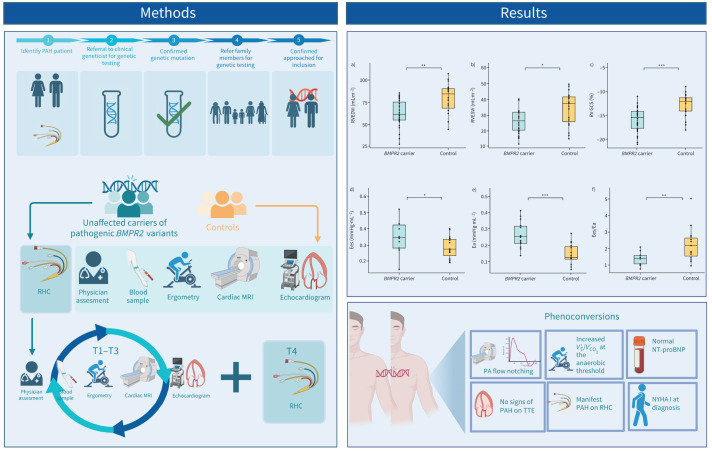
Graphical abstract
Summary of study protocol and main study findings. Unaffected carriers (UCs) of pathogenic BMPR2 variants and healthy family members (controls) were recruited for study participation after genetic counselling. A multimodality screening approach was employed with UCs undergoing an additional right heart catheterisation (RHC) at baseline and at the 4-year follow-up (T4). Main study findings include lower indexed right ventricular (RV) end-systolic volume (ESVi) and RV end-diastolic volume (EDVi) in UCs as well as a higher RV global circumferential strain (GCS) (red dots indicate phenoconverters). Haemodynamic and pressure–volume loop analysis showed higher RV end-systolic elastance (Ees), RV afterload (arterial elastance (Ea)) and altered RV pulmonary artery (PA) coupling (Ees/Ea) in UCs. During the study, two participants developed pulmonary arterial hypertension (PAH). MRI: magnetic resonance imaging; NT-proBNP: N-terminal pro-brain natriuretic peptide; NYHA: New York Heart Association; TTE: transthoracic echocardiography; V′E: minute ventilation; V′CO2: carbon dioxide production.
Abstract
Introduction
Pathogenic variants in the gene encoding for BMPR2 are a major genetic risk factor for heritable pulmonary arterial hypertension. Owing to incomplete penetrance, deep phenotyping of unaffected carriers of a pathogenic BMPR2 variant through multimodality screening may aid in early diagnosis and identify susceptibility traits for future development of pulmonary arterial hypertension.
Methods
28 unaffected carriers (44±16 years, 57% female) and 21 healthy controls (44±18 years, 48% female) underwent annual screening, including cardiac magnetic resonance imaging, transthoracic echocardiography, cardiopulmonary exercise testing and right heart catheterisation. Right ventricular pressure–volume loops were constructed to assess load-independent contractility and compared with a healthy control group. A transgenic Bmpr2Δ71Ex1/+ rat model was employed to validate findings from humans.
Results
Unaffected carriers had lower indexed right ventricular end-diastolic (79.5±17.6 mL·m−2 versus 62.7±15.3 mL·m−2; p=0.001), end-systolic (34.2±10.5 mL·m−2 versus 27.1±8.3 mL·m−2; p=0.014) and left ventricular end-diastolic (68.9±14.1 mL·m−2 versus 58.5±10.7 mL·m−2; p=0.007) volumes than control subjects. Bmpr2Δ71Ex1/+ rats were also observed to have smaller cardiac volumes than wild-type rats. Pressure–volume loop analysis showed that unaffected carriers had significantly higher afterload (arterial elastance 0.15±0.06 versus 0.27±0.08 mmHg·mL−1; p<0.001) and end-systolic elastance (0.28±0.07 versus 0.35±0.10 mmHg·mL−1; p=0.047) in addition to lower right ventricular pulmonary artery coupling (end-systolic elastance/arterial elastance 2.24±1.03 versus 1.36±0.37; p=0.006). During the 4-year follow-up period, two unaffected carriers developed pulmonary arterial hypertension, with normal N-terminal pro-brain natriuretic peptide and transthoracic echocardiography indices at diagnosis.
Conclusion
Unaffected BMPR2 mutation carriers have an altered cardiac phenotype mimicked in Bmpr2Δ71Ex1/+ transgenic rats. Future efforts to establish an effective screening protocol for individuals at risk for developing pulmonary arterial hypertension warrant longer follow-up periods.
Shareable abstract
Unaffected BMPR2 mutation carriers have an altered cardiac phenotype, with slightly increased right ventricular contractility. In the screening of individuals susceptible to developing PAH, echocardiography and NT-proBNP are insufficient. https://bit.ly/3xtcD0w
Introduction
Pulmonary arterial hypertension (PAH) is a rare disease marked by elevated pulmonary vascular resistance (PVR), resulting from pulmary vascular remodelling. This leads to increased right ventricular (RV) afterload, initiating extensive cardiac remodelling that ultimately results in right heart failure [1]. It is now well established that 80% of familial PAH cases and 11–40% of sporadic cases are associated with a pathogenic variant in BMPR2 [2]. Heritable PAH shows incomplete penetrance, and carriers have a lifetime risk of developing PAH ranging from 14% in males to 42% in females [3]. Current guidelines advise notifying PAH patients about the potential for a causative genetic condition and its presence in family members, allowing for early screening and diagnosis in carriers identified by cascade genetic screening [4, 5].
The 2015 European Society of Cardiology (ESC)/European Respiratory Society (ERS) guidelines recommended annual echocardiography for healthy carriers of a pathogenic variant in BMPR2 to aid in early identification and treatment of PAH [6]. However, the prospective DELPHI-2 study [7] showed through their longitudinal screening programme that unaffected carriers (UC) of a pathogenic variant in BMPR2 mutation, who were identified as having PAH at baseline, would not have been identified with echocardiography alone [7]. Updated directives emphasise the importance of a multimodal screening approach [4], but evidence-based screening programmes for UCs at risk for developing PAH are still lacking.
The presence of a pathogenic variant in BMPR2 is a significant risk factor but large variability in disease manifestation remains. It has not yet been established what sets apart BMPR2 carriers who develop PAH from those who do not. Experiments with induced pluripotent stem cells from UCs and familial PAH patients show that UCs have increased BMPR2 activators and decreased inhibitors allowing for preserved BMPR2 signalling, supporting the hypothesis that individual-specific protective mechanisms may be at play [8].
Our study outlines a comprehensive screening programme of UCs for a pathogenic variant in BMPR2 and of healthy control subjects aimed to aid in early diagnosis and offer a detailed phenotypic description of UCs. We additionally include a Bmpr2+/− transgenic rat model to explore whether potential phenotypic differences are reflected in animal models [9].
Methods
DOLPHIN-GENESIS study
Participant selection
The DOLPHIN-GENESIS study (Detection Of Latent Pulmonary Hypertension In Genetically Susceptible Individuals, 2017.318; CCMO registry with trial ID NL61732.029.17) is a longitudinal prospective cohort study performed at Amsterdam UMC, the Netherlands. The study was approved by the Medical Ethical Review Committee of the Amsterdam UMC location VUmc and conducted in accordance with the principles of the Declaration of Helsinki. All participants were prospectively recruited between 2017 and 2023 and written informed consent was obtained for all. Patients in whom a pathogenic variant was identified were provided with a family letter, containing information about the identified genetic predisposition for PAH, general information on the disease and on heritability and information on the option to inform their close relatives about genetic testing. In addition, a form was provided to the general practitioner for referral to the outpatient clinic of clinical genetics. Relatives who wished to undergo genetic testing were tested for the familial pathogenic variant after receiving genetic counselling. Unaffected adults between the ages of 18 and 80 years carrying a class 5 pathogenic variant in the BMPR2 gene according to the American College of Medical Genetic and Genomics criteria [10] and healthy non-BMPR2 mutation carrier relatives were included in the study. Further information regarding genetic testing can be found in the supplementary methods. All participants were in New York Heart Association (NYHA) functional class I. Exclusion criteria included comorbidities such as left heart failure, chronic respiratory disease, uncontrolled systemic hypertension, renal failure, chronic inflammatory disorders and chronic liver disease. Additionally, pregnancy, the use of immunosuppressive medications and a total lung capacity of <70% were exclusion criteria. An overview of all pathogenic variants represented in our study can be found in supplementary table S1.
