Abstract
Dopamine (DA) neurons play a crucial role in the development and manifestation of depression, as well as in response to antidepressant treatments. While the function of the predominantly distributed DA neurons in the ventral tegmental area (VTA) is well established, the contribution of a small fraction of DA neurons in the dorsal raphe nucleus (DRN) during depression remains unclear. In this study, we found that chronic unpredictable stress (CUS) induces depression-related behaviors and decreases spontaneous firing rates, excitatory and inhibitory postsynaptic currents of DA neurons in the DRN associated with reduced excitatory synaptic transmission in male and female mice. The chemogenetic inhibition of DA neurons in the DRN produces depressive phenotypes. Conversely, their activation completely reversed the anhedonic and despair behaviors induced by CUS. Furthermore, we showed that a DRN dopaminergic projecting to the dorsal bed nucleus of the stria terminalis (dBNST) selectively controls depressive behaviors by influencing the neural activity and N-methyl-D-aspartate receptor (NMDAR) mediating EPSC of calcium/calmodulin-dependent protein kinase II+ (CaMKII+) target neurons by regulating dopamine neurotransmitter and dopamine receptor 2 (DR2) in the dBNST. Overall, these findings highlight the essential role of the DRNDA → dBNSTCaMKII+ neural circuit in bi-directionally mediating stress-induced depression-related behaviors. Our findings indicate that DRN DA neurons are a key component of the neural circuitry involved in regulating depression-related behaviors, making them a potential therapeutic target for depression.
Subject terms: Diseases, Depression
Introduction
Depression is a common mental disorder characterized by a variety of symptoms, including prominent and persistent low mood, loss of pleasure (anhedonia), and suicidal thoughts and attempts. It is one of the leading causes of disability and imposes a severe burden on society and families [1–3]. Despite extensive clinical and basic research conducted over the past few decades, several key factors, such as the absence of objective assessments and high rates of refractory and drug-resistant, continue to hinder the therapeutic progress of depression [4–8]. Therefore, there is an urgent need for a better understanding of the pathogenesis and new neural substrates underlying depression.
The mesolimbic dopamine system, mainly involving dopamine (DA) neurons in the ventral tegmental area (VTA), has been reported as a key neural substrate for reward-related hedonic and emotional behaviors. This has been supported by increasing evidence from human brain imaging and animal studies, especially in the context of depression [9–12]. In addition to the widely distributed DA neurons in the VTA, there is also a small fraction of DA neurons (~1000 in mice) expressing the DA markers tyrosine hydroxylase and DA transporter in the dorsal raphe nucleus (DRN) [13, 14]. Traditionally, these neurons have been recognized as dorsal-caudal extensions of VTA DA neurons [15]. Recent studies have shed new light on the modulation of memory expression, addiction, pain sensitivity, arousal, and sociability for the DRN DA neurons [15–18]. Unlike the VTA DA neurons, which project widely to the limbic and cortical regions, DRN DA neurons exhibit much more focused projections, especially innervating the bed nucleus of the stria terminalis (BNST) and the central amygdala (CeA) [19, 20]. This confirms that DRN DA neurons may function as a distinct midbrain DA subsystem. Nevertheless, an existing gap regarding the involvement of DRN DA neurons in depression, and the potential roles and mediating neural circuits of DRN DA neurons in the regulation of depression-related behaviors remain unclear.
In this study, we investigated the function of DRN DA neurons and their neural projections targeting the dorsal bed nucleus of the stria terminalis (dBNST) in depression-related behaviors. We employed various approaches, including patch clamp electrophysiology, neural tracing/ablation, immunohistochemistry (IHC), in vivo chemogenetics, fiber photometry recording, and a variety of behavioral paradigms, to examine the dysfunctional activity of DRN DA neurons during stress exposure and the impact of modulating these neurons on depression-related behaviors via specific projecting in the dBNST. Our results demonstrate that this separate cell population is involved in the progression of stress-induced depression and that dBNST neural projections are critical for this process. In summary, our data indicate the essential role of DRN → dBNST neural projections in regulating depression-related behaviors.
Materials and methods
Animals
Male and female wild-type (WT) C57BL/6 J mice (Stock No. 000664), Ai14-tdTomato (Stock No. 007914) and DAT-Cre (Stock No. 006660) transgenic mice were obtained from Jackson Laboratory (Bar Harbor, ME, USA) [21]. The genotyping protocol was descripted previously [22, 23]. All mice were housed under controlled environmental conditions (12 h light/12 h dark cycle, lights on at 7 a.m.; ambient temperature 22-24°C) with food and water ad libitum.
Drugs
Clozapine-N-oxide (CNO, C0832, Sigma-Aldrich, St. Louis, MO, USA) was dissolved in 100% dimethyl sulfoxide (DMSO), diluted with 0.9% saline to a final dose of 1 mg/ml, and administered intraperitoneally (i.p.) 45 min before the behavioral tests. A dose of 0.3 mg/kg body weight was administered to AAV-DIO-hM3D-mCherry-injected mice, while a dose of 2 mg/kg body weight was administered to hM4D treated or both hM3D and hM4D treated mice based on previously published studies [24–26]. For whole-cell patch-clamp recordings, the slices were perfused with CNO (10 μM) or D2 agonist Quinpirole (10 μM), (Q102, Sigma-Aldrich, St. Louis, MO, USA) based on our previous reports [24, 27].
Stereotaxic surgery and adeno-associated viruses infusion
Stereotaxic surgeries and AAV infusions of DRN and dBNST were performed under anesthesia (sodium pentobarbital, P3761, Sigma-Aldrich, St. Louis, MO, USA). The AAVs used in this study included AAV2/9-hSyn-DIO-hM4D(Gi)-mCherry (AAV-DIO-hM4D-mCherry), AAV2/9-hSyn-DIO-hM3D(Gq)-mCherry (AAV-DIO-hM3D-mCherry), AAV2/9-hSyn-DIO-mCherry (AAV-DIO-mCherry), AAV2/9-hSyn-DIO-GFP-WPRE-pA (AAV-DIO-GFP), AAV2/Retro-CAG-GFP (AAV-Retro-GFP) and AAV2/Retro-CAG-Cre-GFP (AAV-Retro-Cre-GFP) viruses that were all provided and verified by Hanheng Biotechnology (Shanghai, China). AAV2/1-TH-Cre, AAV2/9-CMV-DIO-taCasp3-TEVp-GFP (AAV-DIO-taCasp3), AAV2/9-EF1a-DIO-DA4.4-WPRE-hGH polyA (AAV-EF1a-DIO-DA4.4), AAV2/9-Vgat-DIO-GFP (AAV-Vgat-DIO-GFP) and AAV2/9-CaMKII-DIO-GFP (AAV-CaMKII-DIO-GFP) were purchased from BrainVTA (BrainVTA Co., Ltd., Wuhan, China). In brief, the target virus (0.30 μL for single or 1:1 mixed virus, viral titer >1 × 1012 vg/mL) was injected into the DRN or dBNST (bilaterally, 0.30 μL/side; DRN coordinates: anteroposterior (AP) = −4.0 mm, mediolateral (ml) = ± 0.4 mm, dorsoventral (DV) = −3.0 mm from the bregma, with a 15° angle); dBNST coordinates: AP = 0.30 mm, ML = ± 2.00 mm, DV = −4.20 mm from the bregma, 15° angle) of adult WT or transgenic mice (8 weeks old) with a 33-gauge stainless steel injector connected to a UMP3 micro syringe pump (World Precision Instruments, Sarasota, FL). The viruses were microinjected at a rate of 0.10 μL/min, and the injection needles were kept in place for an additional 5 min to allow for diffusion and prevent backflow. The behavioral tests were performed 3 weeks after AAV injection.
Immunofluorescence histochemistry
The mice were deeply anesthetized with sodium pentobarbital (40 mg/kg, P3761, Sigma-Aldrich) and transcardially perfused with precooled 4% paraformaldehyde. The brains were then removed, post-fixed overnight, and cryoprotected in a 30% sucrose solution until they sank to the bottom of the container. Coronal sections (40 μm thick) containing the DRN and dBNST regions were cut using a cryostat microtome (Leica Microsystems, Wetzlar, Germany). The brain sections were washed three times in 0.01 M PBS. After blocking in a solution of 1% bovine serum albumin (diluted in 0.01 M PBS containing 0.3% goat serum and 3% Triton X-100) for 1 h at room temperature, all sections were incubated overnight at 4 °C with rabbit anti-GABA primary antibody (1:500, A2052, Sigma-Aldrich, Saint Louis, MO, USA) or mouse anti-CaMKII primary antibody (1:400, ab134041, Abcam, Cambridge, UK), rabbit anti-TH primary antibody (1:500, Proteintech, 25859-1-AP), anti-TPH2 (1:400, ab184505, Abcam, Cambridge, UK), anti-c-Fos primary antibody (1:1000, 2250S, CST, Danvers, MA), anti-D1 receptor (1:500, NB110-60017, Littleton, CO, USA) anti-D2 receptor (1:500, B-10, Santa Cruz Biotechnology, Santa Cruz, CA) in the blocking solution after washing in 0.01 M PBS for three times, the sections were incubated with Alexa Fluor® 488 goat anti-rabbit IgG (1:400, A21206, Invitrogen, Carlsbad, CA, USA), Alexa Fluor®488 goat anti-mouse IgG antibodies (1:400, A21202, Invitrogen) or Alexa Fluor® 546 goat anti-rabbit IgG antibodies (1:400, A11035, Invitrogen) for 4 h at room temperature protected from light. In addition, after washing in 0.01 M PBS for three times, the stained sections were wet mounted onto polylysine-coated glass slides, counterstained with fluorescent mounting medium, and covered with cover slips. Images were visualized using an Olympus FV1000 laser scanning confocal microscope (Olympus, Shinjuku, Tokyo, Japan). The immuno-positive cells in 2–5 sections per brain containing DRN (approximately –4.84 to -4.48 mm relative to bregma) or dBNST (approximately –0.10 to 0.26 mm relative to bregma) were manually counted by an observer blind to the treatment. The immunofluorescence intensity of neural fibers in 2–5 sections per brain containing BNST and CeA (approximately 1.70 to 0.74 mm relative to bregma) and VTA (approximately -3.80 to -2.92 mm relative to bregma) were analyzed using ImageJ (http://imagej.nih.gov/ij/) and normalized to 100% as described previously [25, 28].
RNA fluorescence in situ hybridization
Biotin-labeled TH probe (5′-ATCACGGGCAGACAGTAGACCGTCTCTAAGTGGTGGATTTTGGAATTTCCTCCTTTGTGTATTCC-3′) and negative control probe (5′-TGCTTTGCACGGTAACGCCTGTTTT-3′) were designed and synthesized (GenePharma, Shanghai, China). The probe signals were determined using the RNA fluorescence in situ hybridization (FISH) Probe Kit (SA-Biotin system) (F32101, GenePharma, Shanghai, China) according to the manufacturer’s instructions. Briefly, the slice were fixed with 4% paraformaldehyde, and permeabilized with 0.5% Triton X-100 for 15 min at room temperature. 1% proteinase K was added for 20 min at 37 °C, then, incubated with blocking buffer 30 min at 37 °C, The sections were incubated with denaturation solution for 8 min at 78 °C, followed by rehydration in descending concentrations of ethanol (70%∼100%). The sections were subsequently incubated with mixed Streptavidin-Cy3 and Biotin-labeled TH probe working solution at 37 °C overnight. DAPI was used to stain nuclei for 15 min. Images were visualized using an Olympus FV1000 laser scanning confocal microscope (Olympus, Shinjuku, Tokyo, Japan).
