoglobulin and mucin domain-containing protein 3; PD-1, programmed cell death protein 1; ERK, extracellular signal-regulated kinase; PI3K, phosphoinositide 3-kinase; Akt, protein kinase B; cAMP, cyclic adenosine monophosphate
GPR171 (H963)
GPR171, also known as H963 or Platelet Activating Receptor Homolog, is a class A rhodopsin-like receptor initially characterized for its role within the central nervous system in modulating feeding and reward behaviors in mice [21–23]. Recently, work has shown GPR171 to be expressed on CD8 + and CD4 + T cells, natural killer (NK) cells, and directly on certain cancers cells [24, 25].
GPR171 is predominantly activated by BigLen, an amino acid neuropeptide, and GPR83, a colocalized receptor mainly expressed by cells of the nervous system [26]. Binding of BigLen to GPR171 has been shown to inhibit T cell activation via abrogation of the TCR signaling cascade, reducing T cell proliferation, and decreasing release of cytokines important for T cell activation and differentiation, such as IL-2, IFNγ, TNFα, IL-10, IL-17, and IL-22 [25]. In preclinical studies, T cells of GPR171 knockout (KO) mice were less susceptible to T cell inhibition [25]. Pertinent to the TME, GPR171 has found to be upregulated on tumor infiltrating lymphocytes (TILs) and to inhibit T cell-mediated antitumor immunity [25]. GPR171 ablation in mice resulted in enhanced T cell activity, enabling improved antigen stimulation in an acute graft-versus-host model. Mice adoptively transferred with splenocytes from GPR171 negative mice demonstrated a nearly 2.5-fold increase in donor CD4 + and CD8 + T cells compared to wildtype (WT) counterparts [25]. Not only has GPR171 been found to be expressed directly on TILs, but directly on malignant cells such as breast and small cell lung cancer, providing a path to evade the host immune system and support their own proliferation and metastatic dissemination [24, 27]. The role of GPR83, a colocalized receptor to GPR171 expressed throughout the CNS and immune system, in conjunction with GPR171 to influence the immune response is subject to ongoing investigation [26, 28, 29].
Preclinical studies have demonstrated promising antitumor effect of GPR171 blockade. In murine colorectal cancer (CRC) and melanoma models, treatment with small molecule GPR171 inhibitor resulted in significant reductions in tumor growth of up to 40% [25]. Treated tumors showed not only an increased intratumoral CD8 + T cells but concomitant upregulation of pro-inflammatory cytokines such IFNγ and TNFα with upregulation of immune checkpoint receptors PD-1 and T cell immunoreceptor with immunoglobulin and ITIM domain (TIGIT). Furthermore, GPR171 antagonist appears to offer a potential avenue to bolster ICB efficacy. Combination of GPR171 antagonist with anti-PD-L1 treatment of mice inoculated with either murine melanoma or CRC tumors resulted in significant enhancement of T cell activity, reduction of tumor burden, and improved survival compared to anti-PDL-L1 therapy alone [25]. Furthermore, GPR171 can be utilized in conjunction with other tumor modifying treatment in creating a more robust response. A 2016 study combining anti-GPR171 antibodies with epidermal growth factor receptor inhibitors reduced human lung cancer cell viability by 66.3%, nearly triple the effect observed with each agent individually [24]. In addition, the same study found that these mice also demonstrated decreased metastatic burden compared to control mice, up to 87% depending on the tumor cell line [24]. While epidermal growth factor itself is not an immune checkpoint, studies have reported that epidermal growth factor receptors do play a role in modulating PD-L1 expression through the activation of the receptor pathway. The synergistic combination of both EGFR and GPR171 serves as an important possibility for further research that would enable both targeting both tumor apoptosis directly and enhancement of the innate immune system. Taken together, there is mounting evidence that while epithelial tumors can upregulate GPR171 to evade the host immune response, antagonizing GPR171 activity results in promising antitumor activity.
Currently, no human clinical trials investigating anti-GPR171 treatment in patients with solid tumors have been initiated. As one of the most recently found GPCRs that enable tumor immunosuppression, much investigation remains to be conducted to definitively define GPR171’s role in the TME and immune cell inactivation.
Adenosine A2A receptor (A2AR/ADORA2)
Adenosine 2A receptor (A2AR), also known as ADORA2 and RDC8, is a rhodopsin-like class A GPCR akin to the adenosine deaminase (ADA) receptors first reported in the 1990s [30]. Adenosine is A2AR’s most well-characterized ligand; however, other ligands, such as neuronal calcium-binding protein and caffeine, have also been reported to activate the receptor [31–35]. Though A2AR activation has previously been shown to alter neutrophil migration through inhibition of mTOR and mitochondrial signaling, only recently has A2AR been found to be highly expressed on CD8 + T cells with substantial roles in mediating T cell function [36–39].
Adenosine, a purine nucleoside base and by-product of energy metabolism, is predominantly secreted by the liver but has been shown to be produced by both effector CD8 + T cells and immunosuppressive T regulatory (Treg) cells to attenuate the effector immune response [40, 41]. Furthermore, high concentrations of adenosine have been found in the TME of both blood-borne cancers (leukemias) and solid tumors (SqCC) via upregulation of the hypoxia–adenosine–A2AR axis [42–45]. Binding of adenosine to A2AR has been shown to downregulate CD8 + T cell immune function as well as augment CD4 + T cell clonal expansion via stimulation of the A2AR-cyclic adenosine monophosphate (cAMP) axis, resulting in downregulation of TCR signaling and IFNγ production [40, 46]. Treg cells can bind to adenosine, increase intracellular cAMP levels, and eventually lead to extracellular adenosine production not only inhibiting effector T cells function via A2AR signaling while promoting its own positive feedback loop. Treg cells deficient in A2AR resulted in reduced number of CD4 + T cells and decreased differentiation into Th17 cells [40].
Adenosine’s role in influencing the immune component within solid is subject of ongoing investigation. Ohta et al. found that silencing adenosine signaling in tumor-specific T cells in lymphoma bearing mice resulted in significantly reduced lung metastasis [46]. In contrast, research has also shown that the absence of adenosine promotes tumor expansion. A 2014 report found that selective knockout of A2AR on T cells in malignant melanoma and urothelial tumors resulted in lower CD8 + T cell populations and effector-memory differentiation in these tumors [43]. The authors postulate that this reduction of CD8 + T cell expansion is predominantly a local rather than global response that is dependent on both the type of tumor and the level of adenosine required for T cell activation. While studies support both the anti- and pro-tumorigenic effects of A2AR signaling on immune activation within the TME, increased activity of the adenosine-A2AR seems to favor increased tumor size, metastasis, and histologic grade by inactivation of CD8 + T cells in the TME of a variety of malignancies [47].
A2AR expression on T cells and the growing body of evidence suggesting adenosine’s pro-tumorigenic effects have led to promising preclinical studies demonstrating the efficacy of A2AR modulation. In a study examining the effects of A2AR agonism, WT mice treated with A2AR agonists demonstrated impaired CD8 + T cell function through downregulation of CD25—a component important for T cell activation — and promotion of FoxP3 + transcription and CD39, CD73, and CTLA-4 expression on CD8 + T cells inhibition activity of CD4 + T cells [48]. Conversely, antagonism of A2AR demonstrated improved CD8 + T cells cytotoxic effects evidenced by higher levels of IFNγ and TNFα and reduced CD4 + Treg cells, particularly when used in combination with classic ICB agents in transforming growth factor beta receptor (TGFBR) and phosphatase tensin homolog (PTEN) double conditional KO mice with SqCC [47]. Leone et al. also found that administration of a different A2AR small molecule antagonist improved density of TNFα + and IFNγ + expressing CD8 + T cell in mice with either B16-OVA melanoma or CRC (CT26 or MC38) tumor models, leading to improved overall survival, and reduced tumor growth in addition to decreased surface cell receptors PD-1, TIM-3, and LAG-3 on both CD8 + and CD4 + tumor draining lymph nodes [49]. Despite this, combination of anti-PD-1 inhibitors still improved tumor regression in the mice which they suggest is likely due to an expanded pool of cytotoxic lymphocytes that are subsequently responsive to anti-PD-1 therapy (Supplementary Figure). Nevertheless, promising preclinical results in murine models have spurred transition to human clinical trials.
Compared to other novel immunomodulatory GPCRs, targeting A2AR has made substantial headway in human clinical trials. Ciforadenant, a selective, reversible small molecule A2AR inhibitor, has demonstrated a favorable response rate of nearly 11% when used in combination with atezolizumab (anti-PD-L1 inhibitor) compared to 3% with ciforadenant monotherapy in patients with refractory renal cell carcinoma [44]. In a recent phase I trial, patients with metastatic non-small cell lung cancer were treated with combination A2AR antagonist and spartalizumab (PD-L1 inhibitor) which showed reactivation of antitumor CD8 + T cells and an improved disease control rate of 67% of the patients compared to 43% with an A2AR antagonist alone [50]. Though their reports reflect the utility of A2AR with standard therapies, a second phase II study showed that combination therapy was not sufficient to alter the standard of care [51]. However, antagonism of A2AR is still being investigated in other tumor types such as breast and CRC. While the combination of A2AR and PD-L1 inhibitors in CRC during a phase II clinical study did not yield a significant response rate to alter standard therapy, a phase II clinical trial showed that the addition of taminadenant, a A2AR antagonist, to PD-L1 inhibitor therapy resulted in a dose-dependent CD8 + T cell response and overall response rate of 11% in 27 patients, higher than when either inhibitor was used alone [52, 53].
Though our knowledge of how A2AR antagonism activates the immune system is in its nascent stages, promising preclinical and clinical data suggests benefit of continued investigation into its mechanisms of action. Given its clinical success thus far, new trials continue to expand upon the use of A2AR treatment with current chemotherapy regimens of malignancies such as renal cell carcinoma. Exploring the interactions and downstream signaling pathways involving A2AR, its ligands, and the TME is paramount to maximizing therapeutic efficacy and safety.
