Abstract
Aims
We aimed to assess the association between Inflammatory Burden Index (IBI) and cardiovascular disease (CVD) in adult Americans.
Methods
This cross-sectional investigation included people with comprehensive data on IBI and CVD from the National Health and Nutrition Examination Survey (NHANES) 2005–2010 database. C-reactive protein (CRP) × neutrophil/lymphocyte (NLR) count was used to calculate IBI. CVD included angina pectoris, stroke, congestive heart failure (CHF), and coronary heart disease (CHD). Subgroup analysis and weighted multivariate regression were utilized to analyze the independent association between CVD and IBI.
Results
A total of 15,325 adult Americans were involved. There were 9.57 % of subjects having CVD, which was increased with increasing IBI quartiles (Tertile 1: 4.64 %; Tertile 2: 7.71 %; Tertile 3: 10.63 %; Tertile 4: 15.29 %; p < 0.001). After full adjustment, multivariate logistic regression analysis demonstrated a positive correlation between IBI level and CVD prevalence (OR = 1.43; 95 % CI, 1.16–1.76, p < 0.001). Subgroup analyses and interaction tests showed that the association between IBI and the prevalence of CVD was not affected by sex, age, body mass index, race, hypertension, and diabetes mellitus.
Conclusions
In adult Americans, There is an association between IBI levels and the prevalence of CVD. More large-scale future research is required to assess the effect of IBI on CVD.
Keywords: Inflammatory Burden Index, Cardiovascular disease, NHANES, Cross-sectional study, Association
1. Introduction
Cardiovascular disease (CVD) is the primary cause of human death and shortened healthy life expectancy. It also plays a major role in health loss and high healthcare expenses, which puts a tremendous strain on the world's health systems [1]. Statistics show that there were 523 million cases of CVD worldwide in 2019 and 271 million cases in 1990. Until 2019, the annual number of deaths from CVD reached 18.6 million [2]. Therefore, it is imperative to explore the related risk factors as soon as feasible to limit the occurrence of CVD.
Accumulated evidence suggests that the activation of the immune system and persistent infection are important factors in the cause of CVD [[3], [4], [5]]. There is a strong correlation between CVD risk and higher c-reactive protein (CRP) levels [6]. It has been demonstrated that neutrophil/lymphocytes (NLR), as a marker of cardiovascular risk, can be utilized to enhance the risk stratification of patients with a variety of cardiometabolic disorders and CVDs [7]. Researchers combined CRP and NLR to create the Inflammatory Burden Index (IBI) metric, which is now commonly used in prognostic studies of cancer, and cerebrovascular disease [[8], [9], [10]]. Compared with CRP and INR, IBI, as a comprehensive inflammation indicator, can provide a more stable assessment of inflammation, more accurately reflect the inflammatory state of the body better evaluate the effectiveness of treatment, and predict the prognosis of patients [10,11].
Since there is currently no research on the correlation between IBI and CVD, we hypothesized that IBI is associated with the prevalence of CVD, and we carried out a current study to explore this relationship.
2. Methods
The Centers for Disease Control and Prevention (CDC) used the National Health and Nutrition Examination Survey (NHANES) to track the health and nutritional status of adults and children in the United States. Every participant provided informed consent, and the National Center for Health Statistics (NCHS) Institutional Review Board authorized the survey. To ensure that the sample chosen for the NHANES is representative of the whole American population, a complex stratified and multistage probability clustering sampling technique was employed throughout its creation. The comprehensive details about the administration and methodology of the NHANES survey can be uploaded at http://www.cdc.gov/nchs/nhanes.htm.
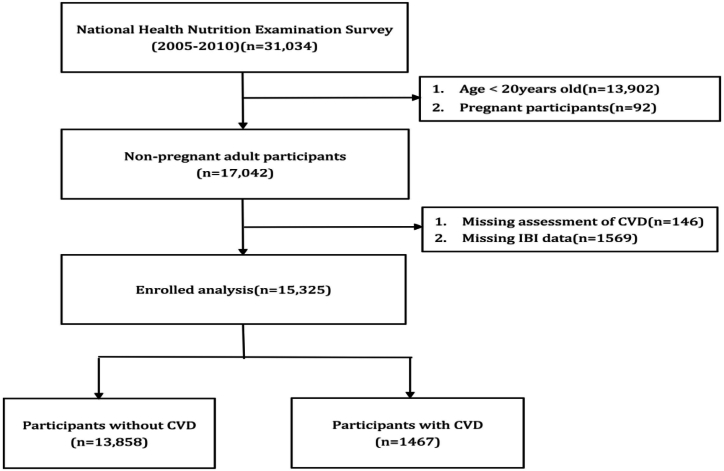
To evaluate their physical and medical conditions, participants performed a standardized home interview, an ambulatory screening center health screening, and laboratory tests. We selected three NHANES cycles from 2005 to 2010 to explore the relationship between IBI and CVD prevalence, because they included the entire set of variables needed to calculate IBI using the same technique. Exclusion criteria for the individuals involved in our analysis were as follows: (1) adult Americans aged <20 years; (2) pregnant individuals; and (3) incomplete IBI and CVD evaluation data. Fig. 1 depicts the selection process. Finally, 15,325 adult Americans were included in the analysis that came after.
