Abstract
Adipose tissue senescence is a precursor to organismal aging and understanding adipose remodelling contributes to discovering novel anti-aging targets. Glutathione peroxidase 3 (GPx3), a critical endogenous antioxidant enzyme, is diminished in the subcutaneous adipose tissue (sWAT) with white adipose expansion. Based on the active role of the antioxidant system in counteracting aging, we investigated the involvement of GPx3 in adipose senescence. We determined that knockdown of GPx3 in adipose tissue by adeno-associated viruses impaired mitochondrial function in mice, increased susceptibility to obesity, and exacerbated adipose tissue senescence. Impairment of GPx3 may cause mitochondrial dysfunction through inner mitochondrial membrane disruption. Adipose reshaping management (cold stimulation and intermittent diet) counteracted the aging of tissues, with an increase in GPx3 expression. Overall metabolic improvement induced by cold stimulation was partially attenuated when GPx3 was depleted. GPx3 may be involved in adipose browning by interacting with UCP1, and GPx3 may be a limiting factor for intracellular reactive oxygen species (ROS) accumulation during stem cell browning. Collectively, these findings emphasise the importance of restoring the imbalanced redox state in adipose tissue to counteract aging and that GPx3 may be a potential target for maintaining mitochondrial homeostasis and longevity.
Keywords: Glutathione peroxidase 3, Adipose tissue, Mitochondrial, Anti-Senescence
Highlights
-
•
GPx3 deregulation by abnormal fat expansion may exacerbate adipose tissue senescence.
-
•
Adipose-specific GPx3 deficiency causes metabolic abnormalities due to mitochondrial derangement.
-
•
GPx3 deficiency partially restricts cold-induced adipose remodelling and anti-aging effect.
-
•
GPx3 may affect adipocyte browning by interacting with UCP1.
Abbreviations
| AUC | Area under the curve |
| CS | Cold Stimulation |
| DEG | Differential Expressed Genes |
| FBS | Fetal Bovine Serum |
| GO | Gene Ontology |
| GPx3 | Glutathione Peroxidase 3 |
| GSH | Glutathione |
| GSSG | Oxidized Glutathione |
| GTT | Glucose tolerance test |
| H2O2 | Hydrogen Peroxide |
| HDL-C | High-Density Lipoprotein Cholesterol |
| H&E | Hematoxylin-eosin |
| HFD | High-Fat Diet |
| HOMA-IR | Homeostatic Model Assessment for Insulin Resistance |
| ITT | Insulin tolerance test |
| KEGG | Kyoto Encyclopedia of The Genome |
| LC-MS | Liquid Chromatography-Mass Spectrometry |
| LDL-C | Low-Density Lipoprotein Cholesterol |
| MDA | Malondialdehyde |
| NEFA | Non-Esterified Fatty Acids |
| rAAV | Recombinant Adeno-Associated Virus |
| S1P | Sphingosine 1-Phosphate |
| SA-β-gal | β-galactosidase |
| SOD | Superoxide dismutase |
| SVF | Vascular Stromal Fractions |
| sWAT | Subcutaneous Adipose Tissue |
| T-CHO | Total Cholesterol |
| TEM | Transmission Electron Microscopy |
| TG | Triglycerides |
1. Introduction
Aging is an inevitable global upsurge, with an increasing population enjoying longer life expectancies. This has become a major demand and added to the economic burden of healthcare [1,2]. Over the past decade, aging has been intensively studied, and a multitude of aging hallmarks have been revealed, including metabolic decline [3], mitochondrial disorders, and cellular senescence [4]. Attention is drawn to the fact that adipose tissue is one of the most sensitive organs in the ageing process, with alterations in a wide range of biological and physiological processes that can affect the overall health of the organism [5]. However, the mechanisms underlying adipose tissue senescence and the causes of accelerated systemic senescence remain poorly understood. Understanding the physiological changes that occur during adipose senescence may provide novel strategies to combat aging. Adipose senescence is characterised by fat redistribution and dysfunction [6], including adipose hypertrophy, brown/beige fat loss, and reduced adipose progenitor/stem cell function, and is highly similar to obesity [7]. The overexpansion of white adipose tissue may be an early risk factor for tissue aging. Metabolic and molecular reorganisation are activated in adipocytes, culminating in a hypertrophic and hypersecretory phenotype that accelerates ageing [8]. Conversely, adipose remodelling strategies, such as cold stimulation and intermittent fasting [9], favour mitochondrial function and mitochondrial reactive oxygen species ([mt]ROS)-mediated signalling with anti-aging potential [[10], [11], [12]], in which the activation of insulin and SIRT1 may be involved. A growing consensus exists that adipose browning, induced by different stresses such as a cold environment and intermittent calorie-restricted diet, represents an adaptive response to counteract exogenous or endogenous changes [13]. In conjunction with the oxidative damage (free radical) theory of aging, which suggests the activation of endogenous antioxidant defences to prolong lifespan [14], a possible hypothesis is that fat browning may ameliorate aging by orchestrating the antioxidant system. Evidence has shown that elevated antioxidant capacity protects against metabolic abnormalities in obesity and age-related diseases by activating SIRT1 and maintaining mitochondrial function [15]. In addition, some of the browning-associated factors (such as UCP1 and PGC1α) are also correlated with antioxidant systems [16]. In this study, we induced adipocyte browning and explored its anti-aging effects.
Under normal conditions, excess superoxide during adipose expansion activates antioxidant systems such as superoxide dismutase (SOD), the glutathione reductase system, and mitophagy to maintain redox homeostasis [17]. However, a decline in the antioxidant defense system leads to an imbalance in oxidative homeostasis, ultimately triggering the onset of aging. As the fat mass expands, white adipose tissue becomes hypoxic, exacerbating oxidative stress, which is thought to underlie the development of inflammation and cellular dysfunction. Under hypoxic conditions, glycolytic pathways are significantly upregulated in adipocytes, resulting in high levels of lactate production and overexpressing HIF-1α, which promotes insulin resistance. Alternatively, a decrease in substrate flux through lipid and oxidative metabolism, which is indicated by a decrease in PPARγ and PGC-1α, has been reported [18]. However, randomised clinical trials on the antiaging effects of antioxidant supplements lack efficacy in numerous cases [19]. This may be related to the lack of reliability and specificity of oxidative stress indicators; therefore, the identification of biomarkers in clinical samples for monitoring oxidative homeostasis is helpful for anti-aging studies.
Glutathione peroxidase 3 (GPx3) is a vital antioxidant enzyme that is widely found in the plasma and other body fluids, especially in adipose tissue, and degrades various hydrogen peroxides through the catalysis of glutathione (GSH) conversion to oxidized glutathione (GSSG) [20,21]. One clinical study of adipose tissue pointed out that GPx3 expression in subcutaneous white adipose tissue (sWAT) was significantly lower in obese than in lean people and was even lower in insulin-resistant obese individuals [22]. GPx3 is engaged in the regulation of insulin receptor expression in pre-adipocytes as a potential novel regulator of insulin sensitivity [23]. Conversely, weight loss induced by bariatric surgery results in a significant reduction in serum GPx3 concentration [22]. The dysregulation of GPx3 in obese individuals is typically accompanied by increased inflammatory signalling and oxidative stress [24,25], which are factors that exacerbate aging. Therefore, we investigated the role of GPx3 in adipose senescence to reveal the possible anti-aging mechanisms. Moreover, adipose browning was induced to further demonstrate the antiaging effects of GPx3.
2. Materials and methods
2.1. Mice
Male C57/BL mice (20–22g) were purchased from Weitong Lihua Experimental Animal Technology Co., Ltd (Beijing, China). The experimental animals were housed in a specific pathogen-free environment at the Laboratory Animal Center of Shanghai University of Traditional Chinese Medicine (SHUTCM), providing 12h light/dark cycles and free diets. Mice were acclimatized for one week before the experiment. All animal experiments follow the requirements of the SHUTCM Laboratory Animal Ethics Committee. (Ethics No. PZSHUTCM2303030001).
Adoption of an ad libitum high-fat diet (HFD) to induce obesity model in mice. 60 % fat supply HFD (D12492, Research diet) was changed at 3-day intervals during the modeling period, with regular recordings of mouse body weights. Cold stimulation (CS) and intermittent dietary induction were performed to observe the effects of the two weight loss measures on HFD-induced obese mice. The mice in the CS group were first subjected to one week of 18 °C cold acclimatization before being given 4 °C cold exposure [26]. The mice in the intermittent diet group were then subjected to a cyclic cycle of 24 h ad libitum feeding and 24 h fasting, with free access to water [27].
Recombinant adeno-associated virus (rAAV) was utilized to specifically knock down GPx3 expression in adipose tissue by subcutaneous site-specific injection. Briefly, in order to achieve the packageing of rAAV and replace its entire genomic genes, we produced AAV vectors based on the AAV 293T cell line in vitro and additionally supplied the required components for rAAV, which mainly included the packageing plasmid, assistant plasmid, and Helper plasmid. The plasmid backbone carries the AdipoQ promoter to act specifically on lipids, and GPx3 knockdown was achieved by inserting the mir30-shGPX3 sequence into the vector (AdipoQ-AAV8-shGPX3). After sufficient rAAV was produced from the cell supernatant, the cells were lysed and purified by ultracentrifugation before freezing at −80 °C. Adipose tissues were transfected with knockout GPx3 by subcutaneous targeted injection following the end of mouse acclimatization. Peer control groups were given injections of AdipoQ-AAV8-shVeh.
2.2. Serum biochemistry index assay
Prior to the experimental endpoint, mice were fasted for 6h and subjected to tail-tip blood sampling for fasting blood glucose measurement using a glucometer. Measurement of blood glucose at different time points to reflect glucose tolerance and insulin tolerance in mice after gavage of insulin (0.75 U/kg) and glucose (1 g/kg) solutions, respectively [28]. After anesthesia, mice were subjected to blood sampling and centrifuged to separate the serum following resting. Total Cholesterol (T-CHO), Triglycerides (TG), Non-esterified fatty acids (NEFA), High-density lipoprotein cholesterol (HDL-C), and Low-density lipoprotein cholesterol (LDL-C) were measured by using commercial kits (Nanjing Jiancheng, China) as described. Mouse serum insulin concentration was measured by ELISA, and HOMA-IR was calculated by comparing the measured values with the corresponding mouse blood glucose [29].
2.3. Malondialdehyde (MDA) and hydrogen peroxide (H2O2) assays
After mice were sacrificed, the subcutaneous adipose tissue was rapidly isolated and then frozen. MDA and H2O2 levels in tissues were measured by specific commercialized kits (Cat. S0131 M for MDA and Cat. S0038 for H2O2) from Beyotime. All tissues used in the assay were weighed and applied to the final concentration calibration.
2.4. Measurement of total GPx and SOD enzymes
Antioxidant enzyme (GPX and SOD) activities were measured individually with specific commercial kits (Cat. S0101S for SOD and Cat. S0058 for GPX) from Beyotime. Briefly, the level of GPx enzyme activity was responded to by the reduction in NADPH during the GPx-catalysed production of GSSG from GSH. The disproportionation of superoxide anions was utilized for the detection of SOD by the WST-8 method of reaction. Furthermore, commercial ELISA kits were used to detect GPx3 concentration in serum and tissues.
3. Method detail
3.1. Gene Ontology (GO) and KEGG pathway enrichment analysis
The mRNA profiles of adipose tissue from obese mice (GSE24637) and the gene annotation platform of GPL1261 were searched from the Gene Expression Omnibus (GEO) database (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE24637). Based on R Studio, differentially expressed genes (DEGs) were detected by applying the LIMMA algorithm according to exon-modeled fragments per kilobase per million mapped fragments (FPKM) data. In this case, genes with an adjusted P value of <0.05 and |log 2 fold change (FC)| >1.0 in obese mice were selected as obesity-related genes. Pathway enrichment analysis was performed for differential expressed genes (DEG), using the “clusterProfiler” packages to identify hub maps for selected Gene Ontology (GO) and Kyoto Encyclopedia of the Genome (KEGG) pathway enrichment analysis.
