Highlights
-
•
Home-based neurofeedback is feasible for people with chronic neuropathic pain.
-
•
Accessibility was 48% (11/23) with a 2:1 ratio of participant screening to enrollment.
-
•
Overall safety was good with no serious adverse events recorded.
-
•
Tolerability and acceptability were measured as 26% (6/23).
-
•
Improvements were detected in pain levels, mood, and central sensitization scores.
KEYWORDS: Brain computer interface, Chronic neuropathic pain, Feasibility, Home-based neurofeedback, Rehabilitation, Safety
Abstract
Objective
To evaluate the feasibility, safety, and potential health benefits of an 8-week home-based neurofeedback intervention.
Design
Single-group preliminary study.
Setting
Community-based.
Participants
Nine community dwelling adults with chronic neuropathic pain, 6 women and 3 men, with an average age of 51.9 years (range, 19-78 years) and with a 7-day average minimum pain score of 4 of 10 on the visual analog pain scale.
Interventions
A minimum of 5 neurofeedback sessions per week (40min/session) for 8 consecutive weeks was undertaken with a 12-week follow-up baseline electroencephalography recording period.
Main Outcome Measures
Primary feasibility outcomes: accessibility, tolerability, safety (adverse events and resolution), and human and information technology (IT) resources required. Secondary outcomes: pain, sensitization, catastrophization, anxiety, depression, sleep, health-related quality of life, electroencephalographic activity, and simple participant feedback.
Results
Of the 23 people screened, 11 were eligible for recruitment. One withdrew and another completed insufficient sessions for analysis, which resulted in 9 datasets analyzed. Three participants withdrew from the follow-up baselines, leaving 6 who completed the entire trial protocol. Thirteen adverse events were recorded and resolved: 1 was treatment-related, 4 were equipment-related, and 8 were administrative-related (eg, courier communication issues). The human and IT resources necessary for trial implementation were identified. There were also significant improvements in pain levels, depression, and anxiety. Six of 9 participants perceived minimal improvement or no change in symptoms after the trial, and 5 of 9 participants were satisfied with the treatment received.
Conclusions
It is feasible and safe to conduct a home-based trial of a neurofeedback intervention for people with chronic neuropathic pain, when the human and IT resources are provided and relevant governance processes are followed. Improvements in secondary outcomes merit investigation with a randomized controlled trial.
Neuropathic pain (NP) has been defined as pain arising as a result “of a lesion or disease affecting the somatosensory system.”1 It is frequently described as a “lancinating, shooting, electrical-like, burning, stabbing” pain,2 and in contrast to many types of nociceptive pain and acute nerve injury, chronic neuropathic pain (CNP) is always dysfunctional. Accurate epidemiologic statistics for CNP are hampered by the lack of simple diagnostic criteria within the general population, and therefore, estimates of CNP prevalence vary.3 A systematic review of 21 epidemiologic studies conducted in Europe, United States, Brazil, Taiwan, and Canada reported an estimate of NP in those populations of 6.9%-10%.4 About 15%-25% of chronic pain (CP) is thought to be of neuropathic origin,2 and the estimated prevalence of CP cases in the United Kingdom is 8.9%.5
Pain is considered to be chronic when it lasts or recurs for >3 to 6 months.6 Diagnosis of CNP is a complex physician-led process incorporating medical history, physical examination, and various tests. Standard UK treatment is pharmacological, involving close monitoring and referral to specialist pain services, depending on severity.7 Pharmacological treatments for CP conditions carry risks of dependence and potential misuse, which supports research into non-pharmacological options to assist in the management of CNP.
Research into a home-based self-managed intervention for central NP related to spinal cord injury suggests that neurofeedback (NFB) training may be a feasible complementary treatment option.8 This study builds on a previous proof of concept trial using the Axon Electroencephalogram (EEG) NFB systema manufactured by Exsurgo Ltd for people diagnosed with general CP9 and aimed to assess the feasibility and safety of the same system for people diagnosed with CNP.
The primary aim of this trial was to assess the feasibility, safety, accessibility, and tolerability of this NFB intervention. Feasibility was assessed in accordance with the metrics identified by the research literature10,11 to investigate the important aspects of trial process, management, resources, and relevance of scientific outcomes. Safety was assessed by the number and nature of adverse events (AEs) and how effectively these were identified, managed, and resolved. AEs are defined by the Good Clinical Practice regulations as “any untoward or unintended response in a subject to whom an Investigational Medicinal Product has been administered, including occurrences which are not necessarily caused by or related to that product.”12 AEs were recorded and collected, after consent and enrollment of participants, as required for clinical trials of all medicinal products and devices in the United Kingdom.12 Accessibility was defined as the proportion of all participants who subsequently entered the trial after screening relative to those who failed screening (reasons identified and recorded), in alignment with previous research.13
In addition, secondary outcome measures were used to identify any potential health benefits associated with this intervention and included measurement of EEG activity throughout the trial. Participants were also asked for simple feedback.
Methods
The study used a prospective, open-label, single-arm, nonrandomized design and was approved by the London - Central Research Ethics Committee Health Research Authority Ethics (reference: 20/LO/0523) via the Integrated Research Application System (IRAS reference: 310674). The trial was registered with ClinicalTrials.gov (NCT05464199), and the Clinical Trials Unit of the sponsor organization, East Kent University Hospitals National Health Service Foundation Trust (reference: 2022/CTU9/NEURO).
