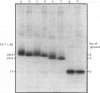
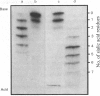
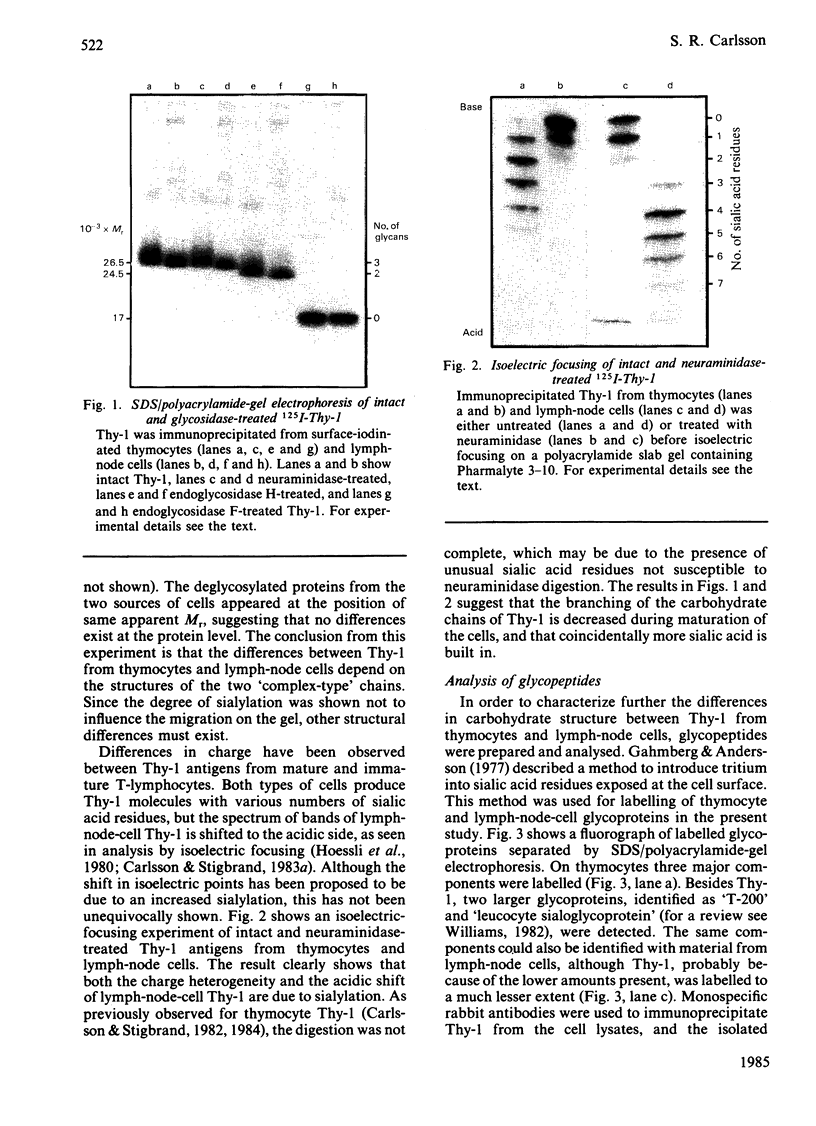
Abstract
The glycans of the Thy-1 antigen present on thymocytes and lymph-node T-lymphocytes were investigated after external labelling of the cells. Neuraminidase, endoglycosidase H and endoglycosidase F were used in combination with sodium dodecyl sulphate/polyacrylamide-gel electrophoresis and isoelectric focusing in order to characterize the nature of the glycans on 125I-labelled and immunoprecipitated Thy-1. Glycopeptides were prepared from Thy-1 obtained from cells labelled by periodate/boro[3H]hydride treatment. The glycopeptides were separated by affinity chromatography on concanavalin A-Sepharose and analysed by gel filtration. The results show that both types of cells possess Thy-1 molecules with three N-linked carbohydrate chains, of which one is of 'high-mannose' type and the other two of triantennary and biantennary 'complex' type. The ratio of triantennary/biantennary chains was decreased on Thy-1 of mature cells compared with that of immature cells, but instead more sialic acid was present on these chains. Deglycosylated Thy-1 appeared to be of the same size regardless of origin, indicating that only the carbohydrate moiety differs between Thy-1 molecules of the two cell types.
