Abstract
Woodchuck hepatitis virus (WHV) and hepatitis B virus (HBV) are closely similar with respect to genomic organization, host antiviral responses, and pathobiology of the infection. T-cell immunity against viral nucleocapsid (HBcAg or WHcAg) has been shown to play a critical role in viral clearance and protection against infection. Here we show that vaccination of healthy woodchucks by gene gun bombardment with a plasmid coding for WHcAg (pCw) stimulates proliferation of WHcAg-specific T cells but that these cells do not produce significant levels of gamma interferon (IFN-γ) upon antigen stimulation. In addition, animals vaccinated with pCw alone were not protected against WHV inoculation. In order to induce a Th1 cytokine response, another group of woodchucks was immunized with pCw together with another plasmid coding for woodchuck interleukin-12 (IL-12). These animals exhibited WHcAg-specific T-cell proliferation with high IFN-γ production and were protected against challenge with WHV, showing no viremia or low-level transient viremia after WHV inoculation. In conclusion, gene gun immunization with WHV core generates a non-Th1 type of response which does not protect against experimental infection. However, steering the immune response to a Th1 cytokine profile by IL-12 coadministration achieves protective immunity. These data demonstrate a crucial role of Th1 responses in the control of hepadnavirus replication and suggest new approaches to inducing protection against HBV infection.
Hepatitis B virus (HBV) is a partially double-stranded DNA virus encoding envelope (pre-S1, pre-S2, HBsAg), nucleocapsid (HBcAg, HBeAg), trans-activating (X), and polymerase (Pol) proteins (46). Clinical manifestations of HBV infection range from acute and chronic hepatitis to cirrhosis and hepatocellular carcinoma in humans (6). There are more than 350 million chronic HBV carriers worldwide (54), and HBV is an important cause of morbidity and mortality in the Third World.
Several reports have shown that the immune response plays a significant role in both HBV clearance and pathogenesis of hepatitis B (13). A vigorous polyclonal and multispecific cellular response to HBV has been associated with recovery from acute infection. Cytotoxic T lymphocytes (CTLs) and T-helper (Th) cells with specificity for HBcAg and most HBV proteins can be detected in the circulation during acute infection (22, 34, 37). In contrast, defective CTL and Th cell responses are observed in chronic HBV carriers, except during exacerbation (48). As has been reported for several viral infections, a preferential induction of a Th1 type T-helper response with production of gamma interferon (IFN-γ) may be crucial in the control of HBV infection (2). Interleukin 12 (IL-12) is a heterodimeric molecule, produced by activated antigen-presenting cells (APCs), which plays a key role in the activation of Th1 responses. IL-12 promotes the maturation of Th1 cells from an uncommitted T-cell pool and stimulates the production of IFN-γ by Th1 cells, CTLs, and NK cells (47).
The woodchuck (Marmota monax) is readily infected with woodchuck hepatitis virus (WHV), providing a valuable animal model in which to study the pathogenesis, prevention, and treatment of HBV infection (7, 38, 45). The genomic organization and replicative cycle of WHV and the proteins encoded by WHV DNA are very similar to those of HBV (12, 14). Like HBV, WHV induces acute self-limited hepatitis in adult animals in most cases, while perinatal infection may cause chronic hepatitis which may evolve to hepatocellular carcinoma (35). Because of similarities between HBV and WHV infections (49), the woodchuck model is a useful system for the study of prophylactic and therapeutic vaccines (19, 28, 39, 40).
The current HBV vaccine contains HBs protein and confers protection via anti-HBs antibodies (18, 23). However, approximately 5 to 10% of vaccinees do not develop humoral immunity (5, 24), and, on the other hand, HBsAg escape viral mutants may occur in infected patients (4, 5). Moreover, the HBs vaccine is of little efficacy in controlling established chronic HBV infection (10; Y. M. Wen, X. H. Wu, D. C. Hu, Q. P. Zhang, and S. Q. Guo, Letter, Lancet 345:1575–1576, 1995).
Immunization with HBcAg or WHV core antigen (WHcAg) can efficiently induce CTL responses and prevent infection in the chimpanzee and in woodchucks (21, 25, 28, 32, 39, 40). These viral protein antigens, although unable to induce neutralizing antibodies, may stimulate protective immunity by priming Th cells that support a specific CTL response, leading to early clearance of virus (29, 42).
Lu et al. (28) demonstrated that intramuscular injection of plasmids coding for WHV surface antigen (WHsAg) or WHcAg elicited immune responses which protected woodchucks against challenge with WHV. However, protective immunity was obtained after three immunizations with 1 mg of plasmid DNA (28). Gene gun technology has gained acceptance as a method for genetic vaccination using very low doses of immunogen. This method consists of a biolistic device that propels gold particles coated with plasmid DNA into the skin by means of pressurized helium. It allows efficient delivery of DNA to APCs of the skin, which after synthesis and processing present the antigen to major histocompatibility complex (MHC) class I and class II molecules (36, 43). This immunization system can elicit cellular and humoral immune responses which mimic those to live attenuated virus vaccines while avoiding some of the possible problems associated with live vaccines. Gene gun vaccination is a safe and inexpensive procedure which has been shown to be effective against various pathogens including HBV (11, 30, 50–53). In the present work, we have investigated the effects of gene gun immunization with WHcAg to elicit T-cell immunity. We found that although this vaccination induced proliferation of WHcAg-specific T cells, these T cells were unable to produce IFN-γ and animals did not show protection against WHV inoculation. However, gene gun immunization with WHcAg plus woodchuck IL-12 generated a Th1 immune response and efficient protective immunity.
MATERIALS AND METHODS
Animals.
Twelve uninfected woodchucks (purchased from Northeastern Wildlife, Ithaca, N.Y.), handled according to the guidelines of our institution (Centro de Investigación en Farmacobiología Aplicada [CIFA], Pamplona, Spain), were used. All animals were negative for WHsAg, WHV DNA, anti-WHV surface antibodies (anti-WHs) and anti-WHV core antibodies (anti-WHc). Blood was collected from the saphena vein of the hind legs under anesthesia.
Female BALB/c mice (10 to 20 weeks old) were purchased from Charles River (Barcelona, Spain) and housed according to our institution's guidelines.
Construction and characterization of expression vectors pCw and pIL12w.
A DNA fragment (nucleotides 2022 to 2586) coding for WHcAg was amplified by PCR from plasmid pBR325-WHV (16) using specific primers (Table 1). PCR was performed by following standard protocols. PCR products were purified by the Concert Rapid Gel Extraction System (Life Technologies, Gibco BRL, Eggestein-Leopoldshafen, Germany), cloned into the pcDNA3-T vector (Invitrogen, San Diego, Calif.), and sequenced with an ABI Prism Cycle Sequencing kit (Applied Biosystems, Foster City, Calif.), followed by automated capillary electrophoresis in an ABI Prism 310 Genetic Analyzer. The resulting plasmid was named pCw.
TABLE 1.
