Abstract
The current observational, prospective study enrolled 65 patients with gout, diagnosed according to 2015 ACR/EULAR criteria [17], evaluated in Rheumatology Clinic, Emergency County Hospital Craiova, and 40 healthy subjects. This research aimed to determine the presence of subclinical carotid atherosclerosis, revealed by an increased intima media thickness and carotid plaques in gout patients, by US examination. Secondary, we aimed to search for the possible correlations displayed between the presence of subclinical carotid atherosclerosis and several disease variables. CCAIMT over 0.9mm was identified for 19 patients (29.23%), percentage statistically significant different compared to controls (7; 17.5%), p=0,0428. For 23 patients (35.38%) carotid plaques were present at US examination, more prevalent compared to controls (19; 29.23%), p=0.002. Using multivariate logistic regression, we pointed out that SUA (OR 2,103; p=0.0002), age (OR=1,051; p<0.001), disease duration (OR=1.740; p=0.0039) and LDLc (OR=1,003; p=0.0029) were independently associated to an increased IMT in patients with gout, similar results being obtained for carotid plaques. MSKUS was performed for all patients, with important results. The presence of deposits associated with an increased risk of a thick IMT; similar results were obtained for double contour sign, aggregates and tophi. A statistically significant risk was noticed for the presence of deposits (p=0.002). Regarding the presence of carotid atheroma plaques, a higher risk was associated to deposits identification, double contour sign, aggregates, tophi and PD signal. Our results sustain that carotid ultrasound is an easily accessible imagistic method that offers important predictors of atherosclerotic status
Keywords: Gout , subclinical atherosclerosis , carotid ultrasound
Introduction
Gout is a chronic articular pathology consequent to the deposition of monosodium urate crystals into and around the articular structures [1].
Hyperuricaemia and gout are known to be associated with a wide variety of severe comorbidities, such as cardiovascular (CVD) and renal disorders. Increased serum levels of uric acid (SUA) have shown to contribute to inflammation, oxidative stress and vascular disease, with subsequent atherosclerotic microvascular changes, cardiovascular and chronic kidney diseases [2, 3].
Atherosclerosis is the major pathologic cause that leads to cardiovascular disease (CVD), mentioning myocardial infarction (MI) and stroke, the main major cardiovascular events and mortality factors in general population [4, 5].
Subclinical carotid atherosclerosis, and notably atheroma plaques, associate an increased cardiovascular risk with future development of ischemic cerebral and coronary disease [6, 7, 8, 9].
The prevalence of carotid atherosclerosis in people with gout is reported between 29.1 to 48.9% [10, 11, 12, 13], increased compared to the non-gouty population.
Over the past years, the interplay between CVD risk and gout, employed by epidemiological and clinical studies, was stated to be etiologically established, with a causative strong association [11].
The relationship is multifaced and involves multistep interactions between inflammation, genetic input and oxidative stress, with incompletely revealed act of cardiovascular and renal components [11, 14].
Urate has the initial role, by activation of immune responses and gout flares. Regarding oxidative stress, urate seems to alter endothelial function and rises future atherosclerotic risk [14, 15].
Several research studies showed the interplay between hyperuricaemia and a several risk factors associated to atherosclerotic process, as the reduction in urate levels is proven to be related to improvement these risk factors [16].
However, it is not completely described the certain SUA level that can notably be considered out of risk for cardiovascular events and future researches involving newer urate-lowering agents are being awaited to improve therapeutic management for both gout and atherosclerosis.
The present study aimed to describe the type and prevalence of subclinical carotid atherosclerosis, revealed by an increased intima media thickness and carotid plaques in gout patients, by US examination. Secondary, we aimed to search for correlations between the presence of subclinical carotid atherosclerosis and several disease variables. Thus, the results can provide a possible multistep evaluation pattern of gout patients, in order to optimize the early detection of inherent cardiovascular risk and improve the management of these patients.
Materials and Methods
The current observational, prospective study enrolled 65 patients diagnosed with gout according to 2015 ACR/EULAR criteria [17], evaluated in Rheumatology Clinic, Emergency County Hospital Craiova, and 40 healthy subjects, with no abnormalities in recent clinical examination and no history of rheumatic conditions, with similar demographic characteristics. We collected demographic data, clinical and laboratory parameters from each patient.
The presence of MetS was established in agreement to the National Cholesterol Education Program (NCP) Adult Treatment Panel (ATP) III by the presence of three or more of the following conditions: increased waist circumference (>102cm in men or >88cm in women, modified with cutoff points for Asian population, which are the same cutting points used in the National Health Surveys of our country), hypertriglyceridemia (triglycerides >=150mg/dL), high-density lipoprotein cholesterol (HDL-C) (<40mg/dL in men or <50mg/dL in women), hypertension (blood pressure >=130/85mmHg), and hyperglycemia (fasting levels of serum glucose >=100mg/dL) [18].