3.2. Real-time PCR analysis
Total RNA was extracted from tissue or cells using TRIzol reagent (9108, Takara). Isolated RNA was measured for purity and concentration using a NanoDrop spectrophotometer, followed by cDNA synthesis using a reverse transcriptase kit (RR037A, Takara). The Real-Time PCR analysis was conducted using SYBR Green (QPK-201CH, TOYOB), of which were analyzed using the 2-ΔΔCT method. The gene expressions were normalized to β-actin expression. Fold change values were calculated for genes expressed in experimental versus control conditions. Primer sequences used are listed in Table S1.
3.3. Western blotting analysis
Tissue or cell samples were lysed by RIPA buffer (Cat. WB0102 from Wellbi) for protein quantification and the protein structure was reverted with loading buffer. The adjusted concentration of the samples was added to SDS-PAGE gels for electrophoresis and followed by transferring the proteins to PVDF membranes. After sealing the membrane with 5 % BSA solution, incubation with the primary antibodies (overnight at 4 °C) and secondary antibodies (2h at room temperature) was performed sequentially. Finally, the ECL visualizer was added dropwise to the membrane and visualized by enhanced chemiluminescence. Protein bands were analyzed with Image J software and normalized to the expression of β-actin or β-tubulin.
3.4. Histological staining
After anesthesia, the adipose and liver tissue were fixed in 4 % paraformaldehyde for 48 h. Tissues were paraffin-embedded and cut into 5 μm sections for hematoxylin-eosin staining (Cat. 0105 M from Beyotime). Whereas senescent β-galactosidase-stained tissues were not paraffin-embedded and were washed several times with PBS and stained as described in the commercial kit (Cat. C0602 from Beyotime).
3.5. Immunofluorescence staining
Fat pads were removed and fixed in 4 % paraformaldehyde for 48 h. Tissues were paraffin-embedded and cut into 5 μm sections for immunofluorescence staining (IF). sWAT samples were immunofluorescence staining to detect GPx3 (primary antibody: Rabbit Anti GPx-3, abcam, CAT: ab256470, dilution 1:500; secondary antibody: Donkey anti-rabbit Alexa Fluor 488, Invitrogen, CAT: A21206, dilution 1:500), Ucp1 (primary antibody: Mouse anti-UCP1, R&D systems, CAT: MAB6158, dilution 1:100; secondary antibody: Donkey anti-mouse Alexa Fluor 555, Invitrogen, CAT: A31570, dilution 1:500), Ucp2 (primary antibody: Mouse anti-UCP2, Santa cruz, CAT: sc-390189, dilution 1:100; secondary antibody: Donkey anti-mouse Alexa Fluor 555, Invitrogen, CAT: A31570, dilution 1:500). For cell staining, 4 % paraformaldehyde was used to fix the cells for 20 min to cell slides. Pre-fixed cell slides were washed in 1 % FBS-PBS. Sections were incubated overnight at 4 °C with primary antibody, fully washed, and then incubated with secondary antibody for 1h at room temperature. All slides were covered with Antifade mounting medium containing DAPI. Stained sections were imaged using OLYMPUS IX83. The exposure time of each channel was kept consistent for different samples. The images were subsequently analyzed using Fiji image processing software.
3.6. Multiple immunohistochemistry
Pre-fixed adipose tissue were paraffin-embedded and cut into 5 μm sections for multiple immunohistochemistry (mIHC). Briefly, the sections were incubated successively with primary and secondary antibodies after antigen repair. The staining is performed by TSA signal amplifying dye and heated for re-antigen repair to remove the existing antibody binding. The primary antibodies of GPx3 and Ucp1 are used in a manner consistent with that in the IF. Rabbit anti-Tom 20 (CST, CAT: 42406, dilution 1:200) were used to mark mitochondrial. Stained sections were imaged using nikon csu-w1 sora confocal microscopy, and the XY resolving power of nikon csu-w1 sora confocal microscopy is approx. 120 nm. The exposure time of each channel was kept consistent for different samples. The images were subsequently analyzed using Fiji image processing software.
3.7. Transmission electron microscopy (TEM) specimen preparation and analysis
After mouse execution, adipose tissue was rapidly isolated and immediately cut into cubes (∼1 mm3) on cold with a clean scalpel. Samples were transferred to a fixative (2.5 % glutaraldehyde and 0.1 M sodium phosphate, pH 7.4) and fixed at 4 °C for 24 h. Subsequent samples were dehydrated with graded alcohol and fixed in 1 % OsO4 solution for 1 h before embedding in epoxy resin. Specimens were made into ultrathin sections (50 nm) using an ultramicrotome (Leica, 705902) stained with uranyl acetate and lead citrate, and then observed under TEM (FEI Tecnai G2 spirit).
3.8. Tandem mass tags (TMT)-labeled liquid chromatography-mass spectrometry (LC-MS) detection
The sWAT in the shVeh-CS and shGpx3-CS groups were conducted TMT-based proteomics to elucidate the role of adipose GPx3 in browning. Briefly, tissue samples are lysed to extract proteins and then lyophilized. The lyophilized samples were re-suspended in 100 mM tetraethylammonium bromide and transferred to new tubes, acetonitrile was added and 10 μl of TMT reagent was added to each sample and incubated for 1 h for labeling. Hydroxylamine was added to each sample and incubated for 15 min to terminate the reaction and then lyophilized for subsequent LC-MS detection.
3.9. Off-target lipid metabolome
Serum from HFD-fed shVeh and shGPx3 groups of mice was collected and detected by Q-TOF mass spectrometry after sample pre-treatment in order to analyze lipid metabolite changes. Mass spectrometry was performed using Agilent 6530B Q-TOF mass spectrometry in positive ion scan mode with the following parameters set for the electrospray ionization source: Mass Range (m/z): 50–1700; Acquisition Rate/Time: 3.00 spectra/sec; Gas Temp: 350 °C; Drying Gas: 11 L/min; Nebuilzer: 45 psi; Sheath Gas Temp: 350 °C; Sheath Gas Flow: 11 L/min; VCap: 4000 V; Nozzle Voltage: 500 V; Fragmentor: 140 V; Skimmer: 65 V, CE: 0–40 V. Data acquisition was performed using a Masshunter. Data acquisition was performed using Masshunter 8.0 software with a mass error of ≤20 ppm. To visualize the raw data, an analysis software (MS-Dial, version 4.24 https://prime.psc.riken.jp/compms/msdial/main.html) was used. The calculated ions were constructed from the MS/MS spectra of the standard and detected internal compounds, where isomers were further distinguished by the abundance ratio of the MS/MS spectra. Using the MS-Finder, additional candidate ions were identified by searching Scifinder, HMDB, LipidMAPS and other databases.
3.10. Cell culture and treatment
The multipotent stem cell C3H/10T1/2 cells were cultured in Dulbecco's Modified Eagle Medium (DMEM, Gibco) containing 10 % fetal bovine serum (FBS) and 1 % antibiotic–antimycotic. This cell could be induced to differentiate to form beige adipocytes after stimulation by a mixed cocktail method. Briefly, after the cells had grown all over the culture disc, the induction medium containing indomethacin, dexamethasone, rosiglitazone, 3,3′,5-Triiodo-l-thyronine, 3-Isobutyl-1-methylxanthine, and insulin was replaced to induce cell differentiation for two days. Additional insulin, rosiglitazone, and 3,3′,5-Triiodo-l-thyronine were added to the medium to maintain cell differentiation status for six days [30]. During induction, the state of cell differentiation was observed under the microscope. The siGPx3 was added to the cells into transfection overnight after incubation with Lipofectamine 3000 and Opti-MEM mix. Peer controls were given siNC.
3.11. Ribonucleic acid sequencing (RNA-seq) analysis
RNA was extracted from cells using TRIzol reagent, and RNA samples from each set of triplicates were subjected to RNA sequencing and analysis. Purified RNA was quantified using the Qubit RNA wide range assay and RNA quality was assessed using an RNA nano-chip on an Agilent 2100 Bioanalyzer. The rRNA-removed RNA was converted to cDNA and a final sequence-ready library using the NEBNext Ultra II RNA Library Prep Kit. The final libraries were sequenced on a NextSeq 500 (Illumina) using paired 40 bp reads.
3.12. Flow cytometry analysis
Cells were initially seeded in 12-well plates and after cell adhesion, siNC and siGPx3 were transfected separately. Cells were stained for 30 min at 4 °C, with antibodies including Mito-Tracker Red CMXRos (C1035, Beyotime), Bodipy 493/503 (C2053S, Beyotime), UCP1 Alexa Fluor® 647-conjugated antibody (IC6158R, R&D Systems), then washed and resuspended in FACS buffer. Finally, cells were analyzed immediately on a Beckman CytoFLEX LX flow cytometer for data acquisition.
3.13. Statistical analysis
All data were expressed as the mean ± SEM, and statistical analysis was performed using GraphPad Prism software 10.0. Differences between the two groups were analyzed using an unpaired Student's t-test, and multiple comparisons were assessed using a one-way analysis of variance (ANOVA) with Tukey's multiple comparison test. p < 0.05 was considered a significant difference and is labeled as ∗, while p < 0.01 is labeled as ∗∗ and p < 0.001 is labeled as ∗∗∗. In addition, no significant differences were labeled as “ns”.
4. Results
4.1. A weakened antioxidant system exacerbated adipose tissue senescence, especially GPx3
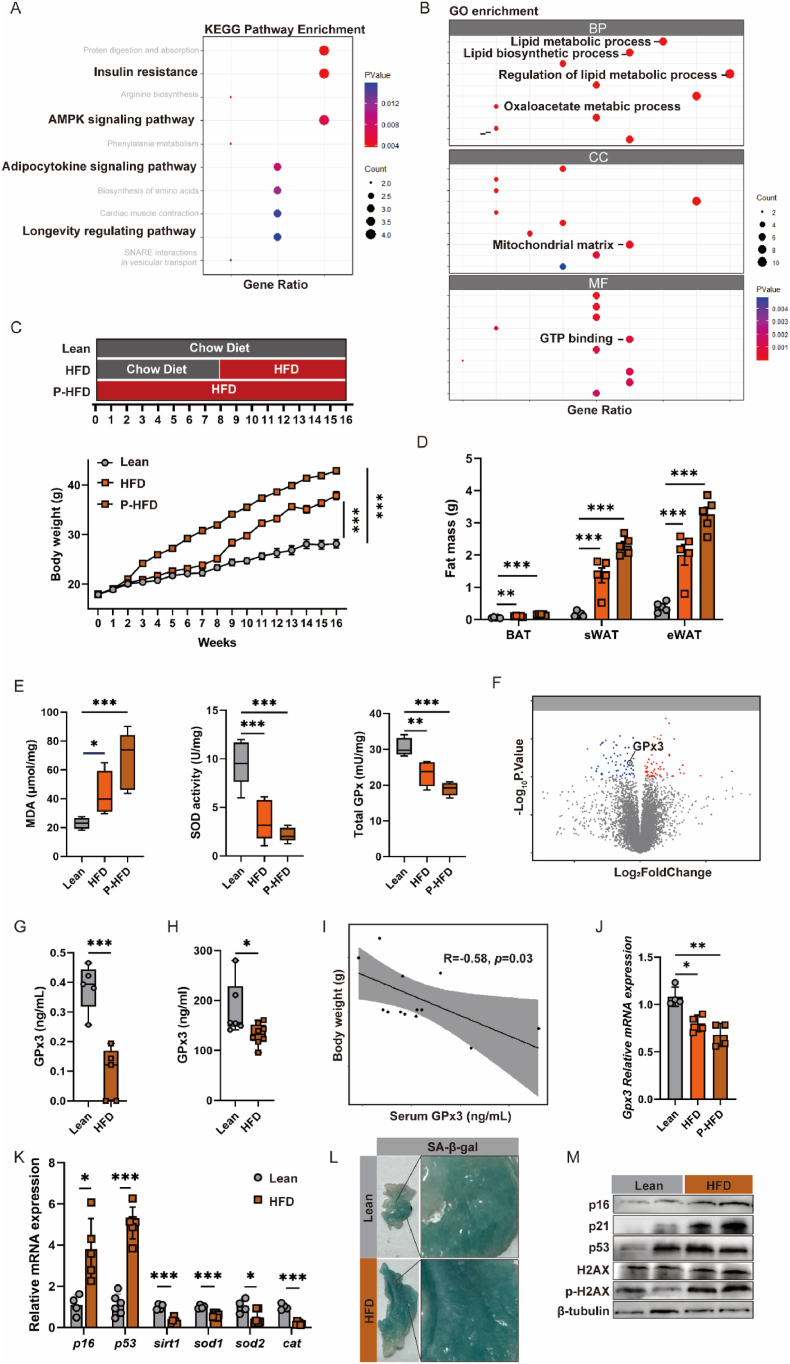
To elucidate the changes in the antioxidant system involved in accelerated tissue senescence caused by adipose expansion, we performed a combination of adipose Gene Expression Omnibus (GEO) database analyses and a variable-time high-hat diet (HFD)-fed mouse model. Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analyses indicated that obesity affects “longevity regulatory pathways”, in addition to the insulin resistance and adipocytokine model pathways, which are closely associated with aging (Fig. 1A). The aggravation of senescence by adipose expansion may occur through multiple pathways, such as upregulation of lipid synthesis, decline of the mitochondrial matrix, and attenuation of GTP binding (Fig. 1B). We detected oxidative homeostasis in the adipose tissue of obese mice, in which the HFD group was fed a high-fat diet for 8 weeks and the prolonged HFD (P-HFD) group for 16 weeks (Fig. 1C). Accompanying the HFD-induced fat mass surge (Fig. 1D), oxidative contingency damage was significantly elevated in sWAT, which may be closely related to the downregulation of antioxidant enzymes (SOD and GPx) (Fig. 1E). Moreover, as the HFD feeding cycle increased, fat mass increased, along with a further increase in MDA and a decrease in antioxidant enzyme profiles in sWAT. This suggests that adipose tissue expansion is typically coupled with an intra-tissue oxidative imbalance.
