Abstract
Analytical treatment interruptions (ATIs) are widely used to evaluate HIV cure-related research interventions. However, sex partners of cure-related trial participants might be at risk of acquiring HIV during ATIs. Addressing this risk is key to ensuring the continued success of trials involving ATIs and offer greater acceptability across multiple trials sites. In 2022, the Advancing Clinical Therapeutics Globally (ACTG) Network convened a Partner Protections Working Group (PPWG) to update the 2020 HIV transmission risk toolkit developed by Peluso and colleagues. In our review of the original toolkit, we identified new challenges and needs at the participant, partner and study levels, as well as new evidence on measures to address these needs and more advanced ethical thinking on partner protections in HIV cure-related trials with ATIs. Based on these findings, we developed an updated toolkit that will provide trial participants and their partners with better support to address new and unfamiliar situations and protect partners from undue harm. We present this toolkit, make it available as a resource for cure-related trials with ATIs and discuss possible future directions.
Keywords: HIV, HIV cure-related research, Analytical treatment interruptions, Partner protections, Risk mitigation, HIV criminalization
Abbreviations
| ACTG | Advancing Clinical Therapeutics Globally |
| ART | Antiretroviral Treatment |
| ATI | Analytical Treatment Interruption |
| CAB | Community Advisory Board |
| DAIDS | Division of AIDS |
| DARE | Delaney AIDS Research Enterprise |
| FAQ | Frequently Asked Questions |
| IPV | Intimate Partner Violence |
| IRB | Institutional Review Board |
| LGBTQIA+ | Lesbian, Gay, Bisexual, Transgender, Intersex, or Questioning |
| NIH | National Institutes of Health |
| P3 | Partner Protection Package |
| PEP | Post-Exposure Prophylaxis |
| PrEP | Pre-Exposure Prophylaxis |
| PWH | People with HIV |
| PPWG | Partner Protections Working Group |
| STI | Sexually Transmitted Infection |
| SOP | Standard Operating Procedure |
| UCSF | University of California San Francisco |
| U = U | Undetectable = Untransmittable |
| WHO | World Health Organization |
1. Introduction
Analytical treatment interruptions (ATIs) are currently used as the standard for determining the effect of experimental interventions that aim to achieve sustained antiretroviral treatment (ART)-free suppression in HIV cure-related trials.1 The temporary pause of ART implies potential risks to both trial participants and their sex partner(s) (henceforth partners).2, 3, 4 Previously, the report of two HIV transmission events in the context of therapeutic HIV vaccine trials5,6 prompted the development of a practical approach to HIV transmission risk mitigation during ATI trials, published in the Journal of Virus Eradication2 in 2020. Although no additional transmission has been reported since that time, the risk remains, especially as ATI trials become more common, last longer, tolerate higher viremia, and occur in more diverse settings and among more diverse participants. Considering these challenges, evolving evidence on how to address them and recent ethical advances, a time-limited (2022–2023) effort within the Advancing Clinical Therapeutics Globally (ACTG) Network – the Partner Protections Working Group (PPWG) – was convened to refine the HIV transmission risk mitigation toolkit (henceforth toolkit). In the present manuscript, we summarize updates made to the toolkit since 2020.
1.1. ACTG Partner Protections Working Group
In 2022, the authors established the ACTG PPWG to create a practical framework for partner protections in ACTG-initiated ATI trials. The ACTG PPWG was composed of researchers (including ATI trialists), trial sponsors, people with HIV (PWH), former ATI trial participants, ACTG community representatives, bioethicists, and socio-behavioral scientists. The main objective of the ACTG PPWG was to support HIV cure-related research teams and participants in their efforts to mitigate the risk of unintended HIV transmission to partners during ATI trials at ACTG sites. In doing so, we hoped to support the continued success and safety of HIV cure-related trials as these are scaled up in number, including larger numbers and greater diversity of participants, as they assess more complex interventions with ATIs of longer durations and are conducted in more diverse geographic locations where the burden of HIV is greatest, such as Sub-Saharan Africa.7,8 Of note, we conducted our work at a critical juncture in the field of HIV cure-related research that coincided with the re-opening ATI trials following SARS-CoV-2 restrictions,9 representing a natural break point at which to re-examine and refine approaches to trials following the COVID-19 pandemic, during which many ATI trials had been paused.