Setting and participants
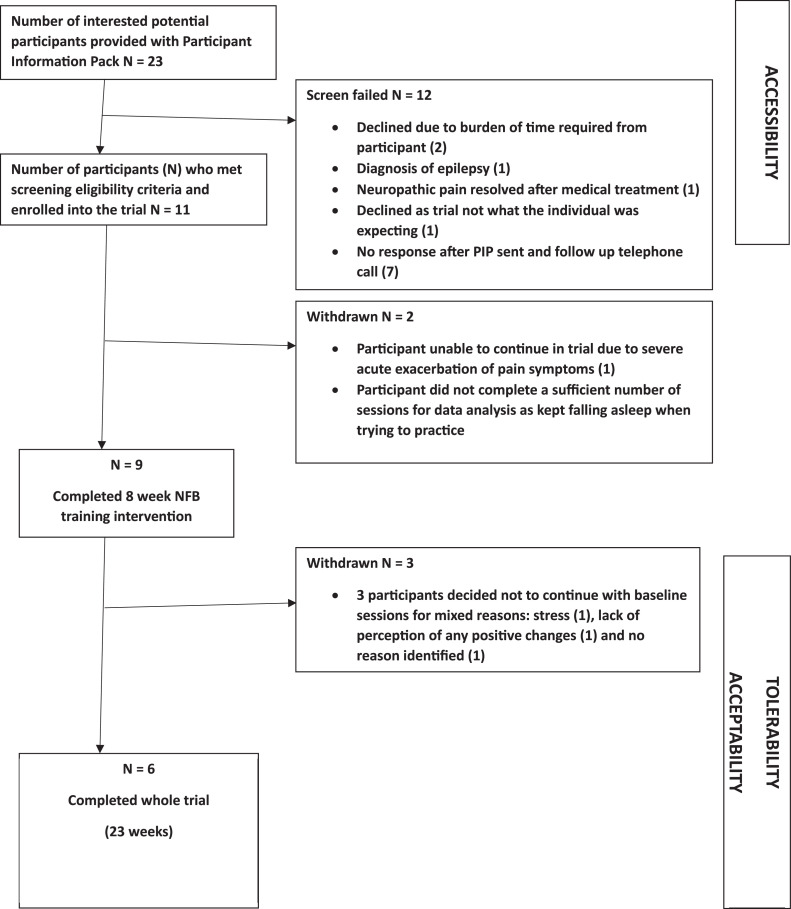
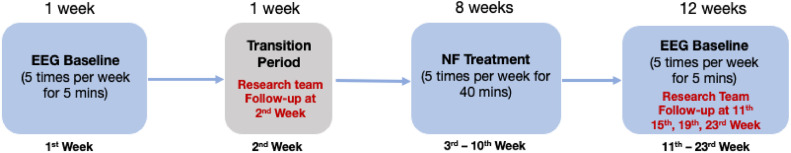
A single cohort of 11 patients was recruited via Principal Investigator (PI, MS) Out-patient clinics at East Kent Hospitals University National Health Service Foundation Trust and use of a flyer via social media (fig 1). Recruitment started in September 2022 and ended in March 2023. Table 1 details the trial eligibility criteria. Individuals who expressed interest were sent a Participant Information Pack (PIP) by email. This comprised a participant information sheet, consent form, headset measurement guide, trial flyer, and prescreening questionnaire (see supplemental appendices, available online only at http://www.archives-pmr.org/). After review of the PIP, interested participants completed an electronic version of the prescreening questionnaire to confirm trial eligibility, provided their head circumference measurement, and confirmed their interest in proceeding to trial enrollment via an initial assessment appointment with the Co-investigator (Co-I, KS) using Zoom.b The initial appointment comprised going through the informed consent process, completion of baseline outcome measurement questionnaires, and verification of the headset measurement provided. Subsequently, participants were enrolled into the trial, and 5 Zoom appointments were scheduled with the Co-I (KS) to complete secondary outcome measurement questionnaires at the 5 predefined follow-up (FU) time points (TPs), as per protocol (fig 2). Table 2 shows the participant demographic and clinical characteristics.
Fig 1.
Study flow diagram.
Table 1.
Trial inclusion and exclusion criteria.
| Type of Criteria | Details |
|---|---|
| Inclusion |
|
| Exclusion |
|
Fig 2.
NFB trial intervention process diagram.
Table 2.
Demographic and clinical characteristics of participants.