Fig. 1.
Bioinformatics analysis and experimental evaluation of GPx3 in obese mice; (A) KEGG pathway and (B) GO enrichment of DEG based on GSE24637; (C) Body weight and (D) fat mass, where the HFD group was fed with high-fat diet for 8 weeks and the prolonged HFD (P-HFD) group for 16 weeks; (E) Malondialdehyde (MDA) concentration, total SOD and total GPx enzyme activity assays in sWAT; (F) Gene expression (GSE24637) shown as a volcano plot, where down-regulated genes in the obese group are labeled in blue and up-regulated genes are labeled in red; (G) GPx3 concentration in sWAT and (H) serum; (I) Correlation analysis between body weight and serum GPx3 levels; (J) Gpx3 mRNA expression in Swat (fold change of Lean); (K) Senescence and antioxidant-related gene mRNA expressions in sWAT. Relative expression of target genes for β-actin (fold change of Lean); (L) β-galactosidase staining of sWAT to assess cellular senescence; (M) Ageing-related protein expressions in sWAT. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Evidence from clinical studies have suggested that GPx3 expression is significantly higher in the sWAT of lean individuals than in obese individuals, particularly in patients with insulin-resistant obesity [5]. In line with the population samples, a significant decrease in GPx3 was observed in high-fat- and sugar-fed mouse samples (Fig. 1F). This suggests a correlation between GPx3 and unhealthy adipose expansion (with no significant changes in its cognate family members) and its role as a key antioxidant enzyme deserves further exploration in obesity-accelerated aging. The reduced levels of GPx3 in obese mice were corroborated by ELISA (Fig. 1G). As an important endocrine organ, a decrease in GPx3 in adipose tissue was associated with a simultaneous reduction in GPx3 levels in peripheral blood (Fig. 1H), which was negatively correlated with mouse body weight (R = −0.58, p = 0.03) (Fig. 1I). Extending the feeding time of the HFD further reduced GPx3 mRNA expression in sWAT (Fig. 1J). The reduced antioxidant capacity of fats may lead to metabolic imbalance and tissue senescence. In comparison to the lean group, p16 and p53 mRNA levels were significantly increased in the HFD group, with Sirt1, Sod1, Sod2, and Cat decreasing (Fig. 1K), suggesting the possible involvement of the antioxidant system in this aging process. The enzyme assay for β-galactosidase (SA-β-gal) is the most commonly used method for detecting senescence. Briefly, active lysosomal hydrolases in senescent cells produce a blue precipitate upon exposure to a chromogenic substrate, with a darker blue colour reflecting more severe cellular senescence [31]. The SA-β-gal staining results showed that HFD resulted in a significantly larger and deeper blue area of sWAT (Fig. 1L). The significant increase in p16 and p21 protein expression and phosphorylation of H2AX within the HFD group validated accelerated adipose senescence (Fig. 1M). In conclusion, excessive hypertrophic adipose tissue accompanied by dysfunction of the antioxidant system may be a risk factor for the acceleration of the aging process in adipose tissue, and GPx3 is a potential key target.
4.2. Adipose-specific GPx3 deficiency led to mitochondrial dysfunction
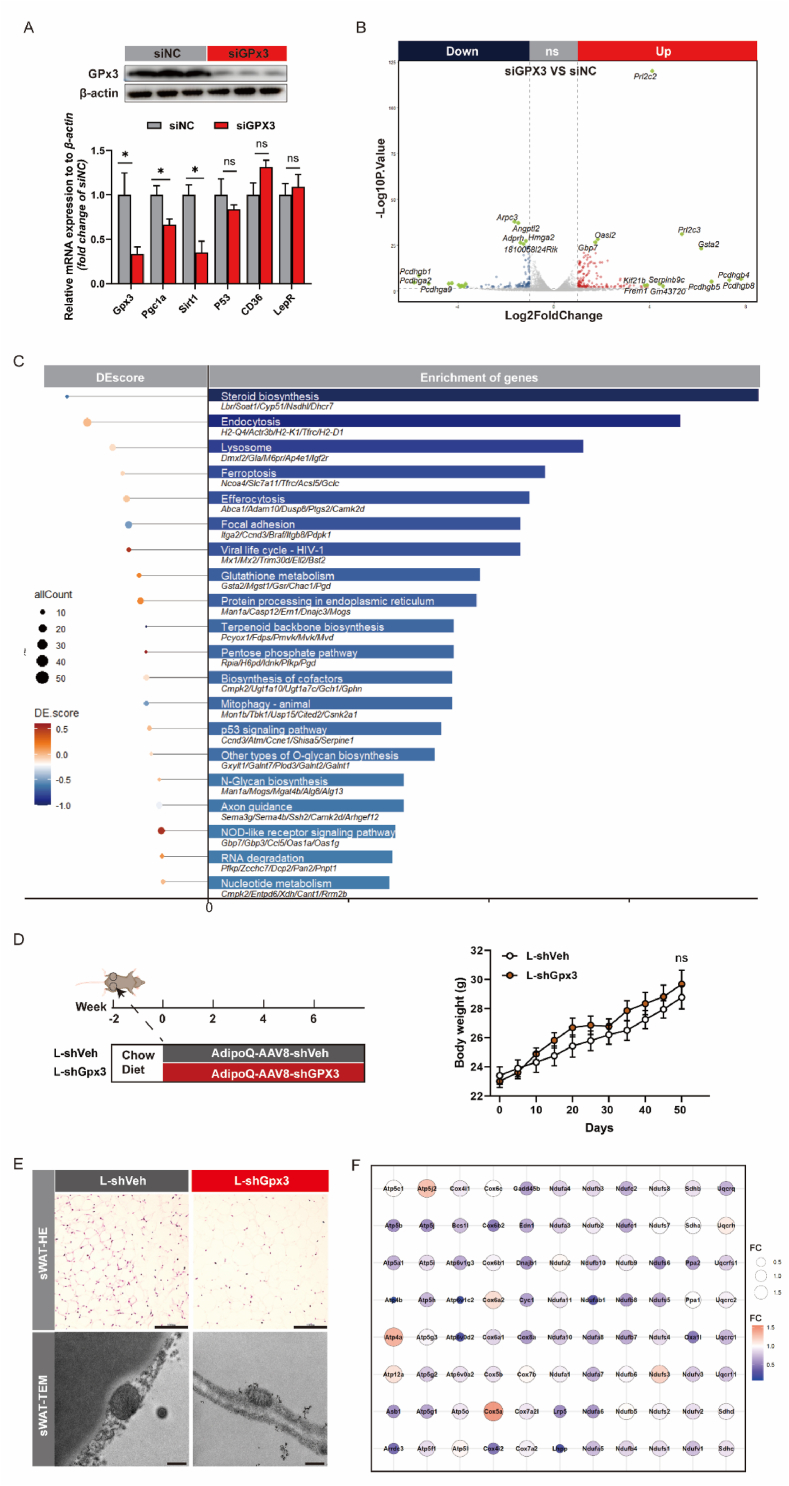
To explore the role of GPx3 in adipose senescence, we performed in vitro siGPx3 transfection to knockdown GPx3 expression. According to the Human Genetic Database of adipose tissue (Supplementary Fig. S1A), the GPx3 levels in adipose stem cells were notably higher than those in other cell types, including mature adipocytes, T cells, and fibroblasts; therefore, we selected C3H/10T1/2 cells (C3H) for mechanical validation. Gpx3 knockdown leads to decreased Pgc1α mRNA expression, suggesting reduced mitochondrial function, whereas decreased Sirt1 suggested the risk of cellular senescence (Fig. 2A). HMGA2 was directly associated with the development of obesity by maintaining stem cell renewal capacity, which [32] was an observed reduction in the siGPx3 group (Fig. 2B). We calculated DEsore based on the number of relevant differential proteins upregulated and downregulated in each pathway after performing KEGG enrichment analysis on the DEG (Fig. 2C). We found that although siGPx3 induced multi-pathway variations, the number of associated proteins upregulated and downregulated was equivalent in most signalling pathways, including glutathione metabolism and the p53 signalling pathway. Cells may compensate for the GPx3 reduction by upregulating Gsta2 to maintain glutathione metabolism. Interestingly, the expression of mitophagy pathway-related genes significantly decreased. A reduction in mitochondrial autophagy is characterised by reduced mitochondrial size, disturbed cristae folding, and reduced ATP production. To verify the potential impact of GPx3 on mitochondrial, mice were subcutaneously injected with AdipoQ-AAV8-shGPx3 (Fig. 2D) to specifically knock down GPx3 in sWAT, which was confirmed by measuring Gpx3 mRNA expression (Supplementary Fig. S1B). The morphology of adipocytes in sWAT was markedly hypertrophied, and the mitochondria in adipocytes shrunk, indicating the effect of GPx3 on mitochondrial and adipose functions (Fig. 2E). Consequently, we performed MitoArray polymerase chain reaction (PCR) on the sWAT of the L-shVeh and L-shGPx3 groups, which showed that the overall mitochondrial complex-related mRNA levels tended to decrease in the L-shGpx3 group, especially Atp4b, Atp6v1c2, Atp6v0d2, Ndufa1, and Lhpp (Fig. 2F). Therefore, the absence of GPx3 may lead to reduced mitochondrial autophagy, causing accumulation of senescent cells and increasing their susceptibility to aging.
Fig. 2.
GPx3 deficiency resulted in impaired mitochondrial respiration. (A) GPx3 protein expression in C3H/10T1/2 cells transfected with siGPx3 and the mRNA expression of senescence- and obesity-related genes in siGPx3-transfected cells; (B) The DEG were set to |Fold change| > 2; adjusted p < 0.05 by RNA-seq; (C) Differential gene KEGG enrichment analysis and DEsore scoring; (D) Body weight evaluation of mice receiving chow diet after subcutaneous spot injections of AdipoQ-AAV8-shGPx3/shVeh; (E) Representative hematoxylin and eosin (H&E) staining (scale bars: 100 μm) from sWAT sections, and transmission electronic microscopy (TEM) images (magnification: 17500×, scale bars: 5 μm) of sWAT from L-shVeh or L-shGPx3 group; (F) MitoArray PCR of sWAT between L-shVeh and L-shGPx3 group.