Study design
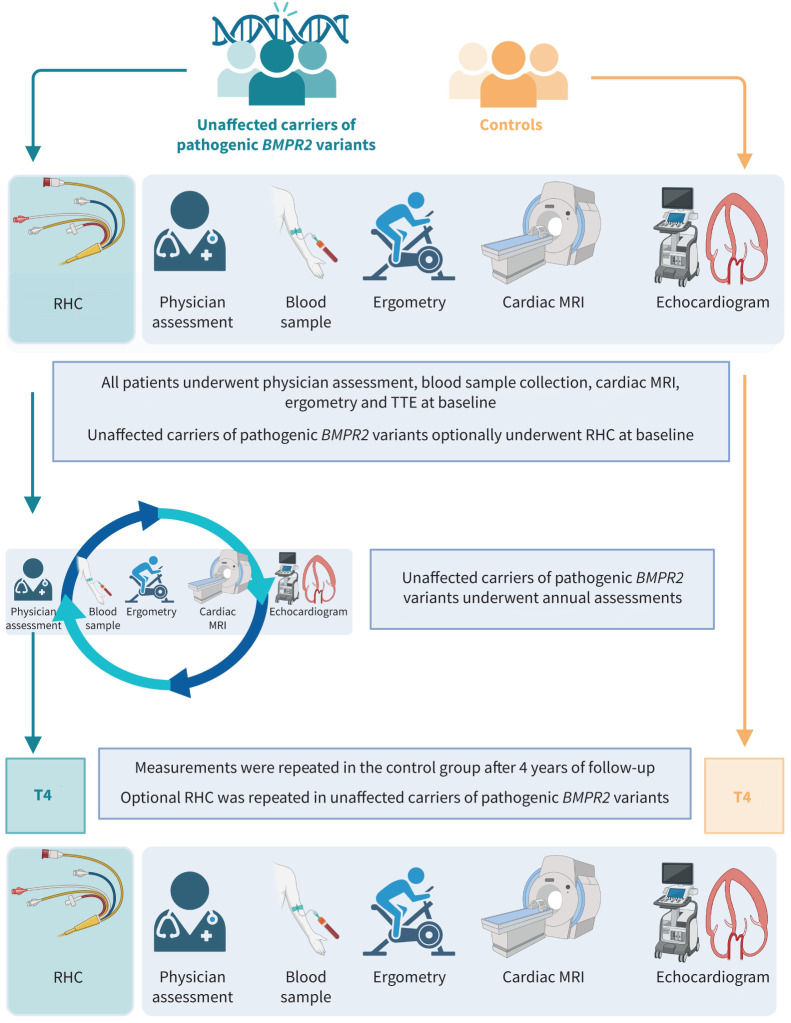
Participants performed cardiopulmonary exercise testing (CPET) and underwent cardiac magnetic resonance imaging (MRI) and transthoracic echocardiography (TTE) upon inclusion. UCs were offered an optional right heart catheterisation (RHC) at the baseline visit and at the fourth year of follow-up. UCs were evaluated annually by a pulmonologist and assessed with a detailed symptom review, physical examination, TTE, cardiac MRI, CPET and a plasma N-terminal pro-brain natriuretic peptide (NT-proBNP) test. Healthy control subjects were evaluated on a 4-year basis. Participants were considered to have PAH based on the criteria defined in the 2015 ERS/ESC guidelines for the diagnosis and treatment of pulmonary hypertension (PH), namely a mean pulmonary artery pressure (mPAP) ≥25 mmHg, pulmonary artery wedge pressure (PAWP) ≤15 mmHg and a PVR ≥3 Wood units (WU) as assessed by RHC. The 2022 ERS/ESC guidelines for the diagnosis and treatment of PAH were published during the inclusion period of our study. Updated directives included lowering the mPAP threshold for diagnosis to >20 mmHg and of PVR to >2 WU. Following the adoption of the 2022 protocol, haemodynamic data from UCs was re-examined and none of the participants fit the updated definition of PAH at baseline. Risk stratification and treatment of participants diagnosed with PAH during the study was done according to 2022 ESC/ERS guidelines. A complete methodological description of each modality can be found in the supplementary methods and figure S1. An overview of the study design is provided in figure 1.
FIGURE 1.
Schematic overview of the DOLPHIN-GENESIS study. MRI: magnetic resonance imaging; RHC: right heart catheterisation; T4: 4-year follow-up; TTE: transthoracic echocardiography.
Transgenic Bmpr2 rat model
The animal experiments were approved by the Imperial College London Ethical Committees in accordance with national regulations, the UK Home Office Animals (Scientific Procedures) Act 1986 (London, UK). The rats were housed in 12 h light/dark cycles with ad libitum access to water at standard laboratory conditions. Transgenic rats (Bmpr2Δ71Ex1/+) with a 71 bp monoallelic deletion in exon 1 of Bmpr2 [9] were examined via haemodynamic measurements, histological evaluation and cardiac MRI. The noninvasive cardiac MRI examinations for the rat cohort were performed at 14 weeks old (wild type (WT) n=5, Bmpr2Δ71Ex1/+ n=6), and haemodynamic data (n=5 per group) and tissues (WT n=6, Bmpr2Δ71Ex1/+ n=5) were collected at 16 weeks old. A methodological description of cardiac MRI examinations, haemodynamic measurements and histological analysis can be found in the supplementary methods.
Statistical analysis
All analyses were performed using R version 4.1.2 (www.r-project.org). Histograms and Q-Q plots were used to visually assess normal distribution for all variables. Unpaired t-tests and logistical regressions were used where appropriate. Continuous data are reported as mean±sd or median (interquartile range (IQR)) depending on distribution, unless stated otherwise. Categorical variables are presented as absolute numbers and relative frequencies (%). Differences in cardiac metrics for the animal model were only assessed in the 14-week-old rats. General characteristics of participants and results of all screening modalities at baseline were analysed and stratified according to the presence of a pathogenic variant. A p-value of <0.05 was considered statistically significant. No imputations were made for any missing data.