| ID | Ethnicity | Age (y) | Sex | NP Conditions | Other Conditions | Medication, Dosage (Condition/Purpose) |
|---|---|---|---|---|---|---|
| EKT02 | English or British | 78 | Female | Poststroke central nerve pain syndrome | Depression Anxiety/panic attack |
Bisoprolol, 1.25 mg once a day (blood pressure) Paracetamol, 4-6 500-mg capsules once a day (pain) Clopidogrel, 75 mg once a day (blood thinner) Vitamin D, 800 IU once a day (supplement) Levothyroxine 100, 75 mg every other day (underactive thyroid) Atorvastatin, 800 mg once a day (cholesterol) Candesartan, 32 mg once a day (blood pressure) Allopurinol, 200 mg once a day (gout) Rescue herbal medicine, once a day (sleep) |
| EKT03 | English or British | 58 | Female | Poststroke NP in right hemiplegic side of body | Depression | Paracetamol, 1000 mg every 6 h (pain) Fluoxetine, 20 mg once a day (low mood) Tizanidine, 2 mg twice a day (spasticity) Lercanidipine, 10 mg once a day (blood pressure) Lansoprazole, 30 mg once a day (reflux) |
| EKT04 | English or British | 19 | Female | Chronic back pain and chronic headache | ADHD Migraine |
Diclofenac sodium SR, 75 mg 1-2 times once a day (back pain) Melatonin, 6 mg once a day in the evening (sleep) Lansoprazole, 30 mg once a day (stomach protection) Methylphenidate hydrochloride, 54 mg in the morning and 18 mg in the evening (ADHD) Cetirizine hydrochloride, 10 mg once a day (allergy) Mometasone nasal spray, when necessary (allergy) |
| EKT05* | English or British | 44 | Female | Fibromyalgia | IBS Migraine Neck injury (including whiplash) Anxiety/panic attack Depression |
Pregabalin, 200 mg 2 times once a day (CP) Etoricoxib, 120 mg once a day (fibromyalgia) Omeprazole, 20 mg 2 times once a day (IBS) Duloxetine, 30 mg once a day (fibromyalgia/pain) Paracetamol, 1200 mg every 4-6 h (headache, CP, back pain) CosmoCol, once a day (constipation) Promethazine, 25 mg once a day in the evening (sleep) Evening primrose, 3 mg once a day in the evening (hormones) Robaxin, 750 mg (back and rib pain) Sumatriptan, 50 mg (migraine) |
| EKT06 | English or British | 45 | Female | Severe nerve damage in L4 and L5 Nerve, tendon, and muscle damage in C4, C5, and C6 |
Migraine IBS Neck injury (including whiplash) Anxiety/panic attack |
Clonidine, 25 mg twice a day (menopause) Nortriptyline, 2 10-mg capsules once a day in the evening (nerve damage) Naproxen, 500 mg twice a day (anti-inflammatory) Naratriptan, 2.5 mg once a day (nerve damage) Tramadol, 50 mg 3 times a day (pain) Co-codamol, 30 mg/500 mg 2 capsules 4 times a day (pain) Sertraline, 50 mg once a day (nerve pain) Omeprazole, 20 mg twice a day (stomach protection) Sage, 100 mg once a day (vitamins and supplements) Perfectil nail, once a day (supplements) |
| EKT07 | Mixed Ethnicity | 38 | Female | Nerve damage because of stroke | Gabapentin, 600 mg 3 times a day (nerve damage) Gabapentin, 2 100-mg capsules 3 times a day (nerve damage) Amitriptyline, 50 mg once a day in the evening (nerve damage) Ibuprofen gel 5%, three times a day (nerve damage) |
|
| EKT08 | English or British | 28 | Male | Disc degeneration (bulging disc at L4/L5) Spinal stenosis |
Omeprazole, 20 mg (acid reflux) | |
| EKT09 | English or British | 69 | Female | Myelopathy myelitis Constant pain in lower legs and feet |
Restless leg syndrome | Lansoprazole, 30 mg once a day (indigestion) Gabapentin, 2 100-mg capsules every 6 h (nerve pain) Folic acid, 5 mg once a day in the morning (blood) Amlodipine, 5 mg once a day in the morning (blood) Clopidogrel, 75 mg once a day in the morning (blood) Adcal D3, once a day in the morning (bones) Gliclazide, 40 mg once a day in the morning (diabetes) Atorvastatin, 40 mg once a day in the evening (cholesterol) |
| EKT10 | English or British | 72 | Male | Cervical myelopathy C3 and C4 Central cord syndrome |
IBS Neck injury (including whiplash) |
Amitriptyline, 10 mg (pain) Ibuprofen gel 5% (back pain) Ibuprofen, 400 mg (pain) Co-codamol, 8/500 mg (pain) |
| EKT11† | English or British | 59 | Male | High electric constant pain in the back | Neck injury (including whiplash) Anxiety/panic attack Depression Suicidal thoughts |
Aspirin, 75 mg once a day Betamethasone, 10 mL 4 times a day Co-codamol, 30 mg/500 mg, 2 capsules 4 times a day Carbocisteine, 375 mg Lansoprazole, 15 mg twice a day Gabapentin, 100 mg 2 or 3 times a day |
| EKT12 | English or British | 60 | Male | Poststroke central neuropathy | Anxiety/panic attack Depression |
Atorvastatin, 80 mg once a day (cholesterol) Bisoprolol, 5 mg once a day (heart) Omeprazole, 20 mg once a day (stomach acid) Ramipril, 2.5 mg once a day (heart) Sertraline, 125 mg once a day (anxiety) Warfarin, 6 mg once a day (anticoagulation) |
NOTE. Sample was 66.7% women and 33.3% men. Median age of women: 51.17 y (range, 19-78y); median age of men: 53.33 y (range, 28-60y). Rows highlighted in gray are the participants not included in the data analysis.
Abbreviations: ADHD, attention-deficit/hyperactivity disorder; IBS, irritable bowel syndrome.
Excluded because of insufficient sessions.
Withdrew because of hospital admission for acute episode of back pain.