Lysophosphatidic acid receptor family
The lysophosphatidic acid receptor (LPAR) family is a group of six receptors that possess diverse roles within the biological system [54]. LPARs are expressed on a variety of cells from neuronal ganglia to cardia to intestinal, and most notably on immune cells [55, 56]. LPAR1-3 are a part of the endothelial differentiation gene receptors, and LPAR4-6 are homologous to those of the P2-type purine receptors [54, 57]. Within the immune system, LPAR1-6 have been found to be expressed on CD8 + and CD4 + T cells as well as directly on tumor cells of the prostate, breast, and endometrium [55, 58–61]. LPAR’s main ligand is lysophosphatidic acid (LPA), a pleiotropic bioactive signaling lipid acting primarily as a growth factor when bound to its receptor [62].
Activation of LPAR on CD8 + T cells following ligand binding generally leads to two pathways: firstly, an LPA-LPAR5-Gαq path, resulting in reduction of calcium mobilization, increases in cAMP levels, and impairing target-cell immune synapse formation, the inositol 1,4,5-triphosphate receptor (IP3R1) specifically; and secondly, initiation of the LPA-LPAR5-Gα12/Gα13 pathway that alters both stress fibers of the cell and the actin-microtubule cytoskeleton.[63] Both pathways result in interference with TCR signaling preventing CD8 + T cell inactivation and ERK activation [60, 61]. Furthermore, LPA signaling appears to induce a host of negative effects on CD8 + T cells via LPAR5. In vitro studies demonstrate that LPA via LPAR5 induces upregulation of CD8 + T cell exhaustion markers PD-1 and Tim-3, disrupts expression of other proteins and receptors such as Nur77 and CD69, as well as hinders actual cytotoxic functions of CD8 + T cells [58, 60]. Further in in vivo tumor models, Tox and Lag-3 expressions were decreased in LPAR5 KO mice compared to WT counterparts. Functional assays demonstrate that LPAR5 deficiency improved in vivo antigen-specific killing of immunized target cells in addition to decreased gross tumor size in murine lung cancer models. Lastly, LPA signaling via LPAR5 appears to limit metabolic capacity of CD8 + T cells rendering them more susceptible to oxidative stress and fatty acid oxidation/metabolic exhaustion [58]. Other investigations have corroborated these findings as LPAR5 deficient CD8 + T cells demonstrated significant improvement in tumor control with increases in CD8 + proliferation and hyperactivity compared to WT controls in in vitro and in vivo B16 murine melanoma [64, 65]. Similarly, inhibition of LPAR5 with a small molecule antagonist attenuated LPA-driven dampening of TCR signaling, resulting in an overall improved antitumor response and enhanced CD8 + T cell function in murine melanoma and non-small cell lung cancer models [60, 66, 67]. Collectively, these lines of preclinical evidence suggest an important role of LPA-LPAR5 signaling in regulating T cell function and metabolism.
LPAR6 is the most recently characterized GPCR of the LPAR family and is highly expressed on CD8 + T cells. Activation of LPAR6 alters the proliferation, survival, and cytoskeleton of CD8 + T cells via Gα12/13-coupled signaling pathway [54, 68, 69]. In patient ex vivo derived TILs, LPAR6 has been shown to play an inhibitory role in T cell migration causing chemorepulsion of TILs in melanoma tumors with addition of ligands LPA and autotaxin (ATX) [68]. However, rescue of ex vivo TIL to overcome chemorepulsion was observed with administration of XAA, a novel xanthylene-based LPAR6 antagonist [68]. As a prognostic marker, LPAR6 expression appears to be associated with variable outcomes across solid tumors. For hepatocellular carcinoma (HCC) and PDAC, higher LPAR6 expression has been shown to be associated with tumor progression and worse survival compared to higher LPAR6 expression being protective in lung adenocarcinoma. LPAR6 has been found to have a prognostic association with various cancers [70, 71].
While significant progress has been made in understanding the immunomodulatory roles of LPAR5/6 on CD8 + T cells, the impact of LPAR activation on CD4 + T cells is less well characterized. Current evidence suggests that LPAR1/2 activation facilitates CD4 + T cell migration as in vivo studies have demonstrated impaired CD4 + T cell migration and infiltration from lymphoid structures in LPAR1/2 KO murine models [72–74]. Knowlden et al. demonstrated that although LPAR2 deficiency on CD4 + T cells did not attenuate chemotaxis to adhesion molecules such as ICAM-1 and CCL21, these LPAR-deficient lymphocytes demonstrated overall reduced migratory speed [75]. Interestingly, Mata-Rico et al. demonstrated that ATX-expressing tumors excluded CD8 + T cells but not conventional nor regulatory CD4 + T cells [68]. From these studies, it appears that LPARs may influence CD4 + T cell function, yet definitive studies are needed to clarify such interactions.
Despite strong preclinical evidence supporting LPAR mediation of T cell activity, there are no active clinical trials examining LPAR antagonists in the treatment of cancer. Given the well-characterized upregulation of LPARs on cancer cells that mediate proliferation and migration of tumor cells as well as emerging evidence demonstrating LPAR modulation of immune cell activation, opportunities to target the LPAR cascade in the clinic are warranted [76]. An LPAR antagonist has recently shown promise in reducing the rate of decline in lung function in patients with idiopathic pulmonary fibrosis which may offer avenues for application in cancer-centered clinical trials [77].
Ovarian cancer G-protein coupled receptor 1 (OGR1)
Ovarian Cancer GPCR 1 (OGR1, aka sphingosylphosphorylcholine (SPC), GPR12A, A12A6, and GPR68), despite its name, is expressed on smooth muscle and epithelial cells of the respiratory system, gastrointestinal tract, renal tubules, placenta, and pertinently, CD8 + T cells [78, 79]. Furthermore, OGR1 has evolved to function optimally in acidic environments, a characteristic feature of the TME known to impair T cell activation [80–82]. OGR1 expression, while low at baseline, has also been found to be upregulated in several solid cancers such as CRC, pancreatic ductal adenocarcinoma (PDAC), cutaneous melanoma, SqCC, and prostate [78, 83]. Within the immune system, the receptor appears to be expressed on CD8 + and CD4 + T cells [79, 84]. While main ligand for OGR1 is thought to by hydrogen ions, other ligands such as SPC, isoxazoles, and metal ion such as zinc or copper have been found to initiate downstream pathways of OGR1 such as phospholipase C (PLC)/inositol phosphate (IP)/Ca2 + /extracellular signal-regulated kinases (ERK), thereby increasing intracellular cAMP concentrations [85–87].
OGR1’s role in tumorigenesis is the subject of ongoing investigation. Preclinical murine melanoma models in OGR1 KO mice exhibited improved survival accompanied by a significant increase in tumor infiltrating CD8 + T cells [84]. A proposed mechanism driving improved antitumor response in OGR1 KO mice is increased release of effector cytokines such as IFNγ from intratumoral CD8 + T cells and myeloid-derived cells [88, 89]. Interestingly, only male mice deficient in GPR68 experienced an increase in CD8 + T cell activities and thereby a decrease in tumor burden compared to female mice that showed no difference between the KO and WT strains. In prostate cancer, Yan et al. demonstrated that prostate cancer tumors in GPR68 deficient mice had increased number of tumor infiltrating CD8 + and CD4 + T [89]. To date, there are no active trials testing OGR1 manipulation as an anticancer therapy in the clinic; however, mounting evidence suggests that it holds promise as a potential therapeutic target capable of reinvigorating cytotoxic CD8 + T cells within the TME.
GPR18
GPR18, a class A GPCR now recognized as a receptor for several endogenous neurotransmitters, namely endocannabinoid N-arachidonyl glycine (NAGly), was initially investigated for its ability to alter microglial migration; however, it has recently been found to play a regulatory role on CD8 + T cell lymphocytes [90, 91]. Upon activation, GPR18 helps to establish and maintain CD8 + effector memory T cells, especially within the lymphoid and reproductive organs such as spleen and testis, respectively [90–93].
Apart from NAGly and resolvin-D2, GPR18 can also bind to imidazothiazinones, and tricyclic xanthines resulting in ERK1/2 phosphorylation and PI3K/Akt signaling, a signaling pathway that will inhibit cAMP and phosphorylation of ERK important for assisting in CD8 + T cell migration, accumulation, and differentiation [94–96]. This was found during an in vivo study of GPR18 KO mice that the presence of GPR18 was required for CD8 + T cells migration from intraepithelial compartments to other parts of the tissue such as lamina propria. Flow cytometry assessment of the tissue reflected that T cells deficient in GPR18 had impaired migration abilities compared to their WT counterparts [93]. In addition, GPR18 KO mice have been shown to have reduced CD8 + memory T cells and decreased expression of the cytotoxic granule granzyme B on cytotoxic T cells within lymphoid tissue [90, 93]. Due to its similar homology to the cannabinoid receptors, there have also been reports of 9-tetrahydrocannabinol (Δ9THC) though its exploration has primarily been found on its effects to endothelial cells rather than on T cells [94].
There have been several suggested antagonists of GPR18, including amauromine, synthetic cannabinoid O-1918, and a bicyclic imidazole-4-one derivative, that facilitate a range of functions including vasodilation and treatment of obesity [96, 97]. However, no formal therapeutics manipulating GPR18 have been explored to alter T cell response in the context of solid organ cancers. Preliminary assessments have also suggested that GPR18 has a possible pro-oncogenic role in protecting malignancies such as melanoma [98–100]. In melanoma, it was found through quantitative expression profiling that GPR18 was highly active within in vitro cultures of melanoma from human cell lines and has an anti-apoptotic effect for these tumorous cells [101]. In contrast to other genomic profile studies, the presence of GPR18 has a more meaningful prognostic value against HCC and breast cancer from T cell responses [100]. Antagonism of GPR18 has yet to reach clinical trials. Despite this, GPR18’s ability to facilitate CD8 + T cell migration and activation provides a potential avenue for future antitumor research.