4.3. Adipose GPx3 deficiency increased obesity susceptibility and accelerated abnormal glucolipid metabolism
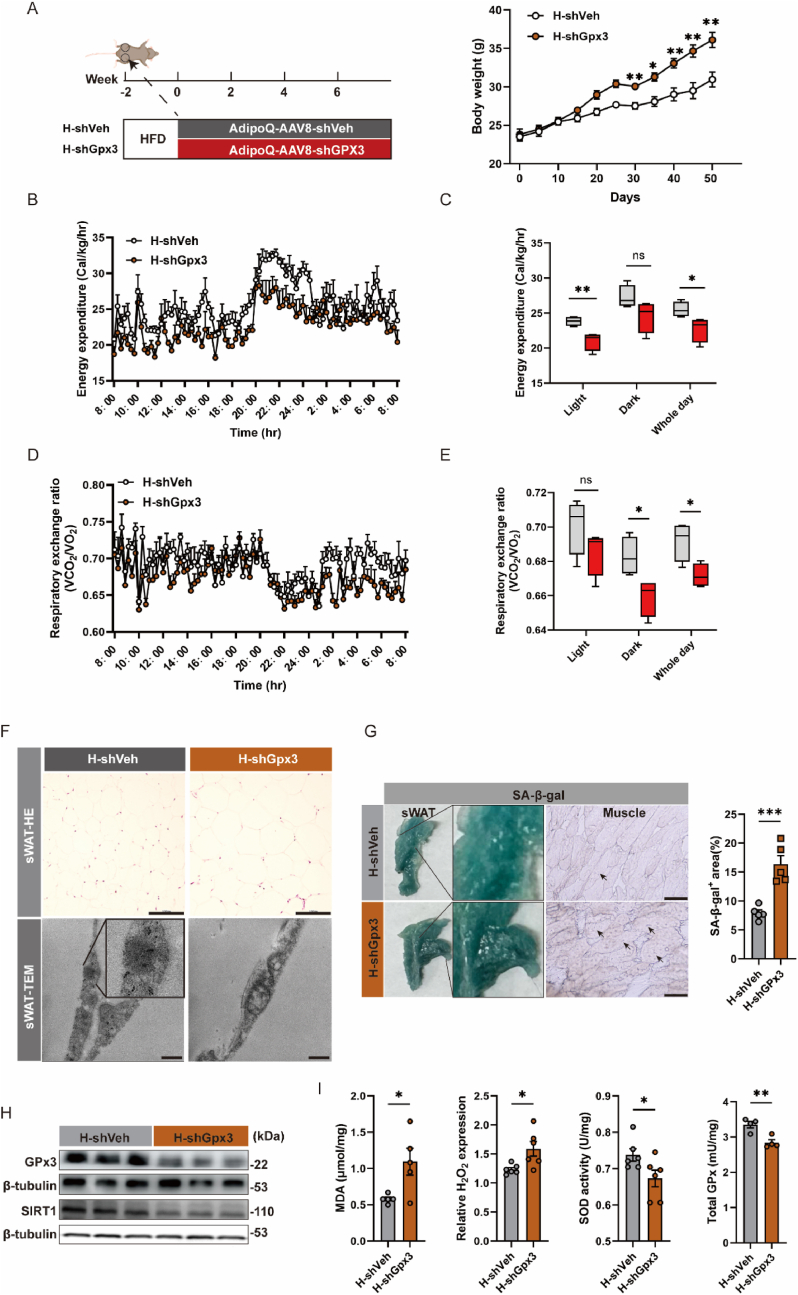
Impairment of the mitochondria, the primary site of energy metabolism, could affect energy expenditure, possibly related to the obesity correlation of GPx3. Hence, we subjected adipose-deficient GPx3 mice to HFD induction and observed the effects on obesity. Interestingly, when GPx3-deficient mice were fed a high-fat diet (H-shGpx3), weight gain was significantly exacerbated (Fig. 3A). Compared to the high-fat control group (H-shVeh), the daily dietary intake of the H-shGpx3 group did not exhibit any significant changes (Supplementary Fig. S1C). The overall energy expenditure of mice in the H-shGpx3 group was reduced (Fig. 3B and C), which may be closely related to a decline in respiratory activity (Fig. 3D and E and Supplementary Fig. S2). Excess energy tended to be stored in the adipose tissue in the form of lipids, with increased adipocyte size in the sWAT (Fig. 3F). The absence of GPx3 caused mitochondrial swelling during adipose expansion (Fig. 3F). sWAT in the shGPx3 group presented more intense β-galactosidase staining (Fig. 3G), and the results of cryosection staining of muscle tissue from adjacent sWAT also reflected more blue deposits in the H-shGPx3 group. Moreover, the expression of SIRT1 in the sWAT was significantly reduced (Fig. 3H), indicating increased senescence. GPx3 deficiency also caused elevated hydrogen peroxide levels and lipid peroxidation in sWAT, accompanied by decreased SOD and GPx enzyme activities (Fig. 3I).
Fig. 3.
GPx3 deficiency increased obesity susceptibility via impaired mitochondrial energy expenditure. (A) Body weight of mice receiving HFD after subcutaneous spot injections of AdipoQ-AAV8-shGPx3/shVeh; (B) Energy expenditure was evaluated and expressed as Kcal/kg/h per animal; (C) The bar graphs represent the energy expenditure average for each group; (D) Respiratory exchange ratio was evaluated by monitoring CO2 and O2 consumption; (E) The bar graphs represent the respiratory exchange ratio average for each group; (F) Representative H&E staining (scale bars: 100 μm) from sWAT sections, and TEM images (magnification: 17500×, scale bars: 5 μm) of sWAT from H-shVeh or H-shGPx3; (G) β-galactosidase staining of sWAT and cryosections of sWAT-adjacent muscle (scale bars: 100 μm); (H) GPx3 and SIRT1 protein expression in sWAT; (I) MDA, H2O2 concentration, total SOD and total GPx enzyme activity in sWAT.
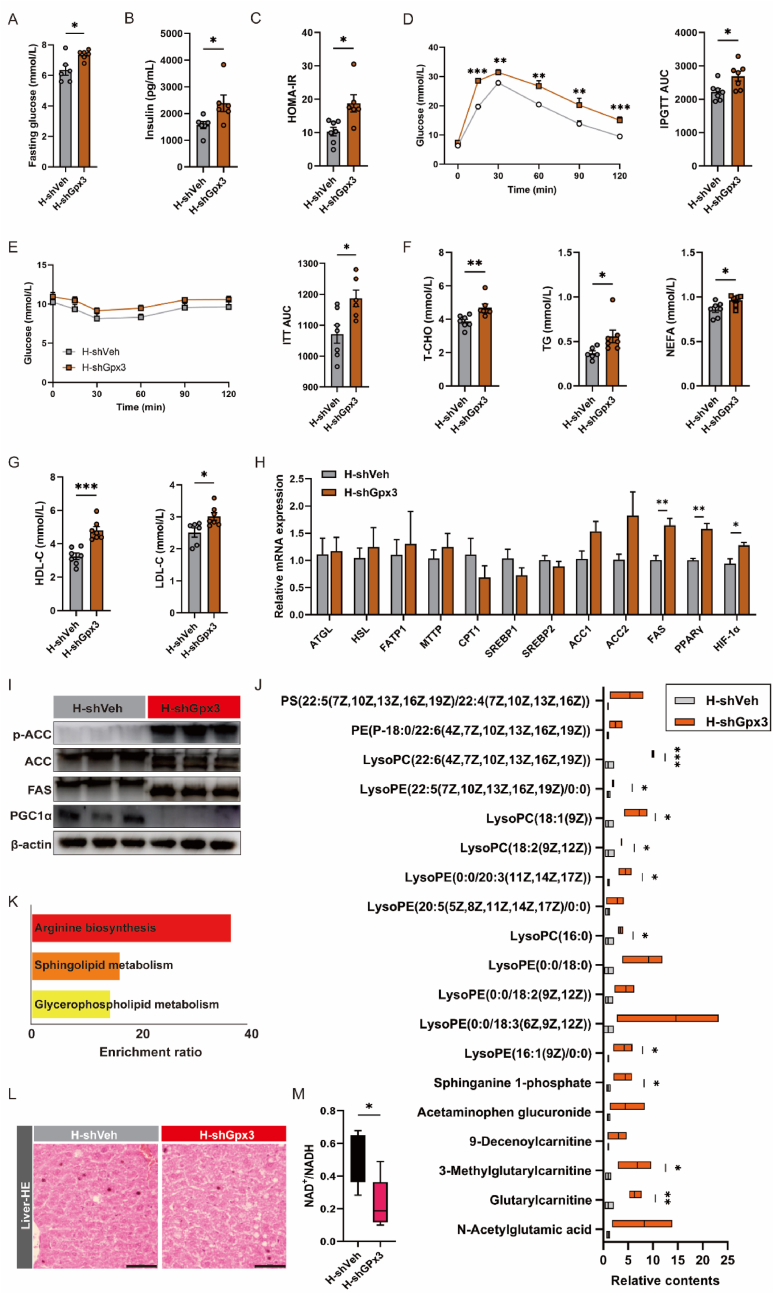
Adipose tissue senescence initiates systemic metabolic dysregulation. Fasting blood glucose was significantly increased in the H-shGpx3 group (Fig. 4A). H-shGpx3 mice presented higher serum insulin concentrations (Fig. 4B) and a higher homeostatic model assessment for insulin resistance (HOMA-IR) (Fig. 4C), with impaired glucose tolerance and lower insulin sensitivity (Fig. 4D and E). GPx3 deficiency significantly increased serum T-CHO, TG, NEFA, and LDL-C (Fig. 4F and G), suggesting exacerbated lipid dysfunction. To determine whether GPx3 triggers the susceptibility to obesity directly related to lipid metabolism, we performed PCR for lipolysis (ATGL and HSL), lipid transport (FATP1 and CPT1), and lipid synthesis (SREBP, ACC, and FAS). The results indicate that low levels of GPx3 do not seem to directly cause significant changes in lipolysis processes (Fig. 4H), but the mRNA level of FAS and PPARγ was significantly elevated, suggesting increased lipid synthesis. Elevated lipid synthesis in the H-shGPx3 group is validated by higher expression of ACC phosphorylation and FAS proteins with decreased PGC-1α (Fig. 4I). Purified serum samples were assayed by off-target metabolomics, and the results suggested that compared to the H-shVeh group, multiple lipid components were upregulated in the H-shGPx3 group (Fig. 4J). Among these, the upregulation of sphingosine 1-phosphate (S1P) is thought to be associated with obesity. Differential metabolite enrichment analyses focused on arginine biosynthesis and sphingolipid and glycerophospholipid metabolism (Fig. 4K). An imbalance in lipid metabolism may lead to the ectopic deposition of lipids, and H&E staining of the liver showed a small amount of lipid accumulation in the H-shGpx3 group (Fig. 4L). In conclusion, the absence of GPx3 accelerates adipose enlargement and senescence with an oxidative imbalance, which, in turn, affects systemic metabolism.
Fig. 4.
GPx3 deficiency worsened the glycolipid metabolism on HFD-fed mice. Glucose metabolism profiles in the H-shVeh and H-shGPx3 mice, including (A) fasting blood glucose levels, (B) serum insulin levels, and (C) assessment of the index of insulin resistance (HOMA-IR). HOMA-IR was calculated by the corresponding insulin and fasting blood glucose of each mouse; (D) Glucose tolerance test (GTT), and (E) insulin tolerance test (ITT) in H-shVeh and H-shGPx3 mice at day 50, with quantification of area under the curve (AUC); (F–G) Serum lipid metabolism profiles in the H-shVeh and H-shGPx3 mice, including T-CHO, TG, NEFA, LDL-C and HDL-C levels; (H) The mRNA levels of lipid metabolism-related genes; (I) Protein expressions of p-ACC, ACC, FAS and PGC1α in sWAT of H-shVeh and H-shGPx3 mice; (J) Serum lipid metabolite concentrations of H-shVeh and H-shGPx3 mice, which were measured by the untargeted lipid metabolome as described in Methods; (K) Enrichment analysis of lipid differential metabolites in H-shVeh and H-shGPx3 serum; (L) H&E staining for liver sections (scale bars: 50 μm) from H-shVeh and H-shGPx3 mice; (M) The NAD⁺/NADH ratio in serum from H-shVeh and H-shGPx3 mice.