Results
Baseline characteristics
In total, 28 UCs of a pathogenic variant in BMPR2 and 21 healthy non-carrier family members of participants with PAH from 14 families were included in the study. At baseline, 22 UCs underwent RHC. Six UCs refused RHC due to the invasive nature of the procedure. In the UC group, 57% were female with an average age of 44±16 years. In the healthy control group, 48% were females with an average age of 44±18 years. Two participants (7%) in the UC group and two (10%) in the control group had a medical history of controlled systemic hypertension. One participant among the control subjects had a history of a venous thromboembolic event. All UCs had a normal NT-proBNP level at baseline (19 ng·L−1, IQR 10–26 ng·L−1) (table 1). At baseline, echocardiography revealed a significantly lower but well within physiological range RV S’ wave in UCs when compared to controls (13.2±2.0 cm·s−1 versus 12.1±1.7 cm·s−1; p=0.041). There were no significant differences between other echocardiographic measures, such as tricuspid annular plane systolic excursion (22.8±3.1 mm versus 23.5±2.9 mm; p=0.415), pulmonary arterial acceleration time (135.6±29.9 cm·s−1 versus 132.1±17.7 cm·s−1) and tricuspid regurgitation velocity (TRV) (2.0±0.3 m·s−1 versus 2.2±0.2 m·s−1). It is of note, however, that TRV measurements were not possible in every participant owing to a lack of signal. Echo-derived pulmonary artery (PA) flow notching was not observed at baseline. Subtle differences were observed using CPET. Both groups showed adequate effort during CPET with a mean maximum respiratory exchange ratio of 1.2±0.1 for both controls and UCs. Remarkably, UCs had a significantly lower end-tidal carbon dioxide partial pressure (4.7±0.3 kPa versus 4.3±0.4 kPa; p<0.001) and lower ventilatory efficiency, as shown by a higher ratio of minute ventilation to carbon dioxide production (V′E/V′CO2) at rest (30.0±3.4 versus 33.3±5.0; p=0.011) than controls (table 1).
TABLE 1.
Baseline characteristics of DOLPHIN-GENESIS cohort
| Control | BMPR2 carrier | p-value | |
|---|---|---|---|
| Participants (n) | 21 | 28 | |
| Female | 10 (48) | 16 (57) | 0.710 |
| Age | 44±18 | 44±16 | 0.937 |
| Serum NT-proBNP (ng·L−1), median (IQR) | NA | 19 (10–26) | NA |
| Medical history | |||
| Hypertension | 2 (9.5) | 2 (7.1) | 1.000 |
| Diabetes mellitus | 0 (0.0) | 1 (3.6) | 1.000 |
| Obesity | 1 (4.8) | 2 (7.1) | 1.000 |
| Malignancy | 0 (0.0) | 2 (7.1) | 0.348 |
| Prior VTE | 1 (4.8) | 0 (0.0) | 0.884 |
| CPET | |||
| V′O2max (mL·min−1) | 2355±682 | 2094±595 | 0.159 |
| RERmax | 1.2±0.1 | 1.2±0.1 | 0.426 |
| PETCO2max (kPa) | 4.7±0.4 | 4.5±0.8 | 0.375 |
| PETCO2rest (kPa) | 4.7±0.3 | 4.3±0.4 | <0.001*** |
| V′E/V′CO2rest | 30.0±3.4 | 33.3±5.0 | 0.011* |
| V′E/V′CO2max | 31.0±2.9 | 33.0±6.1 | 0.160 |
| V′E/V′CO2AT | 26.0±3.2 | 27.6±3.4 | 0.092 |
| Max O2 pulse (mL·beat−1) | 13.6±3.7 | 12.9±3.6 | 0.476 |
| Echocardiography | |||
| TRV (m·s−1) | 2.0±0.3 | 2.2±0.2 | 0.590 |
| TI | 6 (28.6) | 6 (22.0) | 0.867 |
| RV hypertrophy | 1 (4.8) | 0 (0.0) | 1.000 |
| Septal bulging | 0 (0.0) | 0 (0.0) | 1.000 |
| PAAT (cm·s−1) | 135.6±29.9 | 132.1±17.7 | 0.643 |
| PASP (mmHg) | 19.8±5.2 | 19.7±5.0 | 0.975 |
| TAPSE (mm) | 22.8±3.1 | 23.5±2.9 | 0.415 |
| RV S’ wave (cm·s−1) | 13.2±2.0 | 12.1±1.7 | 0.041* |
| Cardiac MRI | |||
| Participants (n) | 21 | 26 | |
| RAimin (mL·m−2) | 20.2±7.2 | 15.9±7.0 | 0.051 |
| RAimax (mL·m−2) | 38.1±10.6 | 29.5±10.8 | 0.011* |
| LAimin (mL·m−2) | 12.2±6.1 | 11.1±3.4 | 0.435 |
| LAimax (mL·m−2) | 34.7±11.6 | 29.6±6.2 | 0.063 |
| RVEF (%) | 58.6±4.8 | 57.1±6.2 | 0.366 |
| RVEDVi (mL·m−2) | 79.5±17.6 | 62.7±15.3 | 0.001** |
| RVESVi (mL·m−2) | 34.2±10.5 | 27.1±8.3 | 0.014* |
| RV mass index (g·m−2) | 23.5±4.8 | 20.2±5.0 | 0.031* |
| LVEF (%) | 68.7±7.2 | 68.7±7.2 | 0.976 |
| LVEDVi (mL·m−2) | 68.9±14.1 | 58.5±10.7 | 0.007* |
| LVESVi (mL·m−2) | 22.4±8.2 | 19.1±7.3 | 0.155 |
| LV mass index (g·m−2) | 64.8±10.8 | 57.5±9.5 | 0.020* |
| RV GCS (%) | −12.8±2.4 | −15.9±2.6 | <0.001*** |
| LV GCS (%) | −18.8±2.4 | −19.6±2.1 | 0.206 |
| RHC | |||
| Participants (n) | 22 | ||
| Heart rate (bpm) | NA | 72.7±12.1 | NA |
| mPAP (mmHg) | NA | 16.5±2.3 | NA |
| mRAP (mmHg) | NA | 5.7±2.1 | NA |
| Cardiac output (L·min−1) | NA | 5.7±1.2 | NA |
| Cardiac index (L·min−1·m−2) | NA | 3.1±0.6 | NA |
| PVR (WU) | NA | 1.1±0.5 | NA |
| PCWP (mmHg) | NA | 10.4±3.1 | NA |
Data are presented as mean±sd or n (%), unless otherwise indicated. AT: anaerobic threshold; EDVi: indexed end-diastolic volume; EF: ejection fraction; ESVi: indexed end-systolic volume; GCS: global circumferential strain; IQR: interquartile range; LAi: left atrial indexed volume; LV: left ventricular; max: maximum; min: minimum; mPAP: mean pulmonary arterial pressure; mRAP: mean right atrial pressure; NA: not applicable; NT-proBNP: N-terminal pro-brain natriuretic peptide; PAAT: pulmonary artery acceleration time; PASP: pulmonary artery systolic pressure; PCWP: pulmonary capillary wedge pressure; PETCO2: end-tidal carbon dioxide tension; PVR: pulmonary vascular resistance; RAi: right atrial indexed volume; RER: respiratory exchange ratio; RV: right ventricular; RV S’ wave: peak systolic velocity of the tricuspid annulus; TAPSE: tricuspid annular plane systolic excursion; TI: tricuspid insufficiency; TRV: tricuspid regurgitation velocity; V′E/V′CO2: minute ventilation to carbon dioxide production ratio; V′E/V′CO2max: minute ventilation to carbon dioxide production ratio at maximum exercise; V′O2max: maximal oxygen consumption during exercise; VTE: venous thromboembolic event; WU: Wood unit. *: p<0.05; **: p<0.01; ***: p<0.001.