Whole-cell patch-clamp recordings
Electrophysiological recordings were performed following previously published methods [24, 29, 30]. Mice were anesthetized with isoflurane and decapitated, then brain was rapidly dissected out and quickly transferred to ice-cold artificial cerebrospinal fluid (aCSF) containing (in mM): 206 sucrose, 2 MgSO4, 2 KCl, 1 MgCl2, 1 CaCl2, 1.25 NaH2PO4, 0.40 Vitamin C, 10 D-glucose, and 24 NaHCO3. Coronal slices (300 μm thick) containing the recording area were prepared with a Leica VT1200 vibratome (Leica Microsystems, Bannockburn, IL, USA) and transferred to a water bath incubator for at least 1 hour at 30 °C in oxygenated (95% O2/5% CO2) extracellular bath aCSF containing (in mM): 124 NaCl, 2 KCl, 2 MgSO4, 1.25 NaH2PO4, 2 CaCl2, 24 NaHCO3, 10 d-dextrose, and 0.40 vitamin C before recording. Slices were viewed using an infrared differential interference contrast optics under an upright microscope (BX51WI, Olympus, Tokyo, Japan) fitted with a 40× water immersion objective. The slice was then transfered to recording chamber and recorded under room temperature. The chamber was continuously perfused with the extracellular bath aCSF at a flow rate of 1.5–2 ml/min during whole-cell patch clamp recordings. Recording pipettes with tip resistances of 3–5 MΩ were pulled from thin-walled borosilicate glass capillaries (BF-150, Sutter Instrument, USA) using the Sutter P-97 (Sutter Instrument, Novato, CA, USA). For neuronal excitability recording, pipettes were filled with a potassium gluconate-based internal solution containing (in mM): 120 potassium gluconate, 20 KCl, 2 MgCl2, 10 HEPES, 0.1 EGTA, 2 Na2•ATP, 0.25 Mg•GTP, and 12 phosphocreatine, with a pH of 7.40 and osmolarity of 295–300 mOsm, and current injection steps ranged from 10 to 200 pA with a 500 ms duration. Twenty sweeps were recorded, and then switched to input resistance protocol to detect the hyperpolarization current. Data on spike amplitude, half-width, fast after-hyperpolarization (fAHP), and spike threshold were collected. The spontaneous firing of DA-capable neurons was recorded at zero current mode (I = 0) and the resting membrane potential was measured immediately after obtaining whole-cell access.
To record spontaneous excitatory postsynaptic currents (sEPSC), brain slice was perfused with extracellular bath aCSF in the presence of picrotoxin (100 μM, CAS: 124-87-8, Sigma-Aldrich) to isolate excitatory transmission. Fluorescent labeled neurons were selected in the recording area, and the recording electrode was filled with an intracellular solution containing (in mM): 120 CsMeSO4, 20 CsCl, 2 MgCl•6H2O, 10 HEPES, 1 EGTA, 2.20 QX-314, 12 phosphocreatine, 4 Mg•ATP, and 0.50 Na2•GTP, adjusted to pH 7–7.20, osmolarity 295–300 mOsm, set holding potential at −70 mV. Miniature EPSC (mEPSC) were recorded in the presence of tetrodotoxin (1 μM, CAS: 4368-28-9, Tocris Bioscience, Bristol, United Kingdom) at a holding potential of −70 mV. For the spontaneous inhibitory postsynaptic currents (sIPSC) recording, CNQX (10 μM, CAS: 115066, Sigma-Aldrich) and DL-AP5 (50 μM, CAS: 79055-68-8, Tocris Bioscience) were added to the aCSF, and the holding potential was set at −70 mV. The recording electrode was filled with an internal solution containing (in mM): 130 CsCl, 2 MgCl2•6H2O, 10 HEPES, 2 EGTA, 5 QX-314, 10 phosphocreatine, 5 Mg•ATP, and 0.50 Na2•GTP with an osmolarity of 295-300 mOsm. During miniature IPSC (mIPSC) recording, tetrodotoxin (1 μM) was added to the perfused extracellular bath aCSF and the membrane potentials were held at −70 mV.
To evoked responses, a bipolar tungsten stimulating electrode was placed locally in the dBNST and positioned within 100 μm of the recorded cell. The current was controlled by a stimulus isolator (ISO-Flex, AMPI, Israel) and the stimulus intensity was adjusted to elicit monosynaptic responses in the range of 100-300 pA. The NMDAR-eEPSC were recorded at a holding of +40 mV with AMPA receptor antagonist CNQX (10 μM) and GABAA receptor antagonist picrotoxin (100 μM) added to the perfused aCSF. Pharmacologically isolated the AMPA receptor-mediated synaptic responses, the neurons were held at −70 mV, NMDAR antagonist DL-AP5 (50 μM) and GABAA receptor antagonist picrotoxin (100 μM) were also added into the perfusate. The amplitude and frequency of sEPSC, mEPSC, sIPSC and mIPSC were determined from all detected events in each cell of last 1 min of 3 min recording; The recording was successful with the criteria: Ra <30 MΩ or <25 MΩ, leak current <100 pA), if the series resistance changed > 20%, the recording was discarded. All the data were collected with an Axon700B amplifier (Molecular Devices, LLC., CA) and filtered at 2 kHz and digitized at 10 kHz with Axon Digidata1550 Low-Noise Data Acquisition System(Axon Instruments). All data collected were analyzed with Clampfit 10.7 Microsoft software.
Fiber photometry recording
Dopamine neurotransmitters were recorded by a three-color fiber photometry system (RWD Life Science, Shenzhen, China). The mice, injected with AAV-hSyn-DA4.4-WPRE-hGH-polyA (BrainVTA Technology, Wuhan, China) into dBNST were then implanted an optical fiber (with 1.25 mm Ceramic Ferrule, 200 μm Core, 0.39 numerical aperture) above the injection site of dBNST. Three weeks later, mice were intraperitoneal injection CNO, the fluorescence signals were recorded with 470 nm excitation and data was analyzed by the recording software (RWD Life Science, Shenzhen, China). The values of dopamine signal changes (ΔF/F) were derived by calculating(F-F0)/F0 (baseline fluorescence signal) [31].
Chronic unpredictable stress
The Chronic unpredictable stress (CUS) procedure was carried out as described previously [23, 32, 33]. Briefly, mice were subjected to two different stressors at different times of every day for 14 consecutive days. The stressors included restraint (2-h), tail pinch (15-min), constant light(24-h), wet bedding (24-h) with 45° tilt, inescapable foot shocks (10-min, 0.3 mA, 2 s duration, 16 s interval), elevated platform (30-min), and social isolation.
Behavioral tests
All behavioral tests were conducted during the late light phase of the lighting cycle, except for the SPT, which was measured during the first 2 h of the dark phase. Before each test, all the mice underwent a 4-hour habituation period in the testing room. A 2–3-day interval between two consecutive tests was observed to prevent possible carryover effects. Furthermore, to eliminate any potential subjective bias, the behavioral performance of each mouse in each test was recorded in a double-blind manner.
Female urine sniffing test
The FUST was performed to assess sex-related reward-seeking behavior by observing the attraction of male mice to pleasurable pheromones in female urine. Prior to the test, mice were placed in an individual cage for 2–3 h to acclimate to their surroundings. Male mice were given at least 1 h to adapt to the presence of the cotton swab applicator used in the experiment. The testing room was dimly lit with ambient lighting of approximately 3 lx. During the test, each individually housed mouse was exposed to a sterile cotton swab applicator soaked in water for 3 min. This was followed by exposure to another cotton swab applicator soaked in fresh urine (80 μL) collected from estrous female mice of the same strain 45 minutes later. The time spent by each mouse sniffing the sterile water and female urine was recorded. Mice that did not move or sniff the swab in the cage during the test were excluded from statistical analysis [24].
Sucrose preference test
The SPT was performed to investigate anhedonia [23, 33]. Briefly, the mice underwent a 7-day habituation period during which they had access to two tubes (50 mL) equipped with fitted stoppers and ball-point sipper to drink water 4 h before the test, mice were separately transferred to a testing room and deprived of food and water. During the testing period, each single-housed mouse was allowed a free choice between a tube of water and a tube of 1% sucrose solution for 2 h. The mice were returned to their home cages. Liquid tubes containing water and 1% sucrose solution were weighed. The preference for the sucrose solution was calculated as (weight of sucrose solution consumed/weight of total liquid consumed) × 100.
Forced swim test
The FST was performed under normal lighting conditions. During the test, each mouse underwent a 6-minute swimming session in a transparent Plexiglas cylinder measuring (10 cm in diameter × 25 cm in height). The cylinder was filled with tap water (23-25 °C, 15 cm in depth). The swimming sessions were videotaped. The immobility time during the last 4 min of the 6-minute swim session was later determined by a blind review of the video files. Immobility was defined as passive floating without any additional movement except was necessary to keep the head above the water [34].
Novelty-suppressed food test
The NSFT was performed in a plastic box (50 × 50 × 20 cm3) covered with bedding under dimly light conditions. Before the test, the mice were restricted from food for 24 h. During the test, a single pellet of food was placed on a round filter paper (11 cm in diameter) positioned at the center of the box. A single mouse was then placed in one of the corners. The latency to feeding was measured for 10 min. Non-feeding behaviors such as touching and smelling were ignored. Subsequently, mice were returned to their home cages. Food consumption in the home cage was measured at 5, 10, and 30 min intervals [28, 33].
Open field test
The entire test was performed in a nontransparent square box (60 × 60 × 40 cm3) area with even illumination. Mice were placed in the center of the open field area, and their activity was recorded for 5 min. The arena was thoroughly cleaned with 20% ethanol between tests. The open field arena was divided into nine equal squares using a 3 × 3 grid. The assessed parameters were time spent in the center zone and total distance traveled was quantified and analysised using Any-maze software (Stoelting, Wood Dale, IL, USA) [32].
Elevated plus maze
This is a cross-shaped device consisting of four arms (30 cm long and 5 cm wide). During the test, the mice were placed in the middle area with their head facing the junction of the open arm and the closed arm, to allow the mice to explore the open arm or the closed arm freely; mice was recorded for five minutes with a camera mounted above the maze. The time spent in the open arm, the number of entries into the open arm, and the numbers of entries into each arm were recorded manually [32, 35].
Locomotor activity
The locomotor activity was tested using SuperFlex Fusion open-field cages (40 × 40 × 40 cm, Omnitech Electronics Inc., Columbus, OH). At the beginning of the test, a single mouse was placed in the center of the cage and allowed to explore freely for 30 min. The total distance traveled was monitored by an infrared motion sensor positioned on top of the cage and quantified using the Fusion Software (Omnitech Electronics Inc., Columbus, OH, USA) [25].
Quantification and statistical analysis
All data are presented as the mean (SEM) and were analyzed using GraphPad Prism version 8. The assumptions of normality and equal variance were tested using the Shapiro-Wilk test and F test, respectively. Differences between two experimental groups were assessed using unpaired two-tailed t test (for equal variance) and Mann–Whitney tests (for unequal variance), while differences between three experimental groups were assessed by one-way analysis of variance (ANOVAs) with or without repeated measures followed by Sidak post hoc tests. For the analysis of multiple groups, a two-way ANOVA with or without repeated measures was conducted, followed by Holm-Sidak’s test. Cumulative probabilities of amplitude and interevent interval distributions were analyzed with the two-tailed Kolmogorov–Smirnov test. Statistical significance for all analyses was set at p < 0.05.