4.4. Adipose remodelling hindered adipose senescence accompanied by elevated GPx3 levels
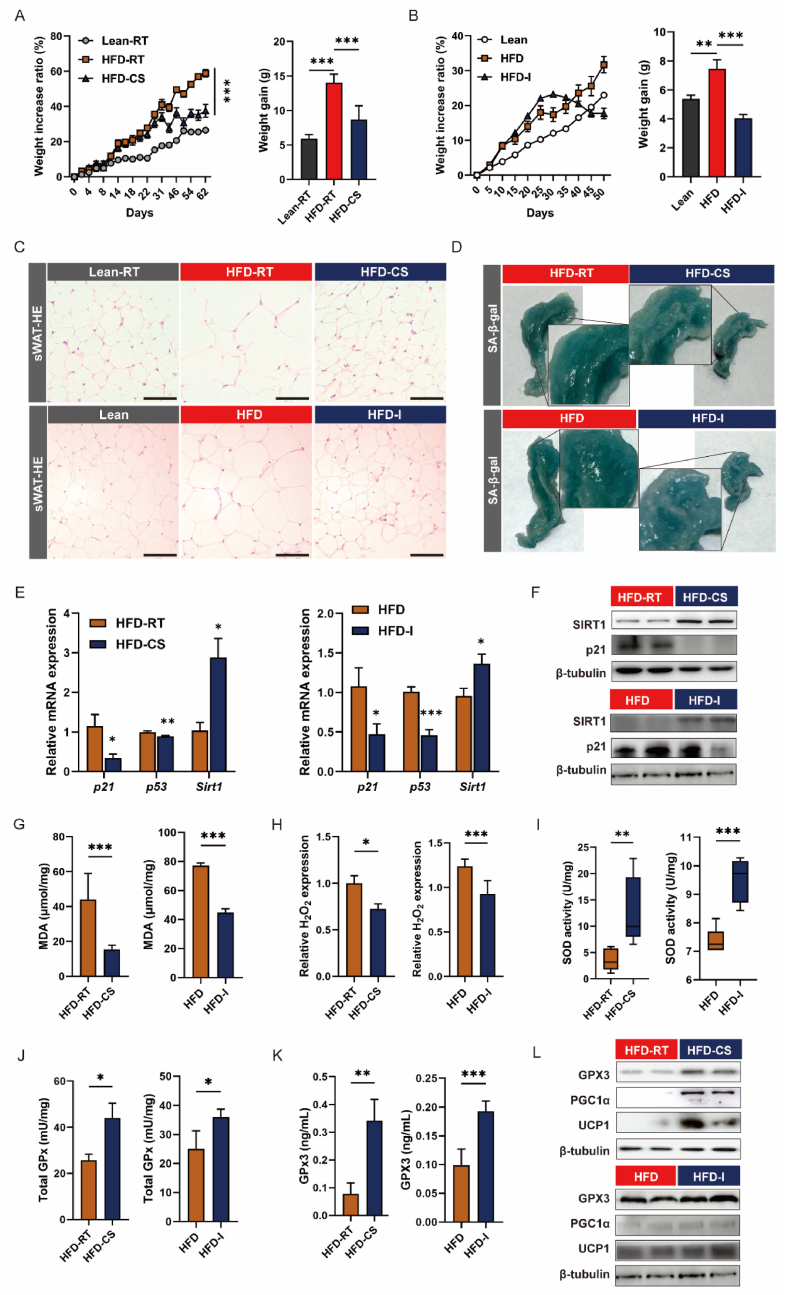
Based on the intensified senescence resulting from fat expansion, we questioned whether fat remodelling could alleviate senescence and whether GPx3 was involved. We employed two well-recognised methods to reconstruct adipose tissue: cold stimulation (HFD-CS) and intermittent fasting (HFD-I). Both approaches were effective in alleviating HFD-induced weight gain (Fig. 5A and B) and reducing adipocyte volume in sWAT (Fig. 5C). The fat mass at all sites, including BAT, sWAT, and eWAT, was reduced (Supplementary Figs. S3A and B). Concurrently, both methods were effective in improving abnormal lipid metabolism in obese mice, as evidenced by the downregulation of serum T-CHO, NEFA, and LDL-C, and the upregulation of HDL-C (Supplementary Fig. S3C). Notably, both approaches achieved a reduction in the extent of β-galactosidase staining (Fig. 5D), as well as increased SITR1 expression and decreased p21 (Fig. 5E and F), indicating potential improvement in adipose senescence. Both moderate cold stimulation or intermittent fasting were effective in alleviating the obesity-induced surge in MDA and peroxides in the adipose tissue (Fig. 5G and H), which was likely closely correlated with the activation of the antioxidant system (elevated total SOD activity and total GPx levels shown in Fig. 5I and J). Among the GPx family members, only Gpx3 mRNA expression was significantly upregulated in response to CS and IF (Supplementary Fig. S3D). ELISA and WB results also demonstrated elevated GPx3 levels (Fig. 5K and L), suggesting that GPx3 may be a vital antioxidant enzyme mediating adipose senescence under cold conditions and intermittent fasting.
Fig. 5.
Cold stimulation (HFD-CS) and intermittent feeding (HFD-I) enhanced antioxidant capacity to counteracting ageing. (A) Body weight increase ratio and final weight gain of Lean-RT, HFD-RT and HFD-CS groups; (B) Body weight increase ratio and final weight gain of Lean, HFD and HFD-I groups; (C) H&E staining of sWAT from Lean-RT, HFD-RT and HFD-CS (upper panel), and H&E staining of sWAT (scale bars: 100 μm) from Lean, HFD and HFD-I (bottom panel); (D) The β-galactosidase staining of sWAT from HFD-RT and HFD-CS (upper panel), and β-galactosidase staining of sWAT from HFD and HFD-I (bottom panel) with partial magnification of the deepest part of the staining to assess senescence; (E–F) The mRNA and protein expression of senescence-related indicators in sWAT (HFD-RT vs HFD-CS, and HFD vs HFD-I, respectively); (G–J) Evaluation of oxidative stress indicator levels, including MDA, H2O2, SOD, and GPx in sWAT (HFD-RT vs HFD-CS, and HFD vs HFD-I, respectively); (K) The concentration of GPx3 in sWAT (HFD-RT vs HFD-CS, and HFD vs HFD-I, respectively); (L) GPx3, PGC1α and UCP1 protein levels in sWAT (HFD-RT vs HFD-CS, and HFD vs HFD-I, respectively).
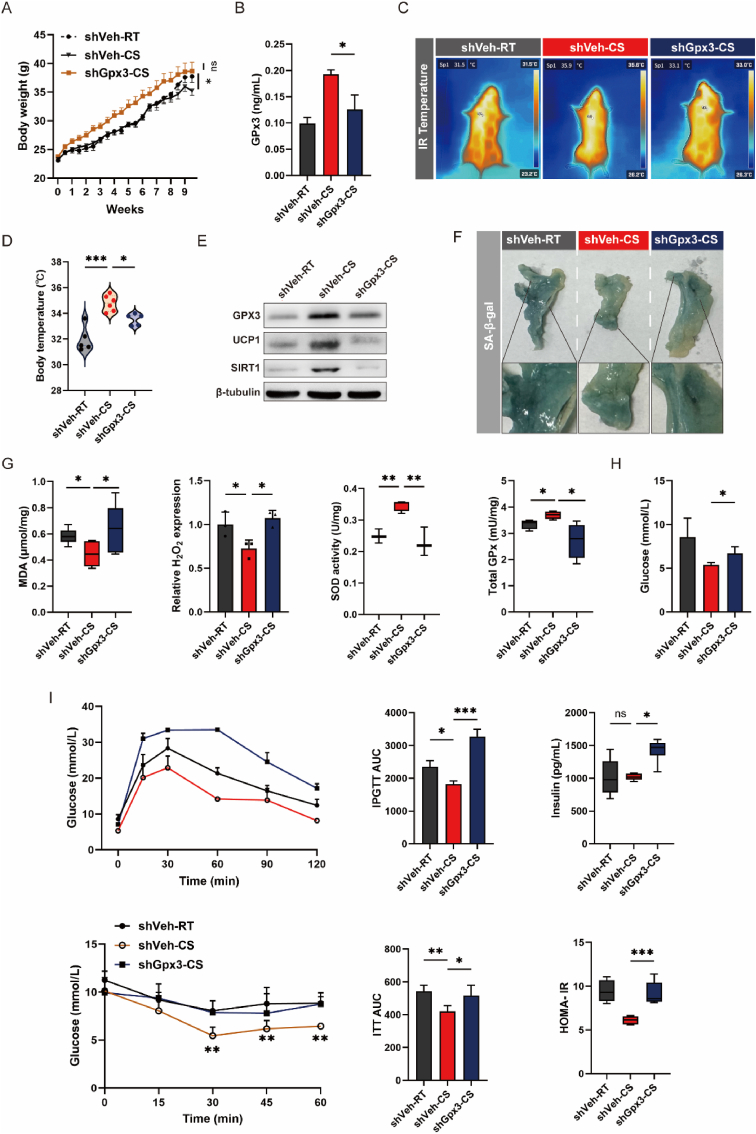
4.5. Cold-induced adiposity improvement was partially abolished by GPx3 knockdown
Because of the elevation of GPx3 in cold-stimulated mice, we cold-treated GPx3−/− mice to further validate the relationship between the GPx3 shift and senescence improvement. The cold-induced weight loss was limited in the shGPx3 group (Fig. 6A). The upregulation of GPx3 in sWAT by CS was restricted in the shGPx3 group (Fig. 6B). In addition, we detected GPx3 levels in eWAT and BAT, and found that GPx3 expression was decreased in BAT (Supplementary Fig. S4A). The knockdown of GPx3 partially limited cold-induced adipocyte volume reduction in BAT and WAT and constrained the amelioration of liver lipid accumulation (Supplementary Figs. S4B and C). The shGPx3 group showed diminished warming variability after cold exposure (Supplementary Figs. 6C and D), accompanied by a decrease in UCP1 (Fig. 6E). This partly indicates a diminished capacity for thermogenesis. We noted that GPx3 knockdown aggravated tissue senescence in comparison to the shVeh-CS group (Fig. 6F), which may have been caused by the impaired cold-induced enhancement of SOD and total GPx and increased oxidative stress (Fig. 6G). In addition, the beneficial effect of CS on metabolic abnormalities (fasting blood glucose, TC, and LDL-C levels) was restricted to some extent (Supplementary Fig. S4D). shGPX3-CS mice showed reduced glucose tolerance and insulin sensitivity, with upregulated serum insulin and HOMA-IR levels (Fig. 6H and I).
Fig. 6.
GPx3 knockdown partly abolished the CS-induced thermogenesis and metabolic improvement. (A) Body weight evaluation of shVeh-RT, shVeh-CS, and shGPx3-CS mice; (B) GPx3 concentration in sWAT from shVeh-RT, shVeh-CS, and shGPx3-CS mice; (C) Thermal imaging photos of shVeh-RT, shVeh-CS and shGPx3-CS mice; (D) The body temperatures of each group were visualized in a bar graph; (E) Protein expression of GPx3, UCP1 and SIRT1 in sWAT from shVeh-RT, shVeh-CS, and shGPx3-CS mice; (F) The β-Galactosidase staining of sWAT from shVeh-RT, shVeh-CS, and shGPx3-CS mice with partial magnification of the deepest part of the staining to assess senescence; (G) Evaluation of oxidative stress indicators levels, including MDA, H2O2, SOD activity, and total GPx activity in sWAT from shVeh-RT, shVeh-CS, and shGPx3-CS mice; (H–I) Evaluating glucose metabolism among shVeh-RT, shVeh-CS, and shGPx3-CS mice by measuring, fasting blood glucose level, GTT, ITT, serum insulin level, and HOMA-IR.