Modulators of CD4 + T cells
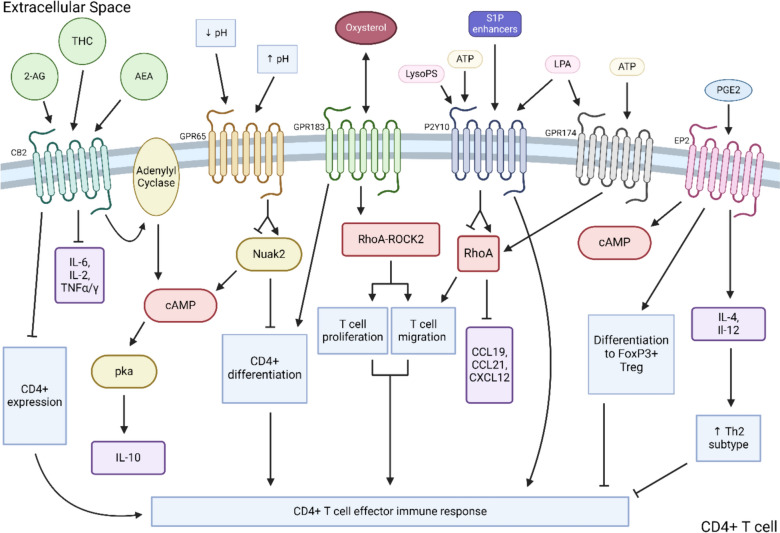
In contrast to the direct cytotoxic functions of CD8 + T cells, CD4 + T cells predominantly facilitate antitumor function indirectly via the release of soluble cytokines and priming of effector lymphocytes such as CD8 + T cells and macrophages to target tumor cells [102, 103]. CD4 + T cells differentiate into effector subtypes known as T helper (Th) cells through the signaling of soluble cytokines secreted by APCs that induce transcription of subset-dictating transcription factors. Among the several Th subsets, Th1, Th9, Th17, and T follicular helper cells are suggested to have anti-solid tumor activity while T regulatory cells are largely considered pro-tumor [104, 105]. The following sections review the mechanisms by which select GPCRs—cannabinoid receptor 2 (CB2), T cell death-associated gene 8 protein (GPR65), GPR183, putative P2Y purinoceptor 10 (P2Y10), GPR174, and Prostaglandin E Receptor 2 and 4 (EP2 and EP4)—regulate CD4 + T cell function in the context of cancer. Figure 2 displays a schematic summary of intracellular signaling pathways and downstream function of CD4 + T cell immunomodulatory GPCRs.
Fig. 2.
Summary of GPCR signaling modulating CD4 + T cell activity. 2-AG, 2-arachidonoylglycerol; THC, 9-tetrahydrocannabinol; AEA, anandamide; CB2, cannabinoid receptor 2; LysoPS, lysophosphatidylserine; LPA, lysophosphatidic acid; ATP, adenosine triphosphate; S1P, sphingosine-1-phosphate; LPA, lysophosphatidic acid; PGE2, prostaglandin E2; EP2, prostaglandin receptor 2; CCL, chemokine ligand; CXCL, chemokine (C-X-C motif) ligand; Treg, T regulatory cell; ROCK2, Rho-associated coiled-coil containing protein kinase 2; TNF, tumor necrosis factor
Cannabinoid receptor 2 (CB2)
Cannabis use has been on the rise in the past decade particularly in patients with cancer as a pain management strategy and an alternative treatment for cancer treatment-related side effects [106, 107]. Recent data suggest that increasing cannabinoid metabolite levels (Δ9THC), the main psychoactive metabolite in marijuana, can impede the efficacy of ICB therapy in murine CRC and melanoma tumor models [108]. Cannabinoid receptor 2 (CB2), reported to be expressed on a wide variety of lymphoid cells such as macrophages, NK cells, dendritic cells (DCs), and eosinophils, has emerged as an important modulator of CD4 + T cell activity [106, 109–111].
CB2 is a plasma membrane receptor thought to interact with many ligands, including Δ9THC, anandamide (AEA), and 2-arachidonoylglycerol (2-AG), which lead to negative effects on CD4 + T cells [111]. Interaction with these ligands leads to the activation of a Gi/o receptor, leading to a series of downstream, intracellular events such as initiation of the adenylate cyclase pathway and activation of protein kinase A (PKA) that increase the secretion of cytokines such as IL-10 which in turn strengthen the development of immunosuppressive Treg cells [112–114]. Furthermore, mice immunized with bovine serum albumin as an immune trigger demonstrated that the addition of exogenous 2-AG impaired the secretion of pro-inflammatory Th1- and Th17-associated cytokines such as IL-6, IL-2, TNFα, and IFNγ [111]. Furthermore, in a cecal ligation and puncture model of sepsis, mice with CB2 KO specific CD4 + T cells demonstrated improved mortality compared to the WT controls [115]. Interestingly, even high levels of endogenous cannabinoid ligands such as AEA have been correlated with worse survival in cancer patients and depressed tumor-specific T cell function via inhibition of the JAK1/STAT pathway [116]. CB2 selective agonists have been shown to inhibit nuclear transcription factors NF-kB and NFAT as well as mRNA expression of CD40L and CyclinD3, which was associated with decreased CD4 + expression of FoxP3 + Treg transcription factor in a model of graft-versus-host reaction. In vitro assays show downregulation of IL-2 production in a dose-dependent matter, impaired chemotaxis, and suppression of T cell proliferation with CB2 signaling [114, 117, 118].
CB2 also appears to mediate CD8 + T cell function [110, 114]. CB2 KO mice inoculated with lung carcinoma demonstrated an increase in CD8 + T cell infiltration within tumors with a concomitant increase in expression of PD-1 [110]. Furthermore, although application of a CB2 agonist exhibited no difference in overall tumor burden or degree of tumor infiltrating lymphocytes within tumor tissue, CB2 antagonism significantly reduced tumor weights by nearly 100 mg compared to controls. Similarly, Xiong et al. found that CB2 deficient mice had increased CD8 + TILs, slowed tumor growth and prolonged survival rate across various tumor models, including CRC, melanoma, and Lewis lung carcinoma (LLC) [116]. Modulation of CB2 continues to show promise as a potential antitumor agent via with increasing evidence supporting its importance in influencing T cell function in other fields of study such as transplant immunology that have found CB2 agonists reduce or slow immune rejection [117]. Investigation into CB2 remains confined to preclinical trials; however, given the available evidence, CB2 manipulation may be an effective modality to shift host immune response toward antitumor phenotype of both CD4 + and CD8 + T cells.
GPR65 (T cell death-associated gene 8 protein)
GPR65, originally known as T cell death-associated gene 8 (TDAG8), like OGR1, is a member of the acid-sensing family of GPCRs mainly expressed on immune cells such as CD4 + T cells, macrophages, and eosinophils but also epithelial cells of the intestinal villi. GPR65 has been found to be upregulated on several solid tumor cells (glioblastoma, esophageal, and PDAC) while downregulated on others (lung adenocarcinoma, adrenocortical carcinoma) [119–121]. Unlike OGR1, GPR65 appears to predominantly attenuate rather than propagate inflammatory responses; however, evidence suggests that this may differ in autoimmune compared to tumor scenarios. Like OGR1, GPR65 activity is inversely related to environmental pH, resulting in increased activity/intracellular cAMP concentrations as the pH decreases. A well-characterized, physiologic role for GPR65 is in the thymus where it is expressed on immature thymocytes to promote self-tolerance by inducing thymocyte apoptosis upon TCR binding [122, 123].
In an autoimmune disease setting, there is evidence that GPR65 promotes contrasting effects on immune response compared to its immunosuppressive role in the TME. Employing an inflammatory bowel disease murine model, Lin et al. demonstrated that expression of GPR65 on CD4 + T cells was positively correlated with IBD activity and that GPR65 on peripheral CD4 + T cells promotes differentiation into Th1 and Th17 subtypes implicated in driving IBD severity [120–122, 124]. The authors revealed that activation of GPR65 on CD4 + T cells suppresses expression of NUAK family kinase 2 (Nuak2) which enables CD4 + T cell differentiation into Th1 and Th17 subsets which ultimately promote secretion of pro-inflammatory cytokines [125]. Inversely, GPR65-deficient T cells resulted in activation of an alternate pathway (cAMP‐PKA‐C‐Raf‐ERK1/2‐LKB1) which lead to increased NUAK2 expression. This led to reduced T cell proliferation and differentiation into pro-inflammatory Th cell subtypes as well as reduced CD4 + T cell infiltration into mucosal tissue [121]. While the results from Lin et al. join a chorus of studies supporting a pro-inflammatory role of GPR65 in autoimmune pathologies, others have shown that GPR65 deficiency is associated with worse disease severity in chemical-induced colitis and dermatitis murine models [126]. While the role of GPR65 in autoimmune pathologies appears variable and specific to disease biology, evidence strongly indicates an impact of GPR65 on the immune response.
Regarding the function of GPR65 in the TME, recent preclinical work supports a key role of GPR65 in inhibiting antitumor immune response. A small molecule GPR65 inhibitor has shown promising results in revitalizing of the host immune response against tumor cells [127]. In syngeneic murine CRC and melanoma models, GPR65 inhibition with a small molecule agent has been shown to counteract the pro-tumor effects of an acidic environment on tumor-associated macrophages (TAM). This is executed by re-polarization of TAMs toward an antitumor phenotype, stimulating the release of pro-inflammatory cytokines and promoting the infiltration of antitumor effector cells such as T and NK cells into the TME. This stimulation of TAMs and subsequent antitumor effects resulted in substantially reduced tumor burden as a monotherapy and combined with conventional immune checkpoint blockade. Together, this line of evidence suggests that GPR65 inhibition is a promising solo or adjunct antitumor therapeutic avenue that has potential for success in human clinical trials.
