Abstract
Clinical outcomes after a first-episode of psychosis (FEP) are heterogeneous. Many patient-related factors such as gender and comorbidity have been studied to predict symptomatic outcomes. However, psychiatrist-related factors such as prescription behaviour and gender have received little attention. We assessed the relationship between patients’ psychiatrists, psychosis severity and daily functioning in 201 patients remitted from an FEP for a duration of one year, treated by 18 different psychiatrists. We controlled for baseline severity, dose and type of antipsychotic medication, frequency of visits, and patients’ education. Symptom severity, daily functioning, and antipsychotic drug use were assessed at baseline and at 3, 6, and, 12 months follow-up. We found that psychiatrists accounted for 9.1% of the explained variance in patients’ symptom severity and 10.1% of the explained variance in daily functioning.These effects persisted even when controlling for factors such as baseline severity and the prescribed dose. The effect of prescribed dose on symptom severity and daily functioning differed between psychiatrists. Treatment centre, session frequency, and medication nonadherence were not related to symptom severity. Our results emphasize the importance of individual psychiatrist factors in symptomatic outcomes after an FEP. Further identification of psychiatrist-related factors such as the quality of therapeutic alliances and shared decision-making, may optimize psychiatrists’ training with the goal of improving patient outcomes.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-72678-4.
Keywords: Antipsychotic agents, Psychotic disorders, Treatment outcome, Severity of illness, Psychiatrists
Subject terms: Psychosis, Clinical pharmacology
Introduction
Outcomes during the first year after a first-episode of psychosis (FEP) are an important determinant of long-term prognosis for people with schizophrenia-spectrum disorders1. To better understand the heterogenous outcomes during the first year after remission from an FEP, most studies have focused on patient-related predictors, such as gender, age, duration of untreated psychosis, and comorbidity. However, the potential effect of the psychiatrist providing pharmacotherapy on patient’s outcomes may also be relevant. The quality of the therapeutic relationship and shared decision-making process, as well as prescription behaviour and frequency of monitoring visits or gender may influence outcomes. To the best of our knowledge psychiatrist-related factors affecting treatment outcomes after a first psychosis are currently only studied as a determinant of medication adherence. Yet, psychiatrist-related factors can influence many other aspects relevant for recovery and are therefore important to explore as these may inspire interventions to improve treatment collaboration and ultimately benefit patients’ outcomes.
Therapist effects have mainly been studied in psychotherapy. In these studies, therapeutic alliance and communication skills of therapists have been shown to influence patient outcomes2,3. For instance, Berry et al. (2016) have shown in a study on motivational interviewing and cognitive behavioural therapy for psychosis that lower patients-rated therapeutic alliance predicted more negative symptoms, poor insight, and more cannabis use, while lower therapist-rated alliance related to the daily use of cannabis4. McCabe et al. et al. (2016) showed that psychiatrists who had received communication training for discussing auditory verbal hallucinations improved their communication skills, which increased both psychiatrist- and patient-rated therapeutic relationships5.
A better therapeutic alliance between psychosis patients and their clinicians has been associated with lower symptom severity, greater medication adherence6–8, improved vocational functioning9, and lower symptom severity10. A recent systematic review by Da Costa et al. (2020) indicated that the quality of the therapeutic alliance between clinicians and psychosis patients related to symptom severity, support by family and surroundings, the degree of shared decision-making, and hospitalization types6. Also during acute admissions, the quality of the relationship between clinicians, in particular the physician prescriber, and patients with schizophrenia-spectrum disorders has been shown to be an important factor in medication adherence and attitudes toward treatment7. A systematic review by Shattock et al. (2018) indicated that SSD patients rated the therapeutic alliance higher when the therapist appeared more genuine and empathic11. It has been concluded that some psychotherapists are more effective than others12, with 15–20% of the therapists being consistently more effective and another 15–20% consistently less effective than most therapists13. The effect of psychotherapists was found to be stronger in patients with high symptom severity14. This might indicate that a therapist’s skills are challenged more when working with patients who show severe psychotic symptoms.
The patient-therapist relationship is also of key importance within pharmacotherapeutic treatment15,16, as these treatments are never purely pharmacological, but also involve interpersonal communication, information, support, and guidance. Currently, there is a remarkable paucity of research on the role of the psychiatrist in the outcomes of pharmacotherapy. Only two studies17,18 have assessed the influence of psychiatrists on patients’ outcomes for pharmacotherapy in depression, with contradictory results. McKay et al. (2006) found that psychiatrists explained 6.7% of the variance on the Hamilton Rating Scale for Depression (HAMD) scores (p = 0.053), while the effect of the pharmacotherapeutic intervention (placebo versus imipramine hydrochloride) could explain 5.9% of the variance (p < 0.05)17. In contrast, no significant effect of psychiatrists (p > 0.30) on depression severity (HAMD) was observed by Strunk et al. (2010) in an RCT comparing placebo and paroxetine in 120 patients treated by five psychiatrists18.
Various aspects of psychotic disorders could complicate the therapeutic bonding and shared decision-making process, such as paranoid tendencies, reduced insight, negative symptoms, and impaired social cognition. Communication skills of psychiatrists may impact the collaboration with patients and maybe even more important in psychosis than in depression19. Adherence to pharmacological guidelines by physicians has been shown to relate to lower severity of psychosis symptoms in schizophrenia patients20. This underscores the importance of following treatment protocols to manage symptom severity effectively and suggests that psychiatrists’ influence on patient outcomes may be partly mediated by the appropriate choice and dosage of antipsychotic medication20. Interestingly, gender of the medical specialist in relation to that of the patient, has also been reported to impact outcomes21. Lombarts and Verghese (2022) found that female patients tend to have worse outcomes with male versus female medical specialists across several medical disciplines.
Given the importance of the first year of treatment after an FEP on long-term outcomes, including symptom severity and daily functioning, psychiatrist-related factors may be particularly relevant in this period. During the first year after remission from an FEP, many patients taper their medication or switch to another antipsychotic drug to relieve side-effects. The current study assessed the effect of 18 treating psychiatrists on positive symptom severity and daily functioning in 201 patients remitted from an FEP receiving pharmacotherapy over a 12-month period in a nationwide discontinuation trial in The Netherlands22. We hypothesize a relationship between psychiatrists and patients’ daily functioning and symptom severity in the first year after remission from an FEP, which is expected to relate to the frequency of psychiatrist-patient contacts, to antipsychotic prescription behaviour, and to baseline symptom severity of patient’s symptoms. In line with Lombarts and Verghese (2022), we expected female patients to display worse outcomes with male versus female psychiatrists.
