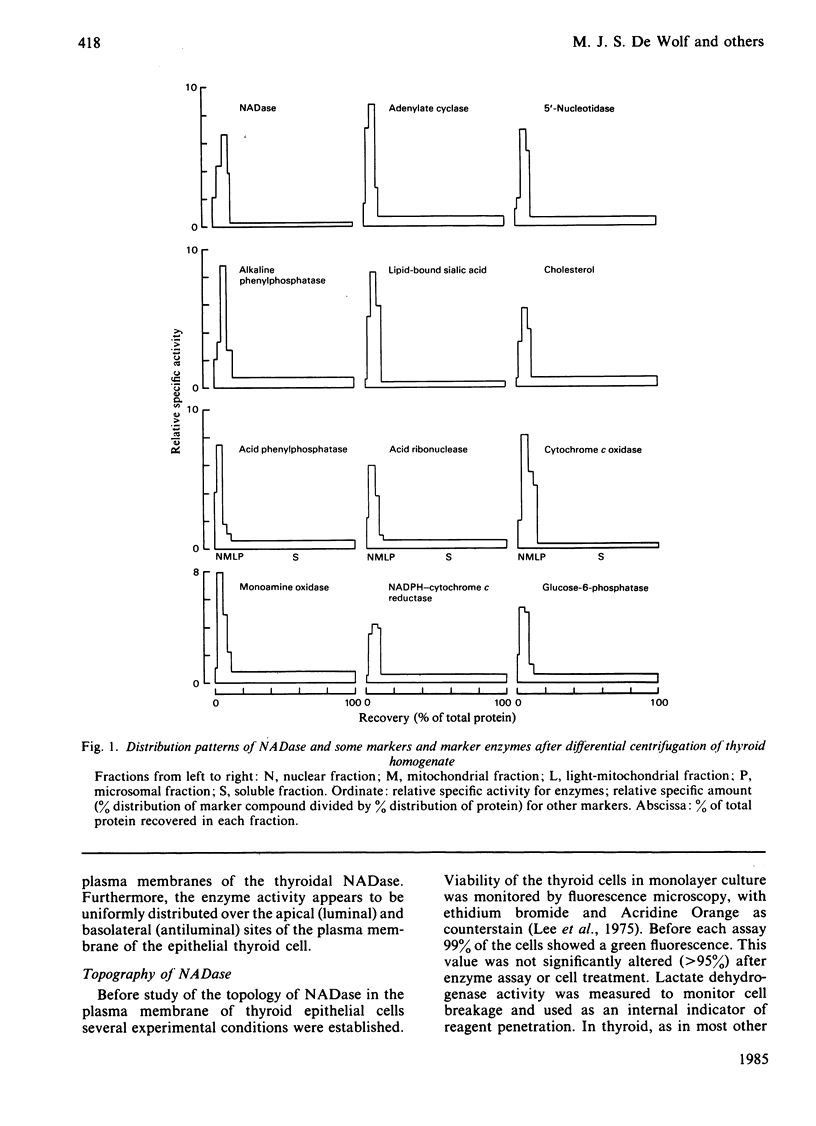
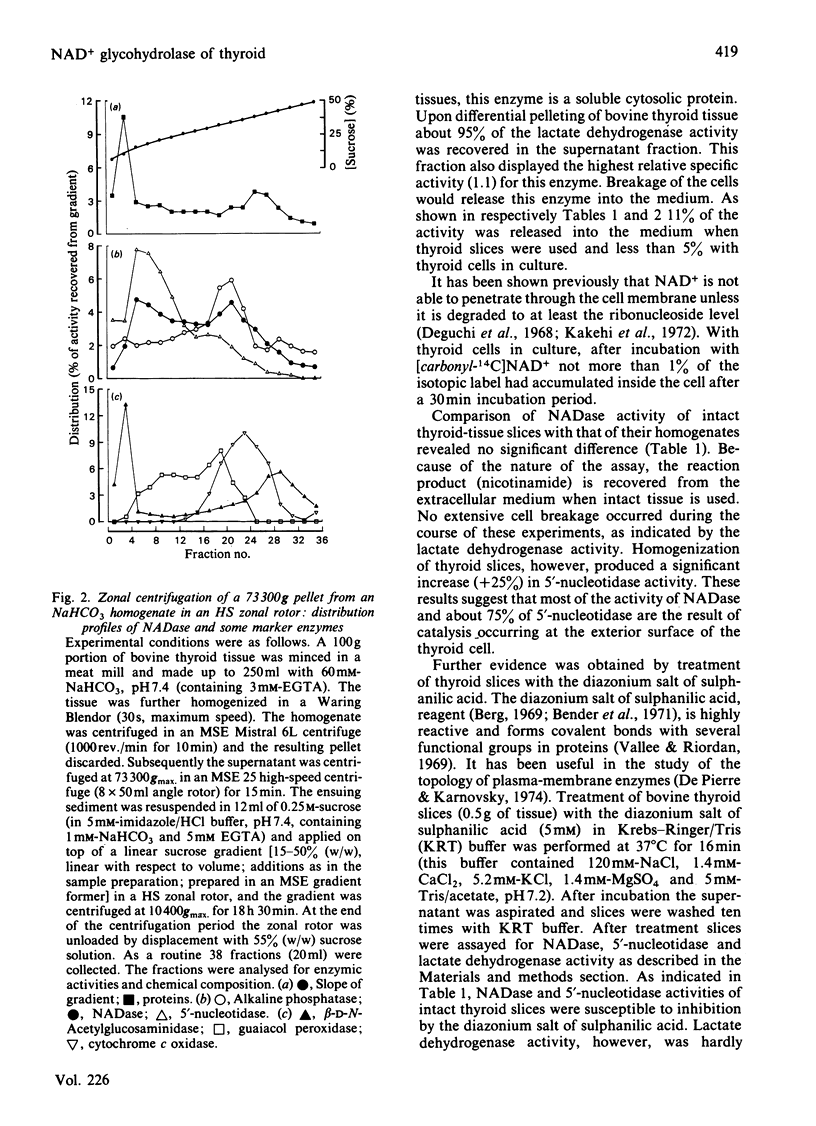
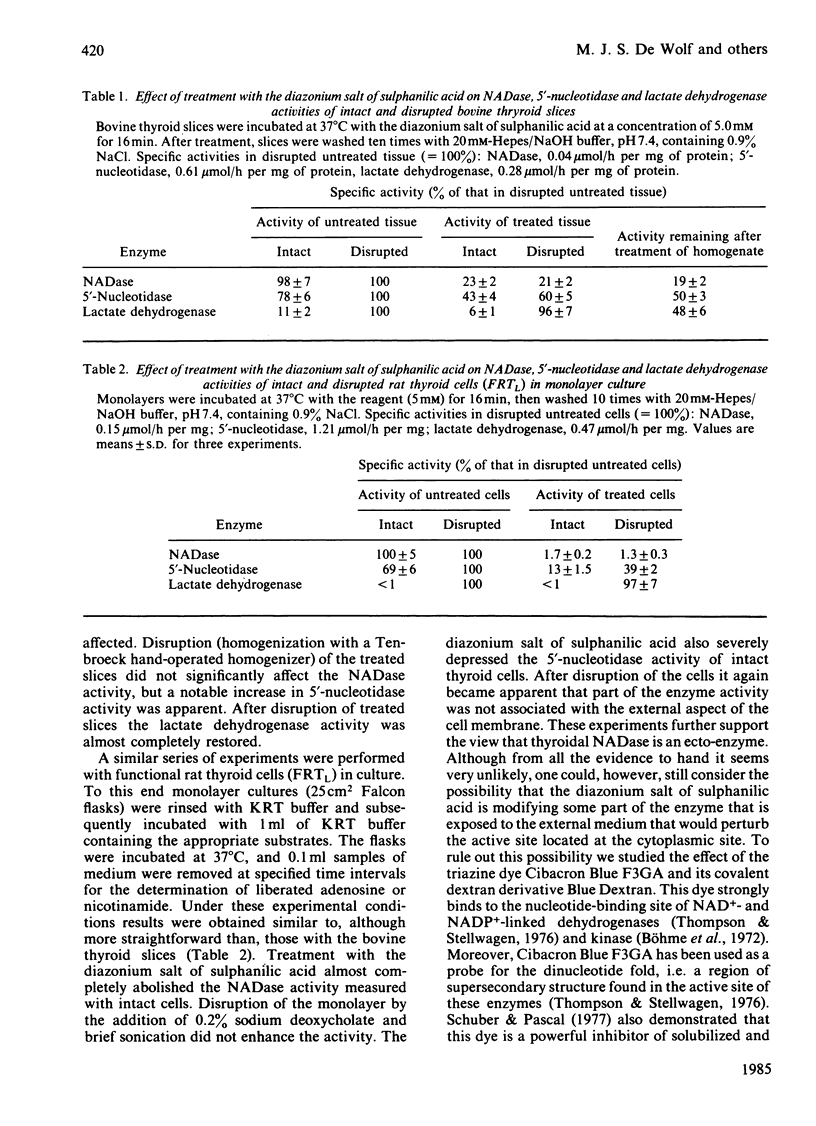
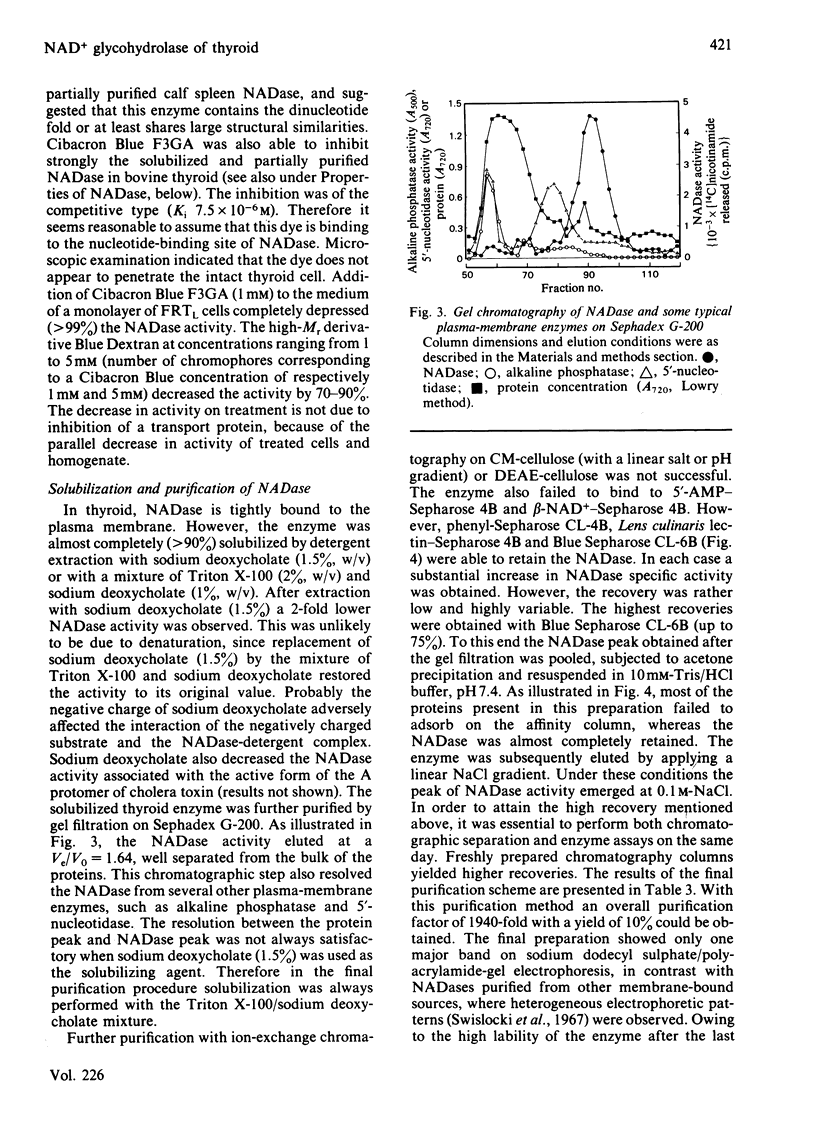
Abstract
Subcellular fractionation of bovine thyroid tissue by differential pelleting and isopycnic gradient centrifugation in a zonal rotor indicated that NAD+ glycohydrolase is predominantly located and rather uniformly distributed in the plasma membrane. Comparison of NAD+ glycohydrolase activities of intact thyroid tissue slices, functional rat thyroid cells in culture (FRTl) and their respective homogenates indicated that most if not all of the enzyme (catalytic site) is accessible to extracellular NAD+. The reaction product nicotinamide was predominantly recovered from the