Smaller cardiac volumes and mass in UCs
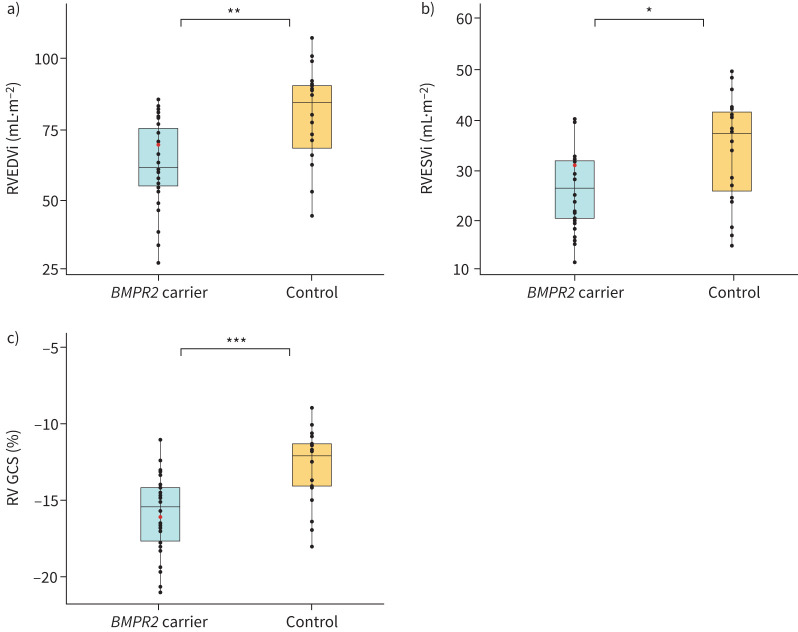
To further elucidate potential differences in RV morphology in UCs, we performed cardiac MRI. Volumetric analyses revealed that UCs had significantly smaller indexed RV end-diastolic volume (EDVi) (79.5±17.6 mL·m−2 versus 62.7±15.3 mL·m−2; p=0.001) and RV end-systolic volume (ESVi) (34.2±10.5 mL·m−2 versus 27.1±8.3 mL·m−2; p=0.014) when compared to healthy control subjects (figure 2a, b). Interestingly, UCs also had a significantly lower indexed left ventricular (LV) EDVi (68.9±14.1 mL·m−2 versus 58.5±10.7 mL·m−2; p=0.007) but no differences were observed for LVESVi (22.4±8.2 mL·m−2 versus 19.1±7.3 mL·m−2; p=0.155). In addition to smaller volumes, we observed a lower mass index in both the right ventricle (23.5±4.8 g·m−2 versus 20.2±5.0 g·m−2; p=0.031) and left ventricle (64.8±10.8 g·m−2 versus 57.5±9.5 g·m−2; p=0.020) in the UC versus control population. This difference in volume was seen not only in the ventricles, but also in the indexed right atrial (RA) maximum volume (38.1±10.6 mL·m−2 versus 29.5±10.8 mL·m−2; p=0.011). Indexed RA minimum volume and both minimum and maximum indexed left atrial (LA) volumes were similar in both groups. All volumetric and mass analyses are summarised in table 1. Linear regression analysis showed that carrier status was predictive of smaller RVEDVi, RVESVi and RV mass despite age and gender (supplementary table S2).
FIGURE 2.
Cardiac magnetic resonance imaging indices for unaffected carriers of a pathogenic BMPR2 variant compared to control participants. a) Indexed right ventricular (RV) end-diastolic volume (EDVi). b) Indexed RV end-systolic volume (ESVi). c) RV global circumferential strain (GCS). Red dots indicate phenoconverters. *: p<0.05; **: p<0.01; ***: p<0.001.
To investigate the effect of smaller volumes and mass on functional parameters, we performed strain analyses. UCs had a higher peak RV global circumferential strain (GCS) (−12.8±2.4% versus −15.9±2.6%; p<0.001) than control subjects (figure 2c). No significant differences were seen in peak LV regional circumferential strain or GCS (−18.8±2.4% versus −19.6±2.1%; p=0.206). There were no significant differences observed in longitudinal strain. All strain analyses can be found in table 1. Again, carrier status was predictive of higher RV GCS on linear regression despite age and gender (supplementary table S2).
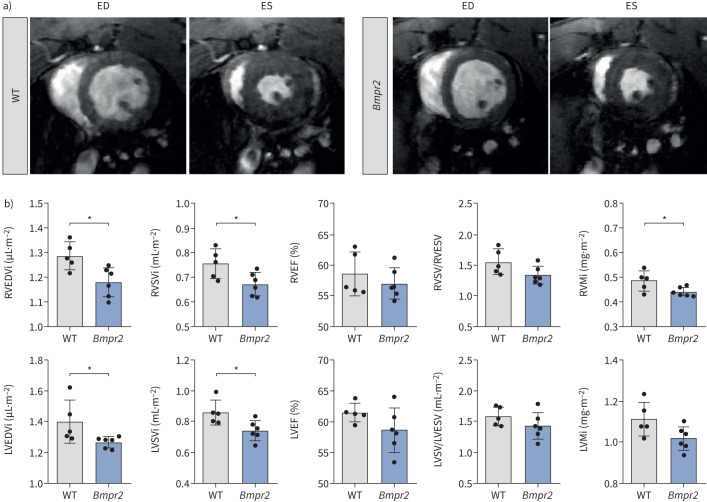
To validate our observations in human subjects using an experimental model, we conducted cardiac MRI on Bmpr2Δ71Ex1/+ transgenic rats. None of the rats showed signs of PAH at the time of analysis. Consistent with the findings from clinical cardiac MRI analysis, these transgenic rats exhibited a significant reduction in RVEDVi (p<0.015), RV stroke volume (p<0.05), RV mass (p<0.05), LVEDVi (p<0.05) and LV stroke volume (p<0.05) after correction for body surface area (figure 3; supplementary tables S3 and S4, supplementary figures S2 and S3).
FIGURE 3.
Cardiac volumetric and functional values for wild-type (WT) and Bmpr2 (Bmpr2Δ71Ex1/+) rats. a) Mid-ventricular cardiac magnetic resonance imaging images from WT (n=5) and Bmpr2Δ71Ex1/+ transgenic (n=6) rats at end-diastole (ED) and end-systole (ES). b) Right ventricular (RV) and left ventricular (LV) indexed end-diastolic volume (EDVi), indexed stroke volume (SVi), ejection fraction (EF), ventricular–arterial coupling index (SV/ESV) and mass index (Mi) values, all corrected for body surface area. *: p<0.05.
Increased mean RA pressure and RV afterload resulting in altered RV–PA coupling in UCs in comparison to healthy controls
In total, 22 UCs underwent RHC upon inclusion. Haemodynamic characteristics, presented in table 1, were compared to a historical control group of healthy subjects. These subjects had been referred for RHC between 2013 and 2021 but did not receive a final diagnosis of PAH or have a history of cardiovascular disease. We compared our historical control group to the haemodynamic survey of healthy volunteers used by Kovacs et al. [11] and concluded that our control group adequately reflected a healthy population.