Full text
PDF


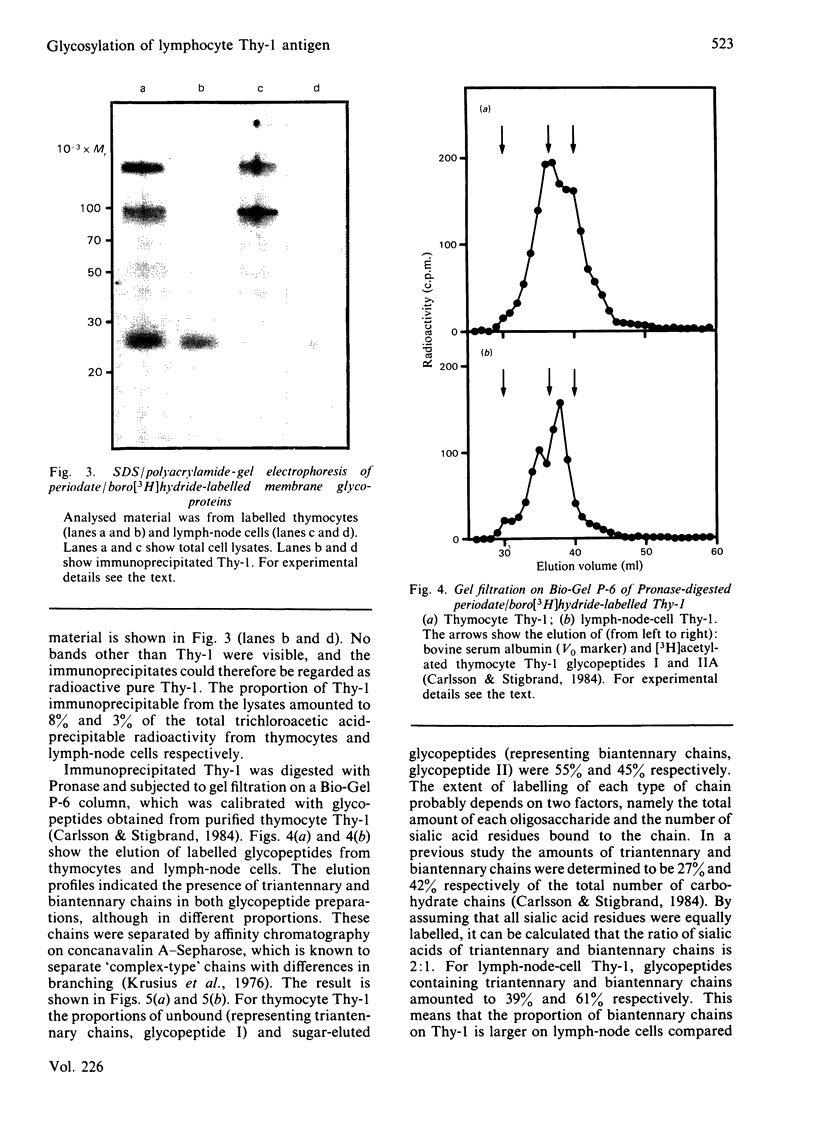
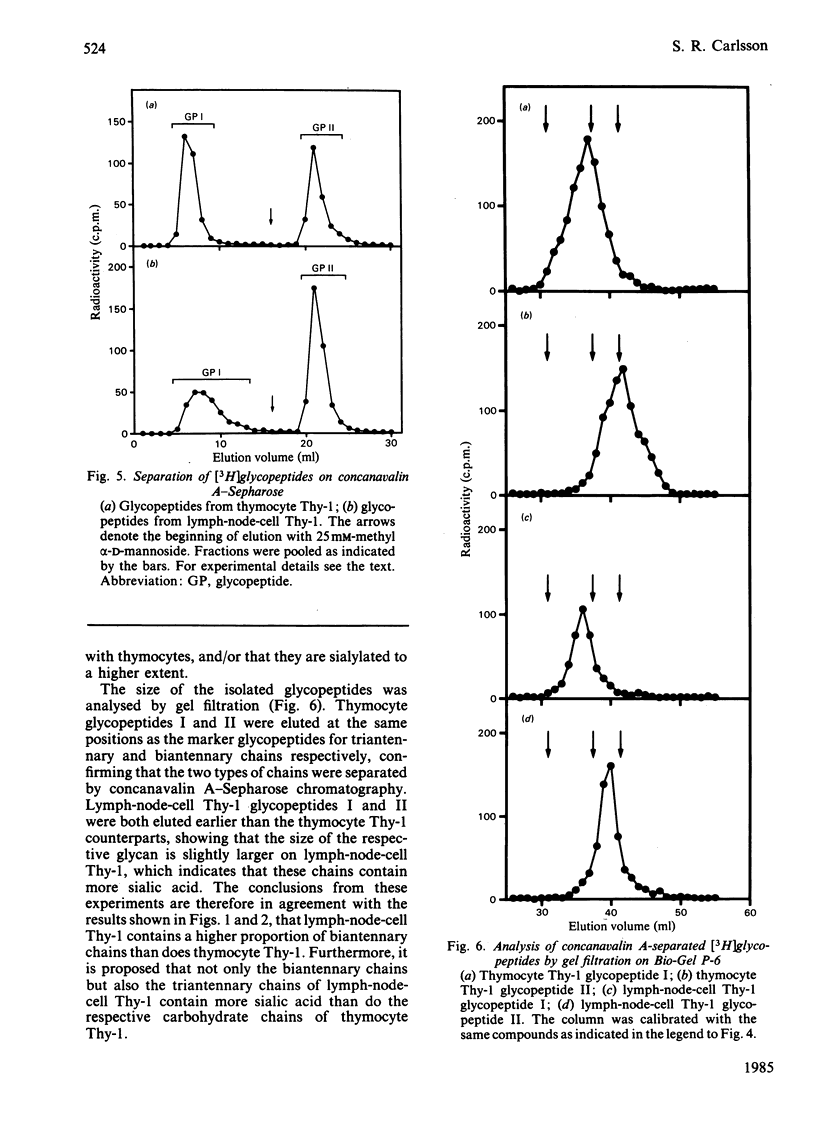

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Campbell D. G., Gagnon J., Reid K. B., Williams A. F. Rat brain Thy-1 glycoprotein. The amino acid sequence, disulphide bonds and an unusual hydrophobic region. Biochem J. 1981 Apr 1;195(1):15–30. doi: 10.1042/bj1950015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsson S. R., Stigbrand T. I. Alterations in expression and glycosylation pattern of the Thy-1 glycoprotein during maturation and transformation of mouse T lymphocytes. J Immunol. 1983 Apr;130(4):1837–1842. [PubMed] [Google Scholar]
- Carlsson S. R., Stigbrand T. I. Purification and characterization of the mouse thymocyte Thy-1 glycoprotein. Biochem J. 1983 Jun 1;211(3):641–647. doi: 10.1042/bj2110641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsson S. R., Stigbrand T. Partial characterization of the oligosaccharides of mouse thymocyte Thy-1 glycoprotein. Biochem J. 1984 Jul 15;221(2):379–392. doi: 10.1042/bj2210379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsson S., Stigbrand T. Carbohydrate complexity of the mouse thymocyte Thy-1 glycoprotein as demonstrated by lectin affinity and isoelectric focusing. Eur J Biochem. 1982 Mar;123(1):1–7. doi: 10.1111/j.1432-1033.1982.tb06490.x. [DOI] [PubMed] [Google Scholar]