Ultrasound examination was performed using an Esaote MyLab machine with a high frequency linear probe (10-18MHz). The two sonographers who carried out the vascular and musculoskeletal ultrasound examinations and were blinded of patients' clinical data.
Common carotid arteries were symmetrically evaluated for measurement of IMT and detection of atheroma plaques, according to the Mannheim consensus [19].
A value over 0.9mm defined an increased IMT.
Musculoskeletal ultrasound (MSKUS) examination was performed following the domains of elementary lesions of gout, according to OMERACT definitions [20].
Inflammatory changes were assessed by the presence of a local PD signal and were graded in a semiquantitative score as 0-3 [21].
We evaluated the wrists regions (radiocarpal and midcarpal), second metacarpophalangeal (MCPs) and first metatarsophalangeal (MTPs) joints, and triceps and patellar tendons and their entheses [22].
For statistical analysis we employed GraphPad Prism 5.5. Descriptive statistics are presented as frequencies (n) and percentages (%) for qualitative variables and as mean (standard deviation) for parametric or non-parametric quantitative variables, respectively. In order to compare groups, we used t-test. Logistic regression models were established for factors screening and a multivariate logistic regression model was developed using a forward-stepwise mode. The variables screened by a multivariate logistic regression model were combined with following determination of receiver operating characteristic (ROC) curve and areas under curve (AUC). p<0.005 was considered significant.
The research has received the approval of local Ethics Committee and all patients signed an informed consent, after receiving a standard form in which was specified the exclusive use of the results for research purposes.
Results
Demographic and clinical characteristics of the study group
The study group included 65 patients, 8 (12.30%) female and 57 (87.70%) males, with a mean age of 58.61+8.12 years. For controls, we registered a mean age of 55.3+6.21 years; most of the participants of the control group were men (37; 92.5%).
The characteristics of the study groups are described in Table 1.
Table 1.
General characteristics of the study groups
|
Gout (N=65) |
controls (N=40) |
p |
|
|
Age (years) mean; SD |
58.61; 8.12 |
55,3; 6.21 |
0.686 |
|
Disease duration (years) mean; SD |
5.21; 7.32 |
- |
- |
|
Gender Male (N; %) Female (N; %) |
57; 87.70 8; 12.30% |
37;92.5% 3; 7.5% |
0.788 |
|
BMI (Kg/m2sc) mean; SD |
26.2; 3.9 |
25.9; 4.1 |
0.072 |
|
Smoking (N; %) |
32; 49.23% |
18; 45% |
0.041 |
|
Hypertension (N; %) |
25; 38.45% |
11; 27.5% |
0.521 |
|
Blood glucose (mg/dl) |
123.75 |
101.65 |
0.432 |
|
Diabetes (N; %) |
15; 23.07% |
6;15% |
0.031 |
|
Metabolic syndrome (N; %) |
32; 49.23% |
10; 25% |
0.073 |
|
ALT (U/L) mean; SD |
35; 10.23 |
31; 8.75 |
0.854 |
|
AST (U/L) mean; SD |
33; 7.85 |
31; 8.91 |
0.756 |
|
GGT (mg/dl) mean; SD |
46.75; 71.23 |
41.32; 61.32 |
0.852 |
|
FAL (mg/dl) mean; sd |
98.8; 56.21 |
91.23; 32.21 |
0.853 |
|
Creatinine (mg/dl) mean; SD |
1.21; 2.23 |
1.09; 1.01 |
0.041 |
|
SUA (mg/dl) mean; SD |
4.21; 3.22 |
4.41; 2.23 |
0.043 |
|
TC (mg/dl) mean; SD |
264. 0; 48.30 |
244. 2; 55.12 |
0.055 |
|
LDLc (mg/dl) mean; SD |
191.1; 43.10 |
172. 1; 55.99 |
0.053 |
|
HDLc (mg/dl) mean; SD |
47.51; 47.51 |
43.21; 39.21 |
0.076 |
|
TG (mg/dl) mean; SD |
151.45; 43.22 |
143.32; 45.32 |
0.071 |
|
ESR (mm/h) mean; SD |
11.02; 19.12 |
8.04; 11.23 |
0.044 |
|
CRP (mg/dl) mean; SD |
8.9; 11.27 |
3.6; 3.21 |
0.021 |
BMI: body mass index; ALT: alanine aminotransferase; AST: aspartate aminotransferase; GGT: gamma-glutamyl-transferase; ALP: alkaline phosphatase; TG: triglycerides; TC: total cholesterol; HDLC: high-density lipoprotein cholesterol; LDLC: low-density lipoprotein cholesterol; CRP: C-reactive protein; ESR: erythrocyte sedimentation rate.