extracellular medium. The diazonium salt of sulphanilic acid, not penetrating into intact cells, was able to decrease the activity of intact thyroid tissue slices to the same extent as in the homogenate. Under the same conditions this reagent almost completely abolished NAD+ glycohydrolase activity associated with intact thyroid cells in culture. The triazine dye Cibacron Blue F3GA and its high-Mr derivative Blue Dextran respectively completely eliminated or caused a severe depression in the NAD+ glycohydrolase activity of FRTl cells. The enzyme could be readily solubilized from bovine thyroid membranes by detergent extraction, and was further purified by gel filtration and affinity chromatography on Blue Sepharose CL-6B. The overall procedure resulted in a 1940-fold purification (specific activity 77.6μmol of nicotinamide released/h per mg). The purified enzyme displays a Km of 0.40mm for β-NAD+, a broad pH optimum around pH7.2 (0.1 m-potassium phosphate buffer) and an apparent Mr of 120000. Nicotinamide is an inhibitor (Ki 1.9mm) of the non-competitive type. The second reaction product ADP-ribose acts as a competitive inhibitor (Ki 2.7mm). The purified enzyme splits β-NAD+, β-NADP+, β-NADH and α-NAD+ at rates in the relative proportions 1:0.75:<0.02:<0.02 and exhibits transglycosidase (pyridine-base exchange) activity. Anionic phospholipids such as phosphatidylinositol and phosphatidylserine inhibit the partially purified enzyme. A stimulating effect was observed upon the addition of histones.