Primers used for construction of pCw and pIL12w, and primers and probes used for real-time quantitative PCR of cytokines and WHV-DNA
| Template DNA | Oligonucleotide sequences | Probe | Genome localization or GenBank accession no. |
|---|---|---|---|
| p35a | Forward, AAGCTT GCCACC ATG TGTCCATCAGC | AF288520 | |
| Reverse, AAGCTT CTA AGAAGAATTCAGATAGCTCATCACC | |||
| p40a | Forward, TTCATA CCATGG CCATGTGTCTTCAGCAGTTG | AF288519 | |
| Reverse, ACGCGT TCTAGA GAATTC CTAAGGGCAGGAC | |||
| WHcAgb | Forward, TTTTCAAGAAGCCTCCAAGCTGT | 1956–1976d | |
| Reverse, GTTTTATGTACCCATTGAAG | 2585–2605d | ||
| WHcAgc | Forward, AGAAGACGCACTCCCTCTCCT | AGAAGATCTCAATCACCGCGTCGCAG | 2501–2580d |
| Reverse, TGGCAGATGGAGATTGAGAGC | |||
| IFN-γc | Forward, CAGATGGCGGGTCTCTCCTTC | TGGATATTTTGGATAAATGGAAAGAGGA | 137–244e |
| Reverse, GACAACTTGGCTCTGGATTACTTTT | |||
| β-Actinc | Forward, TCACCCACACTGTGCCCATCTACGA | ATGCCCTCCCCCATGCCATCCTGCGT | 2141–2381e |
| Reverse, CAGCGGAACCGCTCATTGCCAATGG |
Expression of WHcAg was confirmed by a coupled transcription-translation reaction using the TNT T7 Quick Transcription/Translation System (Promega, Madison, Wis.) according to the manufacturer's instructions. Proteins were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and autoradiography, following standard protocols (1). A band corresponding to the putative molecular weight of WHcAg was obtained (data not shown).
Plasmid coding for woodchuck interleukin 12 (pIL12w) was prepared by reverse transcription (RT)-PCR amplification from 3 × 106 peripheral blood mononuclear cells (PBMC), which were stimulated with lipopolysaccharide (LPS) (20 ng/ml) at 37°C and 5% CO2 for 5 h. Primers for p35 and p40 cloning (Table 1) were designed according to previously reported sequences (GenBank accession number X97018 for p35 and X97019 for p40). RTs (1 h at 37°C and 5 min at 95°C) for p35 and p40 were performed in a final volume of 20 μl containing 1 μg of RNA and 500 nM reverse primer, 1 × RT buffer, 5.5 mM MgCl2, 2 mM deoxynucleoside triphosphates (dNTPs), 0.4 U of RNAse inhibitor/μl, and 1.25 U of murine leukemia virus (MuLV) reverse transcriptase (Applied Biosystems)/μl. The resulting cDNA was then amplified by following standard protocols (annealing temperatures, 48.7°C for p35 amplification and 58°C for p40). PCR-derived products, 691-bp (p35) and 1,026-bp (p40) fragments, were cloned into pCR2.1 TOPO vectors (Invitrogen) according to the manufacturer's guidelines. p40 was then removed by NcoI and XbaI digestion, and the fragment was cloned into a similarly digested pCITE-1 vector (Novagen, Madison, Wis.), a plasmid containing an internal ribosome entry site (IRES). An EcoRI fragment containing an IRES followed by p40 was then excised, blunt ended, and subcloned into pcDNA3 opened with EcoRV. The final plasmid, named pIL12w, was constructed by inserting (by blunt-end ligation) a HindIII fragment containing p35 into pcDNA3 IRESp40 linearized with BamHI.
pIL12w was characterized by sequencing and by a coupled transcription-translation reaction, using the TNT T7 Quick Transcription/Translation System (Promega) according to the manufacturer's instructions.
To determine the biological activity of pIL12w, we transfected 293 cells with pIL12w or pEGFP-N1 (Clontech, Palo Alto, Calif.), a plasmid coding for enhanced green fluorescent protein, using polyethylenimine (PEI; generously provided by J. P. Behr) as described elsewhere (3). Supernatants (500 μl/well) from the transfected cells were added to woodchuck PBMC (3 × 106 cells/well) extracted as previously described (19). After 24 h of culture, induction of IFN-γ mRNA was determined by quantitative PCR as described below.
Immunization protocol.
Plasmids pIL12w and pCw were used to prepare complexes of DNA with gold beads (diameter, 1.6 μm) as described elsewhere (20). Gene gun immunizations were delivered to shaved abdominal skin of mice or woodchucks using the Helios Gene Gun (Bio-Rad, Hercules, Calif.) at a helium discharge pressure of 400 to 450 lb/in2.
Female BALB/c mice were immunized with different doses of pCw. Each animal received two nonoverlapping shots with bullets containing 0.5 mg of gold particles at a concentration of 2 μg of DNA/mg of Au (group 1) or 4 μg of DNA/mg of Au (group 2) in the abdominal skin. Mice were immunized three times at 2-week intervals for a total dose of 6 μg (group 1) or 12 μg (group 2) of pCw.
Three groups of woodchucks were immunized using the Gene Gun with bullets containing 0.5 mg of gold particles at a concentration of 4 μg of DNA/mg of Au. The protocol consisted of three immunization sessions, separated by 2-week intervals, with four shots per session, on different areas of the abdominal skin. Group 1 woodchucks (WC1, WC2, WC3, and WC4) were immunized with pCw (8 μg per session). Group 2 woodchucks (WILC1, WILC2, WILC3, WILC4, and WILC5) were immunized sequentially with pCw and pIL12w (8 μg of each plasmid per session). To ensure colocalized expression of WHcAg and IL12w, every shot of pCw was followed by a shot of pIL12w delivered into exactly the same area of the skin. Group 3 was used as a control and consisted of two nonimmunized woodchucks (WN1 and WN2) and a woodchuck (WN3) that was subjected to the group 1 immunization protocol but received 8 μg of empty plasmid pcDNA3 in each immunization session.
Challenge with WHV.
Four weeks after completion of the vaccination protocol, immunized and non-immunized animals were challenged by subcutaneous injection of an inoculum containing 1010 WHV genomic particles. The inoculum was a serum sample obtained from a chronically infected woodchuck. The titer was calculated by dot blot hybridization (19), using a digoxigenin-labeled WHsAg probe obtained by PCR amplification using a commercially available labeling mix (Boehringer Mannheim, GmbH, Mannheim, Germany).
Immunological studies, determations of levels of viral DNA in serum, and identification of serological markers were carried out using blood samples obtained weekly during a 10-week follow-up period and also at week 24 postinfection.
Serology.
Enzyme-linked immunosorbent assay (ELISA) techniques were used to determine levels of anti-WHc, anti-WHs, and WHsAg in serum as previously reported (19, 39). WHsAg was isolated from a serum pool of a chronically infected woodchuck as previously reported (15, 19). Recombinant WHcAg was produced by expression of a plasmid containing the WHc gene kindly provided by M. Roggendorf (40). Antibody titers are given as the serum dilutions at which the absorbance value was twice the value of a negative control (a 1/10 dilution of a serum sample from an uninfected woodchuck).
Determination of WHV DNA levels.
Serum WHV DNA was quantified by real-time quantitative PCR. WHV DNA was isolated from 100 μl of serum using the High Pure Viral Nucleic Acid Kit (Boehringer Mannheim) according to the following manufacturer's instructions. Primers and a TaqMan probe (Applied Biosystems) for WHV core were designed using Primer-express software (Applied Biosystems) (Table 1). The reaction was performed in a 25-μl volume with 2 μl of sample, 1× Taqman buffer, 0.4 mM dNTPs, 0.01 U of Amperase/μl, 0.05 U of BioTaq DNA polymerase (Bioline, London, United Kingdom)/μl, MgCl2 at 5.5 mM, 300 nM each primer, and 100 nM probe. After PCR using the LightCycler system (Roche Diagnostics), the copy number of WHV DNA in each sample was determined by interpolation, using an external standard consisting of serial dilutions (108 to 103) of pCw.
IL-2 production bioassay.
