Abstract
Atopic Dermatitis (AD), recognized as one of the most prevalent chronic inflammatory skin disorders among children, is characterized by skin barrier dysfunction and immune system abnormalities. Historically viewed as a childhood condition, recent findings underscore a notable prevalence of AD in adults, prompting a critical examination of this demographic. Diagnosis hinges largely on subjective clinical assessments due to the absence of universally accepted biomarkers. Consequently, efforts are underway to identify dependable biomarkers to enhance diagnostic precision. This paper underscores the scarcity of AD diagnoses in adults despite its pediatric prominence, emphasizing the need for heightened awareness and tailored diagnostic approaches in adult populations. Severity scores such as SCORing Atopic Dermatitis (SCORAD) and dermatological life quality index (DLQI) play pivotal roles in evaluating disease severity and its impact on quality of life, guiding the development of personalized treatment strategies for adult AD patients. In this study, we aim to present four compelling cases of adult-onset atopic dermatitis, each offering unique insights into this increasingly recognized phenomenon. What makes these cases particularly noteworthy is the absence of any prior atopic history in two out of four patients, challenging the conventional understanding of AD as a condition predominantly linked to childhood. Moreover, the clinical presentation in all four cases was markedly atypical, underscoring the elusive nature of adult-onset AD diagnosis. In our investigation, interleukin 4 (IL-4), interleukin 13 (IL-13), and Immunoglobulin E (IgE) were utilized as diagnostic biomarkers for our patient cohort. Given the established pivotal roles of IL-4 and IL-13 in AD pathogenesis, elevated serum levels of these biomarkers, although not universally endorsed, hold potential for diagnostic utility. Furthermore, heightened levels of IgE, indicative of allergic responses and inflammation inherent to the condition, emphasize its significance as a key biomarker and therapeutic target in AD management.
Keywords: Atopic dermatitis , peripheral arterial disease , adult onset , IL-4 , IL-1 , IgE , biomarker
Introduction
AD, a persistent skin condition, is characterized by impairments in skin barrier function and abnormalities in the immune system.
It affects approximately 20% of children and 2-5% of adults worldwide [1].
Traditionally seen as a childhood condition that often resolves before adulthood, recent observations show a significant prevalence of AD in adults [2].
The cause of this rise remains uncertain, prompting the need for a deeper understanding of the adult AD population.
The diagnosis of AD primarily depends on the modified Hanifin and Rajka criteria [3], introducing subjectivity, particularly as it hinges on the physician's subjective interpretation of the clinical manifestations exhibited by a given patient.
Currently, there is a lack of universally accepted and employed paraclinical biomarkers in routine medical practice.
Despite being the most prevalent chronic inflammatory skin disorder, ongoing efforts are being directed towards identifying reliable biomarkers that can accurately reflect the severity of the disease, gauge treatment response, and function as distinctive and dependable diagnostic elements.
The main tools commonly used to assess the severity of AD include the SCORAD score and the DLQI score.
These metrics serve as pivotal clinical tools, offering comprehensive assessments of the extent and impact of AD on both the physical manifestation of the condition and the overall quality of life experienced by individuals affected by it.
The SCORAD score provides a quantitative measure, considering factors such as lesion severity, extent, and subjective symptoms, to offer a holistic perspective on disease severity.
Simultaneously, the DLQI score delves into the psychosocial aspects, examining the influence of AD on various aspects of daily life, emotions, and social interactions.
Together, these assessments provide a thorough and multifaceted evaluation of the clinical severity and broader implications of AD, aiding healthcare professionals in tailoring effective treatment strategies.
While IL-4 and IL-13 are not universally recognized as biomarkers for AD, their pivotal roles in the pathogenesis of this condition have been extensively documented [4].
However, IgE levels, although not exclusive to AD, are presently employed as indicators, albeit not diagnostic biomarkers.
In our four cases, all three aforementioned biomarkers were utilized as diagnostic adjuncts due to the profoundly atypical clinical presentations, which did not align entirely with the established clinical criteria for diagnosis.