Comparison of IMT and carotid plaque between patients with gout and controls
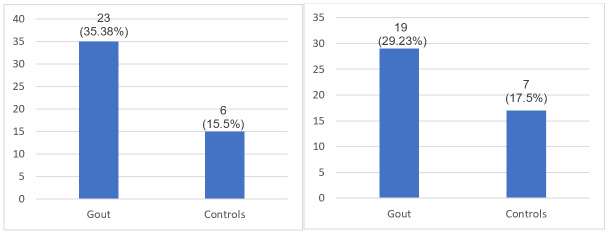
CCAIMT over 0.9mm was identified for 19 patients (29.23%), percentage statistically significant different compared to controls (7; 17.5%), p=0,0428 (Figure 1A).
Figure 1.
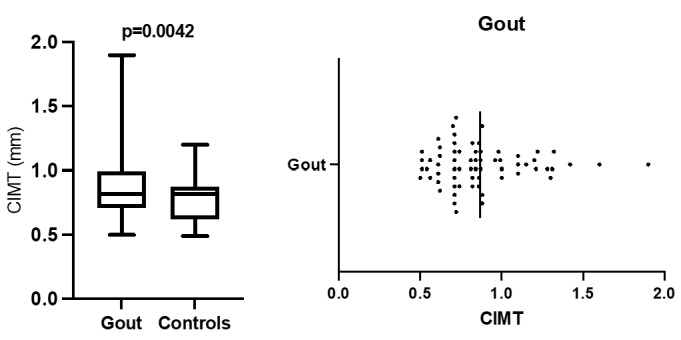
A.Comparison of mean IMT for gout and controls; B. IMT distribution in gout patients
Mean value registered for gout was 0,8663 mm (limits 0.5-1.9mm) (Figure 1B) as for controls 0,7763 mm (0.49-1.2mm).
For 23 patients (35.38%) carotid plaques were present at US examination, more prevalent compared to controls (19; 29.23%), p=0.002 (Figure 2A).
Figure 2.
A. Carotid plaques distribution for gout and controls. B. IMT>0.9mm for gout and controls.
Factors related to an increased IMT and carotid plaques in patients with gout
Analyzing the possible input of certain variables on the presence of an increased IMT, we reckoned an OR of 1.218 (CI 0,219-6,547) for CRP, 1.050 for ESR (CI 0.319-5.217), 1.83 for the presence of HTA (CI 0.217-4.371) and 3.38 for metabolic syndrome (CI 0.371-6.512), p=0.0243). We didn't obtain any association between SUA and augmented risk for an increased IMT in our study group.
Multivariate logistic regression pointed out that SUA (OR 2.103; p=0.0002), age (OR=1.051; p<0.001), disease duration (OR=1.740; p=0.0039) and LDLc (OR=1,003; p=0.0029) were independently associated to an increased IMT in patients with gout (Table 2).
Table 2.
Multivariate logistic regression for IMT>0.9mm
|
OR |
95% CI |
p |
|
|
Age (years) |
1.051 |
0,9582-1,157 |
<0.001 |
|
Disease duration (years) |
1.7401 |
0,3936-1,342 |
0.0391 |
|
SUA (mg/dl) |
2.103 |
1,424-3,435 |
0.0002 |
|
LDLc (mg/dl) |
1.003 |
0,9889-1,017 |
0. 0029 |
The association of these independent factors (age, disease duration, SUA and LDLc) was able to perform the differentiation between gout patients with an increased IMT from those with a normal value (AUC 0.823; 95% CI 0.7068-0.9410; p<0.0001) (Figure 3A).
Figure 3.
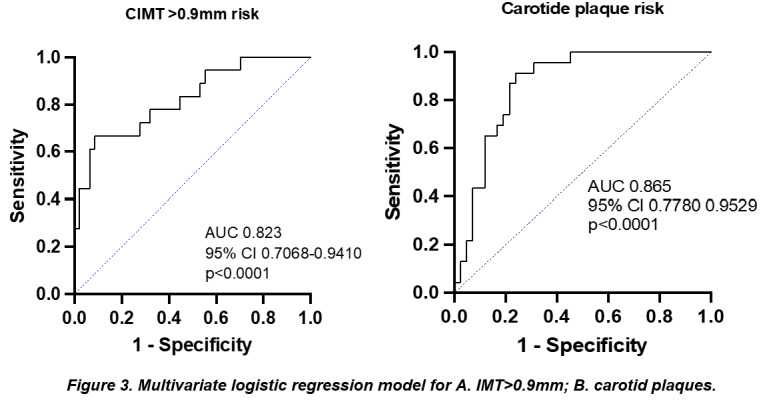
Multivariate logistic regression model for A. IMT>0.9mm; B. carotid plaques
In regards to the presence of carotid plaques, our statistical analysis indicated that hypertension (OR 2.25), diabetes mellitus (OR 2.25), ESR (OR 1.21) and blood glucose (OR 1.71) were associated to this finding. In contrast, SUA and BMI showed an association with a lower risk for the presence of carotid atheroma plaques.
Multivariate logistic regression showed that SUA (OR 2.013; CI 1.424-3.435), age (or 1.051; CI 0.9582-1.157), TG (OR 1.003; 0.9889-1.017) and the presence of tophi (OR 0.7401; CI 0.393-1.342) were independently associated to the presence of carotid plaques (Table 3).
