Summary
Background
The tobacco-free generation aims to prevent the sale of tobacco to people born after a specific year. We aimed to estimate the impact of eliminating tobacco smoking on lung-cancer mortality in people born during 2006–10 in 185 countries.
Methods
For this population-based birth-cohort simulation study, we proposed a scenario in which tobacco sales were banned for people born between Jan 1, 2006, and Dec 31, 2010, and in which this intervention was perfectly enforced, quantified until Dec 31, 2095. To predict future lung-cancer mortality rates, we extracted lung-cancer mortality data by sex, 5-year age group, and 5-year calendar period for countries with at least 15 years of data from the WHO Mortality Database. For countries for which mortality data were not available, we extracted data on lung-cancer incidence from the Cancer Incidence in Five Continents. To establish the number of lung-cancer deaths that could be prevented in the birth cohort if tobacco smoking was eliminated, we subtracted reported age-specific rate of deaths in people who had never smoked tobacco (hereafter referred to as never smokers) from a previous study from the expected rate of lung-cancer deaths in our birth cohort and applied this difference to the size of the population. We computed population impact fractions (PIFs), the percentage of lung-cancer deaths that could be prevented, by dividing the number of preventable lung-cancer deaths by the expected lung-cancer deaths in the birth cohort. We also aggregated expected and prevented deaths into the four World Bank income groups (ie, high-income, upper-middle-income, lower-middle-income, and low-income). The primary outcome was the impact on lung-cancer mortality of implementing a tobacco-free generation.
Findings
Our birth cohort included a total population of 650 525 800 people. Globally, we predicted that 2 951 400 lung-cancer deaths could occur in the population born during 2006–10 if lung-cancer rates continue to follow trends observed during the past 15 years. Of these deaths, 1 842 900 (62·4%) were predicted to occur in male individuals and 1 108 500 (37·6%) were expected to occur in female individuals. We estimated that 1 186 500 (40·2%) of 2 951 400 lung-cancer deaths in people born during 2006–10 could be prevented if tobacco elimination (ie, a tobacco-free generation) was achieved. We estimated that more lung-cancer deaths could be prevented in male individuals (844 200 [45·8%] of 1 842 900 deaths) than in female individuals (342 400 [30·9%] of 1 108 500 deaths). In male individuals, central and eastern Europe had the highest PIF (48 900 [74·3%] of 65 800 deaths) whereas in female individuals, western Europe had the highest PIF (56 200 [77·7%] of 72 300 deaths). Middle Africa was the region with the lowest PIF in both male individuals (180 [2·1%] of 8600 deaths) and female individuals (60 [0·9%] of 6400 deaths). In both sexes combined, PIF was 17 400 (13·5%) of 128 900 deaths in low-income countries, 104 900 (15·8%) of 662 800 deaths in lower-middle-income countries, 650 100 (43·9%) of 1 482 200 deaths in upper-middle-income countries, and 414 100 (61·1%) of 677 600 deaths in high-income countries.
Interpretation
The implementation of a tobacco-free generation could substantially reduce global lung-cancer mortality. However, data from low-income countries were scarce and our estimates should be interpreted with caution.
Funding
Spanish Society of Pneumology and Thoracic Surgery.
Introduction
Lung cancer is the second most diagnosed cancer and the leading cause of cancer death worldwide, responsible for approximately 1·8 million deaths per year.1 Tobacco smoking is the most important risk factor for lung cancer and, in 2019, was estimated to cause more than 67% of lung-cancer deaths globally.2
Tobacco control is a priority for global public health. Strategies such as the WHO MPOWER measures (ie, monitoring tobacco use; protecting people from tobacco smoke; quitting smoking; warning about the dangers of tobacco; enforcing bans on tobacco advertising, promotion, and sponsorship; and increasing tobacco taxes) have been developed to control poor health outcomes as a result of tobacco use. However, few countries have the highest level of achievement in the measures.3 In the past 10 years, a new strategy to combat poor health outcomes as a result of tobacco use, known as the tobacco endgame, has emerged. The aim of tobacco-endgame strategies is to accelerate tobacco control by rapidly, equitably, and profoundly reducing the prevalence of tobacco smoking and eliminating it among the population. One of the measures included in many proposed tobacco-endgame strategies is the tobacco-free generation, which would restrict the sale and supply of tobacco to people born after a specific year.4 New Zealand was a pioneer in the tobacco-free generation strategy by proposing to ban the sale of tobacco to people born on or after Jan 1, 2009.5 Although some other cities have implemented a similar strategy, such as Balanga City (Philippines)6 and Brookline (MA, USA),7 and countries such as the UK8 are considering implementing one, few studies have analysed the impact of a tobacco-free generation on population health.9, 10, 11
Research in context.
Evidence before this study
Tobacco smoking is the leading cause of preventable deaths worldwide. Identifying and implementing effective tobacco-prevention strategies is essential to reduce the prevalence of smoking and to increase control of poor health outcomes as a result of tobacco use. In the past 10 years, new proposals have emerged as part of comprehensive tobacco-elimination strategies, such as the tobacco-free generation, which aims for a substantial reduction in prevalence of tobacco smoking and elimination of it among younger generations. To identify studies that estimated the impact of eliminating tobacco smoking in a population by restricting the sale of tobacco to people born after a specific year, we searched PubMed for articles published between database inception and Oct 26, 2023. We used the search terms (“tobacco-free generation” OR “smoke-free generation” OR “tobacco endgame”) AND “lung cancer”, without language restrictions. Four simulation studies analysed the impact of a tobacco-free generation on the population of a specific country, but most focused on health gains rather than mortality.
Added value of this study
To our knowledge, our simulation study is the first to evaluate the impact of a tobacco-free generation on lung-cancer mortality in any country. We estimated the impact of a tobacco-free generation on lung-cancer mortality in a birth cohort of people from 185 countries. Globally, the implementation of a tobacco-free generation could prevent more than 1·1 million lung-cancer deaths in people born between Jan 1, 2006, and Dec 31, 2010. In sensitivity analyses, we estimated that 961 500 lung-cancer deaths could be prevented in this birth cohort if the effectiveness of smoking control was reduced by 25% and, if lung-cancer mortality rates remained stable from 2022 onwards, the estimates differ from those using the predicted rates up to 2040–44.
