Abstract
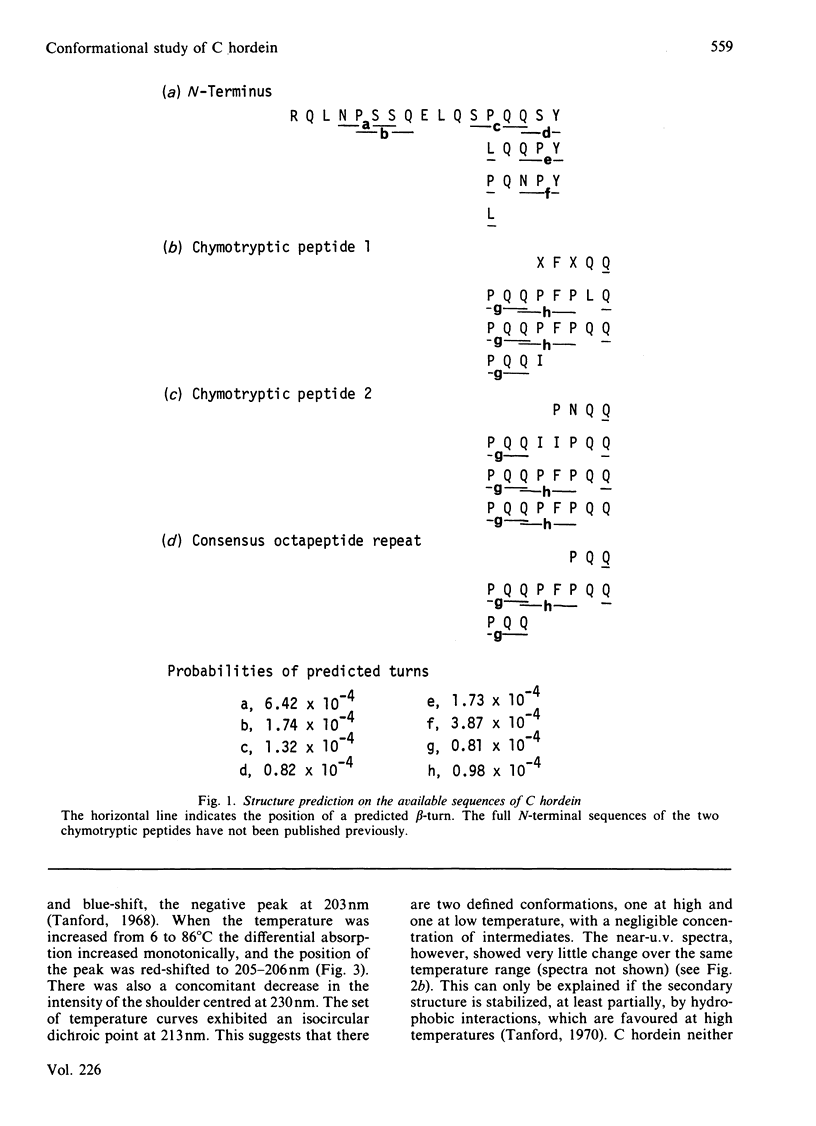
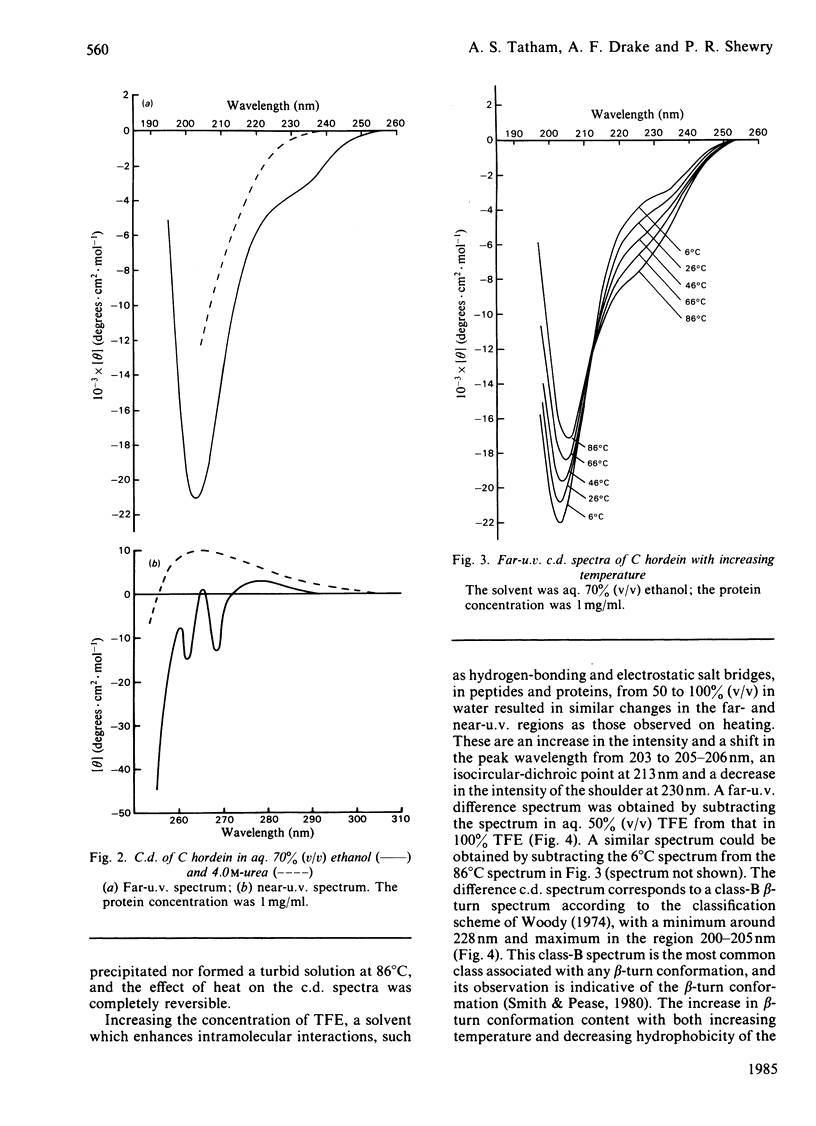
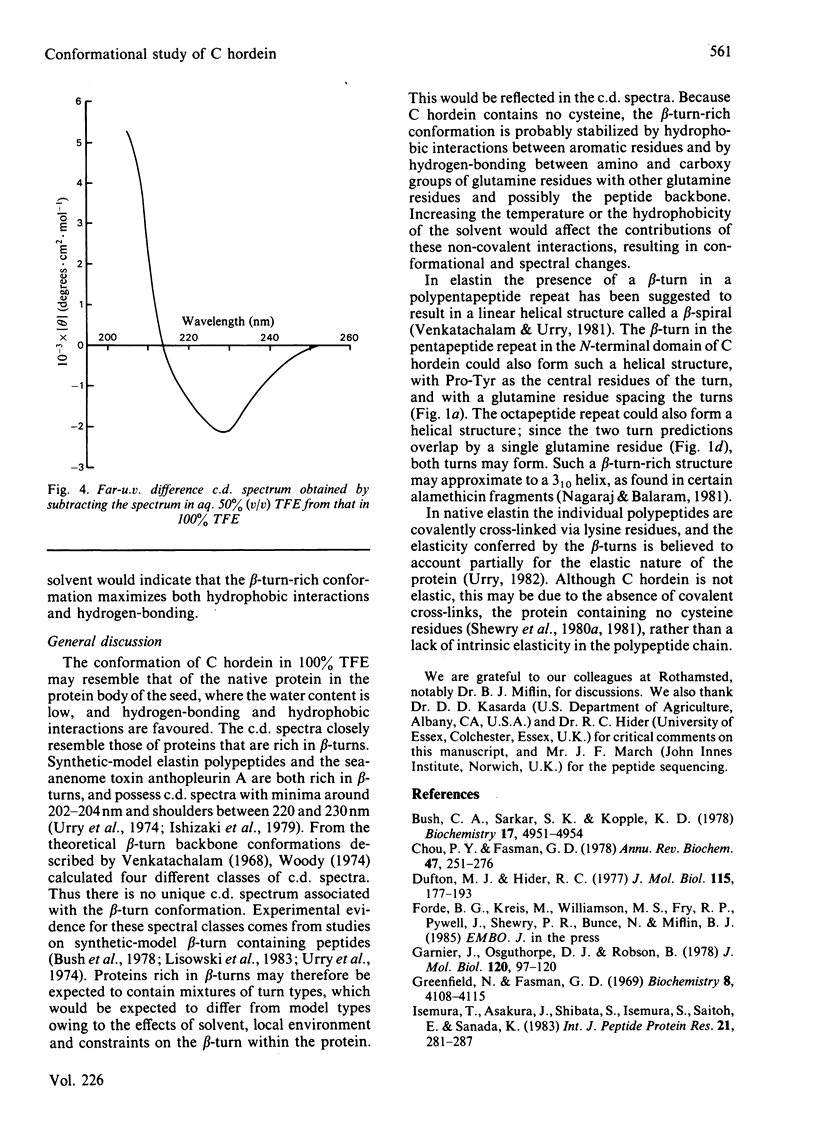
A combination of c.d. spectroscopy and computer prediction is used to show that C hordein has an unusual secondary structure with an absence of alpha-helix and beta-sheet, but the presence of regularly repeated beta-turns. This is associated with a repetitive primary structure based mainly on blocks of eight residues. Similar spectral changes occurred when the protein was heated from 6 to 86 degrees C in aq. 70% (v/v) ethanol or dissolved in increasing concentrations (50-100%, v/v) of trifluoroethanol in water. The studies indicated that the conformation is stabilized by strong hydrophobic interactions and by extensive hydrogen-bonding.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bush C. A., Sarkar S. K., Kopple K. D. Circular dichroism of beta turns in peptides and proteins. Biochemistry. 1978 Nov 14;17(23):4951–4954. doi: 10.1021/bi00616a015. [DOI] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Empirical predictions of protein conformation. Annu Rev Biochem. 1978;47:251–276. doi: 10.1146/annurev.bi.47.070178.001343. [DOI] [PubMed] [Google Scholar]
- Dufton M. J., Hider R. C. Snake toxin secondary structure predictions. Structure activity relationships. J Mol Biol. 1977 Sep 15;115(2):177–193. doi: 10.1016/0022-2836(77)90095-x. [DOI] [PubMed] [Google Scholar]
- Garnier J., Osguthorpe D. J., Robson B. Analysis of the accuracy and implications of simple methods for predicting the secondary structure of globular proteins. J Mol Biol. 1978 Mar 25;120(1):97–120. doi: 10.1016/0022-2836(78)90297-8. [DOI] [PubMed] [Google Scholar]