GPR183
GPR183, initially identified as Epstein–Barr virus-induced G-protein coupled receptor 2 (EIB2) in light of its upregulation on EBV-infected B cells, is an immunomodulatory GPCR that mainly acts within the lymphatic system [128]. GPR183 is expressed on B cells and CD4 + T cells and binds oxysterols, an interaction which facilitates migration, differentiation, and maturation of both B and T cells [129–133].
GPR183 is functionally unique for two reasons: 1. it has been found to predominantly mediate B, T, and DCs interactions in the lymph system and 2. GPR183 is thought to synthesize its own ligand—oxysterol—initiating the Gα-RhoA-ROCK2 axis, a cascade that facilitates rearrangement of the cytoskeletal proteins controlling cell migration and proliferation [134–136]. Upregulation and downregulation of GPR183 expression on B and T cells in lymph nodes facilitate migration of these cells to various regions within the lymph node, for example, upregulation of GPR183 on B cells stimulates interaction with oxysterol which facilitates migration of B cells toward the marginal zone to increase exposure to T cells [137]. For T cells specifically, GPR183 upregulation allows migration to the periphery of the T cell zone which maximizes interaction with antigen-presenting DCs facilitating differentiation into helper subsets [131, 132]. Further mechanistic investigation found that CD4 + T cells deficient in GPR183 have decreased sensitivity to IL-2, a molecule that promotes Th cell differentiation [130]. As a result, these GPR183-deficient T cells displayed impaired chemotaxis and poor differentiation to Th2 subtype. Baptista et al. also demonstrated impaired CD8 + T cell-mediated memory response and prolonged antigen recognition by CD4 + T cells in the absence of GPR183 [138]. Similar studies in GPR183 KO mice have found both reduced secretion of cytokines that promote the differentiation of T cells such as IL-6R and IL-2 and worse disease severity of mice in a tuberculosis model [131]. Lack of the pro-inflammatory signaling resulted in diminished Th cell responses, interference with activation, and alteration of several other receptor expression [135]. Collectively, normal GPR183 function appears to positively facilitate immune cell activation and effector differentiation.
Although there are no officially approved GPR183-targeting therapeutics successfully altering T cell for clinical cancer treatment, a GPR183 antagonist has been shown to successfully attenuate immune cell response utilizing macrophage receptors to attenuate the viral load of viruses such as COVID and RSV [132]. Most recently, a 2023 pan-cancer analysis of GPR183 found that high GPR183 expression was associated with poor prognosis in chronic lymphocytic leukemia, lung SqCC, glioma, and uveal melanoma while serving as a positive prognostic indicator for urothelial carcinoma and cutaneous melanoma [139]. Thus, this has opened possibilities into investigation of the influence GPR183 has on tumor immunity that can be continued to be investigated, particularly as new antagonists that can target GPR183 are uncovered.
Putative P2Y purinoceptor 10 (P2Y10)
P2Y purinoceptor 10 (P2Y10; aka GPR10, LYPSR2) is a recently deorphanized, class A GPCR with similar homology to the purinergic (P2Y)-like family of receptors, although conflicting evidence as to its true origins has prevented it from formally being included as a P2Y subtype [140]. P2Y10 is normally expressed on a diverse range of cells including those of the cardiac endothelia, smooth muscle, and brain [141]. Pathologic P2 expression has been found to be upregulated directly on tumor cells of select malignancies, including melanoma and various adenocarcinomas [140]. The physiologic roles of P2Y receptors are diverse—it facilitates platelet aggregation to hepatocyte apoptosis and neuronal outgrowth [142, 143]. CD4 + T cells, along with other leukocytes, basally express P2Y10, but is significantly upregulated on activated CD4 + T cells that have strong migratory function suggesting a role of P2Y10 in regulating CD4 + T cell activity [144–146].
In addition to nucleotide binding, P2Y10 is unique among its purinergic receptor relatives in its ability to bind lysophospholipids such as lysophosphatidic acid (LPA), lysophosphatidylserine (LysoPS), and sphingosine-1-phosphate (S1P) enhancers [144, 146]. Regarding CD4 + T cells, P2Y10 receptor enhances T cell migration via binding LysoPS, S1P, and adenosine triphosphate (ATP). In vivo studies demonstrated that binding of P2Y10 with LysoPS, S1P, and ATP, released in a chemokine-induced fashion, led to increase migratory ability of CD4 + T cells via RhoA protein activation, a mechanism believed to be also conserved in humans [147]. This aligned with findings that spinal-cord infiltrating CD4 + T cells had upregulated P2Y10 expression in a murine model of neuroinflammation, and that WT mice had significantly greater disease severity compared to P2Y10 KO controls. High expression of P2Y10 on CD4 + T cells has been shown to enhance T cell function and migration, and overall plays a crucial role in fine tuning T cell activation [148].
In a pan-cancer analysis, Liu et al. outlined significant associations between abnormal P2Y receptor expression, immune cell scores, and prognosis across several types of cancers [140]. Although P2Y10 was not included in this analysis, given its role in mediating CD4 + T cell activity and the relationships with other P2Y receptors and cancer, investigation into modulating P2Y10 as a cancer therapeutic seems warranted. Further, LPA, a bioactive lipid expressed by cancer cells to induce immunosuppression (discussed in previous sections), is a known ligand for P2Y10; however, the interaction between LPA and additional P2Y10 ligands with P2Y10 in the context of cancer has not been explored. Identifying relationships between CD4 + T cell migratory ability, P2Y10 expression, and tumor-induced exclusion of immune infiltrating lymphocytes may represent area of future research.
Prostaglandin E receptor 2 and 4 (EP2 and EP4)
Prostaglandin E Receptor 2 and 4 (EP2/4), like the majority of immunomodulatory GPCRs, are class A GPCRs. EP2/4 are expressed ubiquitously across cell types, pertinently on T lymphocytes, facilitating signal transduction via Gs coupling and subsequent increases in intracellular cAMP upon binding to its ligand, prostaglandin E2 (PGE2), a well-characterized bioactive signaling lipid [149, 150]. Upregulation of PGE2 production as a result of increased activity of cyclooxygenase-2 (COX-2) and breakdown of PGE2 precursor, arachidonic acid, is a well-characterized process in tumor tissue that has a variety of pro-tumor consequences, one of which is downregulation of effector CD4 + T cell activity. For T cells specifically, EP2 expression has been localized to CD4 + T cells while EP4 predominantly on CD8 + T cells [151, 152].
PGE2 plays a plethora of roles in normal physiology such as mediating fever, pain, and blood platelet function. PGE2 is also released directly by tumor and host cells of several solid tumors such as lung, prostate, breast, and pancreas. In both clinical and preclinical investigations, PGE2-EP2/3 interaction has been shown to promote tumor progression via enhancement of primary tumor growth, angiogenesis, and metastasis [153, 154]. PGE2 also facilitates tumor immune evasion by altering macrophages, NK cells, myeloid-derived suppressor cells (MDSCs), and DCs within the TME toward an immunosuppressive, pro-tumor phenotype [155]. Regarding CD4 + T cells, PGE2, via upregulation of IL-4 relative to IFNy, has been shown to promote differentiation to the Th2 subtype, dampening the effector T cell response [156, 157]. Additionally, studies report that PGE2 downregulates the IL-12 Th1 subtype-activating pathway, further enhancing the proportion of Th2 cells [158]. PGE2 has also been shown to induce CD4 + T cells into immunosuppressive FoxP3 + Treg cells, a process that is attenuated in EP2 and four deficient cells [159, 160]. There is evidence that the effect of PGE2 on CD4 + T cell differentiation (pro-inflammatory Th17 vs immunosuppressing Th2 subtypes) is dependent on PGE2 concentration; however, this remains an area of activity research. In translational studies, enhanced PGE2 signaling has been correlated with upregulation of immune checkpoints such as PD-1 on immune cells [151, 161]. Collectively, several lines of evidence suggest that PGE2 via interaction with EP2 and 4 results in suppression of effector CD4 + T cell function within the TME, thus limiting the host adaptive immune response.
Mounting evidence in the last two decades suggests that downregulation of PGE2 via pharmaceutical COX inhibition (i.e., aspirin) may reduce the risk of developing various solid tumors [162, 163]. However, support for this approach applied to a therapeutic setting is less convincing and has not been substantiated consistently in clinical trials. Furthermore, long-term COX inhibition is known to have adverse side effects that may limit such practices [164]. Thus, specific targeting of EP2 and EP4 has emerged as an appealing antitumor therapeutic alternative in manipulating the PGE2 axis. In murine prostate cancer models, EP4 small molecule inhibitor resulted in significant improvements in anticancer effector CD8 + T cells as well as concomitant downregulation of MDSCs within the TME. Aside from the TME, He et al. demonstrated that blockade of PGE2-EP4 signaling by a small molecule inhibitor of EP4 significantly impairs murine PDAC migration and invasion in vitro and abrogates hepatic metastatic dissemination in vivo [165]. In humans, an interim report of an active phase I trial investigating single-agent dual-antagonist small molecule inhibitor of EP2 and 4 or in combination with a PD-L1 blockade in a variety of advanced solid tumors such as has demonstrated acceptable safety profile across escalating dosing regimens with a 43% disease control rate with mono and combination therapy for over a median study time of 2 and 3 months, respectively [166]. Formal results following completion of enrollment in April 2024 are highly anticipated.
Perspective and conclusions
The advent of immunotherapy has altered the therapeutic landscape for cancer at-large. Reigniting the host immune response via T cell reactivation offers promising avenues toward specific and durable antitumor efficacy, evidenced by the success of immune checkpoint blockade in the clinic. The roles of GPCRs in tumorigenesis have largely been overlooked; however, as we have reviewed, recent work has revealed that several GPCRs play important immunomodulatory functions, particularly among T cells, serving as alternative immune modulators within the TME (Table 1). As such, targeting these GPCRs represents a potentially meaningful antitumor strategy apart from conventional ICBs.
Table 1.