Table 3.
Multivariate logistic regression for carotid plaques
|
OR |
95% CI |
p |
|
|
Age (years) |
1.051 |
0.9582-1.157 |
<0.001 |
|
Tophi |
0.7401 |
0.3936-1.342 |
0.0391 |
|
SUA (mg/dl) |
2.103 |
1.424-3.435 |
0.0002 |
|
TG (mg/dl) |
1.003 |
0.9889-1.017 |
0. 0029 |
The combination of the aforementioned factors was an important discriminator for the presence of atheroma carotid plaques (AUC 0.865; 95% CI 0.7780-0.9529; p<0.0001) (Figure 3B).
Musculoskeletal ultrasound (MSKUS) characteristics
We performed ultrasound examination to all patients, following OMERACT definitions. The presence of crystal deposits was revealed for all patients, with a mean sum of locations of 3.646 for deposits (limits 0-10), 1.477 for double contour sign (limits 0-5), 2.8 for aggregates (limits 0-10), 3.50 for tophi (limits 0-11) and 0.907 for PD signal (limits 0-5).
The presence of deposits related to an increased risk of a thick IMT (calculated OR. 1.061; 95% CI 0.95-1.27); similar results were obtained for double contour sign (OR 1.112; 95% CI 0.78-1.71), aggregates (OR 1.051; 95% CI 0.9-1.21) and tophi (OR 1.214; 95% CI 1.05-1.64. A statistically significant risk was noticed for the presence of deposits (p=0.002).
Regarding the presence of carotid atheroma plaques, a higher risk was associated to deposits identification (OR 2.112; 95% CI 0.91-2.78), double contour sign (OR 1.1; 95% CI 0.59-1.61), aggregates (OR 1.02; 95% CI 0.88-1.19), tophi (OR 1.740; 95% CI 1.05-1.64) and PD signal (OR 1.57; 95% CI 1.09-2.41). Statistically significant results must be mentioned for tophi (p=0.002) and PD signal (p=0.001) (Table 4).
Table 4.
Comparison of MSKUS characteristics for patients with IMT>0.9mm and carotid plaques
|
IMT>0.9mm (19 patients) |
Atheroma plaques (23 patients) |
|||||
|
Mean (SD) |
Range |
OR (95% CI) |
p |
OR (95% CI) |
p |
|
|
Deposits |
3,646 (2,786) |
0-10 |
1.061 (0.95-1.27) |
0.002 |
2.112 (0.91-2.78) |
0.723 |
|
Double contour sign |
1.477 (1.511) |
0-5 |
1.112 (0.78-1.71) |
0.821 |
1.1 (0.59-1.61) |
0.621 |
|
Aggregates |
2,800 (2,711) |
0-10 |
1.051 (0.9-1.21) |
0.071 |
1.02 (0.88-1.19) |
0.021 |
|
Tophi |
3,508 (3,098) |
0-11 |
1.214 (0.99-1.46) |
0.082 |
1,740 (1.05-1.64) |
0.002 |
|
PD signal >1 |
0,9077 (1,378) |
0-5 |
0.756 (0.46-1.25) |
0.281 |
1.57 (1.09-2.41) |
0.001 |
Discussion
Gout represents a complex pathology, unraveled with each investigation, with potential severity consequent not only from its continuous destructive evolution, but also via its several comorbidities that share a common pathogenesis [23, 24, 25].
The complex orchestration between gout and CVD has been extensively studied and researches have focused on the synergic effect of both disease related and demographic variables of gout, with fundamental results that enable a proper evaluation and prompt prevention of future cardiovascular events [25, 26, 27, 28, 29].
Cutting edge studies revealed that the percentage of an increased IMT varies from 18% to 33% [30, 31]. Our results showed a prevalence of 29.23%, percentage statistically significant different compared to controls, p=0,0428. Statistical analysis also noticed the presence of carotid plaques for 23 patients (35.38%) at US examination, more prevalent compared to controls (19; 29.23%), p=0.002.
In contrast to our findings, the study of Calaburg et al. reported a higher percentage (59.2%) [30], similar to Hammer et al. in 2021 (54.9%) [31]; similar results (34.6%) were published in 2024 by Dang et al. [32] and in 2017 by Gancheva et al. [33].
Our data also pointed out a significant difference between patients and controls, underlining the effect of systemic inflammation and associated comorbidities on atherosclerosis pathways and the occurrence of both carotid plaques and an increased IMT.
Analyzing the possible input of certain variables on the presence of an increased IMT, we reckoned an increased risk associated to high levels of inflammatory markers, hypertension 3.38 for MetS. The serum level of UA was not related to a higher risk of an increased IMT in our study group. Multivariate logistic regression pointed out that SUA, age, disease duration and LDLc constitute independently factors associated to an increased IMT in patients with gout. The association of these independent factors (age, disease duration, SUA and LDLc) was able to perform the differentiation between gout patients with an increased IMT from those with a normal value.
