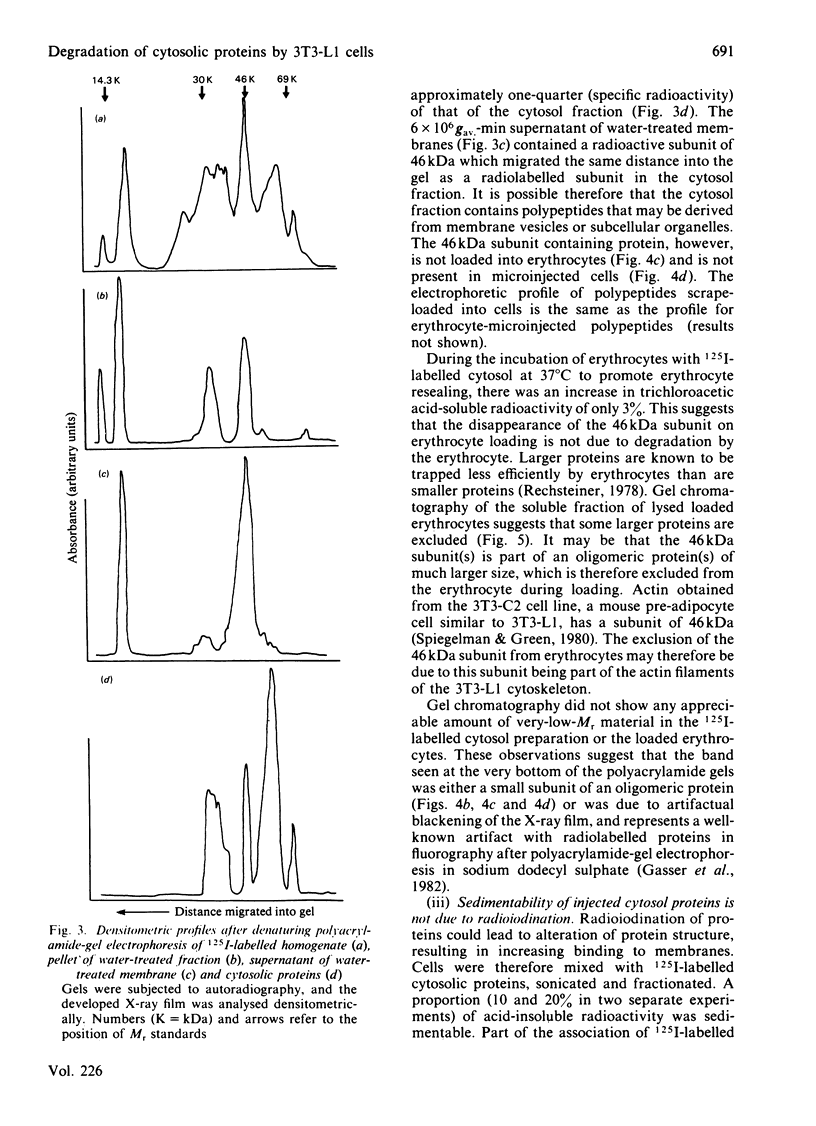
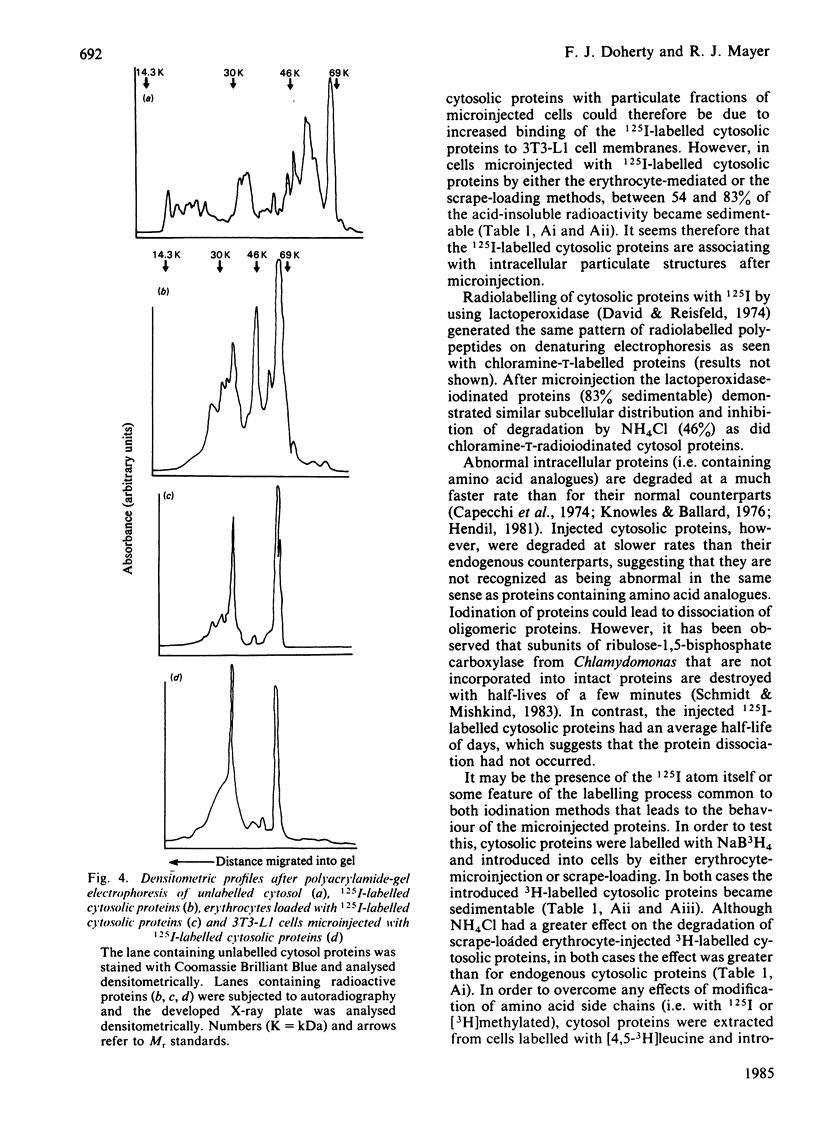