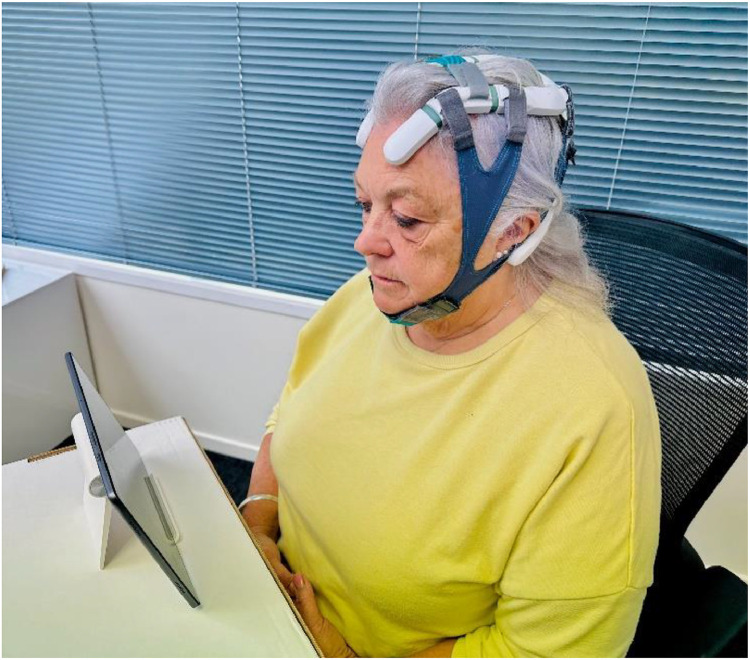
The Axon NFB device, comprising a headset and tablet (fig 3), was sent to participants via courier. Once received, all participants had 2 training sessions with the supervising researcher (CO) from the industrial partner, Exsurgo Ltd. These sessions covered: guidance on fitting the device, orientation, usage, how to perform and complete initial EEG baselines, and all aspects of self-administering NFB training. To monitor and improve participant adherence, email reminders were sent to participants when system records showed a lack of usage, and technical support was provided when needed. All data were collected remotely and stored securely in Exsurgo's cloud-based storage service (AWSc) using end-to-end encryption. The trial was conducted remotely at participants’ homes.
Fig 3.
Axon NFB system equipment. Headset worn by individual and tablet on stand.
Intervention
Each baseline session commenced with patients rating their pain, mood, and sleep via the Axon application (already installed on the tablet provided to each participant), followed by 2 EEG baseline recordings: 2 minutes with eyes open followed by 2 minutes with eyes closed for week 1, completing at least 5 baselines. The NFB intervention period began in week 3 and continued for 8 weeks, involving a minimum of 5 sessions per week, each lasting 40 minutes (see fig 2). Each session commenced with an EEG eyes open baseline recording, where relative alpha, theta, and high-beta activity was recorded and averaged to calculate the threshold for each session, after which an EEG eyes closed baseline recording was performed. Each session consisted of 5 × 5-minute blocks with a 1-minute rest period in between. Participants viewed a gamified representation of their brain activity as the interface for self-regulation, with the option of choosing from a selection of five “games,” for example, the jigsaw game (assembling the pieces of a randomized sequence of nature pictures), balloon game (enabling a hot air balloon to ascend from the ground into the sky), and paint game (painting a picture by numbers). After the intervention period (week 11 onward), participants continued with EEG baseline recordings for 12 weeks.
Study outcomes
Primary outcomes measured were accessibility (screening loss analysis), number of participants who completed the trial protocol, number of AEs and resolution, human resource time needed, and information technology (IT) resources required. Secondary outcomes measured were pain level and severity (assessed using the visual analog scale for pain14 VAS (Pain) and brief pain inventory [BPI]15), anxiety and depression (Depression, Anxiety and Stress Scale-21 items16), catastrophization (Pain Catastrophizing Scale17), sensitization components (Central Sensitization Inventory [CSI]18), sleep (Pittsburgh Sleep Quality Index19), health-related quality of life (EQ-5D-5L20), and resting state EEG activity. Simple feedback from participants regarding perception of change during the trial and satisfaction with the treatment was obtained.
Data collection and statistical analysis
Data were collected at 6 TPs in the trial: pre-EEG baselines (Week 0, to establish a baseline before the intervention), preintervention (Week 2, to measure any potential change during the 2-week EEG baseline recording period), postintervention (Week 11, to measure and evaluate any change because of the intervention), and 3 FU TPs (Weeks 15, 19, 23, to monitor the duration and persistence of any measured changes). Because some FU data were missing, data were analyzed at 4 TPs: Weeks 0, 2, 11, and 15, which provided the most comprehensive data set to assess the intervention's immediate and short-term effect.
All data were analyzed using SPSS version 29.0,21,d and descriptive statistics were used to summarize the data. Power analyses were not conducted because of the preliminary nature of the study; however, future studies should include them. To assess the effects of training sessions on different outcome measures, repeated measures analysis of variance was performed, using Bonferroni adjusted post hoc analysis for comparison between different TPs. A P value <.05 was considered significant.
Results
The study flow diagram (see fig 1) summarizes the screening loss analysis, and table 2 presents participant demographic and clinical characteristics.
Primary outcomes
Accessibility was just below average at 48% (11/23) with the main loss of potential participants occurring after the PIP had been sent out and after the FU telephone call (see fig 1). It is possible that applicants self-screened and did not respond further after reviewing the PIP. Tolerability and acceptability were measured as 26% (6/23), with identified reasons shown in fig 1. Five participants required email FU with respect to noncompliance with the number of sessions identified in the PIP. The reasons given for this included illness of a family member, complications with their own condition, hospital admission, and time shortage.
Overall safety was good with no serious AEs. There were 13 AEs in total: 1 was treatment-related, 4 were equipment-related, and 8 were related to courier deliveries. These AEs were resolved by the trial team and Exsurgo Ltd (table 3). In terms of human resources, an estimated minimum time needed for trial implementation is presented in Table 4, Table 5, which identify time input from Exsurgo Ltd and time input from the Co-I (KS), respectively. The total trial time estimates were 18.5 hours for Exsurgo Ltd and 77 hours for the Co-I (KS), with the latter not including appointments not attended by participants, rescheduling and booking appointments, and the administrative time required to send out PIPs, complete AE forms, and telephone participants. Time was also required from the PI (MS) on occasion to call participants to discuss a particular trial-related issue.
Table 3.