In regards to the presence of carotid plaques, our statistical analysis indicated that hypertension, diabetes mellitus, ESR and blood glucose were associated to this finding. In contrast, SUA and BMI showed an association with a lower risk for the presence of carotid atheroma plaques. Multivariate logistic regression showed that SUA (OR, age, disease duration, TG were independent variables related to the presence of carotid plaques. The combination of the aforementioned factors was an important discriminator for the presence of atheroma carotid plaques in gout. Similar observations were reported in literature, by Dang et al. in 2024 [32] or Friedlander et al. in 2017 [34].
MetS, a cluster of metabolic and cardiovascular risk factors is found with variable percentages among patients with gout, as well as in autoimmune rheumatic diseases [36].
Metabolic alterations represent another start point for systemic inflammation state, with an altered pattern of adipokines secretion, that inputs an additional risk of cardiovascular disease and conducts the appearance of future morbidity and mortality rates [36].
Pivotal research unfolded the connection between cardiovascular diseases and gout, via its both inflammatory and metabolic components, with hyperuricemia as a promoter of a continuous inflammatory status, hypertension and inherent cardiovascular pathology, lipid and glucose metabolism disorders [35].
As SUA was not proven to be directly related to either an increased IMT or the presence of atheroma carotid plaques in our cohort, the proper therapeutic management of hyperuricemia is certainly an indicator of this finding. Other published reports are inconsistent regarding the direct association of SUA with carotid atherosclerosis, with registered results indicating an association by Cicero et al. [37], or, in contrast, no association reported in 2020 by Drivelegka et al. [38] or Dang et al. [32].
However, as the studies enrolled patients using hyperuricemia as a criterion, and not the diagnosis of gout, the divergent results might be biased by lack of certain metabolic comorbidities and risk factors. Hyperuricemia is closely related to CVD risk, without being completely elucidated its only input on the traditional risk factors or a causative role of itself.
MSKUS evaluation is at utmost importance for clinical practice and offers the advantage of being an easily reproducible cost-effective method, with valuable findings both for diagnostic purposes as for therapeutic response follow up [39].
Ultrasound was performed for all of our patients, revealing certain important data. A point of our research focused on identifying the MSKUS parameters associated to the presence of subclinical carotid atherosclerosis. The presence of deposits was related to an increased risk of a thick IMT, with a notable risk (p=0.002) and similar results were obtained for double contour sign, aggregates and tophi. Regarding the presence of carotid atheroma plaque, a higher risk was associated to deposits identification, double contour sign, aggregates, tophi and PD signal. Statistically significant results must be mentioned for tophi (p=0.002) and PD signal (p=0.001), findings that sustain the impact of disease burden and inflammatory status on cardiovascular risk and future outcome. Literature research showed semblable reports [30, 40].
The obtained results sustain a certain relationship between the presence of crystals revealed by US and subclinical carotid atherosclerosis, findings advocated by current state of art regarding atherosclerosis. It is difficult to state a certain interdependence between the variables, as larger cohorts and multiple follow-up evaluations are required in order to validate the results.
Conclusion
The interplay between inflammatory status and metabolic disturbances found in gout patients inputs a certain caution and early detection of associated pathologies, with focus on cardiovascular ones, associated with major cardiovascular events.
Carotid ultrasound is an easily accessible imagistic method that offers important predictors of atherosclerotic status.
Ultrasonographic detection of urate crystals along with inflammatory changes, indicated by the presence of PD signal and their relationship to carotid ultrasound features underline the occurrence of subclinical atherosclerosis and sustain the close connectiveness between gout and future cardiovascular events.
Although a remarkable upturn has been made in preventing and therapeutic approach of gout, the early and prompt identification of comorbidities, especially cardiovascular ones, remains challenging and future research remains imperative.
Conflict of interests
The authors have no conflict of interest to declare.
