Abstract
The Paramyxovirus respiratory syncytial virus (RSV) is the primary etiologic agent of serious epidemic lower respiratory tract disease in infants, immunosuppressed patients, and the elderly. Lower tract infection with RSV is characterized by a pronounced peribronchial mononuclear infiltrate, with eosinophilic and basophilic degranulation. Because RSV replication is restricted to airway epithelial cells, where RSV replication induces potent expression of chemokines, the epithelium is postulated to be a primary initiator of pulmonary inflammation in RSV infection. The spectrum of RSV-induced chemokines expressed by alveolar epithelial cells has not been fully investigated. In this report, we profile the kinetics and patterns of chemokine expression in RSV-infected lower airway epithelial cells (A549 and SAE). In A549 cells, membrane-based cDNA macroarrays and high-density oligonucleotide probe-based microarrays identified inducible expression of CC (I-309, Exodus-1, TARC, RANTES, MCP-1, MDC, and MIP-1α and -1β), CXC (GRO-α, -β, and -γ, ENA-78, interleukin-8 [IL-8], and I-TAC), and CX3C (Fractalkine) chemokines. Chemokines not previously known to be expressed by RSV-infected cells were independently confirmed by multiprobe RNase protection assay, Northern blotting, and reverse transcription-PCR. High-density microarrays performed on SAE cells confirmed a similar pattern of RSV-inducible expression of CC chemokines (Exodus-1, RANTES, and MIP-1α and -1β), CXC chemokines (I-TAC, GRO-α, -β, and -γ, and IL-8), and Fractalkine. In contrast, TARC, MCP-1, and MDC were not induced, suggesting the existence of distinct genetic responses for different types of airway-derived epithelial cells. Hierarchical clustering by agglomerative nesting and principal-component analyses were performed on A549-expressed chemokines; these analyses indicated that RSV-inducible chemokines are ordered into three related expression groups. These data profile the temporal changes in expression by RSV-infected lower airway epithelial cells of chemokines, chemotactic proteins which may be responsible for the complex cellular infiltrate in virus-induced respiratory inflammation.
Respiratory syncytial virus (RSV), named by its ability to induce fusion of infected epithelial cells (49), is a leading cause of epidemic respiratory tract illness in children (28). Spread primarily by contact with contaminated secretions, RSV replicates in the nasopharyngeal epithelium and spreads to the lower respiratory tract via epithelial cell-to-cell transfer along intracytoplasmic bridges (27). Although only two RSV serotypes, A and B, circulate in RSV epidemics (29), immunity to naturally acquired infection is incomplete, resulting in repeated infections through adulthood (24; reviewed in reference 28). In humans, RSV infection produces a spectrum of airway involvement ranging from otitis media to lower tract infection.
Clinically severe RSV infections involving the lower respiratory tract are primarily seen in young children with naïve immune systems and/or genetic predispositions (32), patients with suppressed T-cell immunity (such as heart transplant recipients [41]), and the elderly (48). In autopsy studies of fatal disease, RSV infection is characterized by the presence of cytoplasmic eosinophilic inclusion bodies, characteristic of viral replication, in airway epithelial cells; sloughing and necrosis of the epithelial surface; and concomitant mucous plugging of the airways with trapping of air (1, 18, 19). In addition to these manifestations of direct epithelial involvement, RSV infection produces a pronounced perivascular infiltrate of mononuclear cells and lymphocytes (1, 18) and a neutrophil-rich exudate detected by bronchoalveolar lavage (16). Finally, the presence of eosinophil cationic protein (20, 30) and histamine (64) in nasal secretions at concentrations that correlate with disease severity suggests the participation of eosinophils and basophils in the pathology of RSV infection.
The mechanisms responsible for recruitment of circulating leukocytes, mononuclear cells, and lymphocytes into the lung as a consequence of RSV infection are largely unknown. Cellular recruitment into inflamed tissues is a multistep process in which circulating leukocytes first demarginate, adhere to stimulated endothelial cells, and subsequently become activated. Activated leukocytes then migrate through the vascular endothelium toward chemical gradients of chemoattractant peptides or antigens (reviewed in reference 58). Recent attention has focused on the important role of chemokines in mediating immune cell chemotaxis into the airways. Chemokines are a superfamily of proteins divided into functionally distinct groups: three groups of small basic (heparin-binding) proteins, termed the C, CC, and CXC chemokines (based on the number and spacing of highly conserved NH2-terminal cysteine residues), and a fourth, distantly related group, the CX3C chemokines, composed of large, membrane-bound glycoproteins attached through a COOH-mucin-like domain (4; reviewed in references 3 and 53). That receptors for the chemokines are expressed in a cell type-restricted fashion has allowed specificity in chemokine action—for example, members of the C group primarily activate lymphocyte chemotaxis, members of the CXC group induce neutrophil chemotaxis, and the CC group stimulates monocyte, lymphocyte, and eosinophil chemotaxis (3, 53). These data suggest that pulmonary inflammation associated with neutrophilic and monocytic infiltration is the result of coordinate expression of diverse chemokines with distinct cellular specificities.
Although a number of cell types inducibly secrete chemokines in inflamed tissues, a body of evidence supports a central role for the airway epithelium as an important initiator and modifier of pulmonary inflammation after exposure to environmental irritants or infectious agents (reviewed in reference 42). The epithelium plays a central role in initiating pulmonary inflammation, particularly in the case of RSV, since this virus productively replicates only in the respiratory mucosa, inducing secretion of interleukins, growth factors, cytokines, and chemokines in vitro (2, 8, 21, 51, 54) and in vivo (26). These data, in conjunction with the recent demonstration by Haeberle et al. that mice deficient in MIP-1α, a CC chemokine expressed predominantly in airway epithelial cells, show reduced inflammatory cell recruitment (26), strongly indicate that epithelial cell-derived chemokine expression plays an important role in pulmonary inflammation. However, a more comprehensive examination of the pattern and secretion of chemokine expression by RSV-infected epithelial cells has not been performed.
We and others have shown that RSV infection of well-differentiated type II-like A549 cells produces a time- and dose-dependent induction of mRNA for CC (RANTES) and CXC (interleukin-8 [IL-8]) chemokines (21, 52). In this report we globally profile the kinetics and patterns of chemokine expression in RSV-infected airway cells. In type II alveolar cell-like A549 cells, membrane-based cDNA macroarrays and high-density oligonucleotide probe-based microarrays identified inducible expression of CC chemokines (I-309, Exodus-1, thymus and activation-regulated chemokine [TARC], RANTES, monocyte chemotactic protein 1 [MCP-1], macrophage-derived chemokine [MDC], and macrophage inflammatory protein 1α and 1β [MIP-1α and -1β]), CXC chemokines (growth-regulated gene α, β, and γ [GRO-α, -β, and -γ], epithelial cell-derived neutrophil attractant 78 [ENA-78], IL-8, and interferon-inducible T-cell α chemoattractant [I-TAC]), and CX3C chemokines (Fractalkine). Expression of these chemokine genes was confirmed by independent techniques. In small airway epithelial (SAE) cells, a similar pattern of CC chemokine (Exodus-1, RANTES, and MIP-1α and -1β), CXC chemokine (I-TAC, GRO-α, -β, and -γ, and IL-8), and CX3C chemokine (Fractalkine) expression was detected, yet TARC, MDC, and MCP-1 were not inducible. Clustering analysis indicated that the chemokine genes are contained within three major expression groups. These data suggest the involvement of lymphotactic, NK, and dendritic cell-activating chemokines as potential mediators of the RSV-initiated immune response.
MATERIALS AND METHODS
RSV preparation.
The A2 strain of RSV was propagated on HEp-2 cells (American Type Culture Collection [ATCC], Manassas, Va.) and purified by polyethylene glycol precipitation followed by centrifugation in a 35-to-65% discontinuous sucrose gradient (21, 61). The titer of the sucrose cushion-purified RSV (pRSV) was determined by methylcellulose plaque assay (39), and the pRSV was snap-frozen until use. No contaminating cytokines, including IL-1, IL-6, IL-8, tumor necrosis factor, granulocyte-macrophage colony-stimulating factor, or interferons, were detectable in these preparations (36). UV-inactivated pRSV (UV-pRSV) was prepared as described previously(36).
Cell culture and viral infection.
Human A549 cells with characteristics of type II alveolar cells were obtained from ATCC and grown under standard conditions (36). Primary human SAE cells were from Clonetics and were grown as previously described (52). For pRSV infection, 90% confluent cells were infected with pRSV at a multiplicity of infection (MOI) of 1. Frozen RSV stock was rapidly thawed and diluted with Dulbecco's modified Eagle medium containing 2% fetal bovine serum. The virus was added immediately to flasks (0.04 ml of diluted virus per cm2) after removal of culture medium. An equivalent amount of sucrose solution was added to the control culture (which received no RSV). After addition of virus, the flasks were rocked mechanically for 1 h at 37°C, and then 0.2 ml of Dulbecco's modified Eagle medium containing 2% fetal bovine serum was added to the culture flasks. The infection was continued for the indicated times at 37°C.
RPA.
Chemokine mRNA levels were analyzed by a multiprobe RNase protection assay (RPA) using the RiboQuant multiprobe set (PharMingen, San Diego, Calif.). Total RNA was extracted from control or RSV-infected A549 cells by acid guanidium-phenol extraction (TRI reagent; Sigma, St. Louis, Mo.), and RNA abundance was quantitated spectrophotometrically. Five micrograms of total RNA was hybridized overnight to an α-32P-labeled RNA template (RiboQuant; PharMingen) containing individual riboprobes for the human lymphotactin, RANTES, MIP-1α, MCP-1, IL-8, and I-309 genes and the L32 and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) housekeeping genes. Samples were hybridized overnight at 42°C, and single-stranded RNA and free probe were digested by RNase A and T1. Subsequently, protected RNA was phenol-chloroform extracted, ethanol precipitated, and analyzed on a QuickPoint Pre-Cast Gel (Novex, San Diego, Calif.). The quantity of protected RNAs was determined using a PhosphorImager and ImageQuant software (both from Molecular Dynamics, Sunnyvale, Calif.). The chemokine transcripts were identified by the lengths of the respective fragments. For quantitation, chemokine levels were expressed as percentages of the mean levels of the L32 and GAPDH housekeeping genes for each RNA sample.