The 2020 toolkit prepared by Peluso and colleagues, in collaboration with the Delaney AIDS Research Enterprise (DARE) Community Advisory Board (CAB), was inspired by discussions around a ‘standard of prevention’ in HIV prevention trials.10, 11, 12, 13 The initial toolkit was implemented at the University of California, San Francisco (UCSF) clinical trial site,14 in the context of two clinical studies involving ATIs (NCT04357821 and NCT04359186).
The ACTG PPWG drew on several sources in updating this toolkit. This included the experience of implementing the toolkit at UCSF, discussions at an August 2021 virtual meeting titled “Addressing Challenges that Limit Participation in HIV Trials with ATIs,” organized by the U.S. National Institutes of Health (NIH) Divisions of AIDS (DAIDS) in collaboration with PWH and former ATI trial participants, a paper that built on this meeting and two focused literature reviews (one on partner protection approaches in ATIs, one on the ethics of third-party risks in health-related research) to propose a systematic, ethically robust and evidence-based partner protection package (P3) for trials involving ATIs,4 additional focused literature reviews on HIV prevention, and the PPWG's diverse backgrounds, experiences, scholarship and advocacy.
Here, we summarize updates made to the 2020 toolkit, which stemmed from a collaborative effort of the ACTG PPWG in 2022–2023 to ensure greater applicability of the toolkit across multiple ACTG ATI trial sites and other research centers. In updating the toolkit, we identified new challenges and needs at the participant, partner and study levels, as well as new evidence on measures to address these needs and more advanced ethical thinking on partner protections in HIV cure-related trials with ATIs. Based on these findings, we developed an updated toolkit that will hopefully provide ATI trial participants and their partners with better support to address new and unfamiliar situations and protect partners from undue harm. The toolkit offers key practically oriented and evidence-based materials that aim to reduce the likelihood of unintended HIV transmission during ATI trials and ensure prompt management of any acquired HIV during ATIs. These measures should be part of a systematic P3 approaches, described in detail elsewhere.4 The toolkit is available on the ACTG website in the Community section (see Supplementary materials).
2. Partner protections in ATI trials toolkit updates
Drawing on the above sources, the ACTG PPWG reviewed the 2020 toolkit2 and found that all the included tools remained important for protecting partners of ATI trial participants.
2.1. Participant-level challenges and risk mitigations
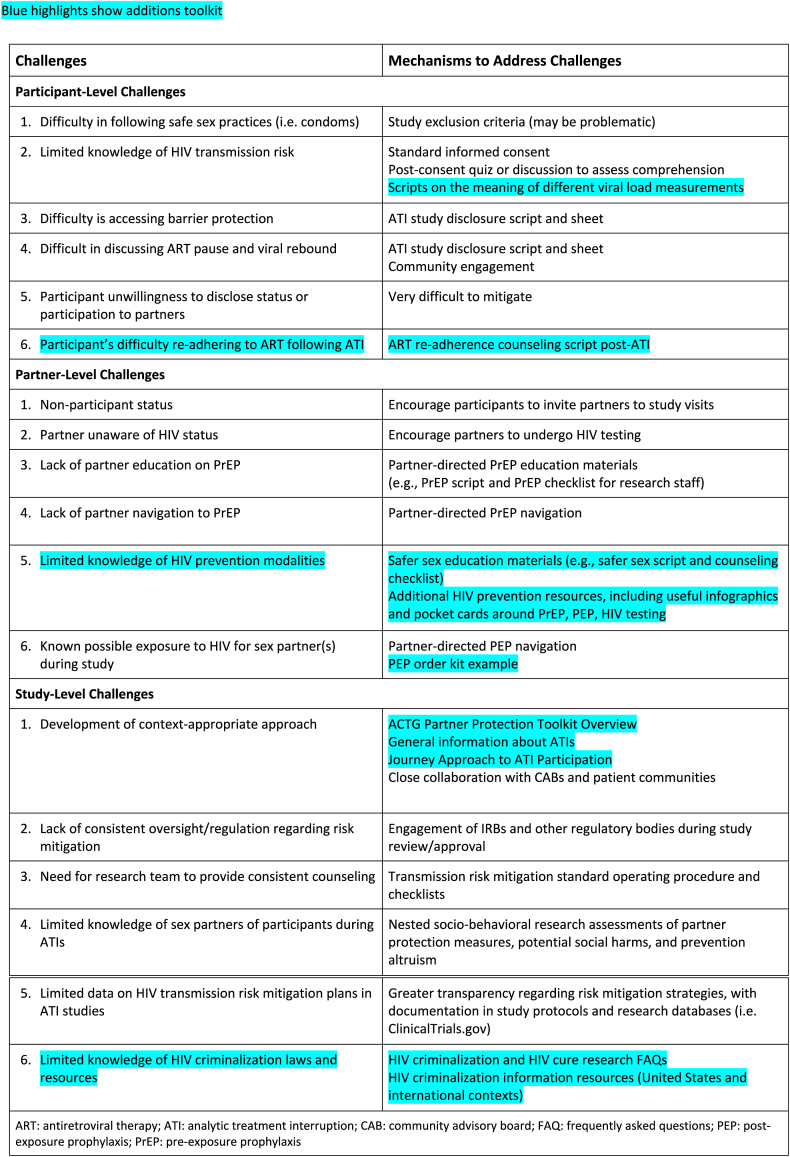