Case Report
Case 1
A 62-year-old woman with a three-year history of moderate-to-severe AD presented to our dermatology clinic.
The patient reported an onset age of approximately 58 years.
Previous treatments involved high-potency topical corticosteroids (TCS), systemic corticosteroids (SCS), and narrowband ultraviolet-B (UVB) phototherapy, which initially showed positive responses but often led to rapid flare-ups when the treatment frequency was reduced or discontinued.
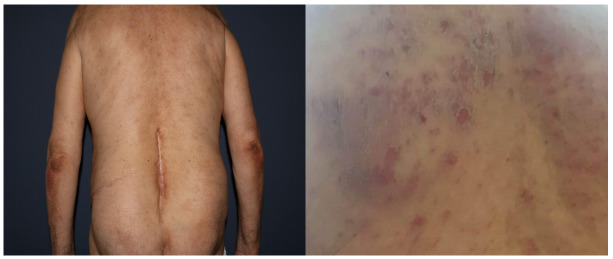
Upon examination, diffuse AD covered about 68% of her body surface, featuring papulo-nodular lesions, post-grating hematic crusts, excoriations, lichenifications, and numerous postlesional hyperpigmentations throughout her body (Figure 1).
Figure 1.
Post-grating hematic crusts, excoriations, lichenifications, and multiple postlesional hyperpigmentations on the posterior thoracic region
The DLQI score was 18 and SCORAD was 51.
Additionally, the patient had a history of arterial hypertension and chronic kidney disease but was not receiving any current treatment.
Laboratory results showed elevated inflammatory markers: IL-4 at 8,321 pg/mL, IL-13 at 10,356 pg/mL, and IgE at 302 UI/mL.
Case 2
A 64-year-old retired male patient, presented with a disseminated, intensely pruritic maculopapular skin rash.
Despite a history of various comorbidities, including allergic bronchial asthma and chronic obstructive pulmonary disease, the cutaneous lesions had emerged around 4 years ago.
Temporary remissions had been observed with SCS therapt, but the rash had reappeared upon discontinuation.
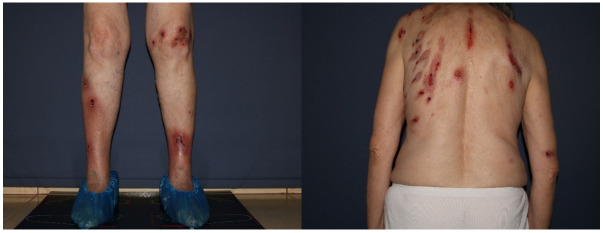
Dermatological examination had revealed marked cutaneous xerosis and a maculopapular eczematous skin rash, particularly pronounced on the anterior and posterior chest, thighs, and flexor surfaces of the limbs (Figure 2).
Figure 2.
Eczematous lesions, erythematous, infiltrated, with fine scales on the surface, scratch marks and erosions covered by crusts on the anterior and posterior thoracic regions
The SCORAD and DLQI were 59 and 15, respectively.
Laboratory analysis revealed significant elevations in inflammatory markers: IL-4 (6,234 pg/mL), IL-13 (21,014 pg/mL), and IgE (391 UI/mL).
Case 3
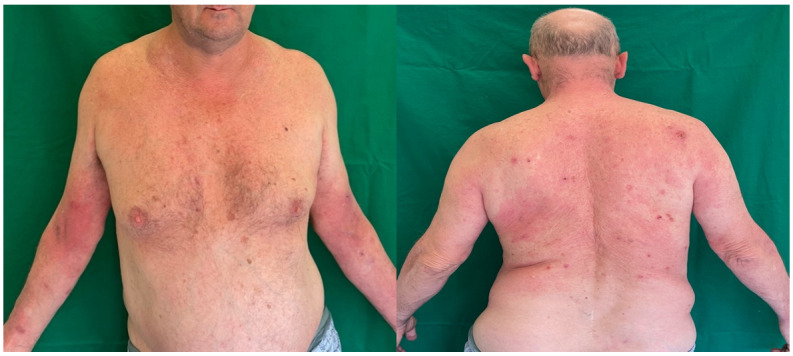
A 67-year-old male patient presented with an eruption consisting of maculopapular lesions, with diameters ranging from 0.2 to 1 cm.