Membrane-based cDNA macroarrays
Total RNA was extracted from control or pRSV-infected A549 cells by acid guanidium-phenol extraction (TRI reagent; Sigma). RNA was treated with RNase-free DNase to remove contaminating genomic DNA, and RNA integrity was confirmed by gel electrophoresis and reverse transcription-PCR (RT-PCR). Five micrograms of total RNA was reverse transcribed in the presence of 35 μCi of [α-33P]dATP, and cDNA was purified by column chromatography (Chroma spin-200; Clontech). The final probe concentration was adjusted to 106 cpm/ml, and hybridization was performed overnight at 68°C. The membrane was washed three times in 2× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate)–1% sodium dodecyl sulfate (SDS) at 68°C for 30 min and twice in 0.1× SSC–0.5% SDS at 68°C for 30 min. Membranes were exposed to a PhosphorImager cassette, and relative changes in hybridization intensity were determined with AtlasImage 1.01 software (Clontech, Palo Alto, Calif.). Comparisons of mRNA populations between control and RSV-infected A549 cells were performed with two different sets of Atlas Array membrane lots in two independent experiments.
Hybridization intensity was determined after the array was aligned to the reference grid template to determine the target location for each of the duplicate 268 human cDNAs, nine housekeeping cDNAs, and negative controls. For each gene, local background was determined and subtracted, and the average signal intensity was determined for duplicate spots. Data were discarded when significant deviations between duplicates were found, or if signal intensity was not above background. For assessment of differences in gene expression between arrays, data were examined in several different ways. Changes in raw signal intensity were compared for the treated and control samples; the intensity value of each cDNA was normalized to those of designated housekeeping genes; and finally, the intensity values were normalized to the sum of the intensity values of all the genes represented on the chip. Because these comparisons yielded equivalent information, only the raw values are presented. Only those genes which showed at least an average twofold upregulation or downregulation across duplicate membrane lots were listed as differentially expressed.
Oligonucleotide probe-based microarrays.
For the oligonucleotide probes, the Hu95A Gene Chip (Affymetrix Inc., Santa Clara, Calif.), containing 12,626 sequenced human genes, was used. First-strand cDNA synthesis was performed using total RNA (10 to 25 μg), a T7–(dT)24 oligomer (5′-GGCCAGTGAATTGTAA TACGACTCA CTATAGGGAGGC GG–dT24-3′), and SuperScript II reverse transcriptase (Life Technologies). Second-strand synthesis converted the cDNA into a double-stranded DNA template for use in an in vitro transcription reaction. The T7 promoter introduced during first-strand cDNA synthesis provided the necessary sequence for directing the synthesis of cRNA using bacteriophage T7 RNA polymerase. The cRNA or target RNAs were labeled with biotin during the in vitro transcription reaction. Biotin-labeled target RNAs were fragmented to a mean size of 200 bases to facilitate their hybridization to probe sequences on the Gene Chip array. Each target RNA sample was initially hybridized to a test array to confirm the successful labeling of the target RNAs and to prevent the use of degraded or nonrepresentative target RNA samples. The test array contained a set of probes representing genes that are commonly expressed in the majority of cells (actin, GAPDH, transferrin receptor, transcription factor ISGF-3, 18S RNA, 28S RNA, and Alu genes).
Hybridization of the Hu95A Gene Chip arrays was performed at 45°C for 16 h in hybridization buffer (0.1 M morpholineethanesulfonic acid [pH 6.6], 1 M NaCl, 0.02 M EDTA, and 0.01% Tween 20). Four prokaryotic genes (bioB, bioC, and bioD from the Escherichia coli biotin synthesis pathway and cre, the recombinase gene from bacteriophage P1) were added to the hybridization cocktail as internal controls. Arrays were washed using both nonstringent (1 M NaCl, 25°C) and stringent (1 M NaCl, 50°C) conditions prior to staining with phycoerythrin-streptavidin (final concentration, 10 μg/ml).
Gene Chip arrays were scanned using a Gene Array Scanner (Hewlett-Packard) and analyzed using the Gene Chip Analysis Suite 3.3 software (Affymetrix Inc.). For each gene, 16 to 20 probe pairs were immobilized as ∼25-mer oligonucleotides that hybridized throughout the mRNA; each probe pair is represented as a perfect match (PM) oligonucleotide and a mismatch (MM) oligonucleotide used as a hybridization control. The average intensity of each probe cell was calculated after subtraction of the local background (the lowest 2% intensity of each sector; each probe cell is divided into 16 sectors). The normalized average intensity value was used to determine the number of positive and negative probe pairs. Based on the positive/negative ratio, the positive fraction, and the log average ratio of the PM to the MM, the absolute call (i.e., the gene is detected ([“present”] or not [“absent”]) was determined (68). Finally, the average difference was determined by calculating the difference in intensity between the PM and MM of every probe pair and averaging the differences over the entire probe set. The average difference statistic was retrieved for quantification of mRNA abundance in those samples in which the absolute call indicated that the gene was present. Probe set data were deposited into our data warehouse and relational database server.
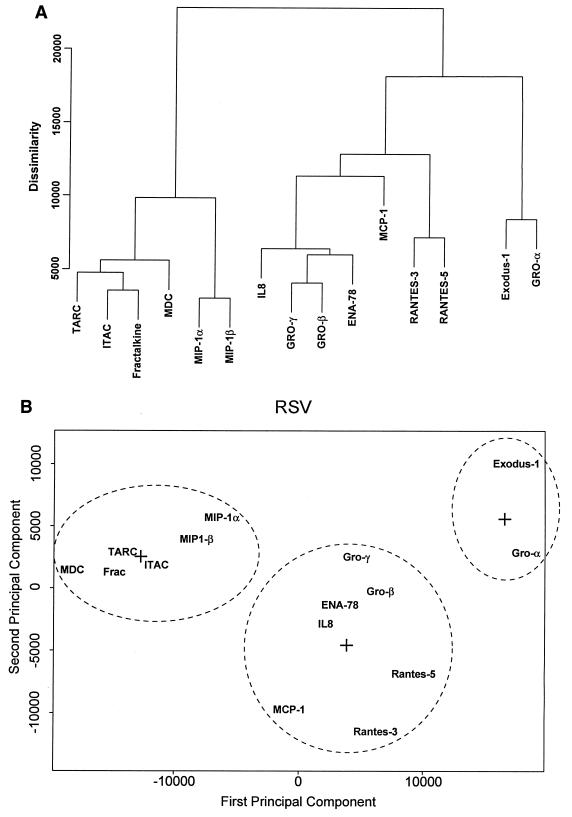
Among the several clustering methods that were tried, agglomerative nesting (AGNES [Splus version 5 for Unix; Mathsoft, Inc.]) (38) gave the most consistent results. AGNES calculates a hierarchy of clusterings that form a single hierarchical tree. This “bottom-up” approach uses each gene as a cluster itself, merging with its nearest neighbor until only one large cluster, containing all the objects, remains. At each stage the two “nearest” clusters are combined to form one larger cluster. While the “average” method (where the distance between two clusters is determined by the average of the dissimilarities between the points in one cluster and the points in the other cluster) was used for data presentation in this report, no qualitative difference was seen between this method and that using minimal or maximal dissimilarity to distinguish clusters. The dissimilarity matrix was constructed using Euclidean distances. The dendrogram in Fig. 7 was calculated using raw gene chip data (the average difference); however, no qualitative difference in the dendrogram was produced after the raw data were standardized to z scores. Principal-components analysis (PCA) of the chemokine data was also carried out using the k means method to test if a different technique would reproduce the hierarchical cluster result. The k means method is an exploratory multivariate statistical technique which chooses a prespecified number of cluster centers and minimizes the within-class sum of the squares from those centers. This allows each probe set to be assigned to one of the k groups, where group membership is determined by calculating the centroid for each group and then assigning each probe set to the group with the closest centroid, continuing iteratively until convergence is reached. We used the calculated chemokine dissimilarity matrix based on Euclidean distances as the input to the PCA for a k value of 2 to 5; the best fit was produced for a k value of 3.
FIG. 7.
Clustering analysis of chemokine expression patterns. (A) Hierarchical clustering, performed by AGNES on data for 19 probe sets belonging to the chemokine group. The “agglomerative coefficient,” a figure of merit that measures the amount of clustering structure found, was 0.74, indicating the best cluster relationship of the methods tried. For each gene, the dissimilarity value is plotted. The degree of dissimilarity is indicated by the height of a common line which connects the two nodes. In the array, RANTES was detected by three independent probe sets; the data for RANTES probe set 3 (no. 1403_s_at), and probe set 5 (no. 1405_i_at) correspond to the behavior of the endogenous gene (compare Fig. 3A and 4A). (B) PCA of the chemokine gene set. Shown is the result of iterative convergence of a k means PCA where three centroids (represented by plus signs) are specified on the chemokine gene set. Dashed ovals each contain the members of a group.
Northern blotting and RT-PCR.
Twenty micrograms of total RNA was fractionated on a 1.2 or 1.5% agarose-formaldehyde gel and transferred to Zeta-Probe GT blotting membranes (Bio-Rad, Hercules, Calif). RNA was UV cross-linked to the blots and prehybridized as described previously (37). The blots were hybridized overnight at 60°C in 5% SDS hybridization buffer with the indicated cDNA probes at a final concentration of 2 × 106 cpm/ml (62, 63). cDNA probes were labeled with [α-32P]dATP in the PCR using cDNA templates and the following primers. For Exodus-1, a 359-nucleotide (nt) fragment was produced using sense primer 5′-ACCAAGAGTTTGCTCCTGGCT-3′ and antisense primer 5′-TGCAAGTGAAAC CTCCAACCC-3′; for MDC, a 357-nt fragment was produced using sense primer 5′-GCCAACATGGA AGACAGCG-3′ and antisense primer 5′-ACAGCACGGAGGTGACCAA-3′; for I-TAC, a 369-nt fragment was produced using sense primer 5′-TGTCTTTGCA TAGGCCCTGG-3′ and antisense primer 5′-CCTTTCACCCAC CTTTCATCC-3′; for TARC a 340-nt fragment was produced using sense primer 5′-AGAGGGACCTG CACACAGAGA-3′ and antisense primer 5′-GGTGAGGAGG CTTCAAGACCT-3′; for thymosin-β10 a 359-nt fragment was produced using sense primer 5′-TGGCAGACAA ACCAGACATGG-3′ and antisense primer 5′-AAACCGGAGA ATTTGGCAGTC-3′; for RSV nucleocapsid the sense primer was 5′-CAAATGG ATCC ATGGCTCT TAGCAAAG TCAAG-3′ and the antisense primer was 5′-TTCCCGGGT CAAAGCTCTACATCATTATC-3′. After hybridization, the membrane was washed with a buffer containing 5% SDS and 1× SSC for 15 min at room temperature, followed by 20 min at 60°C. The membranes were exposed to XAR film (Kodak) for 24 to 48 h.