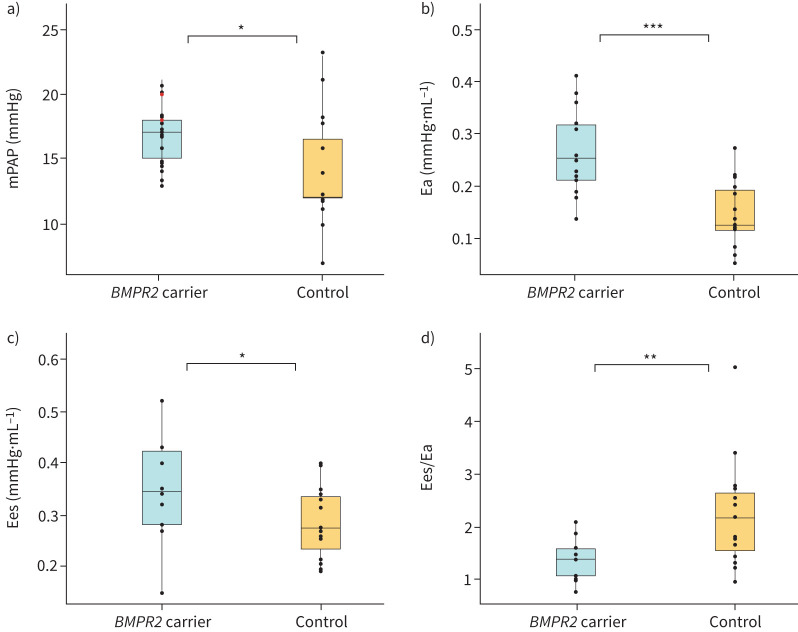
While none of the BMPR2 carriers was diagnosed with PAH at baseline, UCs had a significantly higher mPAP (14.1±4.3 mmHg versus 16.5±2.3 mmHg; p=0.036) (figure 4a), mean RA pressure (3.9±2.4 mmHg versus 5.7±2.1 mmHg; p=0.024) and a lower cardiac index (4.4±0.8 L·min−1·m−2 versus 3.1±0.6 L·min−1·m−2; p<0.001) than healthy controls. To further assess potential load-independent changes in RV function, single beat pressure–volume analyses were performed in a subgroup of participants. RV afterload was increased in UCs in comparison to healthy controls (arterial elastance (Ea): 0.15±0.06 mmHg·mL−1 versus 0.27±0.08 mmHg·mL−1; p<0.001) (figure 4b). Additionally, an increased RV end-systolic elastance (Ees) (0.28±0.07 mmHg·mL−1 versus 0.35±0.10 mmHg·mL−1; p=0.047), a measure of RV contractility, and reduced RV–PA coupling were observed in UCs (2.24±1.03 versus 1.36±0.37; p=0.006) (figure 4c, d). Haemodynamic data and pressure–volume loop analyses can be found in table 2.
FIGURE 4.
Comparison of haemodynamic parameters and pressure–volume relationships between unaffected carriers of a pathogenic BMPR2 variant and healthy control patients. a) Mean pulmonary artery pressure (mPAP). b) Arterial elastance (Ea). c) End-systolic elastance (Ees). d) Ees/Ea ratio. Red dots indicate phenoconverters. *: p<0.05; **: p<0.01; ***: p<0.001.
TABLE 2.
Haemodynamic characteristics and pressure–volume loop analysis of BMPR2 carriers versus control group
| Control | BMPR2 carrier | p-value | |
|---|---|---|---|
| Participants (n) | 15 | 22 | |
| Age (years) | 40±12 | 45±16 | 0.273 |
| Female (%) | 11 (73) | 14 (64) | 0.794 |
| Haemodynamic parameters | |||
| mPAP (mmHg) | 14.1±4.3 | 16.5±2.3 | 0.036* |
| mRAP (mmHg) | 3.9±2.4 | 5.7±2.1 | 0.024* |
| PCWP (mmHg) | 7.0±2.7 | 10.4±3.1 | 0.001** |
| PVR (WU) | 0.9±0.5 | 1.1±0.5 | 0.168 |
| Cardiac output (L·min−1) | 8.1±1.5 | 5.7±1.2 | <0.001*** |
| Cardiac index (L·min−1·m−2) | 4.4±0.8 | 3.1±0.6 | <0.001*** |
| Pressure–volume relationship | |||
| Participants (n) | 15 | 14 | |
| Maximal pressure (mmHg) | 42±6 | 38±7 | 0.105 |
| RVEDV (mL) | 130±29 | 110±33 | 0.080 |
| RVESV (mL) | 52±13 | 45±19 | 0.249 |
| Stroke volume (mL) | 79±21 | 65±16 | 0.056 |
| Eed (mmHg·mL−1) | 0.19±0.06 | 0.20±0.13 | 0.846 |
| Ea (mmHg·mL−1) | 0.15±0.06 | 0.27±0.08 | <0.001*** |
| Ees (mmHg·mL−1) | 0.28±0.07 | 0.35±0.10 | 0.047* |
| RV coupling | 2.24±1.03 | 1.36±0.37 | 0.006** |
Data are presented as mean±sd, unless otherwise indicated. Control group is derived from a historical control group of healthy subjects. Ea: arterial elastance; Eed: end-diastolic elastance; Ees: end-systolic elastance; EDV: end-diastolic volume; ESV: end-systolic volume; mPAP: mean pulmonary arterial pressure; mRAP: mean right atrial pressure; PCWP: pulmonary capillary wedge pressure; PVR: pulmonary vascular resistance; RV: right ventricular; WU: Wood unit. *: p<0.05; **: p<0.01; ***: p<0.001.
Longitudinal follow-up
During the 4-year follow-up period, two UCs were diagnosed with PAH. At the date of this manuscript, a total of 15 UCs underwent RHC after 4 years. A comparison between the diagnostic modalities comparing the conversions with the overall UC population at baseline can be found in supplementary table S5. Excluding the two participants who developed PAH during the 4 years, volume and strain measurements were stable in all participants during the follow-up period (supplementary figure S4). MRI-derived PA flow notching, a nonspecific sign often observed in PAH, was only noted in two BMPR2 carriers throughout the study, both of whom developed PAH several years after it first appeared [12]. To further explore this signal, we compared acceleration time, ejection time and acceleration time/ejection time ratio based on PA flow curves between control subjects and UCs but did not find any significant differences between the groups [13].
Conversion 1
The first phenoconverter, a 62-year-old man, had a medical history of systemic hypertension and a family history of heart failure of unknown aetiology on the paternal side (suggestive of PAH) and a sibling with confirmed PAH. Both the participant and sibling with confirmed PAH were carriers of the pathogenic variant c.1454A>G (p.Asp485Gly) in BMPR2. There were no abnormalities on RHC, CPET or echocardiography at the time of inclusion. At baseline, cardiac MRI was refused due to claustrophobia. From the second year of follow-up onwards, he agreed to undergo cardiac MRI, which showed no signs of RA or RV dilation but did show PA notching on flow analysis.
RHC was performed at the fourth follow-up visit, at which an mPAP of 42 mmHg, PVR of 5.2 WU and a pulmonary capillary wedge pressure (PCWP) of 8 mmHg were measured. There were no signs of PAH on echocardiography and the plasma NT-proBNP was 24 ng·L−1. Flow analysis at the 4-year follow-up revealed distinct PA notching but no right heart dilation or hypertrophy. CPET showed an elevated V′E/V′CO2 at the anaerobic threshold. Initially, the participant denied any new-onset symptoms and was categorised as NYHA functional class I, but recalled mild exercise intolerance on a recent hiking trip after receiving the diagnosis. The participant was classified as low risk, according to the three-strata model, and started on macitentan and tadalafil. Although there was slightly worsened RV dilation at the 6-month follow-up, the patient remained in the low-risk category according to the four strata risk assessment model [6].
Conversion 2
The second conversion was a 56-year-old male UC carrying the pathogenic BMPR2 variant c.1978G>T (p.Glu660X). He had a previous diagnosis of epilepsy for which he used carbamazepine. At baseline, there were no abnormalities on CPET or echocardiography. During the follow-up period, a slightly decreased RV ejection fraction of 45% and PA flow notching were observed on cardiac MRI. At the 4-year follow-up visit, RHC was repeated and showed an mPAP of 31 mmHg, PCWP of 11 mmHg and PVR of 3 WU. TTE showed no signs of PAH and NT-proBNP was 4 ng·L−1. CPET showed a slightly decreased end-tidal carbon dioxide tension of 3.4 kPa at peak exercise. MRI-derived PA flow measures revealed persistent PA flow notching. The participant did not report any new-onset symptoms or exercise intolerance, and was thereby classified as NYHA functional class I. A risk assessment was performed, and the participant was categorised as low risk. The participant started on macitentan and tadalafil with an excellent response in exercise capacity. At 6-month follow-up, NT-proBNP remained low with a 6-min walk distance of 639 m and NYHA II, thereby categorised as low risk.