Novel immunomodulatory GPCRs with associated T cell modulation. NECAB, neuronal calcium-binding protein; LPA, lysophosphatidic acid; ATX, autotaxin; NAGly, N-arachidonoyl glycine; Δ9THC, delta-9-tetrahydrocannabinol; AEA, anandamide; 2-AG, 2-arachidonoylglycerol; LysoPS, lysophosphatidylserine; S1P; sphingosine-1-phosphate; PGE2, prostaglandin-E2
| GPCR | Gene name | Ligand(s) | Expression | Function in T cells | Clinical development | Reference |
|---|---|---|---|---|---|---|
| GPR171 |
GPR171 H963 |
BigLen |
CD4 + T cells CD8 + T cells |
Negative regulator T lymphocytes | Preclinical | 31, 32 |
| A2AR |
ADORA2A RDC8 |
Adenosine, NECAB, caffeine |
CD4 + T cells CD8 + T cells |
Positive regulation CD4 + T cells Negative regulator CD8 T cells |
Phase 1 Phase 2 |
46, 47, 51–53 |
|
LPAR1 LPAR2 LPAR5 LPAR6 |
LPAR1 LPAR2 LPAR5 LPAR6 |
LPA, ATX |
CD4 + T cells CD8 + T cells |
Promote CD4 + /CD8 + T cells migration Inhibit CD8 + T cell activation/migration |
Preclinical |
69, 70 61, 66, 74, 76 |
| GPR68 | OGR1 | Hydrogen ions |
CD8 + T cells CD4 + T cells |
Suppress CD8 + T cell activation | Preclinical | 83, 88 |
| GPR18 | GPR18 | NAGly, resolvin-D2, imidazothiazinones, tricyclic xanthines | CD8 + T cells | Promote CD8 + expansion and differentiation | Preclinical | 73, 98, 99 |
| CB2 | CN2 | Δ9THC, AEA, 2-AG | CD4 + T cells |
Promote CD4 + T cells Suppress CD8 + T cell activation |
Preclinical | 115–117 |
| GPR65 | GPR65 | H + | CD4 + T cells | Promote differentiation into Th1 and Th17 | Preclinical | 123–125, 127 |
| GPR183 | GPR183 | Oxysterol |
CD4 + T cells CD8 + T cells |
Promote CD4 + T cell activation | Preclinical | 137–139 |
| P2Y10 | P2RY10 | LPA, LysoPS, S1P enhancers, ATP |
CD4 + T cells CD8 + T cells |
Regulate CD4 + T cell migration | Preclinical | 145–147 |
| GPR174 | GPR174 | Class A rhodopsin-Like | T reg cells |
Suppress T cell proliferation Suppress T reg generation |
Preclinical | 116, 153, 156 |
|
EP2 EP4 |
PTGER2 PTGER4 |
PGE2 |
CD4 + T cells CD8 + T cells |
Suppress CD8 + T cell infiltration | Phase 1 | 161, 162, 166 |
Questions regarding the mechanisms and applications of targeting GPCRs in the context of solid tumors remain. Outstanding mechanistic investigations include further elucidation of endogenous binding ligands for novel GPCRs such as GPR18, OGR1, and GPR65 and associated downstream intracellular signaling pathways. Furthermore, careful examination of the relationship between tumor cells with the expression and activity of immunomodulatory GPCRs would assist in understanding the cross talk between tumor and host that promote tolerogenic immune responses. Regarding therapeutic applications, the ubiquity of GPCRs such as LPARs, A2AR, and their associated ligands in normal physiologic processes present challenges in specifically targeting GPCRs of the TME. One potential strategy to enhance specificity of such agents could be elucidation of conformational changes of GPCRs that may occur secondary to malignant transformation/signaling as this could potentially facilitate development of small molecule inhibitors or monoclonal antibodies specific to TME-related GPCRs. Furthermore, wide-scale genomic sequencing of solid and liquid tumors alike have elucidated patterns of aberrantly produced or dysfunctional GPCRs characteristic of certain malignancies [167]. Such patterns have been described for cutaneous melanoma, lung, prostate, and breast cancer [14]. Downstream of the GPCRs themselves, certain signaling pathways, such as Wnt and PI3-Akt, are dysregulated in response to pathologic GPCR activity. Targeting both GPCR molecule interaction in combination with manipulation of downstream signaling with small molecules is a potentially amplified approach to targeting cancer-related GPCR pathways. Lastly, preclinical studies should evaluate the use of combined conventional T cell ICB and GPCR modulation in activating host cytotoxic immune response and if GPCR manipulation can serve as a rescue therapy in the setting of ICB resistance/exhaustion as this remains a significant barrier to widespread success of current immune checkpoint inhibitors.
Although this review summarizes only some of the most current and promising immunomodulatory GPCRs, the enormous breadth of GPCR family ensures that there are still GPCRs with meaningful roles in tumorigenesis that we have not uncovered. The success, but also limitations, of current immunotherapy in the clinic has highlighted the need to characterize new pathways and mechanisms by which cancer evades the cytotoxic immune system. As we have begun to understand, GPCRs, while ubiquitous, have a unique role in modulating the adaptive immune response, most pertinently the activity of T cells as we discuss in this review, but may seemingly influence immune cells of the TME writ large. Examining the clinical safety and efficacy of GPCR manipulation via clinical trials is the next step for GPCR-based therapies. Like conventional immune checkpoints, such as PD-L1 and CTLA-4, GPCRs may provide the next revolutionary target in cancer care.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
BioRender.com was used to create Figures 1-3.
Abbreviations
- Δ9THC
9-Tetrahydrocannabinol
- 2-AG
2-Arachidonoylglycerol
- A2AR
Adenosine 2A receptor
- AEA
Anandamide
- APCs
Antigen-presenting cells
- cAMP
Cyclic adenosine monophosphate
- COX
Cyclooxygenase;
- CRC
Colorectal cancer
- CTLA-4
Cytotoxic T-lymphocyte-associated protein-4
- DC
Dendritic cell
- GPCRs
G-coupled protein receptor
- ICB
Immune checkpoint blockade
- KO
Knockout
- LPAR
Lysophosphatidic acid receptor
- lysoPS
Lysophosphatidylserine
- mAb
Monoclonal antibody
- MDSC
Myeloid-derived suppressor cell
- NK
Natural killer
- PD-L1
Programmed death ligand 1
- PDAC
Pancreatic cell adenocarcinoma
- SqCC
Squamous cell cancer
- Treg
T regulatory cell
- TAM
Tumor-associated macrophage
- Th
T helper cell
- TIGIT
T cell immunoreceptor with immunoglobulin and ITIM domain
- TILs
Tumor infiltrating lymphocytes
- TME
Tumor microenvironment
- WT
Wildtype
Authors’ contributions
KD, EJY, and IV collected and reviewed relevant literature; KD, EJY, and IV constructed manuscript, figures, and table; RDS and YZ critically reviewed and edited manuscript. All authors read and approved the final version of the manuscript for submission.
Funding
This work was supported by NIH T32 Training Grant in Cancer Immunotherapy and Experimental Therapeutics (E.J.Y.), the Wings of Hope for Pancreatic Research (Y.Z.), NIH R01CA269644 (Y.Z.), R01CA258302 (Y.Z.), and R01CA279398 (Y.Z.).
Data availability
No datasets were generated or analyzed during the current study.
Declarations
Conflict of interests
R.D.S and Y.Z. are coinventors of a patent licensed to DynamiCure (managed by the University of Colorado) outside the submitted work. Y.Z. consults for DynamiCure Biotechnology. All other authors declare no competing financial interests.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Footnotes
Kaitlyn Dickinson and Elliott J. Yee have Shared first authorship.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- [1].Zhang Y, Zhang Z (2020) The history and advances in cancer immunotherapy: understanding the characteristics of tumor-infiltrating immune cells and their therapeutic implications. Cell Mol Immunol 17(8):807–821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Sharpe AH, Freeman GJ (2002) The B7-CD28 superfamily. Nat Rev Immunol 2(2):116–126 [DOI] [PubMed] [Google Scholar]
- [3].Kammerer-Jacquet SF et al (2019) Targeting the PD-1/PD-L1 Pathway in Renal Cell Carcinoma. Int J Mol Sci 20(7):1692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Huang AC, Zappasodi R (2022) A decade of checkpoint blockade immunotherapy in melanoma: understanding the molecular basis for immune sensitivity and resistance. Nat Immunol 23(5):660–670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Cheng C et al (2022) Overcoming resistance to PD-1/PD-L1 inhibitors in esophageal cancer. Front Oncol 12:955163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Dobosz P et al (2022) Challenges of the immunotherapy: perspectives and limitations of the immune checkpoint inhibitor treatment. Int J Mol Sci 23(5):2847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Yang D et al (2021) G protein-coupled receptors: structure- and function-based drug discovery. Signal Transduct Target Ther 6(1):7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Sun L, Ye RD (2012) Role of G protein-coupled receptors in inflammation. Acta Pharmacol Sin 33(3):342–350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Wang D (2018) The essential role of G protein-coupled receptor (GPCR) signaling in regulating T cell immunity. Immunopharmacol Immunotoxicol 40(3):187–192 [DOI] [PubMed] [Google Scholar]
- [10].Dorsam RT, Gutkind JS (2007) G-protein-coupled receptors and cancer. Nat Rev Cancer 7(2):79–94 [DOI] [PubMed] [Google Scholar]
- [11].Fares CM et al (2019) Mechanisms of resistance to immune checkpoint blockade: Why does checkpoint inhibitor immunotherapy not work for all patients? Am Soc Clin Oncol Educ Book 39:147–164 [DOI] [PubMed] [Google Scholar]
- [12].Wu V et al (2019) Illuminating the Onco-GPCRome: Novel G protein-coupled receptor-driven oncocrine networks and targets for cancer immunotherapy. J Biol Chem 294(29):11062–11086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Kobilka BK (2007) G protein coupled receptor structure and activation. Biochim Biophys Acta 1768(4):794–807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Chaudhary PK, Kim S (2021) An Insight into GPCR and G-Proteins as cancer drivers. Cells 10(12):3288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Wu VH et al (2023) The GPCR-Galpha(s)-PKA signaling axis promotes T cell dysfunction and cancer immunotherapy failure. Nat Immunol 24(8):1318–1330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Wang X et al (2022) The role of CXCR3 and its ligands in cancer. Front Oncol 12:1022688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Zhou P et al (2009) B7 blockade alters the balance between regulatory T cells and tumor-reactive T cells for immunotherapy of cancer. Clin Cancer Res 15(3):960–970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Zhang N, Bevan MJ (2011) CD8(+) T cells: foot soldiers of the immune system. Immunity 35(2):161–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Raskov H et al (2021) Cytotoxic CD8(+) T cells in cancer and cancer immunotherapy. Br J Cancer 124(2):359–367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Philip M, Schietinger A (2022) CD8(+) T cell differentiation and dysfunction in cancer. Nat Rev Immunol 22(4):209–223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Kakarala KK, Jamil K (2014) Sequence-structure based phylogeny of GPCR Class A Rhodopsin receptors. Molecul Phylogen Evolution 74:66–96 [DOI] [PubMed] [Google Scholar]
- [22].GeneCards, GPR171. (2023).