PBMC from each woodchuck were isolated at different time points as previously described (19). Cells were added in triplicate to a 96-well flat-bottom plate (4 × 105 cells/well) and cultured at 37°C and 5% CO2 in complete culture medium (RPMI 1640, 10% fetal bovine serum, 100 U of penicillin/ml, 100 μg of streptomycin/ml, and 2 × 10−5 M β-mercaptoethanol) in the presence or absence of 10 μg of WHcAg/ml (recombinant WHcAg was obtained as mentioned above). Fifty microliters of culture supernatant was collected after 24 h of culture and tested for IL-2 content using the CTL-L bioassay as previously described (26). Results are expressed in terms of the stimulation index (SI), which is the ratio of the mean counts · min−1 incorporated in the presence of antigen to the mean counts · min−1 obtained in the absence of antigen.
Quantitation of IFN-γ mRNA in PBMC.
PBMC were cultured at 37°C and 5% CO2 in flat-bottom 24-well plates (3 × 106 cells/well) in complete medium containing 10 μg of WHcAg/ml. After 24 h of culture, total RNA was extracted using the Ultraspec-II system (Biotecx Laboratories. Houston, Tex.) following the manufacturer's instructions and finally diluted in 40 μl of RNase-free water. An RT reaction was performed in a final volume of 105 μl containing 840 ng of RNA and 1× RT buffer, 5.5 mM MgCl2, 2 mM dNTPs, 2.5 μM random hexamers, 0.4 U of RNAse inhibitor/μl, and 1.25 U of MuLV reverse transcriptase (Applied Biosystems)/μl. Parameters used were 10 min at 25°C, 30 min at 48°C, and 5 min at 95°C. Primers and Taqman probes (Applied Biosystems) for IFN-γ and β-actin were designed according to the published cDNA sequences of woodchuck genes (33) using Primer-Express software (Table 1). PCR was carried out in 25 μl with 12.5 μl of the RT reaction product (containing cDNA corresponding to 100 ng of the original RNA), 1× Taqman buffer, 0.4 mM dNTPs, 0.01 U of Amperase/μl, 0.05 U of BioTaq DNA polymerase (Bioline)/μl, 300 nM each primer, and 200 nM probe. PCR was performed according to the following parameters: 2 min at 50°C for Amperase digestion, initial denaturation for 15 at 94°C, and 45 cycles of 0.1 s at 95°C and hybridization/elongation for 20 s at 60°C. Fluorescent signals generated during PCR amplification were monitored using the LightCycler System (Roche Diagnostics). Numbers of copies of IFN-γ and β-actin cDNAs were determined by interpolation, using external RNA standards. The threshold for IFN-γ and β-actin detection was 100 copies. For preparation of the standard curves, IFN-γ and β-actin fragments were cloned into pCR2.1. TOPO (Invitrogen), containing a T7 promoter and in vitro transcribed with T7 RNA polymerase. The RNA concentration was estimated by optical density, and the copy number was calculated from the concentration, mean molecular weight of nucleotides (330 g/mol), and RNA length.
Statistical analysis.
Statistical analysis for anti-WHc, anti-WHs, and viremia was performed using the Kruskal-Wallis test and the Mann-Whitney U test. Differences were considered significant at a P value of <0.05. Pearson's χ2 was used in the analysis of IL-2 and IFN-γ production and was considered significant at a P value of <0.05.
RESULTS
Cloning and characterization of pIL12w.
RNA extracted from LPS-stimulated woodchuck PBMC was used to amplify the p35 and p40 subunits of the IL-12 gene. PCR primers derived from a previously reported sequence for the p35 and p40 subunits of woodchuck IL-12 (GenBank accession number X97018 for p35 and X97019 for p40) were used to obtain the corresponding PCR products. Alignment of the previously reported sequences with those obtained from our group (AF288520 for p35 and AF288519 for p40) revealed two amino acid changes for the p35 subunit and three for the p40 subunit. Plasmid pIL12w was constructed based on the sequences that we obtained with an IRES between p35 and p40 (see Materials and Methods). The protein encoded by pIL12w was characterized by in vitro transcription and translation, which revealed two fragments with sizes consistent with p40 and p35 subunits without glycosylation (data not shown).
The biological activity of the resulting protein was tested by the ability of supernatants from 293 cells, transfected with pIL12w or a control plasmid (pEGFP-N1), to induce the production of IFN-γ by woodchuck PBMC. The number of copies of IFN-γ mRNA per copy of β-actin mRNA in PBMC stimulated with supernatants from pIL12w-transfected cells was 46.36, while IFN-γ mRNA remained undetectable in PBMC incubated with supernatants from pEGFP-N1-transfected cells. These results indicate that pIL12w encodes biologically active woodchuck IL-12; to our knowledge, this is the first study in which functional woodchuck IL-12 gene has been used.
Induction of a specific immune response against WHV core by gene gun immunization.
In order to see whether gene gun bombardment with pCw was able to elicit an immune response against WHcAg, we vaccinated two groups of mice (n = 3) with three doses of pCw at 2-week intervals: the first group received 2 μg of pCw per immunization session, and the second received 4 μg per session. We found that both immunization protocols were effective at inducing anti-WHc antibodies; the titers in the two groups of animals were similar (data not shown).
After the ability of pCw to act as an immunogen was confirmed, woodchucks were vaccinated by gene gun bombardment of the skin with pCw alone or together with pIL12w. Animals WC1 to WC4 (group 1) received three vaccination sessions at 2-week intervals with pCw alone, and woodchucks WILC1 to WILC5 (group 2) were immunized in a similar way with pCw and pIL12w, the two plasmids being delivered at exactly the same points of the skin. A control group (group 3) of three woodchucks (WN1, WN2, and WN3) was also included. WN1 and WN2 were not immunized, and WN3 received empty plasmid pcDNA3 by the same protocol as group 1.
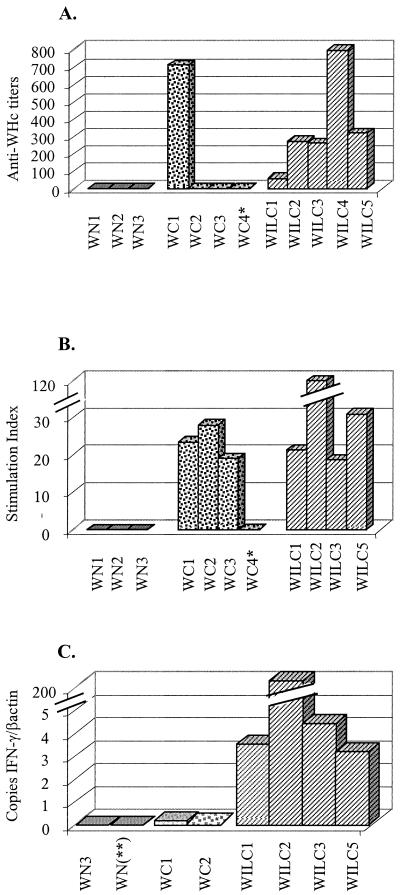
Two weeks after completion of the vaccination protocol, anti-WHc was undetectable in control animals as well as in three (WC2, WC3, and WC4) of the four woodchucks immunized with pCw alone. Only WC1 developed a high titer of anti-WHc antibodies, with values of 711 after the third dose of the immunogen. By contrast, all animals immunized with pCw plus pIL12w produced anti-WHc antibodies 2 weeks after completion of the immunization protocol (Fig. 1A).
FIG. 1.