For RT-PCR, oligo(dT)-primed cDNA was synthesized from 5 μg of A549 RNA and used as a template to amplify a 373-bp Fractalkine cDNA using the upstream primer 5′-GGCTCCGATA TCTCTGTCGTG-3′ and the downstream primer 5′-CCACAGACT CGTCCATTCCC-3′. Cycling conditions were as follows: 40 cycles of denaturation at 94°C for 45 s, annealing at 62°C for 60 s, and extension at 72°C for 90 s, and a final extension at 72°C for 10 min. As an internal control, a 359-nt thymosin-β10 cDNA fragment was amplified using the primers given above. Cycling conditions were as follows: 25 cycles of denaturation at 94°C for 20 s, annealing at 68°C for 30 s, extension at 72°C for 30 s, and a final extension at 72°C for 10 min. After PCR, samples were fractionated by agarose gel electrophoresis, denatured, transferred, and blotted with a radiolabeled Fractalkine or thymosin cDNA probe.
RESULTS
Temporal profile of CC chemokine expression.
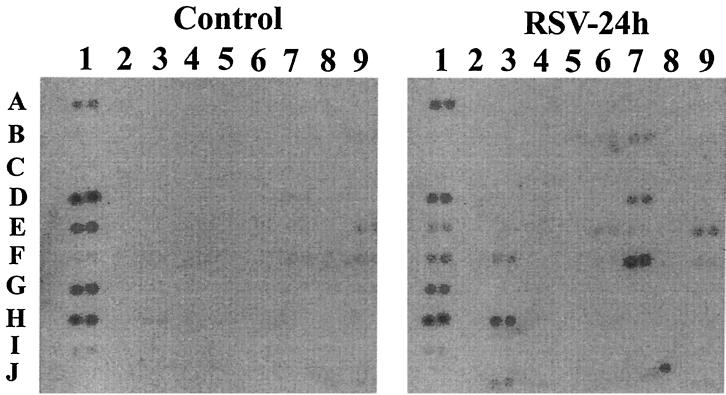
Type II alveolar epithelial cells (A549) infected with pRSV support viral replication, as demonstrated by recovery of infectious virus in the supernatant (9 to 12 h after infection), viral antigen production, and syncytium formation (18 to 24 h after infection) (7, 21). We have previously shown that pRSV infection of A549 cells induces a time- and dose-dependent induction of IL-8, RANTES, MCP, and MIP-1 expression (21, 52). Epithelial chemokine expression is dependent on viral replication, because treatment with nonreplicative UV-pRSV is unable to induce IL-8 or RANTES expression (12, 21). To obtain a more comprehensive profile of gene expression induced by pRSV infection, we first profiled control and infected A549 cellular mRNA using nylon membrane-based cDNA macroarrays representing 268 chemokine, cytokine, and cytokine receptor genes. A representative autoradiogram of the pairwise comparison is shown in Fig. 1. In these experiments, 73 genes of a total of 268 (27%) represented on the membrane were changed twofold or more (either increased or decreased) as a consequence of pRSV infection: 7 chemokine genes, 17 cytokine genes, 9 cytokine receptor genes, 15 peptide growth factor genes, 12 growth factor receptor genes, 3 structural genes, 2 antiviral genes, and 8 genes of unknown function. Because our purpose was to profile chemokine expression, these data were analyzed in detail. Table 1 presents the mean data from duplicate hybridizations. We observed increased expression of all seven chemokines present on the array; these included four previously characterized chemokines, the CC chemokines RANTES, MIP-1α, and MCP-1 and the CXC chemokine IL-8 (21, 52). However, we also noted expression of three unanticipated chemokines, including robust expression of the CC chemokine I-309 and the CXC chemokines ENA-78 and GRO-β/MIP-2. As is apparent from the autoradiogram, for many of the chemokines the signals in the control samples were so close to background that calculation of the fold increase due to pRSV infection was unreliable.
FIG. 1.
Membrane-based array to identify RSV-inducible genes. A Clontech membrane-based cDNA expression array (“cytokine chip”) was used to hybridize radiolabeled RNA from uninfected (control) and pRSV-infected (24 h) cells. Shown is an autoradiogram of the exposure. For orientation, probe set 7F is RANTES (see Table 1 for further orientation information). For each sample, hybridization intensity was determined by exposure to a PhosphorImager cassette for each gene (represented in duplicate spots), normalized to local background, and analyzed by normalizing either to an internal control (thymosin-β10) or to total hybridization signals, with essentially identical results (raw values are given in Table 1).
TABLE 1.
RSV-inducible chemokines detected by membrane-based DNA macroarraysa
| Chemokine | Locationb | Hybridization intensityc in:
|
Δd | Fold Δe | |
|---|---|---|---|---|---|
| Control cells | RSV-infected cells | ||||
| RANTES | 7F | 307 | 1,550 | 1,243 | 5 |
| I-309/SCYA1 | 7I | 7 | 331 | 324 | 47.3 |
| MIP-2α | 7B | 52 | 205 | 153 | 3.9 |
| MCP-1 | 7D | 82 | 234 | 152 | 2.9 |
| IL-8 | 3J | 70 | 140 | 70 | 2 |
| ENA-78 | 7E | 47 | 101 | 54 | 2.9 |
| MIP-1α/LD78 | 6N | 7 | 37 | 30 | 5.3 |
Uninfected A549 cells and those infected with RSV (at an MOI of 1) were harvested 24 h after infection and hybridized to an Atlas cytokine chip. Data for C, CC, and CXC cytokines were obtained and sorted according to changes in hybridization intensity.
Reference location of duplicate spot on Clontech membrane. See Fig. 1.
Expressed as signal intensity (arbitrary units) above local background. For each gene, pairwise comparison to the control was performed. Data are average values from two independent viral infections and membrane hybridizations.
Arithmetic difference in corrected hybridization intensity (corrected for local background) between RSV-infected and control samples.
Fold difference between RSV-infected and control samples.
Oligonucleotide probe-based microarray of RSV-infected A549 cells.
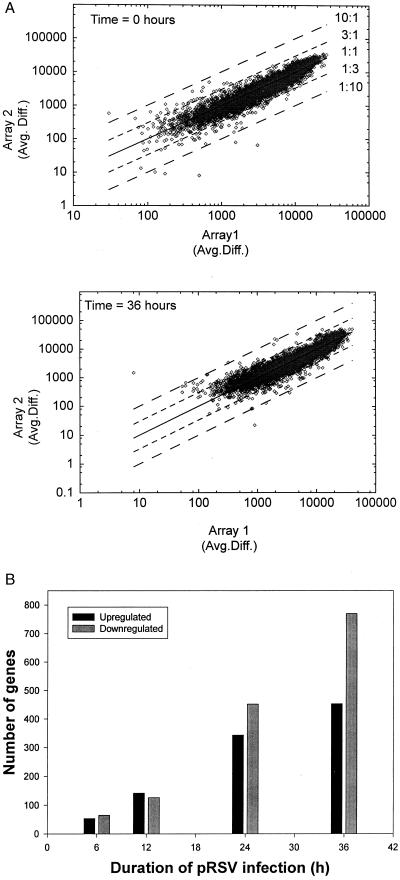
To more reliably identify additional chemokines induced by pRSV, we profiled global changes in gene expression using an oligonucleotide probe set containing ∼12,626 sequenced human genes (Affymetrix Hu95A Gene Chip). With this methodology, the detection of hybridized biotinylated target mRNA after binding of a phycoerythrin-streptavidin conjugate has a large dynamic range of more than 5 orders of magnitude, and in practice, this method can detect 1 to 103 copies of mRNA per cell. In Fig. 2A, we analyze the reproducibility of the data generated in two independent time courses by log-log plots of the average difference signal for the independently performed arrays for each time point of pRSV infection. The data were tightly clustered within ±3-fold changes in expression. At each time point, linear regression analysis was performed. In aggregate, the data were described by a slope of 1.22 (n = 5) with a Pearson correlation coefficient greater than 0.9 for each pairwise comparison (Fig. 2A). This indicates that the data can be described as a linear relationship and that data points from the two experiments were highly reproducible. In Fig. 2B, we plot the number of genes whose expression changed by a factor of 3 (conservatively chosen to minimize the number of false positives) relative to that in uninfected cells. At the early time points (6 and 12 h), the number of genes upregulated was approximately equal to the number of genes downregulated and corresponded to ∼1% of all the genes on the chip. At the later times, downregulated genes predominated, with nearly twice as many genes downregulated as upregulated at 36 h. Nearly 10% of the total genes on the chip showed significant changes in expression at 36 h, indicating that pRSV infection had a significant effect on global gene expression (Fig. 2B).
FIG. 2.
Analysis of oligonucleotide-based microarrays. (A) Reproducibility of experimental time courses. Pairwise comparisons of the average differences for the data in time series 1 (Array 1) versus those in time series 2 (array 2) are plotted. The only criterion for inclusion was that the probe set was designated “present” in both time series. Least-squares linear regression was used to determine the fit to a straight line. For the 0-h data set, the regression was described by the equation y = 1.172x − 715.408 (r2 = 0.935), and for the 36-h data set, the equation was y = 1.198x − 741.671 (r2 = 0.911). (B) Kinetics of changes in total gene expression after pRSV infection. The average difference for each probe set is proportional to its level of expression. Genes whose average difference values differed threefold from those of uninfected cells were determined by algorithm. Shown are individual values for genes upregulated and genes downregulated by RSV infection, taken as means of two independent time courses. The total number of genes influenced was 118 after 6 h of pRSV infection (∼5% of the total genes expressed [scored “present”] at that time and 1% of all the genes on the chip), 267 after 12 h of infection, 796 after 24 h, and 1,200 after 36 h (∼10% of all genes on the chip).
We further analyzed the data by classifying threefold-regulated genes by their primary functions. However, no single biochemical process could be identified; of genes significantly influenced by RSV, we could identify the following functional groups: chemokines (16 unique members), cytokines (14 genes), growth factors (39 genes), putative antiviral factors (30 genes), receptors (87 genes), cytoskeletal genes (165 genes), DNA repair and chromosomal maintenance genes (104 genes), histocompatibility and cell surface markers (15 genes), metabolic genes (264 genes), oncogenes (91 genes), genes involved in RNA processing, protein translation, or metabolism (78 genes), secreted peptides (49 genes), signaling molecules and kinases (226 genes), transcription factors (209 genes), and those of unknown function (543 genes). These observations suggest that RSV infection does not affect expression of genes belonging to a single biological pathway but causes significant perturbation of global gene expression controlling multiple cellular processes.
