Key Points
Question
What is the effect of 8 weeks of treatment with at least 4.69 × 109 colony-forming units/d of Bifidobacterium animalis subsp lactis HN019 on complete spontaneous bowel movements (CSBMs) in individuals with constipation?
Findings
This triple-blindrandomized clinical trial including 229 participants in 5 hospitals in China demonstrated that CSBMs were improved in both the placebo and HN019 groups, with no meaningful differences between groups.
Meaning
Daily consumption of B lactis HN019 at the tested dose of 4.69 × 109 CFU did not outperform placebo to increase CSBMs.
Abstract
Importance
Probiotic supplementation may improve bowel movements. However, large, properly designed studies are lacking.
Objective
To evaluate the potential benefit of Bifidobacterium animalis subsp lactis HN019 on constipation, expressed as complete spontaneous bowel movements (CSBMs).
Design, Setting, and Participants
This randomized triple-blind placebo-controlled clinical trial with 2 weeks of run-in and 8 weeks of intervention was conducted from December 25, 2020, to February 28, 2022, at 5 hospitals in Shanghai, China. Participants included healthy volunteers with functional constipation according to Rome III criteria, 18 to 70 years of age, and a body mass index (calculated as the weight in kilograms divided by the height in meters squared) of less than 30.0. Eligibility after the run-in phase required the randomized participants to have 3 or fewer CSBMs/wk. Data were analyzed from September 29, 2022, to March 23, 2023, and reported as intention to treat.
Intervention
Participants were randomized to receive probiotic (B lactis HN019, 7.0 × 109 colony forming units (CFU)/d in maltodextrin at the start of the study and 4.69 × 109 CFU/d at the end of the study or maltodextrin placebo once a day for 8 weeks.
Main Outcomes and Measures
Primary outcome was change in CSBMs. Secondary outcomes included use of rescue medication, stool consistency, degree of straining for each bowel movement, abdominal pain, and bloating. Further, dietary habits and physical activity were recorded. Fecal samples were analyzed for moisture content, short-chain fatty acids, branched-chain fatty acids, microbiota composition, and calprotectin.
Results
Of the 283 individuals assessed for eligibility, 229 were randomized to either the placebo (n = 117) or the HN019 (n = 112) group. One participant in the placebo group discontinued due to COVID-19 restrictions. The 229 participants (194 [84.7% female) had a median age of 45 (38-52) years, mean (SD) BMI of 22.8 (2.5), and a mean (SD) of 0.77 (1.0) CSBM/wk. There was no difference in the change of weekly CSBMs from baseline to the end of study between the HN019 (least-square mean change, 0.80 [95% CI, 0.54-1.05]) and placebo (least-square mean change, 0.66 [95% CI, 0.41-0.90]) groups.
Conclusions and Relevance
Although probiotics have been reported to improve bowel function, this large, well-conducted randomized clinical trial did not confirm such results. Daily consumption of B lactis HN019 at the tested dose of 4.69 × 109 CFU did not outperform placebo to increase CSBMs.
Trial Registration
Chinese Clinical Trial Registry Identifier: ChiCTR2000029215
This randomized clinical trial evaluates the effect of HN019 supplementation in the management of functional constipation in a Chinese population as determined by complete spontaneous bowel movements per week.
Introduction
Constipation is a common gastrointestinal tract disorder affecting 14% to 16% of the general Western population.1 In China, the prevalence in adults ranges from 4% to 10%.2 The cause of constipation is multifactorial. Bowel movements alone are not a reliable indicator for constipation severity, as they might be the successive outcome from laxatives without describing the sensation during defecation or the repeated passage of hard stools and straining.1,3 Complete spontaneous bowel movements (CSBMs) are a more reliable indicator.4
Bifidobacterium animalis subsp lactis HN019 (HN019) has been shown to improve constipation and gastrointestinal tract symptoms in adults at doses ranging from 1.0 × 109 to 17.2 × 109 colony-forming units (CFU)/d for 2 to 4 weeks.5,6 Nevertheless, no studies have been performed using HN019 for functional constipation treatment with bowel movement frequency (BMF) as primary outcome. The aim of this study was to evaluate the effect of HN019 in the management of functional constipation as determined by CSBMs among patients in China.
Methods
Protocol
This randomized clinical trial followed the Consolidated Standards of Reporting Trials (CONSORT) reporting guideline. This trial was conducted in the gastroenterology units at Shanghai 9th People’s Hospital (coordinating site), Shanghai Tongji Hospital, Shanghai Zhongshan Hospital, Shanghai Tongren Hospital, and Shanghai 6th People’s Hospital, Shanghai, China, from December 25, 2020, to February 28, 2022. Ethical approval was received from each hospital’s Ethics Committee and the China Human Genetic Resources Management Office. The trial protocol and statistical analysis plan are found in Supplement 1. In short, eligible participants were enrolled and received placebo for 2 weeks. After this, compliant participants were randomized to receive either probiotic or placebo for 8 weeks.
Participants were prescreened via telephone call for self-evaluated symptoms of functional constipation, followed by main inclusion and exclusion criteria assessments at visit 1. Only eligible participants signed the informed consent and were enrolled in the study (eFigure 1 in Supplement 2). At visit 2, confirmation of BMF (≤3/wk) and treatment adherence (100%) was required. At baseline (visit 3), final eligibility was examined, randomization was undertaken, and assessments of clinical, exploratory, ancillary and safety outcomes were performed. Intermediate visit 4 and end of study (EOS) visit 5 recorded clinical, exploratory, ancillary, and safety outcomes. The 3-day dietary record and fecal samples were only collected at EOS (eFigure 1 in Supplement 2).