Reported AEs detailing severity and relationship to the treatment during the trial.
| AE No. | PI Grade* | Treatment-Related Events | Equipment-Related Events | Admin-Related Events | Event Description† | Support and Resolution | Outcome |
|---|---|---|---|---|---|---|---|
| 1 | Minor | - | - | Yes | Participant received unexpected text message from courier company requesting payment of export duty charge fee prior to delivery of trial equipment, which caused anxiety. Participant emailed study team to inform them. | Study team emailed Exsurgo Ltd to request they resolve the payment issue and informed participant not to pay the charge. Exsurgo Ltd addressed the matter with the company by paying the charge. | Equipment was successfully delivered to the participant. |
| 2 | Moderate | Yes | - | - | Participant reported experiencing a heavy feeling, tingling, and weakness on her left side during the session, accompanied by fatigue. She reported that she also gets the same symptoms, localized to her left hemiplegic side, when she is anxious. | The PI telephoned the participant to discuss the issues reported. After discussion, the PI determined that the reported symptoms were associated with intense concentration and underlying anxiety issues. | Participant was reassured and wished to continue in the trial. |
| 3 | Minor | - | Yes | - | Participant reported to the PI and CoI that she was unable to do the baseline sessions because the headset device was faulty. | Study team forwarded emails to Exsurgo Ltd and requested support. Exsurgo Ltd set up a support call with participant and confirmed the headset as faulty. Replacement headset sent to participant. | Participant received a new headset to resume the trial. |
| 4 | Minor | - | - | Yes | Participant received unexpected text message from courier company requesting payment of export duty charge fee prior to delivery of trial equipment, which caused anxiety. Participant emailed study team to inform them. | Study team emailed Exsurgo Ltd to request they resolve the payment issue and informed participant not to pay the charge. Exsurgo Ltd addressed the matter with the company by paying the charge. | Equipment was successfully delivered to the participant. |
| 5 | Minor | - | Yes | - | Participant's husband reported to CoI that headset failed to illuminate and was unable to establish a connection with tablet, so participant was unable to do the NFB sessions. | Study team forwarded emails to Exsurgo Ltd support for awareness and resolution. Exsurgo Ltd conducted a support call with participant and found out that one electrode felt pad had fallen out. | Participants replaced the missing pad using instructions provided by Exsurgo Ltd and continued with NFB sessions. |
| 6 | Minor | - | - | Yes | Participant received unexpected text message from courier company requesting payment of export duty charge fee prior to delivery of trial equipment, which caused anxiety. Participant emailed study team to inform them. | Exsurgo Ltd addressed the matter with the company by paying the charge. | Equipment was successfully delivered to the participant. |
| 7 | Minor | - | - | Yes | Participant received an unexpected telephone call from a delivery person at the courier company, who called the participant on her mobile telephone number to inform her that they have a parcel from Exsurgo Ltd to be delivered but could not deliver it to her because the export duty charge fee had not been paid. Participant contacted PI and CoI by email to inform them of the incident and to ask about what she should do. | PI apologized to the participant by email and informed her that she did not have to pay the duty import fee. PI also informed participant that the CoI had raised this as an ongoing issue of concern to Exsurgo Ltd and had requested that Exsurgo Ltd stop the courier from sending inappropriate text messages and contacting participants inappropriately in this manner. Exsurgo Ltd addressed the matter with the company by paying the charge. | Equipment was successfully delivered to the participant. |
| 8 | Minor | - | - | Yes | Participant emailed CoI to report that she had received a letter and telephone call from the courier company informing her that the export duty charge fee for the trial equipment had not been paid. The letter stated that if Exsurgo Ltd can send the courier company a letter or email saying that they will pay the money, then her name would be taken off their list. | The CoI sent an email back to the participant, copied to the PI and Exsurgo Ltd, to enable both to be made aware of this and enable investigation and resolution by Exsurgo Ltd. Exsurgo Ltd addressed the matter with the company by paying the charge. | Equipment was successfully delivered to the participant. |
| 9 | Minor | - | - | Yes | Participant reported to CoI that one of the headset electrode pads kept falling out, which meant that she could not do her baseline EEG recordings. | Study team emailed Exsurgo Ltd to inform them of the issue. Exsurgo Ltd sent a new electrode pad with replacement instructions to the participant. | Participant replaced electrode pad and continued with the trial. |
| 10 | Minor | - | - | Yes | Participant reported to CoI that the headset was not working. | CoI replied by email and copied Exsurgo Ltd support to request support for the participant. Participant sent screenshots of the app to Exsurgo Ltd support. Exsurgo Ltd support informed the participant that the Axon app had failed to install on the tablet and sent further technical advice. | Participant followed Exsurgo Ltd advice and informed study team that the equipment was working again. |
| 11 | Minor | - | Yes | - | Participant informed that the headset was not working, which prevented her from progressing beyond the eyes open baseline recordings. | Exsurgo Ltd arranged delivery of the new headset device and extended the training period by 1 more week to take delivery period into account. | Participant received a new headset device and continued with the trial. |
| 12 | Minor | - | - | Yes | Participant reported one of the headset electrode pads had fallen out. | Study team emailed Exsurgo Ltd to inform them of the issue. Exsurgo Ltd sent a new electrode pad with replacement instructions to the participant. | Participant replaced electrode pad and continued with the trial. |
| 13 | Minor | - | Yes | - | Participant reported a joint on the headset seemed to be broken and was not recording EEG activity properly. | Exsurgo Ltd arranged for delivery of a new headset. | Participant received new headset and continued with the trial. |
PI grading of AEs as minor, moderate, or serious.