4.6. GPx3 deficiency disrupted the inner mitochondrial membrane
To elucidate the engagement of GPx3 in adipose remodelling, we performed TMT-based proteomic analysis of sWAT in the shVeh-CS and shGpx3-CS groups. The relationship between samples was determined using Principal Component Analysis (PCA), which revealed similar protein expression in the same group of samples (Supplementary Fig. S5A). Differentially expressed proteins between these two groups were screened according to a 1.2-fold difference threshold (FC > 1.2 and FC < 0.83, p < 0.05). Compared to the shVeh group, 154 upregulated and 208 downregulated proteins were found in the shGpx group (Fig. 7A and Supplementary Fig. S5B). Subsequently, we predicted the subcellular localisation of differentially expressed proteins using the MultiLoc package (Fig. S5C), suggesting that it was primarily concentrated in the cytoplasm (47.79 %), mitochondria (12.15 %), and nucleus (9.12 %) (Fig. 7A and B). GPx3 deficiency caused cellular dysfunction, which was largely concentrated in the mitochondria, even though nearly half of the differentially expressed proteins were present in the cytoplasm. Gene Ontology (GO) enrichment analyses showed that, in terms of cellular components, the differentially expressed proteins were primarily concentrated in the mitochondrial inner membrane protein complex and oxidoreductase complex. Ribonucleoside triphosphate biosynthesis, nucleotide biosynthesis, and the electron transport chain are affected in biological processes. The functions of molecules closely related to mitochondrial function, such as NADH dehydrogenase (quinone) and electron transport, were also altered (Supplementary Fig. S5D). This is consistent with the apparent changes in mitochondria upon deletion of GPx3 in the adipose tissue. Gene set enrichment analysis (GSEA) were conducted, and multiple mitochondria-associated pathways were enriched, such as the inner mitochondrial membrane, mitochondrial membrane, and respiratory chain complexes (Fig. 7C). Interestingly, mitochondrial inner membrane pathway-associated proteins were reduced, whereas mitochondrial membrane proteins were elevated, suggesting a possible association between GPx3 and mitochondrial inner membrane dysfunction. By examining the mtDNA copy number and MitoTracker, we confirmed that the shGpx3-CS group had a reduced number of mtDNAs relative to the shVeh-CS group (Fig. 7D and E). The above experimental findings were verified by the altered mitochondrial morphology (Fig. 7F); disorganisation of mitochondrial cristae and thickening of mitochondrial membranes were observed in the shGpx3-CS group. KEGG enrichment analysis showed that GPx3 deficiency resulted in significant changes in oxidative phosphorylation and thermogenesis (Fig. 7G). GPx3 deficiency altered reactive oxygen species and may be associated with multiple metabolic and neurodegenerative diseases (Supplementary Fig. S5E).
Fig. 7.
GPx3 deficiency weaken CS-induced thermogenesis due to mitochondrial disorganisation. (A) Differential proteins in shGpx3-CS and shVeh-CS groups presented as volcano plots, in which down-regulated proteins were marked in blue in the shGPX3-CS group and up-regulated in red; (B) The subcellular localisation of the differential proteins was predicted based on the MultiLoc package; (C) GSEA analysis of differential proteins and display of mitochondria-associated pathways; (D) The mtDNA copy number in sWAT of shVeh-RT, shVeh-CS, and shGpx3-CS mice; (E) MitoTracker staining of SVF isolated from sWAT by Flow Cytometry analysis; (F) TEM images of sWAT from shVeh-RT, shVeh-CS, and shGpx3-CS mice with magnified demonstration of localized mitochondria (magnification: 17500×, scale bars: 5 μm); (G) KEGG enrichment analysis of differential proteins in sWAT between shGpx3-CS and shVeh-CS mice. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Thermogenesis and oxidative phosphorylation of beige adipose tissue are crucial for CS-induced energy consumption and metabolic improvement. Proteins related to these two pathways exhibited a decreasing trend (Fig. 8A). As we previously observed significant changes in UCP1 (a classic marker of beige/brown adipose tissue) in the CS group, we proceeded to validate the possibility of GPx3 interacting with UCP1. In addition, we profiled proteins associated with the inner mitochondrial membrane (Supplementary Fig. S5F) and observed the downregulation of Slc25a11, a UCP2 protein, in the shGpx3-CS group. We hypothesized that adipose browning differentiation under cold exposure may be impeded by the absence of GPx3, thereby affecting thermogenesis. Thus, we performed immunofluorescence (IF) staining of sWAT for GPx3 and UCP proteins. GPx3 and UCP share a high degree of fluorescence overlap. Moreover, the intensity of UCP1, a classic marker of fat browning, and UCP2 fluorescence signals in the shGPx3-CS group was weakened (Fig. 8B). Subsequently, molecular docking was performed to fit GPx3 with UCP1 and UCP2, and the link between UCP1 and GPx3 exhibited high molecular bond energies (Fig. 8C). In addition, we performed multiple immunohistochemical (mIHC) staining for GPx3, UCP1, and Tomm20 (mitochondrial outer membrane markers) to analyze their cellular localisation and co-expression (Supplementary Fig. S6A). High expression of GPx3 and Ucp1 proteins can be observed in Tom 20-labeled mitochondrial regions (Supplementary Fig. S6B). Colocalisation analysis of GPx3 and UCP1 showed a correlation with an Rcoloc value of 0.3902 ± 0.0377 (Supplementary Figs. S6C–D). We then enriched potential binding proteins in sWAT using an anti-GPx3 antibody for immunoprecipitation (IP) validation, and the results indicated the possibility that GPx3 interacted with UCP1 (Fig. 8C). Then, we performed flow cytometry on UCP1+ cells in vascular stromal fractions (SVF) of sWAT, and showed that UCP1+ SVF had higher levels of GPx3 expression compared to UCP1- part after flow cytometric sorting (Fig. 8D). GPx3 deficiency led to a decrease in the total number of SVF cells isolated from sWAT as well as a decrease in the number of UCP1+ cells. GPx3 deficiency resulted in a reduction in mitochondria in UCP1+ cells from the SVF (Fig. 8E). To assess the interactions between differentially expressed proteins, we constructed PPI networks of differentially expressed proteins using the STRING database (Supplementary Fig. S5G).
Fig. 8.
GPx3 may interact with UCP proteins participating in adipose browning. (A) GSEA analysis of oxidative phosphorylation and thermogenesis, following the KEGG enrichment analysis of differential proteins in sWAT of shGpx3-CS and shVeh-CS mice; (B) GPx3 and Ucp1/Ucp2 immunofluorescence staining (Green fluorescence for GPx3, red for UCP1) of sWAT sections from shVeh-CS and shGPx3-CS mice (scale bars: 50 μm); (C) Molecular docking of UCP1 and GPx3 and results of co-IP experiments; (D) GPx3 expression in UCP1- and UCP+ fraction of SVF from sWAT; (E) Quantification of total cell numbers and UCP1+ cells in the SVF between shVeh-CS and shGPx3-CS mice, as well as MitoTracker analysis in UCP1+ cells; (F) Schematic illustrating the proposed interactions between GPx3 and UCP1 and their effects on adipose tissue browning. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Collectively, the proteomic results indicated that the absence of Gpx3 likely impedes CS-promoted adipose browning through UCP1 interactions. This process leads to dysfunction of the inner mitochondrial membrane, limiting thermogenesis, and the resulting mitochondrial damage may be a causative factor for tissue senescence (Fig. 8F).
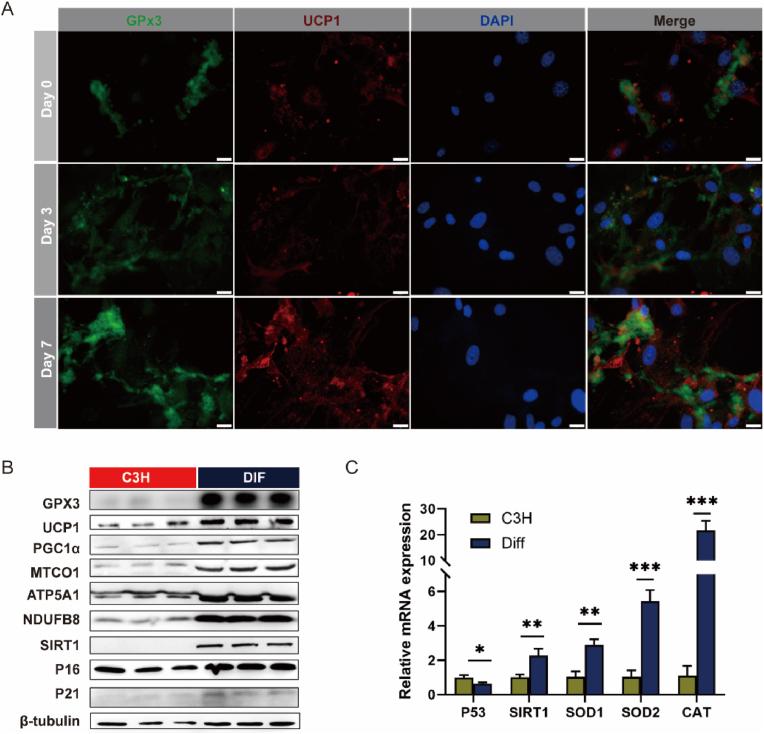
4.7. GPx3 deficiency impaired the browning differentiation of stem cells
Mouse embryonic fibroblast C3H/10T1/2 cells (C3H) were induced to differentiate into beige adipocytes using an in vitro cocktail method, and the expression of GPx3 and Ucp1 were significantly increased after differentiation (Fig. 9A and B). Beige adipocyte differentiation increased expression of Gpx3, Ucp1, PGC1α, and Sirt1 (Fig. 9B), suggesting enhanced mitochondrial and anti-ageing effects of browning. P53 mRNA levels were significantly reduced, and SIRT1 was significantly increased after differentiation (Fig. 9C). Antioxidant-related genes (Sod1, Sod2, and Cat) expression was also elevated, which was consistent with the results of in vivo adipose browning.
Fig. 9.
Adipocyte browning possesses anti-ageing potential accompanied by elevated antioxidative activity. (A) C3H/10T1/2 cells were induced into the brown adipocytes by giving a mixed cocktail of stimuli. Immunofluorescence staining of cells for GPx3 and UCP1 during induction of differentiation (Day 0, 3, and 7) (scale bars: 20 μm); (B) Protein expression of senescence and antioxidant-related indicators in C3H- and DIF-group cell; (C) mRNA expression levels of senescence- and antioxidant-related genes between C3H- and DIF-group cell, with relative gene expression compared to β-actin. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
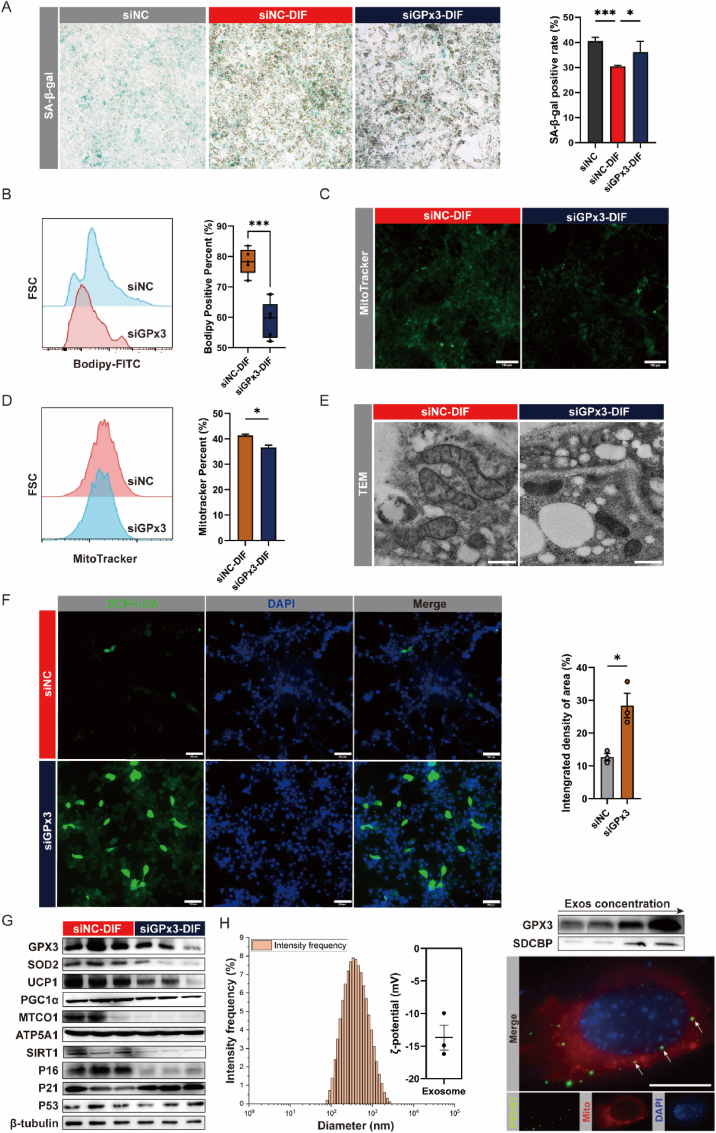
The lack of GPx3 weakened the browning-induced anti-senescence effect (Fig. 10A). Reduced beige lipogenesis (Fig. 10B) and mitochondrial numbers (Fig. 10C–E) in the siGPx3 group, which may be associated with an increase in ROS accumulation (Fig. 10F). TEM revealed mitochondrial disorders, including abnormal mitochondrial morphology, blurred cristae structures, and a reduction in the number of mitochondria (Fig. 10E). GPx3 deficiency may inhibit mitochondrial neogenesis by impeding the process of mitochondrial autophagy, resulting in reduced clearance of cellular senescent or disordered mitochondria through reduced PGC1α and mitochondrial complex MTCO1 (Fig. 10G). In addition, we isolated and examined the exosomes in cell supernatants that expressed GPx3, which may be responsible for its systemic effects. (Fig. 10H). Furthermore, to evaluate whether these exogenous GPx3 could enter adipocytes, these exosomes were co-incubated with C3H cells and detected using PKH67 as shown in Fig. 10H and Figs. S6E–F.