Participants
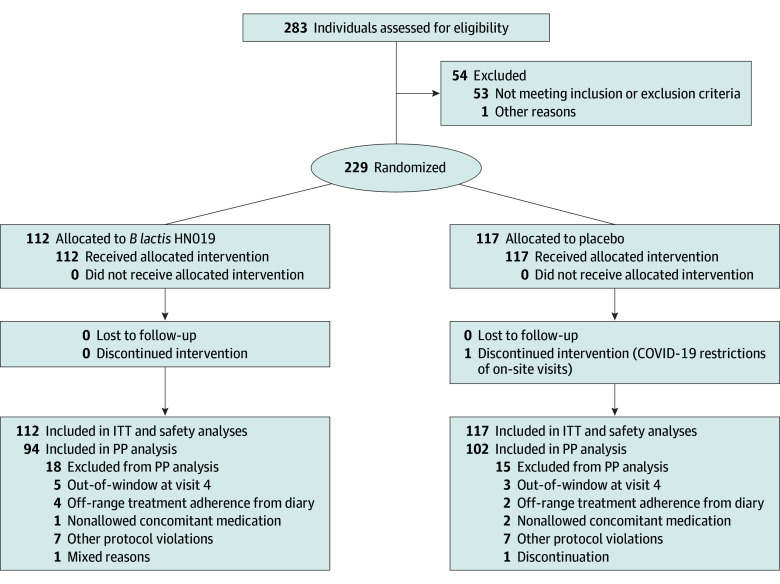
Participants were otherwise healthy (judged by physicians), free-living individuals with functional constipation (excluding constipation-dominated irritable bowel syndrome) by Rome III criteria, 18 to 70 years of age, with a body mass index (BMI; calculated as the weight in kilograms divided by the height in meters squared) of less than 30.0. Rome III was selected over Rome IV criteria to benefit from experiences of earlier studies.5,6 Additional eligibility criteria at visits 1 to 3 required the randomized participants to have 3 or fewer CSBMs/wk, 100% adherence for both investigational treatment intake and diary data entry, and no safety concerns (Figure 1). Detailed eligibility criteria are provided in eMethods in Supplement 2.
Figure 1. The CONSORT Participant Flow Diagram.
B lactis HN019 indicates Bifidobacterium animalis subsp lactis HN019; ITT, intention to treat; and PP, per protocol.
Randomization and Blinding
Participants were equally, randomly allocated to receive probiotic or placebo at the baseline visit (visit 3). A centralized randomization list was prepared using SAS software, version 9.4 (SAS Institute Inc), by a statistician who had no other role in the study. Although each study site was originally intended to be stratified by sex (30% men), this was abandoned due to recruitment difficulties. Block sizes of 4, 6, and 8 were randomly assigned to each site. All products were masked and labeled with a randomization number by representatives of the sponsor who were responsible for product manufacturing and labeling, but had no other involvement in the study. The randomization numbers were uploaded into the electronic case report form (eCRF; Viedoc Clinic) from which the investigator received the treatment codes for the intervention assigned to the enrolled participants. Trial participants, clinicians, outcome assessors, and data analysts were blinded until all data were analyzed.
Study Products
The probiotic was freeze-dried HN019 (7.0 × 109 CFU at first participant visit and 4.69 × 109 CFU at last participant visit) mixed with maltodextrin and silicon dioxide in a 2-g sachet. The comparator was a matching placebo containing the same formulation except the HN019. Participants ingested 1 sachet daily of investigational treatment (placebo during run-in and probiotic or placebo during treatment period), orally with still water at room temperature at least 15 minutes before breakfast.
Study Outcomes, Data Collection, and Management
Clinical Outcomes
The primary end point was the mean change in the number of CSBMs reported each week from baseline to EOS. The secondary clinical outcomes were use of rescue medication, stool consistency using the Bristol Stool Scale (BSS),7,8 and the intensity of the degree of straining for each bowel movement. The BSS scores ranged from 1 (separate hard lumps) to 7 (watery), with 3 to 5 indicating optimal. Abdominal pain and bloating were rated using a visual analogue scale (VAS; range, 0-100 mm, with higher scores indicating more severe symptoms).5,9 Allowed rescue medication was bisacodyl, 5 to 10 mg/d (CPU-Pharma). All outcome measures were recorded in a daily diary. The severity of constipation symptoms and quality of life were assessed at visits 3 to 5 using the Patient Assessments of Constipation–Symptoms (PAC-SYM) and the PAC–Quality of Life (PAC-QoL), respectively10 with a 5-point Likert scale ranging from 0 (absent) to 4 (severe).
Ancillary Outcomes
Diet and physical activity have a major influence on bowel function and were therefore assessed at visits 3 to 5. Dietary habits were captured with a self-declared 3-day dietary record to quantify total intake of calories, carbohydrates, fat, protein, and fiber. Nutrition Star software, version 5.2.0 (Zhending Inc), was used for analysis. Physical activity levels were quantified by the International Physical Activity Questionnaire–Short Version.11 Treatment and diary adherence was determined using eCRF data and derived from diaries.
Exploratory Outcomes
Fresh stool samples were collected from 50% of the participants at home within 3 days before visits 3 and 5. Participants stored the stool samples immediately in the freezer (−18 °C) before sending them frozen to the coordinating laboratory within 24 hours. Fecal analyses of moisture content, short-chain fatty acids, branched-chain fatty acids (BCFAs), microbiota composition, and calprotectin, as well as the quantification of B lactis before and after the intervention were assessed as detailed in the eMethods in Supplement 2.
Safety Outcomes
Adverse events (AEs) were collected in daily diaries, coded according to Medical Dictionary of Regulatory Authorities, version 24.1, and summarized according to the System Organ Class and Preferred Term. Laboratory safety tests were performed for whole blood hematology, serum variables, and urine analyses at visit 1 and EOS (eMethods in Supplement 2).
Data Collection and Management
The participant data, including demographic characteristics, medical history, physical examination results, BMI, vital signs, pregnancy, safety values, and data from the PAC-SYM, PAC-QoL, and International Physical Activity Questionnaire–Short Form were entered directly into the eCRF by site personnel, whereas information from daily diaries, such as clinical outcomes and AEs, use of investigational treatment, rescue medication, and concomitant medication, and most of the efficacy outcomes, such as the number of bowel movements, the completeness and degree of straining of each defecation, and stool consistency were collected by electronic patient-reported outcome diary (Viedoc Me) or paper diaries as backup and entered into the eCRF at sites during the following visit.
Sample Size Calculation
The power calculation was based on a 2-sample t test assuming equal variance using a meta-analysis on the effects of probiotics for total bowel movements per week, which was the only available and reliable reference.12 The sample size was estimated at 178 evaluable participants (89 per group), assuming an estimated mean (SD) difference for stool frequency (total No. of bowel movements per week) of 0.83 (1.69), considering a 2-sided α = .05 and a power of 90%. Accounting for 20% attrition, a total number of 224 randomized participants (112 per group) was needed.