Methods
Study design and participants
This study includes a post-hoc analysis of data from 201 FEP patients from the ongoing HAMLETT study (Handling Antipsychotic Medication Long-term Evaluation of Targeted Treatment), a nationwide single-blind randomised controlled trial on antipsychotic medication discontinuation in The Netherlands22. Participants were randomised with a 1:1 ratio to either continue, or taper/discontinue antipsychotic medication in the first year after initial remission of an FEP. Participants and psychiatrists were notified of the randomization outcome and were provided with recommended tapering schedules based on gradual hyperbolic discontinuation. The type and dose of antipsychotic medication were determined in a shared decision-making process between the patient, family or partner, and the psychiatrist. If relapse symptoms emerged during the tapering process, the dose was stabilized or increased.
FEP patients were recruited from 26 specialized outpatient psychosis centres in the Netherlands. Clinicians from the psychosis centres screened, approached, and informed potential participants. Eligible participants were aged between 16 and 60 years, had achieved symptomatic remission for 3–6 months after an FEP (e.g. sustained improvement of psychotic symptoms, any remaining psychotic symptoms did not interfere with daily functioning), and had a DSM-5 or ICD-10 diagnosis of the first-episode of schizophrenia, schizoaffective disorder, schizophreniform disorder, brief psychotic disorder, delusional disorder, or Unspecified Schizophrenia Spectrum and Other Psychotic Disorder. Patients were excluded from the study if they had demonstrated severe or life-threatening violence or self-harm or had needed coercive treatment during the psychosis. Eligibility criteria were assessed by trained researchers with the Comprehensive Assessment of Symptoms and History interview (CASH)23 and in case further information was needed, the treating psychiatrist was contacted. Ethical approval was obtained from the research and ethics committee of the University Medical Centre Groningen, the Netherlands (protocol number: NL 62202.042.17, trial registration EudraCT number: 2017-002406-12). Recruitment and study procedures are described in detail in Begemann et al. (2020).
Procedures
We examined psychotic symptom severity and daily functioning in 201 FEP patients at four different time points: at baseline (3–6 months after remission) and 3, 6, and, 12 month follow-up. At baseline, all patients were in symptomatic remission and used antipsychotic medication. During the course of one year, 82.4% of the participants reduced or fully discontinued antipsychotic medication, while 17.6% of the patients continued on the same dose. Data were collected between November 2017 and July 2022.
Outcomes
To assess the relationship between patients’ psychiatrists and both clinical and functional recovery, the present study focused on psychosis symptom severity and daily functioning during the year after remission from an FEP. Psychosis symptom severity was assessed with the positive symptoms subscale of the Positive and Negative Syndrome Scale (PANSS)24. PANSS scores were assessed by a trained rater from the central study team, who has no role in the patient’s treatment and was blind to tapering condition. Daily functioning was measured with the World Health Organization Disability Assessment Schedule 2.0 (WHO-DAS)25.
We aimed to assess the extent of psychiatrist-, patient-, or psychosis centre-related factors on patient outcomes, in particular the severity of positive symptoms. Hence, we analysed whether and to which degree the explained variance of the association between patient outcomes and the psychiatrist changed in response to adding individual factors to the model. Psychiatrist-related factors included the prescribed antipsychotic type and dose, frequency of contact, and gender of the psychiatrist in relation to that of the patient. Information on antipsychotic drug use/prescription was based on self-report questionnaires and dispensation data from pharmacies provided by the Dutch Foundation for Pharmaceutical Statistics (SFK). The types of antipsychotic drugs were categorized as olanzapine, aripiprazole, and other antipsychotics, as the drugs of this latter category were not used frequently enough to generate separate categories. To compare doses of various types of antipsychotics, all daily doses were converted to olanzapine equivalents (mg/day)26. Patient-related factors included years of education, medication nonadherence, and baseline symptom severity. Patients’ demographic characteristics were measured at baseline with the Comprehensive Assessment of Symptoms and History (CASH)23. Medication nonadherence was measured as the number of days the participant used less or no antipsychotic drugs while these had been prescribed, in the two weeks before the study assessment with a self-report medication questionnaire. Healthcare centre-related factors included the psychosis centre itself and the frequency of contact with the nurse and psychologist, which was measured with the Trimbos and iMTA Cost questionnaire associated with Psychiatric illness (TiC-P)27. Data were only included from psychiatrists who treated at least 6 FEP patients, to adequately assess the relationship between the psychiatrists and patient outcomes.
Statistical analysis
To assess the association between psychiatrists and patients’ symptom severity or daily functioning, linear mixed-effects models were performed with patients’ PANSS positive items subscale score or WHO-DAS II score, respectively, on four measurements (baseline, and 3, 6, and 12 month follow-ups) as dependent variables, with psychiatrists and time as fixed effect, and random intercepts for patients28. The relative importance of predictors was assessed with 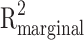 29. The likelihood ratio test (LRT) was used to assess the significance of psychiatrists as predictors in each model. The models were adjusted for the patient’s age, gender, years of education, treatment site, and baseline symptom severity or daily functioning. For this last factor, baseline severity or daily functioning were categorized as ‘mild’ (baseline score below mean), ‘moderate’ (baseline scores between the mean and 3rd quantile), or ‘severe’ (baseline scores between the 3rd quantile and maximum score). Statistical analyses were performed in R (version 4.2.0) via Rstudio (version 2022.07.0)30. To assess whether the association between the psychiatrist and symptom severity or daily functioning during the one-year period after study inclusion could be partly explained by the type and dose of antipsychotic, both were entered as fixed effects in the linear mixed effects models. The effect of baseline patient-related covariates age, gender and years of education on symptom severity or daily functioning were individually assessed. Only baseline covariates that significantly affected outcomes were included in the analyses. The relationship between the gender of the psychiatrist and the patient, and outcome was modelled as a fixed effect in linear mixed effects analyses. To test whether gender of the psychiatrist and patient related to the prescribed dose of antipsychotic medication, a linear mixed effects analysis was performed with dose as dependent variable, and time and gender (both of psychiatrist and patient) as fixed effects factors. Reciprocal transformation was applied to the data when the assumptions of normality and homogeneity were violated. Missing data were imputed with chained random forest imputations and a sensitivity analysis was performed on the non-imputed data only (Supplementary Table 1)31.