Fig. 1.
Flowchart of participants included in the analysis.
During the personal interview, participants answered the following question about their medical conditions: “Has your doctor ever told you that you have angina/stroke/congestive heart failure/coronary heart disease?” The results of the questionnaire, which were validated, included self-reported diagnoses of CVD. Any individual who answered “yes” to any of the questions was deemed to have a positive diagnosis of CVD. Angina, stroke, congestive heart failure, and coronary heart disease were included in the CVD questionnaire.
The methods used to derive CBC parameters are based on the Beckman Coulter method of counting and sizing, in combination with an automatic diluting and mixing device for sample processing, and a single beam photometer for hemoglobinometry. The NLR for each participant was determined by dividing the total absolute neutrophil count by the total absolute lymphocyte count. For a detailed description of laboratory methods, please visit:https://wwwn.cdc.gov/Nchs/Nhanes/2009-2010/CBC_F.htm.For CRP processing, quantification was performed using latex-enhanced turbidimetry and particle-enhanced assays. Measurements were performed on a Behring turbidimeter to determine quantitative CRP levels. For a detailed description of laboratory methods, please visit:https://wwwn.cdc.gov/Nchs/Nhanes/2009-2010/CRP_F.htm.IBI is defined as CRP × NLR count as previously reported [9]. Our analysis used IBI as the exposure variable. IBI as a continuous variable will be treated as quartiles in our analysis.
The study's covariates included diabetes mellitus (DM) and hypertension, High blood pressure was classified as a self-reported diagnosis of hypertension, the self-reported intake of hypertension drugs, or a recorded systolic blood pressure ≥140 mmHg or a diastolic blood pressure≥ 90 mmHg. Diabetes was identified as a self-reported diagnosis of diabetes, taking diabetes medication or insulin, an HbA1c level of 6.5 % or higher, fasting plasma glucose of 7.0 mmol/L or above, or a 2-h postprandial blood glucose level of 11.1 mmol/L or more following an oral glucose challenge. demographic traits (body mass index (BMI), age, gender, education level, race, marital status, poverty/income ratio (PIR), tobacco use, and alcohol); and laboratory results (hemoglobin (Hb), white blood cells (WBC), platelets (PLT), albumin (Alb), alanine albumin (Alb), alanine aminotransferase (ALT), aspartate aminotransferase (AST), triglycerides (TG), total cholesterol (TC), blood urea nitrogen (BUN), uric acid (UA) and creatinine (Cr)).
Mexican Americans, Other Hispanics, Non-Hispanic Whites, and Non-Hispanic Blacks were among the racial classifications. Less than high school, high school, and more than high school were the categories for education levels. Three classifications for marital status included married, separated, and single. A higher poverty/income ratio (PIR) denoted a better household income status. PIR was calculated by dividing income for the family (or individual) by the specific poverty line applicable for the study year. Based on raw results, PIR was classified as 0–1, 1–3, and >3 in this study. Physical examinations were used to measure height and weight. BMI was calculated by splitting weight in kilograms by height in meters squared. Individuals who had smoked less than 100 cigarettes in their lifetime were considered to have never smoked. Adults who were not smoking at the time of the survey but had smoked more than 100 cigarettes were labeled as former smokers, while those who had smoked more than 100 cigarettes but were actively smoking at that time were classified as current smokers. Drinking habits were divided into abstainers, light to moderate drinkers (less than one drink for ladies, more than two for men), and heavy drinkers (more than one drink for ladies, more than two for men) [12]. The definitions of DM and hypertension were both based on self-reported histories. The public can get all comprehensive measurement protocols for these variables at www.cdc.gov/nchs/nhanes/.
Statistical analyses were carried out in compliance with the CDC's suggested analytical criteria. Our statistical analyses included sample weights, stratification, and clustering due to the complex, multistage probability sampling architecture of the NHANES. Based on the survey methodology, continuous variables were compared using t-tests after being provided as weighted means and standard errors (SEs). Weighted percentages were used to show and compare categorical variables, and the Rao-Scott χ2 Statistics were employed. We estimated the odds ratios (ORs) and 95 % confidence intervals (CIs) for IBI levels and CVD incidence by using logistic regression analyses. Quartiles were utilized to classify the IBI, with the lowest quartile serving as the standard. Age, gender, and race were added as covariates in model 2, while no covariates were included in crude model 1. Every covariate was included in Model 3. We also investigated the relationship between four CVD subtypes in Model 3 and IBI. In several populations, subgroup analyses stratified by age, gender, BMI, DM, and hypertension were carried out. The Empower software and R version 3.4.3 served as the foundation for all statistical analyses. Statistical significance was attained when p < 0.05.