Statistical Analysis
Data were analyzed from September 29, 2022, to March 23, 2023. All efficacy end points were analyzed using intention-to-treat (ITT) and per protocol (PP) populations. The ITT analysis was designated as primary whereas the PP analysis was considered supportive. Safety analyses were conducted using the safety population. The ITT set consisted of all randomized participants, while the safety set included the participants who consumed at least 1 dose of investigational treatment and had a subsequent safety test. The PP set included the ITT population without relevant protocol violations. Missing data were assumed to be missing at random, and no imputations were performed. Results are shown as mean (SD) unless otherwise stated. We applied the mixed model for repeated measurements (MMRM) to normally distributed variables, and otherwise implemented the Friedman nonparametric test and Cochran-Mantel-Haenszel statistic. Normality was assessed by graphical display. The MMRM included fixed effects for baseline value, sex, study center, treatments, week (repeating factor), and the interaction between treatment group and week. When the response variable was categorical, it was analyzed with logistic regression, including treatment group, sex, and center as fixed effects.
The primary analysis for the mean change in CSBMs per week from baseline to week 8 used MMRM for the ITT population. The 2 hypotheses to be tested were (1) HN019 supplementation is not superior to placebo as measured by CSBMs per week across the 8-week treatment period (the null hypothesis) and (2) HN019 supplementation is superior to placebo as measured by CSBMs per week across the 8-week treatment period (the alternative hypothesis).
The significance was measured as 2-sided P = .05. To control the type I error, a hierarchical testing procedure was used for multiplicity testing, which started with the primary end point and continuing with the several secondary end points in order: degree of straining, bloating, and overall BSS, PAC-SYM, and PAC-QoL scores. The testing procedure continued if the observed P value was statistically significant. Additionally, sensitivity and responder analyses were performed for the primary end point to support the primary evaluation (eMethods in Supplement 2).
Secondary end points, bowel movements and spontaneous bowel movements with repeated multiple measurements were analyzed using MMRM based on restricted maximum likelihood. The P values are regarded as descriptive measures. Statistical analyses were performed with SAS Enterprise Guide, version 8.3 for Windows (SAS Institute Inc).
Results
The participant flow diagram is presented in Figure 1. In total, 283 participants were enrolled for screening, 229 were randomized, and 228 completed the intervention.
Study Populations
Baseline demographic characteristics and constipation symptoms were similar between the intervention groups (Table 1). Participants were middle-aged adults (median age, 45 [28-52] years) with mild constipation (mean of 0.77 [1.0] bowel movements/wk) and normal to overweight BMI (mean, 22.8 [2.5]). A total of 194 participants (84.7%) were female and 35 (15.3%) were male. Participants were otherwise healthy as judged by results of blood and urine analyses and vital signs (eTable 1 in Supplement 2).
Table 1. Baseline Characteristics of Study Population.
| Characteristics | HN019 group (n = 112) | Placebo group (n = 117) |
|---|---|---|
| Age, mean (SD), y | 46.0 (10.2) | 44.1(10.2) |
| Age group, No. (%) | ||
| ≤45 y | 54 (48.2) | 66 (56.4) |
| >45 y | 58 (51.8) | 51 (43.6) |
| Sex, No. (%) | ||
| Female | 97 (86.6) | 97 (82.9) |
| Male | 15 (13.4) | 20 (17.1) |
| BMI | ||
| Mean (SD) | 22.8 (2.6) | 22.8 (2.4) |
| Range | 18.5-29.7 | 18.7-28.9 |
| Smoking status, No. (%) | ||
| Ever | 1 (0.9) | 2 (1.7) |
| Never | 111 (99.1) | 115 (98.3) |
| Stool frequency, mean (SD) | ||
| CSBM/wk | 0.73 (0.98) | 0.81 (1.03) |
| SBM/wk | 2.53 (1.46) | 2.51 (1.34) |
| BM/wk | 2.57 (1.44) | 2.54 (1.34) |
| Stool consistency, mean (SD) BSS scorea | 3.2 (1.1) | 3.1 (1.1) |
| Gut symptoms, mean (SD) VAS score, mmb | ||
| Abdominal pain | 4.8 (7.5) | 4.8 (8.8) |
| Degree of straining | 49.3 (24.9) | 51.0 (24.6) |
| Bloating | 10.8 (15.1) | 10.0 (12.2) |
| PAC-SYM, total scorec | 0.8 (0.5) | 0.8 (0.5) |
| PAC-QoL, total scorec | 1.3 (0.6) | 1.2 (0.6) |
| Use of rescue medication, No. (%) | ||
| Yes | 5 (4.5) | 5 (4.3) |
| No | 107 (95.5) | 112 (95.7) |
Abbreviations: BMI, body mass index (calculated as the weight in kilograms divided by the height in meters squared); BM, bowel movement; BSS, Bristol Stool Scale; CSBM, complete spontaneous BM; PAC-QoL, Patient Assessment of Constipation–Quality of Life; PAC-SYM, Patient Assessment of Constipation–Symptoms; SBM, spontaneous BM; VAS, visual analogue scale.
Scores range from 1 (separate hard lumps) to 7 (watery), with 3 to 5 indicating optimal.
Scores range from 0 to 100 mm, with greater scores indicating more severe symptoms.
Scores range from 0 (absent) to 4 (severe).
Clinical Outcomes
Stool Frequency
Mean baseline CSBMs per week were 0.73 (0.98) for the HN019 group and 0.81 (1.03) for the placebo group. After 8 weeks of supplementation, mean CSBMs per week increased to 1.58 ( 1.58) in the HN019 group and 1.50 (1.66) in the placebo group. However, there was no statistically significant difference between the groups in change from baseline to EOS in CSBMs per week (least square mean in probiotic group, 0.80 [95% CI, 0.54-1.05]; least square mean in placebo group, 0.66 [95% CI, 0.41-0.90]; P = .37). Similar observations were made for spontaneous bowel movements and bowel movements (Table 2). In eTable 2 in Supplement 2, the evolution of the bowel movements is presented, while in eTable 3 in Supplement 2 the weekly change from baseline and the statistical difference between groups are presented. On a weekly basis, there was no significant difference in change from baseline between the groups (Figure 2A). The sensitivity analysis, which included baseline BMI and age as covariates in the MMRM analysis, confirmed the results for the primary outcome (CSBMs) (eTable 4 in Supplement 2).