Full text
PDF


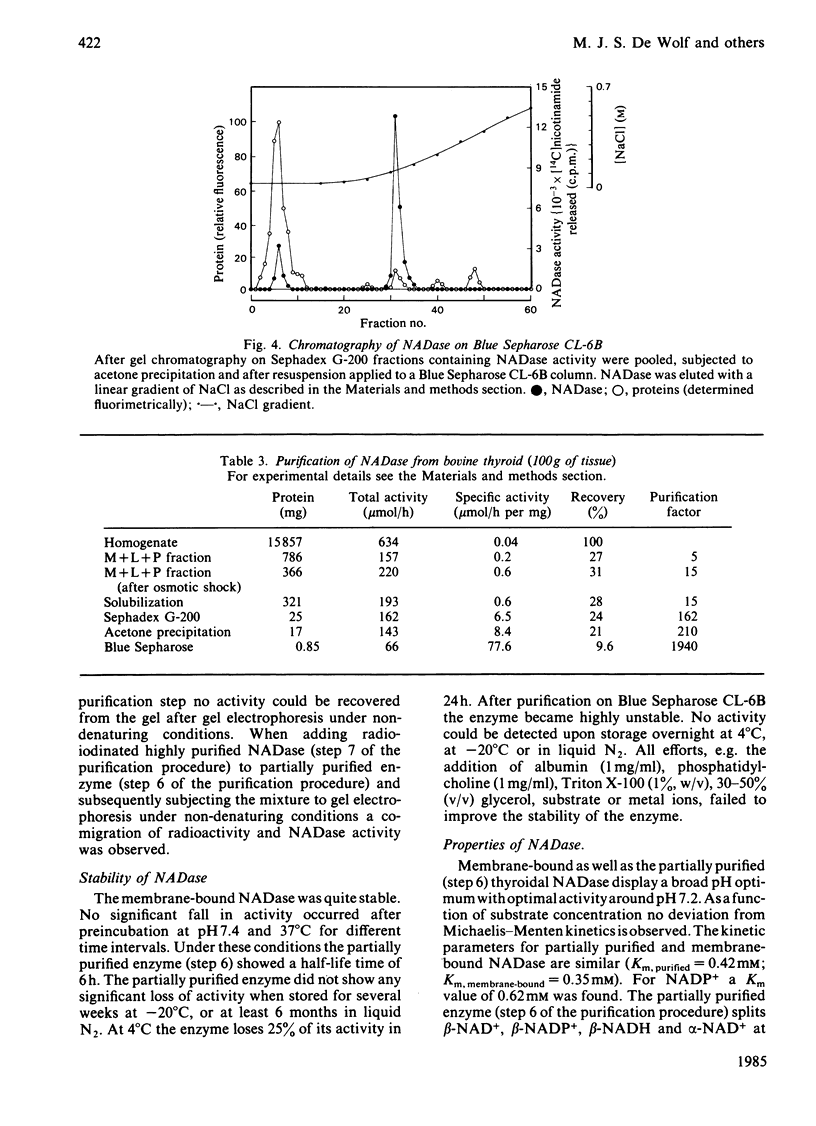
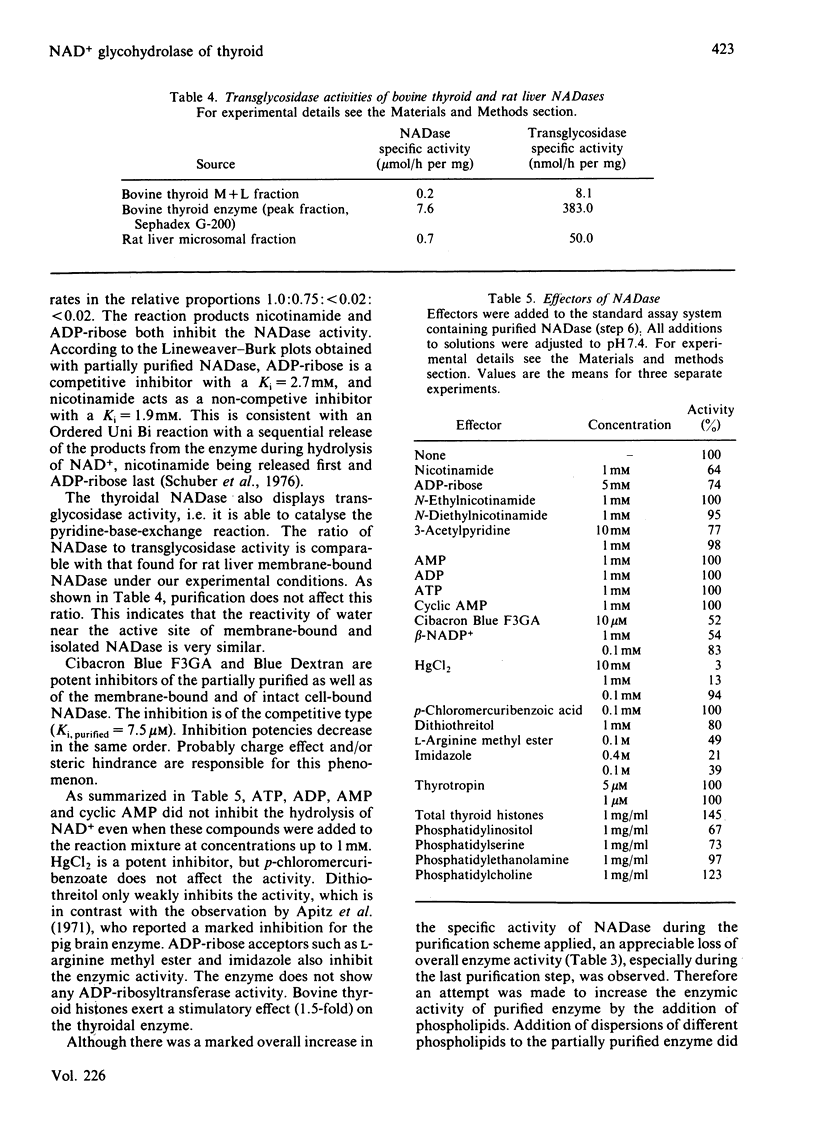




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alivisatos S. G., Ungar F., Gerber M., Arora R., Levitt L. P., Tabakoff B. Cellular distribution of nicotinamide adenine dinucleotide glycohydrolase in the central nervous system. Biochem Pharmacol. 1974 Jul 15;23(14):2060–2062. doi: 10.1016/0006-2952(74)90265-2. [DOI] [PubMed] [Google Scholar]
- Amar-Costesec A., Beaufay H. The association of NAD glycohydrolase with the plasma membrane in rat liver [proceedings]. Arch Int Physiol Biochim. 1977 Dec;85(5):949–950. [PubMed] [Google Scholar]
- Ambesi-Impiombato F. S., Parks L. A., Coon H. G. Culture of hormone-dependent functional epithelial cells from rat thyroids. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3455–3459. doi: 10.1073/pnas.77.6.3455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apitz R., Mickelson K., Shriver K., Cordes E. H. Some properties of reactions catalyzed by pig brain NAD glycohydrolase. Arch Biochem Biophys. 1971 Apr;143(2):359–364. doi: 10.1016/0003-9861(71)90222-0. [DOI] [PubMed] [Google Scholar]
- Artman M., Seeley R. J. Nicotinamide adenine dinucleotide splitting enzyme: a plasma membrane protein of murine macrophages. Arch Biochem Biophys. 1979 Jun;195(1):121–127. doi: 10.1016/0003-9861(79)90333-3. [DOI] [PubMed] [Google Scholar]
- Artman M., Seeley R. J. Nicotinamide adenine dinucleotide-splitting enzyme in normal, elicited and activated peritoneal macrophages of the mouse. J Reticuloendothel Soc. 1979 May;25(5):479–487. [PubMed] [Google Scholar]
- Bender W. W., Garan H., Berg H. C. Proteins of the human erythrocyte membrane as modified by pronase. J Mol Biol. 1971 Jun 28;58(3):783–797. doi: 10.1016/0022-2836(71)90040-4. [DOI] [PubMed] [Google Scholar]
- Berg H. C. Sulfanilic acid diazonium salt: a label for the outside of the human erythrocyte membrane. Biochim Biophys Acta. 1969 Jun 3;183(1):65–78. doi: 10.1016/0005-2736(69)90130-8. [DOI] [PubMed] [Google Scholar]
- Bock K. W., Gäng V., Beer H. P., Kronau R., Grunicke H. Localization and regulation of two NAD nucleosidases in Ehrlich ascites cells. Eur J Biochem. 1968 Apr;4(3):357–363. doi: 10.1111/j.1432-1033.1968.tb00219.x. [DOI] [PubMed] [Google Scholar]
- Bock K. W., Siekevitz P., Palade G. E. Localization and turnover studies of membrane nicotinamide adenine dinucleotide glycohydrolase in rat liver. J Biol Chem. 1971 Jan 10;246(1):188–195. [PubMed] [Google Scholar]