3. Results
We assessed 15,325 adult Americans in total, of which 7303 (47.7 %) were male. Based on the IBI quartile grouping, they were separated into four groups equally, including Q1 (<0.143), Q2 (0.143–0.390), Q3 (0.390–1.046), and Q4 (≥1.046). The overall prevalence of CVD was 9.57 %, and the prevalence of CVD increased as IBI quartiles increased (Tertile 1: 4.64 %; Tertile 2: 7.71 %; Tertile 3: 10.63 %; Tertile 4: 15.29 %;P < 0.001). The IBI quartiles were found to differ statistically (all P < 0.05) in the following categories, including gender, age, BMI, education level, married status, smoking, drinking, hypertension, DM, PIR, WBC, PLT, Hlb, Alb, AST, BUN, UA, TG and TC (Table 1).
Table 1.
Baseline characteristics of IBI among the general adult population in the National Health and Nutrition Examination Survey (NHANES), 2005–2010 (weighted).
| Variables | Quartiles of IBI |
p-Value | |||
|---|---|---|---|---|---|
| Q1(<0.143) | Q2(0.143–0.390) | Q3(0.390–1.046) | Q4(≥1.046) | ||
| Participants,n | 3830 | 3824 | 3839 | 3832 | |
| Gender(%) | <0.001 | ||||
| Male | 2004(52.33) | 2050(53.63) | 1777(46.30) | 1472(38.42) | |
| Female | 1826(47.67) | 1774(46.37) | 2062(53.70) | 2360(61.58) | |
| Age (mean ± SD) (years) | 43.16 ± 15.92 | 47.41 ± 16.39 | 48.64 ± 16.65 | 48.66 ± 16.88 | <0.001 |
| Race (%) | <0.001 | ||||
| Mexican-American | 266(6.96) | 324(8.49) | 338(8.80) | 349(9.10) | |
| Other Hispanic | 165(4.30) | 173(4.54) | 177(4.63) | 160(4.19) | |
| Non-Hispanic White | 2668(69.68) | 2719(71.10) | 2710(70.60) | 2719(70.95) | |
| Non-Hispanic Black | 412(10.77) | 370(9.68) | 403(10.50) | 466(12.16) | |
| Other Race | 319(8.29) | 238(6.19) | 211(5.47) | 138(3.60) | |
| Education level (%) | <0.001 | ||||
| Below high school | 600(15.66) | 687(17.96) | 1121(20.92) | 809(21.12) | |
| High school | 786(20.54) | 945(24.72) | 986(25.69) | 1013(26.44) | |
| Above high school | 3154(63.80) | 2192(57.32) | 1732(53.59) | 2010(52.44) | |
| Marital status (%) | <0.001 | ||||
| Married | 2207(57.62) | 2282(59.68) | 2243(58.43) | 2083(54.36) | |
| Separated | 520(13.58) | 653(17.07) | 793(20.66) | 882(23.02) | |
| Never married | 1103(28.80) | 889(23.25) | 803(20.91) | 867(22.62) | |
| Smoking status (%) | 0.002 | ||||
| Never smoker | 2832(73.95) | 2711(70.89) | 2783(72.51) | 2707(70.66) | |
| Former smoker | 130(3.40) | 144(3.76) | 124(3.24) | 111(2.91) | |
| Current smoker | 868(22.65) | 969(25.34) | 932(24.26) | 1014(26.43) | |
| Drinking status (%) | <0.001 | ||||
| Non-drinker | 2068(53.99) | 2118(55.40) | 2193(57.13) | 2343(61.16) | |
| Low-to-moderate drinker | 1358(35.46) | 1306(34.16) | 1306(34.03) | 1157(30.21) | |
| Heavy drinker | 404(10.55) | 400(10.44) | 340(8.84) | 332(8.63) | |
| Hypertension (%) | <0.001 | ||||
| Yes | 768(20.06) | 1095(28.65) | 1294(33.72) | 1506(39.30) | |
| No | 3062(79.94) | 2729(71.35) | 2545(66.28) | 2326(60.70) | |
| DM (%) | <0.001 | ||||
| Yes | 189(4.95) | 271(7.08) | 332(8.65) | 485(12.65) | |
| No | 3641(95.05) | 3553(92.92) | 3507(91.35) | 3347(87.35) | |
| PIR (%) | <0.001 | ||||
| 0–1 | 411(10.73) | 435(11.38) | 490(12.77) | 587(15.32) | |
| 1–3 | 1169(30.52) | 1225(32.04) | 1362(35.48) | 1401(36.56) | |
| >3 | 2250(58.75) | 2164(56.58) | 1987(51.75) | 1844(48.12) | |