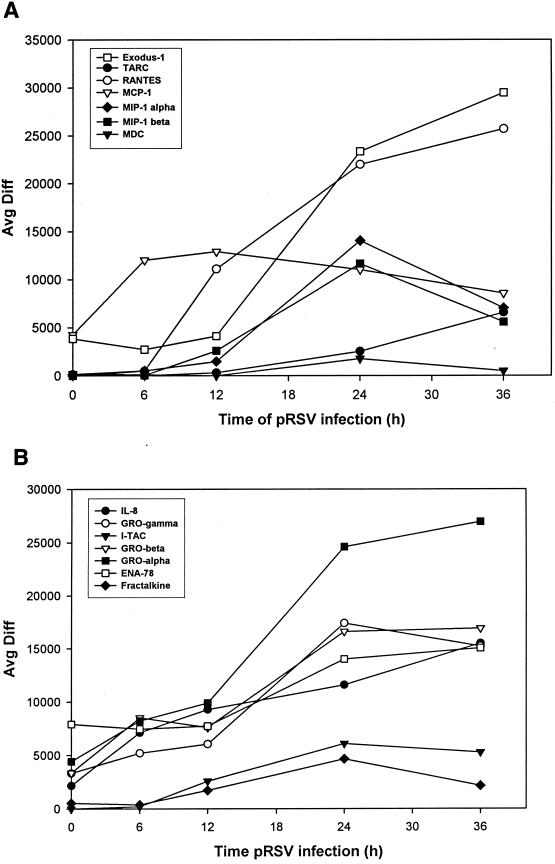
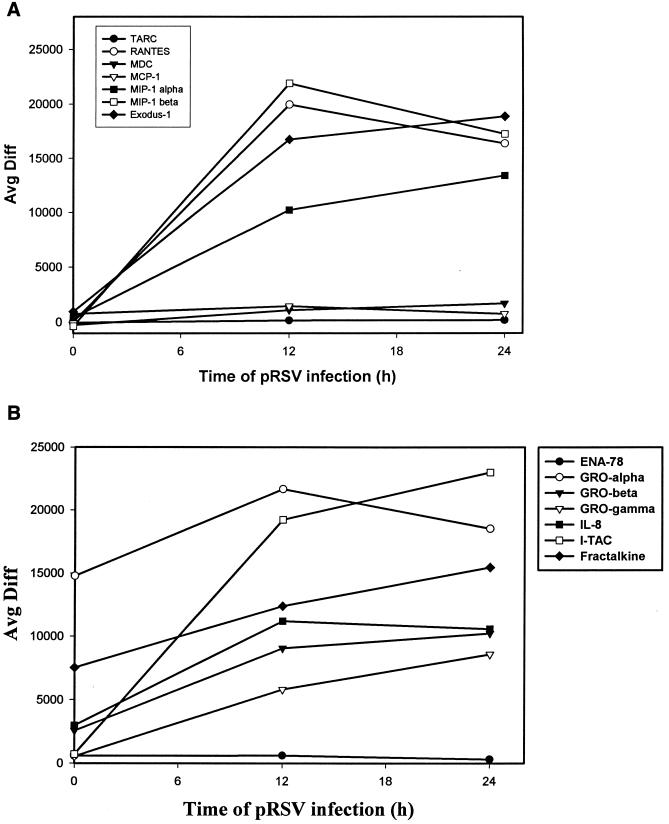
Individual records for the time course were retrieved for CC, CXC, and CX3C chemokines, and changes in hybridization intensity as a function of time were analyzed (no C chemokines were detected). Figure 3A displays observations for the CC chemokines where the average difference statistic is plotted as a function of time of pRSV infection. As expected, robust induction of RANTES, MIP-1α, MCP, and IL-8 was detected (compare with Table 1). In addition, expression of the CC chemokines Exodus-1/LARC/MIP-3α (31, 33), TARC (40), and MDC (reference 25 and references therein) was detected. Of these, expression of Exodus-1 was strongly induced at 24 and 36 h of pRSV infection, in a sigmoidal curve quite similar to that of RANTES. TARC was also induced at later times of pRSV infection, with a distinct nonsigmoidal induction profile. Figure 3B displays the changes in expression of the CXC subgroup of chemokines. As previously described, IL-8 is induced with a linear profile with respect to time (compare Fig. 3B with Table 1). In addition, the neutrophilic chemokines GRO-α, -β, and -γ and ENA-78 and the lymphocyte chemoattractant I-TAC (65) were strongly induced. Finally, we detected inducible expression of Fractalkine, a distantly related CX3C-containing member of the chemokine superfamily (4). Fractalkine expression peaked 24 h after pRSV infection in A549 cells and fell thereafter, producing an expression profile similar to that seen with I-TAC (Fig. 3B).
FIG. 3.
Changes in chemokine expression after RSV infection. (A) CC chemokines. The hybridization intensity (average difference) for each chemokine is plotted as a function of time of pRSV infection. Data shown here are means for independent RANTES probe set 3 (Affymetrix probe set no. 1403_s_at) and probe set 5 (no. 1405_i_at). At time zero, the absolute call for TARC, RANTES, MDC, and MIP-1α and -1β was “absent,” indicating that the signal hybridization was below the background noise of the microarray. (B) CXC chemokines. Data are plotted as described for Fig. 3A. The CX3C chemokine Fractalkine is included.
Confirmation of chemokine expression: multiprobe RPA.
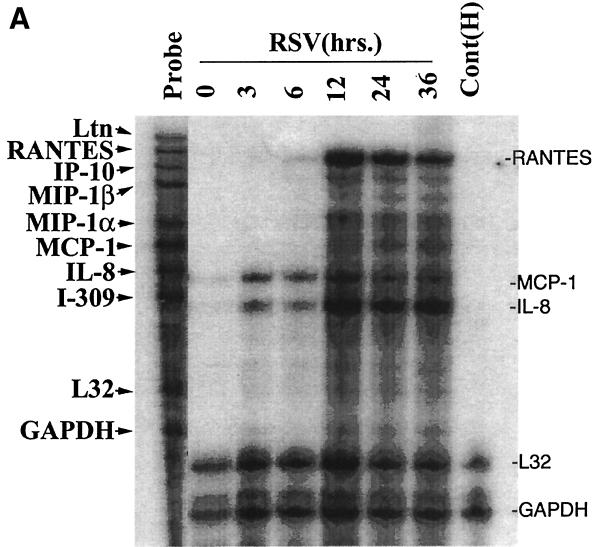
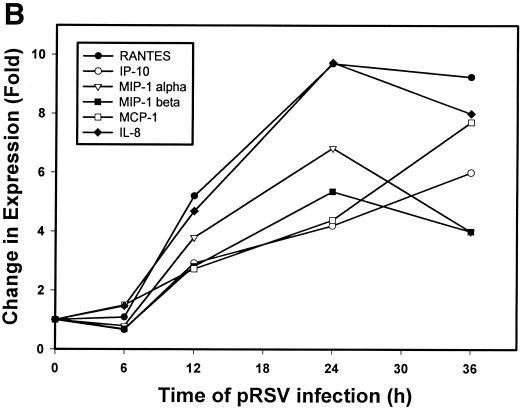
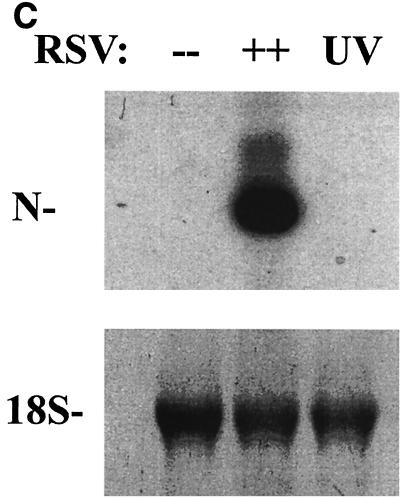
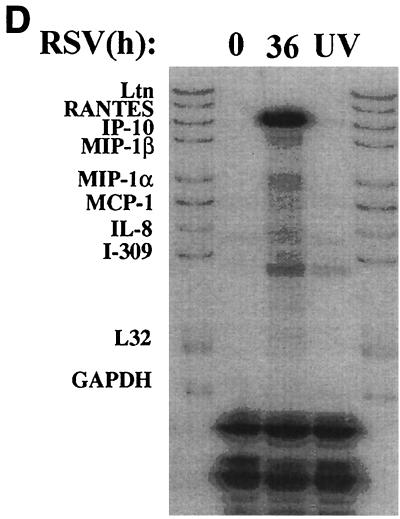
To independently confirm the induction of chemokine gene expression suggested by the microarray analyses, we first analyzed temporal changes in chemokine expression by multiprobe RPA. Steady-state levels of mRNA were measured with templates for the CC chemokines lymphotactin, RANTES, MIP-1α, MCP-1, IL-8, and I-309 and for the L32 and GAPDH housekeeping genes at various times (3, 6, 12, 24, and 36 h) after pRSV infection (MOI = 1). Figure 4A shows the kinetics of chemokine expression in a representative time course. The mean induction of genes in triplicate time courses assayed by RPA is shown in Fig. 4B. The CC chemokine RANTES was the most strongly induced chemokine, with ∼9.5-fold induction (relative to the control) peaking 24 h after pRSV infection. Interferon-inducible protein 10 (IP-10), MIP-1α and -1β, MCP-1, and the CXC chemokine IL-8 were also induced. In contrast, we did not consistently detect expression of the C chemokine lymphotactin. To determine whether induction of these chemokines was dependent on viral transcription and replication, the effect of UV-pRSV on chemokine expression was determined. Figure 4C shows that A549 cells adsorbed with UV-pRSV for 36 h had no detectable expression of the transcript encoding the nucleocapsid protein. Multiprobe RPA was performed on the same RNA samples (Fig. 4D); although abundant expression of RANTES, IP-10, MIP-1β, MIP-1α, MCP, and IL-8 was detected in cells adsorbed with replication-competent pRSV, no significant induction was observed with UV-pRSV. These data indicate that, at least for these chemokines, RSV replication and transcription are required for inducible expression.
FIG. 4.
Confirmation of chemokine expression. (A) Multiprobe RPA of CC chemokines. A549 cells were infected with pRSV for the indicated times (3, 6, 12, 24, and 36 h). Uninfected cells treated with sucrose alone served as a control (“0 RSV”). Total RNA was extracted and analyzed for changes in expression of the CC chemokines lymphotactin, RANTES, MIP-1α, MCP-1, IL-8, and I-309 and the L32 and GAPDH housekeeping genes by multiprobe RPA. Shown is a representative autoradiogram with the identities of the undigested CC chemokine probes given. Cont(H), human RNA control; Ltn, lymphotactin. (B) Quantitation of CC chemokine induction. The RPA autoradiogram was exposed to a PhosphorImager cassette, and the intensity of each band was determined relative to local background. For each sample, the signal was normalized to the internal control GAPDH, present in each lane. The normalized signal is plotted as a function of time of pRSV infection. Data are means of triplicate experiments. (C) UV-pRSV is deficient in viral transcription. Shown is a Northern blot of RSV nucleocapsid (N) expression in A549 cells exposed for 36 h to nothing (−−), replication-competent pRSV (++), or UV-pRSV at an MOI of 1. The N transcript is undetectable in UV-pRSV-treated cells. (D). Multiprobe RPA of CC chemokines after UV-pRSV exposure. A549 cells were exposed to replication-competent pRSV (36) or to UV-pRSV (UV) as described for Fig. 4C. Total RNA was extracted and analyzed for changes in CC chemokine expression by RPA. Shown is a representative autoradiogram. First and last lanes, input probe for transcript identification.