Table 2. Stool Frequency in ITT Population.
| Bowel movement type | No. per week, mean (SD) | MMRM analysis, LSM (95% CI) | P valuea | |||
|---|---|---|---|---|---|---|
| HN019 group (n = 112) | Placebo group (n = 117) | Change (week 8 − week 0) | HN019 vs placebo | |||
| HN019 group (n = 112) | Placebo group (n = 117) | |||||
| Complete spontaneous | ||||||
| Baseline (visit 3, wk 0) | 0.73 (0.98) | 0.81 (1.03) | 0.80 (0.54 to 1.05) | 0.66 (0.41 to 0.90) | 0.14 (−0.17 to 0.45) | .37 |
| End of study (visit 5, wk 8) | 1.58 (1.58) | 1.50 (1.66) | ||||
| Change (wk 8 − wk 0) | 0.85 (1.39) | 0.68 (1.54) | ||||
| Spontaneous | ||||||
| Baseline (visit 3, wk 0) | 2.53 (1.46) | 2.51 (1.34) | 0.49 (0.25 to 0.74) | 0.45 (0.21 to 0.68) | 0.05 (−0.25 to 0.34) | .76 |
| End of study (visit 5, wk 8) | 3.00 (1.36) | 2.95 (1.62) | ||||
| Change (wk 8 − wk 0) | 0.47 (1.38) | 0.44 (1.47) | ||||
| Any | ||||||
| Baseline (visit 3, wk 0) | 2.57 (1.44) | 2.54 (1.34) | 0.46 (0.21 to 0.70) | 0.42 (0.18 to 0.65) | 0.04 (−0.26 to 0.34) | .78 |
| End of study (visit 5, wk 8) | 3.01 (1.35) | 2.95 (1.62) | ||||
| Change (wk 8 − wk 0) | 0.44 (1.36) | 0.41 (1.50) | ||||
Abbreviations: ITT, intention to treat; LSM, least square mean; MMRM, mixed model for repeated measurements.
Estimated from MMRM model.
Figure 2. Study Outcomes.
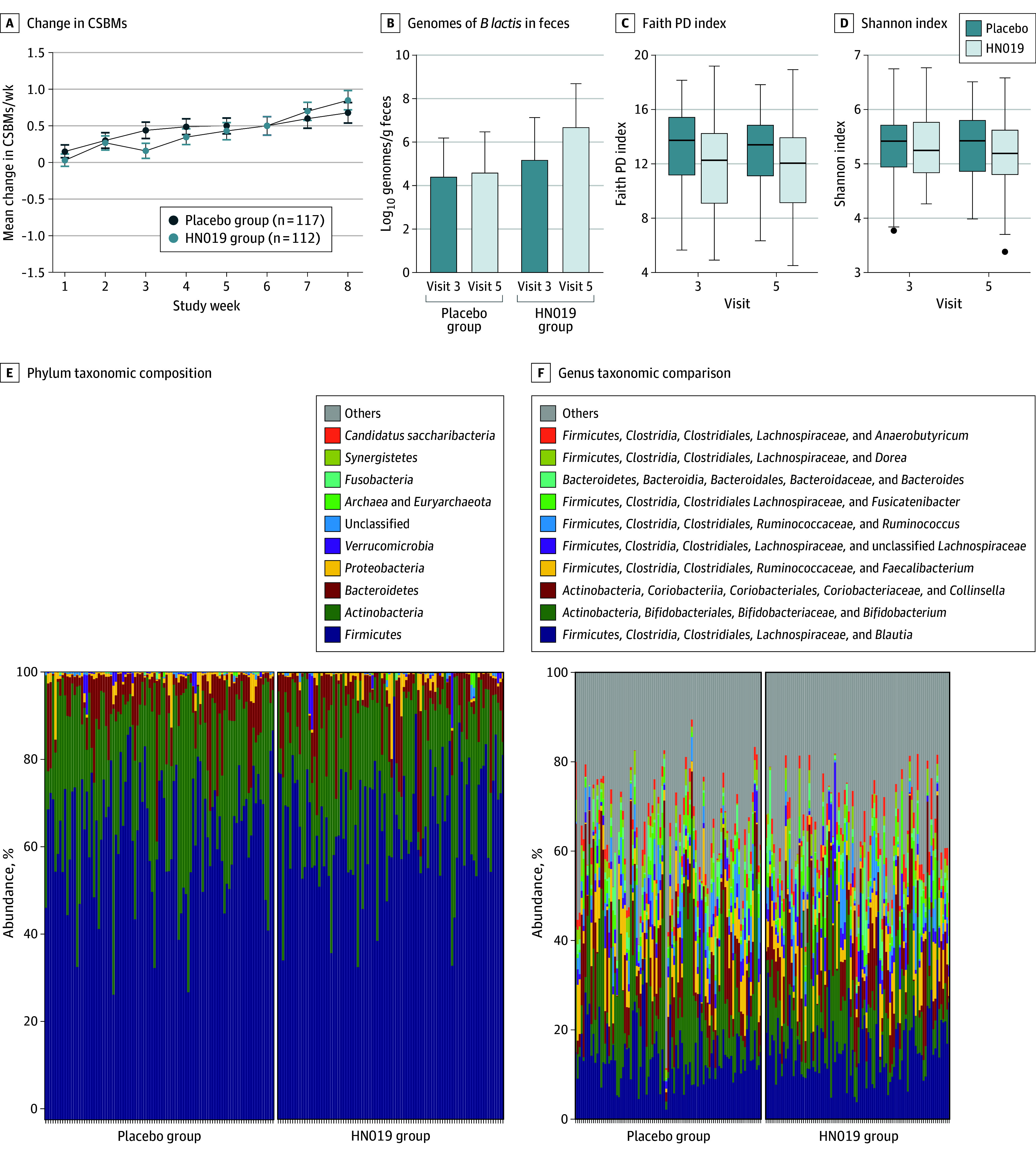
A, Mean change in complete spontaneous bowel movements (CSBMs). B, Mean log10 genomes of Bifidobacterium animalis subsp lactis HN019 in feces at baseline (visit 3) and at the end of the study (visit 5). Error bars indicate SD. C, Fecal microbiota alpha diversity measured by Faith phylogenetic diversity (PD) index (range, 4.51-19.20, with higher values indicating greater diversity). Horizontal bars indicate the median, first and third quartiles; boxes, the interquartile range; and error bars, the minimum and maximum values. D, Fecal microbiota alpha diversity measured by Shannon index (range, 3.39-6.77, with higher values indicating greater diversity). Horizontal bars indicate the median, first and third quartiles; boxes, the interquartile range; error bars, the minimum and maximum values; and data points, outliers. E, Taxonomic composition of fecal microbiota at phylum level. F, Taxonomic composition of fecal microbiota at genus level.