Results
CUS impaired the neuronal activity and neuronal transmission of DA neurons in DRN
Our previous studies reported that CUS induces depression-related behaviors, characterized by attenuated anhedonia and aggravated despair behaviors in both male and female mice [32, 33]. These behaviors were accompanied by a lower preference for sucrose in the SPT and higher immobility in the FST (Fig. 1A). Initially, to explore the potential relationship between depression and dopamine neurons’ activity in DRN, we adopted CUS to both male and female DAT-CretdTomato mice (Fig. 1B), which generated by crossing DAT-Cre mice with Ai14tdTomato to label DA neurons [22, 36]. Immunofluorescent colocalization analysis revealed that the majority of tdTomato-expressing neurons across the DRN were tyrosine hydroxylase -immunopositive (TH + ) (≈78.51%) (Fig. 1B), which was in line with the published report [37]. We then conducted electrophysiological recordings to measure the neuronal excitability of dopamine neurons, displaying spontaneous firing properties under basal conditions (Fig. 1C) [38]. We found that the spontaneous firing frequency was reduced, and the membrane potential was hyperpolarized in both male and female mice after CUS (Fig. 1D); however, other membrane properties, such as amplitude, remained unchanged in both sexes, while the half-width was increased in CUS-treated female mice, but remains no changed in CUS-treated male mice compared to control mice (Fig. S1A).
Fig. 1. CUS decreased the neuronal activity and impaired the synaptic inputs of dopamine neurons in the DRN of both male (blue color) and female (red color) mice.
A The depression-related behaviors induced by CUS. Sucrose preference test (SPT) (Male: unpaired two-tailed t test, t(14) = 2.6000, p = 0.0210; female: unpaired two-tailed t test, t(14) = 3.1990, p = 0.0064); Forced swim test (FST) (Male: unpaired two-tailed t test, t(14) = 2.3030, p = 0.0371; female: unpaired two-tailed t test, t(14) = 2.8850, p = 0.0120). n = 8 per group. B Schematic illustrations demonstrating the DAT-Cre and tdTomato reporter system and representative images and percentage of mCherry co-expressing with TH. n = 12 sections from five mice. Scale bars = 100 μm. C Timeline of the CUS procedure, electrophysiological recordings of dopamine neurons in the DRN of DAT-CretdTomato mice, and representative recording images showing the patched dopamine neurons under fluorescence and brightfield conditions. D Representative traces of action potentials of dopamine neurons, and quantitative analysis of AP frequency (male: Unpaired t test with Welch’s correction, p < 0.0010; female: unpaired two-tailed t test, t(34) = 8.2760, p < 0.0010) and membrane potential (male: Mann–Whitney test, p < 0.0010; female: unpaired two-tailed t test, t(34) = 11.3800, p < 0.0010). Male: n = 18 cells from 4 mice in CUS group, n = 19 cells from 4 mice in control group; female: n = 18 cells from 4 mice per group. E Representative traces of mEPSC in dopamine neurons and cumulative probability plots for the amplitude (male: left, Kolmogorov–Smirnov test, D = 0.4636, p < 0.0010; female: left, Kolmogorov–Smirnov test, D = 0.6071, p < 0.0010) and interevent interval (male: right, Kolmogorov–Smirnov test, D = 0.0287, p = 0.1692; female: right, Kolmogorov–Smirnov test, D = 0.0498, p = 0.0518) of mEPSC. The insets show the average amplitude (male: unpaired two-tailed t test, t(25) = 2.9470, p = 0.0069; female: unpaired two-tailed t test, t(25) = 3.4110, p = 0.0022) and frequency (male: unpaired two-tailed t test, t(25) = 1.4440, p = 0.1612; female: unpaired two-tailed t test, t(25) = 0.2339, p = 0.8170) of mEPSC. Male: n = 12 cells from 4 mice in control group, n = 15 cells from 4 mice in CUS group. Female: n = 13 cells from 4 mice in control group, n = 14 cells from 3 mice in CUS group. F Representative traces of mIPSC in dopamine neurons and cumulative probability plots for the amplitude (male: Kolmogorov–Smirnov test, D = 0.0417, p = 0.0849; female: Kolmogorov–Smirnov test, D = 0.1759, p < 0.0010) and interevent interval (male: Kolmogorov–Smirnov test, D = 0.0388, p = 0.0570; female: Kolmogorov–Smirnov test, D = 0.0382, p = 0.0510). The insets show the average amplitude (male: unpaired two-tailed t test, t(29) = 0.6186, p = 0.5410; female: unpaired two-tailed t test, t(28) = 4.0120, p < 0.0010) and frequency (male: unpaired two-tailed t test, t(29) = 1.2410, p = 0.2245; female: unpaired two-tailed t test, t(28) = 0.5536, p = 0.5843). Male: n = 15 cells from 4 mice in control group, n = 16 cells from 4 mice in CUS group. Female: n = 15 cells from 4 mice in control group, n = 15 cells from 4 mice in CUS group. *p < 0.050, **p < 0.010, ***p < 0.0010, compared to the control group.
Next, mEPSC, sEPSC, mIPSC and sIPSC were recorded to determine whether CUS could modify excitatory or inhibitory synaptic transmission/inputs. Our results showed that CUS exposure significantly decreased the amplitude of mEPSC in dopamine neurons in the DRN, as shown by the reduced average amplitude and left-shifted cumulative frequency distribution of the amplitude in both male and female mice, however, no significant change was observed in the mean values of mEPSC frequency between the groups (Fig. 1E). Moreover, the same results for sEPSC were also revealed with the decreased average amplitude and left-shifted cumulative frequency distribution of the amplitude after CUS (Fig. S1B). In addition, the amplitude of mIPSC and sIPSC in dopamine neurons from male mice remained unaltered, the cumulative amplitude distribution was increased and right-shifted in female mice after CUS exposure (Figs. 1F and S1C). The mean values of the sIPSC and mIPSC frequency of male and female mice between the groups showed no significant alteration (Figs. 1F and S1C). These results indicate that CUS impairs excitatory or inhibitory synaptic inputs in a sex-dependent manner.
Inhibition of DRN dopamine neurons produced depression-related behaviors
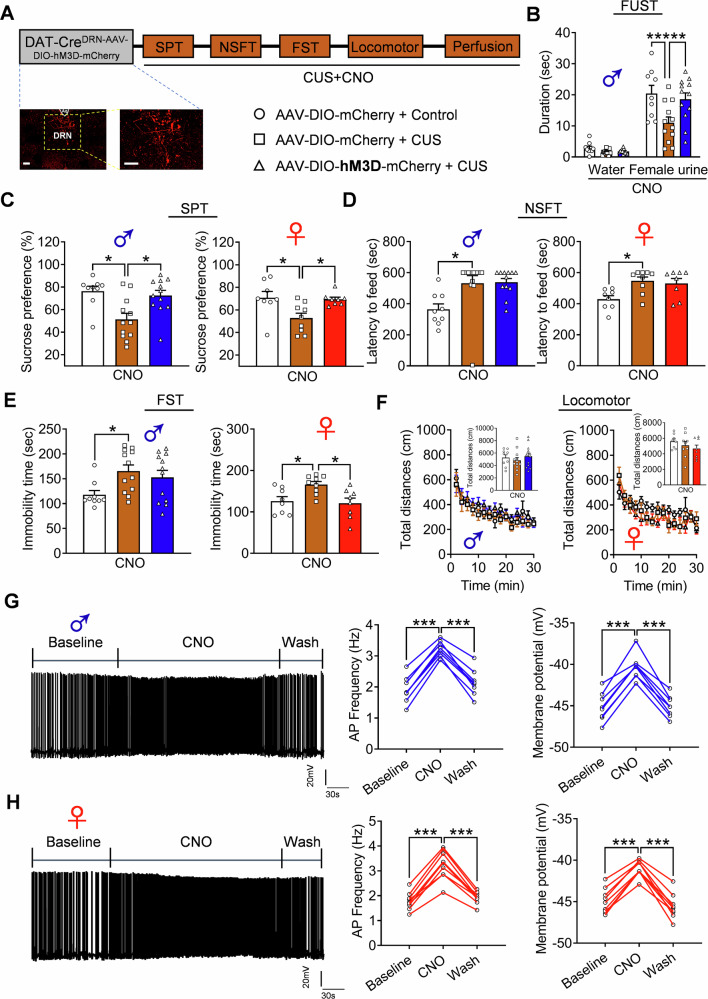
The causal relationship between the decreased activity of dopaminergic neurons in the DRN and CUS-induced depressive behaviors was explored. Adeno-associated virus (AAV) vectors expressing Cre-dependent hM4D (AAV-DIO-hM4D-mCherry) or mCherry (AAV-DIO-mCherry) were injected bilaterally into the DRN of DAT-Cre mice of both sexes (Fig. 2A). To exclude the side effects of surgery, sucrose preference test (SPT) was performed after saline injection, and both male and female AAV-DIO-hM4D-mCherry treated mice showed no difference in preference for 1% sucrose compared to AAV-DIO-mCherry mice (Fig. 2B). Clozapine-N-oxide (CNO) was injected intraperitoneally and the behaviors were evaluated 45 min after injection. We observed the reduced sucrose preference in AAV-DIO-hM4D-mCherry mice in both sexes compared with AAV-DIO-mCherry mice (Fig. 2C), indicating the anhedonia deficient phonotypes. Female urine sniffing test (FUST) was performed to examine whether inhibition of DRN DA neurons could attenuate sex-related reward-seeking behavior. Male AAV-DIO-hM4D-mCherry mice spent less time sniffing female urine compared to control AAV-DIO-mCherry mice (Fig. 2D). Both male and female AAV-DIO-hM4D-mCherry mice showed no difference in the latency to food in the novelty-suppressed food test (NSFT), an approach-avoidance paradigm, but displayed significant despair behavior characterized by increased immobility time during the forced swim test (FST) (Fig. 2E, F). To exclude the possible effect of motor activity on behaviors, locomotor activity was measured in the SuperFlex Fusion open-field cages, and the results showed no significant differences of travelled distance between the groups (Fig. 2G). Additionally, anxiety-related behaviors were investigated in these mice, and the results demonstrated no significant differences in the open field test (Fig. S2A) and elevated plus maze test (Fig. S2B) between mice of both sexes. Finally, the inhibitory effects of AAV-DIO-hM4D-mCherry on dopaminergic neurons in the DRN of both male and female mice were verified, and the results showed that the action potential (AP) frequency of the recorded dopaminergic neurons in AAV-DIO-hM4D-mCherry mice of both sexes were significantly decreased after CNO infusion, which was recovered after washout (Fig. 2H, I). These findings indicate that inhibition of DRN dopamine neurons produced depression-related behaviors in both male and female mice.
Fig. 2. Inhibition of DRN dopamine neurons induces depression-related behaviors.