Northern blot confirmation of novel chemokine gene expression.
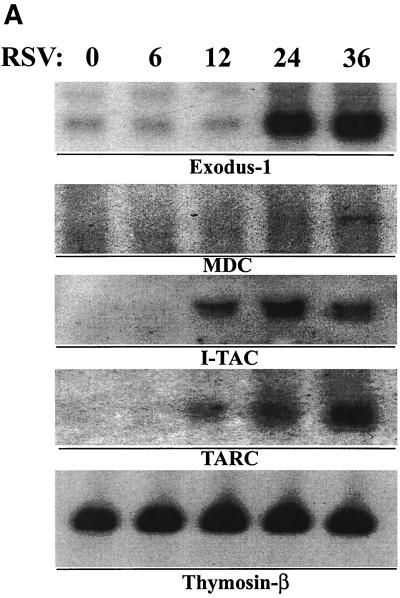
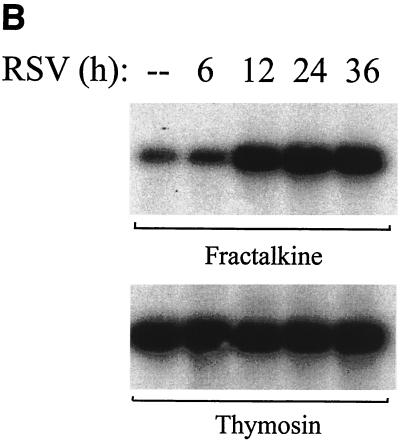
To confirm expression of the newly identified RSV-induced CC and CXC chemokine genes suggested by the oligonucleotide array, we performed Northern blot assays to detect changes in the steady-state expression of the lymphocyte and monocyte chemoattractants, TARC, MDC, Exodus-1, and I-TAC in pRSV-infected A549 cells (Fig. 5A). Expression of 0.8-kb Exodus-1 transcripts was only faintly detectable in control cells; however, robust increases in expression occurred 24 and 36 h after pRSV infection. The pattern of Exodus-1 mRNA induction and its changes in hybridization intensity on the oligonucleotide array are quite similar (compare Fig. 5A and 3A). At the limits of the Northern blot detection, the 2.9-kb MDC transcript was only faintly detectable after 36 h of infection. In contrast to that of Exodus-1, a 1.4-kb I-TAC transcript was not detectable in control cells but was induced after 12 h of pRSV infection, peaking at 24 h postinfection and falling thereafter. Levels of the internal control houskeeping transcript thymosin-β10 were not altered at these times of pRSV infection (Fig. 5A, bottom panel). Fractalkine expression was detectable by semiquantitative RT-PCR assay (Fig. 5B), where the steady-state abundance of the specific 373-nt product was induced after 12 h of pRSV infection and apparently peaked after 24 h of infection; this induction pattern was similar to the pattern of changes in the average difference statistic (compare to Fig. 3B). These data validate the expression of lymphocyte-specific CXC and CC chemokines predicted by the oligonucleotide array.
FIG. 5.
Profile of novel chemokines induced by pRSV. (A) Northern blot analysis. Total RNA was fractionated by morpholinepropanesulfonic acid (MOPS)-formaldehyde-agarose gel electrophoresis and transferred to nylon membranes. cDNA probes specific for Exodus-1, TARC, MDC, I-TAC, and the internal control thymosin β-10 were used to hybridize individual membranes. Shown is an autoradiographic exposure of the washed membranes. These results were repeated in three independent time courses. (B) RT-PCR for Fractalkine. Total mRNA was reverse transcribed and PCR amplified under linear cycling conditions using Fractalkine-specific primers (top) or primers specific for the internal control β-thymosin (bottom). The specific product is shown.
Oligonucleotide-based microarrays of RSV-infected bronchiolar cells.
SAE cells are well-differentiated primary cells obtained from normal human bronchioli (52). By immunohistochemistry, SAE cells are positive for cytokeratin 19, negative for vimentin (indicating epithelial origin), and negative for alkaline phosphatase (a marker of lung type II epithelial cells). To confirm and extend our studies to other types of lower respiratory tract epithelial cells, SAE cells were pRSV infected, and RNA was extracted and analyzed by oligonucleotide array. The records for the CC and CXC chemokines were retrieved and plotted as a function of time (Fig. 6). Over the 24-h period examined in this confirmatory assay, highly inducible expression of Exodus-1, MIP-1α and -1β, and RANTES was observed. Levels of MCP-1, MDC, and TARC, though detectable, were not appreciably increased by viral infection at these early points (Fig. 6A). Analysis of the CXC chemokine profile indicated that SAE cells have strongly inducible expression of I-TAC and less strongly inducible expression of GRO-α, -β, and -γ and IL-8. The CX3C chemokine Fractalkine was detectable in uninfected cells and was further induced in a linear manner by pRSV infection (Fig. 6B). Together these data indicate that inducible expression of the CC chemokines Exodus-1, MIP-1α and -1β, and RANTES, the CXC chemokines GRO-α, -β, and -γ, IL-8, and I-TAC, and the CX3C chemokine Fractalkine is a common feature of pRSV-infected lower airway epithelial cells.
FIG. 6.
Changes in chemokine expression in type I alveolar cells. SAE cells were cultured and infected with pRSV for 0 to 24 h, and oligonucleotide array analysis was performed. The hybridization intensity (average difference) for each chemokine is plotted as a function of time of pRSV infection. (A) Time course for the CC chemokine set; (B) time course for the CXC chemokine set (also including Fractalkine).
Cluster analysis of chemokine gene networks.
Inspection of the oligonucleotide array profiles (Fig. 3 and 6) suggested highly distinct profiles of mRNA accumulation for various members of the chemokine superfamily. We further analyzed the expression patterns for the chemokine gene networks in the complete A549 data set using various hierarchical clustering methods. The AGNES technique clustered genes with common expression patterns in the most meaningful way (Fig. 7). By this technique, a dendrogram that assembles all of the chemokines into a single tree is computed. Each gene is grouped with its nearest neighbor, and the mathematical proximity in expression is shown by the degree of dissimilarity (determined by the height of a common line that connects the two nodes). Three general subgroups were suggested by this analysis. The first subgroup includes the CC chemokines TARC, MDC, I-TAC, and MIP-1α and -1β and the CX3C chemokine Fractalkine. Genes in this subgroup were linked by the lowest dissimilarity score, and we noted that they had similar profiles of expression (compare Fig. 4A and B), with a maximum relative increase at 24 h after pRSV infection. The second and third subgroups were distantly similar. The second subgroup was primarily composed of the CXC chemokines IL-8, GRO-β and -γ, and ENA-78 and the CC chemokines MCP-1 and RANTES (probe sets 3 and 5). The third subgroup is composed of Exodus-1 and GRO-α.
A PCA treatment of the chemokine data was also carried out using the k means method to test if a different technique would reproduce the clusters produced by the hierarchical method described above. The k means method chooses a prespecified number of cluster centers and minimizes the within-class sum of the squares from those centers. This allows each probe set to be assigned to one of the k groups, where group membership is determined by calculating the centroid for each group and then assigning each probe set to the group with the closest centroid. As shown in Fig. 7B, the member composition of the chemokine groups is the same by PCA as by the AGNES method of hierarchical clustering.
DISCUSSION
The airway epithelium forms a cellular barrier between the external environment and the internal milieu and plays an initiating role in pulmonary inflammation after exposure to environmental irritants or infectious agents (reviewed in reference 42). In naturally acquired infections in humans, and in experimental infections of rodents, RSV is a potent inducer of airway inflammation and postinfectious airway hyperreactivity (1, 26; reviewed in reference 28). However, the spectrum of immunomodulatory molecules produced by RSV-infected epithelial cells is presently unresolved. Here we have profiled the kinetics and pattern of chemokine expression in RSV-infected type II (A549) and bronchiolar (SAE) cells by high-density arrays. Oligonucleotide arrays have been used to analyze global gene expression changes in genetic networks activated by signal-transducing molecules (17), interferon stimulation (14), and replication of viruses, including cytomegalovirus (68) and influenza virus (23), in permissive cell lines. In contrast to the observations made for cytomegalovirus-infected human fibroblasts (68), where the expression of only 258 genes was changed, we have found that pRSV infection caused more than 1,200 genes, representing approximately 10% of the genome, to change expression (either increase or decrease). These data suggest that profound global responses in gene expression occur in the context of this Paramyxovirus infection. Interestingly, the analysis of the effects of influenza virus infection on global gene expression in HeLa epithelial cells did not reveal induction of chemotactic cytokines (23), and there was no expression of interferon-inducible genes, features distinct from the patterns we observe in RSV-infected epithelial cells (37). Although more systematic analyses need to be undertaken to identify pathogen-specific genetic signatures, these data suggest that such signatures indeed exist. In addition, although inactivated influenza virus influenced expression of many early genes, RSV induces expression of many chemokine genes in a replication-dependent manner (12, 21, 35) (Fig. 4D). At present we cannot exclude the possibility that a subset of genes are inducible by virus binding; this question will require additional work. Undoubtedly, much more information about the biology of cellular responses to pRSV infection is contained in this data set. Our purpose, however, was to characterize the pattern of expression in chemotactic cytokines. We note that RSV is a potent inducer of CC, CXC, and CX3C chemokine subgroups in lower airway cells, which suggests potential mechanisms for the epithelial cell-initiated immune response and immunopathogenic injury.
Newly identified RSV-inducible CC chemokines.
Expression of the CC chemokines MIP-1, MCP-1, and RANTES has been previously described by our group and others (6, 52). Here we report that RSV-infected A549 cells inducibly express the CC chemokine I-309, a potent chemotactic peptide for monocytes (46) (other salient features of inducible chemokines are shown in Table 2). I-309 is the human homolog of murine thymus-derived chemotactic agent (mTCA), based on conservation of coding sequences, gene topology (conservation of intron-exon organization), 70% sequence identity in 5′ regulatory sequences, and chromosomal mapping to human chromosome 17, a region syntenic with the mTCA locus on mouse chromosome 11 (47). In a murine model of RSV-induced pulmonary inflammation, we previously reported the expression of mTCA in whole-lung homogenates, however, the cell types producing mTCA were not determined (26). We suggest that the airway epithelial cell may be one source of mTCA/I-309 expression. The mechanism for pRSV-induced I-309 expression is presently unknown; preliminary sequence analysis of the I-309 promoter has identified an NF-κB site 201 nt upstream from the transcription initiation site (47), a feature common to two other well-characterized RSV-inducible genes, the IL-8 (21) and RANTES (12) genes. We speculate that one mechanism for induction of I-309 may be mediation by NF-κB; however, this will require direct experimentation. The monocyte-recruiting property of I-309, in conjunction with the mononuclear and T-cell chemotactic properties of MCP-1 and the monocyte and neutrophil chemotactic properties of MIP-1α and -1β (Fig. 1) (52), may account for the spectrum of monocytic cells recruited into RSV-infected lungs.