29. The likelihood ratio test (LRT) was used to assess the significance of psychiatrists as predictors in each model. The models were adjusted for the patient’s age, gender, years of education, treatment site, and baseline symptom severity or daily functioning. For this last factor, baseline severity or daily functioning were categorized as ‘mild’ (baseline score below mean), ‘moderate’ (baseline scores between the mean and 3rd quantile), or ‘severe’ (baseline scores between the 3rd quantile and maximum score). Statistical analyses were performed in R (version 4.2.0) via Rstudio (version 2022.07.0)30. To assess whether the association between the psychiatrist and symptom severity or daily functioning during the one-year period after study inclusion could be partly explained by the type and dose of antipsychotic, both were entered as fixed effects in the linear mixed effects models. The effect of baseline patient-related covariates age, gender and years of education on symptom severity or daily functioning were individually assessed. Only baseline covariates that significantly affected outcomes were included in the analyses. The relationship between the gender of the psychiatrist and the patient, and outcome was modelled as a fixed effect in linear mixed effects analyses. To test whether gender of the psychiatrist and patient related to the prescribed dose of antipsychotic medication, a linear mixed effects analysis was performed with dose as dependent variable, and time and gender (both of psychiatrist and patient) as fixed effects factors. Reciprocal transformation was applied to the data when the assumptions of normality and homogeneity were violated. Missing data were imputed with chained random forest imputations and a sensitivity analysis was performed on the non-imputed data only (Supplementary Table 1)31.
Results
Data were available for 201 participants (30.8% female, 69.2% male) who received antipsychotic medication from 18 different psychiatrists (on average 11 patients per psychiatrist) in 14 treatment centres. Patients were assessed at baseline (3–6 months after remission) and had an average follow-up duration of 271 days. Follow-up assessments were completed by 170 participants (85%) at 3 months, 161 (80%) at 6 months, and 131 (65%) at 12 months after baseline. Lower completion rates were partly due to dropout (n = 45 at 12 months after baseline), and partly because newly recruited patients had not yet completed all follow-up visits (n = 22 at 12 months after baseline). Participants’ baseline sociodemographic and clinical characteristics are shown in Table 1. Patients had an average age of 27.5 (SD = 8.4) years of age, and a mean duration of illness of 151.2 (SD = 203.1) days. Male patients were younger at FEP onset, obtained a lower educational level, and showed more severe positive symptoms at baseline compared to female patients. Eighty-two percent of patients tapered off antipsychotic medication at some point between baseline and 12 months later, with an average dose reduction of 64.1% (SD = 56.1). Eighteen participants (10.6%) switched the type of antipsychotic drug at some point during the study period. We studied the period after remission when patients were visiting their psychiatrist at outpatient facilities. In the month before baseline, 48.7% of the FEP patients visited a psychologist on average 1.2 times (SD = 1.6), and 51.9% had contact with a nurse on average 1.6 times (SD = 4.5).
Table 1.
Baseline sociodemographic, clinical, and healthcare use characteristics.
| Characteristic | Total (n = 201) | Female patients (n = 62, 30.8%) | Male patients (n = 139, 69.2%) | p value | Missing N |
|---|---|---|---|---|---|
| Age at onset of psychosis: mean (SD) | 26.3 (8.4) | 28.0 (8.4) | 25.5 (8.2) | 0.048 | 2 |
| Age: mean (SD) | 27.5 (8.3) | 29.0 (8.4) | 26.8 (8.2) | 0.082 | 1 |
| Years of education: mean (SD) | 14.2 (2.3) | 15.2 (1.5) | 13.8 (2.4) | < 0.001 | 3 |
| PANSS score: mean (SD) | 1 | ||||
| Positive scale | 9.1 (2.6) | 8.5 (2.3) | 9.4 (2.8) | 0.035 | |
| Negative scale | 11.8 (4.2) | 11.5 (4.3) | 11.9 (4.2) | 0.529 | |
| General scale | 22.8 (5.2) | 22.6 (4.9) | 22.9 (5.3) | 0.719 | |
| Total | 43.7 (9.5) | 42.6 (8.9) | 44.2 (9.8) | 0.290 | |
| WHO-DAS 2.0 score: mean (SD) | 17.8 (15.0) | 19.1 (16.9) | 17.2 (14.1) | 0.400 | 2 |
| GAF: mean (SD) | 66.1 (12.1) | 68.0 (12.3) | 65.3 (12.0) | 0.158 | 4 |
| Antipsychotic medication: n (%) | 0 | ||||
| Aripiprazole | 61 (30.3%) | 20 (32.3%) | 41 (29.5%) | 0.446 | |
| Olanzapine | 69 (34.3%) | 24 (38.7%) | 45 (32.4%) | ||
| Other types | 71 (35.3%) | 18 (29.0%) | 53 (38.1%) | ||
| Dose of antipsychotic medication (Olanzapine equivalent): mean (SD) | 9.5 (6.0) | 9.1 (6.6) | 9.7 (5.7) | 0.495 | 1 |
| Participants who tapered antipsychotic medication between baseline and 12 months follow-up: n (%) | 145 (82.9%) | 42 (85.6) | 94 (81.7) | 0.664 | 37 |
| Dose reduction (%) of antipsychotic medication between baseline and 12 months: mean (SD) | 64.1% (56.1) | 71.9 (44.3) | 60.7 (60.4) | 0.231 | 28 |
Abbreviations: PANSS, Positive and Negative Syndrome Scale; WHO-DAS, World Health Organisation Disability Assessment Schedule; GAF, Global Assessment of Functioning.