Peluso and colleagues initially identified five ATI-related participant-level challenges and proposed ways of addressing these challenges (Table 1): 1) difficulty in following safe sex practices (i.e., condoms), which might be addressed by excluding participants at increased risk of transmitting HIV; 2) limited knowledge of HIV transmission risk, which can be addressed by informing participants during the informed consent process and testing for their understanding; 3) difficulty in following safe sex practice (i.e., condoms), which can be addressed by implementing an ATI study disclosure script/sheet, 4) difficulty in discussing ART pause and viral rebound, which can be addressed by implementing an ATI study disclosure script/sheet and community engagement, and 5) participant unwillingness to disclose status or participation to partners, which is difficult to mitigate.
Table 1.
Updated toolkit to mitigate HIV transmission risks to sex partner(s) of ATI trial participants adapted from Peluso and Colleagues.2
Under challenge 2 (limited knowledge of HIV transmission risk), the ACTG PPWG underscored the importance of providing additional information to ATI trial participants around the meaning of different viral load measurements during ATIs and the associated risk of HIV transmission. The ACTG PPWG developed scripts on the meaning of different viral load measurements based on a focused review of the literature to help explain to ATI trial participants the potential risk of HIV transmission based on different viral load measures before, during, and after ATIs. The scripts use a green-light, yellow-light, and red-light system (resembling traffic lights), which helps research teams explain to ATI trial participants when more protection and caution are needed. The World Health Organization (WHO) embraced a similar messaging approach in its July 2023 policy brief titled “The role of HIV viral suppression in improving individual health and reducing transmission,” published in the context of HIV care.15
Additionally, we identified a sixth participant-level challenge, which was the difficulty of resuming ART and maintaining treatment adherence following an ATI. Our socio-behavioral research has shown that the periods involving viral rebound and ART restart can be stressful and challenging for ATI trial participants, and that additional psychosocial support is needed.14,16 The ACTG PPWG created a new ART-adherence counseling script post-ATI to help mitigate this challenge. The adherence script helps research staff assess a participant's readiness to restart ART and provides counseling steps in case a participant is not ready to restart ART and needs additional support following an ATI. The counseling script also explains the importance of the viral load re-suppression window to help protect the sex partners of ATI trial participants.
3. Partner-level challenges and risk mitigations
Peluso and colleagues initially identified five partner-level challenges: 1) non-participant status of the partner(s), which might be mitigated by encouraging ATI trial participants to invite partners to study visits, 2) partner(s) unaware of HIV status, which might be mitigated by encouraging partners to undergo HIV testing, 3) lack of partner education on pre-exposure prophylaxis (PrEP), which can be mitigated by partner-directed PrEP education materials, 4) lack of partner navigation to PrEP, which could be mitigated by partner-directed PrEP navigation, and 5) known possible exposure to HIV for sex partner(s) during the study, which could be mitigated by partner-directed PEP navigation.