Immune response against WHcAg following gene gun immunization with pCw (stippled bars) or pCw plus pIL12w (hatched bars). Nonimmunized woodchucks (shaded bars) served as controls. (A) Humoral immune response. (B) Production of IL-2 by woodchuck PBMC incubated in the presence of WHcAg. (C) IFN-γ mRNA levels (as determined by real-time quantitative RT-PCR) in woodchuck PBMC incubated in the presence of WHcAg. ∗, animal in which a second round of immunizations with pCw induced anti-WHcAg antibodies (titer = 90) and IL-2 production (SI = 21). ∗∗, healthy control animal that was not further infected or followed up.
Production of IL-2 (Fig. 1B) and IFN-γ (Fig. 1C) by PBMC in response to WHcAg was measured in some animals from each group. While no IL-2 synthesis was found in control animals, specific IL-2 production against WHcAg was observed in three (WC1, WC2, and WC3) of four animals immunized with pCw and in four of four tested animals immunized with pCw plus pIL12w. SI values ranged between 10 and 30 in the two immunized groups. WC4 developed a proliferative response (SI = 21) and low levels of anti-WHc antibodies after a second round of three immunizations with pCw.
At the end of the immunization protocol, IFN-γ mRNA production was low in the two nonimmunized animals tested (0.02 and 0.3 copies of IFN-γ mRNA per copy of β-actin mRNA) and also in the two tested animals which had been immunized with pCw alone (0.017 copy in one and undetectable levels in the other) (Fig. 1C). In contrast, substantial amounts of IFN-γ were found in animals immunized with the combination of pCw plus pIL12w, ranging from 3 to 4 copies in WILC1, WILC3, and WILC5 to as many as 200 copies in WILC2 (Fig. 1C).
Influence of vaccination on the evolution of WHV infection.
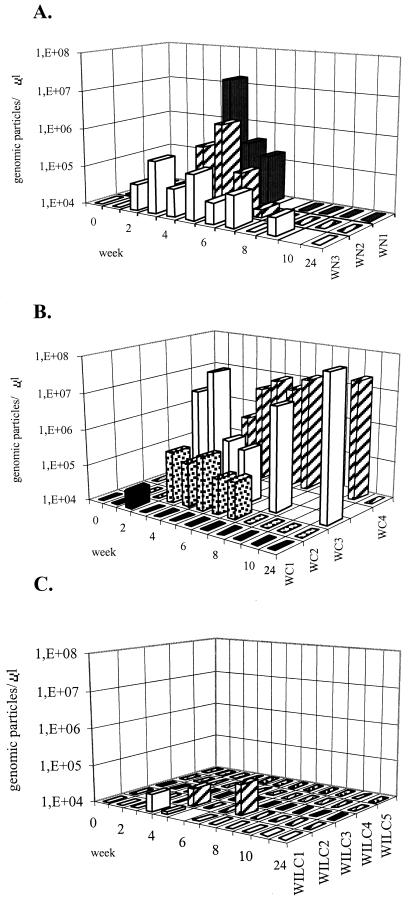
To test the protective effect of gene gun vaccination against WHV infection, woodchucks from the three experimental groups were challenged subcutaneously with an inoculum containing 1010 genomic copies of WHV. Viral serology was investigated weekly during a follow-up period of 10 to 12 weeks and at week 24.
As shown in Fig. 2A, all nonvaccinated animals developed acute infections, with viremia lasting for 4 to 6 weeks starting at week 2 or 3 after infection. WHV DNA levels were about 105 genomic copies/μl, with peaks of 106 or 107 copies/μl in woodchucks WN2 and WN1, respectively. WHsAg was detected in serum for 7, 1, and 4 weeks in woodchucks WN1, WN2, and WN3 respectively (Table 2).
FIG. 2.
Evolution of viremia after challenge with WHV. (A) Control nonimmunized animals; (B) animals immunized with pCw, (C) woodchucks immunized with pCw plus pIL12w. Inoculation of WHV was performed at week 0. WHV DNA was quantified by real-time quantitative PCR. WHV DNA is expressed as genomic particles per microliter of serum.
TABLE 2.
Influence of different immunization protocols on serum WHsAg after WHV challenge
| Treatment | Woodchuck | Period of WHsAg positivity in serum (wks) | |
|---|---|---|---|
| Immunization with pCw (group 1) | WC1 | 0 | |
| WC2 | 2 (wk 2 and 3 postinfection) | ||
| WC3 | 24 (wk 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, and 24 postinfection) | ||
| WC4 | 9 (wk 4, 5, 6, 7, 8, 9, 10, and 12 postinfection) | ||
| Immunization with pCw plus pIL12w (group 2) | WILC1 | 0 | |
| WILC2 | 0 | ||
| WILC3 | 1 (wk 1 postinfection) | ||
| WILC4 | 0 | ||
| WILC5 | 0 | ||
| Healthy controls (group 3) | WN1 | 7 (wk 1, 2, 3, 4, 5, 6, and 7 postinfection) | |
| WN2 | 1 (wk 8 postinfection) | ||
| WN3 | 4 (wk 4, 5, 6, and 7 postinfection) |
No protection against infection was observed in animals immunized with pCw (Fig. 2B) except for animal WC1, which showed short-lived viremia of low intensity (a sporadic value of 3 × 104 copies/μl) and remained WHsAg negative (Table 2). Two woodchucks from this group showed higher levels of WHV DNA and viremia of longer duration than those observed in nonimmunized animals (Fig. 2A and B). Serum samples were positive for WHsAg for 2, 9, and 24 weeks for WC2, WC4, and WC3, respectively. Woodchuck WC3 developed chronic infection, an event which is exceptional when WHV infection occurs after the perinatal period.
In contrast with animals immunized with pCw alone, all woodchucks vaccinated with a combination of pCw and pIL12w showed protection against WHV infection (Fig. 2C). Three of these animals (WILC3, WILC4, and WILC5) exhibited undetectable levels of WHV-DNA in serum after infection, whereas the other two (WILC1 and WILC2) presented low-level viremia (below 105 copies/μl) at only one or two time points during follow-up. While no significant differences in postinfection WHV DNA levels were found between controls and pCw-vaccinated woodchucks, the levels of viremia at weeks 4, 5, and 6 in animals immunized with pCw plus pIL12w were significantly lower (p<0.05) than those in the other two groups. Only one of the five woodchucks (WILC3) immunized with pCw plus pIL12w showed a single positive WHsAg value at week 1 (Table 2), which might result from the inoculum used for challenge. The other four animals remained WHsAg negative during follow-up.
Antiviral immunity after WHV infection.
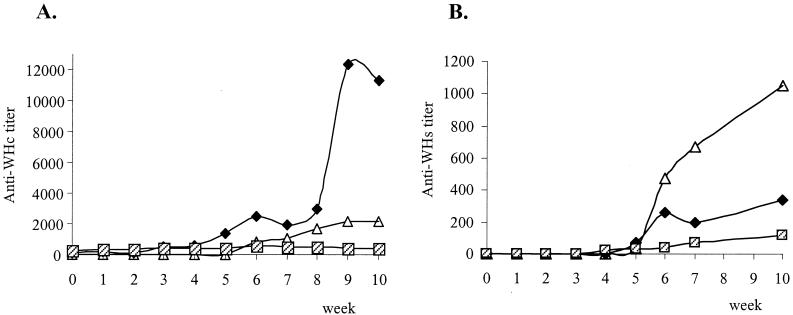
Figure 3A shows the mean levels of anti-WHc antibodies in the three experimental groups. It is noteworthy that while anti-WHc titers were high in pCw-vaccinated woodchucks, they were low in animals immunized with pCw in combination with pIL12w. In the last group the levels of anti-WHc antibodies at weeks 8, 9, and 10 were significantly lower than those in controls (P < 0.05) and in pCw-vaccinated animals (P < 0.02).