Symptom severity
Psychiatrist-related factors
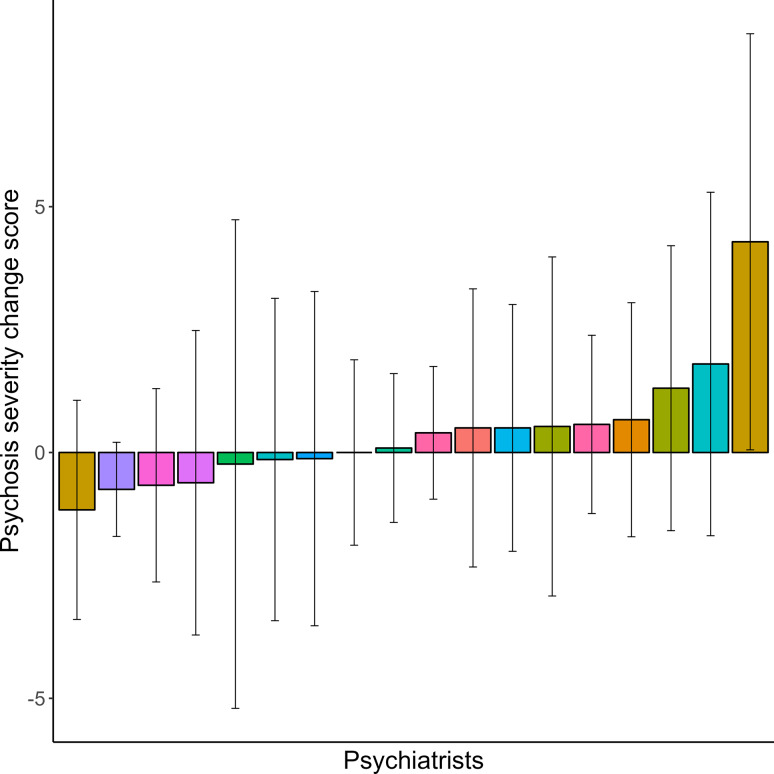
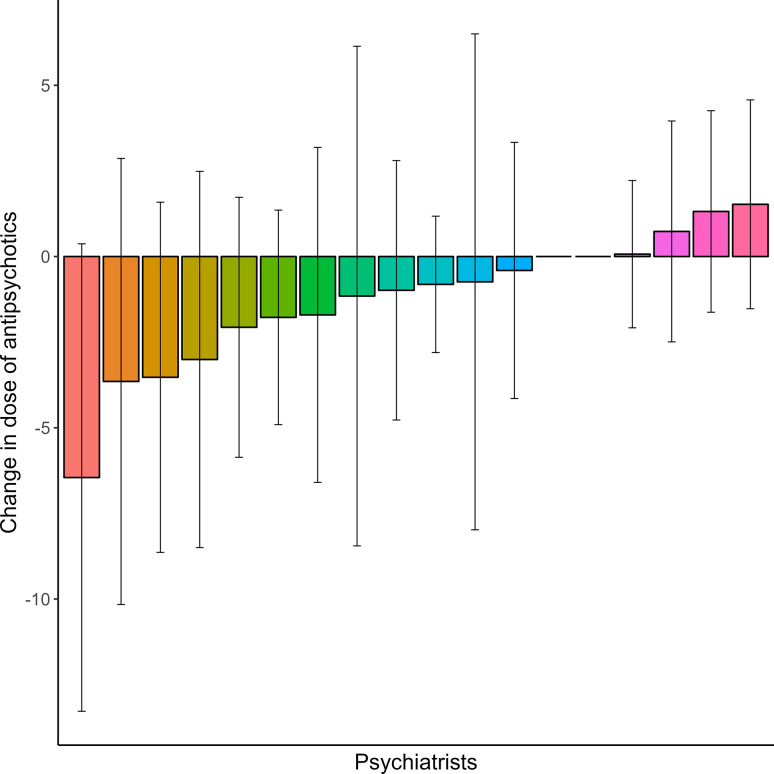
Linear mixed effects models showed that positive symptom severity, measured with the PANSS positive items subscale, slightly increased over time from 9.1 (SD = 2.6) at baseline to 9.6 (SD = 3.4) at 12-month follow-up (χ2 = 4.387, p = 0.036). A significant association between psychiatrists and positive symptom severity during the study period was found, which explained 9.1% of the variance (χ2 = 29.067, p = 0.034) (Figs. 1 and 2, Supplementary Material Table S1). Positive symptom severity was related to antipsychotic dose during the study period (χ2 = 6.315, p = 0.012) (Fig. 3), but not to the type of medication (χ2 = 0.858, p = 0.835). When adjusted for the dose of antipsychotic medication, psychiatrists still explained 9.0% of the variance in symptom severity (LRT = 29.322, p = 0.033). The significant interaction between psychiatrists and antipsychotic dose (χ2 = 27.928, p = 0.046) indicated that the effect of the prescribed dose on symptom severity during the study period differed between psychiatrists, accounting for an additional 2.4% of the variance (Figs. 1, 2, 3, 4).
Fig. 1.
Change score in psychosis symptom severity per psychiatrist. The average change in psychotic symptom severity, measured with the Positive and Negative Syndrome Scale (PANSS), during the study period averaged over all patients per psychiatrist. The bars are differently coloured per treatment centre.
Fig. 2.
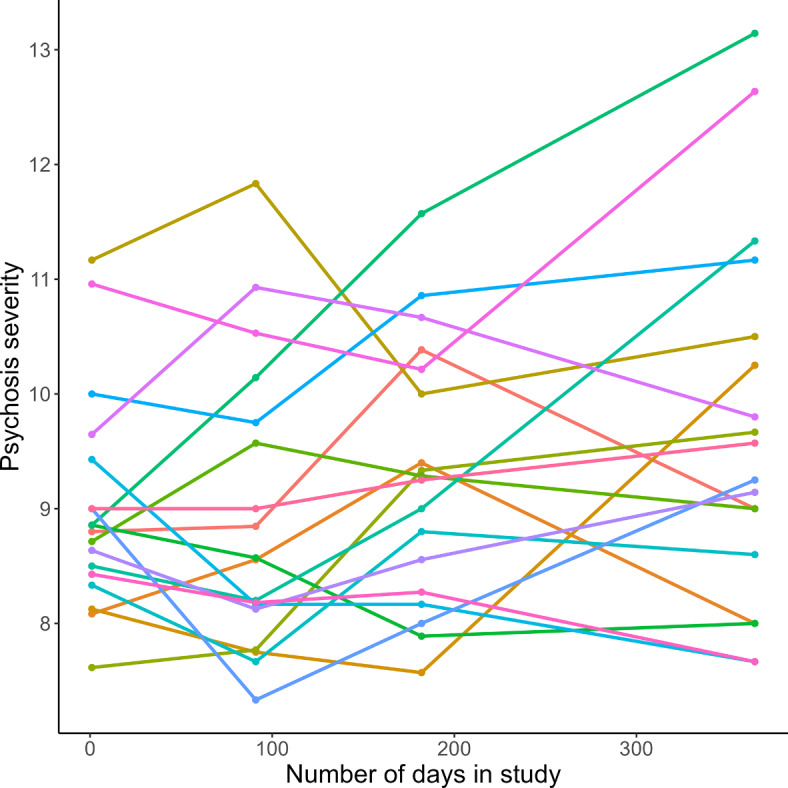
Symptom severity of FEP patients averaged per psychiatrist during the study period. Mean psychotic symptom severity, measured with the Positive and Negative Syndrome Scale (PANSS), during the study period, averaged over all patients per psychiatrist. The lines are coloured per psychiatrist.
Fig. 3.
Mean change in dose of antipsychotics per psychiatrist. The average change in prescribed antipsychotic dose (in olanzapine equivalents) per psychiatrist during the study period.
Fig. 4.