A Schematic diagram of depression-and anxiety-related behavioral tests and a representative image showing the expression of AAV-DIO-hM4D-mCherry in the DRN dopamine neurons of male (blue color) and female (red color) DAT-Cre mice. Scale bar is 100 μm. B SPT after saline injection (Two-way ANOVA: treatment: F(1, 37) = 0.5700, p = 0.4551, gender: F(1, 37) = 2.8470, p = 0.1000, interaction: F(1, 37) = 0.0007, p = 0.9979; male: p = 0.8525; female: p = 0.8184). C SPT after CNO injection (Two-way ANOVA, treatment: F(1, 37) = 19.2000, p < 0.0010, gender: F(1, 37) = 4.3890, p = 0.0431, interaction: F(1, 37) = 0.2801, p = 0.5998; male: 0.0113; female: p = 0.0244). D FUST after CNO injection (Two-way repeated ANOVA: treatment: F(1, 16) = 31.1700, p < 0.0010, sniffing object: F(1, 16) = 151.2000, p < 0.0010, interaction: F(1, 16) = 26.9900, p < 0.0010; water: p = 0.9610; female urine: p < 0.0010). E NSFT after CNO injection (Two-way ANOVA, treatment: F(1, 37) = 1.7090, p = 0.1991, gender: F(1, 37) = 6.7970, p = 0.0131, interaction: F(1, 37) = 0.0004, p = 0.9835; male: 0.6896; female: p = 0.6896). F FST after CNO injection (Two-way ANOVA, treatment: F(1, 37) = 19.8700, p < 0.0010, gender: F(1, 37) = 2.7550, p = 0.1054, interaction: F(1, 37) = 0.0178, p = 0.8946; male: 0.0160; female: p = 0.0119). G Locomotor activity after CNO injection (male: Two-way repeated ANOVA: treatment: F(1, 16) = 2.6890, p = 0.1205, timepoints: F(14, 224) = 45.4400, p < 0.0010, interaction: F(14, 224) = 0.6839, p < 0.7891; total distance: unpaired two-tailed t test, t(16) = 1.6400, p = 0.1205; female: Two-way repeated ANOVA: treatment: F(1, 21) = 0.8493, p = 0.3672, timepoints: F(14, 294) = 21.9000, p < 0.0010, interaction: F(14, 294) = 0.7094, p < 0.7648; total distance: unpaired two-tailed t test, t(21) = 0.9216, p = 0.3672). Male: n = 9 per group; female: AAV-DIO-mCherry group, n = 11, AAV-DIO-hM4D-mCherry group, n = 12. Representative traces of spontaneous action potentials of dopamine neurons, and quantitative analysis of AP frequency (male: p < 0.001 and p < 0.001; one-way repeated ANOVA, F(2, 14) = 135.9000, p < 0.0010; female: p < 0.001 and p < 0.001; one-way repeated ANOVA, F(2, 16) = 111.5000, p < 0.0010) and membrane potential (male: p < 0.001 and p < 0.001; one-way repeated ANOVA, F(2, 14) = 95.1400, p < 0.0010; female: p < 0.001 and p < 0.001; one-way repeated ANOVA, F(2, 16) = 79.4700, p < 0.0010) from (H) male and (I) female mice. Male: n = 8 cells from 3 mice per group. Female: n = 9 cells from 3 mice per group.
The antidepressant effects of activating DRN dopamine neurons
Further experiments were conducted to demonstrate whether the activation of DRN dopaminergic neurons could reverse the behaviors induced by CUS. AAV vectors expressing Cre-dependent hM3D (AAV-DIO-hM3D-mCherry) or mCherry (AAV-DIO-mCherry) were injected bilaterally into the DRN of DAT-Cre mice of both sexes (Figs. 3A, S3A, and S4A). We first assessed whether the activation of DRN dopamine neurons in male and female mice could exert antidepressant effects under basal conditions (Figs. S3A and S4A). The SPT was performed after saline injection, and both male and female AAV-DIO-hM3D-mCherry mice showed no difference in preference for 1% sucrose compared to their control AAV-DIO-mCherry mice (Figs. S3B and S4B). Similarly, the activation of DRN dopamine neurons in AAV-DIO-hM3D-mCherry mice of both sexes also showed no altered preference for 1% sucrose under basal conditions (Figs. S3C and S4C). Additionally, the behavioral tests, such as the FUST, NSFT, FST, and locomotor activity were conducted, and no significant changes were observed between AAV-DIO-hM3D-mCherry and AAV-DIO-mCherry mice after CNO injection in either sex, except for the decreased immobility of FST in females (Figs. S3D–G and S4D–F). Moreover, no significant changes in anxiety-related behaviors were observed in elevated plus maze test and open field test (Figs. S3H–I and S4G, H).
Fig. 3. The antidepressant effect of activating DRN dopamine neurons.
A Schematic diagram of depression-related behavioral tests and a representative image showing the expression of AAV-DIO-hM3D-mCherry in the DRN dopamine neurons of DAT-Cre mice. Scale bar is 100 μm. B FUST after CNO injection (Two-way repeated ANOVA: treatment: F(2, 30) = 6.2050, p = 0.0056, sniffing object: F(1, 30) = 139.7000, p < 0.0010, interaction: F(2, 30) = 4.5870, p = 0.0183; p < 0.0010 and p = 0.0032). C SPT after CNO injection (Male: Kruskal-Wallis test, p = 0.0094; p = 0.0206 and p = 0.0360. Female: One-way ANOVA, F(2, 22) = 5.6440, p = 0.0104; p = 0.0177 and p = 0.0291). D NSFT after CNO injection (Male: Kruskal–Wallis test, p = 0.0012; p = 0.0014. Female: Kruskal–Wallis test, p = 0.0153; p = 0.0222). E FST after CNO injection (Male: Kruskal–Wallis test, p = 0.0422; p = 0.0357. Female: F(2, 22) = 5.9510, p = 0.0086; p = 0.0294 and p = 0.0132). F Locomotor activity after CNO injection (Male: Two-way repeated ANOVA: treatment: F(2, 30) = 0.5154, p = 0.6024, timepoints: F(14, 420) = 19.4700, p < 0.0010, interaction: F(28, 420) = 0.7247, p = 0.8485; total distance: F(2, 30) = 0.5154, p = 0.6024. Female: Two-way repeated ANOVA: treatment: F(2, 22) = 1.0420, p = 0.3694, timepoints: F(14, 308) = 12.8900, p < 0.0010, interaction: F(28, 308) = 1.2020, p = 0.2264; total distance: F(2, 22) = 0.0420, p = 0.3694). Male: AAV-DIO-mCherry + Control group: n = 9; AAV-DIO-mCherry + CUS: n = 12; AAV-DIO-hM3D-mCherry + CUS: n = 12. Female: AAV-DIO-mCherry + Control group: n = 8; AAV-DIO-mCherry + CUS: n = 9; AAV-DIO-hM3D-mCherry + CUS: n = 8. Representative traces of spontaneous action potentials of dopamine neurons, and quantitative analysis of AP frequency (male: p < 0.001 and p < 0.001; one-way repeated ANOVA, F(2, 16) = 190.2000, p < 0.0010; female: p < 0.001 and p < 0.001; one-way repeated ANOVA, F(2, 16) = 286.2000, p < 0.0010) and membrane potential (male: p < 0.001 and p < 0.001; one-way repeated ANOVA, F(2, 16) = 137.7000, p < 0.0010; female: p < 0.001 and p < 0.001; one-way repeated ANOVA, F(2, 16) = 127.4000, p < 0.0010) from (G) male and (H) female mice. Male: n = 9 cells from 3 mice per group. Female: n = 9 cells from 3 mice per group.*p < 0.050, ***p < 0.0010, compared to control group.
Subsequently, the antidepressant effects of the activation of DRN dopaminergic neurons in male and female mice were evaluated under stress conditions. Male and female mice were divided into two groups: AAV-DIO-hM3D-mCherry and AAV-DIO-mCherry. The mice were then subjected to CUS, and a series of behaviors were assessed 45 min after CNO injection (Fig. 3A). The FUST results showed that the sniffing duration of female urine decreased in AAV-DIO-mCherry treated male mice after CUS. However, this decrease was restored in male mice treated AAV-DIO-hM3D-mCherry (Fig. 3B). The SPT results showed that CUS-exposed AAV-DIO-mCherry mice exhibited a significant reduction in sucrose preference compared to the controls. Nevertheless, this reduction was significantly attenuated by activating DRN DA neurons in both male and female mice (Fig. 3C). The NSFT results showed that the CUS caused an extension in the latency to feed in male and female AAV-DIO-mCherry mice. However, this effect was not altered in either sex by the activation of DRN DA neurons (Fig. 3D). The FST results showed that CUS caused an increase in the immobility time in male and female AAV-DIO-mCherry mice, and only a significant attenuation was observed in female AAV-DIO-hM3D-mCherry mice after CNO injection (Fig. 3E). Subsequently, locomotor activity was measured, and the results showed no significant difference between the groups in either sex (Fig. 3F). These results indicate that the activation of DRN dopaminergic neurons under stress conditions produces an antidepressant effect in both male and female mice. Finally, electrophysiological recordings in slices verified the stimulatory effect of AAV-DIO-hM3D-mCherry on dopaminergic neurons in DRN of both male and female mice (Fig. 3G, H).
Identification of dopaminergic neuronal projections from the DRN to the dBNST
Subsequently, the dopaminergic neuronal projections from the DRN to the dBNST were examined. The AAV-DIO-GFP virus was infused into the DRN of DAT-Cre mice. After 3 weeks, we observed GFP-labeled cell bodies in the DRN (Fig. S5A). There were more GFP-labeled fibers predominantly distributed in the dBNST than ventral BNST (vBNST), accompanied with extensive co-expression with TH+ fibers (Fig. S5A). At the same time, GFP-labeled fibers was also observed in CeA and VTA as previously reported [19, 36], but with relative lower fluorescence intensity than that in dBNST, but higher intensity than that in vBNST. To further confirm the DRN-dBNST projections, we infused the retrograde AAV-Retro-GFP virus into the dBNST of WT mice, and after 3 weeks, we observed GFP-expressing neurons in the DRN, approximately 51.89% of which were co-labeled with TH immunofluorescence (Fig. S5B).
Inhibiting the DRN-dBNST neural projection induces depression-related behaviors
We then explored whether the inhibition of dBNST projected DRN neurons could lead to depression-related behaviors. A retrograde monosynaptic AAV-Retro-Cre-GFP virus was injected into the dBNST, and Cre-dependent hM4D (AAV-DIO -hM4D-mCherry) or Cre-dependent mCherry control virus (AAV-DIO-mCherry) was infused into the DRN of male WT mice. Multiple behaviors were evaluated 45 min after intraperitoneal injection of CNO (Fig. S5C). The SPT results showed that AAV-DIO-hM4D-mCherry mice exhibited a reduced preference for 1% sucrose compared with AAV-DIO-mCherry mice (Fig. S5D). The FUST results showed that male AAV-DIO-hM4D-mCherry mice spent less time sniffing female urine than control AAV-DIO-mCherry mice (Fig. S5E). Furthermore, the FST results showed that AAV-DIO-hM4D-mCherry mice displayed increased immobility compared to AAV-DIO-mCherry mice (Fig. S5F). In addition, there were no significant differences in locomotor activity between the groups (Fig. S5G). These results indicate that inhibition of DRN-dBNST neural projections leads to depression-related behaviors.