The ACTG PPWG considered it important to adopt a broader view of HIV prevention that encompassed not only barrier protection and PrEP but also safer sex, defined as actions taken before and during sex to reduce the risk of passing or acquiring HIV. The inclusion of safer sex was deemed important to provide more options to ATI trial participants. A new challenge identified (new challenge 5) was the limited knowledge of HIV prevention modalities. To mitigate this challenge, the ACTG PPWG expanded the toolkit by adding safer sex education materials and additional HIV prevention resources, such as useful infographics and pocket cards around PrEP, PEP, and HIV testing. While the Undetectable = Untransmittable (U = U) movement has relied on treatment as prevention, we believe it has also led to a decline in the practice of safer sex in the HIV community, specifically around precautions related to other sexually transmitted infections (STIs). The ACTG PPWG wanted to reintroduce the concept of safer sex to participants and their partners before, during, and after the ATI phase of the study. The safer sex script and counseling tool provides ways to discuss alternatives to higher-risk (e.g., penetrative) sex during ATIs.
Under the partner-directed PEP navigation, the ACTG PPWG also added a recommendation to always have at least two PEP kits available with standard operating procedures (SOPs) in place and local clinicians available to provide HIV testing and dispense PEP and/or on call during an ATI trial.
3.1. Study-level challenges and risk mitigations
Peluso and colleagues identified five study-level challenges: 1) development of a context-appropriate approach, which could be mitigated by closely collaborating with CABs and patient communities, 2) lack of consistent oversight/regulation regarding risk mitigation, which could be mitigated by engaging institutional review boards (IRBs) and other regulatory bodies during study review/approval, 3) need for the research team to provide consistent counseling, which could be mitigated by developing transmission risk mitigation SOPs and checklists, 4) limited knowledge of sex partners of participants during ATIs, which might be mitigated by nesting socio-behavioral research assessments, 5) and limited data on HIV transmission risk mitigation plans in ATI studies, which could be mitigated by greater transparency regarding risk mitigation strategies.
To further help alleviate the first study-level challenge, the ACTG PPWG created an overview to facilitate the development of context-appropriate approaches, in addition to general information about ATIs that could be adapted for specific trials. In addition, the ACTG PPWG emphasized the trajectory approach to ATI trial participation and provided a general map of the participant journey throughout an ATI (before, during, and after an ATI).
The ACTG PPWG recognized a sixth study-level challenge: limited knowledge of HIV criminalization laws and site-level resources. This was deemed necessary given the increased attention to HIV criminalization and anti-lesbian, gay, bisexual, transgender, intersex, or questioning (LGBTQIA+) legislation in recent years.17 Two members of the ACTG PPWG developed HIV criminalization and HIV cure research Frequently Asked Questions (FAQs) and additional information resources. Community members underscored the importance of increasing understanding of how the HIV criminalization landscape may affect ATI trials and partner protections in general. The goal of providing HIV criminalization information was to share information, not to provide legal advice to participants. The ACTG PPWG emphasized learning about legal aid organizations operating in the trial area. The ACTG PPWG also recommends familiarity with the legal context around HIV criminalization in each country or setting, particularly for LGBTQIA + communities at higher risk of HIV criminalization in some settings. We believe providing HIV criminalization information to participants will not deter participation in ATI trials because this criminalization is already a fact of life for PWH.17
4. Possible future directions
The ACTG PPWG also envisioned possible future directions for the updated toolkit. It may be helpful to create a systematic mapping of stakeholders to optimize dissemination and use of the toolkit. Various tools will also need careful site review and adaptation, and participant-facing materials will need local language translations and IRB approval. We recommend additional pilot testing of the tools and generation of lessons learned and accumulation of best practices around HIV transmission mitigation in the context of ATI trials in the U = U era. We have also started integrating socio-behavioral research assessments around partner protections in ongoing and planned ACTG and non-ACTG ATI protocols.14 Like HIV standard of prevention package updates in the field of HIV prevention research,10 we anticipate that the toolkit will benefit from periodic updates over time based on the evolving state of the science.