These lesions were erythematous, pruritic, and covered with post-scratching hemorrhagic crusts.
They were disseminated over the abdomen and the anterior and posterior thorax (Figure 3).
Figure 3.
Maculo-papular erythematous, pruritic lessions and post-scratching hemorrhagic crusts disseminated over the abdomen and the anterior and posterior thorax
The patient lacked a personal history of atopy; however, he confirmed a familial predisposition, citing both parents as having a history of atopic conditions.
The patient had no other comorbidities necessitating continuous treatment, only a history of surgically excised prostate adenoma.
The cutaneous lesions emerged around 6 months ago.
Temporary remissions were observed with SCS and TCS therapy, but the rash reappeared upon discontinuation.
Despite inadequate improvement with conventional treatments, such as corticosteroids and antihistamines, the patient's condition remained unchanged, with a SCORAD score of 61 and a DLQI of 16.
The laboratory findings for the patient indicated notable increases in inflammatory markers: IL-4 at 14,825 pg/mL, IL-13 at 22,406 pg/mL, and IgE at 442 UI/mL.
Case 4
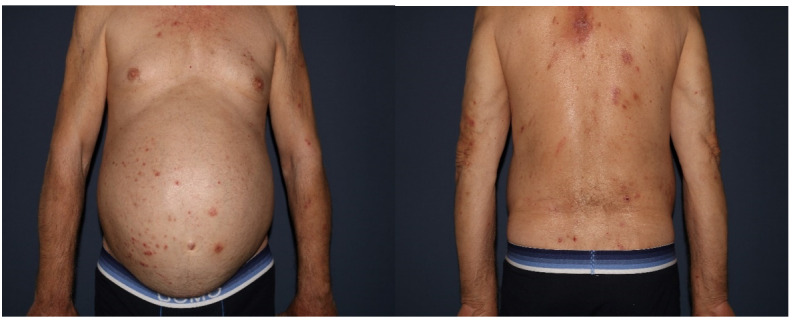
A 67-year-old female patient presented with an eruption consisting of multiple severe, bleeding erosions, postinflammatory melanoderma located on the posterior thorax, as well as on the lower extremities.
Physical examination revealed generalized cutaneous xerosis, and despite the clinical appearance of the lesions being nonspecific for AD, the patient stated that they were self-induced due to unbearable pruritus. (Figure 4).
Figure 4.
Multiple severe, bleeding erosions, postinflammatory melanoderma located on the posterior thorax, as well as on the lower extremities
The patient had a history of bronchial asthma and allergic rhinitis, for which she was currently not undergoing treatment.
Other comorbidities included grade 3 primary hypertension and type 2 diabetes mellitus, both under treatment.
The cutaneous lesions emerged around 2 years ago.
Temporary remissions were observed with SCS therapy, but the rash reappeared upon discontinuation, afterwards, the patient declined any form of treatment due to fear of adverse effects.
She had a SCORAD of 55 and a DLQI o 22.
The patient's lab results showed elevated inflammatory markers: IL-4 (9,376 pg/mL), IL-13 (18,332 pg/mL), and IgE (502 UI/mL).
In this series of clinical cases, we encountered a spectrum of presentations of AD among elderly patients, highlighting the multifaceted nature of this chronic skin condition in later stages of life.
Firstly, the patients' mean age was 65 years-old.
Despite their age, these patients experienced the onset of AD symptoms around their late fifties.
Past treatments in all cases revealed a history of attempts to manage the condition with conventional treatments such as the use of potent topical corticosteroids, systemic corticosteroids, and narrowband ultraviolet-B phototherapy.
While these interventions initially provided relief, discontinuation or reduction in frequency led to rapid flare-ups.
Clinically, all patients presented with severe manifestations of AD, characterized by widespread skin involvement.
The presence of papulo-nodular lesions, excoriations, lichenifications, and postlesional hyperpigmentations underscored the chronicity and severity of their disease.