Implications of all the available evidence
Our results provide global and national insights into the burden of lung-cancer mortality that could be prevented by implementing a restriction on tobacco sales to people born during 2006–10. The burden of lung-cancer mortality attributable to tobacco smoking remains high in many countries and is expected to increase in others. Our findings indicate that implementation of a tobacco-free generation could reduce the impact of tobacco smoking on lung-cancer mortality in future generations. Furthermore, our methods could be used to assess the impact of tobacco-free generations on other cancers or causes of death related to tobacco smoking.
We aimed to estimate the impact of eliminating tobacco smoking on lung-cancer mortality in people born during 2006–10 in 185 countries.
Methods
Study design
To analyse the impact of implementing a tobacco-free generation on lung-cancer mortality in this population-based birth-cohort simulation study, we proposed a scenario in which tobacco sales were banned for people born between Jan 1, 2006, and Dec 31, 2010, and in which this intervention was perfectly enforced. We quantified the number of preventable lung-cancer deaths in this birth cohort until Dec 31, 2095.
Population and procedures
To predict future lung-cancer mortality rates, we extracted lung-cancer mortality data (ICD-10 codes C33–34) by sex, 5-year age group (eg, 0–4 years, 5–9 years, 10–14 years, until age 85 years or older), and 5-year calendar period (eg, 2005–09, 2010–14, 2015–19) for countries with at least 15 years of data (ie, three 5-year calendar periods) from the WHO Mortality Database. For countries for which mortality data were not available, we extracted data on lung-cancer incidence from the Cancer Incidence in Five Continents database, which are collected via population-based cancer registries. Sufficient lung-cancer data over time were available for 82 countries (appendix pp 2–4). We obtained current and predicted population sizes from the UN World Population Prospects 2019 medium-fertility variant.12
We predicted sex-specific and age-specific lung-cancer rates up to 2040–44 for each of the 82 countries using the Nordpred software package in R version 4.3.0, which is based on an age-period cohort model.13 To improve our estimation, we calculated Nordpred predictions for a period of five blocks of 5 years, the first period being 2020–24 and the fifth being 2040–44. We incorporated an attenuation effect using drift parameters of 25% in the second prediction period (2025–29), 50% in the third prediction period (2030–34), and 75% in the fourth (2035–39) and fifth (2040–44) prediction periods (appendix pp 2–4).
From the predicted age-specific lung-cancer rates, we established lung-cancer rates for the cohort born between Jan 1, 2006, and Dec 31, 2010, using a Lexis diagram (appendix pp 5–7). Although cohort-specific rates up to 2040–44 were derived from the predicted rates, we assumed the rates remained constant from this block onwards, as the predictions become more uncertain after this time period.
Although data were available for 82 countries, we decided to expand our estimates to 185 countries to evaluate potential global benefits of the implementation of a tobacco-free generation across regions with different smoking prevalences. Thus, after deriving cohort-specific mortality rates, we calculated the ratio between lung-cancer rates in the birth cohort and the baseline period (ie, 2006–10) by age and sex for each of the 82 countries for which the prediction was done, to allow us to transform period rates to cohort rates for additional countries. We then applied this period/cohort ratio to lung-cancer mortality rates from the Global Cancer Observatory 2022 database, which was modelled and adjusted for missing data, to obtain birth-cohort rates by country and sex for 185 countries. In countries where data to predict cohort-specific lung-cancer rates were not available, a regional mean period/cohort ratio was applied. We derived the regional mean period/cohort ratio from countries that were in the same geographical region or had similar lung-cancer mortality rates in 2022 (appendix pp 8–14).
To calculate the number of lung-cancer deaths expected in the birth cohort as per business as usual, we constructed the population structure of this cohort using age-specific, sex-specific, 5-year-period-specific, and country-specific population sizes from Jan 1, 2006, to Dec 31, 2095, from the UN Population Prospects 2019 medium-fertility variant.12 At the end of the simulation, all age groups older than 85 years were included. We obtained expected lung-cancer deaths in the birth cohort by multiplying lung-cancer mortality rates by the size of the population.
To establish the number of lung-cancer deaths that could be prevented in the birth cohort if tobacco smoking was eliminated, we subtracted reported age-specific rate of deaths in people who had never smoked tobacco (hereafter referred to as never smokers) from a previous study14 from the expected rate of lung-cancer deaths in our birth cohort and applied this difference to the size of the population. We used the Cancer Prevention Study phase II15 for all countries and regions with a high lung-cancer rate (eg, Europe, North America, Australia, and New Zealand) and the Korean Cancer Prevention Study16 for all countries and regions with a low lung-cancer rate (eg, Asia [excluding female individuals from China and North Korea], Africa, and Latin America). We categorised countries with more than 35 deaths per 100 000 population as having high lung-cancer mortality rates. For female individuals from China and North Korea, in whom lung-cancer rates are much higher than for female individuals in other countries, we used lung-cancer incidence rates from four cities in China during 1983–87.14 The rates of never smokers used for each country are provided in the appendix (pp 15–17). We computed population impact fractions (PIFs), interpreted as the percentage of lung-cancer deaths that could be prevented by stopping the generation born during 2006–10 from tobacco smoking, by dividing the number of preventable lung-cancer deaths by the expected lung-cancer deaths in the birth cohort (appendix p 18).
Statistical analysis
The primary outcome was the impact on lung-cancer mortality of implementing a tobacco-free generation.
Prediction of lung-cancer mortality rates was done in R version 4.3.0 and the remaining statistical analyses were done in Stata version 17.0. Our results are presented in absolute numbers rounded to the nearest 100, proportions, and age-standardised rates (ASRs; Segi-Doll world standard)17, 18 by sex and 19 UN world regions.19 In addition, we aggregated expected and prevented deaths into the four World Bank income groups (ie, high-income, upper-middle-income, lower-middle-income, and low-income).20
We conducted two sensitivity analyses (appendix pp 19–24). In the first sensitivity analysis, we quantified a conservative reduction in lung-cancer mortality in a scenario in which the tobacco-free generation reduced lung-cancer mortality rates to 25% more than those of never smokers. We chose this scenario to assess what would happen if implementation of the tobacco-free generation was not fully achieved. We set this percentage at 25% on the basis of a study published by Ait Ouakrim and colleagues,9 in which they assumed that implementation of a tobacco-free generation would result in a 90% (95% uncertainty interval 78·5–97·4) reduction in rates of initiating tobacco smoking. In the second sensitivity analysis, we held lung-cancer mortality rates stable from 2022 onwards to assess whether the results of the primary analysis differed depending on the evolution of rates over time. Based on reported stabilisation of lung-cancer rates in several upper-middle-income countries, such as Brazil21, 22 and South Africa,23 we also assessed whether the results obtained for these two countries differed if we assumed that lung-cancer mortality rates remained stable after 2022. For this purpose, instead of using lung-cancer mortality rates for the birth cohort, we used lung-cancer mortality rates for the 2022 period from GLOBOCAN 2022 to calculate both prevented lung-cancer deaths and PIFs.
Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Results
Our birth cohort included all births occurring between Jan 1, 2006, and Dec 31, 2010, in 185 countries (appendix pp 3, 26–34), with a total population of 650 525 800 people. Of the 82 countries with sufficient lung-cancer data, 46 (56%) were high-income countries with mortality registries or cancer registries. In the remaining 103 (56%) of 185 countries, data to predict cohort-specific lung-cancer rates were not available.
Globally, we predicted that 2 951 400 lung-cancer deaths could occur in the population born during 2006–10 if lung-cancer rates continue to follow trends observed during the past 15 years. Of these deaths, 1 842 900 (62·4%) were predicted to occur in male individuals and 1 108 500 (37·6%) were expected to occur in female individuals (table 1). Asian regions were expected to have the highest number of lung-cancer deaths in both sexes, at 1 863 640 (63·1%) of the global total. Upper-middle-income countries were expected to have the highest number of lung-cancer deaths, at 1 482 200 (50·2%) of the global total (table 2).
Table 1.
Numbers, PIFs, and ASRs of expected and prevented lung-cancer deaths in male individuals, female individuals, and both sexes combined, by world region
|
Male individuals |
Female individuals |
Total |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Expected deaths | Prevented deaths | PIF | ASR of expected deaths | ASR of prevented deaths | Expected deaths | Prevented deaths | PIF | ASR of expected deaths | ASR of prevented deaths | Expected deaths | Prevented deaths | PIF | ASR of expected deaths | ASR of prevented deaths | ||
| Africa | ||||||||||||||||
| Eastern Africa | 32 600 | 1800 | 5·4% | 3·5 | 0·2 | 32 400 | 1300 | 4·0% | 2·6 | 0·1 | 65 000 | 3100 | 4·7% | 3·0 | 0·1 | |
| Middle Africa | 8600 | 180 | 2·1% | 2·8 | 0·1 | 6400 | 60 | 0·9% | 1·6 | 0·0 | 15 000 | 240 | 1·6% | 2·1 | 0·1 | |
| Northern Africa | 90 400 | 45 000 | 49·8% | 17·0 | 8·6 | 26 500 | 3300 | 12·6% | 4·1 | 0·2 | 116 900 | 48 400 | 41·4% | 10·4 | 4·3 | |
| Southern Africa | 14 300 | 6500 | 45·8% | 17·7 | 8·6 | 10 900 | 4600 | 42·1% | 8·1 | 2·9 | 25 200 | 11 100 | 44·2% | 12·3 | 5·5 | |
| Western Africa | 17 200 | 1100 | 6·3% | 2·7 | 0·2 | 10 000 | 320 | 3·2% | 1·5 | 0·1 | 27 200 | 1400 | 5·1% | 2·1 | 0·1 | |
| America | ||||||||||||||||
| Latin America and the Caribbean | 115 100 | 17 000 | 14·7% | 8·3 | 1·4 | 120 800 | 49 800 | 41·2% | 7·4 | 2·8 | 236 000 | 66 800 | 28·3% | 7·8 | 2·1 | |
| North America | 91 200 | 45 000 | 49·3% | 10·3 | 6·1 | 94 900 | 59 700 | 63·0% | 9·1 | 5·7 | 186 100 | 104 700 | 56·3% | 9·7 | 5·9 | |
| Asia | ||||||||||||||||
| Eastern Asia | 702 800 | 422 000 | 60·0% | 28·2 | 18·6 | 369 600 | 45 300 | 12·2% | 13·3 | 0·5 | 1 072 400 | 467 200 | 43·6% | 21·1 | 10·0 | |
| China* | 628 300 | 379 500 | 60·4% | 29·4 | 19·7 | 329 900 | 30 400 | 9·2% | 14·0 | 0·4 | 958 100 | 409 900 | 42·8% | 22·2 | 10·6 | |
| South central Asia | 251 700 | 15 000 | 5·9% | 7·1 | 0·6 | 122 300 | 9600 | 7·8% | 3·5 | 0·2 | 374 000 | 24 600 | 6·6% | 5·2 | 0·4 | |
| India* | 153 600 | 3100 | 2·0% | 6·5 | 0·4 | 81 500 | 1100 | 1·3% | 3·6 | 0·1 | 235 100 | 4200 | 1·8% | 5·1 | 0·3 | |
| Southeast Asia | 161 400 | 49 300 | 30·6% | 16·2 | 6·9 | 78 800 | 11 600 | 14·7% | 6·6 | 1·5 | 240 200 | 60 900 | 25·4% | 11·3 | 4·2 | |
| Western Asia | 134 337 | 86 375 | 64·3% | 21·4 | 14·0 | 42 702 | 18 372 | 43·0% | 7·3 | 3·3 | 177 040 | 104 747 | 59·2% | 14·3 | 8·6 | |
| Europe | ||||||||||||||||
| Central and eastern Europe | 65 800 | 48 900 | 74·3% | 22·6 | 18·4 | 34 000 | 19 900 | 58·4% | 7·9 | 4·7 | 99 800 | 68 700 | 68·9% | 14·9 | 11·2 | |
| Northern Europe | 30 700 | 18 500 | 60·3% | 13·1 | 9·0 | 35 600 | 26 400 | 74·2% | 11·8 | 8·5 | 66 200 | 44 900 | 67·8% | 12·5 | 8·8 | |
| Southern Europe | 51 200 | 37 000 | 72·3% | 18·7 | 14·5 | 40 700 | 29 400 | 72·2% | 13·7 | 10·3 | 91 900 | 66 400 | 72·2% | 16·2 | 12·4 | |
| Western Europe | 64 200 | 44 200 | 68·9% | 19·6 | 15·4 | 72 300 | 56 200 | 77·7% | 17·2 | 13·8 | 136 400 | 100 400 | 73·6% | 18·4 | 14·6 | |
| Oceania | ||||||||||||||||
| Australia and New Zealand | 9900 | 5600 | 56·2% | 13·0 | 8·8 | 9500 | 6100 | 63·8% | 10·5 | 7·1 | 19 400 | 11 600 | 60·0% | 11·7 | 7·9 | |
| Melanesia, Micronesia, and Polynesia | 1700 | 940 | 56·7% | 10·1 | 6·2 | 1200 | 500 | 42·6% | 5·7 | 2·6 | 2800 | 1400 | 50·8% | 7·9 | 4·3 | |
| World | 1 842 900 | 844 200 | 45·8% | 13·2 | 6·6 | 1 108 500 | 342 400 | 30·9% | 6·7 | 1·8 | 2 951 400 | 1 186 500 | 40·2% | 9·9 | 4·2 | |
ASRs are per 100 000 population. ASR=age-standardised rate. PIF=population impact fraction.