Fig. 10.
GPx3 may be required for the maintenance of differentiated browning potential of adipocytes. (A) Galactosidase staining of siNC, si–NC–DIF and siGPx3-DIF cells to assess cellular senescence; (B) Evaluating beige adipogenesis levels in si–NC–DIF and siGPx3-DIF cells using bodipy staining; (C) Immunofluorescence staining of MitoTracker in siNC-DIF and siGPx3-DIF cells to visualize mitochondria (scale bars: 100 μm); (D) Flow cytometric analysis of MitoTracker+ cell percentages in siNC-DIF and siGPx3-DIF cells; (E) TEM representative images of siNC-DIF and siGPx3-DIF cells (magnification: 17500×, scale bars: 5 μm); (F) Immunofluorescence staining with DCFH-DA in siNC and siGPx3 cells to detect oxidative stress, with a bar graph showing the integrated density of area (%); (G) Expression profiling of mitochondrial and senescence-related proteins in siNC-DIF and siGPx3-DIF cells; (H) Nanoparticle tracking analysis and GPx3 protein expression of C3H-released exosomes. Cellular uptake of exosomes labeled with PKH67 was assessed, with mitochondria visualized using MitoTracker (scale bars: 10 μm).
In addition, we performed JC-1 staining to reflect cellular mitochondrial membrane potential and activity, while detecting intracellular mitochondrial superoxide anion levels using MitoSOX. The ratio of JC-1 polymer (red fluorescence) to monomer (green fluorescence) reflects mitochondrial membrane potential and mitochondrial stress, and a decrease in this ratio indicates a risk for mitochondrial impairment. First, a significant reduction in the JC-1 red/green ratio reduction upon GPx3 knockdown was observed, reflecting mitochondrial damage. Direct cellular cold exposure stimulation has been shown to induce mitochondrial dysfunction [33,34]. Similar findings were observed in our assay, which were further amplified in the shGPx3-CS group (Supplementary Fig. S7A), suggesting a reduction in mitochondrial activity. The mitoSOX results indicated that GPx3 knockdown increased mitochondrial oxidative stress and the risk of oxidative stress injury in cells under cold conditions (Supplementary Fig. S7B).
5. Discussion
As a compartment for bioenergy storage and release, the premature senescence of adipose tissue is rapidly recognised as an influential mediator of stress-induced tissue dysfunction. Enlargement of the adipose tissue activates cell cycle programs that promote the emergence of a premature aging transcriptome and secretory phenotype in adipocytes [35]. Modulation of oxidative homeostasis by reshaping the adipose tissue has anti-aging potential. However, the molecular mechanisms of redox systems in adipose tissue remodelling remain poorly understood. The present study suggests that GPx3 is likely functions as an essential antioxidant enzyme in sWAT to maintain mitochondrial function in hypertrophic adipocytes to combat senescence. GPx3 deficiency increases susceptibility to weight gain and exacerbates aberrant glucolipid metabolism. Adding to this, GPx3 may function as a “plasticity gene”, predisposing carriers of putative risk alleles to be vulnerable to environmental influences, such as dietary patterns changes [36]. GPx3 deficiency contributes to mitochondrial disruption, which, in turn, amplifies adipose expansion under high-energy intake stress. We further showed that GPx3 is actively involved in adipose reshaping procedures, such as cold stimulation, and that it may mediate UCP1 activity, affecting mitochondrial endomembrane function and fat browning. These findings highlight the value of the antioxidant system in adipose aging and reveal a partial physiological function of GPx3 in adipose tissue, which may serve as a potential anti-aging target.
We observed increased oxidative stress and decreased antioxidant enzyme levels in sWAT in response to increased adiposity. Oxidative imbalance may be an early sign of premature fat senescence. The degradation of mitochondrial H2O2 is reliant, in part, on the glutathione reduction system to avoid active ·OH production reducing the risk of oxidative damage and senescence [37,38]. Population studies have shown that GPx3 presented decreases with adipose tissue expansion, whereas surgical remodelling increases its expression. Our studies in mice validated the above findings and identified that selective knockdown of GPx3 in adipose tissue may affect cell cycle processes by interfering with mitochondria. In addition, GPx3 may participate effectively in the survival and self-renewal of adipose-differentiated stem cells through ROS-mediated inactivation of p38 MAPK [21], which in turn affects aging. GPx3 deficiency led to reduced mitochondrial volume and cristae disorganisation in adipocytes. Mitophagy is correlated with the restructuring of mitochondrial content, thereby modulating adipocyte differentiation and differentiated adipocyte maintenance [39]. Decreased mitophagy exacerbates the accumulation of dysfunctional mitochondria, and abnormalities in electron respiratory chain conductance lead to a vicious cycle of oxidative damage.
Systematic metabolic abnormalities occur as a result of oxidative damage to adipose tissue. A previous study suggested that GPx3 engages in the regulation of insulin receptor expression in 3T3-L1 preadipocytes and is a potential novel regulator of insulin sensitivity [20]. Therefore, we explored glucose and lipid metabolism in HFD-induced obese mice after GPx3 knockout. Reduced levels of GPx3 lead to significant upregulation of adipose synthesis-associated proteins (ACC, p-ACC, and FAS) in mouse adipose tissue, resulting in enhanced adipose expansion. The serum lipidome revealed significantly upregulated lipid components, including S1P. Sphingolipid metabolism plays a role in inducing adipose tissue dysfunction by blocking insulin signalling and promoting chronic inflammation to induce defective adipose tissue phenotypes [40]. Upregulated S1P inhibits apoptosis and affects the cell cycle by limiting histone deacetylase activity and promoting p53 acetylation [41]. S1P is one of the lipids involved in mitochondrial fusion and autophagy [42]. This suggests the possibility of insulin resistance and adipose tissue senescence in H-shGPx3 mice. Hypertrophic adipocytes release additional free fatty acids, contributing to the ectopic accumulation of lipids, which, in turn, triggers systemic metabolic disorders such as fatty liver and exacerbates aging.
The induction of adipose browning is a promising method for reshaping adiposity in clinical applications. In humans and rodents, a portion of adipose cells respond to environmental changes (cold or fasting) with the ability to “temporarily” transform into brown adipose cells, called browning. We further verified elevated GPx3 levels in cold-stimulated (CS) and intermittently fed mice. Interestingly, CS-induced browning and anti-aging effects were partially hampered when GPx3 was deleted. Our proteomic analyses suggest that GPx3 effects on mitochondria may be associated with a reduction in mitochondrial inner membrane proteins. The thermogenesis and oxidative phosphorylation signalling pathways were significantly downregulated, suggesting that adipocyte browning was impaired. The uncoupling protein family, a key protein family in browning and thermogenesis, promotes cellular autophagy to counteract tissue damage through lipid consumption [43]. Lower GPx3 levels are accompanied by lower UCP1 expression in sWAT, which may be involved in the browning process by interacting with UCP1. UCP1 is found in the inner mitochondrial membrane of brown adipocytes and releases heat directly by “leaking” protons from the inner mitochondrial membrane gap back into the mitochondrial matrix, bypassing ATP synthase [44]. Increased UCP1 expression leads to increased proton leakage, which decreases the mitochondrial membrane potential and the proton gradient in the inner membrane. Increased GPx3 contributes to H+ transport and relieves mitochondrial stress and the accumulation of excess ROS byproducts [45]. Increased levels of GPx3 and UCP1 during differentiation into brown adipocytes may be associated with changes in mitochondrial activity and proton leakage from the electron transport chain. In addition, the modification of UCP1 by sulfenylation at Cys 253 and an increase in ROS-modulated organismal thermogenesis under cold stress. Dynamic changes in GPx3 during thermogenesis can eliminate the potential risk of cellular injury due to elevated ROS levels caused by UCP1. One possible explanation is that GPx3 neutralises additional ROS to prevent the overoxidation of UCP1. The need for antioxidant proteins to be involved in UCP1 thermogenesis has been gradually revealed; for example, the interaction between UCP1 and the antioxidant protein GSTM1 [46]. Our study provides further evidence that antioxidant proteins such as GPx3 are actively involved in the physiological function of adipose tissue and maintenance of mitochondrial homeostasis, contributing to the fight against aging.
MSCs in adipose tissue are an important cellular source reservoir for browning adipocytes and deserve investigation for this alteration in GPx3. Controlling oxidative status significantly affects the survival, proliferation, and differentiation of MSC [47]. Appropriate physiological levels of ROS are considered secondary messengers of stem cell function, whereas excessive intracellular oxidative levels are detrimental to stem cell proliferation and survival [48]. GPx3 is an important enzyme involved in the elimination of ROS and may be involved in MSC survival and differentiation. Our results showed that GPx3 deficiency led to a decrease in the total number of SVF cells isolated from sWAT as well as to a decrease in the number of UCP1+ cells. The anti-aging potential and concomitant GPx3 elevation during browning were further validated using in vitro multipotent embryonic stem cells (C3H/10T1/2). Whether the GPx3 deficiency affects the number and survival status of MSCs requires further investigation. In addition, vascular stromal fractions with high UCP1+ appear to feature higher GPx3 expression, which may release GPx3 for intercellular communication in the form of exosomes.
In conclusion, the present study highlights the involvement of GPx3 in the adipose remodelling processes, including adipose expansion and browning. Lower GPx3 promotes hypertrophy leading to accelerated aging. GPx3 deficiency restricts the occurrence of fat browning and impairs the anti-aging effect of cold exposure, which may, in part, be related to the interplay between GPx3 and UCP1, leading to mitochondrial dysregulation and oxidative stress. GPx3 is likely a pivotal junction in oxidative stress regulation in adipose tissue, maintaining adipocyte browning potential and delaying adipose aging.
Funding source
This work was supported by the Guangdong Province Key Area R&D Program of China [grant numbers 2020B1111110003], the NSFC-Joint Foundation of Yunnan Province [grant numbers U1902213], and Launch fee for talent introduction and research at Shanghai University of Traditional Chinese Medicine (A1U23205020411).
CRediT authorship contribution statement
Yijie Song: Writing – original draft, Visualization, Software, Methodology, Investigation, Formal analysis, Data curation. Mengjie Zhu: Validation, Software, Methodology, Data curation. Md Ariful Islam: Writing – review & editing, Investigation. Wenyi Gu: Data curation. Kavsar Alim: Data curation. Chien-shan Cheng: Resources, Methodology, Data curation. Jingxian Chen: Data curation. Yu Xu: Writing – review & editing, Supervision, Project administration, Conceptualization. Hongxi Xu: Resources, Funding acquisition, Conceptualization.
Declaration of competing interest
None. The authors declare that there are no known financial interests or personal relationships that might interfere with work reported in this paper.
Acknowledgements
The authors are grateful to those who participated in the study.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.redox.2024.103365.
Contributor Information
Yu Xu, Email: xyzjh2021@shutcm.edu.cn.