Despite variations in specific lesion patterns and distributions, the overarching theme was one of significant cutaneous involvement and distressing symptoms.
Paraclinical findings emphasized the systemic involvement of AD in the studied cases.
Elevated levels of inflammatory markers, including IL-4 (mean value 9,689 pg/mL, normal range <0.5 pg/mL), IL-13 (mean value 17,777 pg/mL, normal range <2.3 pg/mL), and IgE (mean value 409.25 IU/mL, normal range 0-100 IU/mL), were consistently observed, indicating a dysregulated immune response contributing to chronic inflammation and disease progression.
Additionally, high mean scores on the SCORAD and DLQI scales highlighted significant disease burden and impaired quality of life among the patients.
The mean SCORAD was 56.5, suggesting very severe disease (a score over 51), while the mean DLQI was 17.75, indicating a substantial impact on quality of life (a score between 11-20).
However, no correlation between higher levels of these biomarkers could be established with the SCORAD score.
Interestingly, the patient with the highest levels of all three measured biomarkers had a lower SCORAD score compared to other two patients in the cohort.
All four previously presented cases underwent screening for the initiation of Dupilumab treatment, with no observed pathological findings contraindicating the biological therapy.
Routine bloodwork returned normal results for all cases.
As a result, all patients were administered Dupilumab at a loading dose of 600mg followed by 300 mg every two weeks.
Subsequently, all cases exhibited improvement, with complete resolution of symptoms observed at the three-month follow-up assessment.
The authors affirm that written consent has been acquired directly from each patient involved.
In these consent forms, patients have granted permission for the reporting of images and other clinical information in the journal.
They understand that efforts will be made to safeguard confidentiality by refraining from publishing names or initials.
Discussion
In summary, the pathophysiology of AD begins with the penetration of allergens through a weakened epidermal barrier, triggering the release of pro-inflammatory cytokines by dermal cells, which initiates a T cell-mediated immune response.
The onset of AD is marked by increased levels of T helper cells type 2 (Th2), such as IL-4, IL-5, IL-13, IL-31, and T helper cells type 22 (Th22), particularly IL-22, with IL-4 and IL-13 recognized as key contributors to AD pathogenesis among Th2 immune mediators [4, 5].
In early childhood, AD lesions typically manifest on the face and extensor areas, whereas in adults, particularly those with adult-onset or recurrent cases, lesions tend to occur more commonly on the head/neck and hands.
Furthermore, the clinical course of AD is diverse, encompassing different ages of onset, varying severity and extent of lesions, periods of remission and recurrence, and the enduring presence of AD signs and clinical manifestations [6, 7].
However, identifying genuine adult-onset AD proves challenging through historical data, given that adult patients might not recollect childhood AD.
Furthermore, there's a recurring observation that adults who develop AD often have no personal or family history of atopy.
This complicates the process of distinguishing AD from other possible diagnoses [8].
Few high-quality national and international studies assess adult AD prevalence, showing varied criteria [9].
Prevalence in international adult AD studies ranges from 2.0% to 17.6%, exhibiting regional and methodological differences [10, 11, 12, 13].
In a recent meta-analysis comprising 17 studies documenting AD onset after the age of 16, the combined proportion of cases with adult-onset AD was determined to be 26.1% [14].
In summary, it's crucial to acknowledge that AD extends beyond its traditional link with childhood, challenging the notion that it solely affects pediatric populations.
Notably, a considerable proportion, approximately one in four adults affected by AD, experiences the onset of symptoms during adulthood [14].
This observation underscores the dynamic nature of AD, necessitating a shift in focus towards understanding and addressing the complexities of adult-onset cases.
The recognition of AD as a condition that can manifest at any age is crucial for comprehensive healthcare approaches and underscores the need for tailored management strategies to cater to the unique challenges presented by adult-onset AD.
Regarding the pathophysiology of adult-onset AD, the exact mechanisms remain uncertain.
While it's possible that the genetic and immunologic factors are similar and established early in life for both adult-onset and childhood-onset AD, environmental triggers may vary over the lifespan.