FIG. 3.
Anti-WHc (A) and anti-WHs (B) responses in woodchucks after challenge with 1010 WHV genomic particles. Symbols: ▵, nonvaccinated animals; ⧫, animals immunized with pCw alone;  , animals immunized with pCw plus pIL12w. Mean values at each time point are shown.
, animals immunized with pCw plus pIL12w. Mean values at each time point are shown.
Similarly to what occurs in HBV infection (23), the development of anti-WHs antibodies provides immunity to WHV (9). As represented in Fig. 3B, animals from the control group developed high levels of anti-WHs antibodies after infection, coinciding with the decline of viremia. Anti-WHs levels tended to be lower in the two groups of vaccinated animals, especially in the group receiving the combined immunization with pCw and pIL12w (in this group anti-WHs levels at week 6 were significantly lower than those in the other two experimental groups taken together; P < 0.05). These data indicate that animals vaccinated with pCw plus pIL12w were able to clear WHV with little contribution of humoral immunity.
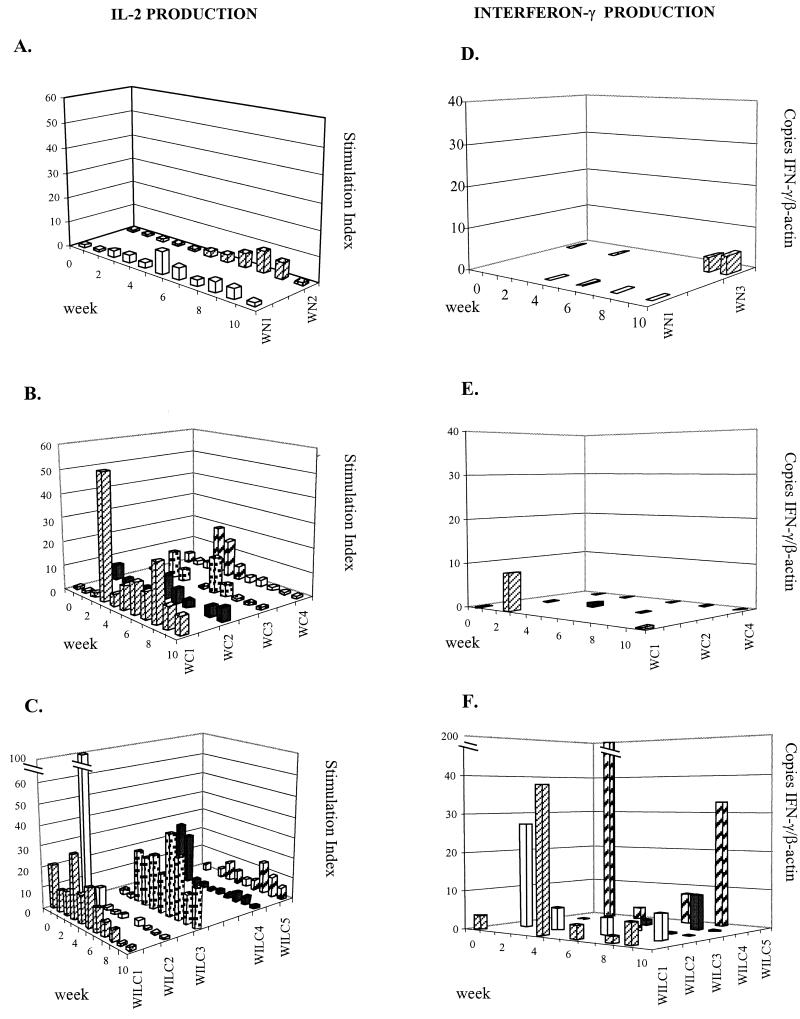
T-cell responses against WHcAg were analyzed for the three groups of animals at different time points after infection by determining the production of IL-2 and IFN-γ (a Th1 cytokine) by PBMC incubated in the presence of recombinant WHcAg. As shown in Fig. 4A, after WHV challenge the two control woodchucks tested (WN1 and WN2) showed low levels of IL-2 production in response to WHcAg (SI values between 2 and 8) and only a modest elevation in the production of IFN-γ (maximal values, 4.5 copies of IFN-γ mRNA/copy of β-actin mRNA at week 10 in WN3 and 0.24 at week 6 in WN1) (Fig. 4D).
FIG. 4.
Cellular immunes response of woodchucks after challenge with 1010 WHV genomic particles at week 0. Data for nonimmunized woodchucks (WN1 and WN2) (A and D), woodchucks previously vaccinated with pCw (WC1, WC2, WC3, and WC4) (B and E), and woodchucks previously immunized with pCw plus pIL12w (WILC1, WILC2, WILC3, and WILC4) (C and F) are shown. Determinations of IL-2 production (A, B, and C) and IFN-γ mRNA levels (D, E, and F) in PBMC after stimulation with recombinant WHcAg were performed at different time points during follow-up after infection. IL-2 levels are expressed in terms of the SI. IFN-γ levels are expressed as the number of IFN-γ mRNA copies per copy of β-actin mRNA.
Figure 4B shows the production of IL-2 in response to WHcAg in woodchucks vaccinated with pCw. In this group SI values above 9 were present at several time points in different animals (P < 0.05 versus values for controls). However, despite substantial IL-2 production, the generation of IFN-γ in response to WHcAg in this group of animals was poor (Fig. 4E). Very low transcriptional levels of this cytokine were found in WC2 and WC4, and a single peak of 8 IFN-γ mRNA copies per copy of β-actin mRNA was observed at week 2 in WC1, the only woodchuck in this group which showed protection against infection.
Woodchucks vaccinated with pCw plus pIL12w and exposed to WHV showed marked increases in IL-2 production in response to WHcAg; the frequency of SI values above 9 was significantly higher than that in controls (P < 0.05), and occasional peaks above 20 were observed in four animals (Fig. 4C). Interestingly, in this group of woodchucks, the increased IL-2 response was associated with high IFN-γ production. Thus, woodchucks that received the combined vaccination (and that were protected against WHV infection) showed values above 5 copies of IFN-γ mRNA per copy of β-actin mRNA with a frequency significantly higher (P < 0.05) than that in the sum of the animals in the other two experimental groups (which were unprotected against WHV challenge). High peaks of IFN-γ production (more than 25 copies of IFN-γ mRNA per copy of β-actin) were found in three of the woodchucks that received the combined immunization (Fig. 4F).
DISCUSSION
It has been shown that specific cellular immunity against HBcAg plays a crucial role in the control of HBV infection (13). Several studies have tested the efficacy of vaccines aimed at enhancing the host's cellular immune response against WHcAg, as a means to prevent WHV infection (28, 29, 40). DNA immunization is an efficient method to stimulate both cellular and humoral immunity. A previous report (28) has shown that intramuscular injection of plasmid DNA was able to induce anti-WHc immunity and protection against infection. However, a large amount of DNA (2 doses of 100 μg followed by 3 doses of 1 mg) was needed to induce specific anti-WHc antibodies and protection against virus challenge. In the present study we used gene gun vaccination because this strategy uses very little amount of plasmid DNA. In experiments carried out on mice, it was observed that three doses of 2 or 4 μg of the plasmid coding for WHcAg (pCw) were equally effective at inducing anti-WHc antibodies. With woodchucks, three doses of 8 μg of pCw induced antibodies in only one of the four vaccinated animals, but T-cell proliferation in response to WHcAg was found in all vaccinated woodchucks except WC4 (this animal showed T-cell proliferation and low titers of anti-WHc antibodies after three additional doses of the immunogen). However, as shown in Fig. 1B and C, the proliferative T-cell response elicited by gene gun vaccination with pCw alone was associated with no or very poor IFN-γ production by proliferating T cells, indicating that the type of Th response generated by this form of vaccination was not Th1.