The hierarchical testing procedure was stopped as CSBMs were not found to be significantly different between the 2 treatment groups (P = .37). Additional statistical analysis of the secondary outcomes only showed small nominal differences between the 2 groups (eTable 9 in Supplement 2).
Stool Consistency
Stool consistency was moderately hard in both groups, with a mean BSS score of 3.20 (1.11) in the HN019 group and 3.10 (1.14) in the placebo group. Over the course of the study, mean BSS score increased slightly in both groups, with less improvement in the HN019 group (3.43 [0.96]) compared with the placebo group (3.71 [1.02]; P = .08) (Table 3). In eTable 2 in Supplement 2, the evolution of the BSS rating is presented. eTable 3 in Supplement 2 presents the weekly change from baseline and the statistical difference of this between the groups.
Table 3. Stool Consistency, Gut Symptoms, and Quality of Life in Intention-to-Treat Population.
| Secondary outcome | Population | MMRM analysis | P value | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| HN019 (n = 112) | Placebo (n = 117) | Change (wk 8 – wk 0) | HN019 vs placebo, LSM (95% CI) | |||||||
| No. | Mean (SD) | No. | Mean (SD) | HN019 (n = 112) | Placebo (n = 117) | |||||
| No. | LSM (95% CI) | No. | LSM (95% CI) | |||||||
| Stool consistencya | ||||||||||
| Baseline (visit 3, wk 0) | 47 | 3.20 (1.11) | 53 | 3.10 (1.14) | 42 | 0.20 (−0.10 to 0.50) | 45 | 0.54 (0.25 to 0.82) | −0.335 (−0.707 to 0.036) | .08 |
| End of study (visit 5, wk 8) | 71 | 3.43 (0.96) | 71 | 3.71 (1.02) | ||||||
| Change (wk 8 – wk 0) | 42 | 0.16 (1.16) | 45 | 0.73 (1.21) | ||||||
| Abdominal pain, VAS scoreb,c | ||||||||||
| Baseline (visit 3, wk 0) | 112 | 4.8 (7.5) | 117 | 4.8 (8.8) | 112 | −0.04 (−1.20 to 1.13) | 116 | 1.42 (0.30 to 2.53) | −1.46 (−2.84 to −0.07) | .04 |
| End of study (visit 5, wk 8) | 112 | 4.4 (8.1) | 117 | 5.9 (10.5) | ||||||
| Change (wk 8 – wk 0) | 112 | −0.4 (6.1) | 116 | 1.1 (6.9) | ||||||
| Degree of straining, VAS scorec | ||||||||||
| Baseline (visit 3, wk 0) | 112 | 49.3 (24.9) | 116 | 51.0 (24.6) | 112 | −11.9 (−15.8 to −8.0) | 115 | −13.9 (−17.6 to −10.1) | 1.98 (−2.61 to 6.53) | .40 |
| End of study (visit 5, wk 8) | 112 | 37.5 (26.7) | 116 | 36.3 (24.5) | ||||||
| Change (wk 8 – wk 0) | 112 | −11.9 (18.6) | 115 | −14.6 (23.8) | ||||||
| Bloating, VAS scorec | ||||||||||
| Baseline (visit 3, wk 0) | 112 | 10.8 (15.1) | 117 | 10.0 (12.2) | 112 | −0.52 (−2.45 to 1.42) | 116 | 2.13 (0.27 to 3.98) | −2.64 (−4.92 to −0.36) | .02 |
| End of study (visit 5, wk 8) | 112 | 10.0 (15.1) | 116 | 11.8 (15.9) | ||||||
| Change (wk 8 – wk 0) | 112 | −0.8 (7.0) | 116 | 1.9 (11.3) | ||||||
| Abdominal symptoms scored | ||||||||||
| Baseline (visit 3, wk 0) | 112 | 0.40 (0.48) | 117 | 0.39 (0.47) | 112 | −0.16 (−0.24 to −0.08) | 116 | −0.23 (−0.31 to −0.15) | 0.07 (−0.03 to 0.16) | .17 |
| End of study (visit 5, wk 8) | 112 | 0.43 (0.48) | 116 | 0.38 (0.41) | ||||||
| Change (wk 8 – wk 0) | 112 | 0.02 (0.41) | 116 | −0.02 (0.39) | ||||||
| Rectal symptoms scored | ||||||||||
| Baseline (visit 3, wk 0) | 112 | 0.40 (0.49) | 117 | 0.39 (0.60) | ||||||
| End of study (visit 5, wk 8) | 112 | 0.37 (0.50) | 116 | 0.32 (0.39) | ||||||
| Change (wk 8 – wk 0) | 112 | −0.03 (0.41) | 116 | −0.08 (0.56) | ||||||
| Stool symptoms scored | ||||||||||
| Baseline (visit 3, wk 0) | 112 | 1.41 (0.84) | 117 | 1.32 (0.77) | ||||||
| End of study (visit 5, wk 8) | 112 | 1.01 (0.78) | 116 | 0.86 (0.65) | ||||||
| Change (wk 8 – wk 0) | 112 | −0.39 (0.64) | 116 | −0.46 (0.75) | ||||||
| Overall scored | ||||||||||
| Baseline (visit 3, wk 0) | 112 | 0.82 (0.51) | 117 | 0.78 (0.51) | ||||||
| End of study (visit 5, wk 8) | 112 | 0.66 (0.53) | 116 | 0.56 (0.43) | ||||||
| Change (wk 8 – wk 0) | 112 | −0.16 (0.39) | 116 | −0.22 (0.46) | ||||||
| Physical discomfort scored | ||||||||||
| Baseline (visit 3, wk 0) | 112 | 0.99 (0.80) | 117 | 0.91 (0.74) | 112 | −0.22 (−0.31 to −0.13) | 116 | −0.25 (−0.34 to −0.16) | 0.03 (−0.08 to 0.13) | .62 |
| End of study (visit 5, wk 8) | 112 | 0.72 (0.70) | 116 | 0.67 (0.64) | ||||||
| Change (wk 8 – wk 0) | 112 | −0.28 (0.68) | 116 | −0.24 (0.71) | ||||||