The disparate function and molecular mechanisms of DRN DA neuron in the synaptic activity of the dBNST-targeted neurons
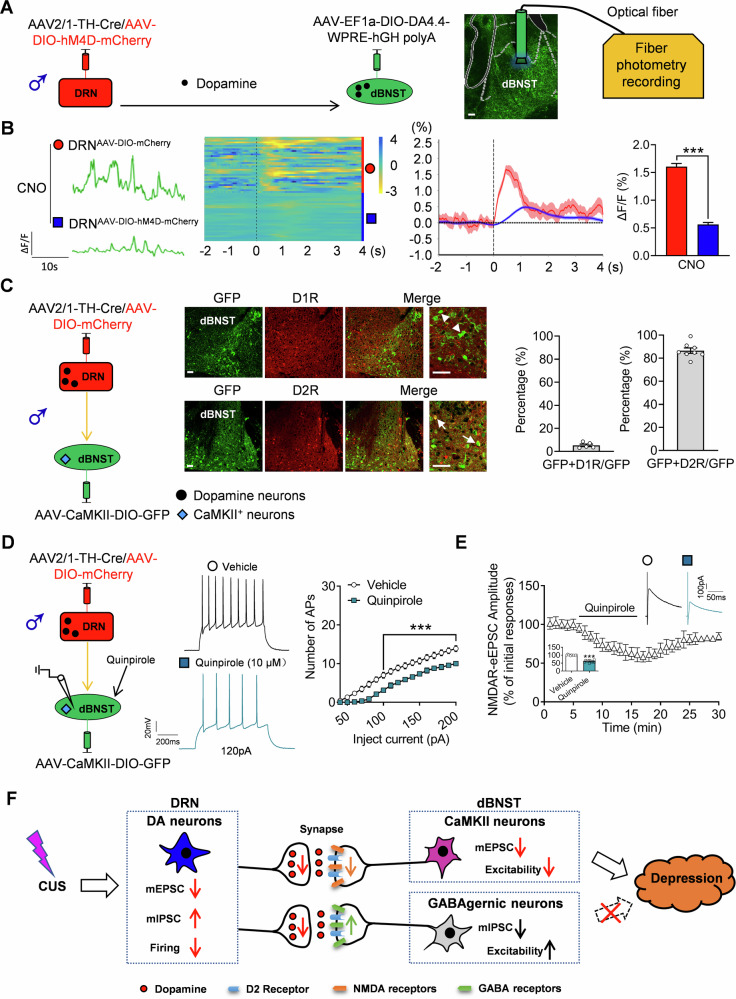
Next, the existing synaptic connection between the neuronal terminals of DRN and dBNST neurons was demonstrated using an AAV-mediating tracing strategy [25]. A mixture of anterograde and monosynaptic AAV2/1-TH-Cre with AAV-DIO-mCherry was infused into the DRN [39–41], while Cre-dependent AAV-DIO-GFP was injected into the dBNST. After 3 weeks, we observed that ~94% of red fluorescence-labeled neurons in the DRN were tyrosine hydroxylase (TH) positive (Figs. 4A and S6A), but rarely was 5-HT neurons (Fig. S6B), indicative of high specificity of viral targeting for dopaminergic neurons. Meanwhile, numerous GFP-labeled neurons in the dBNST were also observed, because AAV2/1-TH-Cre virus exhibit anterograde transsynaptic spread properties [42], which caused the Cre enzyme to be expressed in the soma of neurons in dBNST under the active TH promoter, because the TH mRNA were found in the dBNST targeted neurons, resulting in the co-localization of GFP with the TH mRNA (Fig. S6C), but nearly no TH protein were found in the cell body of GFP-labeled targeted neurons (Fig. S6D). Further analysis indicated that approximately 67.2% of the GFP-labeled neurons were GABAergic neurons, and ~23.8% of the GFP-labeled neurons were CaMKII+ neurons (Fig. 4A).
Fig. 4. Identification of dopaminergic neuronal projections and activities from DRN to BNST.
A Representative images showing AAV2/1-TH-Cre/AAV-DIO-mCherry co-infusion in the DRN and AAV-DIO-GFP infusion in the dBNST; and co-localization and the percentile analysis of TH labeled DA neurons, GFP expression GABAergic neurons marker (GABA+ ) and excitatory neurons marker (CaMKII+) in the dBNST. n = 9–12 slice from 4 mice. n = 2486 cells from 15 slice of 4 mice. Scale bar is 100 μm at low magnification and 50 μm at high magnification. B Schematic diagram of co-infusing of virus and electrophysiological recordings. Representative images and percentage of GFP co-expressing with GABA (arrow markers). Scale bars = 100 μm. C Representative traces of action potentials of spontaneously active (SA) GABAergic neurons in the dBNST, and quantitative analysis of AP frequency (unpaired two-tailed t test, t(45) = 4.0640, p < 0.0010) and membrane potential (unpaired two-tailed t test, t(45) = 5.6580, p < 0.0010) after inhibiting the activity of dopamine neurons in DRN. n = 23 cells from 7 mice in DRNAAV-DIO-mCherry group, n = 24 cells from 7 mice in DRNAAV-DIO-hM4D-mCherry group. D Representative traces of mIPSC in GABAergic neurons and cumulative probability plots (Kolmogorov–Smirnov test, D = 0.3157, p < 0.0010) for the amplitude of mIPSC. The insets show the amplitude (unpaired two-tailed t test, t(21) = 2.6410, p = 0.0153) of mIPSC. n = 18 cells from 5 mice in DRNAAV-DIO-mCherry group, n = 19 cells from 5 mice in DRNAAV-DIO-hM4D-mCherry group. E Schematic diagram of virus co-infusion, and the electrophysiological recordings of CaMKII+ neurons in dBNST. Representative images and percentage of GFP co-expressing with CaMKII (arrow markers). Scale bars = 100 μm. F Representative traces of action potentials of CaMKII+ neurons in the dBNST, and quantitative analysis of AP after injected currents (Two-way repeated ANOVA: treatment: F(1, 39) = 34.6100, p < 0.0010, currents: F(19, 741) = 577.7000, p < 0.0010, interaction: F(19,741) = 16.5300, p < 0.0010) after inhibiting the activity of dopamine neurons in DRN. n = 22 cells from 6 mice in DRNAAV-DIO-mCherry group, n = 19 cells from 6 mice in DRNAAV-DIO-hM4D-mCherry group. G Representative traces of mEPSC in CaMKII+ neurons and cumulative probability plots (Kolmogorov–Smirnov test, D = 0.3106, p < 0.0010) for the amplitude of mEPSC. The insets show the amplitude (unpaired two-tailed t test, t(42) = 2.8730, p = 0.0063) of mEPSC. n = 22 cells from 6 mice per group. H Summary graph of the time course and the magnitude of the potentiation of AMPAR-eEPSC after CNO infusion (Two-way repeated ANOVA: treatment: F(1, 19) = 0.1977, p = 0.6616, timepoints: F(29, 551) = 2.0022, p = 0.0016, interaction: F(29, 551) = 0.8576, p = 0.6826). n = 11 cells from 3 mice in DRNAAV-DIO-mCherry, n = 10 cells from 3 mice in DRNAAV-DIO-hM4D-mCherry group. Right graph illustrates averaged AMPAR-eEPSC traces taken at the time point indicated in the left graph. I Summary graph of the time course and the magnitude of the potentiation of NMDAR-eEPSC after CNO infusion in DRNAAV-DIO-mCherry and DRNAAV-DIO-hM4D-mCherry groups (two-way repeated ANOVA: treatment: F(1, 20) = 95.4100, p < 0.0010, timepoints: F(29, 580) = 29.9400, p < 0.0010, interaction: F(29, 580) = 16.8400, p < 0.0010). n = 12 cells from 4 mice in AAV-DIO-mCherry group, n = 10 cells from 4 mice in AAV-DIO-hM4D-mCherry group. The right graph illustrates averaged NMDAR-eEPSC traces taken at the time point indicated in the left graph. J Representative AMPAR-eEPSC traces and analyzed amplitudes after CUS (Two-way repeated ANOVA: treatment: F(1, 8) = 1.0650, p = 0.3323, stimulation intensity: F(4, 32) = 154.5000, p < 0.0010, interaction: F(4, 32) = 0.5055, p = 0.7320). n = 5 cells from 3 mice per group. K Representative NMDAR-eEPSC traces and analyzed amplitudes after CUS (Two-way repeated ANOVA: treatment: F(1, 8) = 511.7000, p < 0.0010, stimulation intensity: F(4, 32) = 176.5000, p < 0.0010, interaction: F(4, 32) = 16.4800, p = 0.7320). n = 5 cells from 3 mice per group. **p < 0.010, ***p < 0.0010, compared to control group.
In addition, we investigated whether DRN DA neurons affect the activity of these two types of targeted neurons in the dBNST. To achieve this purpose, we microinjected AAV2/1-TH-Cre with the AAV-DIO-hM4D-mCherry virus into the DRN and Cre-dependent AAV-Vgat-DIO-GFP into the dBNST to label GABAergic targeting neurons (Fig. 4B). Immunofluorescent staining results showed that the most of GFP-expressing neurons in dBNST were GABA-immunopositive (94.55%). The patch clamp test results showed that two types GABAergic neurons was observed, the spontaneously active (SA) (75.8%) and low-threshold bursting (LTB) neurons (24.2%), and the ratio of these two types GABAergic neurons remains no significant changes between the DRNAAV-DIO-mCherry and DRNAAV-DIO-hM4D-mCherry groups (Fig. S7A). Furthermore, increased neural activity of GABAergic targeting neurons following the inhibition of the axonal inputs of DRN DA neurons was observed with an increased level of AP, decreased membrane potential for SA neurons (Fig. 4C), and a greater number of AP corresponding to the same amount of current injections (Fig. S7B). Additionally, we observed higher input resistance, lower rheobase current, higher fAHP amplitude and AP half width; however, the AP amplitude and threshold did not show any significant differences between these groups for LTB neurons (Fig. S7C). Moreover, the hyperactivity of GABAergic target neurons could be mediated via changes in synaptic inputs. Therefore, we examined synaptic transmission at excitatory and inhibitory synapses of these GABAergic neurons after the inhibition of the axonal inputs of DRN DA neurons. The results indicated that neither the amplitude nor the frequency of mEPSC was altered between the groups (Fig. S7D). Nevertheless, recordings of mIPSC revealed a decreased amplitude and a left-shifted cumulative distribution of the amplitude in AAV-DIO-hM4D-mCherry injected mice compared to controls (Fig. 4D), along with a negative effect on frequency (Fig. S7E).
Furthermore, we also microinjected AAV2/1-TH-Cre with the AAV-DIO-hM4D-mCherry virus into the DRN and Cre-dependent AAV-CaMKII-DIO-GFP into the dBNST to label CaMKII+ targeting neurons, immunofluorescent results showed that the most of GFP-expressing neurons in dBNST were CaMKII-immunopositive (97.24%) (Fig. 4E). Then, the excitability of CaMKII+ targeting neurons was also measured. Interestingly, we found a decreased neural activity after suppressing the axonal inputs of DRN DA neurons, with fewer AP (Fig. 4F), lower input resistance, higher rheobase current and AP amplitude half-width, and unchanged fAHP, AP amplitude, and threshold (Fig. S7F). Next, whole-cell voltage-clamp recordings showed that the amplitude, but not the frequency, of mEPSC was reduced in AAV-DIO-hM4D-mCherry injected mice compared to that in controls (Figs. 4G and S7G). The amplitude and frequency of mIPSC remained unchanged between groups (Fig. S7H). Furthermore, we examined the potential mechanism underlying the decreased mEPSC of CaMKII+ targeting neurons by monitoring the α-Amino-3-hydroxy-5-methylisoxazole-4-propionic acid subtype glutamate receptor (AMPAR) or N-methyl-D-aspartate receptor (NMDAR) mediating EPSC, and found that NMDAR-eEPSC was attenuated by suppressing the axonal inputs of DRN DA neurons, with unaltered AMPAR-eEPSC compared to the control treatment (Fig. 4H and Fig. 4I). In parallel, we evaluated the potential roles of AMPAR-eEPSC and NMDAR-eEPSC in CUS induced depression by monitoring the input/output curves for differences in stimulation efficiency [43, 44]. The results demonstrated similar changes of AMPAR-eEPSC, but reduced amplitudes of NMDAR-eEPSC in the CUS treated CaMKII+ targeting neurons (Fig. 4J, K).
Specific modulation of dBNST distinct targeting neurons blocked the depression-related behaviors induced by inhibiting DRN dopamine neurons
To determine whether BNST-targeting neurons mediate the role of DRN DA neurons in depression. We co-injected AAV2/1-TH-Cre and AAV-DIO-hM4D-mCherry viruses into the DRN and AAV-CMV-DIO-taCasp3-TEVp-GFP virus into the dBNST (Fig. 5A). This resulted in the specific expression of hM4D in the DRN DA neurons (92.37%) and the ablation of DRN projected targeting neurons in the dBNST, with the dramatically decreased number of GFP positive neurons [25, 45, 46] (Fig. 5B). Tests of behaviors showed that the decreased time spent sniffing female urine and reduced sucrose preference in AAV-DIO-hM4D-mCherry-injected mice were rescued by dBNST neuronal ablation in the FUST and SPT (Fig. 5C, D), and neuronal ablation in the dBNST prevented increased immobility in the FST (Fig. 5E), but the locomotor activity remains unchanged (Fig. 5F). We next examined whether vBNST-targeting neurons mediate the effects of DRN DA neurons in depression using the same strategy (Fig. S8A), the behavioral examination results revealed that ablation of DRN projected targeting neurons in the vBNST did not affect the decreased time spent sniffing female urine, reduced sucrose preference and increased immobility in AAV-DIO-hM4D-mCherry-injected mice in the FUST, SPT, and FST (Fig. S8B–D), and the locomotor activity is nearly the same among the groups (Fig. S8E).