- [23].Ram A et al (2021) GPR171 agonist reduces chronic neuropathic and inflammatory pain in male, but not female mice. Front Pain Res. 10.3389/fpain.2021.695396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Dho SH et al (2016) GPR171 expression enhances proliferation and metastasis of lung cancer cells. Oncotarget 7(7):7856–7865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Fujiwara Y et al (2021) The GPR171 pathway suppresses T cell activation and limits antitumor immunity. Nat Commun 12:5857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Gomes I et al (2016) Identification of GPR83 as the receptor for the neuroendocrine peptide PEN. Sci Signal. 10.1126/scisignal.aad0694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Dai J et al (2022) DIRAS3, GPR171 and RAC2 were identified as the key molecular patterns associated with brain metastasis of breast cancer. Front Oncol. 10.3389/fonc.2022.965136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Hansen W et al (2006) G protein-coupled receptor 83 overexpression in naive CD4+CD25- T cells leads to the induction of Foxp3+ regulatory T cells in vivo. J Immunol 177(1):209–215 [DOI] [PubMed] [Google Scholar]
- [29].Lu LF et al (2007) G protein-coupled receptor 83 is dispensable for the development and function of regulatory T cells. Mol Cell Biol 27(23):8065–8972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Blay J, White T, Hoskin D (1997) The extracellular fluid of solid carcinomas contains immunosuppressive concentrations of adenosine. Can Res 57(13):2602–2605 [PubMed] [Google Scholar]
- [31].Korkutata M et al (2019) Enhancing endogenous adenosine A2A receptor signaling induces slow-wave sleep without affecting body temperature and cardiovascular function. Neuropharmacology 144:122–132 [DOI] [PubMed] [Google Scholar]
- [32].Padilla KM et al (2018) Behavioral changes induced through adenosine A2A receptor ligands in a rat depression model induced by olfactory bulbectomy. Brain Behavior. 10.1002/brb3.952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Shang P et al (2021) Emerging Nondopaminergic medications for Parkinson’s disease: focusing on A2A receptor antagonists and GLP1 receptor agonists. J Moving Disorders 14:193–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Canela L et al (2007) The neuronal Ca(2+) -binding protein 2 (NECAB2) interacts with the adenosine A(2A) receptor and modulates the cell surface expression and function of the receptor. Molecul Cell Neurosci 36(1):1–12 [DOI] [PubMed] [Google Scholar]
- [35].Borroto-Escuela D et al (2017) Cocaine self-administration specifically increases A2AR-D2R and D2R-sigma1R heteroreceptor complexes in the rat nucleus accumbens shell. Relevance for cocaine use disorder. Pharmacol Biochem Behavior 155:24–31 [DOI] [PubMed] [Google Scholar]
- [36].Bao Y et al (2015) mTOR and differential activation of mitochondria orchestrate neutrophil chemotaxis. J Cell Biol 210(7):1153–1164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Koshiba M et al (1999) Patterns of A2A extracellular adenosine receptor expression in different functional subsets of human peripheral T cells. Flow cytometry studies with anti-A2A receptor monoclonal antibodies. Molecul Pharmacol 7:614–624 [PubMed] [Google Scholar]
- [38].Mastelic-Vagillet B et al (2019) Adenosine mediates functional and metabolic suppression of peripheral and tumor-infiltrating CD8+ T cells. J ImmunoTherapy Cancer 7:257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Mediavilla-Varela M et al (2013) Antagonism of adenosine A2A receptor expressed by lung adenocarcinoma tumor cells and cancer associated fibroblasts inhibits their growth. Cancer Biol Therapy 14:860–868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Ohta A, Sitkovsky M (2014) Extracellular adenosine-mediated modulation of regulatory T cells. Front Immunol 5:304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Schneider E et al (2021) CD73-mediated adenosine production by CD8 T cell-derived extracellular vesicles constitutes an intrinsic mechanism of immune suppression. Nat Commun 12(1):5911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Briceno P et al (2021) CD73 ectonucleotidase restrains CD8+ T Cell metabolic fitness and anti-tumoral activity. Front Cell Develop Biol. 10.3389/fcell.2021.638037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Cekic C, Linden J (2014) Adenosine A2A receptors intrinsically regulate CD8+ T cells in the tumor microenvironment. Cancer Res 74:7239–7249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Fong L et al (2020) Adenosine A2A receptor blockade as an immunotherapy for treatment-refractory renal cell cancer. Cancer Discov 10(1):40–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Ohta A et al (2006) A2A adenosine receptor protects tumors from antitumor T cells. Proceed t Nati Acad Sci. 10.1073/pnas.0605251103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Sorrentino C et al (2019) Adenosine A2A receptor stimulation inhibits TCR-induced notch1 activation in CD8+T-cells. Front Immunol 10:162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Ma SR et al (2017) Blockade of adenosine A2A receptor enhances CD8+ T cells response and decreases regulatory T cells in head and neck squamous cell carcinoma. Molecul Cancer 16:99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Ohta AKR et al (2012) The development and immunosuppressive functions of CD4+ CD25+ FoxP3+ regulatory T cells are under influence of the adenosine-A2A adenosine receptor pathway. Front Immunol 3:190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Leone RD et al (2018) Inhibition of the adenosine A2a receptor modulates expression of T cell coinhibitory receptors and improves effector function for enhanced checkpoint blockade and ACT in murine cancer models. Cancer Immunol Immunotherapy 67(8):1271–1284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Chiappori A et al (2018) Phase I/II study of the A2AR antagonist NIR178 (PBF-509), an oral immunotherapy, in patients (pts) with advanced NSCLC. J Clinic Oncol 36(15):9089 [Google Scholar]
- [51].Lin CC, Joerger M, Grell P, Chiappori AA, Leal TA, Kasper S, Tan DS (2020) Continuous vs intermittent adenosine 2A receptor (A2AR) inhibition in preclinical colon cancer (CC) models and in a Phase (Ph) II study of taminadenant (NIR178) + spartalizumab (PDR001) in patients (pts) with non-small cell lung cancer (NSCLC). European J cancer 138:S12–S13 [Google Scholar]
- [52].Gadea OSS et al (2021) 436P Phase (Ph) II study of taminadenant (NIR178) + spartalizumab (PDR001) in patients (pts) with microsatellite stable (MSS) colorectal cancer (CRC. Ann Oncol 32:552–55333352201 [Google Scholar]
- [53].Jerusalem G et al (2022) 201P Phase II study of taminadenant (A2AR antagonist) + spartalizumab (anti PD-1 antibody) in patients with triple-negative breast cancer (TNBC. Immuno-Oncol Technol 16:100312 [Google Scholar]
- [54].Geraldo LHM et al (2021) Role of lysophosphatidic acid and its receptors in health and disease: novel therapeutic strategies. Signal Transduct Target Ther 6(1):45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Kotarsky K et al (2006) Lysophosphatidic acid binds to and activates GPR92, a G protein-coupled receptor highly expressed in gastrointestinal lymphocytes. J Pharmacol Exp Ther 318:619–628 [DOI] [PubMed] [Google Scholar]
- [56].Plastira I et al (2020) MAPK signaling determines lysophosphatidic acid (LPA)-induced inflammation in microglia. J Neuroinflammation 17:127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].An S et al (1997) Molecular cloning of the human Edg2 protein and its identification as a functional cellular receptor for lysophosphatidic acid. Biochem Biophys Res Commun 231:619–622 [DOI] [PubMed] [Google Scholar]
- [58].Turner JA et al (2023) Lysophosphatidic acid modulates CD8 T cell immunosurveillance and metabolism to impair anti-tumor immunity. Nat Commun 14(1):3214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Xiang H et al (2020) Lysophosphatidic acid receptors: biochemical and clinical implications in different diseases. J Cancer 11:3519–3535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Mathew D et al (2019) LPA5 is an inhibitory receptor that suppresses CD8 T-cell cytotoxic function via disruption of early TCR signaling. Front Immunol. 10.3389/fimmu.2019.01159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Lundequist A, Boyce JA (2011) LPA5 is abundantly expressed by human mast cells and important for lysophosphatidic acid induced MIP-1β release. PLoS ONE. 10.1371/journal.pone.0018192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Llona-Minguez S, Ghassemian A, Helleday T (2015) Lysophosphatidic acid receptor (LPAR) modulators: The current pharmacological toolbox. Prog Lipid Res 58:51–75 [DOI] [PubMed] [Google Scholar]
- [63].Kremer KN et al (2022) LPA suppresses T cell function by altering the cytoskeleton and disrupting immune synapse formation. Proc Natl Acad Sci U S A 119(15):e2118816119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Kanda H et al (2008) Autotaxin, an ectoenzyme that produces lysophosphatidic acid, promotes the entry of lymphocytes into secondary lymphoid organs. Nat Immunol 9:415–423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Oda SK et al (2013) Lysophosphatidic acid inhibits CD8 T cell activation and control of tumor progression. Cancer Immunol Res 1:245–255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Komachi M et al (2012) Orally active lysophosphatidic acid receptor antagonist attenuates pancreatic cancer invasion and metastasis in vivo. Cancer Sci 103:1099–1104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Konen JM et al (2023) Autotaxin suppresses cytotoxic T cells via LPAR5 to promote anti-PD-1 resistance in non-small cell lung cancer. J Clin Invest. 10.1172/JCI163128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Matas-Rico E et al (2021) Autotaxin impedes anti-tumor immunity by suppressing chemotaxis and tumor infiltration of CD8+ T cells. Cell Rep 5:704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Murai N et al (2017) Analgesic effects of novel lysophosphatidic acid receptor 5 antagonist AS2717638 in rodents. Neuropharmacology 126:97–107 [DOI] [PubMed] [Google Scholar]