| BMI (mean ± SD) (kg/m2) | 25.08 ± 4.37 | 27.84 ± 5.30 | 29.73 ± 6.09 | 32.90 ± 8.27 | <0.001 |
| WBC (mean ± SD) (1000 cells/μL) | 6.46 ± 2.24 | 6.97 ± 1.89 | 7.47 ± 2.02 | 8.44 ± 2.52 | <0.001 |
| PLT (mean ± SD) (1000 cells/μL) | 248.01 ± 59.90 | 256.81 ± 65.46 | 266.17 ± 67.56 | 283.46 ± 78.37 | <0.001 |
| Hb (mean ± SD) (g/dL) | 14.42 ± 1.45 | 14.52 ± 1.44 | 14.37 ± 1.50 | 14.02 ± 1.54 | <0.001 |
| Alb (mean ± SD) (g/dL) | 4.39 ± 0.29 | 4.31 ± 0.30 | 4.23 ± 0.31 | 4.04 ± 0.37 | <0.001 |
| ALT (mean ± SD) (U/L) | 24.19 ± 14.27 | 26.44 ± 15.96 | 27.25 ± 18.54 | 25.94 ± 24.18 | 0.096 |
| AST (mean ± SD) (U/L) | 25.52 ± 11.91 | 25.89 ± 13.66 | 26.31 ± 13.21 | 25.70 ± 19.13 | <0.001 |
| BUN (mean ± SD) (mg/dL) | 12.72 ± 4.59 | 13.12 ± 4.97 | 13.00 ± 5.23 | 13.05 ± 6.52 | 0.004 |
| Cr (mean ± SD) (mg/dL) | 0.89 ± 0.29 | 0.90 ± 0.26 | 0.90 ± 0.29 | 0.90 ± 0.47 | 0.351 |
| UA (mean ± SD) (mg/dL) | 5.12 ± 1.32 | 5.45 ± 1.36 | 5.56 ± 1.40 | 5.67 ± 1.53 | <0.001 |
| Triglycerides (mean ± SD) (mg/dL) | 129.31 ± 118.57 | 155.96 ± 117.66 | 173.35 ± 128.61 | 169.87 ± 123.28 | <0.001 |
| Total cholesterol | 192.31 ± 38.82 | 198.69 ± 41.11 | 202.87 ± 42.55 | 198.94 ± 42.26 | <0.001 |
| (mean ± SD) (mg/dL) | |||||
| CHF(%) | <0.001 | ||||
| Yes | 44(1.16) | 47(1.23) | 85(2.21) | 162(4.24) | |
| No | 3786(98.84) | 3777(98.87) | 3754(97.79) | 3670(95.76) | |
| CHD(%) | <0.001 | ||||
| Yes | 82(2.14) | 112(2.93) | 158(4.13) | 147(3.83) | |
| No | 3748(97.86) | 3712(97.07) | 3681(95.87) | 3685(96.17) | |
| Angina pectoris(%) | <0.001 | ||||
| Yes | 59(1.55) | 56(1.46) | 93(2.43) | 119(3.19) | |
| No | 3771(98.45) | 3768(98.54) | 3746(97.57) | 3713(96.81) | |
| Stroke(%) | <0.001 | ||||
| Yes | 65(1.69) | 87(2.29) | 117(3.05) | 166(4.35) | |
| No | 3765(98.31) | 3737(97.71) | 3722(96.95) | 3666(95.65) | |
| CVD(%) | <0.001 | ||||
| Yes | 178(4.64) | 295(7.71) | 408(10.63) | 586(15.29) | |
| No | 3652(95.36) | 3529(92.29) | 3431(89.37) | 3246(84.71) | |
1. Values are weighted means ± SE for continuous variables or weighted percentages for categorical variables. 2. PIR: poverty/income ratio; DM: diabetes mellitus; BMI: body mass index; WBC: white blood cells; PLT: platelets; Hb: hemoglobin; Alb: albumin; ALT: alanine transaminase; AST: aspartate transaminase; BUN: blood urea nitrogen; Cr: creatinine; UA: uric acid; CVD: cardiovascular disease; CHF: congestive heart failure; CHD: coronary heart disease.3. The significance of differences between quartiles is indicated by the p-value. A p-value <0.05 was considered statistically significant.
Table 2 illustrates the correlation between IBI and CVD. It also showed that the prevalence of CVD was higher in the highest quartile of all models when compared to the lowest quartile of IBI (the unadjusted model 1: OR: 2.57, 95 % CI: 2.18–3.02, P for trend<0.001; model 2 adjusted for age, gender, and race: OR: 2.09, 95 % CI: 1.76–2.49, P for trend<0.001; Model 3 after accounting for all covariates: OR: 1.43, 95 % CI: 1.16–1.76, P for trend<0.001.)After thorough adjustment, we noticed that for each unit rise in IBI, the occurrence of CVD increased 1.43 times. Table 3 presents the correlation between IBI and various subtypes of CVDs. Among these subtypes, IBI was related to the prevalences of CHF, angina pectoris, and stroke.
Table 2.