| Psychosocial discomfort scored | ||||||||||
| Baseline (visit 3, wk 0) | 112 | 0.73 (0.75) | 117 | 0.61 (0.70) | ||||||
| End of study (visit 5, wk 8) | 112 | 0.62 (0.69) | 116 | 0.52 (0.61) | ||||||
| Change (wk 8 – wk 0) | 112 | −0.11 (0.60) | 116 | −0.09 (0.54) | ||||||
| Worries and concerns scored | ||||||||||
| Baseline (visit 3, wk 0) | 112 | 1.20 (0.81) | 117 | 1.04 (0.76) | ||||||
| End of study (visit 5, wk 8) | 112 | 0.92 (0.64) | 116 | 0.82 (0.61) | ||||||
| Change (wk 8 – wk 0) | 112 | −0.28 (0.57) | 116 | −0.22 (0.60) | ||||||
| Satisfaction scored | ||||||||||
| Baseline (visit 3, wk 0) | 112 | 2.83 (0.71) | 117 | 2.79 (0.76) | ||||||
| End of study (visit 5, wk 8) | 112 | 2.46 (0.77) | 116 | 2.33 (0.81) | ||||||
| Change (wk 8 – wk 0) | 112 | −0.37 (0.83) | 116 | −0.46 (0.96) | ||||||
| Overall scored | ||||||||||
| Baseline (visit 3, wk 0) | 112 | 1.33 (0.65) | 117 | 1.21 (0.63) | ||||||
| End of study (visit 5, wk 8) | 112 | 1.08 (0.56) | 116 | 0.98 (0.52) | ||||||
| Change (wk 8 – wk 0) | 112 | −0.25 (0.48) | 116 | −0.23 (0.51) | ||||||
Abbreviations: BSS, Bristol Stool Scale; LSM, least square mean; MMRM, mixed model for repeated measurements; NA, not applicable; PAC-QoL, Patient Assessment of Constipation–Quality of Life; PAC-SYM, Patient Assessment of Constipation–Symptoms; VAS, visual analogue scale.
BSS scores range from 1 (separate hard lumps) to 7 (watery), with 3 to 5 indicating optimal.
Gut symptoms in daily diaries.
VAS scores range from 0 to 100 mm, with greater scores indicating more severe symptoms.
PAC-SYM and PAC-QOL scores range from 0 (absent) to 4 (severe).
Constipation Symptoms
Abdominal pain and bloating were low at baseline for both groups. However, they increased slightly in the placebo group (mean pain VAS difference, 1.1 [6.9] mm; mean bloating VAS difference, 1.9 [11.3] mm) but not in the HN019 group (mean VAS pain difference, −0.4 [6.1] mm; mean VAS bloating difference, −0.8 [7.0] mm). Both reached statistical significance for the difference between the groups in change from baseline to EOS (pain, P = .04; bloating, P = .02) (Table 3). There was a significant change in abdominal pain for weeks 6 (−1.57 [95% CI, −2.95 to −0.19]; P = .03) and 8 (−1.46 [95% CI, −2.84 to −0.07]; P = .04) in favor of HN019 (eTable 3 in Supplement 2).
The degree of straining was moderate for both groups at baseline and was slightly reduced for both at EOS (Table 3). Also, based on week-by-week findings, there was no significant difference in change from baseline between the groups (eTable 3 in Supplement 2).
The overall PAC-SYM and PAC-QoL scores were low for both groups at baseline and reduced slightly for both groups. Only small differences between the groups in change from baseline to week 4 (eTable 3 in Supplement 2) and to EOS (Table 3) were observed.
Subgroup Analysis
Subgroup analysis for CSBMs by age, sex, fiber intake, and smoking status did not identify any statistical significance between the treatment groups in the ITT population. Details are found in eTable 5 in Supplement 2.
Lifestyle Comparisons
The nutritional analyses from the 3-day dietary record data are summarized in eTable 7 in Supplement 2 for the ITT population. The HN019 and placebo groups displayed similar values and changes during the study. From the perspective of constipation, the low dietary fiber intake (9 g/d) by the participants is relevant.
Rescue Medication
The use of rescue medication (bisacodyl) was low in both the HN019 (5 of 112 [4.5%]) and placebo group (5 of 117 [4.3%]). Details are found in eTables 6 and 7 in Supplement 2).
Adherence
The proportion of participants who took 6 to 8 sachets per week ranged from 95% to 100% in both groups. Diary adherence was close to 100% in both groups.
Fecal Analysis Outcomes
Fecal moisture content and calprotectin levels did not change significantly over the course of the study (eTable 8 in Supplement 2). Also, short-chain fatty acid levels were not different between the groups and did not change with a trend for increased levels of butyrate in the HN019 group compared with the placebo group (eTable 8 in the Supplement).