- Greenfield N., Fasman G. D. Computed circular dichroism spectra for the evaluation of protein conformation. Biochemistry. 1969 Oct;8(10):4108–4116. doi: 10.1021/bi00838a031. [DOI] [PubMed] [Google Scholar]
- Isemura T., Asakura J., Shibata S., Isemura S., Saitoh E., Sanada K. Conformational study of the salivary proline-rich polypeptides. Int J Pept Protein Res. 1983 Mar;21(3):281–287. doi: 10.1111/j.1399-3011.1983.tb03105.x. [DOI] [PubMed] [Google Scholar]
- Ishizaki H., McKay R. H., Norton T. R., Yasunobu K. T., Lee J., Tu A. T. Conformational studies of peptide heart stimulant anthopleurin A. Laser Raman, circular dichroism, fluorescence spectral studies, and Chou-Fasman calculations. J Biol Chem. 1979 Oct 10;254(19):9651–9656. [PubMed] [Google Scholar]
- Kreis M., Shewry P. R., Forde B. G., Rahman S., Miflin B. J. Molecular analysis of a mutation conferring the high-lysine phenotype on the grain of barley (Hordeum vulgare). Cell. 1983 Aug;34(1):161–167. doi: 10.1016/0092-8674(83)90146-0. [DOI] [PubMed] [Google Scholar]
- Lisowski M., Siemion I. Z., Sobczyk K. Conformation of model, alanine and proline containing tetrapeptides in water. Comparison of 13C-n.m.r. and CD results. Int J Pept Protein Res. 1983 Mar;21(3):301–306. doi: 10.1111/j.1399-3011.1983.tb03108.x. [DOI] [PubMed] [Google Scholar]
- Nagaraj R., Balaram P. Conformations of synthetic alamethicin fragments. Evidence for 310 helical folding from 270-MHz hydrogen-1 nuclear magnetic resonance and circular dichroism studies. Biochemistry. 1981 May 12;20(10):2828–2835. doi: 10.1021/bi00513a019. [DOI] [PubMed] [Google Scholar]