Data from China are included in eastern Asia and data from India are included in south central Asia.
Table 2.
Numbers, PIFs, and ASRs of expected and prevented lung-cancer deaths in male individuals, female individuals, and both sexes combined, by country-income group
|
Male individuals |
Female individuals |
Total |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Expected deaths | Prevented deaths | PIF | ASR of expected deaths | ASR of prevented deaths | Expected deaths | Prevented deaths | PIF | ASR of expected deaths | ASR of prevented deaths | Expected deaths | Prevented deaths | PIF | ASR of expected deaths | ASR of prevented deaths | |
| High-income | 356 000 | 201 900 | 56·7% | 13·4 | 8·4 | 321 600 | 212 300 | 66·0% | 10·5 | 6·9 | 677 600 | 414 100 | 61·1% | 11·9 | 7·7 |
| Upper-middle-income | 959 500 | 541 100 | 56·4% | 22·5 | 14·1 | 522 700 | 109 000 | 20·9% | 10·5 | 1·7 | 1 482 200 | 650 100 | 43·9% | 16·6 | 8·0 |
| Lower-middle-income | 457 600 | 89 900 | 19·6% | 8·7 | 2·2 | 205 200 | 15 000 | 7·3% | 3·8 | 0·4 | 662 800 | 104 900 | 15·8% | 6·2 | 1·3 |
| Low-income | 69 800 | 11 300 | 16·3% | 4·8 | 0·9 | 59 100 | 6100 | 10·3% | 3·0 | 0·3 | 128 900 | 17 400 | 13·5% | 3·8 | 0·6 |
| World | 1 842 900 | 844 200 | 45·8% | 13·2 | 6·6 | 1 108 500 | 342 400 | 30·9% | 6·7 | 1·8 | 2 951 400 | 1 186 500 | 40·2% | 9·9 | 4·2 |
Country-income group was derived from The World Bank. ASRs are per 100 000 population. ASR=age-standardised rate. PIF=population impact fraction.
We estimated that 1 186 500 (40·2%) of 2 951 400 lung-cancer deaths in people born during 2006–10 could be prevented if tobacco elimination (ie, a tobacco-free generation) was achieved. We estimated that more lung-cancer deaths could be prevented in male individuals (844 200 [45·8%] of 1 842 900 deaths) than in female individuals (342 400 [30·9%] of 1 108 500 deaths; table 1).
In both sexes, we estimated the impact of eliminating tobacco smoking measured via PIF to be greater than 50% in eight (47·1%) of 17 world regions (table 1), particularly in Europe. In male individuals, central and eastern Europe had the highest PIF (48 900 [74·3%] of 65 800 deaths) whereas in female individuals, western Europe had the highest PIF (56 200 [77·7%] of 72 300 deaths). Middle Africa was the region with the lowest PIF in both male individuals (180 [2·1%] of 8600 deaths) and female individuals (60 [0·9%] of 6400 deaths). Estimated PIFs for six regions (ie, western Africa, Latin America and the Caribbean, North America, northern Europe, western Europe, and Australia and New Zealand) were higher in female individuals than in male individuals (table 1; appendix p 25).
Eastern Asia (18·6 per 100 000 population) and central and eastern Europe (18·4 per 100 000 population) were the regions with the highest estimated ASRs of prevented lung-cancer deaths in male individuals. By contrast, eastern Africa (0·2 per 100 000 population), western Africa (0·2 per 100 000 population), and middle Africa (0·1 per 100 000 population) had the lowest ASRs in male individuals. Western Europe (13·8 per 100 000 population) and southern Europe (10·3 per 100 000 population) were the regions with the highest estimated ASRs of prevented lung-cancer deaths in female individuals. By contrast, eastern Africa (0·1 per 100 000 population), western Africa (0·1 per 100 000 population), and middle Africa (0·0 per 100 000 population) had the lowest ASRs in female individuals (table 1; appendix p 25).