Fecal Microbiota
The change in fecal microbial alpha diversity between the 2 groups from baseline to EOS as measured by Faith’s phylogenetic diversity index (Figure 2C) and the Shannon index (Figure 2D) was not statistically significant (eTable 10 in the Supplement). Beta diversity did not indicate any clustering (eFigure 2 in Supplement 2). The permutational analysis of variance test indicated the main factor driving sample clustering was participant, which explained 76% of the variation (P = .001). Samples did not cluster significantly according to visit (eTable 11 in Supplement 2). Study samples did not cluster according to the treatment groups at EOS (eFigure 3 in Supplement 2), nor did the beta diversity differ significantly between the treatment groups (eTable 12 in Supplement 2). The overall taxonomic composition of the fecal microbiota summarized at phylum (Figure 2E) and genus (Figure 2F) levels was similar between the 2 study groups. After adjusting for multiplicity of testing, the difference between the 2 groups in change in the abundance at genus level was not significant. The difference in change of B lactis log10 genomes/g between the groups was significant (2.07 log10 genomes/g; P = .003) in favor of the HN019 group (Figure 2B). Also, the occurrence of B lactis log10 genomes/g at the EOS was higher in the HN019 group (DATA; P < .001) (Figure 2B).
Adverse Events
During the intervention, 5 treatment-emergent AEs (TEAEs) were reported by 5 participants (4.3%) in the placebo group, and 6 TEAEs were reported by 5 (4.5%) in the HN019 group (eTable 13 in Supplement 2). None of these were reported as related to the investigational treatment. No severe or serious AEs were reported, and none of the TEAEs reported led to study discontinuation.
Discussion
Probiotics have been reported to improve BMF; particularly strains of the subspecies B lactis.13 HN019, the strain investigated in the present study, has been reported to improve BMF in people with constipation.5,6 The Rome III criteria for functional constipation require a minimum of 2 of 5 criteria to be met for diagnosis, introducing the potential for substantial heterogeneity in a study population.5 To address this, our randomized clinical trial used 3 or fewer CSBMs/wk as an inclusion criterion.
The 2 study groups were very similar with respect to baseline characteristics. The participants had a low dietary fiber intake (9 g/d), which is in line with observations by others (11.7 g/d) and well below recommended levels (25.0 g/d).14 This may be a contributing factor to the bowel movement problems experienced by the participants. Treatment adherence was excellent for both groups. Although the number of CSBMs increased by 0.80/wk in the HN019 group, CSBMs increased by 0.66/wk in the placebo group. This increase in the placebo group is in line with what was observed earlier for the placebo group in the ITT population of Ibarra and coworkers5 and is likely a placebo effect. The results of the primary outcome (CSBMs per week) contrast with earlier reports.5,6 Relief of constipation was evaluated by questionnaire as a secondary end point6 and not by diary, and this difference in reporting may explain the discrepancy. A later study5 investigated BMF as secondary end point; BMF was also investigated as a secondary end point in the present study and not found to be significant between the groups. Why the primary outcome of the present study differs from those of previous studies is thus challenging to explain.
The absence of change in BSS score and fecal moisture would be in line with the observed lack of efficacy of HN019. Other studies included in a meta-analysis13 have reported similar lack of change and observed improvements in bowel movements. Also, the fecal microbiota composition neither changed over time nor differed between the groups. While this could align with the observed lack of efficacy of HN019, it is also commonly observed that probiotics do not change the microbiota composition in healthy adults.15
The number of reported AEs was small and equally distributed over the treatment groups, and none was judged to be related to HN019. The strain was thus well tolerated and safe for consumption, in line with earlier observations.5
COVID-19 has been a global challenge in the past few years and has also affected this trial. The “zero COVID-19” policies of local government brought challenges to the conduct of the study, and mitigation actions were adopted to minimize those. It is unknown whether these actions had any influence on the study outcome and what it was, although a change in diet has been reported because of zero COVID-19 policies.16
Strengths and Limitations
The strengths of this study include adequate size and prolonged duration and the confirmed constipation status of the participants. A limitation of the study is that the participants' lifestyle was severely affected by the COVID-19 pandemic. Furthermore, the study was conducted in Shanghai only, limiting the geographical scope and studied ethnicity.
Conclusions
In this randomized clinical trial, B lactis HN019 did not increase CSBMs per week more than placebo. Daily consumption of B lactis HN019 at the tested dose of 4.69 × 109 CFU did not outperform placebo to increase CSBMs.
Trial Protocol and Statistical Analysis Plan
eMethods. Eligibility Criteria, Sample Processing, and Statistical Analysis
eTable 1. Safety Variables in the Safety Population
eTable 2. Clinical Outcomes of Primary and Secondary End Points in Absolute Values for Intention-to-Treat Population
eTable 3. Clinical Outcomes of Primary and Secondary End Points Analyzed by MMRM for Intention-to-Treat Population
eTable 4. Sensitivity Analysis for the CSBMs in the Intention-to-Treat Population
eTable 5. Subgroup Analysis for the Primary Outcome (Complete Spontaneous Bowel Movements) in the Intention-to-Treat Population
eTable 6. Use of Rescue Medication
eTable 7. Comparison of Diet and Lifestyle in the Intention-To-Treat Population
eTable 8. Outcomes From Fecal Analysis at Baseline, End of Study, and Change During Intervention in Intention-to-Treat Population
eTable 9. Hierarchical Testing Procedure Summary in Intention-to-Treat Population
eTable 10. Alpha Diversity in the Intention-to-Treat Population
eTable 11. PERMANOVA (Adonis) Test Describing the Effect of Various Study Factors on Beta Diversity Sample Clustering From Fecal Microbiota Samples
eTable 12. PERMANOVA (Adonis) Test Describing the Effect of Treatment Group on Beta Diversity Sample Clustering From Fecal Microbiota Samples