Further, the ACTG PPWG believes there remains unfinished business around partner protections in ATI trials, given the limited scope of the PPWG, future updates that will be necessary, and the evolving landscape of HIV cure-related trials with ATIs.18 For example, ACTG clinical trial sites have expressed a greater need to improve the communications and community engagement components supporting ATI trials, including creating engagement materials around ATI trials. Our team strongly supports engaging local CABs and former ATI trial participants around the adaptation of the PPWG toolkit and dedicating resources to ensure the toolkit's acceptability and maintain community willingness to participate in ATI trials. We also recognize the importance of more proactively involving partners in HIV transmission mitigation,19,20 even though they are not formally trial participants. Considering the June 2024 Phase 3 PURPOSE 1 trial results indicating that twice-yearly long-acting PrEP (Lenacapavir) showed 100% efficacy in preventing HIV transmission in cisgender women,21 more attention should be paid to facilitating access to long-acting PrEP for partners of ATI trial participants as an ethical imperative. For this reason, we strongly encourage increased collaboration among the DAIDS-funded clinical trials networks on establishing evidence-based partner protections in ATI trials. Further, in contexts where intimate partner violence (IPV) is prevalent, we recognized the critical importance of trauma-informed and healing-centered frameworks around partner protections, where research teams need to take participants' safety into account.22, 23, 24 We have also advocated for justice-informed approaches to extend ATI trials in communities that remain under-represented in ATI trials and under-served with respect to HIV prevention globally, provided that reasonable safeguards are in place.25 We also anticipate integrating emerging technologies into the toolkit in the future, such as home-based blood collection for viral load testing, to reduce the burden of ATI trials.26
4.1. Limitations
Limitations of the HIV transmission risk mitigation toolkit have been previously acknowledged by Peluso and colleagues, such as reliance on participants’ willingness to disclose information to sex partner(s), and difficulties posed by disclosure with multiple or anonymous partner(s).2 In addition, the ACTG PPWG had a limited duration of one year and was a volunteer effort. The PPWG toolkit remains focused on the United States context and would benefit from greater community engagement and formative socio-behavioral research in diverse contexts. The PPWG toolkit should also be updated based on emerging evidence around partner protections in ATI trials. We anticipate the need to design interventions based on emerging socio-behavioral evidence in the context of actual ATI trials (which has remained limited to date), particularly around the disclosure of HIV and ATIs and PrEP referral and uptake for partners. PrEP and PEP are not equitable, available, or acceptable everywhere. Even with the presence of a toolkit, trial sites will face challenges around partner protections that could not have been anticipated. As such, sites need to have mechanisms in place to monitor for and address unforeseen challenges (e.g., regular team and advisory board meetings). Risk mitigation tools do not replace mental health and psychosocial support throughout the entire ATI trial participation trajectory, and should be part of a broader, systematic package designed to protect both participants and partners.4
5. Conclusions
In summary, the ACTG PPWG updated the toolkit published in the Journal of Virus Eradication in 2020. We identified new challenges and needs at the participant, partner and study levels, and provided additional tools to address these challenges. We hope HIV cure research teams will use the toolkit components they deem most relevant to specific ATI trials and continue to meaningfully engage community members in the ethics and trustworthiness of ATI trials in the U = U era.
Disclaimer
The views expressed here are those of the authors and do not necessarily reflect the policies or positions of the U.S. National Institutes of Health (NIH), or the U.S. Department of Health and Human Services.
Funding
K.D. received separate support from R01-MH126768 and UM1AI126620 (BEAT-HIV Collaboratory) which is co-supported by the National Institute of Allergies and Infectious Diseases (NIAID), the National Institute of Mental Health (NIMH), the National Institute of Neurological Disorders and Stroke (NINDS), the National Institute on Drug Abuse (NIDA), and the Robert I. Jacobs Fund of The Philadelphia Foundation. M.J.P. received support from K23AI157875 and the DARE Collaboratory (UM1 AI164560).
CRediT authorship contribution statement
Karine Dubé: Writing – review & editing, Writing – original draft, Supervision, Conceptualization. Thomas J. Villa: Writing – review & editing. William Freshwater: Writing – review & editing. Brittney Mauk: Writing – review & editing. Annette Rid: Writing – review & editing. Michael J. Peluso: Writing – review & editing, Supervision, Conceptualization.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:
K.D. has received funding from Gilead Sciences, Inc. M.J.P. has received consulting fees from Gilead Sciences, Inc. and is on a Data Safety Monitoring Board for American Gene Technologies. All other authors declare no conflict of interest.