- [70].He J et al (2021) Lysophosphatidic Acid Receptor 6 (LPAR6) Is a Potential Biomarker Associated with Lung Adenocarcinoma. Int Jurnal Environ Res Public Health 18(21):11038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Lin YH, Lin YC, Chen CC (2021) Lysophosphatidic acid receptor antagonists and cancer: the current trends, clinical implications, and trials. Cells 10(7):1629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Shi J et al (2020) LPAR1, correlated with immune infiltrates, is a potential prognostic biomarker in prostate cancer. Front Oncol 10:846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Takeda A et al (2016) Fibroblastic reticular cell-derived lysophosphatidic acid regulates confined intranodal T-cell motility. Elife 5:e10561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Hong JM et al (2023) LPA1-mediated inhibition of CXCR4 attenuates CXCL12-induced signaling and cell migration. Cell Commun Signal 21(1):257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Knowlden SA et al (2014) Regulation of T cell motility in vitro and in vivo by LPA and LPA2. PLoS ONE. 10.1371/journal.pone.0101655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Balijepalli P, Sitton CC, Meier KE (2021) Lysophosphatidic acid signaling in cancer cells: What makes LPA so Special? Cells 10(8):2059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Decato BE et al (2022) LPA(1) antagonist BMS-986020 changes collagen dynamics and exerts antifibrotic effects in vitro and in patients with idiopathic pulmonary fibrosis. Respir Res 23(1):61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Wiley ZZ et al (2019) GPR68: an emerging drug target in cancer. Int J Molecul Sci 20:559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].McAleer JP et al (2018) Cytokine regulation in human CD4 T Cells by the Aryl hydrocarbon receptor and Gq-coupled receptors. Sci Rep 8:10954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Estrella V et al (2013) Acidity generated by the tumor microenvironment drives local invasion. Cancer Res 73:1524–1535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Herzig M et al (2019) Comprehensive assessment of GPR68 expression in normal and neoplastic human tissues using a novel rabbit monoclonal antibody. Int J Molecul Sci 20:5261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Lee SH, Griffiths JR (2020) How and why are cancers acidic? Carbonic anhydrase IX and the homeostatic control of tumour extracellular pH. Cancers 12:1616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Zhang W et al (2020) Clinical data analysis reveals the role of OGR1 (GPR68) in head and neck squamous cancer. Animal Models Experiment Med 3:55–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Cao L et al (2021) Inhibition of host Ogr1 enhances effector CD8+ T-cell function by modulating acidic microenvironment. Cancer Gene Ther 28(10–11):1213–1224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Nayak AP, Penn RB (2020) The proton-sensing receptor ovarian cancer G-protein coupled receptor 1 (OGR1) in airway physiology and disease. Curr Opin Pharmacol 51:1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Vallière C et al (2022) pH-sensing G protein-coupled receptor OGR1 (GPR68) expression and activation increases in intestinal inflammation and fibrosis. Int J Molecul Sci 23:1419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Shore, D., et al., GPR68 limits the severity of chemical-induced oral epithelial dysplasia. Scientific Reports, 2023: p. 353. [DOI] [PMC free article] [PubMed]
- [88].Ye S et al (2023) G protein-coupled receptor GPR68 inhibits lymphocyte infiltration and contributes to gender-dependent melanoma growth. Front Oncol 13(1):353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Yan L et al (2014) Role of OGR1 in myeloid-derived cells in prostate cancer. Oncogene 33(2):157–164 [DOI] [PubMed] [Google Scholar]
- [90].Sumida H, Cyster JG (2018) G-Protein coupled receptor 18 contributes to establishment of the CD8 effector T Cell compartment. Front Immunol 11:660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].McHugh D et al (2010) N-arachidonoyl glycine, an abundant endogenous lipid, potently drives directed cellular migration through GPR18, the putative abnormal cannabidiol receptor. BMC Neurosci 11:44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Penumarti A, Abdel-Rahman AA (2014) The Novel endocannabinoid receptor GPR18 is expressed in the rostral ventrolateral medulla and exerts tonic restraining influence on blood pressure. J Pharamcol Experiment Therapeutics 349(1):29–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Wang X, Sumida H, Cyster JG (2014) GPR18 is required for a normal CD8αα intestinal intraepithelial lymphocyte compartment. J Experiment Med 211:2351–2359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Morales P et al (2020) Therapeutic exploitation of GPR18: beyond the cannabinoids? J Med Chem 63:14216–14227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Finlay DB et al (2016) GPR18 undergoes a high degree of constitutive trafficking but is unresponsive to N-Arachidonoyl Glycine. Peer J. 10.7717/peerj.1835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Simcocks AC et al (2019) Atypical cannabinoid ligands O-1602 and O-1918 administered chronically in diet-induced obesity. Endocr Connect 8(3):203–216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Rempel V et al (2014) Bicyclic imidazole-4-one derivatives: a new class of antagonists for the orphan G protein-coupled receptors GPR18 and GPR55. Med Chem Comm 5(5):632–649 [Google Scholar]
- [98].Irving, A., et al., (2017) Chapter Seven - Cannabinoid Receptor-Related Orphan G Protein-Coupled Receptors. Advances in Pharmacology p. 223–247. [DOI] [PubMed]
- [99].Ren H et al (2020) Identification of Genes with prognostic value in the breast cancer microenvironment using bioinformatics analysis. Med Sci Monthly. 10.12659/MSM.920212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Qiao GJ et al (2019) Identification of an eight-gene signature for survival prediction for patients with hepatocellular carcinoma based on integrated bioinformatics analysis. Peer J 7:e6548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Qin Y et al (2011) Quantitative expression profiling of G-protein-coupled receptors (GPCRs) in metastatic melanoma: the constitutively active orphan GPCR GPR18 as novel drug target. Pigment Cell Melanoma Res 24(1):207–218 [DOI] [PubMed] [Google Scholar]
- [102].Kravtsov DS et al (2022) Roles of CD4+ T cells as mediators of antitumor immunity. Front Immunol 13:972021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Tay RE, Richardson EK, Toh HC (2021) Revisiting the role of CD4(+) T cells in cancer immunotherapy-new insights into old paradigms. Cancer Gene Ther 28(1–2):5–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Luckheeram RV et al (2012) CD4(+)T cells: differentiation and functions. Clin Dev Immunol 2012:925135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Poncette L, Bluhm J, Blankenstein T (2022) The role of CD4 T cells in rejection of solid tumors. Curr Opin Immunol 74:18–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Braile M et al (2021) The Interplay between the Immune and the Endocannabinoid Systems in Cancer. Cells 10:1282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Ward SJ et al (2021) Cannabinoids and Cancer Chemotherapy-Associated Adverse Effects. J Nat Cancer Institute 58:78–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Xiong X et al (2022) Cannabis suppresses antitumor immunity by inhibiting JAK/STAT signaling in T cells through CNR2. Sig Transduct Target Ther. 10.1038/s41392-022-00918-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Acharya N, Sabatos-Peyton C, Anderson AC (2020) Tim-3 finds its place in the cancer immunotherapy landscape. J immunoTher Cancer. 10.1136/jitc-2020-000911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Sarsembayeva A et al (2023) Cannabinoid receptor 2 plays a pro-tumorigenic role in non-small cell lung cancer by limiting anti-tumor activity of CD8+ T and NK cells. Front Immunol. 10.3389/fimmu.2022.997115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Sido JM, Nagarkatti PS, Nagarkatti M (2016) Production of endocannabinoids by activated T cells and B cells modulates inflammation associated with delayed-type hypersensitivity. European J Immunol 46:1472–1479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Basu S, Dittel BN (2011) Unraveling the complexities of cannabinoid receptor 2 (CB2) immune regulation in health and disease. Immunol Res 51(1):26–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Ross SH, Cantrell DA (2019) Signaling and function of interleukin-2 in T lymphocytes. Annual Rev Immunol 36:411–413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Robinson RH et al (2015) A CB2-Selective Cannabinoid Suppresses T-Cell Activities and Increases Tregs and IL-10. J Neuroimmune Pharmacol 10:318–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Chen J et al (2022) Activation of CD4+ T cell-derived cannabinoid receptor 2 signaling exacerbates sepsis via inhibiting IL-10. J Immunol 208(11):215–2522 [DOI] [PubMed] [Google Scholar]
- [116].Xiong X et al (2022) Cannabis suppresses antitumor immunity by inhibiting JAK/STAT signaling in T cells through CNR2. Signal Transduct Target Ther 7(1):99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Robinson RH et al (2013) Cannabinoids inhibit T-cells via cannabinoid receptor 2 in an in vitro assay for graft rejection, the mixed lymphocyte reaction. J Neuroimmune Pharmacol 8:1239–1250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Ghosh S et al (2006) Cannabinoid receptor CB2 modulates the CXCL12/CXCR4-mediated chemotaxis of T lymphocytes. Mol Immunol 43(14):2169–2179 [DOI] [PubMed] [Google Scholar]