Logistic regression analysis of the association between IBI and CVD (weighted).
| Model 1OR (95 % CI) | Model 2OR (95 % CI) | Model 3OR (95 % CI) | |
|---|---|---|---|
| IBI | 1.05(1.04,1.06)a | 1.04(1.03,1.06)a | 1.02(1.01,1.04)a |
| Q1 | reference | reference | reference |
| Q2 | 1.40(1.17,1.68)a | 1.12(0.93,1.35) | 1.05(0.86,1.29) |
| Q3 | 1.89(1.59,2.24)a | 1.41(1.18,1.69)a | 1.19(0.98,1.46) |
| Q4 | 2.57(2.18,3.02)a | 2.09(1.76,2.49)a | 1.43(1.16,1.76)a |
| P for trend | <0.001 | <0.001 | <0.001 |
Model 1: non-adjusted model; Model 2: adjusted age, gender, race; Model 3: adjusted age, gender, race, BMI, education level, marital status, smoking status, drinking status, hypertension, DM, PIR, Wbc, Hb, Plt, Alb, ALT, AST, BUN, Cr, UA, Triglycerides and Total cholesterol. IBI: Q1: <0.143, Q2: 0.143–0.390, Q3: 0.390–1.046, Q4: ≥1.046.
P < 0.001.
Table 3.
Association of IBI with the total and specific CVD.
| Subgroup | OR (95%CI) | P for trend |
|---|---|---|
| Total-CVD | <0.001 | |
| Q1 | Reference | |
| Q2 | 1.05(0.86,1.29) | |
| Q3 | 1.19(0.98,1.46) | |
| Q4 |
1.43(1.16,1.76)c |
|
| CHF | <0.001 | |
| Q1 | Reference | |
| Q2 | 0.98(0.68,1.41) | |
| Q3 | 1.21(0.85,1.71) | |
| Q4 |
1.75(1.25,2.46)b |
|
| CHD | 0.293 | |
| Q1 | Reference | |
| Q2 | 1.07(0.80,1.42) | |
| Q3 | 1.38(1.04,1.82)a | |
| Q4 |
1.25(0.93,1.67) |
|
| Angina pectoris | <0.001 | |
| Q1 | Reference | |
| Q2 | 0.85(0.59,1.21) | |
| Q3 | 1.13(0.81,1.58) | |
| Q4 |
1.48(1.05,2.09)a |
|
| Stroke | 0.024 | |
| Q1 | Reference | |
| Q2 | 1.20(0.88,1.62) | |
| Q3 | 1.25(0.93,1.69) | |
| Q4 | 1.46(1.08,1.99)a |
The model was adjusted for the parameters of age, gender, race, BMI, education level, marital status, smoking status, drinking status, hypertension, DM, PIR, Wbc, Hb, Plt, Alb, ALT, AST, BUN, Cr, UA, Triglycerides and Total cholesterol. IBI: Q1: <0.143, Q2: 0.143–0.390, Q3: 0.390–1.046, Q4: ≥1.046. CVD:cardiovascular disease; CHF: congestive heart failure; CHD: coronary heart disease.
P < 0.05.
P < 0.01.
P < 0.001.
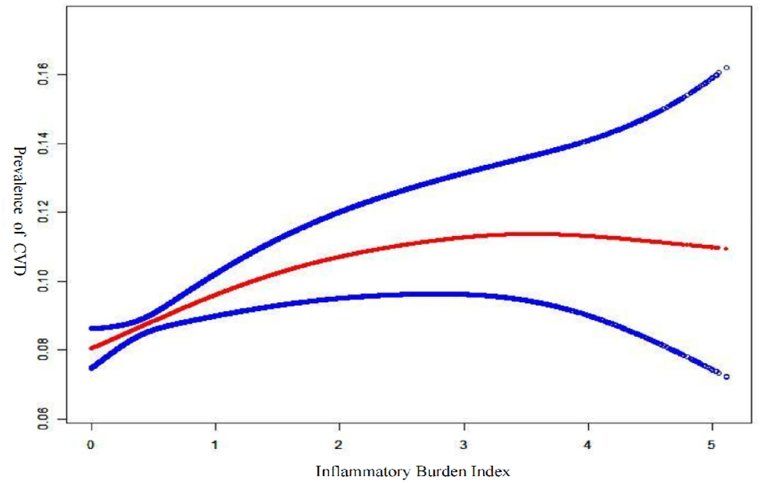
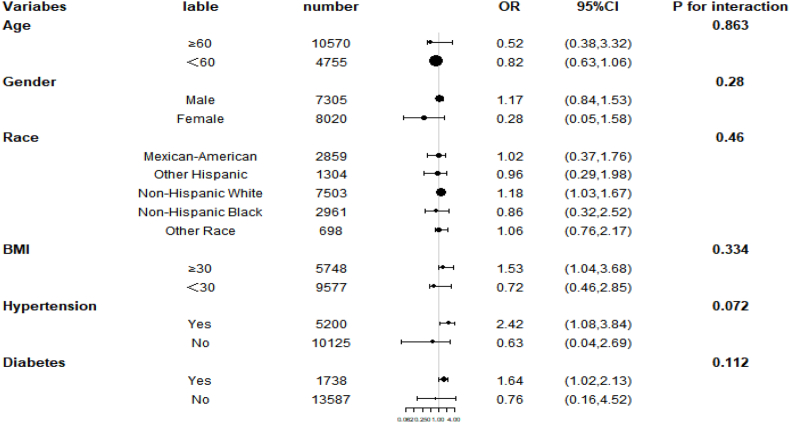
The use of smoothed curve fitting showed a nonlinear positive correlation between IBI and the prevalence of CVD(Fig. 2). The results of our subgroup analyses showed that IBI levels were not consistently associated with the prevalence of CVD (Fig. 3). For subgroups stratified by sex, age, BMI, race, hypertension, and diabetes, the significant association between IBI levels and the occurrence of CVD was significant in Non-Hispanic White, BMI ≥30 kg/m2, hypertension, and diabetes subgroups (all p < 0.05). Interaction tests showed no statistically significant differences between strata for the association between IBI and occurrence of CVD, indicating no significant dependence of gender, age, BMI, race, hypertension, and diabetes on this positive association (all p for interaction >0.05).