TABLE 2.
Summary of RSV-inducible chemokines detected in this study
| Class and gene | GenBank accession no. | Cell typea | Locusb | Detection methodc | Receptord | Target cellse |
|---|---|---|---|---|---|---|
| CC chemokines | ||||||
| I-309 | M57502 | A | 17 | C | CCR-8 | Th2 cells, NK cells |
| MCP-1/JE | M28225 | A | 17q11.2–q21.1 | R, A, C | CCR-2 | Basophils, monocytes |
| MDC | U83171 | A | 16q13 | A, R | CCR-4 | CD4+ T cells, IL-2-activated NK cells, DC |
| MIP-1α/LD78 alpha | D90144 | A, S | 17q11-q21 | R, A, C, N | CCR-1, -5 | Lymphocytes, monocytes, macrophages, immature DC |
| MIP-1β/Act-2 | J04130 | A, S | 17q21 | A, R | CCR-5, -8 | Monocytes, macrophages |
| MIP-3α/LARC/Exodus-1 | U64197 | A, S | 2q33–q37 | A, N, W | CCR-6 | T lymphocytes, naïve B cells, DC |
| RANTES | M21121 | A, S | 17q11.2–q12 | R, A, C, N, E | CCR-1, -3, -4, -5 | Eosinophils, monocytes, T lymphocytes, DC |
| TARC | D43767 | A | 16q13 | A | CCR-4, -8 | Th2 lymphocytes, NK cells |
| CXC chemokines | ||||||
| ENA-78 | X78686 | A | 4q13–q21 | A, C | CXCR-2 | Neutrophils |
| GRO-α/MGSA | X54489 | A, S | 4q21 | A, N | CXCR-2 | Neutrophils, basophils |
| GRO-β/MIP-2 | M36820 | A, S | 4q21 | A, N, C | CXCR-2 | Monocytes, neutrophils, basophils |
| GRO-γ | M36821 | A, S | 4q21 | A, N | CXCR-2 | Monocytes, neutrophils, basophils |
| IL-8 | M28130 | A, S | 4q13–q21 | R, A, C, N, E | CXCR-1, -2 | Neutrophils, eosinophils, T lymphocytes |
| IP-10 | X02530 | A | 4q21 | R | CXCR-3 | Activated Th1 lymphocytes |
| I-TAC | AF030514 | A, S | 4q21.2 | A, N | CXCR-3 | Activated Th1 lymphocytes |
| CX3C chemokine, Fractalkine | U84487 | A, S | 16q | A, R | CXCR-1, -2 | Monocytes, lymphocytes |
Cell expressing the chemokine. A, A549 cells; S, SAE cells.
Human chromosomal locus.
A, oligonucleotide probe array; C, membrane cDNA array; N, Northern blotting; R, RPA; W, Western immunoblotting.
Known high-affinity receptor for the chemokine. CCR, CC chemokine receptor; CXCR, CXC chemokine receptor.
Cells chemotactic for the chemokine. DC, dendritic cells.
Our study is the first to show highly inducible Exodus-1 expression in A549 and SAE cells. Exodus-1/LARC/MIP-3α is a chemokine strongly expressed in epithelial cells overlying mucosal lymphoid organs, including intestinal epithelium covering Peyer's patches (60) and epithelium lining inflamed tonsillar crypts (15). This chemokine has been mapped to human chromosome 2, a genetic locus distinct from that of the CC chemokine cluster at 17q11–32 (9), and is only distantly related to the other CC chemokines. The closest structural relative of Exodus-1 is MIP-1β, with only 28% amino acid identity (31). Interestingly, in previous studies, constitutive Exodus-1 expression was detected in the lung but in no other normal human tissues (its inducible expression in the lung was not examined [33]). Exodus-1 is chemotactic for monocytes (33) and immature dendritic cells (15) and stimulates arrest of memory T cells (10). Dendritic cells are of potential interest in RSV immunopathology because circulating monocytic progenitor cells are recruited from the blood compartment to initially process viral antigen in the pulmonary mucosa. Indeed, in antigen-induced mucosal inflammation, dendritic cells are rapidly recruited into the lung (45). Although their biology is incompletely understood, in later stages of maturation, dendritic cells are thought to migrate to draining lymph nodes, where they present processed antigen in the context of major histocompatibility complex to naïve CD4+ T cells that generate an antigen-specific T-cell response (15, 67; reviewed in reference 59). Interestingly, Exodus-1 competitively binds with β-defensins to a common receptor expressed on immature dendritic cells (CCR6 [66]). The relative roles of Exodus-1 and β-defensins in dendritic cell recruitment in RSV-infected lungs have not been determined.
TARC is a CC chemokine that is chemotactic for primed CD4+ T lymphocytes and immature dendritic cells (40). TARC has been shown to be expressed in human alveolar A549 and BEAS-2B cells, where its protein and mRNA expression can be synergistically induced by IL-4 and tumor necrosis factor alpha administration; this induction is highly sensitive to the inhibitory effects of glucocorticoids (57). Moreover, TARC has also been implicated in human atopic asthma, where increased levels of TARC protein appear on the apical surfaces of epithelial cells by immunohistochemistry (57). We show here that TARC can also be inducible by RSV infection in A549 cells and may play a role in the immunopathology of severe infection.
MDC is chemotactic for monocytes, IL-2-activated NK cells (34), immature dendritic cells, and Th2 lymphocytes (13). MDC has been reported to play a role in allergic ovalbumin-induced pulmonary inflammation in mice, because neutralizing antibodies to MDC block eosinophil recruitment (25). In addition, MDC protein is detected in normal human bronchial epithelium of patients with atopic asthma (57). Binding the CCR4 receptor, a receptor also activated by TARC, MDC is a potent chemotactic factor for immature dendritic cells (55). The relative roles of MDC and TARC in dendritic cell homing in the RSV-infected lung will require further investigation.
Newly identified RSV-inducible CXC chemokines.
The CXC chemokine class is functionally subdivided into two groups based on the presence or absence of a signature ELR (Glu-Leu-Arg) motif upstream of the canonical CXC motif. The ELR-containing group of CXC chemokines includes IL-8, GRO-α, -β, and -γ, and ENA-78; these cytokines primarily activate the bactericidal activity and chemotaxis of neutrophils, an abundant cell type found in the bronchoalveolar lavage fluid of intubated RSV-infected children (16). Additionally, the ELR CXC chemokines can activate other target cells as well. For example, IL-8 activates T cells and eosinophils (44, 50), and GRO-α, -β, and -γ activate basophils (22), which may account for some of the spectrum of cellular infiltration in RSV-infected lungs and the presence of cell-specific degranulation products in nasopharyngeal secretions of patients with naturally acquired RSV infections (20). We and others have shown that IL-8 is highly inducible by RSV infection in airway cells (21, 43, 51). Although constitutive expression of GRO-α, -β, and -γ and ENA-78 in airway epithelial cells and alveolar macrophages has been reported(5), we observe that, perhaps not surprisingly, GRO-α, -β, and -γ and ENA-78 are also highly inducible by RSV infection.
We report the expression of the non-ELR CXC chemokine I-TAC in RSV-infected A549 and SAE cells. I-TAC induces migration of activated T and NK cells (56) and can be inducibly expressed in the lung after experimental endotoxemia (65). I-TAC is encoded on human chromosome 4q21.2, in a cluster with two other non-ELR CXC chemokines, IP-10 and monokine induced by gamma interferon (MIG). The mechanisms for I-TAC induction by RSV are presently unknown.
Induction of the CX3C chemokine Fractalkine.
Fractalkine is encoded as a large, membrane-anchored glycoprotein, with the NH2-terminal cytokine domain being attached by a mucin repeat-containing stalk to the COOH-terminal transmembrane segment. As a membrane-anchored protein, Fractalkine may function in monocyte and T-cell haptotaxis, where cells migrate to regions of high adhesiveness (58), or it may be processed and released in a soluble form (4). Our data suggest that constitutive Fractalkine expression differs between the two cell types: in A549 cells, Fractalkine is expressed at such low levels that it is almost undetectable (except by RT-PCR), whereas in SAE cells, it is abundantly expressed. In both cell types, however, RSV rapidly induces Fractalkine expression; the biological effects of Fractalkine expression on monocyte recruitment in RSV infections and the effect of RSV infection on Fractalkine processing or secretion, if any, will require future investigation.
Members of the chemokine superfamily share common expression patterns.
In lower airway epithelial cells, RSV-induced expression of chemokine genes is mediated through a coordinated effect of virus-inducible transactivating proteins, including NF-κB, activator protein 1, interferon regulatory factor 1, and NF-IL-6 (12). For the genes which have been studied in detail, including IL-8 (11) and RANTES (12), all of these transcription factors are required for fully inducible transcription; they form a multiprotein complex (an “enhanceosome”) on their promoters. We have analyzed empirically the inducible members of the chemokine superfamily by hierarchical clustering and PCA and have consistently observed three subgroups whose members are linked by their expression patterns. We note that members of the most highly related subgroup, that including MDC, TARC, I-TAC, MIP-1α and -1β, and Fractalkine, share functional similarities in their abilities to activate lymphocytes (Table 2). However, neither of the other two subgroups exhibits a single, clearly defined functional activity, so it would be overinterpretation of the data to imply that the members of a cluster are functionally related. Instead, grouping genes in this manner may only suggest common regulatory mechanisms; in this regard, we note that the IL-8 and RANTES genes cluster in the same subgroup. This relationship is significant because both of these promoters share similar regulatory elements, and their transcriptional activation is enhanceosome dependent (12). It will be of interest to determine whether virus-inducible expression of other subgroups is transcriptionally mediated, and if so, whether their expression is dependent on enhanceosome formation with similar or distinct trans-acting factors. Identification of differences in genetic control elements that account for the differences in expression will require further investigation.
In summary, we have used high-density oligonucleotide arrays to profile changes in the expression of CC, CXC, and CX3C chemokines in lower airway cells. Together these data indicate that inducible expression of the CC chemokines Exodus-1, MIP-1α and -1β, and RANTES, the CXC chemokines GRO-α, -β, and -γ, IL-8, and I-TAC, and the CX3C chemokine Fractalkine is a common feature of pRSV-infected lower airway epithelial cells. The activities of these factors suggest mechanisms for recruitment of neutrophils, monocytes, eosinophils, NK cells, T lymphocytes, and dendritic cells into RSV-infected airways. Although undoubtedly important for protective immunity, an exuberant immune response may result in pathological manifestations of inflammation such as acute bronchiolitis or postinfectious airway hyperreactivity. We suggest that modification of the expression or activity of these chemokines may have significant effects on the immunopathology of RSV infection.