- Böhme H. J., Kopperschläger G., Schulz J., Hofmann E. Affinity chromatography of phosphofructokinase using Cibacron blue F3G-A. J Chromatogr. 1972 Jun 28;69(1):209–214. doi: 10.1016/s0021-9673(00)83103-9. [DOI] [PubMed] [Google Scholar]
- Clark J. B., Pinder S. Control of the steady-state concentrations of the nicotinamide nucleotides in rat liver. Biochem J. 1969 Sep;114(2):321–330. doi: 10.1042/bj1140321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Wolf M. J., Fridkin M., Epstein M., Kohn L. D. Structure-function studies of cholera toxin and its A and B protomers. Modification of tryptophan residues. J Biol Chem. 1981 Jun 10;256(11):5481–5488. [PubMed] [Google Scholar]
- De Wolf M. J., Lagrou A. R., Hilderson H. J. Subcellular structure of bovine thyroid gland. The localization of the peroxidase activity in bovine thyroid. Biochem J. 1978 Sep 15;174(3):939–949. doi: 10.1042/bj1740939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Wolf M. J., Vitti P., Ambesi-Impiombato F. S., Kohn L. D. Thyroid membrane ADP ribosyltransferase activity. Stimulation by thyrotropin and activity in functioning and nonfunctioning rat thyroid cells in culture. J Biol Chem. 1981 Dec 10;256(23):12287–12296. [PubMed] [Google Scholar]
- De Wolf M., Lagrou A., Hilderson H. J., Dierick W. Lipolytic enzymes in bovine thyroid tissue. I. Subcellular localization, purification and characterization of acid phospholipase A1. Arch Int Physiol Biochim. 1978 Dec;86(5):1055–1075. doi: 10.3109/13813457809055962. [DOI] [PubMed] [Google Scholar]
- DePierre J. W., Karnovsky M. L. Ecto-enzymes of the guinea pig polymorphonuclear leukocyte. I. Evidence for an ecto-adenosine monophosphatase, adenosine triphosphatase, and -p-nitrophenyl phosphates. J Biol Chem. 1974 Nov 25;249(22):7111–7120. [PubMed] [Google Scholar]
- DePierre J. W., Karnovsky M. L. Plasma membranes of mammalian cells: a review of methods for their characterization and isolation. J Cell Biol. 1973 Feb;56(2):275–303. doi: 10.1083/jcb.56.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deguchi T., Ichiyama A., Nishizuka Y., Hayaishi O. Studies on the biosynthesis of nicotinamide-adenine dinucleotide in the brain. Biochim Biophys Acta. 1968 Jun 24;158(3):382–393. doi: 10.1016/0304-4165(68)90292-4. [DOI] [PubMed] [Google Scholar]
- Dierick W., Hilderson H. Subcellular structure of bovine thyroid. I. Beta-glucuronidase: a marker-enzyme for lysosomal particles. Arch Int Physiol Biochim. 1967 Feb;75(1):1–11. doi: 10.3109/13813456709084913. [DOI] [PubMed] [Google Scholar]