Fig. 2.
Association between IBI and prevalence of CVD.
Fig. 3.
Subgroup analysis for the association between IBI and the prevalence of CVD.
4. Discussion
In this study, we evaluated the relationship between the prevalence of CVD and IBI levels. In a cross-sectional study involving 15,325 adult Americans, We found an association between IBI levels and the prevalence of CVD. This correlation remains when analyzing specific CVDs. After performing subgroup analyses, we found that the association between IBI and the prevalence of CVD was independent of sex, age, race, body mass index, hypertension, and diabetes.
This is the first cross-sectional study that explores the relationship between IBI and the prevalence of CVD. Numerous inflammatory variables are related to CVD risk in previous research. Nowadays, a lot of people utilize SII, a comprehensive index that combines platelet, lymphocyte, and neutrophil counts, to determine their risk of CVD. Based on an analysis of 17,389 participants using the NHANES database, Xiao [13] et al. found a positive relationship, particularly in the prediction that stroke may be more favorable. Our results are supported by the majority of research [[14], [15], [16]]. It is commonly acknowledged that one of the fundamental processes behind the emergence of chronic illnesses like CVD is inflammation [17]. According to current epidemiologic findings, the use of refined cereals, sugar-filled beverages, red meat, margarine, and other foods is related to higher levels of inflammatory markers. Overindulgence in inflammatory foods triggers the body's inflammatory response, which in turn speeds up the development of CVD and induces atherosclerosis due to several inflammatory variables. According to pertinent research, those who follow a pro-inflammatory diet are 38 % more likely to develop CVD than people who follow an anti-inflammatory diet [18]. Additionally, there is a substantial relationship between dietary inflammatory index levels and the beginning of CVD [19]. Our findings also suggest that there is a significant correlation between IBI levels and the development of CVD.
IBI is a marker that is used to forecast the prognosis and evaluate the inflammatory load of various malignancies [9]. It can not only distinguish between individuals with varying degrees of inflammation and their prognosis but also offer significant prognostic classification for the majority of tumor patients. By integrating CRP, neutrophils, and lymphocytes, IBI analyzes the balance between acute inflammation and immune inflammation, which may be advantageous in predicting the prognosis of patients with malignancies. It is unclear what causes the higher IBI levels in CVD patients. CRP is a crucial part of IBI and is a pro-inflammatory circulating cytokine, a stabilizing factor that is frequently tested, and a generally recognized biomarker of extensive inflammation [20]. It occurs in the plasma, and inflammation, infection, or damage can cause its concentration to rise [21]. Apoptotic cells, extracellular matrix elements, cellular receptors, growth factors, and infarct size increase as a result of CRP binding to the complement system, which in turn advances CVD [22]. It has been reported the connection between elevated CRP levels and higher risks of peripheral artery disease, atherosclerosis, ischemia, acute myocardial infarction, and sudden cardiac death [23,24]. Neutrophils and lymphocytes, two types of peripheral inflammatory cells, are important for the development of inflammation and subsequent pathological processes, including vasospasm, disruption of the blood-brain barrier, and vascular endothelial damage [25]. An essential part of the immune system is NLR. NLR is related to CVDs, such as acute coronary syndromes, stroke, and CAD. Furthermore, new studies demonstrate that NLR increases the incidences of acute limb ischemia, recurrent cardiovascular events, and mortality [[26], [27], [28], [29], [30]]. Our study found a strong correlation between higher IBI levels and the development of angina, stroke, and CHF.IBI is a clinical tool that can be used to predict the incidence and prognosis of CVD. It offers a complete image of the pro-inflammatory and immunological status of the human body by using the advantages of both CRP and NLR.
We then modified the variables for subgroup analyses to take into account changes in demographic characteristics. Our findings suggest that the association between IBI and CVD is not affected by sex, age, race, BMI, hypertension, and diabetes. Thus, for the overall population, IBI may be a more reliable predictor of CVD prevalence.