Event description includes relationship to participation in the trial and treatment.
Table 4.
Resource metric measurement - training time for participants from Exsurgo Ltd.
| Participant | Number of Training Sessions* | Total Time in Training and Support Sessions (min) |
|---|---|---|
| EK02 | 2 | 100 |
| EK03 | 2 | 100 |
| EK04 | 2 | 100 |
| EK06 | 2 | 100 |
| EK07 | 2 | 300† |
| EK08 | 2 | 100 |
| EK09 | 2 | 100 |
| EK10 | 2 | 100 |
| EK12 | 2 | 100 |
NOTE. Total time training and supporting participants = 18.5 h.
Training was split into 2 sessions – fitting and baseline, followed by NFB training during the transition week.
Participant required a lot of support and had very high anxiety throughout.
Table 5.
Resource metric measurement – appointment time with the Co-I.
| Number of Participants | Total Number of Zoom Video Appointments per Participant in the Trial | Time (h) for First Zoom Video Assessment Appointment | Total Time (h) for 5 FU Zoom Video Appointments of 1 h | Total Appointment Time in Trial to Support Participants (h) |
|---|---|---|---|---|
| 11 | 6 | 2 | 5 | 77 |
NOTE. The time related to appointment nonattendance by participants, appointment rescheduling, and administrative tasks to support the trial are not included.
Secondary outcome measures
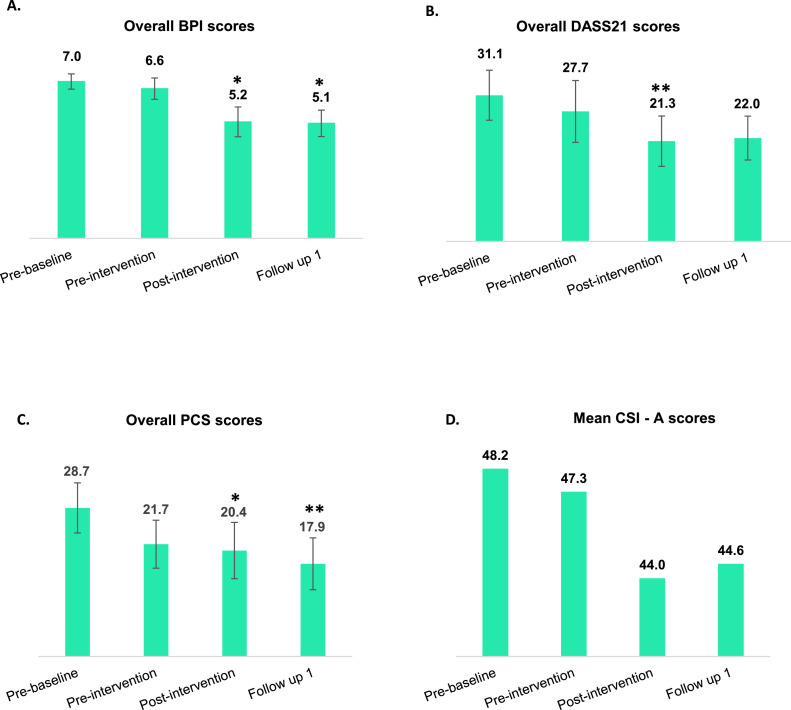
These are presented in table 6 and fig 4. Analysis of pain scores revealed that all participants reported improvements in total BPI scores. The mean total BPI score improved significantly from 7.0±0.5 at baseline to 5.2±0.8 postintervention and 5.1±0.6 (P<.05) at FU. Total Pain Catastrophizing Scale scores improved significantly from prebaseline to postintervention (P<.05) and showed continued improvements (P<.01) at FU (see fig 4). Clinically significant (≥30%) improvements in BPI were reported by 5 participants. Total Depression, Anxiety and Stress Outcome Scale-21 scores significantly improved from prebaseline (31.1) to postintervention (21.3) (P<.01). There was an improvement in mean CSI scores, with participants mostly categorized in the mild category at the postintervention and FU. The mean CSI score decreased from 48.2±6.6 to 44.0±7.2 postintervention and 44.6±6.7 at FU. However, there were no significant improvements in sleep or quality of life scores. Most participants (6/9) perceived minimal improvement or no change in their symptoms, and most participants (5/9) were satisfied with the treatment received. The full data analysis of the secondary outcomes measured in the trial will be published separately.
Table 6.
Overall mean outcome measurement scores.
| Outcome Measurements | Prebaseline (n=9) | Preintervention (n=9) | Postintervention (n=9) | FU 1 (n=9) |
|---|---|---|---|---|
| Overall BPI | 7.0±0.5 | 6.6±0.6 | 5.2±0.8 | 5.1±0.6 |
| DASS-21 | 31.1±5.3 | 27.7±6.6 | 21.3±5.4 | 22.0±4.7 |
| PCS | 28.7±4.8 | 21.7±4.6 | 20.4±5.4 | 17.9±5.0 |
| CSI | 48.2±6.6 | 47.3±6.6 | 44.0±7.3 | 44.6±6.7 |
| PSQI | 10.4±1.5 | 10.7±2.2 | 10.9±2.0 | 9.6±1.6 |
| EQ-5D-5L (VAS) | 52.2±6.3 | 45.7±6.8 | 52.0±7.4 | 51.1±6.0 |
NOTE. Data are shown as overall mean ± SE scores of the trial participants.
Abbreviations: DASS-21, Depression, Anxiety and Stress Scale-21 items; PCS, Pain Catastrophizing Score; PSQI, Pittsburgh Sleep Quality Index.
Fig 4.