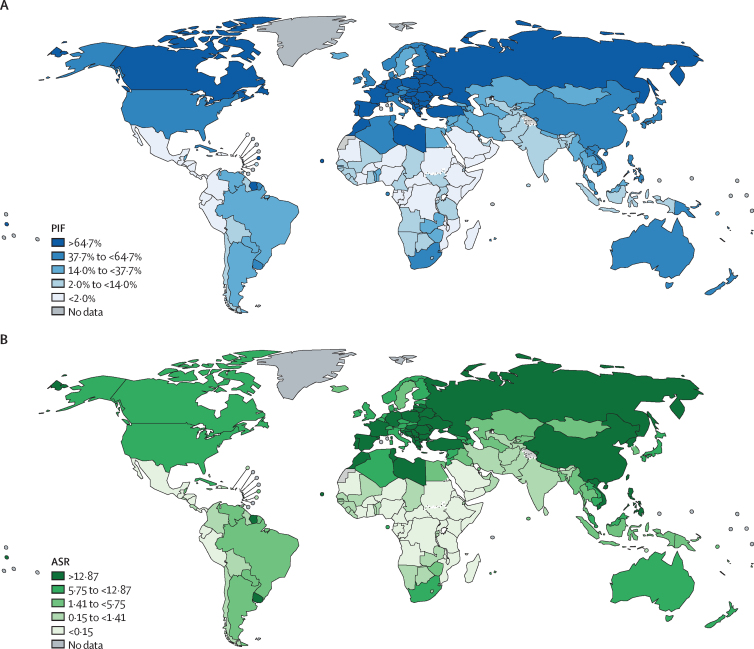
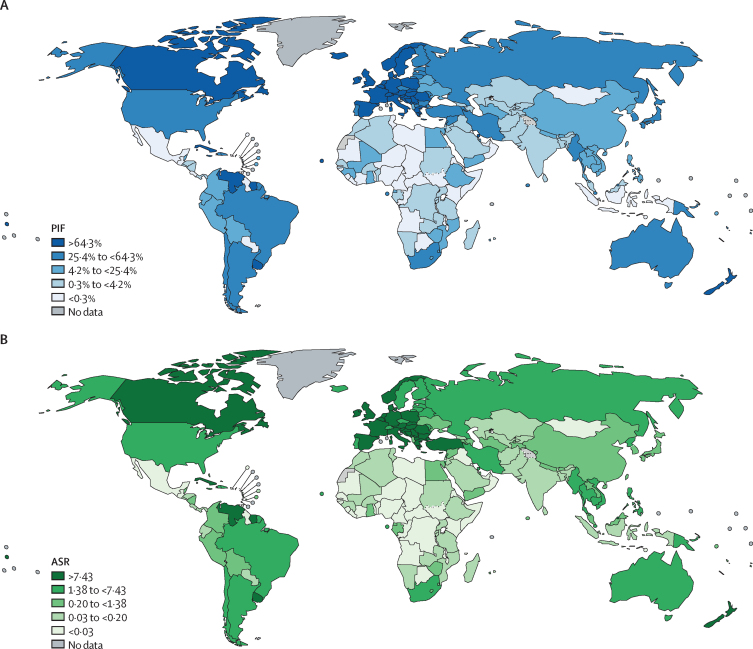
Countries with the highest estimated PIFs in male individuals were Cyprus (1100 [87·3%] of 1200 deaths), Belarus (2600 [82·5%] of 3100 deaths), and Greece (4900 [83·0%] of 5900 deaths) and in female individuals were Hungary (3000 [84·0%] of 3500 deaths), the Netherlands (6400 [82·6%] of 7800 deaths), and Canada (18 300 [81·5%] of 22 400 deaths). The highest estimated ASRs of prevented lung-cancer deaths in male individuals were in Türkiye (41·4 per 100 000 population), Cyprus (37·6 per 100 000 population), and French Polynesia (32·7 per 100 000 population) and in female individuals were in Hungary (17·4 per 100 000 population), France (16·9 per 100 000 population), and Serbia (15·7 per 100 000 population; Figure 1, Figure 2; appendix pp 26–34).
Figure 1.
Percentages and ASRs of prevented lung-cancer deaths in male individuals born 2006–10, by country
ASRs are per 100 000 population. ASR=age-standardised rate. PIF=population impact fraction.
Figure 2.
Percentages and ASRs of prevented lung-cancer deaths in female individuals born 2006–10, by country
ASRs are per 100 000 population. ASR=age-standardised rate. PIF=population impact fraction.
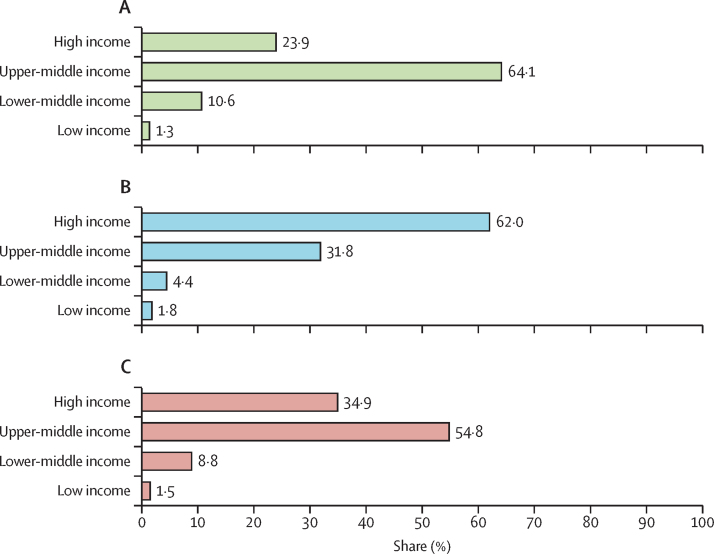
In both sexes combined and each sex separately, PIF was estimated to increase by World Bank country-income group. In both sexes combined, PIF was 17 400 (13·5%) of 128 900 deaths in low-income countries, 104 900 (15·8%) of 662 800 deaths in lower-middle-income countries, 650 100 (43·9%) of 1 482 200 deaths in upper-middle-income countries, and 414 100 (61·1%) of 677 600 deaths in high-income countries. A similar gradient by country-income group was observed for the estimated ASR of prevented lung-cancer deaths in female individuals, with the highest ASR in high-income countries (6·9 per 100 000 population). In male individuals, however, the highest estimated ASR of prevented lung-cancer deaths was in upper-middle-income countries (14·1 per 100 000 population; table 2). Between the four income groups, the largest proportion of prevented lung-cancer deaths in male individuals was estimated to occur in upper-middle-income countries (541 100 [64·1%] of 844 200 deaths), whereas in female individuals it was in high-income countries (212 300 [62·0%] of 342 400 deaths; figure 3).
Figure 3.
Percentage of prevented lung-cancer deaths as a proportion of total prevented deaths in the 2006–10 birth cohort, by country-income group
(A) Male individuals. (B) Female individuals. (C) Total.
In sensitivity analyses, assuming that the tobacco-free generation intervention would reduce lung-cancer mortality rates to 25% more than that of never smokers, we estimated that 961 500 (32·6%) of 2 951 400 total lung-cancer deaths could be prevented. 696 800 (37·8%) of 1 842 900 lung-cancer deaths could be prevented in male individuals and 264 700 (23·9%) of 1 108 500 lung-cancer deaths could be prevented in female individuals. By world region, the smallest difference between PIFs obtained in this scenario and the main analysis was in middle Africa, with an absolute decrease of 0·5 percentage points in both sexes combined. The largest difference between PIFs was observed in southern Africa, with an absolute decrease of 12·7 percentage points in both sexes combined. When assuming that lung-cancer mortality rates remained stable after 2022, there were large differences by sex across world regions (appendix pp 19–24).
Discussion
We estimated that 1 186 500 (40·2%) of 2 951 400 lung-cancer deaths in people born during 2006–10 could be prevented globally if tobacco smoking was eliminated in this cohort. The estimated impact of a tobacco-free generation on lung-cancer deaths varied by sex, country, world region, and country-income group.