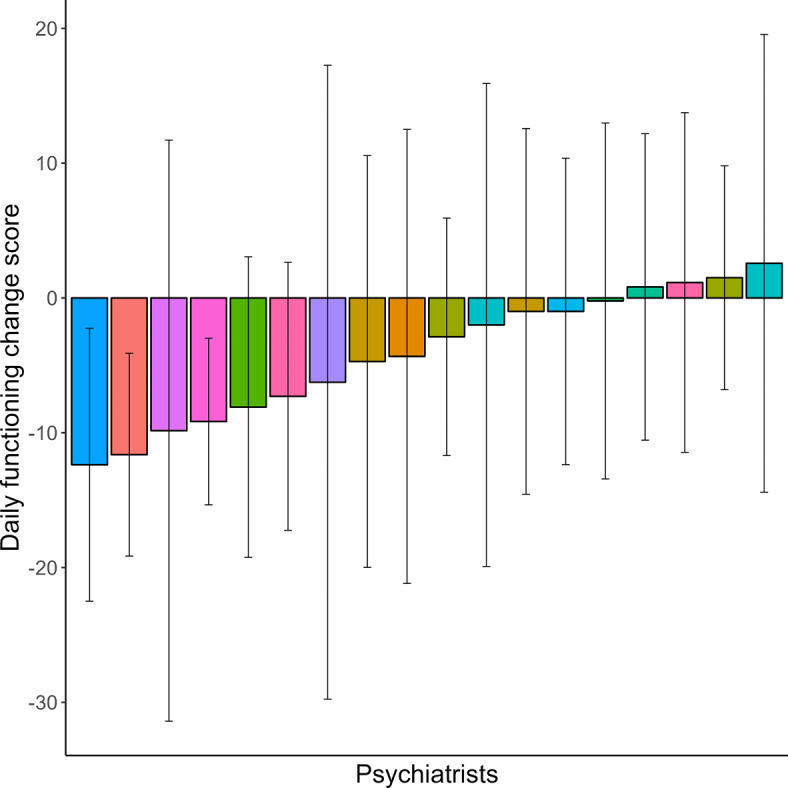
Change score in daily functioning per psychiatrist. The average change in daily functioning, measured with the World Health Organization Disability Assessment Schedule 2.0 (WHO-DAS), during the study period averaged over all patients per psychiatrist. The bars are differently coloured per treatment centre. Lower scores indicate better daily functioning.
Patient-related factors
As expected, baseline symptom severity was also associated with symptom severity at 3, 6, and, 12 months follow-up (χ2 = 206.49, p < 0.001) and explained 38.6% of the variance. When corrected for baseline severity, the psychiatrist association remained significant (accounting for 2.3% of the variance, LRT = 28.801, p = 0.036). The frequency of contact between patients and psychiatrists did not significantly affect the outcome (χ2 = 0.074, p = 0.786), nor did treatment centre (χ2 = 0.053, p = 0.997). Patients with more years of education had lower symptom severity (χ2 = 7.492, p = 0.006). The relationship between psychiatrists and symptom severity remained significant after correcting for years of education (LRT = 31.411 p = 0.018, 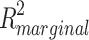 = 0.025). Medication nonadherence did not predict symptom severity (χ2 = 1.515, p = 0.218).
= 0.025). Medication nonadherence did not predict symptom severity (χ2 = 1.515, p = 0.218).
Healthcare centre-related factors
Psychosis symptom severity was associated with the frequency of sessions with the psychologist (χ2 = 5.510, p = 0.019), but not with the nurse (χ2 = 0.015, p = 0.902). The psychiatrist association persisted after additionally correcting for the frequency of sessions with the psychologist (LRT = 31.332, p = 0.018). Similarly, associations were found between psychiatrists and overall symptom severity as measured with the PANSS total score (Supplementary Table S3) during 12 months of follow-up.
Daily functioning
Psychiatrist-related factors
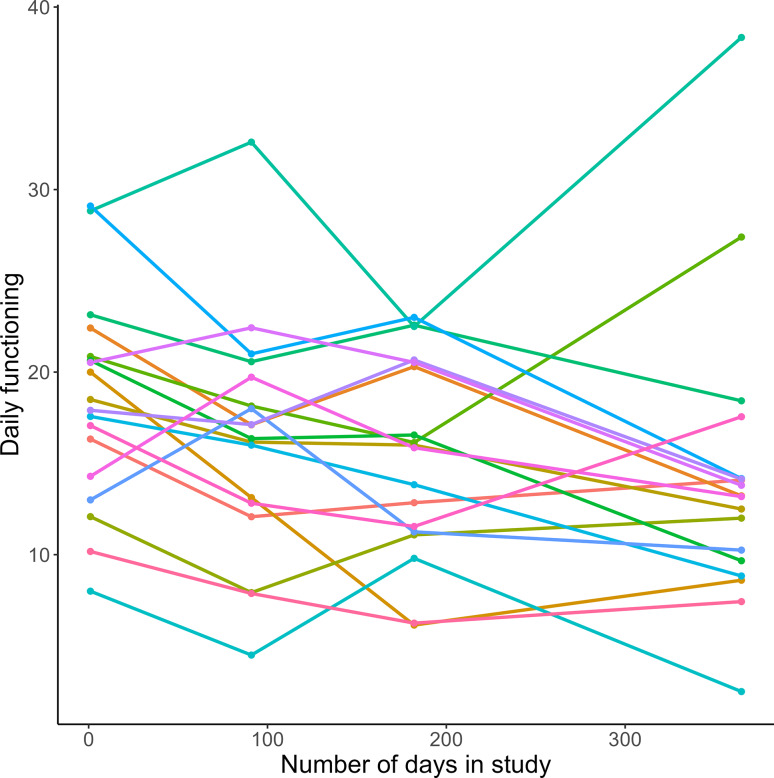
Daily functioning, measured with the WHO-DAS questionnaire, where higher scores indicate lower functioning, improved from 17.794 (SD = 14.997) at baseline to 13.371 (SD = 12.665) at 12 months follow-up (χ2 = 24.058, p < 0.001). Psychiatrists were significantly associated with daily functioning during the study period (χ2 = 33.011, p = 0.011) and explained 10.1% of the variance (Figs. 4 and 5, Supplementary Table S2). Daily functioning was related to antipsychotic dose (χ2 = 23.126, p < 0.001) and type (χ2 = 9.132, p = 0.031). Psychiatrists still accounted for 8.5% of the variance after correcting for the dose and type of antipsychotic drugs (LRT = 28.39, p = 0.041). The effect of antipsychotic dose on daily functioning differed between psychiatrists, as there was a significant interaction effect between psychiatrist and dose (χ2 = 37.749, p < 0.001), which explained an additional 4.3% of the variance.
Fig. 5.
Daily functioning of FEP patients averaged per psychiatrist during the study period. Mean daily functioning, measured with the World Health Organization Disability Assessment Schedule 2.0 (WHO-DAS), during the study period, averaged over all patients per psychiatrist. The lines are coloured per psychiatrist. Lower scores indicate better daily functioning.
Patient-related factors
Baseline functioning was significantly related to daily functioning during the study period (χ2 = 118.96, p < 0.001) and accounted for 24.6% of the variance. The variance in daily functioning explained by psychiatrists decreased to 3.9% after correcting for baseline functioning (LRT = 31.16, p = 0.019). Medication non-adherence did not relate to daily functioning (χ2 = 0.172, p = 0.678).