Significantly, a study in the US indicated a higher prevalence of adult-onset AD among foreign-born individuals compared to those born in the US, hinting at potential associations with different climates or other environmental factors [15].
However, there may be distinct mechanisms underlying adult-onset AD.
This is suggested by a study involving 241 AD patients, which found that the four most common Filaggrin loss-of-function gene mutations were exclusively associated with early childhood-onset AD, rather than late-childhood or adult-onset disease [16].
Furthermore, a study involving 71 adults, 61 children with AD, and 31 adult controls found similar elevations in serum levels of thymic stromal lymphopoietin (TSLP), IL-33, and IgE in both children and adults with AD.
Notably, IL-31 levels were elevated in children, while no significant increases were observed in soluble suppression of tumorigenicity 2 (sST2) levels for either group.
Additionally, IL-31 and IL-33 levels were higher in children compared to adults, whereas TSLP, IgE, and sST2 levels showed similarity between the two age groups [17].
These findings imply possible distinctions in the immune mechanisms of AD between children and adults.
Further research is crucial to uncover the precise mechanisms that govern adult-onset AD.
For AD diagnosis, the utilization of diagnostic criteria based on questionnaires is prevalent in AD diagnosis due to its simplicity in distribution, scalability, and cost-effectiveness.
Nevertheless, there is frequently notable variability in both content and performance of questionnaire-based approaches across different studies.
Nevertheless, in various guidelines, the cataloging of associated criteria often neglects some prevalent characteristics seen in adult-onset AD, such as heightened occurrences of hand and foot dermatitis, facial and neck involvement, an increased prevalence of lichenified lesions, and greater resistance to topical therapies.
This oversight holds significance in the accurate diagnosis and effective treatment of adult AD patients.
Because there's less suspicion for new-onset AD in adults, diagnosing AD in this demographic primarily involves ruling out other potential conditions-a process known as exclusion [18, 19].
In a recent review based on clinical findings derived from various studies, it was identified that the prevailing clinical manifestations in adults with AD encompassed a spectrum of dermatological presentations.
The most frequently reported clinical findings among adults with AD include hand dermatitis, eyelid dermatitis, neck dermatitis, periorificial dermatitis, and involvement of the flexural region of the upper limbs.
This synthesis of clinical evidence sheds light on the diverse and distinctive patterns of AD presentation in adults, providing valuable insights for both clinical practitioners and researchers to enhance their understanding and approach to the management of adult-onset AD [20].
In a recent multicenter study, the prevalent sites affected by adult AD were outlined as follows: the flexural surface of the upper limbs exhibited the highest involvement at 47.8%, followed by the eyelid/periocular area at 37.9%, the hands at 37.2%, and the neck at 32% [21].
Concerning the morphological characteristics of AD lesions, the same study revealed that the predominant findings were in the form of an erythemato-desquamative pattern, succeeded by a lichenified pattern. Exudative lesions were less frequently observed [21].
The diagnostic journey in identifying AD encompasses a thorough exploration of the patient's history and physical presentation.
Commencing with the clinical history, the practitioner delves into the presence of chronic eczema and scrutinizes the patient's personal and family history of atopy.
This initial step sets the foundation for understanding the context of the condition.
Moving on to the physical examination, the healthcare provider meticulously observes the morphology and typical distribution of eczema in adults.
Additionally, they identify distinctive prurigo lesions and note the occurrence of multiple areas of lichenification.
This hands-on assessment aids in painting a comprehensive picture of the dermatological manifestations.
Following the clinical evaluation, the patch test is deployed in various scenarios.
It proves valuable in cases of de novo AD, instances of chronic AD or hand eczema resistant to conventional treatment, and situations where the distribution of dermatitis is atypical or undergoes changes over time.
The patch test becomes particularly instrumental when patterns hint at allergic contact dermatitis.
To further refine the diagnosis, a skin biopsy is employed.
This step is crucial in scenarios involving chronic AD that proves refractory to treatment, the identification of morphological variants such as prurigo, instances of erythroderma, and to rule out alternative diagnoses like psoriasis, cutaneous drug eruptions, or dermatitis herpetiformis [22].