Globally, we estimated that elimination of tobacco smoking in the evaluated birth cohort could prevent a greater number and proportion of lung-cancer deaths in male individuals than in female individuals, likely related to higher prevalence and earlier onset of smoking in male individuals.24 The disparities in our results by sex were particularly important in southern Africa, northern Africa, eastern Asia, southeast Asia, and western Asia, where the proportion of prevented lung-cancer deaths in male individuals was estimated to be 20 percentage points higher than in female individuals. In countries in these regions, smoking prevalence was more than 30–40% in male individuals in 1970 and around 20–40% in 2020, compared with smoking prevalence far less than 10% in female individuals during the same period.24 However, lobbying from the tobacco industry in several countries in these regions is strong25 and smoking prevalence could increase in the near future. Therefore, our estimated impact of a tobacco-free generation could be an underestimate.25 By contrast, in North America, Australia and New Zealand, northern Europe, and western Europe, we estimated that a larger number of lung-cancer deaths could be prevented in female individuals than in male individuals. In these regions, recognition of poor health outcomes as a result of tobacco use started earlier than in other regions.24, 26, 27, 28
A previous study estimated that 80% of lung-cancer deaths are caused by tobacco smoking in high-income countries,29 although this percentage reduced to 64% globally.30 The percentage of lung-cancer deaths attributed to tobacco use in high-income countries has declined during 2010–19, whereas this percentage increased in different regions of Africa during 2010–19.30, 31 The decline in high-income countries is likely to continue in the coming years due to declining smoking prevalence2 and improved lung-cancer diagnostic techniques and survival after diagnosis.32
The deaths that we estimated could not be prevented could be due to other risk factors associated with lung cancer. For example, the GBD 2019 Respiratory Tract Cancers Collaborators30 found that lung-cancer mortality attributed to air pollution and exposure to second-hand smoke decreased during 2010–19 in countries with high sociodemographic index and increased in countries with low sociodemographic index. These diverging trends could have influenced our estimated lung-cancer deaths.
Although we estimated that high-income countries had a larger proportion of prevented lung-cancer deaths, our study also showed that, in terms of number of prevented lung-cancer deaths, the largest contribution came from low-income or middle-income countries (LMICs). LMICs generally have younger populations than high-income countries, so the relative size of our 2006–10 cohort was larger. Furthermore, although youth smoking prevalence has decreased in many high-income countries, it remains high in many LMICs.3 Therefore, measures, such as the tobacco-free generation, that aim to eliminate smoking uptake could have the greatest impact in LMICs.3, 33 Although there has been progress in the implementation of tobacco-control measures since 2020 in LMICs, implementation remains higher in high-income countries.3 Therefore, the long delay in implementation of tobacco-control measures and increasing smoking prevalence in many LMICs24 suggest that LMICs should remain an important focus for global tobacco-control efforts.
The implementation of a tobacco-free generation could be more successful in countries with strong tobacco-control policies, where smoking prevalence is less than 15%, or where there has been a rapid decline in prevalence.34 The implementation of a tobacco-free generation needs to occur alongside other tobacco-control measures, such as setting a nicotine cap, increasing taxes, or reducing the number of retail outlets that sell tobacco products.9, 35, 36 However, the introduction of a tobacco-free generation alone would take many years to have an effect on smoking prevalence9, 36 and, consequently, on lung-cancer mortality rates. Moreover, it is a measure that focuses on preventing initiation of smoking and would not affect current smokers. Therefore, a tobacco-free generation and other measures proposed in the tobacco-endgame strategy should be implemented in conjunction with policies that are effective, such as increased taxes, smoke-free environments, and smoking-cessation support.37, 38 There is still a need to focus on current smokers and smoking cessation.
Our study has multiple strengths, including being, to our knowledge, the first to provide estimates of the impact of a tobacco-free generation on global lung-cancer mortality. Furthermore, we used lung-cancer mortality data from population-based registries, where available.
Our study has multiple limitations. We did not formally consider the multitude of factors that can influence the implementation of tobacco-control programmes, such as the black market or compliance. Other measures based on early smoking bans, such as increasing the minimum legal age of tobacco use, showed variation in compliance between 62% and 94%.39, 40 In our sensitivity analysis, we estimated that if the effectiveness of smoking-control activities was reduced by 25%, the impact of the tobacco-free generation would decrease by between 1 percentage point and 13 percentage points, depending on the region. Furthermore, we mainly studied impacts on the younger generation, for whom the effects of a tobacco-free generation on lung-cancer mortality would only materialise in five or six decades, when rates of lung cancer increase. As such, multiple interventions targeting different cohorts and subsets of populations are key to reducing lung-cancer mortality in the near future. Trends in tobacco smoking and therefore lung cancer might also change due to evolving tobacco and nicotine products, such as the use of non-combustible devices (eg, electronic cigarettes), which has increased in the young population. This development was not captured in our study as the product was introduced to the market only 20 years ago and its impact on lung-cancer mortality is unclear.
We could only estimate lung-cancer prevalence and mortality with local data in 82 countries. For the remaining 103, we extrapolated predictions on the basis of geographical location and lung-cancer burden, which might have led to overestimation or underestimation. Most recorded data came from high-income countries with good health-information systems. Data from low-income countries were scarce and our estimates should be interpreted with caution. We used an established method to estimate long-term cancer trends, but changes in rates during 2010–19 might not be captured in our prediction. One of our sensitivity analyses, with an alternative assumption in which lung-cancer mortality rates remained constant in all regions, showed the importance of including recent data. Furthermore, we assumed that lung-cancer rates would remain constant from 2044 onwards as prediction became increasingly uncertain beyond this period.13 Thus, we could have underestimated expected lung-cancer deaths in countries where the lung-cancer mortality rate could have an upward trend and overestimated them in countries that could have a downward trend.
Another limitation is the scarce data on lung-cancer rates among never smokers, which were only available for the USA, South Korea, and China. The use of lung-cancer mortality rates in never smokers from before the 2000s could influence our estimates, as rates might have changed by 2022—the year in which much of the data used in our calculations was obtained—due to improvements in lung-cancer tumour-detection techniques or changes in exposure to other lung-cancer risk factors. Furthermore, by using lung-cancer mortality rates in never smokers, we did not consider deaths linked to second-hand smoke. If we had included these deaths, the number of preventable lung-cancer deaths due to the tobacco-free generation could be higher.