Healthcare centre-related factors
Daily functioning improved with a higher frequency of sessions between the psychiatrist and patient (χ2 = 5.589, p = 0.016), which explained an additional 0.4% variance, and was related to the treatment site (χ2 = 9.985, p = 0.016), which accounted for an extra 1.2% variance. The frequency of sessions with the psychologist was associated with daily functioning (χ2 = 24.225, p < 0.001), and a trend was seen for the sessions with the nurse (χ2 = 3.684, p = 0.055).
Gender differences
Linear mixed effects models showed that male patients experienced greater positive symptom severity during the study period compared to female patients (χ2 = 6.622, p = 0.010,  = 0.025). Female and male patients did not significantly differ in daily functioning during the study period (χ2 = 0.043, p = 0.837). The gender of the psychiatrist did not significantly relate to positive symptom severity (χ2 = 2.099 p = 0.147), nor daily functioning (χ2 = 1.621, p = 0.203) during 12 months of follow-up. The gender of the psychiatrist in relation to that of the patient did not significantly predict positive symptom severity (χ2 = 0.834, p = 0.361) or daily functioning (χ2 = 0.032, p = 0.858). The prescribed dose of antipsychotic medication did not significantly differ between female versus male patients during the study period (χ2 = 1.233, p = 0.267). The prescribed dose of antipsychotic medication was not associated with the gender of the psychiatrist (χ2 = 1.122, p = 0.290), nor with the interaction between the gender of the psychiatrist and that of the patient (χ2 = 1.958, p = 0.376).
= 0.025). Female and male patients did not significantly differ in daily functioning during the study period (χ2 = 0.043, p = 0.837). The gender of the psychiatrist did not significantly relate to positive symptom severity (χ2 = 2.099 p = 0.147), nor daily functioning (χ2 = 1.621, p = 0.203) during 12 months of follow-up. The gender of the psychiatrist in relation to that of the patient did not significantly predict positive symptom severity (χ2 = 0.834, p = 0.361) or daily functioning (χ2 = 0.032, p = 0.858). The prescribed dose of antipsychotic medication did not significantly differ between female versus male patients during the study period (χ2 = 1.233, p = 0.267). The prescribed dose of antipsychotic medication was not associated with the gender of the psychiatrist (χ2 = 1.122, p = 0.290), nor with the interaction between the gender of the psychiatrist and that of the patient (χ2 = 1.958, p = 0.376).
Discussion
We followed 201 patients remitted from a first episode psychosis, participating in the HAMLETT study for a period of one year, during which 82.4% together with their psychiatrist decided to reduce the dose of their antipsychotic medication and another 10.6% changed the type of antipsychotic drug. We demonstrated that psychiatrists were associated with patients’ outcomes in terms of psychotic symptom severity and daily functioning after one year, which attenuated but remained significant when corrected for baseline severity, antipsychotic dose and type, treatment site, years of education, and number of visits. The gender of the psychiatrist in relation to that of the patient was not related to patient outcomes, nor was patient’s education. These findings support the idea that not only patient-related factors like age, gender, or education, but also psychiatrist-related factors are important for outcomes after an FEP.
The current study is the first to show the association between psychiatrists and long-term patient outcomes in pharmacotherapy after an FEP. Psychiatrists accounted for a 9.1% variance in psychotic symptom severity scores, and a 10.1% variance in daily functioning in the first year after remission from an FEP. This study is also the first to show that the effect of the prescribed antipsychotic dose on clinical outcomes differs between psychiatrists, which could explain an additional 2.4% of the variance in symptom severity and 4.3% in daily functioning. Controlling for the prescribed dose barely affected the explained variance of the psychiatrist on patient outcomes (it lowered from 9.1 to 9.0% for symptoms severity and from 10.1 to 9.2% for daily functioning). This indicates that factors other than antipsychotic dosing may influence the relationship between the psychiatrist and their patient’s outcomes. We explored the psychiatrist-related factor of gender, but this was not significantly associated with symptom severity or daily functioning. While the gender of the doctor in relation to that of the patient related to clinical outcomes in a previous study across medical disciplines21, we did not find this effect on positive symptom severity or daily functioning in FEP patients during the study period. Also, we found similar prescription behaviours in male and female psychiatrists.
Several previous studies6,32 showed that increased therapeutic alliance is associated with better therapy adherence. This alliance might be even more important during the tapering of medication and in finding the lowest possible effective dose. We need to emphasize that this study was not designed to specifically address quality of shared decision-making or therapeutic alliance and hence important factors that might underlie the relationship between the psychiatrist and patient outcomes could not be revealed, nor were we able to test the impact of individual psychiatrist characteristics.
Our results suggest that the relationship between psychiatrists and patient outcomes may be partially related to patients’ baseline symptom severity, as the variance related to the psychiatrist dropped from 9.1 to 2.3% in positive symptom severity and from 10.1 to 3.9% in daily functioning after controlling for baseline severity. However, the exact relationship between psychiatrist-related factors and baseline severity and functioning remains unknown and might actually consist of two different effects. First, some psychiatrists may treat more severe patients than others, which could be related to their position in a treatment centre. Second, psychotherapeutic studies have shown that the therapist effect was largest in the most severe patient, as effective therapeutic bonding may be more demanding with severely psychotic patients. Likewise, psychiatrists might have a greater influence on treatment outcomes during pharmacotherapy in patients with higher dysfunction and severity of psychosis.
The association we observed between psychiatrists and clinical and functional outcomes for pharmacotherapeutic contacts in psychosis is in line with the study of McKay et al. (2006) who found that psychiatrists explained 6.7% of the variance in symptom severity assessed with the Hamilton Rating Scale for Depression (HAMD). Their finding was not replicated by Strunk and colleagues (2010). The present study differs from these two studies in several aspects. First, the present study included nearly twice as many patients and psychiatrists. Second, while the results of McKay et al. (2006) were only corrected for baseline severity, and Strunk et al. (2010) only adjusted for treatment type, our analyses included baseline severity, dose and type of antipsychotic drugs, years of education, treatment site, and number of visits to the psychiatrist. The fact that the influence of the psychiatrist remained significant, points to McKay’s conclusion that the psychiatrist may play an important role in patients’ outcomes during pharmacotherapy. The most important difference, of course, is that we investigated the outcomes of people who have experienced psychosis, while the previous two studies investigated outcomes of depressive patients. Given the occurrence of a paranoid tendency in some people with psychosis, the effect of the psychiatrist could be even more important in this group, as a solid therapeutic relationship with someone with a paranoid tendency may demand specific skills and dedication from the psychiatrist. Indeed, the psychotherapeutic alliance was found to be weaker in patients with more paranoia19. This is in line with our findings, which show that the association between psychiatrists and psychotic symptom severity and daily functioning in FEP patients was partially related to baseline severity.