ACKNOWLEDGMENTS
We thank Shaofei Wang for technical assistance, Helene Haeberle for critical review of the manuscript, and the UTMB Genomics Core Laboratory (T. Wood, Director) for performing high-density oligonucleotide arrays.
This project was supported by NIAID grants R21 AI48163 and, in part, R01 AI40218 (to A.R.B.) and AI 15939 (to R.P.G.), a grant from NICHHD (R30HD 27841), and grant P30 ES06676 from NIEHS (to R. S. Lloyd, UTMB).
REFERENCES
- 1.Aherne W T, Bird T, Court S D B, Gardner P S, McQuillin J. Pathological changes in virus infections of the lower respiratory tract in children. J Clin Pathol. 1970;23:7–18. doi: 10.1136/jcp.23.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arnold R, Humbert B, Werchaus H, Gallati H, Konig W. Interleukin-8, interleukin-6, and soluble tumor necrosis factor receptor type I released from a human pulmonary epithelial cell line (A549) exposed to respiratory syncytial virus. Immunology. 1994;82:126–133. [PMC free article] [PubMed] [Google Scholar]
- 3.Baggiolini M, Dewald B, Moser B. Human chemokines: an update. Annu Rev Immunol. 1997;15:675–705. doi: 10.1146/annurev.immunol.15.1.675. [DOI] [PubMed] [Google Scholar]
- 4.Bazan J F, Bacon K B, Hardiman G, Wang W, Soo K, Rossi D, Greaves D R, Zlotnik A, Schall T J. A new class of membrane-bound chemokine with a CX3C motif. Nature. 1997;385:640–644. doi: 10.1038/385640a0. [DOI] [PubMed] [Google Scholar]
- 5.Becker S, Quay J, Koren H S, Haskill J S. Constitutive and stimulated MCP-1, GRO α, β and γ expression in human airway epithelium and bronchoalveolar macrophages. Am J Physiol. 1994;266:L278–L286. doi: 10.1152/ajplung.1994.266.3.L278. [DOI] [PubMed] [Google Scholar]
- 6.Becker S, Reed W, Henderson F W, Noah T L. RSV infection of human airway epithelial cells causes production of the β-chemokine RANTES. Am J Physiol. 1997;272:L512–L520. doi: 10.1152/ajplung.1997.272.3.L512. [DOI] [PubMed] [Google Scholar]
- 7.Bennett C R, Jr, Hamre D. Growth and serological characteristics of respiratory syncytial virus. J Infect Dis. 1962;110:8–16. doi: 10.1093/infdis/110.1.8. [DOI] [PubMed] [Google Scholar]
- 8.Bitko V, Velazquez A, Yank L, Yang Y-C, Barik S. Transcriptional induction of multiple cytokines by human respiratory syncytial virus requires activation of NF-κB and is inhibited by sodium salicylate and aspirin. Virology. 1997;232:369–378. doi: 10.1006/viro.1997.8582. [DOI] [PubMed] [Google Scholar]
- 9.Boguski M S, Lowe T M, Tolstoshev C M. dbEST—database for “expressed sequence tags.”. Nat Genet. 1993;4:332–333. doi: 10.1038/ng0893-332. [DOI] [PubMed] [Google Scholar]
- 10.Campbell J J, Hedrick J Z A, Siani M A, Thompson D A, Butcher E C. Chemokines and the arrest of lymphocytes rolling under flow conditions. Science. 1998;279:381–383. doi: 10.1126/science.279.5349.381. [DOI] [PubMed] [Google Scholar]
- 11.Casola A, Garofalo R, Jamaluddin M, Vlahopoulos S, Brasier A R. Requirement of a novel upstream response element in respiratory syncytial virus-induced IL-8 gene expression. J Immunol. 2000;164:5944–5951. doi: 10.4049/jimmunol.164.11.5944. [DOI] [PubMed] [Google Scholar]
- 12.Casola A, Garofalo R P, Haeberle H, Elliott T, Lin R, Jamaluddin M, Brasier A R. Multiple cis regulatory elements control RANTES promoter activity in alveolar epithelial cells infected with respiratory syncytial virus. J Virol. 2001;75:6428–6439. doi: 10.1128/JVI.75.14.6428-6439.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chantry D, Romagnani P, Raport C J, Wood C L, Epp A, Romagnani S, Gray P W. Macrophage-derived chemokine is localized to thymic medullary epithelial cells and is a chemoattractant for CD3+, CD4+, CD8low thymocytes. Blood. 1999;94:1890–1898. [PubMed] [Google Scholar]
- 14.Der S D, Zhou A, Williams B R G, Silverman R H. Identification of genes differentially regulated by interferon α, β or γ using oligonucleotide arrays. Proc Natl Acad Sci USA. 1998;95:15623–15628. doi: 10.1073/pnas.95.26.15623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dieu M-C, Vanbervliet B, Vicari A, Bridon J-M, Oldham E, Ait-Yahia S, Briere F, Zlotnik A, Lebecque S, Caux C. Selective recruitment of immature and mature dendritic cells by distinct chemokines expressed in different anatomic sites. J Exp Med. 1998;188:373–386. doi: 10.1084/jem.188.2.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Everard M L, Swarbrick A, Wrightham M, McIntyre J, Dunkley C, James P D, Sewell H F, Milner A D. Analysis of cells obtained by bronchial lavage of infants with respiratory syncytial virus infection. Arch Dis Child. 1994;71:428–432. doi: 10.1136/adc.71.5.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fambrough D, McClure K, Kazlauskas A, Lander E S. Diverse signaling pathways activated by growth factor receptors induce broadly overlapping, rather than independent, sets of genes. Cell. 1999;97:727–741. doi: 10.1016/s0092-8674(00)80785-0. [DOI] [PubMed] [Google Scholar]
- 18.Ferris J A, Aherne W A, Locke W S. Sudden and unexpected deaths to infants: histology and virology. Br Med J. 1973;2:439–449. doi: 10.1136/bmj.2.5864.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gardner P S, McQuillin J, Court S D. Speculation on pathogenesis of death from respiratory syncytial virus infection. Br Med J. 1970;1:327–330. doi: 10.1136/bmj.1.5692.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Garofalo R, Kimpen J L L, Welliver R C, Ogra P L. Eosinophil degranulation in the respiratory tract during naturally acquired respiratory syncytial virus infection. J Pediatr. 1992;120:28–32. doi: 10.1016/s0022-3476(05)80592-x. [DOI] [PubMed] [Google Scholar]
- 21.Garofalo R, Sabry M, Jamaluddin M, Yu R K, Casola A, Ogra P L, Brasier A R. Transcriptional activation of the interleukin-8 gene by RSV infection in alveolar epithelial cells: nuclear translocation of the RelA transcription factor as a mechanism producing airway mucosal inflammation. J Virol. 1996;70:8773–8781. doi: 10.1128/jvi.70.12.8773-8781.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Geiser T, Dewald B, Ehrengruber M U, Clark-Lewis I, Baggiolini M. The interleukin-8 related chemotactic cytokines GROα, GROβ and GROγ activate human neutrophil and basophil leukocytes. J Biol Chem. 1993;268:15419–15424. [PubMed] [Google Scholar]
- 23.Geiss G K, An M C, Bumgarner R E, Hammersmark E, Cunningham D, Katze M G. Global impact of influenza virus on cellular pathways is mediated by both replication-dependent and -independent events. J Virol. 2001;75:4321–4331. doi: 10.1128/JVI.75.9.4321-4331.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Glezen W P, Taber L H, Frank A L. Risk of primary infection and reinfection with respiratory syncytial virus. Am J Dis Child. 1986;140:543–546. doi: 10.1001/archpedi.1986.02140200053026. [DOI] [PubMed] [Google Scholar]
- 25.Gonzalo J-A, Pan Y, Lloyd C M, Jia G-Q, Yu G, Dussault B, Powers C A, Proudfoot A E I, Coyle A J, Gearing D, Gutierrez-Ramos J-C. Mouse monocyte-derived chemokine is involved in airway hyperreactivity and lung inflammation. J Immunol. 1999;163:403–411. [PubMed] [Google Scholar]
- 26.Haeberle H A, Kuziel W A, Dieterich H-J, Casola A, Gatalica Z, Garofalo R P. Inducible expression of inflammatory chemokines in respiratory syncytial virus-infected mice: role of MIP-1α in lung pathology. J Virol. 2001;75:878–890. doi: 10.1128/JVI.75.2.878-890.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hall C B, Douglas R G, Jr, Schnabel K C, Geiman J M. Infectivity of respiratory syncytial virus by various routes of inoculation. Infect Immun. 1981;33:779–783. doi: 10.1128/iai.33.3.779-783.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hall C B, McCarthy C A. Respiratory syncytial virus. In: Mandel G L, Bennett J E, Dolin R, editors. Principles and practice of infectious diseases. New York, N.Y: Churchill Livingstone; 1995. p. 1501. [Google Scholar]
- 29.Hall C B, Walsh E E, Schnabel K C, Long C E, McConnochie K M, Hildreth S W, Anderson L J. Occurrence of groups A and B of respiratory syncytial virus over 15 years: associated epidemiologic and clinical characteristics in hospitalized and ambulatory children. J Infect Dis. 1990;162:1283–1290. doi: 10.1093/infdis/162.6.1283. [DOI] [PubMed] [Google Scholar]
- 30.Harrison A M, Bonville C A, Rosenberg H F, Domachowske J B. Respiratory syncytial virus-induced chemokine expression in the lower airways: eosinophil recruitment and degranulation. Am J Respir Crit Care Med. 1999;159:1918–1924. doi: 10.1164/ajrccm.159.6.9805083. [DOI] [PubMed] [Google Scholar]
- 31.Hieshima K, Imai T, Opdenakker G, Van Damme J, Kusuda J, Tei H, Sakaki Y, Takatsuki K, Miura R, Yoshie O, Nomiyama H. Molecular cloning of a novel human CC chemokine, liver and activation-regulated chemokine (LARC), expressed in liver. J Biol Chem. 1997;272:5846–5853. doi: 10.1074/jbc.272.9.5846. [DOI] [PubMed] [Google Scholar]
- 32.Holberg C J, Wright A L, Martinez F D. Risk factors for respiratory syncytial virus-associated lower respiratory illnesses in the first year of life. Am J Epidemiol. 1991;133:1153. doi: 10.1093/oxfordjournals.aje.a115826. [DOI] [PubMed] [Google Scholar]
- 33.Hromas R, Gray P W, Chantry D, Godiska R, Krathwohl M, Fife K, Bell G I, Takeda J, Aronica S, Gordon M, Cooper S, Broxmeyer H, Klemsz M J. Cloning and characterization of Exodus, a novel β-chemokine. Blood. 1997;89:3315–3322. [PubMed] [Google Scholar]