- Filetti S., Rapoport B. Hormonal stimulation of eucaryotic cell ADP-ribosylation. J Clin Invest. 1981 Aug;68(2):461–467. doi: 10.1172/JCI110276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filetti S., Takai N. A., Rapoport B. Prevention by nicotinamide of desensitization to thyrotropin stimulation in cultured human thyroid cells. J Biol Chem. 1981 Feb 10;256(3):1072–1075. [PubMed] [Google Scholar]
- Filetti S., Takai N., Rapoport B. Influence of cell density on desensitization of the thyroid cell cyclic adenosine 3',5'-monophosphate response to thyrotropin stimulation. Endocrinology. 1981 Oct;109(4):1156–1163. doi: 10.1210/endo-109-4-1156. [DOI] [PubMed] [Google Scholar]
- GREEN S., BODANSKY O. THE SOLUBILIZATION, PURIFICATION, AND PROPERTIES OF NICOTINAMIDE ADENINE DINUCLEOTIDE GLYCOHYDROLASE FROM EHRLICH ASCITES CELLS. J Biol Chem. 1965 Jun;240:2574–2579. [PubMed] [Google Scholar]
- Green S., Dobrjansky A. Relationship of the nicotinamide adenine dinucleotide glycohydrolase activity to nicotinamide adenine dinucleotide content and rate of proliferation of Ehrlich ascites tumor cells. Cancer Res. 1967 Dec;27(12):2261–2266. [PubMed] [Google Scholar]
- Hilderson H. J., De Wolf M. J., Lagrou A. R., Dierick W. S. Subcellular structure of bovine thyroid gland. A study on bovine thyroid membranes by buoyant-density-gradient centrifugation in a B-XIV zonal rotor. Biochem J. 1975 Dec;152(3):601–607. doi: 10.1042/bj1520601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KIND P. R., KING E. J. Estimation of plasma phosphatase by determination of hydrolysed phenol with amino-antipyrine. J Clin Pathol. 1954 Nov;7(4):322–326. doi: 10.1136/jcp.7.4.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakehi K., Shimoyama M., Tanigawa Y., Asamizu J., Ueda I. NAD glycohydrolase from the small intestine of the rat; purification, properties and possible role of the enzyme. Biochim Biophys Acta. 1972 May 12;268(2):506–517. doi: 10.1016/0005-2744(72)90346-4. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lee S. K., Singh J., Taylor R. B. Subclasses of T cells with different sensitivities to cytotoxic antibody in the presence of anesthetics. Eur J Immunol. 1975 Apr;5(4):259–262. doi: 10.1002/eji.1830050408. [DOI] [PubMed] [Google Scholar]
- Levin S. J., Bodansky O. The double pH optimum of 5'-nucleotidase of bull seminal plasma. J Biol Chem. 1966 Jan 10;241(1):51–56. [PubMed] [Google Scholar]
- Li S. C., Li Y. T. Studies on the glycosidases of jack bean meal. 3. Crystallization and properties of beta-N-acetylhexosaminidase. J Biol Chem. 1970 Oct 10;245(19):5153–5160. [PubMed] [Google Scholar]
- Liersch M., Grotelüschen H., Decker K. Zur Frage der Permeation von NAD in die Leberzelle. Hoppe Seylers Z Physiol Chem. 1971 Feb;352(2):267–274. [PubMed] [Google Scholar]