In summary, we estimated that more than 1·1 million lung-cancer deaths in 185 countries could be prevented in one 5-year birth cohort if smoking uptake was eliminated. Europe, North America, and Australia and New Zealand were the regions where more lung-cancer deaths could be prevented by strengthening tobacco-control measures to achieve a tobacco-free generation. However, the impact of tobacco smoking on lung-cancer mortality remains high in many countries and will increase in others. Therefore, the implementation of tobacco-control measures to help reduce the prevalence of tobacco smoking, and its impact, is imperative. Tobacco-control measures, including a tobacco-free generation, should be used in conjunction to achieve the tobacco endgame.
Contributors
Data sharing
All cancer incidence, mortality, and population estimates are available from the Global Cancer Observatory (https://gco.iarc.fr/en).
Declaration of interests
JRB received funding from the Ministry of Universities of Spain for university professorship training (FPU20/00926 and EST23/00725). RE received payments for contribution to the International Tobacco Control Project and the CENIC study (funded by the US National Institutes of Health); received support for conference attendance from the Centre of Research Excellence on Achieving the Tobacco Endgame (funded by the Australian National Health and Medical Research Council) and Hapai te Hauora; received payments for invited presentations at international scientific meetings from the Ontario Public Health Association, the Korean Society for Research on Nicotine and Tobacco, the Malaysian Public Health Physicians’ Association, and the Kedah State Health Department; received payments for Deputy Editor services from Society of Research on Nicotine and Tobacco; received payments for a paper published in Tobacco Control Anniversary Edition 2022 from BMJ Publishing; received payments for writing an Editorial from The Lancet; and has been awarded research funding (payment made to his institution) from the Health Research Council New Zealand, the University of Otago, and the Cancer Society of New Zealand. PCRPC received payments for developing a report on heated tobacco products from Aliança de Promoção da Saúde and received payments for a lecture at a meeting from Boehringer Ingelheim Brazil. MP-R received funding from a competitive grant from the Spanish Society of Pneumology and Thoracic Surgery. All other authors declare no competing interests.
Acknowledgments
This work was funded by the Spanish Society of Pneumology and Thoracic Surgery (reference 1426/23).
Editorial note: The Lancet Group takes a neutral position with respect to territorial claims in published maps.
Acknowledgments
HR and IS conceptualised the study. JRB, HR, and IS designed the study and analysis and interpreted the results. JRB and HR collected, accessed, and verified the data. JRB wrote the first draft of the manuscript. All authors reviewed the manuscript, had full access to all the data in the study, and had final responsibility for the decision to submit for publication.
Supplementary Material
References
- 1.Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.GBD 2019 Tobacco Collaborators Spatial, temporal, and demographic patterns in prevalence of smoking tobacco use and attributable disease burden in 204 countries and territories, 1990–2019: a systematic analysis from the Global Burden of Disease Study 2019. Lancet. 2021;397:2337–2360. doi: 10.1016/S0140-6736(21)01169-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.WHO WHO report on the global tobacco epidemic, 2023. 2023. https://iris.who.int/bitstream/handle/10665/372043/9789240077164-eng.pdf?sequence=1
- 4.Puljević C, Morphett K, Hefler M, et al. Closing the gaps in tobacco endgame evidence: a scoping review. Tob Control. 2022;31:365–375. doi: 10.1136/tobaccocontrol-2021-056579. [DOI] [PubMed] [Google Scholar]
- 5.McCall C. A smoke-free generation: New Zealand's tobacco ban. Lancet. 2022;399:1930–1931. doi: 10.1016/S0140-6736(22)00925-4. [DOI] [PubMed] [Google Scholar]
- 6.WHO Creating a smoke-free city—Balanga City, the Philippines. Nov 29, 2021. https://www.who.int/news-room/feature-stories/detail/creating-a-smoke-free-city-balanga-city-the-philippines
- 7.Berrick J, Bostic C, Chou M, et al. Brookline introduces Tobacco-Free Generation law. Jan 29, 2022. https://blogs.bmj.com/tc/2022/01/29/brookline-introduces-tobacco-free-generation-law/
- 8.Department of Health and Social Care Stopping the start: our new plan to create a smokefree generation. Nov 8, 2023. https://www.gov.uk/government/publications/stopping-the-start-our-new-plan-to-create-a-smokefree-generation
- 9.Ait Ouakrim D, Wilson T, Waa A, et al. Tobacco endgame intervention impacts on health gains and Māori:non-Māori health inequity: a simulation study of the Aotearoa/New Zealand Tobacco Action Plan. Tob Control. 2023 doi: 10.1136/tc-2022-057655. published online Jan 10. [DOI] [PubMed] [Google Scholar]
- 10.Singh A, Petrović-van der Deen FS, Carvalho N, Lopez AD, Blakely T. Impact of tax and tobacco-free generation on health-adjusted life years in the Solomon Islands: a multistate life table simulation. Tob Control. 2020;29:388–397. doi: 10.1136/tobaccocontrol-2018-054861. [DOI] [PubMed] [Google Scholar]
- 11.van der Deen FS, Wilson N, Cleghorn CL, et al. Impact of five tobacco endgame strategies on future smoking prevalence, population health and health system costs: two modelling studies to inform the tobacco endgame. Tob Control. 2018;27:278–286. doi: 10.1136/tobaccocontrol-2016-053585. [DOI] [PubMed] [Google Scholar]
- 12.UN Department of Economics and Social Affairs World population prospects 2019. 2019. https://population.un.org/wpp/Publications/Files/WPP2019_Highlights.pdf
- 13.Møller B, Fekjaer H, Hakulinen T, et al. Prediction of cancer incidence in the Nordic countries: empirical comparison of different approaches. Stat Med. 2003;22:2751–2766. doi: 10.1002/sim.1481. [DOI] [PubMed] [Google Scholar]
- 14.Thun MJ, Hannan LM, Adams-Campbell LL, et al. Lung cancer occurrence in never-smokers: an analysis of 13 cohorts and 22 cancer registry studies. PLoS Med. 2008;5:e185. doi: 10.1371/journal.pmed.0050185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thun MJ, Henley SJ, Burns D, Jemal A, Shanks TG, Calle EE. Lung cancer death rates in lifelong nonsmokers. J Natl Cancer Inst. 2006;98:691–699. doi: 10.1093/jnci/djj187. [DOI] [PubMed] [Google Scholar]