- Elder J. H., Alexander S. endo-beta-N-acetylglucosaminidase F: endoglycosidase from Flavobacterium meningosepticum that cleaves both high-mannose and complex glycoproteins. Proc Natl Acad Sci U S A. 1982 Aug;79(15):4540–4544. doi: 10.1073/pnas.79.15.4540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gahmberg C. G., Andersson L. C. Selective radioactive labeling of cell surface sialoglycoproteins by periodate-tritiated borohydride. J Biol Chem. 1977 Aug 25;252(16):5888–5894. [PubMed] [Google Scholar]
- Hoessli D., Bron C., Pink J. R. T-lymphocyte differentiation is accompanied by increase in sialic acid content of Thy-1 antigen. Nature. 1980 Feb 7;283(5747):576–578. doi: 10.1038/283576a0. [DOI] [PubMed] [Google Scholar]
- Krusius T., Finne J., Rauvala H. The structural basis of the different affinities of two types of acidic N-glycosidic glycopeptides for concanavalin A--sepharose. FEBS Lett. 1976 Nov 15;72(1):117–120. doi: 10.1016/0014-5793(76)80911-8. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Tai T., Yamashita K., Kobata A. The substrate specificities of endo-beta-N-acetylglucosaminidases CII and H. Biochem Biophys Res Commun. 1977 Sep 9;78(1):434–441. doi: 10.1016/0006-291x(77)91273-6. [DOI] [PubMed] [Google Scholar]
- Williams A. F., Gagnon J. Neuronal cell Thy-1 glycoprotein: homology with immunoglobulin. Science. 1982 May 14;216(4547):696–703. doi: 10.1126/science.6177036. [DOI] [PubMed] [Google Scholar]
- Williams A. F. The predominant surface glycoproteins of thymocytes and lymphocytes. Biosci Rep. 1982 May;2(5):277–287. doi: 10.1007/BF01115113. [DOI] [PubMed] [Google Scholar]