In our study, gene gun immunization with pCw was not found to induce protective immunity against WHV infection. Thus, upon WHV infection, viremias in control woodchucks and pCw-vaccinated animals were not statistically different. In fact, one of the woodchucks of the last group (WC3) developed chronic infection, an event which seldom takes place when WHV infection occurs after the perinatal period. Cote et al. (8) reported that the chronicity rate in adult woodchucks was 0% even after inoculation with high doses of the virus.
Interestingly, after WHV infection, woodchucks which had been vaccinated with pCw alone showed high titers of anti-WHc antibodies, and the levels of IL-2 synthesis in response to WHcAg were higher than those in nonimmunized controls (Fig. 3 and 4A and B). However, in pCw-vaccinated woodchucks the synthesis of IFN-γ mRNA by T cells stimulated with WHcAg was poor; the transcriptional levels of this cytokine were appreciable only in WC1, the sole animal from this group which manifested protection against WHV challenge (Fig. 4E). As WHcAg forms the internal nucleocapsid of WHV, humoral antibodies to this antigen are not capable of neutralizing virions, and only T-cell immunity to this antigen can be efficient in the control of viral replication. IFN-γ is an important immunomodulatory cytokine, generated in the Th1 type of immune response, which plays a critical role in orchestrating protective antiviral T-cell immunity (2, 31). Our data suggest that the failure of gene gun immunization with pCw alone to stimulate Th1 reactivity might be responsible for the lack of protection against WHV infection.
The inability of pCw administered by bombardment of the skin to achieve protective immunity is in contrast with the findings of studies (28) showing protection by intramuscular injection of large amounts of plasmid DNA coding for WHcAg. This discrepancy could be explained by the inflammatory reaction caused by intramuscular injection of the plasmid, which could stimulate dendritic cells in the muscle to synthesize IL-12. Also, degradation of injected DNA by extracellular nucleases will generate abundant CpG motifs, which would stimulate APCs to synthesize IL-12 (17). IL-12 is a central cytokine in the induction of IFN-γ production and the Th1 type of responses (47). Gene gun procedures use very little DNA, which is delivered directly to APCs of the skin without any inflammatory reaction, thus facilitating presentation of the coded antigen to T cells by nonactivated dendritic cells. This would prime T cells without IL-12 signaling, leading to a non-Th1 immune reaction. The inability of gene gun immunization to stimulate Th1 responses has also been recently reported by Tanghe et al. (44), who showed that a tuberculosis DNA vaccine was protective when given by intramuscular injection but not when low or high doses of plasmid were administered by gene gun bombardment of the skin.
The efficacy of IL-12 gene administration as a vaccine adjuvant to stimulate Th1 responses has been demonstrated previously by our group (27) as well as by others (41) in murine models of immunization against HCV antigens and herpesvirus. In the present study, in an attempt to promote a Th1 response leading to protection against WHV infection, we vaccinated woodchucks with pCw together with a plasmid coding for woodchuck IL-12 (pIL12w). This strategy proved to be very effective at activating Th1 cells and antiviral immunity, resulting in production of anti-WHc antibodies, T-cell proliferation, and production of IFN-γ in all cases tested (see Fig. 1). These animals were efficiently protected against infection, showing undetectable or very low-level and transient viremia after WHV inoculation. In these woodchucks viral clearance occurred in association with very low titers of anti-WHc and anti-WHs antibodies, indicating little contribution of humoral immunity in the control of viral replication. In contrast to low antibody titers, woodchucks which had received the combined vaccination exhibited, after WHV challenge, high levels of IL-2 and IFN-γ production in response to WHcAg. In comparing Fig. 4B and C with Fig. 4E and F, it is apparent that while animals from the two vaccinated groups showed manifest T-cell proliferation in response to WHcAg, only T cells from the group immunized with pCw plus pIL12w produced significant amounts of IFN-γ. Thus, our findings indicate that the combined genetic vaccination with pCw plus pIL12w results in the preferential induction of a Th1 immune response, leading to effective control of WHV replication.
In summary, we show that gene gun vaccination with WHcAg alone does not protect against WHV challenge. Steering the immune response toward protective immunity by using appropriate cytokines as vaccine adjuvants is a feasible and simple procedure that may prove of value not only in prophylactic but also perhaps in therapeutic vaccination. Our data highlight the role of the Th1 response in the control of hepadnavirus infections and suggest new modalities to induce protection against HBV infection.
ACKNOWLEDGMENTS
J. Ruiz and J. Prieto contributed equally to this work.
This work was supported by grant SAF99/0084 from Comisión Interministerial de Ciencia y Tecnología (CICYT), by a grant from Fundación Ramón Areces, and by personal grants from M. J. Huarte, J. Vidal, and M. Méndez. R. García-Navarro received a grant from Fundación Ramón Areces.
We thank J. P. Behr for helpful instructions on DNA transfection with PEI, C. Suñé and I. Melero for helpful comments, and E. Huarte, M. Zaratiegui, C. Miqueo, and M. Rodriguez for help in preparation of the manuscript. We acknowledge the assistance received from E. Ziordia and J. Guillem with woodchuck handling. We are grateful to N. Casares and A. López for help with IL-2 bioassays.