Secondary outcome measurement scores at 4 TPs in the trial. This figure corresponds to the overall mean scores of the trial participants for (A) Overall BPI, (B) DASS-21, (C) PCS, and (D) CSI-A. The bar graphs are represented as mean ± SE where * denotes P<.05 and ** denotes P<.01. DASS-21, Depression, Anxiety and Stress Scale-21 items; PCS, Pain Catastrophizing Scale.
Discussion
Our objectives were to test the feasibility and safety of an 8-week home-based NFB intervention for people with CNP and to explore if there were any potential secondary health benefits of the intervention. The composition of our sample (66.7% women and 33.3% men) reflected the research literature,4, 5, 6 which indicates that more women than men are diagnosed with NP. The mean age of participants was 51.9 years, which also aligns with the research literature.4, 5, 6 The largest source of CNP in our sample was related to pain experienced by stroke survivors (4/6 who completed the trial). In alignment with the research literature,4,6 most people with CNP in our sample also had associated comorbidities, including fibromyalgia and migraine. Several studies on the use of EEG NFB for various CP conditions (such as fibromyalgia, NP, and migraine) have identified a positive correlation between NFB interventions and pain improvement; however, these studies may have limitations in terms of design and sample sizes, which can potentially lead to a biased conclusion.22 Therefore, randomized controlled trials with more robust methodologies are needed to strengthen the evidence base for the use of NFB in CP management.
In terms of accessibility, this study had a 2:1 ratio for participant screening to trial enrollment and also provided insight into a range of recruitment barriers. These included participants’ access to their own laptop, personal computer, or tablet (financial barrier); dependence on having an email address (an IT literacy barrier); the burden of time required from participants taking part (a 22-week trial commitment period); and the relatively high level of IT literacy required from participants. There is limited research on home-based portable EEG NFB systems, although one center-based study identified that participants often had to travel over 1 hour to the center, which caused fatigue that was counterproductive to the NFB.23 This suggests that it would be advantageous to develop more user-friendly home-based systems, which would also minimize carbon footprint. Usability is an important concept in relation to brain-computer interface systems, and usability research studies have determined that home-based systems should be effective, simple to use, portable, and inexpensive.8 By doing this, the “burden of treatment” is considered to be minimized.24 Technical issues might be more easily resolved if a study was center-based with IT experts on site, although having a study based in a single center would also pose geographic constraints on participant recruitment. Three participants required assistance from a family member to navigate and use the Axon system, and 1 participant had ongoing difficulties with participation because the individual was required to use their own personal IT device for the Zoom appointments, and the 2 devices she had access to (a tablet and smart mobile telephone) did not enable her to participate without difficulty. These digital barriers should be addressed in any future trial.
Logistics were improved during the trial by changing the courier delivery system so that participants were not approached by the courier requesting financial payment of a duty tax charge, which caused anxiety. Equipment-related AEs might be reduced in future trials because the equipment has already been redesigned. The treatment-related AE warrants consideration in terms of the level of concentration required during participation because this was identified as the factor associated with the symptoms reported during intervention use. Further details on AE outcomes is presented in table 3.
Three participants dropped out during the FU period of 12 weeks (see fig 1), which was considered by some to be too long. The length of the FU period was designed to assess the sustainability of NFB treatment effects and would benefit from adjustment in future trials as appropriate. At least 2 participants informed the Co-I (KS) and PI (MS) that they did not know that they should be performing baseline readings for 12 weeks during the FU time period, which may be related to memory issues and the complexity of the participation process. In any future trial, these tolerability issues should be addressed.
In terms of human resources, it would be possible to improve the efficiency of the process and reduce the time burden on the research team with some improvements to the software system used; for example, an automatic email alert sent to the PI (MS) and Co-I (KS) to inform them of when a participant has completed the electronic screening form. Improvements in pain levels, mood, and central sensitization scores were detected, which indicates that these outcome measures used are sensitive and relevant for use in a larger potential future trial. These outcomes and learning insights provide a solid foundation for future trials.
Study limitations
Our results are not generalizable to the larger population of people living with CNP, given the small sample and unblinded single-group design. This was also a convenience sample, which is not representative of the larger population of people living with CNP. The study experienced a notable dropout rate, technical issues, and limited perceived benefits among participants. Our eligibility criteria and screening protocol were also subject to selection bias. Secondary outcome results should be interpreted with caution because of the small sample size. These limitations will be addressed by a larger randomized controlled trial.
Conclusions
It is feasible and safe to conduct a home-based NFB intervention for people with CNP within the National Health Service framework, when the identified human and IT resources are provided and relevant governance processes are followed. The improvements in the secondary outcomes of pain and mood justify further investigation with a randomized controlled trial to confirm the efficacy of the intervention.
Suppliers
-
a.
Axon EEG NFB system; Exsurgo Ltd.
-
b.
Zoom communications platform; Zoom Video Communications, Inc.
-
c.
AWS cloud-based storage service; Amazon.
-
d.
SPSS, version 29.0; IBM.
Disclosures
The investigators have no financial or nonfinancial disclosures to make in relation to this project.
Acknowledgments
We thank all participants for their valuable time and effort; this study would not have been possible without them.
Footnotes
Clinical Trial Registration No.: NCT05464199.
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.arrct.2024.100361.
Appendix. Supplementary materials
References
- 1.International Association for the Study of Pain. What is neuropathic pain? Available at: https://www.iasp-pain.org/wp-content/uploads/2022/10/What-is-Neuropathic-Pain.pdf Accessed August, 2024.