References
- 1.Punzi L, Galozzi P, Luisetto R, Scanu A, Ramonda R, Oliviero F. Gout: one year in review 2023. Clin Exp Rheumatol. 2024;42(1):1–9. doi: 10.55563/clinexprheumatol/uhyzcr. [DOI] [PubMed] [Google Scholar]
- 2.Safiri S, Kolahi AA, Cross M, Carson-Chahhoud K, Hoy D, Almasi-Hashiani A, Sepidarkish M, Ashrafi-Asgarabad A, Moradi-Lakeh M, Mansournia MA, Kaufman JS, Collins G, Woolf AD, March L, Smith E. Prevalence, incidence, and years lived with disability due to gout and its attribuTable risk factors for 195 countries and territories 1990- 2017: a systematic analysis of the Global Burden of Disease Study 2017. Arthritis Rheumatol. 2020;72(11):1916–1927. doi: 10.1002/art.41404. [DOI] [PubMed] [Google Scholar]
- 3.Scanu A, Luisetto R, Ramonda R, Spinella P, Sfriso P, Galozzi P, Oliviero F. Anti-inflammatory and hypouricemic effect of bioactive compounds: molecular evidence and potential application in the management of gout. CIMB. 2022;44(11):5173–90. doi: 10.3390/cimb44110352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jebari-Benslaiman S, Larrea-Sebal A, Benito-Vicente A, Martín C. Cardiovascular Disease, Atherosclerosis and Familial Hypercholesterolemia: From Molecular Mechanisms Causing Pathogenicity to New Therapeutic Approaches. Int J Mol Sci. 2023;24(8):7659–7659. doi: 10.3390/ijms24087659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lechner K, von Schacky, McKenzie AL, Worm N, Nixdorff U, Lechner B, Kränkel N, Halle M, Krauss RM, Scherr J. Lifestyle factors and high-risk atherosclerosis: Pathways and mechanisms beyond traditional risk factors. Eur J Prev Cardiol. 2020;27(4):394–406. doi: 10.1177/2047487319869400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sumpter NA, Takei R, Cadzow M, Topless RKG, Phipps-Green AJ, Murphy R, de Zoysa, Watson H, Qasim M, Lupi AS, Abhishek A, Andrés M, Crișan TO, Doherty M, Jacobsson L, Janssen M, Jansen TL, Joosten LAB, Kapetanovic M, Lioté F, Matsuo H, McCarthy GM, Perez-Ruiz F, Riches P, Richette P, Roddy E, Stiburkova B, So A, Tausche AK, Torres RJ, Uhlig T, Major TJ, Stamp LK, Dalbeth N, Choi HK, Vazquez AI, Leask MP, Reynolds RJ, Merriman TR. Association of gout polygenic risk score with age at disease onset and tophaceous disease in European and Polynesian men with gout. Arthritis Rheumatol. 2023;75(5):816–825. doi: 10.1002/art.42393. [DOI] [PubMed] [Google Scholar]
- 7.Sandoval-Plata G, Morgan K, Abhishek A. Variants in urate transporters, ADH1B, GCKR and MEPE genes associate with transition from asymptomatic hyperuricaemia to gout: results of the first gout versus asymptomatic hyperuricaemia GWAS in Caucasians using data from the UK Biobank. Ann Rheum Dis. 2021;80(9):1220–1226. doi: 10.1136/annrheumdis-2020-219796. [DOI] [PubMed] [Google Scholar]
- 8.Namba S, Konuma T, Wu KH, Zhou W. Global Biobank Meta-analysis Initiative. A practical guideline of genomics-driven drug discovery in the era of global biobank meta-analysis. Cell Genom. 2022;2(10):100190–100190. doi: 10.1016/j.xgen.2022.100190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu H, Xie R, Dai Q, Fang J, XU Y, LI B. Exploring the mechanism underlying hyperuricemia using comprehensive research on multi-omics. Sci Rep. 2023;13:7161–7161. doi: 10.1038/s41598-023-34426-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.ukurova S, Pamuk ÖN, Ünlü E, Pamuk GE, Çakir N. Subclinical atherosclerosis in gouty arthritis patients: a comparative study. Rheumatol Int. 2012;32(6):1769–1773. doi: 10.1007/s00296-011-1900-4. [DOI] [PubMed] [Google Scholar]
- 11.Friedlander AH, Graves LL, Grabich SG, Aghazadehsanai N, Chang TI. Prevalence of calcified carotid artery atheromas on panoramic images of older men with gout: a descriptive retrospective study. Dentomaxillofac Radiol. 2017;46(5):20160406–20160406. doi: 10.1259/dmfr.20160406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gancheva R, Kundurdjiev A, Ivanova M, Kundurzhiev T, Kolarov Z. Evaluation of cardiovascular risk in stages of gout by a complex multimodal ultrasonography. Rheumatol Int. 2017;37(1):121–130. doi: 10.1007/s00296-016-3556-6. [DOI] [PubMed] [Google Scholar]
- 13.Andrés M, Bernal JA, Sivera F, Quilis N, Carmona L, Vela P, Pascual E. Cardiovascular risk of patients with gout seen at rheumatology clinics following a structured assessment. Ann Rheum Dis. 2017;76(7):1263–1268. doi: 10.1136/annrheumdis-2016-210357. [DOI] [PubMed] [Google Scholar]