Several limitations of the current study should be mentioned. As the study was not initially designed to assess psychiatrist-related factors, we did not study the shared decision-making process, nor the therapeutic alliance, which are expected to explain part of the relationship between the psychiatrist and patient outcomes. The limited available sample size did not allow for a more detailed analysis of the relationship between psychiatrists and patient outcomes, for example by determining whether it is different for patients with mild, moderate or severe symptom severity. Due to the nearly 1:1 ratio of the 18 psychiatrists and 14 treatment centres, the individual effects of psychiatrists and treatment centres could not be adequately investigated. Yet, Fig. 1, which displays the psychiatrist-effect colour-coded for treatment centre does not suggest that centre was an important confounder, as the effects of psychiatrists of the same centre (colour) can run in quite different directions. The analyses did not include whether the patient was randomized to the continuation or discontinuation group, because the HAMLETT study protocol was flexible regarding the timing and speed of antipsychotic tapering, and some patients chose to switch from the continuation to the discontinuation group. Therefore, we included the actually prescribed dose of antipsychotic medication in the analyses. Medication adherence was assessed through a questionnaire based on patients’ self-reports, which might not fully capture actual adherence. We studied the effect of the psychiatrists, who are the chief practitioners and together with the patients determine medication prescriptions. While the frequency of visits to psychologists or nurses did not affect the association between psychiatrists and clinical and functional outcomes, we did not assess the possible effects of the combination of other therapists involved in the treatment of FEP patients, such as social workers, physiotherapists, and job coaches. Patients in the present sample often received treatments from specialized early psychosis teams, which included various care professionals. Still, given the central role of the psychiatrist in treatment teams, we cautiously propose that it may be that a psychiatrist’s treatment approach is to some extent also reflected in that of other team members. Previously, the physician-nurse relationship in hospitals has been shown to be predictive of work satisfaction, nurses’ health, and even patients’ outcomes and satisfaction33. Future research may also include effects of other relevant care professionals and the composition of the psychosis treatment team on the outcomes of pharmacotherapy.
The present study highlights the association between the psychiatrist who provides pharmacotherapy and clinical and functional outcomes in remitted first-episode psychosis patients. Although the current study cannot exactly determine which characteristics of the psychiatrist may drive this effect, future studies on these characteristics may inform novel interventions to improve the therapeutic relationship, shared decision making, and prescription behaviour. For instance, even a brief intervention on stimulating communication showed an improved alliance between psychiatrists and psychosis patients5.
Our results emphasize the importance of psychiatrist-related factors to patients’ outcomes after an FEP. Hence detailed identification of potential drivers of these effects could improve psychiatrists’ training, inform optimal psychosis treatment, and ultimately lead to better outcomes for those who experience a first psychotic episode.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We would like to thank the participants of the HAMLETT trial for their voluntary participation and we would like to thank Evan Troelstra for aiding us in the writing process.
Author contributions
FB: methodology, formal analysis, data curation, writing – original draft, visualization, SK: supervision, writing – review & editing, RS: conceptualization, writing – review & editing, WV: writing – review & editing, NB: writing – review & editing, LH: writing – review & editing, NB: writing – review & editing, MK: writing – review & editing, MB: writing – review & editing, HAMLETT-OPHELIA consortium: investigation, resources, JL: methodology, writing – review & editing, IS: funding acquisition, conceptualization, supervision, writing – review & editing.
Funding
The project was funded by the HAMLETT-OPHELIA project, which is financed by the Dutch Medical Science Foundation ZonMW (no. 348041003 and no. 636340001, main applicant: Dr Iris Sommer).
Data availability
The datasets generated during and/or analysed during the current study are not publicly available due to ongoing data collection.
Declarations
Competing interests
The authors declare no competing interests.
Ethical approval
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008. Ethical approval was obtained from the research and ethics committee of the University Medical Centre Groningen, the Netherlands (protocol number: NL 62202.042.17, trial registration EudraCT number: 2017–002406-12).
Informed consent
All participants provided written informed consent prior to study participation.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
A list of authors and their affiliations appears at the end of the paper.
Contributor Information
Franciska de Beer, Email: f.de.beer@umcg.nl.
HAMLETT-OPHELIA Consortium:
Franciska de Beer, Joran Lokkerbol, Iris E. C. Sommer, Iris Sommer, Lieuwe de Haan, Wim Veling, Jim van Os, Filip Smit, Marieke Begemann, Sanne Schuite-Koops, Machteld Marcelis, Martijn Kikkert, Nico van Beveren, Nynke Boonstra, Bram-Sieben Rosema, Sinan Gülöksüz, P. Roberto Bakker, Joran Lokkerbol, Bodyl Brand, Shiral Gangadin, Erna van’t Hag, Priscilla Oomen, Alban Voppel, Iris Hamers, Matej Djordjevic, Toon Scheurink, Therese van Amelsvoort, Maarten Bak, Steven Berendsen, Truus van den Brink, Gunnar Faber, Koen Grootens, Martijn de Jonge, Henderikus Knegtering, Jörg Kurkamp, Gerdina Hendrika Maria Pijnenborg, Anton B. P. Staring, Natalie Veen, Selene Veerman, Sybren Wiersma, Albert Batalla Cases, Ruben Curfs, Jan-Jaap Hage, Ellen Graveland, Joelle Hoornaar, and Inge van der Heijden
References
- 1.Simonsen, C. et al. Early clinical recovery in first-episode psychosis: Symptomatic remission and its correlates at 1-year follow-up. Psychiatry Res.254, 118–125 (2017). [DOI] [PubMed] [Google Scholar]
- 2.Priebe, S., Conneely, M., McCabe, R. & Bird, V. What can clinicians do to improve outcomes across psychiatric treatments: A conceptual review of non-specific components. Epidemiol. Psychiatr. Sci.10.1017/S2045796019000428 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Walwyn, R. & Roberts, C. Therapist variation within randomised trials of psychotherapy: Implications for precision, internal and external validity. Stat. Methods Med. Res.19, 291–315 (2010). [DOI] [PubMed] [Google Scholar]