Discussion
Our study used an extensive, ongoing screening programme of unaffected BMPR2 carriers to reveal the following important findings:
UCs of a pathogenic BMPR2 variant had smaller cardiac volumes and mass alongside increased RV GCS. These findings were confirmed in a transgenic rat model. With regards haemodynamic parameters, PA and RA pressure in UCs were increased but still within physiological range.
Pressure–volume loop analysis revealed increased afterload and RV contractility, with decreased RV–PA coupling.
Echocardiography and NT-proBNP measurements were insufficient to effectively screen individuals susceptible to developing PAH. MRI-derived PA flow notching may be an early, nonspecific predictor of PAH.
Clinical relevance
Characterising and screening of UCs of pathogenic BMPR2 variants is of great clinical importance to identify potential risk factors for disease penetrance and find an effective screening method to aid in early diagnosis. Our approach included the incorporation of a healthy control group, revealing nuanced differences allowing for deep phenotyping of characteristics that have not been previously examined.
UCs of pathogenic BMPR2 variants have smaller cardiac volumes; PA and RA pressure is increased within physiological range
Reduced cardiac volumes and mass in UCs, along with smaller biventricular volumes and mass in transgenic Bmpr2+/− rats, further support existing research implicating BMPR2 in cardiac development. Moreover, it has been observed that Bmpr2Δ71Ex1/+ rats have reduced cardiac output, which has been attributed to the distinct changes in cardiomyocyte morphology, calcium (Ca2+) dynamics and cell contractility within the right ventricle. Specifically, adult RV cardiomyocytes from those rats exhibited diminished diameter, reduced sarcomere sensitivity to Ca2+, lower Ca2+ transient amplitude, decreased sarcoplasmic reticulum Ca2+ content and shorter action potential duration compared to those from WT rats [9]. How this precisely translates to humans is still not understood. It is well known that PAH patients carrying a pathogenic variant in BMPR2 have worse survival and show more impaired RV function than idiopathic PAH patients with a similar afterload [2, 14]. Pathogenic variants in BMPR2 have been shown to contribute to RV lipotoxicity in transgenic murine models, leading to impaired RV hypertrophy and triglyceride and ceramide deposition, which has also been observed in human right ventricle and cultured cardiomyocytes [15–17]. It is conceivable that pathogenic variants in BMPR2 in humans result in smaller cardiac volumes and mass during embryological development. Whether this altered phenotype makes the heart more susceptible to impaired adaptation in the setting of PAH has yet to be explored. Interpretation of these results in our human cohort remains challenging, because any volumetric and mass differences between healthy controls and UCs were still within the normal range and were not associated with functional limitations.
Increased afterload, RV contractility and decreased RV–PA coupling in carriers of pathogenic BMPR2 variants
We also observed an increased GCS and regional RV circumferential strain. Pressure–volume loop analysis revealed a significantly higher Ees and Ea among UCs, possibly indicating altered RV–PA coupling in UCs. Additionally, compromised arterial–ventricular coupling was observed in the transgenic Bmpr2+/− rats, again raising questions regarding the impact of pathogenic variants in BMPR2 on cardiac function. RV function is the primary prognostic factor in patients with PAH. While elevated afterload significantly influences RV function, notable variability in the adaptation of the right ventricle in PH persists [18–21]. A normal RV structure includes a superficial circumferential layer forming approximately a quarter of the wall thickness, followed by a longitudinally oriented subendocardial layer making up the bulk of the myocardium [22]. Animal models have suggested that increased pressure overload in the right ventricle can lead to RV hypertrophy with a large proportion of circumferentially oriented fibres [23]. Haemodynamic measurements revealed a slightly increased mPAP and mean RA pressure among UCs, which could possibly contribute to a subtle increase in circumferential strain through adaptive changes in the myocardium reflective of RV anatomy. Both an altered haemodynamic state and contractility results in altered RV–PA coupling, consistent with findings in transgenic Bmpr2 animals [9].
Increased RV afterload, characterised by higher mPAP and Ea, among pathogenic BMPR2 variant carriers could suggest subclinical changes in the pulmonary vascular bed. BMPR2 is highly expressed in the endothelium and smooth muscle cells of the pulmonary vasculature and has been shown to be one of the pathomechanistic drivers of PAH development via a pro-proliferative process led by endothelial and smooth muscle cell dysfunction [24, 25]. Previous animal studies have shown that Bmpr2 mutant rats without spontaneous PH exhibited higher muscularisation of both smaller and larger vessels as well as accelerated PA smooth muscle cell proliferation rates when compared to WT rats [9]. It is plausible that some degree of endothelial and vascular smooth muscle cell proliferation is present in human UCs of a pathogenic BMPR2 variant even in the absence of disease, leading to mild, but nevertheless distinct, haemodynamic aberrations. The combination of a more vulnerable pulmonary vascular bed and myocardium can potentially contribute to the lower survival and increased disease severity observed in patients with PAH due to a pathogenic variant in BMPR2 [2, 14]. Extended follow-up of UCs is essential to elucidate the clinical relevance of these altered cardiac phenotypes and borderline PA pressures towards the potential development of PAH.
Traditional screening methods are not sufficient for the effective screening of individuals susceptible to developing PAH
Our results further emphasise the difficulty of establishing an effective screening protocol for individuals at risk for PAH, as previously discussed by the investigators of the DELPHI-2 study [7]. NT-proBNP and conventional echocardiographic parameters seem insufficient for the effective screening of individuals susceptible to PAH, thereby confirming the poor discriminative ability of TTE for the early detection of PAH as established by Montani et al. [7]. While we did observe a significantly lower RV S’ among UCs, the values fell within the normal physiological range. It is also of note that NT-proBNP remained within the normal range for all participants, including those diagnosed with PAH during the course of the study. Analysis of PA flow curves revealed notching in both conversions up to 2 years prior to the diagnosis of PAH, suggesting that MRI-derived PA flow notching may be an early, nonspecific predictor of PAH. Expansion of PA flow analyses to integrate four-dimensional cardiac MRI PA flow may allow for the identification of vortex formation, an early sign of PAH which could potentially identify more subtle changes in flow patterns [12]. Exploration of the diagnostic potential of cardiac MRI-derived PA flow analysis warrants further evaluation in larger cohorts.
Additionally, participants who developed PAH displayed changes on CPET which were nonspecific but consistent with a pattern correlating to a circulatory limitation. While these changes on CPET and MRI may foreshadow a diagnosis of PAH, they often do not warrant sufficient suspicion for initiating more invasive testing. Grünig et al. [26] have shown in relatives of PAH patients that a pulmonary hypertensive response to exercise or hypoxia was correlated with but not caused by a BMPR2 mutation, which was also seen in a more recent study by Claessen et al. [27]. Although the presence of this trait was also present in some healthy controls, manifest PAH developed only in patients with both a pathogenic BMPR2 variant in addition to a hypertensive response [28]. An important finding in the DELPHI-2 study was the development of prevalent PAH in UCs with abnormal exercise haemodynamic profiles, indicating it might precede future development of prevalent disease [7]. Unfortunately, in our two conversions, exercising was not yet performed during RHC. The DELPHI-2 also used a noninvasive tool (ECG, low diffusing capacity of the lung for carbon monoxide and CPET probability) to address the likelihood of PH and to justify invasive testing, but exercise PH was still present in three participants with no suspicion of PH. Although this score might not be conclusive, further research should establish a clear set of characteristics of early-stage PAH or exercise PH to perform invasive diagnostics. For now, RHC remains the only diagnostic modality able to detect PAH at an early stage. Conversions could not be identified before significant remodelling of the vascular bed had occurred, further emphasising the importance of continued efforts to identify biomarkers of vascular remodelling. The prognostic implications of early diagnosis accentuate the importance of vigilance and the timely deployment of RHC to establish a definitive diagnosis.