eTable 13. Summary of Adverse Events
eFigure 1. Schematic Representation of the Study Design
eFigure 2. Principal Coordinates Analysis (PCoA) Showing the Beta Diversity (Weighted UniFrac Distance) of the Fecal Microbiota From All Study Samples Colored by Visit 3 (Red) and Visit 5 (Blue)
eFigure 3. Principal Coordinates Analysis (PCoA) Showing the Beta Diversity (Weighted UniFrac Distance) of the Fecal Microbiota From Study Groups B (HN019; Red Circle) and A (Placebo; Blue Square) at End of Study
eReferences
Data Sharing Statement
References
- 1.Camilleri M, Ford AC, Mawe GM, et al. Chronic constipation. Nat Rev Dis Primers. 2017;3:17095. doi: 10.1038/nrdp.2017.95 [DOI] [PubMed] [Google Scholar]
- 2.Chen MH, Hou XH. Comittee NaM. Chinese expert consensus on chronic constipation (2019, GuangZhou). Chin J Digestion. 2019;39(9):22. [Google Scholar]
- 3.Johanson JF, Kralstein J. Chronic constipation: a survey of the patient perspective. Aliment Pharmacol Ther. 2007;25(5):599-608. doi: 10.1111/j.1365-2036.2006.03238.x [DOI] [PubMed] [Google Scholar]
- 4.Harris MS, Daniels OT. Core aspects of clinical development and trials in chronic idiopathic constipation. In: Catto-Smith AG, ed. Constipation—Causes, Diagnosis and Treatment. InTech; 2012:147-172. [Google Scholar]
- 5.Ibarra A, Latreille-Barbier M, Donazzolo Y, Pelletier X, Ouwehand AC. Effects of 28-day Bifidobacterium animalis subsp. lactis HN019 supplementation on colonic transit time and gastrointestinal symptoms in adults with functional constipation: a double-blind, randomized, placebo-controlled, and dose-ranging trial. Gut Microbes. 2018;9(3):236-251. doi: 10.1080/19490976.2017.1412908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Waller PA, Gopal PK, Leyer GJ, et al. Dose-response effect of Bifidobacterium lactis HN019 on whole gut transit time and functional gastrointestinal symptoms in adults. Scand J Gastroenterol. 2011;46(9):1057-1064. doi: 10.3109/00365521.2011.584895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Guidance on the scientific requirements for health claims related to the immune system, the gastrointestinal tract and defence against pathogenic microorganisms. EFSA J. 2016;14:4369. doi: 10.2903/j.efsa.2016.4369 [DOI] [Google Scholar]
- 8.US Department of Health and Human Services. Guidance for industry: irritable bowel syndrome—Clinical Evaluation of Drugs for Treatment. May 2012. Accessed August 27, 2024. https://www.fda.gov/media/78622/download [Google Scholar]
- 9.Bengtsson M, Persson J, Sjölund K, Ohlsson B. Further validation of the visual analogue scale for irritable bowel syndrome after use in clinical practice. Gastroenterol Nurs. 2013;36(3):188-198. doi: 10.1097/SGA.0b013e3182945881 [DOI] [PubMed] [Google Scholar]
- 10.Marquis P, De La Loge C, Dubois D, McDermott A, Chassany O. Development and validation of the Patient Assessment of Constipation Quality of Life questionnaire. Scand J Gastroenterol. 2005;40(5):540-551. doi: 10.1080/00365520510012208 [DOI] [PubMed] [Google Scholar]
- 11.Guidelines for data processing and analysis of the International Physical Activity Questionnaire (IPAQ)–short and long forms. November 2005. Accessed August 27, 2024. https://biobank.ndph.ox.ac.uk/showcase/ukb/docs/ipaq_analysis.pdf
- 12.Miller LE, Ouwehand AC, Ibarra A. Effects of probiotic-containing products on stool frequency and intestinal transit in constipated adults: systematic review and meta-analysis of randomized controlled trials. Ann Gastroenterol. 2017;30(6):629-639. doi: 10.20524/aog.2017.0192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van der Schoot A, Helander C, Whelan K, Dimidi E. Probiotics and synbiotics in chronic constipation in adults: a systematic review and meta-analysis of randomized controlled trials. Clin Nutr. 2022;41(12):2759-2777. doi: 10.1016/j.clnu.2022.10.015 [DOI] [PubMed] [Google Scholar]
- 14.Jin F, Zhang J, Shu L, Han W. Association of dietary fiber intake with newly-diagnosed type 2 diabetes mellitus in middle-aged Chinese population. Nutr J. 2021;20(1):81. doi: 10.1186/s12937-021-00740-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kristensen NB, Bryrup T, Allin KH, Nielsen T, Hansen TH, Pedersen O. Alterations in fecal microbiota composition by probiotic supplementation in healthy adults: a systematic review of randomized controlled trials. Genome Med. 2016;8(1):52. doi: 10.1186/s13073-016-0300-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ding Y, Shi X, Li G, et al. Effects of dynamic zero COVID-19 policy on anxiety status and lifestyle changes of pregnant women in rural South China: a survey-based analysis by propensity score matching method. Front Public Health. 2023;11:1182619. doi: 10.3389/fpubh.2023.1182619 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol and Statistical Analysis Plan
eMethods. Eligibility Criteria, Sample Processing, and Statistical Analysis
eTable 1. Safety Variables in the Safety Population
eTable 2. Clinical Outcomes of Primary and Secondary End Points in Absolute Values for Intention-to-Treat Population
eTable 3. Clinical Outcomes of Primary and Secondary End Points Analyzed by MMRM for Intention-to-Treat Population
eTable 4. Sensitivity Analysis for the CSBMs in the Intention-to-Treat Population
eTable 5. Subgroup Analysis for the Primary Outcome (Complete Spontaneous Bowel Movements) in the Intention-to-Treat Population
eTable 6. Use of Rescue Medication
eTable 7. Comparison of Diet and Lifestyle in the Intention-To-Treat Population
eTable 8. Outcomes From Fecal Analysis at Baseline, End of Study, and Change During Intervention in Intention-to-Treat Population
eTable 9. Hierarchical Testing Procedure Summary in Intention-to-Treat Population
eTable 10. Alpha Diversity in the Intention-to-Treat Population
eTable 11. PERMANOVA (Adonis) Test Describing the Effect of Various Study Factors on Beta Diversity Sample Clustering From Fecal Microbiota Samples
eTable 12. PERMANOVA (Adonis) Test Describing the Effect of Treatment Group on Beta Diversity Sample Clustering From Fecal Microbiota Samples
eTable 13. Summary of Adverse Events
eFigure 1. Schematic Representation of the Study Design
eFigure 2. Principal Coordinates Analysis (PCoA) Showing the Beta Diversity (Weighted UniFrac Distance) of the Fecal Microbiota From All Study Samples Colored by Visit 3 (Red) and Visit 5 (Blue)
eFigure 3. Principal Coordinates Analysis (PCoA) Showing the Beta Diversity (Weighted UniFrac Distance) of the Fecal Microbiota From Study Groups B (HN019; Red Circle) and A (Placebo; Blue Square) at End of Study
eReferences
Data Sharing Statement