Histopathologically, acute lesions of AD typically display intercellular edema in the epidermis (known as spongiosis) and a perivascular mononuclear infiltrate, predominantly consisting of T cells, with increased eosinophils in the dermis.
On the other hand, chronic lesions are characterized by hyperkeratosis, epidermal hyperplasia, irregular elongation of the rete ridges, and varying degrees of spongiosis along with dermal eosinophils [23].
However, as there are presently no definitive histopathological features for diagnosing AD, we opted not to pursue a skin biopsy in our cases.
Complementing these diagnostic procedures, blood testing is usually carried out.
Parameters such as total IgE levels and eosinophilia are evaluated, alongside additional tests like lactate dehydrogenase and antitransglutaminase antibodies.
Employing a multifaceted approach ensures a thorough comprehension of the patient's condition, facilitating a more precise and personalized diagnosis of AD.
The onset of AD is characterized by a T cell-mediated process primarily influenced by Th2 signaling in acute lesions.
As the disease progresses to the chronic phase, there is a transition from Th2 to Th1 signaling.
IL-4 and IL-13, pivotal Th2 immune mediators, have been identified as key players in the pathogenesis of AD, directly stimulating sensory neurons.
Notably, genetic associations between AD and polymorphisms in IL-4 and IL-13 have been established [4, 5].
Therefore, in our investigation, we chose to evaluate IL-4 and IL-13 levels as potential diagnostic biomarkers for AD.
This decision was prompted by a recent study conducted by Bodoor et al., which indicated elevated serum levels of IL-4 and IL-13 in a cohort of 56 patients [24].
As demonstrated in the case report section, all our patients displayed elevated serum levels of these interleukins.
Specific treatment guidelines for adult-onset AD are currently lacking.
In fact, there is a scarcity of studies comparing the efficacy of AD treatments specifically between adults and children, let alone distinguishing between adult-onset and child-onset AD.
As a result, current guidelines recommend consistent treatment approaches for both adult-onset and childhood-onset AD.
All AD treatment guidelines endorse a step-care approach to therapy [25, 26].
In 2017, the approval of the initial biological treatment marked a revolutionary shift from the previously employed strictly symptomatic treatments.
Dupilumab, a humanized monoclonal antibody, specifically targets the α-subunit of the IL-4 receptor, which is a component of the IL-4 and IL-13 receptor complex.
In our patient cohort, we elected to initiate dupilumab at a standard dosage, commencing with a 600mg loading dose, followed by a 300 mg dose administered every two weeks.
This decision was based on the observation that all patients presented elevated serum levels of IL-4 and IL-13, rendering them eligible for biologic therapy.
Encouragingly, all patients exhibited favorable clinical outcomes, and as of now, their conditions remain well-controlled.
Conclusion
Recent research contradicts the long-held notion that AD predominantly affects children, highlighting its significant occurrence in adults and prompting a thorough investigation of this demographic.
Despite their advanced age, pur patients experienced AD onset in late middle age, with conventional treatments offering initial relief but often resulting in rapid flare-ups upon discontinuation.
Clinically, severe AD manifestations were observed, characterized by extensive skin involvement and distressing symptoms.
Paraclinical findings revealed systemic immune dysregulation, yet no clear correlation was found between biomarker levels and disease severity.
However, further research is necessary to establish specific treatment guidelines for adult-onset AD and refine diagnostic protocols to optimize patient care.
Importantly, current biomarkers for AD lack universal acceptance, and treatment guidelines remain variable, highlighting the need for standardized approaches in diagnosis and management strategies.
Funding
The authors did not receive any financial support for the research, authorship, and/or publication of this article.
Availability of Data and Materials
All data generated or analyzed during this study are included in this published article.
Conflict of interests
The authors have no conflict of interest to declare.