REFERENCES
- 1.Ausubel F A, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K, editors. Current protocols in molecular biology. New York, N.Y: John Wiley and Sons; 2000. [Google Scholar]
- 2.Borrow P. Mechanisms of viral clearance and persistence. J Viral Hepat. 1997;4(Suppl. 2):16–24. doi: 10.1111/j.1365-2893.1997.tb00176.x. [DOI] [PubMed] [Google Scholar]
- 3.Boussif O, Lezoualc'h F, Zanta M A, Mergny M D, Scherman D, Demeneix B, Behr J P. A versatile vector for gene and oligonucleotide transfer into cells in culture and in vivo: polyethylenimine. Proc Natl Acad Sci USA. 1995;92:7297–7301. doi: 10.1073/pnas.92.16.7297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carman W F. The clinical significance of surface antigen variants of hepatitis B virus. J Viral Hepat. 1997;4(Suppl. 1):11–20. doi: 10.1111/j.1365-2893.1997.tb00155.x. [DOI] [PubMed] [Google Scholar]
- 5.Carman W F, Zanetti A R, Karayiannis P, Waters J, Manzillo G, Tanzi E, Zuckerman A J, Thomas H C. Vaccine-induced escape mutant of hepatitis B virus. Lancet. 1990;336:325–329. doi: 10.1016/0140-6736(90)91874-a. [DOI] [PubMed] [Google Scholar]
- 6.Chisari F V, Ferrari C. Hepatitis B virus immunopathogenesis. Annu Rev Immunol. 1995;13:29–60. doi: 10.1146/annurev.iy.13.040195.000333. [DOI] [PubMed] [Google Scholar]
- 7.Cote P J, Gerin J L. The woodchuck as a model of hepadnavirus infection, pathogenesis and therapy. Forum Trends Exp Clin Med. 1996;6:131–159. [Google Scholar]
- 8.Cote P J, Korba B E, Miller R H, Jacob J R, Baldwin B H, Hornbuckle W E, Purcell R H, Tennant B C, Gerin J L. Effects of age and viral determinants on chronicity as an outcome of experimental woodchuck hepatitis virus infection. Hepatology. 2000;31:190–200. doi: 10.1002/hep.510310128. [DOI] [PubMed] [Google Scholar]
- 9.Cote P J, Shapiro M, Engle R E, Popper H, Purcell R H, Gerin J L. Protection of chimpanzees from type B hepatitis by immunization with woodchuck hepatitis virus surface antigen. J Virol. 1986;60:895–901. doi: 10.1128/jvi.60.3.895-901.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dienstag J L, Stevens C E, Bhan A K, Szmuness W. Hepatitis B vaccine administered to chronic carriers of hepatitis B surface antigen. Ann Intern Med. 1982;96:575–579. doi: 10.7326/0003-4819-96-5-575. [DOI] [PubMed] [Google Scholar]
- 11.Donnelly J J, Friedman A, Martinez D, Montgomery D L, Shiver J W, Motzel S L, Ulmer J B, Liu M A. Preclinical efficacy of a prototype DNA vaccine: enhanced protection against antigenic drift in influenza virus. Nat Med. 1995;1:583–587. doi: 10.1038/nm0695-583. [DOI] [PubMed] [Google Scholar]
- 12.Feitelson M A, Marion P L, Robinson W S. Antigenic and structural relationships of the surface antigens of hepatitis B virus, ground squirrel hepatitis virus, and woodchuck hepatitis virus. J Virol. 1981;39:447–454. doi: 10.1128/jvi.39.2.447-454.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ferrari C, Penna A, Bertoletti A, Valli A, Antoni A D, Giuberti T, Cavalli A, Petit M A, Fiaccadori F. Cellular immune response to hepatitis B virus-encoded antigens in acute and chronic hepatitis B virus infection. J Immunol. 1990;145:3442–3449. [PubMed] [Google Scholar]
- 14.Galibert F, Chen T N, Mandart E. Nucleotide sequence of a cloned woodchuck hepatitis virus genome: comparison with the hepatitis B virus sequence. J Virol. 1982;41:51–65. doi: 10.1128/jvi.41.1.51-65.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gerin J L, Faust R M, Holland P V. Biophysical characterization of the adr subtype of hepatitis B antigen and preparation of anti-r sera in rabbits. J Immunol. 1975;115:100–105. [PubMed] [Google Scholar]
- 16.Girones R, Cote P J, Hornbuckle W E, Tennant B C, Gerin J L, Purcell R H, Miller R H. Complete nucleotide sequence of a molecular clone of woodchuck hepatitis virus that is infectious in the natural host. Proc Natl Acad Sci USA. 1989;86:1846–1849. doi: 10.1073/pnas.86.6.1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gurunathan S, Klinman D M, Seder R A. DNA vaccines: immunology, application, and optimization. Annu Rev Immunol. 2000;18:927–974. doi: 10.1146/annurev.immunol.18.1.927. [DOI] [PubMed] [Google Scholar]
- 18.Hadler S C, Francis D P, Maynard J E, Thompson S E, Judson F N, Echenberg D F, Ostrow D G, O'Malley P M, Penley K A, Altman N L, et al. Long-term immunogenicity and efficacy of hepatitis B vaccine in homosexual men. N Engl J Med. 1986;315:209–214. doi: 10.1056/NEJM198607243150401. [DOI] [PubMed] [Google Scholar]
- 19.Hervas-Stubbs S, Lasarte J J, Sarobe P, Prieto J, Cullen J, Roggendorf M, Borras-Cuesta F. Therapeutic vaccination of woodchucks against chronic woodchuck hepatitis virus infection. J Hepatol. 1997;27:726–737. doi: 10.1016/s0168-8278(97)80090-6. [DOI] [PubMed] [Google Scholar]
- 20.Irvine K R, Rao J B, Rosenberg S A, Restifo N P. Cytokine enhancement of DNA immunization leads to effective treatment of established pulmonary metastases. J Immunol. 1996;156:238–245. [PMC free article] [PubMed] [Google Scholar]
- 21.Iwarson S, Tabor E, Thomas H C, Snoy P, Gerety R J. Protection against hepatitis B virus infection by immunization with hepatitis B core antigen. Gastroenterology. 1985;88:763–767. doi: 10.1016/0016-5085(85)90148-9. [DOI] [PubMed] [Google Scholar]
- 22.Jung M C, Diepolder H M, Spengler U, Wierenga E A, Zachoval R, Hoffmann R M, Eichenlaub D, Frosner G, Will H, Pape G R. Activation of a heterogeneous hepatitis B (HB) core and e antigen-specific CD4+ T-cell population during seroconversion to anti-HBe and anti-HBs in hepatitis B virus infection. J Virol. 1995;69:3358–3368. doi: 10.1128/jvi.69.6.3358-3368.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kane M A. Global status of hepatitis B immunisation. Lancet. 1996;348:696. doi: 10.1016/S0140-6736(05)65598-5. [DOI] [PubMed] [Google Scholar]
- 24.Karthigesu V D, Allison L M, Fortuin M, Mendy M, Whittle H C, Howard C R. A novel hepatitis B virus variant in the sera of immunized children. J Gen Virol. 1994;75:443–448. doi: 10.1099/0022-1317-75-2-443. [DOI] [PubMed] [Google Scholar]
- 24a.Kodama K, Ogawasara N, Yoshikawa H, Murakami S. Nucleotide sequence of a cloned genome: evolutional relationship between hepadnaviruses. J Virol. 1985;56:978–986. doi: 10.1128/jvi.56.3.978-986.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kuhrober A, Wild J, Pudollek H P, Chisari F V, Reimann J. DNA vaccination with plasmids encoding the intracellular (HBcAg) or secreted (HBeAg) form of the core protein of hepatitis B virus primes T cell responses to two overlapping Kb- and Kd-restricted epitopes. Int Immunol. 1997;9:1203–1212. doi: 10.1093/intimm/9.8.1203. [DOI] [PubMed] [Google Scholar]
- 26.Lai M Z, Ross D T, Guillet J G, Briner T J, Gefter M L, Smith J A. T lymphocyte response to bacteriophage lambda repressor cI protein. Recognition of the same peptide presented by Ia molecules of different haplotypes. J Immunol. 1987;139:3973–3980. [PubMed] [Google Scholar]
- 27.Lasarte J J, Corrales F J, Casares N, Lopez-Diaz de Cerio A, Qian C, Xie X, Borras-Cuesta F, Prieto J. Different doses of adenoviral vector expressing IL-12 enhance or depress the immune response to a coadministered antigen: the role of nitric oxide. J Immunol. 1999;162:5270–5277. [PubMed] [Google Scholar]