There are some advantages in our study. First of all, there is enough sample size and a representative sample selection. Secondly, to make sure that our findings are trustworthy and applicable to a larger group of people, we corrected confounding variables. However, there are also some limitations in this study. First, recall bias is unavoidable, because personal interviews are utilized to detect CVD. Second, as this was a cross-sectional survey, it was not always possible to pinpoint specific causes and draw inferences. Furthermore, the use of a questionnaire diagnosis of CVD as an outcome of the study has some weaknesses, and prospective studies targeting cardiovascular mortality are needed in the future. Finally, we selected data from NHANES 2005–2010 and were unable to use the most recent data due to the constraints of the NHANES database for the IBI. As a result, validation would need a bigger sample size and more surveys.
5. Conclusion
Our cross-sectional analysis revealed a positive association between IBI level and CVD. To support our findings, nevertheless, further extensive prospective investigations are needed.
Data availability statement
Publicly available datasets were analyzed in this study. These data can be uploaded at https://www.cdc.gov/nchs/nhanes/.
Ethics statement
The protocol for data collection was vetted and greenlit by the Research Ethics Review Board of the National Center for Health Statistics. Informed consent in writing was secured from every participant before undertaking the NHANES, and all information was anonymized by the NCHS before it was made accessible to the public.
Funding
None.
CRediT authorship contribution statement
Fei Yu: Data curation. Jiecheng Peng: Supervision.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:nothing If there are other authors, they declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
All authors thank the staff and the participants of this study for their valuable contributions.
References
- 1.Vaduganathan M., Mensah G.A., Turco J.V., Fuster V., Roth G.A. The global burden of cardiovascular diseases and risk: a compass for future health. J. Am. Coll. Cardiol. 2022;80(25):2361–2371. doi: 10.1016/j.jacc.2022.11.005. [DOI] [PubMed] [Google Scholar]
- 2.Roth G.A., Mensah G.A., Johnson C.O., et al. Global burden of cardiovascular diseases and risk factors, 1990-2019: update from the GBD 2019 study. J. Am. Coll. Cardiol. 2020;76(25):2982–3021. doi: 10.1016/j.jacc.2020.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hansson G.K. Inflammation, atherosclerosis, and coronary artery disease. N. Engl. J. Med. 2005;352(16):1685–1695. doi: 10.1056/NEJMra043430. [DOI] [PubMed] [Google Scholar]
- 4.Libby P. Inflammation and cardiovascular disease mechanisms. Am. J. Clin. Nutr. 2006;83(2):456S–460S. doi: 10.1093/ajcn/83.2.456S. [DOI] [PubMed] [Google Scholar]
- 5.Libby P., Ridker P.M., Maseri A. Inflammation and atherosclerosis. Circulation. 2002;105(9):1135–1143. doi: 10.1161/hc0902.104353. [DOI] [PubMed] [Google Scholar]
- 6.Patoulias D., Stavropoulos K., Imprialos K., et al. Inflammatory markers in cardiovascular disease; lessons learned and future perspectives. Curr. Vasc. Pharmacol. 2021;19(3):323–342. doi: 10.2174/1570161118666200318104434. [DOI] [PubMed] [Google Scholar]
- 7.Bhat T., Teli S., Rijal J., et al. Neutrophil to lymphocyte ratio and cardiovascular diseases: a review. Expert Rev. Cardiovasc Ther. 2013;11(1):55–59. doi: 10.1586/erc.12.159. [DOI] [PubMed] [Google Scholar]
- 8.Xie H., Ruan G., Wei L., et al. The inflammatory burden index is a superior systemic inflammation biomarker for the prognosis of non-small cell lung cancer. J Cachexia Sarcopenia Muscle. 2023;14(2):869–878. doi: 10.1002/jcsm.13199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xie H., Ruan G., Ge Y., et al. Inflammatory burden as a prognostic biomarker for cancer. Clin. Nutr. 2022;41(6):1236–1243. doi: 10.1016/j.clnu.2022.04.019. [DOI] [PubMed] [Google Scholar]
- 10.Du M., Xu L., Zhang X., et al. Association between inflammatory burden index and unfavorable prognosis after endovascular thrombectomy in acute ischemic stroke. J. Inflamm. Res. 2023;16:3009–3017. doi: 10.2147/JIR.S419087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Song Z., Lin F., Chen Y., et al. Inflammatory burden index: association between novel systemic inflammatory biomarkers and prognosis as well as in-hospital complications of patients with aneurysmal subarachnoid hemorrhage. J. Inflamm. Res. 2023;16:3911–3921. doi: 10.2147/JIR.S416295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen C., Ye Y., Zhang Y., et al. Weight change across adulthood in relation to all cause and cause specific mortality: prospective cohort study. BMJ. 2019;367 doi: 10.1136/bmj.l5584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xiao S., Wang X., Zhang G., et al. Association of systemic immune inflammation index with estimated pulse wave velocity, atherogenic index of plasma, triglyceride-glucose index, and cardiovascular disease: a large cross-sectional study. Mediat. Inflamm. 2023;2023 doi: 10.1155/2023/1966680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu M., Chen R., Liu L., et al. Systemic immune-inflammation index and incident cardiovascular diseases among middle-aged and elderly Chinese adults: the Dongfeng-Tongji cohort study. Atherosclerosis. 2021;323:20–29. doi: 10.1016/j.atherosclerosis.2021.02.012. [DOI] [PubMed] [Google Scholar]