- [119].Wang L et al (2023) GPR65 as a potential immune checkpoint regulates the immune microenvironment according to pan-cancer analysis. Heliyon 9(2):e13617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Ishii S, Kihara Y, Shimizu T (2005) Identification of T cell death-associated gene 8 (TDAG8) as a novel acid sensing G-protein-coupled receptor. J Biol Chem 280(10):9083–9087 [DOI] [PubMed] [Google Scholar]
- [121].Lin R et al (2022) GPR65 promotes intestinal mucosal Th1 and Th17 cell differentiation and gut inflammation through downregulating NUAK2. Clinic Translation Med. 10.1002/ctm2.771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Ihara Y et al (2010) The G protein-coupled receptor T-cell death-associated gene 8 (TDAG8) facilitates tumor development by serving as an extracellular pH sensor. Proc Natl Acad Sci 107(40):17309–17314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Kyaw H et al (1998) Cloning, characterization, and mapping of human homolog of mouse T-cell death-associated gene. DNA Cell Biol 17(6):493–500 [DOI] [PubMed] [Google Scholar]
- [124].Wang JQ et al (2004) TDAG8 is a proton-sensing and psychosine-sensitive G-protein-coupled receptor. J Biol Chem 279(44):45626–45633 [DOI] [PubMed] [Google Scholar]
- [125].Shakibaei M et al (2010) TNF-α-induced mitochondrial alterations in human T cells requires FADD and caspase-8 activation but not RIP and caspase-3 activation. Antioxid Redox Sig 13(6):821–831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Tcymbarevich I et al (2019) Lack of the pH-sensing receptor TDAG8 [GPR65] in macrophages plays a detrimental role in murine models of inflammatory bowel disease. J Crohns Colitis 13(2):245–258 [DOI] [PubMed] [Google Scholar]
- [127].Cipriani B, Corbin A, Miller D, Naylor A, Khan F, Milne G, Hughes S (2023) The translational biology of small molecule GPR65 inhibitors: shared effects between mouse models and human primary tumors highlight the unique transformative potential of targeting a genetically validated innate immune checkpoint. Cancer Res 83:668–668 [Google Scholar]
- [128].Birkenbach M et al (1993) Epstein-Barr virus-induced genes: first lymphocyte-specific G protein-coupled peptide receptors. J Virol 67(4):2209–2220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Daugvilaite V et al (2014) Oxysterol-EBI2 signaling in immune regulation and viral infection. Eur J Immunol 44(7):1904–1912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Li J et al (2016) EBI2 augments Tfh cell fate by promoting interaction with IL-2-quenching dendritic cells. Nature 533(7601):110–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Bartlett S et al (2020) GPR183 Regulates interferons, autophagy, and bacterial growth during mycobacterium tuberculosis infection and is associated with TB disease severity. Front Immunol 11:601534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Foo CX et al (2022) GPR183 antagonism reduces macrophage infiltration in influenza and SARS-CoV-2 infection. European Respiratory J. 10.1183/13993003.01306-2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Sun S, Liu C (2015) 7α, 25-dihydroxycholesterol-mediated activation of EBI2 in immune regulation and diseases. Front Pharmacol 6:60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Emgard J et al (2018) Oxysterol sensing through the receptor GPR183 promotes the lymphoid-tissue-inducing function of innate lymphoid cells and colonic inflammation. Immunity 48(1):120–132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Kuen DS et al (2021) Critical regulation of follicular helper T cell differentiation and function by Gα13 signaling. Proceed Nat Academy Sci 118(43):e2108376118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Zanin-Shorov A et al (2016) Isoform-specific targeting of ROCK proteins in immune cells. Small GTPases 7(3):173–177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Barington L et al (2018) EBI2 in splenic and local immune responses and in autoimmunity. J Leukoc Biol 104(2):313–322 [DOI] [PubMed] [Google Scholar]
- [138].Baptistia AP et al (2019) The Chemoattractant Receptor Ebi2 Drives Intranodal Naive CD4+ T Cell Peripheralization to Promote Effective Adaptive Immunity. Immunity 50(5):1188–1201 [DOI] [PubMed] [Google Scholar]
- [139].Yang Y et al (2020) A Pan-cancer analysis of GPR183 correlated with tumor immunity. Res Square. 10.21203/rs.3.rs-42118/v1 [Google Scholar]
- [140].Liu C et al (2023) Comprehensive analysis of P2Y family genes expression, immune characteristics, and prognosis in pan-cancer. Translat Oncol 37:101776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].GeneCards, P2RY10. (2023).
- [142].Kugelgen I, Hoffman K (2016) Pharmacology and structure of P2Y receptors. Neuropharmacology 104:50–61 [DOI] [PubMed] [Google Scholar]
- [143].Berchtold S et al (1999) Human monocyte derived dendritic cells express functional P2X and P2Y receptors as well as ecto-nucleotidases. FEBS Letter 458(3):424–428 [DOI] [PubMed] [Google Scholar]
- [144].Gurusamy M et al (2021) G-protein-coupled receptor P2Y10 facilitates chemokine-induced CD4 T cell migration through autocrine/paracrine mediators. Nat Commun 12:6798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Hwang SM, Kim HJ, Kim SM, Jung Y, Park SW, Chung IY (2018) Lysophosphatidylserine receptor P2Y10: A G protein-coupled receptor that mediates eosinophil degranulation. Clin Exp Allergy 48:990–999 [DOI] [PubMed] [Google Scholar]
- [146].Murakami M et al (2008) Identification of the orphan GPCR, P2Y(10) receptor as the sphingosine-1-phosphate and lysophosphatidic acid receptor. Biochem Biophys Res Commun 371(4):707–712 [DOI] [PubMed] [Google Scholar]
- [147].Heasman SJ, Ridley AJ (2010) Multiple roles for RhoA during T cell transendothelial migration. Small GTPases 1(3):174–179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Shinjo Y et al (2017) Lysophosphatidylserine suppresses IL-2 production in CD4 T cells through LPS3/GPR174. Biochem Biophys Res Commun 949(1):332–338 [DOI] [PubMed] [Google Scholar]
- [149].Dey I, Lejeune M, Chadee K (2006) Prostaglandin E2 receptor distribution and function in the gastrointestinal tract. Br J Pharmacol 149(6):611–623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Ying F et al (2021) Prostaglandin E receptor subtype 4 protects against diabetic cardiomyopathy by modulating cardiac fatty acid metabolism via FOXO1/CD36 signalling. Biochem Biophys Res Commun 548:196–203 [DOI] [PubMed] [Google Scholar]
- [151].Peng S et al (2022) Single-cell analysis reveals EP4 as a target for restoring T-Cell infiltration and sensitizing prostate cancer to immunotherapy. Clinic Cancer Res 28:552–567 [DOI] [PubMed] [Google Scholar]
- [152].PTGER4 Gene - Prostaglandin E Receptor 4. GeneCards: The Human Gene Database, (2024).
- [153].De Paz Linares GA et al (2021) Prostaglandin E2 receptor 4 (EP4) as a therapeutic target to impede breast cancer-associated angiogenesis and lymphangiogenesis. Cancers (Basel) 13(5):942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Finetti F et al (2020) Prostaglandin E2 and Cancer: Insight into Tumor Progression and Immunity. Biology (Baseline) 9(12):434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Jin K et al (2023) Cyclooxygenase-2-prostaglandin E2 pathway: a key player in tumor-associated immune cells. Front Oncol 13:1099811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Abe N et al (1997) Prostaglandin E2 and IL-4 provide naive CD4+ T cells with distinct inhibitory signals for the priming of IFN-gamma production. Cell Immunol 181(1):86–92 [DOI] [PubMed] [Google Scholar]
- [157].Bao YS et al (2011) The regulation of CD4+ T cell immune responses toward Th2 cell development by prostaglandin E2. Int Immunopharmacol 11(10):1599–1605 [DOI] [PubMed] [Google Scholar]
- [158].Kalim KW, Groettrup M (2013) Prostaglandin E2 inhibits IL-23 and IL-12 production by human monocytes through down-regulation of their common p40 subunit. Mol Immunol 53(3):274–282 [DOI] [PubMed] [Google Scholar]
- [159].Boniface K et al (2009) Prostaglandin E2 regulates Th17 cell differentiation and function through cyclic AMP and EP2/EP4 receptor signaling. J Explorat Med 206(3):535–548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Okano M et al (2006) E prostanoid 2 (EP2)/EP4-mediated suppression of antigen-specific human T-cell responses by prostaglandin E2. Immunology 118(3):343–352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Wang J et al (2018) Activation of PGE2/EP2 and PGE2/EP4 signaling pathways positively regulate the level of PD-1 in infiltrating CD8(+) T cells in patients with lung cancer. Oncol Lett 15(1):552–558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Elwood P et al (2024) Aspirin and cancer treatment: systematic reviews and meta-analyses of evidence: for and against. Br J Cancer 130(1):3–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Skriver C et al (2023) Long-term aspirin use and cancer risk: a 20-year cohort study. J Natl Cancer Inst. 10.1093/jnci/djad231 [DOI] [PubMed] [Google Scholar]
- [164].Chan AT et al (2005) Long-term use of aspirin and nonsteroidal anti-inflammatory drugs and risk of colorectal cancer. JAMA 294(8):914–923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].He J et al (2022) A novel small molecular prostaglandin receptor EP4 antagonist, l001, suppresses pancreatic cancer metastasis. Molecules 27(4):1209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [166].Ulahannan SV et al (2023) Phase 1a/1b Study of TPST-1495 as a Single Agent and in Combination With Pembrolizumab in Subjects With Solid Tumors. J Clinic Oncol. 10.1200/JCO.2023.41.16_suppl.3107 [Google Scholar]
- [167].Usman S et al (2020) The current status of anti-GPCR drugs against different cancers. J Pharm Anal 10(6):517–521 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No datasets were generated or analyzed during the current study.