- MAAYAN M. L. NAD+-GLYCOHYDROLASE OF THYROID HOMOGENATES. Nature. 1964 Dec 19;204:1169–1170. doi: 10.1038/2041169a0. [DOI] [PubMed] [Google Scholar]
- Maayan M. L., Rosenberg I. N. Effect of thyrotropin injection on pyridine nucleotide content of thyroid in normal and hypophysectomized rat. Endocrinology. 1966 May;78(5):1049–1052. doi: 10.1210/endo-78-5-1049. [DOI] [PubMed] [Google Scholar]
- Moss J., Ross P. S., Agosto G., Birken S., Canfield R. E., Vaughan M. Mechanism of action of choleragen and the glycopeptide hormones: is the nicotinamide adenine dinucleotide glycohydrolase activity observed in purified hormone preparations intrinsic to the hormone? Endocrinology. 1978 Feb;102(2):415–419. doi: 10.1210/endo-102-2-415. [DOI] [PubMed] [Google Scholar]
- Moss J., Vaughan M. Activation of adenylate cyclase by choleragen. Annu Rev Biochem. 1979;48:581–600. doi: 10.1146/annurev.bi.48.070179.003053. [DOI] [PubMed] [Google Scholar]
- Muller H. M., Muller C. D., Schuber F. NAD+ glycohydrolase, an ecto-enzyme of calf spleen cells. Biochem J. 1983 May 15;212(2):459–464. doi: 10.1042/bj2120459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller H., Schuber F. Studies on the association of NAD glycohydrolase with membranes in calf spleen. Eur J Biochem. 1980 Mar;104(2):489–500. doi: 10.1111/j.1432-1033.1980.tb04451.x. [DOI] [PubMed] [Google Scholar]
- Pekala P. H., Anderson B. M. Studies of bovine erythrocyte NAD glycohydrolase. J Biol Chem. 1978 Oct 25;253(20):7453–7459. [PubMed] [Google Scholar]
- Rapoport B., Filetti S., Takai N., Seto P. Studies on the desensitization of the cyclic AMP response to thyrotropin in thyroid tissue. FEBS Lett. 1982 Sep 6;146(1):23–27. doi: 10.1016/0014-5793(82)80697-2. [DOI] [PubMed] [Google Scholar]
- Rebois R. V., Beckner S. K., Brady R. O., Fishman P. H. Mechanism of action of glycopeptide hormones and cholera toxin: what is the role of ADP-ribosylation? Proc Natl Acad Sci U S A. 1983 Mar;80(5):1275–1279. doi: 10.1073/pnas.80.5.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuber F., Pascal M. Interaction of Blue Dextran and and Cibacron Blue F3GA with calf spleen NAD+-glycohydrolase. Biochimie. 1977;59(8-9):735–737. doi: 10.1016/s0300-9084(77)80254-x. [DOI] [PubMed] [Google Scholar]
- Schuber F., Travo P. Calf-spleen nicotinamide--adenine dinucleotide glycohydrolase. Solubilization purification and properties of the enzyme. Eur J Biochem. 1976 May 17;65(1):247–255. doi: 10.1111/j.1432-1033.1976.tb10411.x. [DOI] [PubMed] [Google Scholar]