Hongxi Xu, Email: xuhongxi88@gmail.com.
Appendix A. Supplementary data
The following are the Supplementary data to this article.
Data availability
Data will be made available on request.
References
- 1.Partridge L., Deelen J., Slagboom P.E. Facing up to the global challenges of ageing. Nature. 2018;561:45–56. doi: 10.1038/s41586-018-0457-8. [DOI] [PubMed] [Google Scholar]
- 2.Soerjomataram I., Bray F. Planning for tomorrow: global cancer incidence and the role of prevention 2020-2070. Nat. Rev. Clin. Oncol. 2021;18:663–672. doi: 10.1038/s41571-021-00514-z. [DOI] [PubMed] [Google Scholar]
- 3.Yu L., Wan Q., Liu Q., et al. IgG is an aging factor that drives adipose tissue fibrosis and metabolic decline. Cell Metabol. 2024;36:793–807.e795. doi: 10.1016/j.cmet.2024.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.López-Otín C., Blasco M.A., Partridge L., et al. Hallmarks of aging: an expanding universe. Cell. 2023;186:243–278. doi: 10.1016/j.cell.2022.11.001. [DOI] [PubMed] [Google Scholar]
- 5.Palmer A.K., Jensen M.D. Metabolic changes in aging humans: current evidence and therapeutic strategies. J. Clin. Invest. 2022;132 doi: 10.1172/jci158451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ou M.Y., Zhang H., Tan P.C., et al. Adipose tissue aging: mechanisms and therapeutic implications. Cell Death Dis. 2022;13:300. doi: 10.1038/s41419-022-04752-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Santos A.L., Sinha S. Obesity and aging: molecular mechanisms and therapeutic approaches. Ageing Res. Rev. 2021;67 doi: 10.1016/j.arr.2021.101268. [DOI] [PubMed] [Google Scholar]
- 8.Lettieri Barbato D., Aquilano K. Feast and famine: adipose tissue adaptations for healthy aging. Ageing Res. Rev. 2016;28:85–93. doi: 10.1016/j.arr.2016.05.007. [DOI] [PubMed] [Google Scholar]
- 9.Li Y., Wang D., Ping X., et al. Local hyperthermia therapy induces browning of white fat and treats obesity. Cell. 2022;185:949–966.e919. doi: 10.1016/j.cell.2022.02.004. [DOI] [PubMed] [Google Scholar]
- 10.Gnad T., Navarro G., Lahesmaa M., et al. Adenosine/A2B receptor signaling ameliorates the effects of aging and counteracts obesity. Cell Metabol. 2020;32:56–70.e57. doi: 10.1016/j.cmet.2020.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pereira R.O., Olvera A.C., Marti A., et al. OPA1 regulates lipid metabolism and cold-induced browning of white adipose tissue in mice. Diabetes. 2022;71:2572–2583. doi: 10.2337/db22-0450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tarantini S., Subramanian M., Butcher J.T., et al. Revisiting adipose thermogenesis for delaying aging and age-related diseases: opportunities and challenges. Ageing Res. Rev. 2023;87 doi: 10.1016/j.arr.2023.101912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aldiss P., Betts J., Sale C., et al. Exercise-induced 'browning' of adipose tissues. Metabolism. 2018;81:63–70. doi: 10.1016/j.metabol.2017.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vaiserman A.M., Lushchak O.V., Koliada A.K. Anti-aging pharmacology: promises and pitfalls. Ageing Res. Rev. 2016;31:9–35. doi: 10.1016/j.arr.2016.08.004. [DOI] [PubMed] [Google Scholar]
- 15.Cantó C., Houtkooper R.H., Pirinen E., et al. The NAD(+) precursor nicotinamide riboside enhances oxidative metabolism and protects against high-fat diet-induced obesity. Cell Metabol. 2012;15:838–847. doi: 10.1016/j.cmet.2012.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Besse-Patin A., Léveillé M., Oropeza D., et al. Estrogen signals through peroxisome proliferator-activated receptor-γ coactivator 1α to reduce oxidative damage associated with diet-induced fatty liver disease. Gastroenterology. 2017;152:243–256. doi: 10.1053/j.gastro.2016.09.017. [DOI] [PubMed] [Google Scholar]
- 17.Jones D.P. Radical-free biology of oxidative stress. Am. J. Physiol. Cell Physiol. 2008;295:C849–C868. doi: 10.1152/ajpcell.00283.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Trayhurn P. Hypoxia and adipose tissue function and dysfunction in obesity. Physiol. Rev. 2013;93:1–21. doi: 10.1152/physrev.00017.2012. [DOI] [PubMed] [Google Scholar]
- 19.Luo J., Mills K., le Cessie S., et al. Ageing, age-related diseases and oxidative stress: what to do next? Ageing Res. Rev. 2020;57 doi: 10.1016/j.arr.2019.100982. [DOI] [PubMed] [Google Scholar]
- 20.Jiao Y., Wang Y., Guo S., Wang G. Glutathione peroxidases as oncotargets. Oncotarget. 2017;8:80093–80102. doi: 10.18632/oncotarget.20278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brigelius-Flohé R., Maiorino M. Glutathione peroxidases. Biochim. Biophys. Acta. 2013;1830:3289–3303. doi: 10.1016/j.bbagen.2012.11.020. [DOI] [PubMed] [Google Scholar]
- 22.Langhardt J., Flehmig G., Klöting N., et al. Effects of weight loss on glutathione peroxidase 3 serum concentrations and adipose tissue expression in human obesity. Obes. Facts. 2018;11:475–490. doi: 10.1159/000494295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hauffe R., Stein V., Chudoba C., et al. GPx3 dysregulation impacts adipose tissue insulin receptor expression and sensitivity. JCI Insight. 2020;5 doi: 10.1172/jci.insight.136283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee Y.S., Kim A.Y., Choi J.W., et al. Dysregulation of adipose glutathione peroxidase 3 in obesity contributes to local and systemic oxidative stress. Mol. Endocrinol. 2008;22:2176–2189. doi: 10.1210/me.2008-0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sekine Y., Osei-Hwedieh D., Matsuda K., et al. High fat diet reduces the expression of glutathione peroxidase 3 in mouse prostate. Prostate. 2011;71:1499–1509. doi: 10.1002/pros.21365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lim S., Honek J., Xue Y., et al. Cold-induced activation of brown adipose tissue and adipose angiogenesis in mice. Nat. Protoc. 2012;7:606–615. doi: 10.1038/nprot.2012.013. [DOI] [PubMed] [Google Scholar]
- 27.Li G., Xie C., Lu S., et al. Intermittent fasting promotes white adipose browning and decreases obesity by shaping the gut microbiota. Cell Metabol. 2017;26:672–685.e674. doi: 10.1016/j.cmet.2017.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cózar-Castellano I., Perdomo G. Assessment of insulin tolerance in vivo in mice. Methods Mol. Biol. 2020;2128:217–224. doi: 10.1007/978-1-0716-0385-7_15. [DOI] [PubMed] [Google Scholar]
- 29.Sen P., Qadri S., Luukkonen P.K., et al. Exposure to environmental contaminants is associated with altered hepatic lipid metabolism in non-alcoholic fatty liver disease. J. Hepatol. 2022;76:283–293. doi: 10.1016/j.jhep.2021.09.039. [DOI] [PubMed] [Google Scholar]
- 30.Chouchani E.T., Kazak L., Jedrychowski M.P., et al. Mitochondrial ROS regulate thermogenic energy expenditure and sulfenylation of UCP1. Nature. 2016;532:112–116. doi: 10.1038/nature17399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Childs B.G., Bussian T.J., Baker D.J. Cellular identification and quantification of senescence-associated β-galactosidase activity in vivo. Methods Mol. Biol. 2019;1896:31–38. doi: 10.1007/978-1-4939-8931-7_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Su L., Deng Z., Leng F. The mammalian high mobility group protein AT-hook 2 (HMGA2): biochemical and biophysical properties, and its association with adipogenesis. Int. J. Mol. Sci. 2020;21 doi: 10.3390/ijms21103710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kniewald H., Malcic I., Radosevic K., et al. Application of flow cytometry in the study of apoptosis in neonatal rat cardiomyocytes. Methods Find Exp. Clin. Pharmacol. 2007;29:681–687. doi: 10.1358/mf.2007.29.10.1147767. [DOI] [PubMed] [Google Scholar]
- 34.Lu J., Fu S., Dai J., et al. Integrated metabolism and epigenetic modifications in the macrophages of mice in responses to cold stress. J. Zhejiang Univ. - Sci. B. 2022;23:461–480. doi: 10.1631/jzus.B2101091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li Q., Hagberg C.E., Silva Cascales H., et al. Obesity and hyperinsulinemia drive adipocytes to activate a cell cycle program and senesce. Nat. Med. 2021;27:1941–1953. doi: 10.1038/s41591-021-01501-8. [DOI] [PubMed] [Google Scholar]
- 36.Dalle Molle R., Fatemi H., Dagher A., et al. Gene and environment interaction: is the differential susceptibility hypothesis relevant for obesity? Neurosci. Biobehav. Rev. 2017;73:326–339. doi: 10.1016/j.neubiorev.2016.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cadenas E., Davies K.J. Mitochondrial free radical generation, oxidative stress, and aging. Free Radic. Biol. Med. 2000;29:222–230. doi: 10.1016/s0891-5849(00)00317-8. [DOI] [PubMed] [Google Scholar]
- 38.Liochev S.I. Reactive oxygen species and the free radical theory of aging. Free Radic. Biol. Med. 2013;60:1–4. doi: 10.1016/j.freeradbiomed.2013.02.011. [DOI] [PubMed] [Google Scholar]
- 39.Altshuler-Keylin S., Kajimura S. Mitochondrial homeostasis in adipose tissue remodeling. Sci. Signal. 2017;10 doi: 10.1126/scisignal.aai9248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fang Z., Pyne S., Pyne N.J. Ceramide and sphingosine 1-phosphate in adipose dysfunction. Prog. Lipid Res. 2019;74:145–159. doi: 10.1016/j.plipres.2019.04.001. [DOI] [PubMed] [Google Scholar]
- 41.Gong L., Shen Y., Wang S., et al. Nuclear SPHK2/S1P induces oxidative stress and NLRP3 inflammasome activation via promoting p53 acetylation in lipopolysaccharide-induced acute lung injury. Cell Death Dis. 2023;9:12. doi: 10.1038/s41420-023-01320-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Duan M., Gao P., Chen S.X., et al. Sphingosine-1-phosphate in mitochondrial function and metabolic diseases. Obes. Rev. 2022;23 doi: 10.1111/obr.13426. [DOI] [PubMed] [Google Scholar]
- 43.Xiong W., Xiong Z., Song A., et al. Relieving lipid accumulation through UCP1 suppresses the progression of acute kidney injury by promoting the AMPK/ULK1/autophagy pathway. Theranostics. 2021;11:4637–4654. doi: 10.7150/thno.56082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bertholet A.M., Natale A.M., Bisignano P., et al. Mitochondrial uncouplers induce proton leak by activating AAC and UCP1. Nature. 2022;606:180–187. doi: 10.1038/s41586-022-04747-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ruiz-Ramírez A., Chávez-Salgado M., Peñeda-Flores J.A., et al. High-sucrose diet increases ROS generation, FFA accumulation, UCP2 level, and proton leak in liver mitochondria. Am. J. Physiol. Endocrinol. Metab. 2011;301:E1198–E1207. doi: 10.1152/ajpendo.00631.2010. [DOI] [PubMed] [Google Scholar]
- 46.Cui Z., Liu Y., Wan W., et al. Ethacrynic acid targets GSTM1 to ameliorate obesity by promoting browning of white adipocytes. Protein Cell. 2021;12:493–501. doi: 10.1007/s13238-020-00717-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sart S., Song L., Li Y. Controlling redox status for stem cell survival, expansion, and differentiation. Oxid. Med. Cell. Longev. 2015;2015 doi: 10.1155/2015/105135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li Q., Gao Z., Chen Y., Guan M.X. The role of mitochondria in osteogenic, adipogenic and chondrogenic differentiation of mesenchymal stem cells. Protein Cell. 2017;8:439–445. doi: 10.1007/s13238-017-0385-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.