Fig. 5. Ablation of dBNST neurons or blocking the excitability of CaMKII+ neurons receiving protection from DRN attenuated the depression-related behaviors induced by inhibiting DRN dopamine neurons.
A Schematic diagram of AAVs injections and representative images showing the expression of hM4D-mCherry in the DRN. Scale bars = 100 μm. B The quantitative analysis of GFP-labeled neurons in dBNST (Two-way ANOVA: hM4D treatment: F(1, 18) = 0.3335, p = 0.5708, taCasp3 treatment: F(1, 18) = 180.3000, p < 0.0010, interaction: F(1, 18) = 0.1106, p = 0.7433). n = 5 per group in AAV-mCherry group, n = 6 per group in AAV-hM4D-mCherry group. Scale bars = 100 μm. C FUST (Two-way ANOVA: hM4D treatment: F(1, 36) = 6.1340, p = 0.0181, taCasp3 treatmen: F(1, 36) = 2.0880, p = 0.1571, interaction: F(1, 36) = 9.5070, p = 0.0039). D SPT (Two-way ANOVA: hM4D treatment: F(1, 36) = 9.6340, p = 0.0037, taCasp3 treatment: F(1, 36) = 3.3460, p = 0.0757, interaction: F(1, 36) = 10.7200, p = 0.0023). E FST (Two-way ANOVA: hM4D treatment: F(1, 36) = 31.3500, p < 0.0010, taCasp3 treatment: F(1, 36) = 9.3810, p = 0.0041, interaction: F(1, 36) = 5.8150, p = 0.0211). F Locomotor activity after CNO injection (Two-way ANOVA: hM4D treatment: F(1, 36) = 2.0760, p = 0.1583, taCasp3 treatment: F(1, 36) = 0.2729, p = 0.6046, interaction: F(1, 36) = 0.6377, p = 0.4298). n = 10 per group. G Schematic diagram of AAVs injections and representative images showing the expression of and percentage of hM3D-mCherry co-expressed with TH in the DRN. H The quantitative analysis of c-fos labeled neurons in DRN and dBNST (Two-way ANOVA: hM4D treatment: F(1, 16) = 3.9070, p = 0.0656, hM3D treatment: F(1, 16) = 54.7500, p < 0.0010, interaction: F(1, 16) = 0.0044, p = 0.9480). n = 12-15 slice form 5 mice per group. Scale bar is 100 μm. I FUST (Two-way ANOVA: hM4D treatment: F(1, 28) = 10.9600, p = 0.0026, hM3D treatment: F(1, 28) = 7.8800, p = 0.0090, interaction: F(1, 28) = 7.4260, p = 0.0110). J SPT (Two-way ANOVA: hM4D treatment: F(1, 28) = 11.6300, p = 0.0020, hM3D treatment: F(1, 28) = 31.4500, p < 0.0010, interaction: F(1, 28) = 4.3350, p = 0.0466). K FST (Two-way ANOVA: hM4D treatment: F(1, 28) = 19.0000, p < 0.0010, hM3D treatment: F(1, 28) = 37.8700, p < 0.0010, interaction: F(1, 28) = 2.5280, p = 0.1231). L Locomotor activity after CNO injection (Two-way ANOVA: hM4D treatment: F(1, 28) = 0.0114, p = 0.9159, hM3D treatment: F(1, 28) = 0.0048, p = 0.9455, interaction: F(1, 28) = 2.1750, p = 0.1514). n = 8 per group. M Representative traces of action potentials and quantitative analysis of AP after injected currents (Two-way repeated ANOVA: treatment: F(2, 21) = 27.4000, p < 0.0010, currents: F(19, 399) = 1112.0000, p < 0.0010, interaction: F(38, 399) = 6.5110, p < 0.0010) and rheobase current (F(2, 14) = 34.2600, p < 0.0010) after CNO infusion. n = 8 cells from 3 mice per group. *p < 0.05, **p < 0.01, ***p < 0.001, compared to the control group.
Next, we aimed to identify the potential role of the excitability of individual dBNST target neurons in depression-related behaviors induced by DRN DA neurons. We co-injected AAV2/1-TH-Cre and AAV-DIO-hM4D-mCherry viruses into the DRN, and AAV-CaMKII-DIO-hM3D-mCherry virus into the dBNST (Fig. 5G), which counteracted the decreased excitability that emerged in dBNST CaMKII+ neurons after the inhibition of DRN DA neurons. We observed the specific expression of hM4D in the DRN DA neurons (94.62%), and hM3D mediating elevated neural activity with increased c-fos labeled neuron numbers (Fig. 5H). Moreover, we observed that counteracting the hypoexcitability of dBNST CaMKII+ neurons reversed the depression-related behaviors. These behaviors included reduced sniffing duration (Fig. 5I), sucrose preference (Fig. 5J) and increased immobility (Fig. 5K) caused by inhibiting DRN DA neurons. However, the locomotor activity of these mice remained unchanged (Fig. 5L). Electrophysiological recordings confirmed the stimulatory effect of CNO on CaMKII+ neurons expressing AAV-CaMKII-DIO-hM3D-mCherry (Fig. 5M).
Next, we applied the AAV-Vgat-DIO-hM4D-mCherry virus to the dBNST (Fig. S9A) to counteract the increased excitability that emerged in dBNST GABAergic neurons after the inhibition of DRN DA neurons, as verified by the decreased c-fos labeled neuronstaining (Fig. S9B). Finally, we found that counteracting the hyperexcitability of dBNST GABAergic neurons exerted none effect on depression-related behaviors, including reduced sniffing duration (Fig. S9C), sucrose preference (Fig. S9D), and increased immobility (Fig. S9E), no changed locomotor (Fig. S9F) induced by the inhibition of DRN DA neurons. In conclusion, the depression-related behaviors induced by inhibition of DRN DA neurons were mediated by the hypoexcitability CaMKII+ neurons, not by the hyperexcitability of GABAergic neurons in dBNST.
Inhibiting the D2 receptor can mimic the effect of DRN dopamine neurons on CaMKII+ targeting neurons in dBNST
In order to address the molecular mechanism mediating the changed NMDAR-eEPSC after suppressing the axonal inputs of DRN DA neurons, we first injected AAV2/1-TH-Cre with the AAV-DIO-hM4D-mCherry virus into the DRN and Cre-dependent dopamine probe virus (AAV-EF1a-DIO-DA4.4-WPRE-hGH polyA) into the dBNST, and measured the levels of dopamine neurotransmitter in dBNST using the fiber photometry recording strategy (Fig. 6A). The results revealed there was a decreased dopamine levels in AAV-DIO-hM4D-mCherry injected mice compared to that in controls (Fig. 6B). Next, we measured the distribution of dopamine receptors (D1R and D2R) in these targeting neurons in dBNST, it was interesting to find that there was abundant expression of D2 receptor in the GABAergic or CaMKII+ targeting neurons, while the expression of D1 receptor was scarce (Figs. 6C and S10). Moreover, the effect of D2 receptor in the neural excitability and NMDAR-eEPSC was tested by using the D2 receptor agonist Quinpirole, we observed obviously decreased excitability and NMDAR-eEPSC after Quinpirole treatment compared to the control (Figs. 6D, E and S11).
Fig. 6. The function of D2 receptor in the activity of CaMKII+ targeting neurons in dBNST receiving neural projections from DRN.
A Schematic diagram of AAVs injections and representative images showing the GFP signal of AAV-EF1a-DIO-DA4.4-WPRE-hGH polyA in dBNST. B Representative signal curve, heat maps and average responses showing dopamine transients. C Schematic diagram showing AAV2/1-TH-Cre/AAV-DIO-mCherry co-infusion in the DRN and AAV-CaMKII-DIO-GFP infusion in the dBNST; representative images of co-localization of GFP with D1 or D2 receptor and the percentage analysis. n = 10–12 slice from 5 mice. Arrowhead showing neurons that contain only GFP, arrows indicating neurons that contain both GFP and D2 receptor. Scale bars = 50 μm. D Schematic diagram showing AAV2/1-TH-Cre/AAV-DIO-mCherry co-infusion in the DRN and AAV-CaMKII-DIO-GFP infusion in the dBNST; representative traces of action potentials of dopamine neurons and quantitative analysis of AP frequency after Quinpirole incubation (Two-way ANOVA: treatment: F(1, 16) = 18.7100, p < 0.0010, sniffing object: F(19, 304) = 329.5000, p < 0.0010, interaction: F(19, 304) = 9.3230, p = 0.0183; p < 0.0010). n = 9 cells from 3 mice per group. E Summary graph of the time course and the magnitude of the potentiation of NMDAR-eEPSC after Quinpirole incubation (Two-way repeated ANOVA: treatment: F(1, 20) = 95.4100, p < 0.0010, timepoints: F(29, 580) = 29.9400, p < 0.0010, interaction: F(29, 580) = 16.8400, p < 0.0010). The inserted graph illustrating the averaged NMDAR-eEPSC amplitude (unpaired two-tailed t test, t(10) = 20.1300, p < 0.0010). n = 11 cells from 4 mice per group. F Schematic diagram of the involvement of DRNDA → dBNSTCaMKII+ neural projection in depression-related behaviors. CUS decreases the neural firing of DRN DA neurons, leading to decreased dopamine levels, mEPSC with hyperexcitability of CaMKII+ neurons and decreased mIPSC with hypoexcitability of GABAergic neurons in dBNST and the contributing the development of depression-related behaviors. ***p < 0.001, compared to the control group.
Totally, we discovered that the sEPSC/mEPSC and excitability of DRN DA neurons was decreased by CUS, and these DRN DA neurons reduced the neural activity and mEPSC reactivity of dBNST CaMKII+ neurons through the direct synaptic connections by the decreased dopamine neurotransmitter levels and D2 receptor in these targeting neurons, which resulting in production of depression-related behaviors (Fig. 6F).
Discussion
In this study, we discovered that low excitability of DRN DA neurons was associated with depression-related behaviors induced by CUS. Modulating the excitability of DRN DA neurons in both directions affected the depression-related behaviors. Additionally, dBNST CaMKII+ neurons, which receiving synaptic projections from DRN DA neurons mediate its role in controlling depression-related behaviors.
Several studies have emphasized the crucial role of the dopamine system in the behavioral abnormalities observed in psychiatric disorders [9, 12, 47]. Many researchers have reported enhanced firing rates of DA neurons in the VTA mouse models of depression or anxiety [10, 11, 22]. However, to date, no study has clarified the association between depression and a specific cluster of DA neurons in the DRN, which share basic electrophysiological properties with VTA DA neurons [38]. It was surprising and interesting to observe that DRN DA neurons are activated not only by naturally occurring and rewarding stimuli [16, 17, 36], but also robustly activated by a wide range of aversive stimuli [15, 16, 48], with a pronounced character in males. Our results showed that the spontaneous firing rate of DRN DA neurons decreased under depressive conditions induced by the CUS paradigm in both male and female mice. These studies together with our results indicate that the neural activities of DRN DA neurons are sensitive to the environmental stimuli; furthermore, this discrepancy may arise from different stress conditions (models/durations) and interactive effect of different DRN neurons [14, 49], functional heterogeneity of DRN DA neurons [15]. This suggests that the hypoexcitability of DRN DA neurons may serve as a novel neural basis for depression. Additionally, we explored the possible synaptic transmission mechanisms regulating the spontaneous firing of DRN DA neurons. We found that CUS reduced the amplitude of mEPSC and sEPSC in mice of both sexes, but only increased the amplitude of mIPSC and sIPSC in female mice. Considering that CUS mainly regulated the amplitudes, these results suggest that CUS-impaired synaptic transmission might be due to a postsynaptic mechanism [50–52], with dysfunctional activity or reduced number of postsynaptic receptors clustered at synaptic sites, without affecting the release of neural transmitter in the presynaptic portion. This is because the DRN DA neurons receive extensive excitatory or inhibitory inputs [14]. One interesting findings of our study is revealing a certain difference targeting the amplitude of mIPSC and sIPSC in male and female mice after CUS. Notably, there are also some distinct performances regarding decreased behavioral despair in female mice after chemogenetic activation of DA neurons in the DRN. These sexual difference may be owing to the distinct characters of DA neurons, because previous researchers have indicated the significant differences of DA neurons between males and females in the response to external stress [18, 53].