- 14.Krishnan E. Inflammation, oxidative stress and lipids: the risk triad for atherosclerosis in gout. Rheumatology (Oxford) 2010;49(7):1229–1238. doi: 10.1093/rheumatology/keq037. [DOI] [PubMed] [Google Scholar]
- 15.Jayachandran M, Qu S. Harnessing hyperuricemia to atherosclerosis and understanding its mechanistic dependence. Med Res Rev. 2021;41(1):616–629. doi: 10.1002/med.21742. [DOI] [PubMed] [Google Scholar]
- 16.Kimura Y, Tsukui D, Kono H. Uric Acid in Inflammation and the Pathogenesis of Atherosclerosis. Int J Mol Sci. 2021;17(22):12394–12394. doi: 10.3390/ijms222212394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Neogi T, Jansen TL, Dalbeth N, Fransen J, Schumacher HR, Berendsen D, Brown M, Choi H, Edwards NL, Janssens HJ, Lioté F, Naden RP, Nuki G, Ogdie A, Perez-Ruiz F, Saag K, Singh JA, Sundy JS, Tausche AK, Vazquez-Mellado J, Yarows SA, Taylor WJ. Gout Classification Criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheumatol. 2015;67(10):2557–2568. doi: 10.1002/art.39254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.National Cholesterol, Evaluation undefined, and Treatment. Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106(25):3143–3421. [PubMed] [Google Scholar]
- 19.Touboul PJ, Hennerici MG, Meairs S, Adams H, Amarenco P, Bornstein N, Csiba L, Desvarieux M, Ebrahim S, Hernandez Hernandez, Jaff M, Kownator S, Naqvi T, Prati P, Rundek T, Sitzer M, Schminke U, Tardif JC, Taylor A, Vicaut E, Woo KS. Mannheim carotid intima-media thickness and plaque consensus (2004-2006-2011). An update on behalf of the advisory board of the 3rd, 4th and 5th watching the risk symposia, at the 13th, 15th and 20th European Stroke Conferences, Mannheim, Germany, 2004, Brussels, Belgium, 2006, and Hamburg, Germany, 2011. Cerebrovasc Dis. 2012;34(4):290–296. doi: 10.1159/000343145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Terslev L, Gutierrez M, Schmidt WA, Keen HI, Filippucci E, Kane D, Thiele R, Kaeley G, Balint P, Mandl P, Delle Sedie, Hammer HB, Christensen R, Möller I, Pineda C, Kissin E, Bruyn GA, Iagnocco A, Naredo E, D'Agostino MA. Ultrasound as an outcome measure in gout. A validation process by the OMERACT ultrasound working group. J Rheumatol. 2015;42(11):2177–2181. doi: 10.3899/jrheum.141294. [DOI] [PubMed] [Google Scholar]
- 21.Dougados M, Jousse-Joulin S, Mistretta F, d'Agostino MA, Backhaus M, Bentin J, Chalès G, Chary-Valckenaere I, Conaghan P, Etchepare F, Gaudin P, Grassi W, van der, Sellam J, Naredo E, Szkudlarek M, Wakefield R, Saraux A. Evaluation of several ultrasonography scoring systems for synovitis and comparison to clinical examination: results from a prospective multicentre study of rheumatoid arthritis. Ann Rheum Dis. 2010;69(5):828–833. doi: 10.1136/ard.2009.115493. [DOI] [PubMed] [Google Scholar]
- 22.Naredo E, Uson J, Jiménez-Palop M, Martínez A, Vicente E, Brito E, Rodríguez A, Cornejo FJ, Castañeda S, Martínez MJ, Sanz J, Möller I, Batlle-Gualda E, Garrido J, Pascual E. Ultrasound-detected musculoskeletal urate crystal deposition: which joints and what findings should be assessed for diagnosing gout. Ann Rheum Dis. 2014;73(8):1522–1528. doi: 10.1136/annrheumdis-2013-203487. [DOI] [PubMed] [Google Scholar]
- 23.Singh JA, Gaffo A. Gout epidemiology and comorbidities. Semin Arthritis Rheum. 2020;50(3S):S11–S16. doi: 10.1016/j.semarthrit.2020.04.008. [DOI] [PubMed] [Google Scholar]
- 24.Choi HK, McCormick N, Yokose C. Excess comorbidities in gout: the causal paradigm and pleiotropic approaches to care. Nat Rev Rheumatol. 2022;18(2):97–111. doi: 10.1038/s41584-021-00725-9. [DOI] [PubMed] [Google Scholar]
- 25.Dehlin M, Jacobsson L, Roddy E. Global epidemiology of gout: prevalence, incidence, treatment patterns and risk factors. Nat Rev Rheumatol. 2020;16(7):380–390. doi: 10.1038/s41584-020-0441-1. [DOI] [PubMed] [Google Scholar]
- 26.Cipolletta E, Tata LJ, Nakafero G, Avery AJ, Mamas MA, Abhishek A. Association Between Gout Flare and Subsequent Cardiovascular Events Among Patients with Gout. JAMA. 2022;328(5):440–450. doi: 10.1001/jama.2022.11390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saito Y, Tanaka A, Node K, Kobayashi Y. Uric acid and cardiovascular disease: A clinical review. J Cardiol. 2021;78(1):51–57. doi: 10.1016/j.jjcc.2020.12.013. [DOI] [PubMed] [Google Scholar]