Limitations
The following limitations in our study should be considered. The multimodal characterisation of UCs of pathogenic BMPR2 variants has allowed us to identify cardiac traits not present in healthy controls. However, despite our screening, the low incidence of conversions makes interpretation of these results challenging. The same applies to the 2 mmHg difference in mPAP, because this could be the consequence of limited sample size. However, a comparable small increase in resting state was seen for DELPHI-2 participants with exercise PH, individuals at increased future risk of developing prevalent PAH. Longitudinal assessment in this at-risk population is warranted to address relevancy, potentially in multicentre initiatives to increase power. Obtaining sufficient power through single-centre screening remains a challenge, especially because UCs are excluded in the presence of cardiovascular comorbidities. Second, a different control group was used for our pressure–volume loop analysis. Individuals suspected of PH were used as a control group for the assessment of RV contractility because it is not ethical to perform RHC in healthy volunteers. Also, cardiac phenotyping in this study was performed with MRI, a modality not widely available in other countries. However, PA flow notching might be an early sign of PH and could also be assessed using echocardiography. While we did employ echocardiography, we did not perform exercise echocardiography, which may have allowed for more subtle analysis of cardiac markers for PAH. Lastly, we chose to focus on BMPR2 because this is the most common site of a pathogenic variant causing PAH, resulting in a representative cohort. It remains unanswered whether our findings can be translated to UCs of different pathogenic variants because each affected gene harbours different phenotypic appearances. Overall, our findings suggest that UCs of a pathogenic BMPR2 variant have an altered cardiac phenotype. Future efforts in establishing an effective screening protocol for at-risk individuals warrant longer follow-up periods.
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material ERJ-00442-2024.Supplement (791.2KB, pdf)
Shareable PDF
Footnotes
Ethics statement: The study was approved by the Medical Ethical Review Committee of the Amsterdam UMC location VUmc and conducted in accordance with the principles of the Declaration of Helsinki. All participants were prospectively recruited between 2017 and 2023 and written informed consent was obtained for all.
Author contributions: E.N. Tóth and L.R. Celant had full access to all data and take responsibility for the integrity of the data and accuracy of data analysis and were responsible for writing the manuscript. J.T. Marcus, L.J. Meijboom, A. Vonk Noordegraaf, F.S. de Man, A.C. Houweling, S. Jansen, J. Tramper, J. N. Wessels, E.J. Nossent, J. Aman, D. Montani, J. Grynblat, F. Perros and H.J. Bogaard contributed substantially to the study design, data analysis and interpretation, and reviewing of the manuscript. L. Zhao, J. Grynblat, F. Perros, M. Niglas, A. Ashek and N. Baxan contributed to the animal model design and execution.
This article has an editorial commentary: https://doi.org/10.1183/13993003.01369-2024
Notification of abstract submission: Part of this manuscript has been submitted as an abstract for the ERS 2023 Congress in Milan and the PVRI 2024 congress in London.
Conflicts of interest: F. Perros reports grants from French National Research Agency (ANR-20-CE14-0006) and INSERM (International Research Project (IRP) (Paris – Porto Pulmonary Hypertension Collaborative Laboratory (3PH)). D. Montani reports grants from Janssen, MSD and Acceleron; consultation fees from Janssen, MSD, Ferrer and Acceleron; and payment or honoraria for lectures, presentations, manuscript writing or educational events from Bayer, Janssen, Boehringer, Chiesi, GSK, Ferrer and Merck MSD. A. Vonk Nordegraaf reports research grants from Janssen, MSD and Ferrer. L. Zhao reports grants from British Heart Foundation (PG/18/2/33446 and BHF RE/18/4/34215). F.S. de Man reports support for the present study from Royal Netherlands Academy of Sciences (CVON-2017-10 Dolphin-Genesis); grants from Netherlands CardioVascular Research Initiative: the Dutch Heart Foundation, Dutch Federation of University Medical Centres, Netherlands Organisation for Health Research and Development, The Netherlands Organisation for Scientific Research (NWO-VICI: 918.16.610, NWO-VIDI: 917.18.338), Royal Netherlands Academy of Sciences (CVON-2012-08 PHAEDRA and CVON-2018-29 PHAEDRA-IMPACT) and Dutch Heart Foundation (Post doc Dekker 2018T05). H-J. Bogaard reports support for the present study from Royal Netherlands Academy of Sciences (CVON-2017-10 Dolphin-Genesis); grants from MSD, Ferrer, Janssen, Dutch Federation of University Medical Centres, Netherlands CardioVascular Research Initiative: the Dutch Heart Foundation, Netherlands Organisation for Health Research and Development, and the Royal Netherlands Academy of Sciences (CVON-2012-08 PHAEDRA and CVON-2018-29 PHAEDRA-IMPACT). The remaining authors have no potential conflicts of interest to disclose.
Support statement: H.J. Bogaard, A. Vonk Noordegraaf and F.S. de Man were supported by the Netherlands CardioVascular Research Initiative: the Dutch Heart Foundation, Dutch Federation of University Medical Centres, the Netherlands Organisation for Health Research and Development, and the Royal Netherlands Academy of Sciences (CVON-2012-08 PHAEDRA, CVON-2018-29 PHAEDRA-IMPACT and CVON-2017-10 Dolphin-Genesis). A. Vonk Noordegraaf and F.S. de Man were further supported by The Netherlands Organisation for Scientific Research (NWO-VICI: 918.16.610, NWO-VIDI: 917.18.338). F.S. de Man was supported by the Dutch Heart Foundation Dekker senior post-doctoral grant (2018T059). L. Zhao received funding from British Heart Foundation (PG/18/2/33446, BHF RE/18/4/34215) to support N. Baxan and A. Ashek, and the work at Biological Imaging Center, Imperial College London. M. Niglas was supported by UKRI training grant funding the studentship (EPSRC DTP 2018-19 training grant EP/R513052/1). F. Perros received funding from the French National Research Agency (Agence Nationale de la Recherche, Grant ANR-20-CE14-0006) and from INSERM (International Research Project (IRP): Paris – Porto Pulmonary Hypertension Collaborative Laboratory (3PH)). J. Grynblat is supported by Institut National de la Santé et de la Recherche Médicale (Poste d'accueil INSERM), and a scholarship from the French Paediatric Society. Sponsors (grant funders) had no role in the development of the research or the manuscript. Funding information for this article has been deposited with the Crossref Funder Registry.