Serotonin neurons are the major neuron type in the DRN, widely acknowledged as being involved in depression [54, 55]. In this study, we showed that chemogenetic control of DRN DA neuron activity affects depressive phenotypes. This effect works in concert with the abnormal reactivity observed after CUS treatment and displays no significant sexual difference. These findings indicate that the DRN DA neuron is a remarkable and broad candidate target for depression treatment in both sexes. Although there were only a small number of DA neurons in the DRN, they were not homogeneous. The anatomical studies suggested two possible subpopulations within DA neurons in the DRN with different sizes and distributions (one group consists of a small and round cell body located close to the aqueduct, while the other comprises larger neurons located ventrally in the DRN) [15, 19]. In our study, we used DAT-Cre transgenic mice to label and modulate all DA neurons in the DRN, as previously reported [36]. However, it remains unclear whether these two subpopulations have diverse characteristics and functions related to depression.
It was found that DRN DA neurons project primarily to the CeA and the BNST [36], and our findings further demonstrated that the dBNST was the main projected region of DRN DA neurons. Our results revealed that modulating the activity of dBNST-projecting DRN DA neurons affects depression-related behaviors, validating the role of dBNST as a candidate brain region mediating the activity of DRN DA neurons in depression. The BNST, a subregion of the extended amygdala, serves as a critical node in the regulation of diverse behavioral states [56, 57]. Among the host of distinct types of neurons within the BNST, CaMKII+ and GABAergic neurons have been highlighted their key roles [58, 59]. Our current results also found that the target neurons in dBNST directly receive synaptic input from DRN DA neurons was predominantly occupied with CaMKII+ or GABAergic neurons. However, inhibiting the activity of DRN DA neurons exerted different regulatory effects in these two types of neurons with diverse mechanisms, accompanied by the increased mIPSC and decreased mEPSC independently, and the abnormal changes in amplitude indicates postsynaptic mechanism. Nevertheless, it was interesting to observe that counteracting the abnormal excitability of target CaMKII+ neurons attenuated the depressive phenotypes induced by manipulating DRN DA neurons. This implies that only CaMKII+ neurons mediate the effect of DRN DA neurons in depression. Furthermore, our electrophysiology recordings identified the involvement of NMDA receptors in the postsynaptic membrane function during this specific input for CaMKII+ neurons under CUS and chemogenetic inhibition conditions, which was also supported by other researches showing NMDA receptors in dBNST have been reported to exhibit extensive effects on depression or anxiety-related behaviors [60, 61].
Moreover, in order to explore the potential molecular mechanisms underlying the effect of DRN DA neurons in the hypoexcitability of CaMKII+ targeting neurons, we measured the co-expression status of CaMKII+ neurons with D1R or D2R, respectively, which was widely distributed in dBNST [62], it was unexpected to observe that most of these CaMKII+ targeting neurons was labeled with D2R, then, we also found a reduced excitability of these neurons, along with the decreased NMDAR-eEPSC by activating D2R by quinpirole, a D2-like specific agonist, which is also reported by another research group [62]. All this functional phenomenon of CaMKII+ neurons was absolutely in line with the changes after inhibiting the activity of DRN DA neurons or CUS treatment. It was reported that Quinpirole is associated with the presynaptic response and induces the reduction of dopamine release [63, 64], which is also consistent with our results showing the decreased dopamine levels in dBNST after suppressing DRN DA neurons. One specific question arises: why is the dysfunction of GABAergic neurons in the dBNST not involved in mediating the role of DRN DA neurons? Particularly, we also found the dysfunctions of these GABAergic neurons and its co-expression with D2R, which demonstrated the same expression profile with CaMKII+ targeting neurons, but with opposite phenome. The possible reasons were that the abnormal status of these GABAergic neurons was not the direct controlling effects of DRN DA neurons, even though there are direct synaptic linkages between them, it may be just the adjunctive responses, because there may be some indirect neuronal circuits from DRN DA neurons or internal neural interactions within the dBNST that regulate the excitability of GABAergic neurons in the dBNST. For example, the BNST receiving GABAergic inputs arising from the CeA [65, 66], which also bears neural projections from DRN DA neurons [36]. Furthermore, there are existing two isoforms of D2R, which exerts distinct functions for regulating the neural activity [67], therefore, the exact molecular mechanisms regarding the role of dBNST D2R in depression still needs further investigation.
Taken together, our study has revealed that decreased excitability of DRN DA neurons is associated with depression-related behavior. In addition, we present the first evidence demonstrating the involvement of the DRNDA → dBNSTCaMKII+ neural projection in regulating depression-related behavior. Future studies should explore the role of complex neural connections in the dBNST and investigate possible molecular mechanisms of DRN DA neurons in depression. Based on our current results, we suggest that targeting DRNDA → dBNSTCaMKII+ neural circuit could be an effective approach for depression treatment.
Supplementary information
Supplementary figures and figure legends
Acknowledgements
This work was supported by the National Natural Science Foundation of China (82171521, 82371539 to CL), Special Funds of the Taishan Scholars Project of Shandong Province (No. tsqn202211368 to CL), Shandong Provincial Natural Science Foundation (ZR2022YQ65 to CL, ZR2021MH073 to CL, and ZR2019PH109 to WTW), and Shandong Provincial Key Medical and Health Laboratory of Research in neuropsychiatric disorders (Binzhou Medical University Hospital).
Author contributions
CL and WL proposed the concept and designed the experiments. WTW, DW, DZ, LHX, SJJ, YZ, MHC, JL, FTM, CLL, and DJL performed the experiments. CL, WL, and WTW analyzed the results. CL and WL interpreted the data. CL wrote and edited the manuscript.
Data availability
All data needed to support the conclusions are present in the manuscript and /or supplementary materials.
Competing interests
The authors declare no competing interests.
Ethical approval
All methods and procedures of the animal studies were complied with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 8023, revised in 1996), and approved by the Institutional Animal Care and Use Committee of Binzhou Medical University Hospital (registration number: 20200618-01).
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Wentao Wang, Dan Wang.
Contributor Information
Wei Li, Email: yishengliwei@163.com.
Chen Li, Email: lc_0625@163.com.
Supplementary information
The online version contains supplementary material available at 10.1038/s41398-024-03093-6.
References
- 1.Kupfer DJ, Frank E, Phillips ML. Major depressive disorder: new clinical, neurobiological, and treatment perspectives. Lancet. 2012;379:1045–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hegerl U, Rummel-Kluge C, Varnik A, Arensman E, Koburger N. Alliances against depression - a community based approach to target depression and to prevent suicidal behaviour. Neurosci Biobehav Rev. 2013;37:2404–9. [DOI] [PubMed] [Google Scholar]
- 3.Kessler RC, Berglund P, Demler O, Jin R, Koretz D, Merikangas KR, et al. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R). JAMA. 2003;289:3095–105. [DOI] [PubMed] [Google Scholar]
- 4.Insel TR, Wang PS. The STAR*D trial: revealing the need for better treatments. Psychiatr Serv. 2009;60:1466–7. [DOI] [PubMed] [Google Scholar]
- 5.Kamath J, Leon Barriera R, Jain N, Keisari E, Wang B. Digital phenotyping in depression diagnostics: Integrating psychiatric and engineering perspectives. World J Psychiatry. 2022;12:393–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Vries YA, Roest AM, Bos EH, Burgerhof JGM, van Loo HM, de Jonge P. Predicting antidepressant response by monitoring early improvement of individual symptoms of depression: individual patient data meta-analysis. Br J Psychiatry. 2019;214:4–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Locher C, Koechlin H, Zion SR, Werner C, Pine DS, Kirsch I, et al. Efficacy and safety of selective serotonin reuptake inhibitors, serotonin-norepinephrine reuptake inhibitors, and placebo for common psychiatric disorders among children and adolescents: a systematic review and meta-analysis. JAMA Psychiatry. 2017;74:1011–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Practice Guideline For The Treatment Of Patients With Major Depressive Disorder (revision). American Psychiatric Association. Am J Psychiatry. 2000;157:1-45. [PubMed]
- 9.Tye KM, Mirzabekov JJ, Warden MR, Ferenczi EA, Tsai HC, Finkelstein J, et al. Dopamine neurons modulate neural encoding and expression of depression-related behaviour. Nature. 2013;493:537–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chaudhury D, Walsh JJ, Friedman AK, Juarez B, Ku SM, Koo JW, et al. Rapid regulation of depression-related behaviours by control of midbrain dopamine neurons. Nature. 2013;493:532–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Friedman AK, Walsh JJ, Juarez B, Ku SM, Chaudhury D, Wang J, et al. Enhancing depression mechanisms in midbrain dopamine neurons achieves homeostatic resilience. Science. 2014;344:313–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Russo SJ, Nestler EJ. The brain reward circuitry in mood disorders. Nat Rev Neurosci. 2013;14:609–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Flores JA, El Banoua F, Galan-Rodriguez B, Fernandez-Espejo E. Opiate anti-nociception is attenuated following lesion of large dopamine neurons of the periaqueductal grey: critical role for D1 (not D2) dopamine receptors. Pain. 2004;110:205–14. [DOI] [PubMed] [Google Scholar]
- 14.Luo M, Zhou J, Liu Z. Reward processing by the dorsal raphe nucleus: 5-HT and beyond. Learn Mem. 2015;22:452–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin R, Liang J, Luo M. The Raphe Dopamine System: roles in salience encoding, memory expression, and addiction. Trends Neurosci. 2021;44:366–77. [DOI] [PubMed] [Google Scholar]
- 16.Cho JR, Treweek JB, Robinson JE, Xiao C, Bremner LR, Greenbaum A, et al. Dorsal raphe dopamine neurons modulate arousal and promote wakefulness by salient stimuli. Neuron. 2017;94:1205–19.e1208. [DOI] [PubMed] [Google Scholar]
- 17.Matthews GA, Nieh EH, Vander Weele CM, Halbert SA, Pradhan RV, Yosafat AS, et al. Dorsal raphe dopamine neurons represent the experience of social isolation. Cell. 2016;164:617–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yu W, Pati D, Pina MM, Schmidt KT, Boyt KM, Hunker AC, et al. Periaqueductal gray/dorsal raphe dopamine neurons contribute to sex differences in pain-related behaviors. Neuron. 2021;109:1365–80.e1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hasue RH, Shammah-Lagnado SJ. Origin of the dopaminergic innervation of the central extended amygdala and accumbens shell: a combined retrograde tracing and immunohistochemical study in the rat. J Comp Neurol. 2002;454:15–33. [DOI] [PubMed] [Google Scholar]