- 15.Jin Z., Wu Q., Chen S., et al. The associations of two novel inflammation indexes, SII and siri with the risks for cardiovascular diseases and all-cause mortality: a ten-year follow-up study in 85,154 individuals. J. Inflamm. Res. 2021;14:131–140. doi: 10.2147/JIR.S283835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu Y., Ye T., Chen L., et al. Systemic immune-inflammation index predicts the severity of coronary stenosis in patients with coronary heart disease. Coron. Artery Dis. 2021;32(8):715–720. doi: 10.1097/MCA.0000000000001037. [DOI] [PubMed] [Google Scholar]
- 17.Fernández-Friera L., Fuster V., López-Melgar B., et al. Vascular inflammation in subclinical atherosclerosis detected by hybrid PET/MRI. J. Am. Coll. Cardiol. 2019;73(12):1371–1382. doi: 10.1016/j.jacc.2018.12.075. [DOI] [PubMed] [Google Scholar]
- 18.Walrand S., Guillet C., Salles J., et al. Physiopathological mechanism of sarcopenia. Clin. Geriatr. Med. 2011;27(3):365–385. doi: 10.1016/j.cger.2011.03.005. [DOI] [PubMed] [Google Scholar]
- 19.Xie Z., Wang L., Sun M., et al. Mediation of 10-year cardiovascular disease risk between inflammatory diet and handgrip strength: base on NHANES 2011-2014. Nutrients. 2023;15(4):918. doi: 10.3390/nu15040918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhuang Q., Shen C., Chen Y., et al. Association of high sensitive C-reactive protein with coronary heart disease: a Mendelian randomization study. BMC Med. Genet. 2019;20(1):170. doi: 10.1186/s12881-019-0910-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cao M.Y., Liu D., Zhang X.Y., et al. Association of C-reactive protein with cardiovascular outcomes: a mendelian randomization study in the Japanese population. Biomed. Environ. Sci. 2022;35(2):126–132. doi: 10.3967/bes2022.017. [DOI] [PubMed] [Google Scholar]
- 22.Badimon L., Peña E., Arderiu G., et al. C-reactive protein in atherothrombosis and angiogenesis. Front. Immunol. 2018;9:430. doi: 10.3389/fimmu.2018.00430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Emerging Risk Factors Collaboration, Kaptoge S., Di Angelantonio E., et al. C-reactive protein concentration and risk of coronary heart disease, stroke, and mortality: an individual participant meta-analysis. Lancet. 2010;375(9709):132–140. doi: 10.1016/S0140-6736(09)61717-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kuppa A., Tripathi H., Al-Darraji A., et al. C-reactive protein levels and risk of cardiovascular diseases: a two-sample bidirectional mendelian randomization study. Int. J. Mol. Sci. 2023;24(11):9129. doi: 10.3390/ijms24119129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang B., Lin L., Yuan F., et al. Clinical application values of neutrophil-to-lymphocyte ratio in intracranial aneurysms. Aging (Albany NY) 2021;13(4):5250–5262. doi: 10.18632/aging.202445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Del Turco S., Bastiani L., Minichilli F., et al. Interaction of uric acid and neutrophil-to-lymphocyte ratio for cardiometabolic risk stratification and prognosis in coronary artery disease patients. Antioxidants. 2022;11(11):2163. doi: 10.3390/antiox11112163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Taurino M., Aloisi F., Del Porto F., et al. Neutrophil-to-Lymphocyte ratio could predict outcome in patients presenting with acute limb ischemia. J. Clin. Med. 2021;10(19):4343. doi: 10.3390/jcm10194343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sari I., Sunbul M., Mammadov C., et al. Relation of neutrophil-to-lymphocyte and platelet-to-lymphocyte ratio with coronary artery disease severity in patients undergoing coronary angiography. Kardiol. Pol. 2015;73(12):1310–1316. doi: 10.5603/KP.a2015.0098. [DOI] [PubMed] [Google Scholar]
- 29.Shumilah A.M., Othman A.M., Al-Madhagi A.K. Accuracy of neutrophil to lymphocyte and monocyte to lymphocyte ratios as new inflammatory markers in acute coronary syndrome. BMC Cardiovasc. Disord. 2021;21(1):422. doi: 10.1186/s12872-021-02236-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xu C., Cai L., Yi T., et al. Neutrophil-to-lymphocyte ratio is associated with stroke progression and functional outcome in patients with ischemic stroke. Brain Behav. 2023;13(11) doi: 10.1002/brb3.3261. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Publicly available datasets were analyzed in this study. These data can be uploaded at https://www.cdc.gov/nchs/nhanes/.