- 16.Jee SH, Sull JW, Park J, et al. Body-mass index and mortality in Korean men and women. N Engl J Med. 2006;355:779–787. doi: 10.1056/NEJMoa054017. [DOI] [PubMed] [Google Scholar]
- 17.Doll R, Payne P, Waterhouse J. Springer; Berlin, Germany: 1966. Cancer incidence in five continents: a technical report. [Google Scholar]
- 18.Segi M, Fujisaku S. Department of Public Health, Tohoku University School of Medicine; Sendai, Japan: 1960. Cancer mortality for selected sites in 24 countries. [Google Scholar]
- 19.UN Statistics Division Standard country or area codes for statistical use (M49) 2021. https://unstats.un.org/unsd/methodology/m49/
- 20.World Bank Data Team New country classifications by income level: 2019–2020. 2019. https://blogs.worldbank.org/opendata/new-country-classifications-income-level-2019-2020
- 21.de Lima Costa SN, Gomez de Morais Fernandes FC, Dos Santos CA, Bezerra de Souza DL, Barbosa IR. Gender and regional differences in lung cancer mortality in Brazil. Asian Pac J Cancer Prev. 2020;21:919–926. doi: 10.31557/APJCP.2020.21.4.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fernandes GA, dos Santos Menezes F, Silva LF, Antunes JLF, Toporcov TN. Inequalities in lung cancer mortality trends in Brazil, 2000–2015. Sci Rep. 2020;10 doi: 10.1038/s41598-020-76165-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Winkler V, Mangolo NJ, Becher H. Lung cancer in South Africa: a forecast to 2025 based on smoking prevalence data. BMJ Open. 2015;5 doi: 10.1136/bmjopen-2014-006993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dai X, Gakidou E, Lopez AD. Evolution of the global smoking epidemic over the past half century: strengthening the evidence base for policy action. Tob Control. 2022;31:129–137. doi: 10.1136/tobaccocontrol-2021-056535. [DOI] [PubMed] [Google Scholar]
- 25.Amul GGH, Tan GPP, van der Eijk Y. A systematic review of tobacco industry tactics in southeast Asia: lessons for other low- and middle-income regions. Int J Health Policy Manag. 2021;10:324–337. doi: 10.34172/ijhpm.2020.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Islami F, Torre LA, Jemal A. Global trends of lung cancer mortality and smoking prevalence. Transl Lung Cancer Res. 2015;4:327–338. doi: 10.3978/j.issn.2218-6751.2015.08.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Banks E, Joshy G, Weber MF, et al. Tobacco smoking and all-cause mortality in a large Australian cohort study: findings from a mature epidemic with current low smoking prevalence. BMC Med. 2015;13:38. doi: 10.1186/s12916-015-0281-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Janssen F, El Gewily S, Bardoutsos A. Smoking epidemic in Europe in the 21st century. Tob Control. 2021;30:523–529. doi: 10.1136/tobaccocontrol-2020-055658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kulhánová I, Forman D, Vignat J, et al. Tobacco-related cancers in Europe: the scale of the epidemic in 2018. Eur J Cancer. 2020;139:27–36. doi: 10.1016/j.ejca.2020.07.024. [DOI] [PubMed] [Google Scholar]
- 30.GBD 2019 Respiratory Tract Cancers Collaborators Global, regional, and national burden of respiratory tract cancers and associated risk factors from 1990 to 2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Respir Med. 2021;9:1030–1049. doi: 10.1016/S2213-2600(21)00164-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shiels MS, Graubard BI, McNeel TS, Kahle L, Freedman ND. Trends in smoking-attributable and smoking-unrelated lung cancer death rates in the United States, 1991–2018. J Natl Cancer Inst. 2024;116:711–716. doi: 10.1093/jnci/djad256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shiels MS, Lipkowitz S, Campos NG, et al. Opportunities for achieving the cancer moonshot goal of a 50% reduction in cancer mortality by 2047. Cancer Discov. 2023;13:1084–1099. doi: 10.1158/2159-8290.CD-23-0208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ma C, Xi B, Li Z, et al. Prevalence and trends in tobacco use among adolescents aged 13–15 years in 143 countries, 1999–2018: findings from the Global Youth Tobacco Surveys. Lancet Child Adolesc Health. 2021;5:245–255. doi: 10.1016/S2352-4642(20)30390-4. [DOI] [PubMed] [Google Scholar]
- 34.Thomson G, Edwards R, Wilson N, Blakely T. What are the elements of the tobacco endgame? Tob Control. 2012;21:293–295. doi: 10.1136/tc.2010.040881. [DOI] [PubMed] [Google Scholar]
- 35.Edwards R, Hoek J, Waa A. Smokefree Aotearoa—world-leading developments with profound public health implications. 2023. https://www.phcc.org.nz/briefing/smokefree-aotearoa-world-leading-developments-profound-public-health-implications
- 36.Zeng Z, Cook AR, van der Eijk Y. What measures are needed to achieve a tobacco endgame target? A Singapore-based simulation study. Tob Control. 2023 doi: 10.1136/tc-2022-057856. published online June 6. [DOI] [PubMed] [Google Scholar]
- 37.Gravely S, Giovino GA, Craig L, et al. Implementation of key demand-reduction measures of the WHO Framework Convention on Tobacco Control and change in smoking prevalence in 126 countries: an association study. Lancet Public Health. 2017;2:e166–e174. doi: 10.1016/S2468-2667(17)30045-2. [DOI] [PubMed] [Google Scholar]
- 38.Ngo A, Cheng KW, Chaloupka FJ, Shang C. The effect of MPOWER scores on cigarette smoking prevalence and consumption. Prev Med. 2017;105S:S10–S14. doi: 10.1016/j.ypmed.2017.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang X, Vuong TD, Andersen-Rodgers E, Roeseler A. Evaluation of California's ‘Tobacco 21’ law. Tob Control. 2018;27:656–662. doi: 10.1136/tobaccocontrol-2017-054088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Silver D, Macinko J, Giorgio M, Bae JY, Jimenez G. Retailer compliance with tobacco control laws in New York City before and after raising the minimum legal purchase age to 21. Tob Control. 2016;25:624–627. doi: 10.1136/tobaccocontrol-2015-052547. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All cancer incidence, mortality, and population estimates are available from the Global Cancer Observatory (https://gco.iarc.fr/en).