Acknowledgements
This work was supported in part by the Clinical Center Department of Bioethics, which is in the Intramural Program of the National Institutes of Health (A.R.). We would also like to thank Lawrence (Larry) Fox and Tia Morton from the National Institutes of Health (NIH) Division of AIDS (DAIDS) for organizing the August 2021 meeting titled “Addressing Challenges that Limit Participation in HIV Trials with ATIs” as well as all workshop participants. We would like to thank the Advancing Clinical Therapeutics Globally (ACTG) Network Reservoirs Remission and Cure Transformative Science Group (Cure TSG) as well as all individuals who contributed to the AIDS Clinical Trials Group (ACTG) Partner Protections Working Group (PPWG) from 2022 to 2023, including Belinda Ameterra, Robert (Bob) Bucklew, Stanford Chimutimunzeve, Krista L. Dong, Gail Graham, Lawrence (Larry) Fox, Shelly Karuna, Tia Morton, David Palm, and Wadzanai Samaneka. We would also like to thank Lynda Dee, Jeff Taylor, David Palm and Gail Graham who contributed actively to the generation of the partner protections package (P3) framework.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jve.2024.100386.
Appendix A. Supplementary data
The following are the Supplementary data to this article.
Data availability
No data was used for the research described in the article.
References
- 1.Julg B., Dee L., Ananworanich J., et al. Recommendations for analytical treatment interruptions in HIV research trials. Report of a consensus meeting. Lancet HIV. 2019;6(4):e259–e268. doi: 10.1016/S2352-3018(19)30052-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Peluso M.J., Dee L., Campbell D., et al. A collaborative, multidisciplinary approach to HIV transmission risk mitigation during analytic treatment interruption. J Virus Erad. 2020;6:34–37. doi: 10.1016/S2055-6640(20)30009-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dubé K., Kanazawa J.T., Dee L., et al. Ethical and practical considerations for mitigating risks to sexual partners during analytical treatment interruptions in HIV cure-related research. HIV Res Clin Pract. 2021;Mar;24:1–17. doi: 10.1080/25787489.2021.1902116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dubé K., Morton T., Fox L., et al. A partner protection package for HIV cure-related trials involving analytical treatment interruptions. Lancet Infectious Diseases [Internet] 2023;23(10):e418–e430. doi: 10.1016/S1473-3099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lelièvre J.D., Hocqueloux L. Unintended HIV-1 transmission to a sex partner in a study of a therapeutic vaccine candidate. JID (J Infect Dis) 2019 doi: 10.1093/infdis/jiz012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ugarte A., Romero Y., Tricas A., Casado C., Garcia F., Leal L. Unintended HIV-1 infection during analytical treatment interruption. J Infect Dis. 2020;221(10):1740–1742. doi: 10.1093/infdis/jiz611. [DOI] [PubMed] [Google Scholar]
- 7.Ndung’u T., McCune J.M., Deeks S.G. Why and where an HIV cure is needed and how it might be achieved. Nature [Internet] 2019;576(April):397–405. doi: 10.1038/s41586-019-1841-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deeks S.G., Archin N., Cannon P., et al. Research priorities for an HIV cure: international AIDS society global scientific strategy 2021. Nat Med. 2021 doi: 10.1038/s41591-021-01590-5. https://www.nature.com/articles/s41591-021-01590-5 [DOI] [PubMed] [Google Scholar]
- 9.Peluso M.J., Dee L., Shao S., et al. Operationalizing HIV cure-related trials with analytic treatment interruptions during the SARS-Cov-2 pandemic: a collaborative approach. Clin Infect Dis. 2021;72(10):1843–1849. doi: 10.1093/cid/ciaa1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brown B.J., Sugarman J. Why ethics guidance needs to be updated for contemporary HIV prevention research. J Int AIDS Soc. 2020;23 doi: 10.1002/jia2.25500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haire B., Folayan M.O., Hankins C., et al. Ethical considerations in determining standard of prevention packages for HIV prevention trials: examining PrEP. Develop World Bioeth. 2013;13(2):87–94. doi: 10.1111/dewb.12032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sugarman J., Celum C., Donnell D., Mayer K. Ethical considerations for new HIV prevention trials. Lancet. 2019;6(August):e489–e491. doi: 10.1016/S2352-3018(19)30184-5. [DOI] [PubMed] [Google Scholar]