- Shibata S., Asakura J., Isemura T., Isemura S., Saitoh E., Sanada K. Conformational study of the basic proline-rich polypeptides from human parotid saliva. Int J Pept Protein Res. 1984 Feb;23(2):158–165. doi: 10.1111/j.1399-3011.1984.tb02706.x. [DOI] [PubMed] [Google Scholar]
- Smith J. A., Pease L. G. Reverse turns in peptides and proteins. CRC Crit Rev Biochem. 1980;8(4):315–399. doi: 10.3109/10409238009105470. [DOI] [PubMed] [Google Scholar]
- Strickland E. H. Aromatic contributions to circular dichroism spectra of proteins. CRC Crit Rev Biochem. 1974 Jan;2(1):113–175. doi: 10.3109/10409237409105445. [DOI] [PubMed] [Google Scholar]
- Tanford C. Protein denaturation. C. Theoretical models for the mechanism of denaturation. Adv Protein Chem. 1970;24:1–95. [PubMed] [Google Scholar]
- Tanford C. Protein denaturation. Adv Protein Chem. 1968;23:121–282. doi: 10.1016/s0065-3233(08)60401-5. [DOI] [PubMed] [Google Scholar]
- Urry D. W. Characterization of soluble peptides of elastin by physical techniques. Methods Enzymol. 1982;82(Pt A):673–716. doi: 10.1016/0076-6879(82)82096-x. [DOI] [PubMed] [Google Scholar]
- Urry D. W., Long M. M., Ohnishi T., Jacobs M. Circular dichroism and absorption of the polytetrapeptide of elastin: a polymer model for the beta-turn. Biochem Biophys Res Commun. 1974 Dec 23;61(4):1427–1433. doi: 10.1016/s0006-291x(74)80442-0. [DOI] [PubMed] [Google Scholar]
- Venkatachalam C. M. Stereochemical criteria for polypeptides and proteins. V. Conformation of a system of three linked peptide units. Biopolymers. 1968 Oct;6(10):1425–1436. doi: 10.1002/bip.1968.360061006. [DOI] [PubMed] [Google Scholar]