- 28.Lu M, Hilken G, Kruppenbacher J, Kemper T, Schirmbeck R, Reimann J, Roggendorf M. Immunization of woodchucks with plasmids expressing woodchuck hepatitis virus (WHV) core antigen and surface antigen suppresses WHV infection. J Virol. 1999;73:281–289. doi: 10.1128/jvi.73.1.281-289.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Menne S, Maschke J, Tolle T K, Lu M, Roggendorf M. Characterization of T-cell response to woodchuck hepatitis virus core protein and protection of woodchucks from infection by immunization with peptides containing a T-cell epitope. J Virol. 1997;71:65–74. doi: 10.1128/jvi.71.1.65-74.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Montgomery D L, Shiver J W, Leander K R, Perry H C, Friedman A, Martinez D, Ulmer J B, Donnelly J J, Liu M A. Heterologous and homologous protection against influenza A by DNA vaccination: optimization of DNA vectors. DNA Cell Biol. 1993;12:777–783. doi: 10.1089/dna.1993.12.777. [DOI] [PubMed] [Google Scholar]
- 31.Mosmann T R, Cherwinski H, Bond M W, Giedlin M A, Coffman R L. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986;136:2348–2357. [PubMed] [Google Scholar]
- 32.Murray K, Bruce S A, Wingfield P, van Eerd P, de Reus A, Schellekens H. Protective immunisation against hepatitis B with an internal antigen of the virus. J Med Virol. 1987;23:101–107. doi: 10.1002/jmv.1890230202. [DOI] [PubMed] [Google Scholar]
- 33.Nakamura I, Nupp J T, Rao B S, Buckler White A, Engle R E, Casey J L, Gerin J L, Cote P J. Cloning and characterization of partial cDNAs for woodchuck cytokines and CD3ε with applications for the detection of RNA expression in tissues by RT-PCR assay. J Med Virol. 1997;53:85–95. doi: 10.1002/(sici)1096-9071(199709)53:1<85::aid-jmv15>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 34.Penna A, Chisari F V, Bertoletti A, Missale G, Fowler P, Giuberti T, Fiaccadori F, Ferrari C. Cytotoxic T lymphocytes recognize an HLA-A2-restricted epitope within the hepatitis B virus nucleocapsid antigen. J Exp Med. 1991;174:1565–1570. doi: 10.1084/jem.174.6.1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Popper H, Roth L, Purcell R H, Tennant B C, Gerin J L. Hepatocarcinogenicity of the woodchuck hepatitis virus. Proc Natl Acad Sci USA. 1987;84:866–870. doi: 10.1073/pnas.84.3.866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Raz E, Carson D A, Parker S E, Parr T B, Abai A M, Aichinger G, Gromkowski S H, Singh M, Lew D, Yankauckas M A, et al. Intradermal gene immunization: the possible role of DNA uptake in the induction of cellular immunity to viruses. Proc Natl Acad Sci USA. 1994;91:9519–9523. doi: 10.1073/pnas.91.20.9519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rehermann B, Fowler P, Sidney J, Person J, Redeker A, Brown M, Moss B, Sette A, Chisari F V. The cytotoxic T lymphocyte response to multiple hepatitis B virus polymerase epitopes during and after acute viral hepatitis. J Exp Med. 1995;181:1047–1058. doi: 10.1084/jem.181.3.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Roggendorf M, Tolle T K. The woodchuck: an animal model for hepatitis B virus infection in man. Intervirology. 1995;38:100–112. doi: 10.1159/000150418. [DOI] [PubMed] [Google Scholar]
- 39.Roos S, Fuchs K, Roggendorf M. Protection of woodchucks from infection with woodchuck hepatitis virus by immunization with recombinant core protein. J Gen Virol. 1989;70:2087–2095. doi: 10.1099/0022-1317-70-8-2087. [DOI] [PubMed] [Google Scholar]
- 40.Schodel F, Neckermann G, Peterson D, Fuchs K, Fuller S, Will H, Roggendorf M. Immunization with recombinant woodchuck hepatitis virus nucleocapsid antigen or hepatitis B virus nucleocapsid antigen protects woodchucks from woodchuck hepatitis virus infection. Vaccine. 1993;11:624–628. doi: 10.1016/0264-410x(93)90307-j. [DOI] [PubMed] [Google Scholar]
- 41.Sin J I, Kim J J, Arnold R L, Shroff K E, McCallus D, Pachuk C, McElhiney S P, Wolf M W, Pompa-de Bruin S J, Higgins T J, Ciccarelli R B, Weiner D B. IL-12 gene as a DNA vaccine adjuvant in a herpes mouse model: IL-12 enhances Th1-type CD4+ T-cell-mediated protective immunity against herpes simplex virus-2 challenge. J Immunol. 1999;162:2912–2921. [PubMed] [Google Scholar]
- 42.Sylvan S P, Hellstrom U B, Flehmig B. Characterization of cell mediated immune responses to the hepatitis B core protein in man. Clin Exp Immunol. 1987;68:233–241. [PMC free article] [PubMed] [Google Scholar]
- 43.Tang D C, DeVit M, Johnston S A. Genetic immunization is a simple method for eliciting an immune response. Nature. 1992;356:152–154. doi: 10.1038/356152a0. [DOI] [PubMed] [Google Scholar]
- 44.Tanghe A, Denis O, Lambrecht B, Motte V, van den Berg T, Huygen K. Tuberculosis DNA vaccine encoding Ag85A is immunogenic and protective when administered by intramuscular needle injection but not by epidermal gene gun bombardment. Infect Immun. 2000;68:3854–3860. doi: 10.1128/iai.68.7.3854-3860.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tennant B C, Gerin J L. The woodchuck model of hepatitis B virus infection. In: Aries I M E A, editor. The liver: biology and pathobiology. 3rd ed. New York, N.Y: Raven Press; 1994. pp. 1455–1466. [Google Scholar]
- 46.Tiollais P, Pourcel C, Dejean A. The hepatitis B virus. Nature. 1985;317:489–495. doi: 10.1038/317489a0. [DOI] [PubMed] [Google Scholar]
- 47.Trinchieri G. Interleukin-12: a cytokine at the interface of inflammation and immunity. Adv Immunol. 1998;70:83–243. doi: 10.1016/s0065-2776(08)60387-9. [DOI] [PubMed] [Google Scholar]
- 48.Tsai S L, Chen P J, Lai M Y, Yang P M, Sung J L, Huang J H, Hwang L H, Chang T H, Chen D S. Acute exacerbations of chronic type B hepatitis are accompanied by increased T cell responses to hepatitis B core and e antigens. Implications for hepatitis B e antigen seroconversion. J Clin Investig. 1992;89:87–96. doi: 10.1172/JCI115590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tyler G V, Snyder R L, Summers J. Experimental infection of the woodchuck (Marmota monax monax) with woodchuck hepatitis virus. Lab Investig. 1986;55:51–55. [PubMed] [Google Scholar]
- 50.Ulmer J B, Deck R R, DeWitt C M, Friedman A, Donnelly J J, Liu M A. Protective immunity by intramuscular injection of low doses of influenza virus DNA vaccines. Vaccine. 1994;12:1541–1544. doi: 10.1016/0264-410x(94)90081-7. [DOI] [PubMed] [Google Scholar]
- 51.Xiang Z Q, Spitalnik S, Tran M, Wunner W H, Cheng J, Ertl H C. Vaccination with a plasmid vector carrying the rabies virus glycoprotein gene induces protective immunity against rabies virus. Virology. 1994;199:132–140. doi: 10.1006/viro.1994.1105. [DOI] [PubMed] [Google Scholar]
- 52.Yasutomi Y, Robinson H L, Lu S, Mustafa F, Lekutis C, Arthos J, Mullins J I, Voss G, Manson K, Wyand M, Letvin N L. Simian immunodeficiency virus-specific cytotoxic T-lymphocyte induction through DNA vaccination of rhesus monkeys. J Virol. 1996;70:678–681. doi: 10.1128/jvi.70.1.678-681.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yokoyama M, Zhang J, Whitton J L. DNA immunization confers protection against lethal lymphocytic choriomeningitis virus infection. J Virol. 1995;69:2684–2688. doi: 10.1128/jvi.69.4.2684-2688.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zuckerman J N, Zuckerman A J. Current topics in hepatitis B. J Infect. 2000;41:130–136. doi: 10.1053/jinf.2000.0720. [DOI] [PubMed] [Google Scholar]