- 28.Khituova L, Khabizhanova V, Musayev A, Akhmetova G, Almukhambetova E, Indershiyev V. Risk Factors of Cardiovascular Pathology in Patients with Gout. Curr Rheumatol Rev. 2023;19(1):72–75. doi: 10.2174/1573397118666220802141420. [DOI] [PubMed] [Google Scholar]
- 29.Drosos GC, Vedder D, Houben E, Boekel L, Atzeni F, Badreh S, Boumpas DT, Brodin N, Bruce IN, González-Gay MÁ, Jacobsen S, Kerekes G, Marchiori F, Mukhtyar C, Ramos-Casals M, Sattar N, Schreiber K, Sciascia S, Svenungsson E, Szekanecz Z, Tausche AK, Tyndall A, van Halm, Voskuyl A, Macfarlane GJ, Ward MM, Nurmohamed MT, Tektonidou MG. EULAR recommendations for cardiovascular risk management in rheumatic and musculoskeletal diseases, including systemic lupus erythematosus and antiphospholipid syndrome. Ann Rheum Dis. 2022;81(6):768–779. doi: 10.1136/annrheumdis-2021-221733. [DOI] [PubMed] [Google Scholar]
- 30.Calabuig I, Martínez-Sanchis A, Andrés M. Sonographic Tophi and Inflammation Are Associated with Carotid Atheroma Plaques in Gout. Front Med (Lausanne) 2021;8:795984–795984. doi: 10.3389/fmed.2021.795984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hammer HB, Rollefstad S, Semb AG, Jensen G, Karoliussen LF, Terslev L, Haavardsholm EA, Kvien TK, Uhlig T. Urate crystal deposition is associated with inflammatory markers and carotid artery pathology in patients with intercritical gout: results from the NOR-Gout study. RMD Open. 2022;8(2):1–1. doi: 10.1136/rmdopen-2022-002348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dang W, Hu J, Luo H, Luo D, Xu X, Liu J. The prevalence and independent risk factors of elevated common carotid artery intima-media thickness and carotid plaque in patients with gout. Clin Exp Rheumatol. 2024;42(1):138–144. doi: 10.55563/clinexprheumatol/v1f5yk. [DOI] [PubMed] [Google Scholar]
- 33.Gancheva R, Kundurdjiev A, Ivanova M, Kundurzhiev T, Kolarov Z. Evaluation of cardiovascular risk in stages of gout by a complex multimodal ultrasonography. Rheumatol Int. 2017;37(1):121–130. doi: 10.1007/s00296-016-3556-6. [DOI] [PubMed] [Google Scholar]
- 34.Friedlander AH, Graves LL, Grabich SG, Aghazadehsanai N, Chang TI. Prevalence of calcified carotid artery atheromas on panoramic images of older men with gout: a descriptive retrospective study. Dentomaxillofac Radiol. 2017;46(5):20160406–20160406. doi: 10.1259/dmfr.20160406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Medina G, Vera-Lastra O, Peralta-Amaro AL, Jiménez-Arellano MP, Saavedra MA, Cruz-Domínguez MP, Jara LJ. Metabolic syndrome, autoimmunity and rheumatic diseases. Pharmacol Res. 2018;133:277–288. doi: 10.1016/j.phrs.2018.01.009. [DOI] [PubMed] [Google Scholar]
- 36.Thottam GE, Krasnokutsky S, Pillinger MH. Gout and Metabolic Syndrome: a Tangled Web. Curr Rheumatol Rep. 2017;19(10):60–60. doi: 10.1007/s11926-017-0688-y. [DOI] [PubMed] [Google Scholar]
- 37.Cicero AF, Salvi P, D'Addato S, Rosticci M, Borghi C; Association between serum uric acid, hypertension, vascular stiffness and subclinical atherosclerosis: data from the Brisighella Heart Study. J Hypertens. 2014;32(1):57–64. doi: 10.1097/HJH.0b013e328365b916. [DOI] [PubMed] [Google Scholar]
- 38.Drivelegka P, Forsblad-d'Elia H, Angerås O, Bergström G, Schmidt C, Jacobsson LTH, Dehlin M. Association between serum level of urate and subclinical atherosclerosis: results from the SCAPIS Pilot. Arthritis Res Ther. 2020;22(1):37–37. doi: 10.1186/s13075-020-2119-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yuan H, Fan Y, Mou X, Qing Y, Yan X, Tang X, Yue W, Gu P, Yang Q, He Y. Musculoskeletal Ultrasound in Monitoring the Efficacy of Gout: A Prospective Study Based on Tophus and Double Contour Sign. Balkan Med J. 2023;40(2):104–110. doi: 10.4274/balkanmedj.galenos.2022.2022-7-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Marty-Ané A, Norberciak L, Andrès M, Houvenagel E, Ducoulombier V, Legrand J, Budzik JF, Pascart T. Crystal deposition measured with dual-energy computed tomography: association with mortality and cardiovascular risks in gout. Rheumatology (Oxford) 2021;60(10):4855–4860. doi: 10.1093/rheumatology/keaa920. [DOI] [PubMed] [Google Scholar]