- 2.Cohen SP, Vase L, Hooten WM. Chronic pain: an update on burden, best practices, and new advances. Lancet. 2021;397:2082–2097. doi: 10.1016/S0140-6736(21)00393-7. [DOI] [PubMed] [Google Scholar]
- 3.Colloca L, Ludman T, Bouhassira D, et al. Neuropathic pain. Nat Rev Dis Primers. 2017;3:17002. doi: 10.1038/nrdp.2017.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van Hecke O, Austin SK, Khan RA, Smith BH, Torrance N. Neuropathic pain in the general population: a systematic review of epidemiological studies. Pain. 2014;155:654–662. doi: 10.1016/j.pain.2013.11.013. [DOI] [PubMed] [Google Scholar]
- 5.Fayaz A, Croft P, Langford RM, Donaldson LJ, Jones GT. Prevalence of chronic pain in the UK: a systematic review and meta-analysis of population studies. BMJ Open. 2016;6 doi: 10.1136/bmjopen-2015-010364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Merskey H, Bogduk N. IASP Task Force on Taxonomy. 2nd ed. IASP Press; Seattle: 1994. Classification of Chronic Pain. [Google Scholar]
- 7.National Institute for Health and Care Excellence (NICE). Neuropathic Pain - drug treatment. Last revised February 2024. Available at: https://cks.nice.org.uk/topics/neuropathic-pain-drug-treatment/ Accessed August, 2024.
- 8.Al-Taleb MKH, Purcell M, Fraser M, Petric-Gray N, Vuckovic A. Home used, patient self-managed, brain-computer interface for the management of central neuropathic pain post spinal cord injury: usability study. J Neuroeng Rehabil. 2019;16:128. doi: 10.1186/s12984-019-0588-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Birch N, Graham J, Ozolins C, Kumarasinghe K, Almesfer F. Home-based EEG neurofeedback intervention for the management of chronic pain. Front Pain Res (Lausanne) 2022;3 doi: 10.3389/fpain.2022.855493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Learmonth YC, Motl RW. Important considerations for feasibility studies in physical activity research involving persons with multiple sclerosis: a scoping systematic review and case study. Pilot Feasibility Stud. 2017;4:1. doi: 10.1186/s40814-017-0145-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thabane L, Ma J, Chu R, et al. A tutorial on pilot studies: the what, why and how. BMC Med Res Methodol. 2010;10:1. doi: 10.1186/1471-2288-10-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Good Clinical Practice (GCP). Available at: https://www.nihr.ac.uk/health-and-care-professionals/training/good-clinical-practice.htm. Accessed October 2023
- 13.Kozlowski AJ, Fabian M, Lad D, Delgado AD. Feasibility and safety of a powered exoskeleton for assisted walking for persons with multiple sclerosis: a single-group preliminary study. Arch Phys Med Rehab. 2017;98:1300–1307. doi: 10.1016/j.apmr.2017.02.010. [DOI] [PubMed] [Google Scholar]
- 14.Hawker GA, Mian S, Kendzerska T, French M. Measures of adult pain: Visual Analog Scale for Pain (VAS Pain), Numeric Rating Scale for Pain (NRS Pain), McGill Pain Questionnaire (MPQ), Short-Form McGill Pain Questionnaire (SF-MPQ), Chronic Pain Grade Scale (CPGS), Short Form-36 Bodily Pain Scale (SF-36 BPS), and Measure of Intermittent and Constant Osteoarthritis Pain (ICOAP) Arthritis Care Res (Hoboken) 2011;63(Suppl 11):S240–S252. doi: 10.1002/acr.20543. [DOI] [PubMed] [Google Scholar]
- 15.Cleeland CS, Ryan KM. Pain assessment: global use of the Brief Pain Inventory. Ann Acad Med Singap. 1994;23:129–138. [PubMed] [Google Scholar]
- 16.Henry JD, Crawford JR. The short-form version of the Depression Anxiety Stress Scales (DASS-21): construct validity and normative data in a large non-clinical sample. Br J Clin Psychol. 2005;44(Pt 2):227–239. doi: 10.1348/014466505X29657. [DOI] [PubMed] [Google Scholar]
- 17.Pain Catastrophizing Scale. Physiopedia. Available at: https://www.physio-pedia.com/Pain_Catastrophizing_Scale. Accessed November 2023.
- 18.Teater M, Teater D. The Central Sensitization Inventory (CSI). Available at: https://www.emdr-training.net/wp-content/uploads/2019/09/CSI_Inventory_and_Scoring.pdf. Accessed December 2023.
- 19.Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 20.Balestroni G, Bertolotti G. [EuroQol-5D (EQ-5D): an instrument for measuring quality of life] [Italian] Monaldi Arch Chest Dis. 2012;78:155–159. doi: 10.4081/monaldi.2012.121. [DOI] [PubMed] [Google Scholar]
- 21.IBM SPSS Statistics. Version 29. 2023.
- 22.Diotaiuti P, Corrado S, Tosti B, et al. Evaluating the effectiveness of neurofeedback in chronic pain management: a narrative review. Front Psychol. 2024;15 doi: 10.3389/fpsyg.2024.1369487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hassan MA, Fraser M, Conway BA, Allan DB, Vuckovic A. The mechanism of neurofeedback training for treatment of central neuropathic pain in paraplegia: a pilot study. BMC Neurol. 2015;15:200. doi: 10.1186/s12883-015-0445-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gonçalves AV, Jácome CI, Demain SH, Hunt KJ, Marques AS. Burden of treatment in the light of the international classification of functioning, disability and health: a “best fit” framework synthesis. Disabil Rehabil. 2017;39:1253–1261. doi: 10.1080/09638288.2016.1194898. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.