- Sedgwick B., Hübscher G. Metabolism of phospholipids. IX. Phosphatidate phosphohydrolase in rat liver. Biochim Biophys Acta. 1965 Jul 7;106(1):63–77. doi: 10.1016/0005-2760(65)90096-2. [DOI] [PubMed] [Google Scholar]
- Sims N. R., Carnegie P. R. Use of fluorescamine for the estimation of protein in the presence of detergent Triton X-100. Anal Biochem. 1975 May 12;65(1-2):578–580. doi: 10.1016/0003-2697(75)90550-3. [DOI] [PubMed] [Google Scholar]
- Skidmore J., Trams E. G. Nucleotide pyrophosphatase activity of rat liver plasma membranes. Biochim Biophys Acta. 1970;219(1):93–103. doi: 10.1016/0005-2736(70)90064-7. [DOI] [PubMed] [Google Scholar]
- Soman G., Tomer K. B., Graves D. J. Assay of mono ADP-ribosyltransferase activity by using guanylhydrazones. Anal Biochem. 1983 Oct 1;134(1):101–110. doi: 10.1016/0003-2697(83)90269-5. [DOI] [PubMed] [Google Scholar]
- Swislocki N. I., Kalish M. I., Chasalow F. I., Kaplan N. O. Solubilization and comparative properties of some mammalian diphosphopyridine nucleosidases. J Biol Chem. 1967 Mar 25;242(6):1089–1094. [PubMed] [Google Scholar]
- Thompson S. T., Stellwagen E. Binding of Cibacron blue F3GA to proteins containing the dinucleotide fold. Proc Natl Acad Sci U S A. 1976 Feb;73(2):361–365. doi: 10.1073/pnas.73.2.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallee B. L., Riordan J. F. Chemical approaches to the properties of active sites of enzymes. Annu Rev Biochem. 1969;38:733–794. doi: 10.1146/annurev.bi.38.070169.003505. [DOI] [PubMed] [Google Scholar]
- Van Sande J., Erneux C., Dumont J. E. Negative control of TSH action by iodide and acetylcholine: mechanism of action in intact thyroid cells. J Cyclic Nucleotide Res. 1977 Oct;3(5):335–345. [PubMed] [Google Scholar]
- Vitti P., De Wolf M. J., Acquaviva A. M., Epstein M., Kohn L. D. Thyrotropin stimulation of the ADP-ribosyltransferase activity of bovine thyroid membranes. Proc Natl Acad Sci U S A. 1982 Mar;79(5):1525–1529. doi: 10.1073/pnas.79.5.1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widnell C. C., Tata J. R. A procedure for the isolation of enzymically active rat-liver nuclei. Biochem J. 1964 Aug;92(2):313–317. doi: 10.1042/bj0920313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan J. H., Anderson B. M. Bull semen nicotinamide adenine dinucleotide nucleosidase. I. Purification and properties of the enzyme. J Biol Chem. 1971 Apr 10;246(7):2111–2115. [PubMed] [Google Scholar]
- van Sande J., Pochet R., Dumont J. E. Dissociation by cooling of hormone and cholera toxin activation of adenylate cyclase in intact cells. Biochim Biophys Acta. 1979 Jun 12;585(2):282–292. doi: 10.1016/0304-4165(79)90028-x. [DOI] [PubMed] [Google Scholar]