- 4.Berry, K., Gregg, L., Lobban, F. & Barrowclough, C. Therapeutic alliance in psychological therapy for people with recent onset psychosis who use cannabis. Compr. Psychiatry67, 73–80 (2016). [DOI] [PubMed] [Google Scholar]
- 5.McCabe, R. et al. Training to enhance psychiatrist communication with patients with psychosis (TEMPO): Cluster randomised controlled trial. Br. J. Psychiatry209, 517–524 (2016). [DOI] [PubMed] [Google Scholar]
- 6.Da Costa, H., Martin, B. & Franck, N. Determinants of therapeutic alliance with people with psychotic disorders: A systematic literature review. J. Nerv. Mental Disease208, 329–339 (2020). [DOI] [PubMed] [Google Scholar]
- 7.Day, J. C. et al. Attitudes toward antipsychotic medication: The impact of clinical variables and relationships with health professionals. Arch. Gen. Psychiatry62, 717–724 (2005). [DOI] [PubMed] [Google Scholar]
- 8.Browne, J., Nagendra, A., Kurtz, M., Berry, K. & Penn, D. L. The relationship between the therapeutic alliance and client variables in individual treatment for schizophrenia spectrum disorders and early psychosis: Narrative review. Clin. Psychol. Rev.71, 51–62 (2019). [DOI] [PubMed] [Google Scholar]
- 9.de Jong, S. et al. Longitudinal assessments of therapeutic alliance predict work performance in vocational rehabilitation for persons with schizophrenia. Psychol. Psychother. Theory Res. Pract.94, 915–928 (2021). [DOI] [PubMed] [Google Scholar]
- 10.Priebe, S., Richardson, M., Cooney, M., Adedeji, O. & McCabe, R. Does the therapeutic relationship predict outcomes of psychiatric treatment in patients with psychosis? A systematic review. Psychother. Psychosom.80, 70–77 (2011). [DOI] [PubMed] [Google Scholar]
- 11.Shattock, L., Berry, K., Degnan, A. & Edge, D. Therapeutic alliance in psychological therapy for people with schizophrenia and related psychoses: A systematic review. Clin. Psychol. Psychother.25, e60 (2018). [DOI] [PubMed] [Google Scholar]
- 12.Saxon, D., Firth, N. & Barkham, M. The relationship between therapist effects and therapy delivery factors: Therapy modality, dosage, and non-completion. Adm. Policy Mental Health Mental Health Serv. Res.44, 705–715 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barkham, M., Lutz, W., Lambert, M. J. & Saxon, D. Therapist effects, effective therapists, and the law of variability. In How and Why are Some Therapists Better Than Others?: Understanding Therapist Effects (eds Hill, C. E. & Castonguay, L. G.) 13–36 (American Psychological Association, 2017). [Google Scholar]
- 14.Wampold, B. E. & Brown, G. S. Estimating variability in outcomes attributable to therapists: A naturalistic study of outcomes in managed care. J. Consult. Clin. Psychol.73, 914–923 (2005). [DOI] [PubMed] [Google Scholar]
- 15.Zisman-Ilani, Y., Roth, R. M. & Mistler, L. A. Time to support extensive implementation of shared decision making in psychiatry. JAMA Psychiatry78, 1183–1184 (2021). [DOI] [PubMed] [Google Scholar]
- 16.Wienke Totura, C. M., Fields, S. A. & Karver, M. S. The role of the therapeutic relationship in psychopharmacological treatment outcomes: A meta-analytic review. Psychiatr. Serv.69, 41–47 (2018). [DOI] [PubMed] [Google Scholar]
- 17.McKay, K. M., Imel, Z. E. & Wampold, B. E. Psychiatrist effects in the psychopharmacological treatment of depression. J. Affect. Disord.92, 287–290 (2006). [DOI] [PubMed] [Google Scholar]
- 18.Strunk, D. R. et al. Can pharmacotherapists be too supportive? A process study of active medication and placebo in the treatment of depression. Psychol. Med.40, 1379–1387 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harris, B. A. & Panozzo, G. Therapeutic alliance, relationship building, and communication strategies-for the schizophrenia population: An integrative review. Arch. Psychiatr. Nurs.33, 104–111 (2019). [DOI] [PubMed] [Google Scholar]
- 20.Kodaka, F. et al. Relationships between adherence to guideline recommendations for pharmacological therapy among clinicians and psychotic symptoms in patients with schizophrenia. Int. J. Neuropsychopharmacol.26, 557–565 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lombarts, K. M. J. & Verghese, A. Medicine is not gender-neutral—She is male. N. Engl. J. Med.24, 2368–2375 (2022). [DOI] [PubMed] [Google Scholar]
- 22.Begemann, M. J. H. et al. To continue or not to continue? Antipsychotic medication maintenance versus dose-reduction/discontinuation in first episode psychosis: HAMLETT, a pragmatic multicenter single-blind randomized controlled trial. Trials21, 1–19 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Andreassen, N., Flaum, M. & Arndt, S. The Comprehensive Assessment of Symptoms and History (CASH) an instrument for assessing diagnosis and psychopathology. Arch. Gen. Psychiatry49, 615–623 (1992). [DOI] [PubMed] [Google Scholar]
- 24.Kay, S. R., Fiszbein, A. & Opler, L. A. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr. Bull.13, 261–276 (1987). [DOI] [PubMed] [Google Scholar]
- 25.Chopra, P., Herrman, H. & Kennedy, G. Comparison of disability and quality of life measures in patients with long-term psychotic disorders and patients with multiple sclerosis: An application of the WHO Disability Assessment Schedule II and WHO Quality of Life-BREF. Int. J. Rehabilit. Res.31, 141–149 (2008). [DOI] [PubMed] [Google Scholar]
- 26.Leucht, S. et al. Dose-response meta-analysis of antipsychotic drugs for acute schizophrenia. Am. J. Psychiatry177, 342–353 (2020). [DOI] [PubMed] [Google Scholar]
- 27.Bouwmans, C. et al. Feasibility, reliability and validity of a questionnaire on healthcare consumption and productivity loss in patients with a psychiatric disorder (TiC-P). BMC Health Serv. Res.13, 217 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bates, D., Maechler, M., Bolker, B. & Walker, S. Fitting linear mixed-effects models using lme4. J. Stat. Softw.67, 1–48 (2015). [Google Scholar]
- 29.Nakagawa, S. & Schielzeth, H. A general and simple method for obtaining R2 from generalized linear mixed-effects models. Methods Ecol. Evol.4, 133–142 (2013). [Google Scholar]
- 30.R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing. www.R-project.org Preprint at (2020).
- 31.Wright, M. N. & Ziegler, A. ranger: A fast implementation of random forests for high dimensional data in C++ and R. J. Stat. Softw.10.18637/jss.v077.i01 (2016). [Google Scholar]
- 32.Chang, J. G., Roh, D. & Kim, C. Association between therapeutic alliance and adherence in outpatient schizophrenia patients. Clin. Psychopharmacol. Neurosci.17, 273–278 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tang, C. J., Chan, S. W., Zhou, W. T. & Liaw, S. Y. Collaboration between hospital physicians and nurses: An integrated literature review. Int. Nurs. Rev.60, 291–302. 10.1111/inr.12034 (2013). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analysed during the current study are not publicly available due to ongoing data collection.