References
- 1.Vonk Noordegraaf A, Westerhof BE, Westerhof N. The relationship between the right ventricle and its load in pulmonary hypertension. J Am Coll Cardiol 2017; 69: 236–243. doi: 10.1016/j.jacc.2016.10.047 [DOI] [PubMed] [Google Scholar]
- 2.Evans JD, Girerd B, Montani D, et al. BMPR2 mutations and survival in pulmonary arterial hypertension: an individual participant data meta-analysis. Lancet Respir Med 2016; 4: 129–137. doi: 10.1016/S2213-2600(15)00544-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Larkin EK, Newman JH, Austin ED, et al. Longitudinal analysis casts doubt on the presence of genetic anticipation in heritable pulmonary arterial hypertension. Am J Respir Crit Care Med 2012; 186: 892–896. doi: 10.1164/rccm.201205-0886OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Humbert M, Kovacs G, Hoeper MM, et al. 2022 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Respir J 2023; 61: 2200879. doi: 10.1183/13993003.00879-2022 [DOI] [PubMed] [Google Scholar]
- 5.Eichstaedt CA, Belge C, Chung WK, et al. Genetic counselling and testing in pulmonary arterial hypertension: a consensus statement on behalf of the International Consortium for Genetic Studies in PAH. Eur Respir J 2023; 61: 2201471. doi: 10.1183/13993003.0147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Galie N, Humbert M, Vachiery JL, et al. 2015 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension. Rev Esp Cardiol (Engl Ed) 2016; 69: 177. doi: 10.1016/j.recesp.2016.01.002 [DOI] [PubMed] [Google Scholar]
- 7.Montani D, Girerd B, Jaïs X, et al. Screening for pulmonary arterial hypertension in adults carrying a BMPR2 mutation. Eur Respir J 2021; 58: 2004229. doi: 10.1183/13993003.04229-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gu M, Shao NY, Sa S, et al. Patient-specific iPSC-derived endothelial cells uncover pathways that protect against pulmonary hypertension in BMPR2 mutation carriers. Cell Stem Cell 2017; 20: 490–504. doi: 10.1016/j.stem.2016.08.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hautefort A, Mendes-Ferreira P, Sabourin J, et al. Bmpr2 mutant rats develop pulmonary and cardiac characteristics of pulmonary arterial hypertension. Circulation 2019; 139: 932–948. doi: 10.1161/CIRCULATIONAHA.118.033744 [DOI] [PubMed] [Google Scholar]
- 10.Richards S, Aziz N, Bale S, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med 2015; 17: 405–424. doi: 10.1038/gim.2015.30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kovacs G, Berghold A, Scheidl S, et al. Pulmonary arterial pressure during rest and exercise in healthy subjects: a systematic review. Eur Respir J 2009; 34: 888–894. doi: 10.1183/09031936.00145608 [DOI] [PubMed] [Google Scholar]
- 12.Reiter G, Reiter U, Kovacs G, et al. Magnetic resonance-derived 3-dimensional blood flow patterns in the main pulmonary artery as a marker of pulmonary hypertension and a measure of elevated mean pulmonary arterial pressure. Circ Cardiovasc Imaging 2008; 1: 23–30. doi: 10.1161/CIRCIMAGING.108.780247 [DOI] [PubMed] [Google Scholar]
- 13.Rolf A, Rixe J, Kim WK, et al. Pulmonary vascular remodeling before and after pulmonary endarterectomy in patients with chronic thromboembolic pulmonary hypertension: a cardiac magnetic resonance study. Int J Cardiovasc Imaging 2015; 31: 613–619. doi: 10.1007/s10554-014-0580-z [DOI] [PubMed] [Google Scholar]
- 14.van der Bruggen CE, Happé CM, Dorfmüller P, et al. Bone morphogenetic protein receptor type 2 mutation in pulmonary arterial hypertension: a view on the right ventricle. Circulation 2016; 133: 1747–1760. doi: 10.1161/CIRCULATIONAHA.115.020696 [DOI] [PubMed] [Google Scholar]
- 15.Hemnes AR, Brittain EL, Trammell AW, et al. Evidence for right ventricular lipotoxicity in heritable pulmonary arterial hypertension. Am J Respir Crit Care Med 2014; 189: 325–334. doi: 10.1164/rccm.201306-1086OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brittain EL, Talati M, Fessel JP, et al. Fatty acid metabolic defects and right ventricular lipotoxicity in human pulmonary arterial hypertension. Circulation 2016; 133: 1936–1944. doi: 10.1161/CIRCULATIONAHA.115.019351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Talati MH, Brittain EL, Fessel JP, et al. Mechanisms of lipid accumulation in the bone morphogenetic protein receptor type 2 mutant right ventricle. Am J Respir Crit Care Med 2016; 194: 719–728. doi: 10.1164/rccm.201507-1444OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vonk-Noordegraaf A, Haddad F, Chin KM, et al. Right heart adaptation to pulmonary arterial hypertension: physiology and pathobiology. J Am Coll Cardiol 2013; 62: D22–D33. doi: 10.1016/j.jacc.2013.10.027 [DOI] [PubMed] [Google Scholar]
- 19.van de Veerdonk MC, Kind T, Marcus JT, et al. Progressive right ventricular dysfunction in patients with pulmonary arterial hypertension responding to therapy. J Am Coll Cardiol 2011; 58: 2511–2519. doi: 10.1016/j.jacc.2011.06.068 [DOI] [PubMed] [Google Scholar]
- 20.Ghio S, Gavazzi A, Campana C, et al. Independent and additive prognostic value of right ventricular systolic function and pulmonary artery pressure in patients with chronic heart failure. J Am Coll Cardiol 2001; 37: 183–188. doi: 10.1016/S0735-1097(00)01102-5 [DOI] [PubMed] [Google Scholar]
- 21.Benza RL, Miller DP, Gomberg-Maitland M, et al. Predicting survival in pulmonary arterial hypertension: insights from the Registry to Evaluate Early and Long-Term Pulmonary Arterial Hypertension Disease Management (REVEAL). Circulation 2010; 122: 164–172. doi: 10.1161/CIRCULATIONAHA.109.898122 [DOI] [PubMed] [Google Scholar]
- 22.Sanz J, Sánchez-Quintana D, Bossone E, et al. Anatomy, function, and dysfunction of the right ventricle: JACC state-of-the-art review. J Am Coll Cardiol 2019; 73: 1463–1482. doi: 10.1016/j.jacc.2018.12.076 [DOI] [PubMed] [Google Scholar]
- 23.Tezuka F, Hort W, Lange E, et al. Muscle fiber orientation in the development and regression of right ventricular hypertrophy in pigs. Acta Pathol Jpn 1990; 40: 402–407. [DOI] [PubMed] [Google Scholar]
- 24.Kurakula K, Smolders VFED, Tura-Ceide O, et al. Endothelial dysfunction in pulmonary hypertension: cause or consequence? Biomedicines 2021; 9: 57. doi: 10.3390/biomedicines9010057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Atkinson C, Stewart S, Upton PD, et al. Primary pulmonary hypertension is associated with reduced pulmonary vascular expression of type II bone morphogenetic protein receptor. Circulation 2002; 105: 1672–1678. doi: 10.1161/01.CIR.0000012754.72951.3D [DOI] [PubMed] [Google Scholar]
- 26.Grünig E, Weissmann S, Ehlken N, et al. Stress Doppler echocardiography in relatives of patients with idiopathic and familial pulmonary arterial hypertension: results of a multicenter European analysis of pulmonary artery pressure response to exercise and hypoxia. Circulation 2009; 119: 1747–1757. doi: 10.1161/CIRCULATIONAHA.108.800938 [DOI] [PubMed] [Google Scholar]
- 27.Claessen G, La Gerche A, Petit T, et al. Right ventricular and pulmonary vascular reserve in asymptomatic BMPR2 mutation carriers. J Heart Lung Transplant 2017; 36: 148–156. doi: 10.1016/j.healun.2016.06.018 [DOI] [PubMed] [Google Scholar]
- 28.Hinderhofer K, Fischer C, Pfarr N, et al. Identification of a new intronic BMPR2-mutation and early diagnosis of heritable pulmonary arterial hypertension in a large family with mean clinical follow-up of 12 years. PLoS One 2014; 9: e91374. doi: 10.1371/journal.pone.0091374 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material ERJ-00442-2024.Supplement (791.2KB, pdf)
This one-page PDF can be shared freely online.
Shareable PDF ERJ-00442-2024.Shareable (1.6MB, pdf)