References
- 1.Eichenfield LF, Tom WL, Chamlin SL, Feldman SR, Hanifin JM, Simpson EL, Berger TG, Bergman JN, Cohen DE, Cooper KD, Cordoro KM, Davis DM, Krol A, Margolis DJ, Paller AS, Schwarzenberger K, Silverman RA, Williams HC, Elmets CA, Block J, Harrod CG, Smith Begolka, Sidbury R. Guidelines of care for the management of atopic dermatitis: section 1. Diagnosis and assessment of atopic dermatitis. J Am Acad Dermatol. 2014;70(2):338–51. doi: 10.1016/j.jaad.2013.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sacotte R, Silverberg JI. Epidemiology of adult AD. Clin Dermatol. 2018;36(5):595–605. doi: 10.1016/j.clindermatol.2018.05.007. [DOI] [PubMed] [Google Scholar]
- 3.Hanifin JM, Rajka G. Diagnostic features of AD. Acta Derm Venereol. 1980;92:44–47. [Google Scholar]
- 4.Brunner PM, Guttman-Yassky E, Leung DY. The immunology of AD and its reversibility with broad-spectrum and targeted therapies. J Allergy Clin Immunol. 2017;139:65–S76. doi: 10.1016/j.jaci.2017.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kader HA, Azeem M, Jwayed SA, Al-Shehhi A, Tabassum A, Ayoub MA, Hetta HF, Waheed Y, Iratni R, Al-Dhaheri A, Muhammad K. Current Insights into Immunology and Novel Therapeutics of AD. Cells. 2021;10(7):1392–1392. doi: 10.3390/cells10061392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ozkaya E. Adult-onset AD. J Am Acad Dermatol. 2005;52(4):579–582. doi: 10.1016/j.jaad.2004.11.037. [DOI] [PubMed] [Google Scholar]
- 7.Bannister MJ, Freeman S. Adult-onset AD. Australas J Dermatol. 2000;41(4):225–228. doi: 10.1046/j.1440-0960.2000.00442.x. [DOI] [PubMed] [Google Scholar]
- 8.Hanifin JM. Adult-Onset AD: Fact or Fancy. Dermatol Clin. 2017;35(3):299–302. doi: 10.1016/j.det.2017.02.009. [DOI] [PubMed] [Google Scholar]
- 9.Deckers IA, McLean S, Linssen S, Mommers M, van Schayck, Sheikh A. Investigating international time trends in the incidence and prevalence of atopic eczema 1990-2010: a systematic review of epidemiological studies. PLoS One. 2012;7(7):39803–39803. doi: 10.1371/journal.pone.0039803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Muto T, Hsieh SD, Sakurai Y, Yoshinaga H, Suto H, Okumura K, Ogawa H. Prevalence of AD in Japanese adults. Br J Dermatol. 2003;148(1):117–121. doi: 10.1046/j.1365-2133.2003.05092.x. [DOI] [PubMed] [Google Scholar]
- 11.Saeki H, Tsunemi Y, Fujita H, Kagami S, Sasaki K, Ohmatsu H, Watanabe A, Tamaki K. Prevalence of AD determined by clinical examination in Japanese adults. J Dermatol. 2006;33(11):817–819. doi: 10.1111/j.1346-8138.2006.00187.x. [DOI] [PubMed] [Google Scholar]
- 12.Marks R, Kilkenny M, Plunkett A, Merlin K. The prevalence of common skin conditions in Australian school students: 2. AD. Br J Dermatol. 1999;140(3):468–473. doi: 10.1046/j.1365-2133.1999.02711.x. [DOI] [PubMed] [Google Scholar]
- 13.Herd RM, Tidman MJ, Prescott RJ, Hunter JA. Prevalence of atopic eczema in the community: the Lothian AD study. Br J Dermatol. 1996;135(1):18–19. [PubMed] [Google Scholar]
- 14.Lee HH, Patel KR, Singam V, Rastogi S, Silverberg JI. A systematic review and meta-analysis of the prevalence and phenotype of adult-onset AD. J Am Acad Dermatol. 2019;80(6):1526–1532. doi: 10.1016/j.jaad.2018.05.1241. [DOI] [PubMed] [Google Scholar]