- 34.Inngjerdingen M, Damaj B, Maghazachi A A. Human NK cells express CC chemokine receptors 4 and 8 and respond to thymus and activation-regulated chemokine, macrophage-derived chemokine, and I-309. J Immunol. 2000;164:4048–4054. doi: 10.4049/jimmunol.164.8.4048. [DOI] [PubMed] [Google Scholar]
- 35.Jamaluddin M, Casola A, Garofalo R P, Han Y, Elliott T, Ogra P L, Brasier A R. The major component of IκBα proteolysis occurs independently of the proteasome pathway in respiratory syncytial virus-infected pulmonary epithelial cells. J Virol. 1998;72:4849–4857. doi: 10.1128/jvi.72.6.4849-4857.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jamaluddin M, Garofalo R, Ogra P L, Brasier A R. Inducible translational regulation of the NF-IL6 transcription factor by respiratory syncytial virus infection in pulmonary epithelial cells. J Virol. 1996;70:1554–1563. doi: 10.1128/jvi.70.3.1554-1563.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jamaluddin M, Wang S, Garofalo R, Elliott T, Casola A, Baron S, Brasier A R. IFN-β mediates coordinate expression of antigen-processing genes in RSV-infected pulmonary epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2001;280:L248–L257. doi: 10.1152/ajplung.2001.280.2.L248. [DOI] [PubMed] [Google Scholar]
- 38.Kaufman L, Rousseau P J. Finding groups in data: an introduction to cluster analysis. New York, N.Y: Wiley; 1990. [Google Scholar]
- 39.Kisch A L, Johnson K M. A plaque assay for respiratory syncytial virus. Proc Soc Exp Biol Med. 1963;112:583. doi: 10.3181/00379727-112-28111. [DOI] [PubMed] [Google Scholar]
- 40.Lieberam I, Forster I. The murine β-chemokine TARC is expressed by subsets of dendritic cells and attracts primed CD4+ T cells. Eur J Immunol. 1999;29:2684–2694. doi: 10.1002/(SICI)1521-4141(199909)29:09<2684::AID-IMMU2684>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 41.MacDonald N E, Hall C B, Suffin S C. Respiratory syncytial viral infection in infants with congenital heart disease. N Engl J Med. 1982;307:397–400. doi: 10.1056/NEJM198208123070702. [DOI] [PubMed] [Google Scholar]
- 42.Martin L D, Rochelle L G, Fischer B M, Krunkosky T M, Adler K B. Airway epithelium as an effector of inflammation: molecular regulation of secondary mediators. Eur Respir J. 1997;10:2139–2146. doi: 10.1183/09031936.97.10092139. [DOI] [PubMed] [Google Scholar]
- 43.Mastronarde J G, He B, Monick M M, Mukaida N, Matsushima K, Hunninghake G W. Induction of interleukin (IL)-8 gene expression by respiratory syncytial virus involves activation of nuclear factor (NF)-κB and NF-IL-6. J Infect Dis. 1996;174:262–267. doi: 10.1093/infdis/174.2.262. [DOI] [PubMed] [Google Scholar]
- 44.Matsushima K, Oppenheim J J. Interleukin 8 and MCAF: novel inflammatory cytokines inducible by IL-1 and TNF. Cytokine. 1989;1:2–33. doi: 10.1016/1043-4666(89)91043-0. [DOI] [PubMed] [Google Scholar]
- 45.McWilliam A S, Nelson D, Thomas J A, Holt P G. Rapid dendritic cell recruitment is a hallmark of the acute inflammatory response at mucosal surfaces. J Exp Med. 1994;179:1331–1336. doi: 10.1084/jem.179.4.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Miller M D, Krangel M S. The human cytokine I-309 is a monocyte chemoattractant. Proc Natl Acad Sci USA. 1992;89:2950–2954. doi: 10.1073/pnas.89.7.2950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Miller M D, Wilson S D, Dorf M E, Seuanez H N, O'Brien S J, Krangel M S. Sequence and chromosomal location of the I-309 gene. Relationship to genes encoding a family of inflammatory cytokines. J Immunol. 1990;145:2737–2744. [PubMed] [Google Scholar]
- 48.Morales F, Calder M A, Inglis J M, Murdoch P S, Williamson J. A study of respiratory infections in the elderly to assess the role of respiratory syncytial virus. J Infect. 1983;7:236–247. doi: 10.1016/s0163-4453(83)97142-6. [DOI] [PubMed] [Google Scholar]
- 49.Morris J A, Blount R E, Jr, Savage R E. Recovery of cytopathogenic agent from chimpanzees with coryza. Proc Soc Exp Biol Med. 1956;92:544–549. doi: 10.3181/00379727-92-22538. [DOI] [PubMed] [Google Scholar]
- 50.Murphy P M. The molecular biology of leukocyte chemoattractant receptors. Annu Rev Immunol. 1994;12:593–633. doi: 10.1146/annurev.iy.12.040194.003113. [DOI] [PubMed] [Google Scholar]
- 51.Noah T L, Becker S. Respiratory syncytial virus-induced cytokine production by a human bronchial epithelial cell line. Am J Physiol. 1993;265:L472–L478. doi: 10.1152/ajplung.1993.265.5.L472. [DOI] [PubMed] [Google Scholar]
- 52.Olzewska B, Casola A, Saito T, Alam R, Crowe S, Mei F, Ogra P L, Garofalo R. Cell-specific expression of RANTES, MCP-1, and MIP-1α by lower airway epithelial cells and eosinophils infected with respiratory syncytial virus. J Virol. 1998;72:4756–4764. doi: 10.1128/jvi.72.6.4756-4764.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Oppenheim J J, Zachariae C O C, Mukaida N, Matsushima K. Properties of the novel proinflammatory supergene “intercrine” cytokine family. Annu Rev Immunol. 1991;9:617–648. doi: 10.1146/annurev.iy.09.040191.003153. [DOI] [PubMed] [Google Scholar]
- 54.Saito T, Deskin R W, Casola A, Haeberle H, Olzewska B, Ernst P B, Alam R, Ogra P L, Garofalo R. Respiratory syncytial virus induces selective production of the chemokine RANTES by upper airway epithelial cells. J Infect Dis. 1997;175:497–504. doi: 10.1093/infdis/175.3.497. [DOI] [PubMed] [Google Scholar]
- 55.Sallusto F, Palermo B, Lenig D, Miettinen M, Matikainen S, Julkunen I, Forster R, Burgstahler R, Lipp M, Lanzavecchia A. Distinct patterns and kinetics of chemokine production regulate dendritic cell function. Eur J Immunol. 1999;29:1617–1625. doi: 10.1002/(SICI)1521-4141(199905)29:05<1617::AID-IMMU1617>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 56.Sauty A, Dziejman M, Taha R A, Iarossi A S, Neote K, Garcia-Zepeda E A, Hamid Q, Luster A D. The T cell-specific CXC chemokines IP-10, Mig, and I-TAC are expressed by activated human bronchial epithelial cells. J Immunol. 1999;162:3549–3558. [PubMed] [Google Scholar]
- 57.Sekiya T, Miyamasu M, Imanishi M, Yamada H, Nakajima T, Yamaguchi M, Fujisawa T, Pawankar R, Sano Y, Ohta K, Ishii A, Morita Y, Yamamoto K, Matsushima K, Yoshie O, Hirai K. Inducible expression of a Th2-type CC chemokine, thymus and activation-regulated chemokine, by human bronchial epithelial cells. J Immunol. 2000;165:2205–2213. doi: 10.4049/jimmunol.165.4.2205. [DOI] [PubMed] [Google Scholar]
- 58.Springer T. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multiple paradigm. Cell. 1994;76:301. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- 59.Steinman R M. The dendritic cell system and its role in immunogenicity. Annu Rev Immunol. 1991;9:271–296. doi: 10.1146/annurev.iy.09.040191.001415. [DOI] [PubMed] [Google Scholar]
- 60.Takana Y, Imai T, Baba M, Ihsikawa I, Uehira M, Nomiyama H, Yoshie O. Selective expression of liver and activation-regulated chemokine (LARC) in intestinal epithelium in mice and humans. Eur J Immunol. 1999;29:633–642. doi: 10.1002/(SICI)1521-4141(199902)29:02<633::AID-IMMU633>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 61.Ueba O. Respiratory syncytial virus. I. Concentration and purification of the infectious virus. Acta Med Okayama. 1978;32:265–272. [PubMed] [Google Scholar]
- 62.Virca G D, Northemann W, Shiels B R, Widera G, Broome S. Simplified Northern blot hybridization using 5% sodium dodecyl sulfate. BioTechniques. 1990;8:370–371. [PubMed] [Google Scholar]
- 63.Vlahopoulos S, Boldogh I, Brasier A R. NF-κB-dependent induction of interleukin-8 gene expression by tumor necrosis factor α: evidence for an antioxidant sensitive activating pathway distinct from nuclear translocation. Blood. 1999;94:1878–1889. [PubMed] [Google Scholar]
- 64.Welliver R C, Wong D T, Sun M, Middleton E, Jr, Vaughan R S, Ogra P L. The development of respiratory syncytial virus-specific IgE and the release of histamine in nasopharyngeal secretions after infection. N Engl J Med. 1981;305:841–846. doi: 10.1056/NEJM198110083051501. [DOI] [PubMed] [Google Scholar]
- 65.Widney D P, Xia Y, Lusis A J, Smith J B. The murine chemokine CXCL11 (IFN-inducible T cell α chemoattractant) is an IFN-γ- and lipopolysaccharide-inducible glucocorticoid-attentuated response gene expressed in lung and other tissues during endotoxemia. J Immunol. 2000;164:6322–6331. doi: 10.4049/jimmunol.164.12.6322. [DOI] [PubMed] [Google Scholar]
- 66.Yang D, Chertov O, Bykovskaia S N, Chen Q, Buffo M J, Shogan J, Anderson M, Schroder J M, Wang J M, Howard O M Z, Oppenheim J J. β-Defensins: linking innate and adaptive immunity through dendritic and T cell CCR6. Science. 1999;286:525–528. doi: 10.1126/science.286.5439.525. [DOI] [PubMed] [Google Scholar]
- 67.Yang D, Howard O M Z, Chen Q, Oppenheim J J. Immature dendritic cells generated from monocytes in the presence of TGF-β1 express functional CC chemokine receptor 6. J Immunol. 1999;163:1737–1741. [PubMed] [Google Scholar]
- 68.Zhu H, Cong J-P, Mamtora G, Gingeras T, Shenk T. Cellular gene expression altered by human cytomegalovirus: global monitoring with oligonucleotide arrays. Proc Natl Acad Sci USA. 1998;95:14470–14475. doi: 10.1073/pnas.95.24.14470. [DOI] [PMC free article] [PubMed] [Google Scholar]