- 20.Poulin JF, Zou J, Drouin-Ouellet J, Kim KY, Cicchetti F, Awatramani RB. Defining midbrain dopaminergic neuron diversity by single-cell gene expression profiling. Cell Rep. 2014;9:930–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Backman CM, Malik N, Zhang Y, Shan L, Grinberg A, Hoffer BJ, et al. Characterization of a mouse strain expressing Cre recombinase from the 3’ untranslated region of the dopamine transporter locus. Genesis. 2006;44:383–90. [DOI] [PubMed] [Google Scholar]
- 22.Sun F, Lei Y, You J, Li C, Sun L, Garza J, et al. Adiponectin modulates ventral tegmental area dopamine neuron activity and anxiety-related behavior through AdipoR1. Mol Psychiatry. 2019;24:126–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meng F, Liu J, Dai J, Wu M, Wang W, Liu C, et al. Brain-derived neurotrophic factor in 5-HT neurons regulates susceptibility to depression-related behaviors induced by subchronic unpredictable stress. J Psychiatr Res. 2020;126:55–66. [DOI] [PubMed] [Google Scholar]
- 24.Wang D, Wang W, Jiang S, Ma H, Lian H, Meng F, et al. Regulation of depression-related behaviors by GABAergic neurons in the lateral septum through periaqueductal gray neuronal projections. J Psychiatr Res. 2021;137:202–14. [DOI] [PubMed] [Google Scholar]
- 25.Zhao D, Wang D, Wang W, Dai J, Cui M, Wu M, et al. The altered sensitivity of acute stress induced anxiety-related behaviors by modulating insular cortex-paraventricular thalamus-bed nucleus of the stria terminalis neural circuit. Neurobiol Dis. 2022;174:105890. [DOI] [PubMed] [Google Scholar]
- 26.Zhao D, Liu C, Cui M, Liu J, Meng F, Lian H, et al. The paraventricular thalamus input to central amygdala controls depression-related behaviors. Exp Neurol. 2021;342:113744. [DOI] [PubMed] [Google Scholar]
- 27.Kotecha SA, Oak JN, Jackson MF, Perez Y, Orser BA, Van Tol HH, et al. A D2 class dopamine receptor transactivates a receptor tyrosine kinase to inhibit NMDA receptor transmission. Neuron. 2002;35:1111–22. [DOI] [PubMed] [Google Scholar]
- 28.Wang D, Zhao D, Wang W, Hu F, Cui M, Liu J, et al. How do lateral septum projections to the ventral CA1 influence sociability? Neural Regen Res. 2024;19:1789–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lei Y, Wang J, Wang D, Li C, Liu B, Fang X, et al. SIRT1 in forebrain excitatory neurons produces sexually dimorphic effects on depression-related behaviors and modulates neuronal excitability and synaptic transmission in the medial prefrontal cortex. Mol Psychiatry. 2020;25:1094–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marty VN, Mulpuri Y, Munier JJ, Spigelman I. Chronic alcohol disrupts hypothalamic responses to stress by modifying CRF and NMDA receptor function. Neuropharmacology. 2020;167:107991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sun J, Yuan Y, Wu X, Liu A, Wang J, Yang S, et al. Excitatory SST neurons in the medial paralemniscal nucleus control repetitive self-grooming and encode reward. Neuron. 2022;110:3356–73.e3358. [DOI] [PubMed] [Google Scholar]
- 32.Liu J, Meng F, Wang W, Wu M, Zhang Y, Cui M et al. Medial prefrontal cortical PPM1F alters depression-related behaviors by modifying p300 activity via the AMPK signaling pathway. CNS Neurosci Ther. 2023;29:3624–43. [DOI] [PMC free article] [PubMed]
- 33.Liu J, Meng F, Wang W, Cui M, Wu M, Jiang S, et al. PPM1F in hippocampal dentate gyrus regulates the depression-related behaviors by modulating neuronal excitability. Exp Neurol. 2021;340:113657. [DOI] [PubMed] [Google Scholar]
- 34.Ma X, Li Q, Chen G, Xie J, Wu M, Meng F, et al. Role of hippocampal miR-132-3p in modifying the function of protein phosphatase Mg2+/Mn2+-dependent 1 F in depression. Neurochem Res. 2023;48:2514–30. [DOI] [PubMed]
- 35.Li Q, Meng F, Ma X, Sun Z, Dai J, Liu J, et al. The colonic interleukin-19 aggravates the dextran sodium sulfate/stress-induced comorbidities due to colitis and anxiety. Front Immunol. 2023;14:1153344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lin R, Liang J, Wang R, Yan T, Zhou Y, Liu Y, et al. The raphe dopamine system controls the expression of incentive memory. Neuron. 2020;106:498–514.e498. [DOI] [PubMed] [Google Scholar]
- 37.Cardozo Pinto DF, Yang H, Pollak Dorocic I, de Jong JW, Han VJ, Peck JR, et al. Characterization of transgenic mouse models targeting neuromodulatory systems reveals organizational principles of the dorsal raphe. Nat Commun. 2019;10:4633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dougalis AG, Matthews GAC, Bishop MW, Brischoux F, Kobayashi K, Ungless MA. Functional properties of dopamine neurons and co-expression of vasoactive intestinal polypeptide in the dorsal raphe nucleus and ventro-lateral periaqueductal grey. Eur J Neurosci. 2012;36:3322–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Qi G, Zhang P, Li T, Li M, Zhang Q, He F, et al. NAc-VTA circuit underlies emotional stress-induced anxiety-like behavior in the three-chamber vicarious social defeat stress mouse model. Nat Commun. 2022;13:577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang Q, Hu DX, He F, Li CY, Qi GJ, Cai HW, et al. Locus coeruleus-CA1 projections are involved in chronic depressive stress-induced hippocampal vulnerability to transient global ischaemia. Nat Commun. 2019;10:2942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu D, Tang QQ, Wang D, Song SP, Yang XN, Hu SW, et al. Mesocortical BDNF signaling mediates antidepressive-like effects of lithium. Neuropsychopharmacology. 2020;45:1557–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang XY, Xu X, Chen R, Jia WB, Xu PF, Liu XQ, et al. The thalamic reticular nucleus-lateral habenula circuit regulates depressive-like behaviors in chronic stress and chronic pain. Cell Rep. 2023;42:113170. [DOI] [PubMed] [Google Scholar]
- 43.Dai J, Aoto J, Sudhof TC. Alternative splicing of presynaptic neurexins differentially controls postsynaptic NMDA and AMPA receptor responses. Neuron. 2024;112:515–9. [DOI] [PubMed] [Google Scholar]
- 44.Dai J, Liakath-Ali K, Golf SR, Sudhof TC. Distinct neurexin-cerebellin complexes control AMPA- and NMDA-receptor responses in a circuit-dependent manner. Elife. 2022;11:e78649. [DOI] [PMC free article] [PubMed]
- 45.Zingg B, Chou XL, Zhang ZG, Mesik L, Liang F, Tao HW, et al. AAV-mediated anterograde transsynaptic tagging: mapping corticocollicular input-defined neural pathways for defense behaviors. Neuron. 2017;93:33–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Resch JM, Fenselau H, Madara JC, Wu C, Campbell JN, Lyubetskaya A, et al. Aldosterone-sensing neurons in the NTS exhibit state-dependent pacemaker activity and drive sodium appetite via synergy with angiotensin II signaling. Neuron. 2017;96:190–206.e197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Berry AS, White RL 3rd, Furman DJ, Naskolnakorn JR, Shah VD, D’Esposito M, et al. Dopaminergic mechanisms underlying normal variation in trait anxiety. J Neurosci. 2019;39:2735–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Groessl F, Munsch T, Meis S, Griessner J, Kaczanowska J, Pliota P, et al. Dorsal tegmental dopamine neurons gate associative learning of fear. Nat Neurosci. 2018;21:952–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Luo M, Li Y, Zhong W. Do dorsal raphe 5-HT neurons encode “beneficialness”? Neurobiol Learn Mem. 2016;135:40–9. [DOI] [PubMed] [Google Scholar]
- 50.Wojcik SM, Rhee JS, Herzog E, Sigler A, Jahn R, Takamori S, et al. An essential role for vesicular glutamate transporter 1 (VGLUT1) in postnatal development and control of quantal size. Proc Natl Acad Sci USA 2004;101:7158–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Reese AL, Kavalali ET. Spontaneous neurotransmission signals through store-driven Ca(2+) transients to maintain synaptic homeostasis. Elife. 2015;4:e09262. [DOI] [PMC free article] [PubMed]
- 52.Nathanson AJ, Zhang Y, Smalley JL, Ollerhead TA, Rodriguez Santos MA, Andrews PM, et al. Identification of a core amino acid motif within the alpha subunit of GABA(A)Rs that promotes inhibitory synaptogenesis and resilience to seizures. Cell Rep. 2019;28:670–81.e678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gillies GE, Virdee K, McArthur S, Dalley JW. Sex-dependent diversity in ventral tegmental dopaminergic neurons and developmental programing: a molecular, cellular and behavioral analysis. Neuroscience. 2014;282:69–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mann JJ. Role of the serotonergic system in the pathogenesis of major depression and suicidal behavior. Neuropsychopharmacology. 1999;21:99S–105S. [DOI] [PubMed] [Google Scholar]
- 55.Yohn CN, Gergues MM, Samuels BA. The role of 5-HT receptors in depression. Mol Brain. 2017;10:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Davis M, Walker DL, Miles L, Grillon C. Phasic vs sustained fear in rats and humans: role of the extended amygdala in fear vs anxiety. Neuropsychopharmacology. 2010;35:105–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Avery SN, Clauss JA, Blackford JU. The human BNST: functional role in anxiety and addiction. Neuropsychopharmacology. 2016;41:126–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kim SR, Kim SY. Functional dissection of glutamatergic and GABAergic neurons in the bed nucleus of the stria terminalis. Mol Cells. 2021;44:63–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nguyen AQ, Dela Cruz JA, Sun Y, Holmes TC, Xu X. Genetic cell targeting uncovers specific neuronal types and distinct subregions in the bed nucleus of the stria terminalis. J Comp Neurol. 2016;524:2379–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Salimando GJ, Hyun M, Boyt KM, Winder DG. BNST GluN2D-containing NMDA receptors influence anxiety- and depressive-like behaviors and modulatecell-specific excitatory/inhibitory synaptic balance. J Neurosci. 2020;40:3949–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Glangetas C, Massi L, Fois GR, Jalabert M, Girard D, Diana M, et al. NMDA-receptor-dependent plasticity in the bed nucleus of the stria terminalis triggers long-term anxiolysis. Nat Commun. 2017;8:14456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zheng C, Wei L, Liu B, Wang Q, Huang Y, Wang S, et al. Dorsal BNST DRD2(+) neurons mediate sex-specific anxiety-like behavior induced by chronic social isolation. Cell Rep. 2023;42:112799. [DOI] [PubMed] [Google Scholar]
- 63.Starke K, Gothert M, Kilbinger H. Modulation of neurotransmitter release by presynaptic autoreceptors. Physiol Rev. 1989;69:864–989. [DOI] [PubMed] [Google Scholar]
- 64.Eilam D, Szechtman H. Biphasic effect of D-2 agonist quinpirole on locomotion and movements. Eur J Pharmacol. 1989;161:151–7. [DOI] [PubMed] [Google Scholar]
- 65.Myers B, Mark Dolgas C, Kasckow J, Cullinan WE, Herman JP. Central stress-integrative circuits: forebrain glutamatergic and GABAergic projections to the dorsomedial hypothalamus, medial preoptic area, and bed nucleus of the stria terminalis. Brain Struct Funct. 2014;219:1287–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ch’ng S, Fu J, Brown RM, McDougall SJ, Lawrence AJ. The intersection of stress and reward: BNST modulation of aversive and appetitive states. Prog Neuropsychopharmacol Biol Psychiatry. 2018;87:108–25. [DOI] [PubMed] [Google Scholar]
- 67.Usiello A, Baik JH, Rouge-Pont F, Picetti R, Dierich A, LeMeur M, et al. Distinct functions of the two isoforms of dopamine D2 receptors. Nature. 2000;408:199–203. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary figures and figure legends
Data Availability Statement
All data needed to support the conclusions are present in the manuscript and /or supplementary materials.