- 13.Sugarman J. Ethical considerations regarding oral preexposure prophylaxis in HIV prevention trials. Curr Opin HIV AIDS. 2016;11(1):109–115. doi: 10.1097/COH.0000000000000214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dubé K., Ndukwe S.O., Korolkova A., Dee L., Sugarman J., Sauceda J.A. Participant experiences in a combination HIV cure-related trial with extended analytical treatment interruption in San Francisco, United States. HIV Res & Clin Pract. 2024;25(1):1–15. doi: 10.1080/25787489.2024.2312318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.World Health Organization The role of HIV viral suppression in improving individual health and reducing transmission [Internet] 2023. https://www.who.int/publications/i/item/9789240055179 [cited 2024 Jun 8]
- 16.Bilger A., Plenn E., Barg F.K., et al. Participant experiences in HIV cure-directed trial with an extended analytical treatment interruption in Philadelphia, United States. HIV Res Clin Pract. 2023;24(1) [PMC free article] [PubMed] [Google Scholar]
- 17.Dubé K., Kanazawa J.T., Campbell C., et al. Considerations for increasing racial, Ethnic, Gender and sexual diversity in HIV cure-related research with Analytical treatment interruptions: a qualitative inquiry. AIDS Res Hum Retrovir. 2022;38(1):50–63. doi: 10.1089/aid.2021.0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barr L., Jefferys R. A landscape analysis of HIV cure-related clinical research in 2019. J Virus Erad. 2020;6(4) doi: 10.1016/j.jve.2020.100010. [Internet] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Campbell D., Dubé K., Cowlings P., et al. “It comes altogether as one”: perceptions of analytical treatment interruptions and partner protections among racial, ethnic, sex and gender diverse HIV serodifferent couples in the United States. BMC Publ Health. 2022;22(1317):1–14. doi: 10.1186/s12889-022-13528-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dubé K., Agarwal H., Stockman J.K., et al. “I would absolutely need to know that my partner is still going to be protected”: perceptions of HIV Cure-Related Research among Diverse HIV Serodifferent Couples in the United States. AIDS Res Hum Retrovir. 2022;39(8):400–413. doi: 10.1089/aid.2022.0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gilead Sciences I. Gilead's twice-yearly lenacapavir demonstrated 100% efficacy and superiority to daily Truvada® for HIV prevention. 2024. https://www.gilead.com/news-and-press/press-room/press-releases/2024/6/gileads-twiceyearly-lenacapavir-demonstrated-100-efficacy-and-superiority-to-daily-truvada-for-hiv-prevention [cited 2024 Aug 9]
- 22.Miall A., McLellan R., Dong K., et al. Bringing social context into global biomedical HIV cure-related research: an urgent call to action. J Virus Erad. 2022;8(1):8–10. doi: 10.1016/j.jve.2021.100062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dubé K., Mthimkhulu D., Ngcobo W., et al. “With this study, we have hope that something is coming”: community members' perceptions of HIV cure-related research in Durban, South Africa - a qualitative focus Group study. HIV Res Clin Pract. 2023;24(1):1–13. [PMC free article] [PubMed] [Google Scholar]
- 24.Dubé K., Barr E., Philbin M., et al. Increasing the meaningful involvement of women in HIV cure-related research: a qualitative interview study in the United States. HIV Res Clin Pract [Internet] 2023;24(1) doi: 10.1080/25787489.2023.2246717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dubé K., Perez-Brumer A. Call for justice-informed HIV cure trials with ATIs. Lancet HIV [Internet] 2024 Jan:1–2. doi: 10.1016/S2352-3018(24)00002-X. https://linkinghub.elsevier.com/retrieve/pii/S235230182400002X [Online First] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dubé K., Agarwal H., Carter W.B., et al. Participant experiences using novel home-based blood collection device for viral load testing in HIV cure trials with analytical treatment interruptions. HIV Res Clin Pract [Internet] 2022;23(1):76–90. doi: 10.1080/25787489.2022.2103582. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No data was used for the research described in the article.