- 15.Silverberg JI, Vakharia PP, Chopra R, Sacotte R, Patel N, Immaneni S, White T, Kantor R, Hsu DY. Phenotypical Differences of Childhood-and Adult-Onset AD. J Allergy Clin Immunol Pract. 2018;6(4):1306–1312. doi: 10.1016/j.jaip.2017.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rupnik H, Rijavec M, Korošec P. Filaggrin loss-of-function mutations are not associated with AD that develops in late childhood or adulthood. Br J Dermatol. 2015;172(2):455–461. doi: 10.1111/bjd.13477. [DOI] [PubMed] [Google Scholar]
- 17.Nygaard U, Hvid M, Johansen C, Buchner M, Fölster-Holst R, Deleuran M, Vestergaard C. TSLP, IL-31, IL-33 and sST2 are new biomarkers in endophenotypic profiling of adult and childhood AD. J Eur Acad Dermatol Venereol. 2016;30(11):1930–1938. doi: 10.1111/jdv.13679. [DOI] [PubMed] [Google Scholar]
- 18.Bos JD, Van Leent, Sillevis Smitt. The millennium criteria for the diagnosis of AD. Exp Dermatol. 1998;7(4):132–138. doi: 10.1111/j.1600-0625.1998.tb00313.x. [DOI] [PubMed] [Google Scholar]
- 19.Williams HC, Burney PG, Pembroke AC, Hay RJ. The U.K. Working Party's Diagnostic Criteria for AD. III. Independent hospital validation. Br J Dermatol. 1994;131(3):406–416. doi: 10.1111/j.1365-2133.1994.tb08532.x. [DOI] [PubMed] [Google Scholar]
- 20.Chan AR, Sandhu VK, Drucker AM, Fleming P, Lynde CW. Adult-Onset AD: Presentations and Progress. J Cutan Med Surg. 2020;24(3):267–272. doi: 10.1177/1203475420911896. [DOI] [PubMed] [Google Scholar]
- 21.Megna M, Patruno C, Balato A, Rongioletti F, Stingeni L, Balato N; An Italian multicentre study on adult AD: persistent versus adult-onset disease. Arch Dermatol Res. 2017;309(6):443–452. doi: 10.1007/s00403-017-1739-y. [DOI] [PubMed] [Google Scholar]
- 22.Silvestre Salvador, Romero-Pérez D, Encabo-Durán B. AD in Adults: A Diagnostic Challenge. J Investig Allergol Clin Immunol. 2017;27(2):78–88. doi: 10.18176/jiaci.0138. [DOI] [PubMed] [Google Scholar]
- 23.Silverberg JI. Adult-Onset AD. J Allergy Clin Immunol Pract. 2019;7(1):28–33. doi: 10.1016/j.jaip.2018.09.029. [DOI] [PubMed] [Google Scholar]
- 24.Bodoor K, Al-Qarqaz F, Heis LA, Alfaqih MA, Oweis AO, Almomani R, Obeidat MA. IL-33/13 Axis and IL-4/31 Axis Play Distinct Roles in Inflammatory Process and Itch in Psoriasis and AD. Clin Cosmet Investig Dermatol. 2020;13:419–424. doi: 10.2147/CCID.S257647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boguniewicz M, Fonacier L, Guttman-Yassky E, Ong PY, Silverberg J, Farrar JR. AD yardstick: Practical recommendations for an evolving therapeutic landscape. Ann Allergy Asthma Immunol. 2018;120(1):10–22. doi: 10.1016/j.anai.2017.10.039. [DOI] [PubMed] [Google Scholar]
- 26.Eichenfield LF, Tom WL, Berger TG, Krol A, Paller AS, Schwarzenberger K, Bergman JN, Chamlin SL, Cohen DE, Cooper KD, Cordoro KM, Davis DM, Feldman SR, Hanifin JM, Margolis DJ, Silverman RA, Simpson EL, Williams HC, Elmets CA, Block J, Harrod CG, Smith Begolka, Sidbury R. Guidelines of care for the management of AD: Section 2. J Am Acad Dermatol. 2014;71(1):116–132. doi: 10.1016/j.jaad.2014.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.