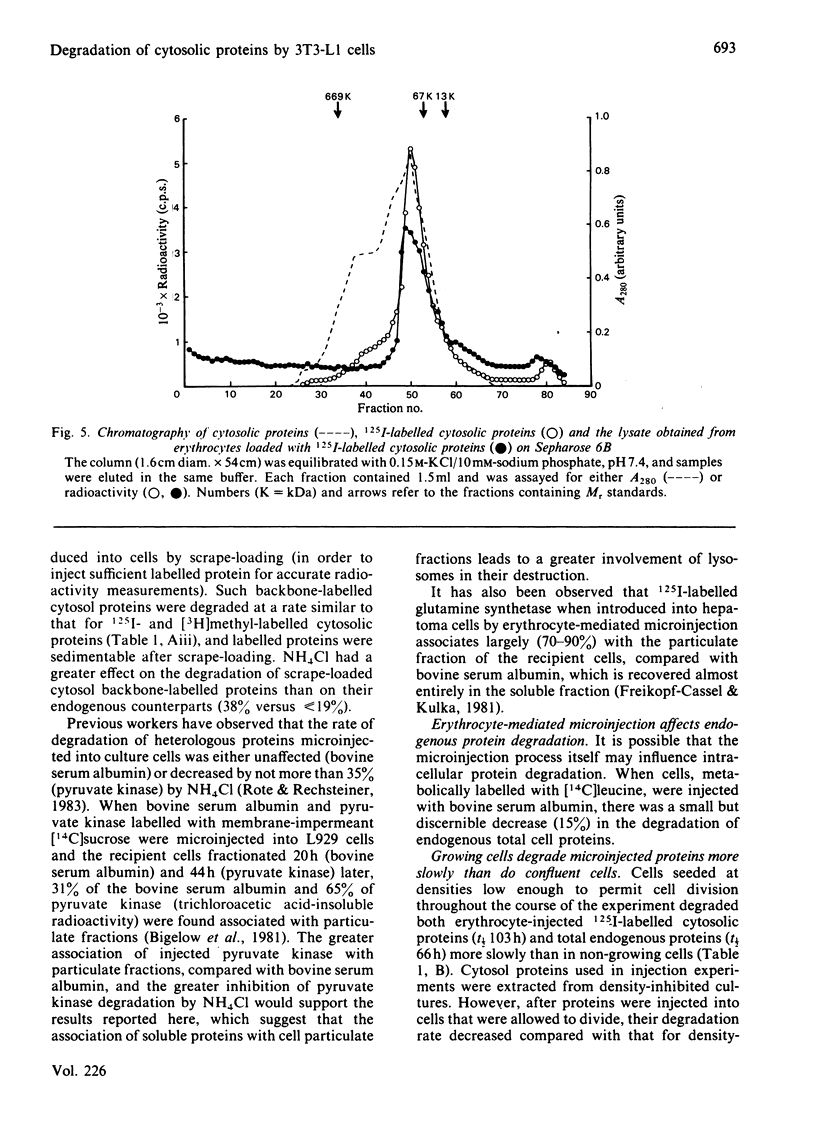
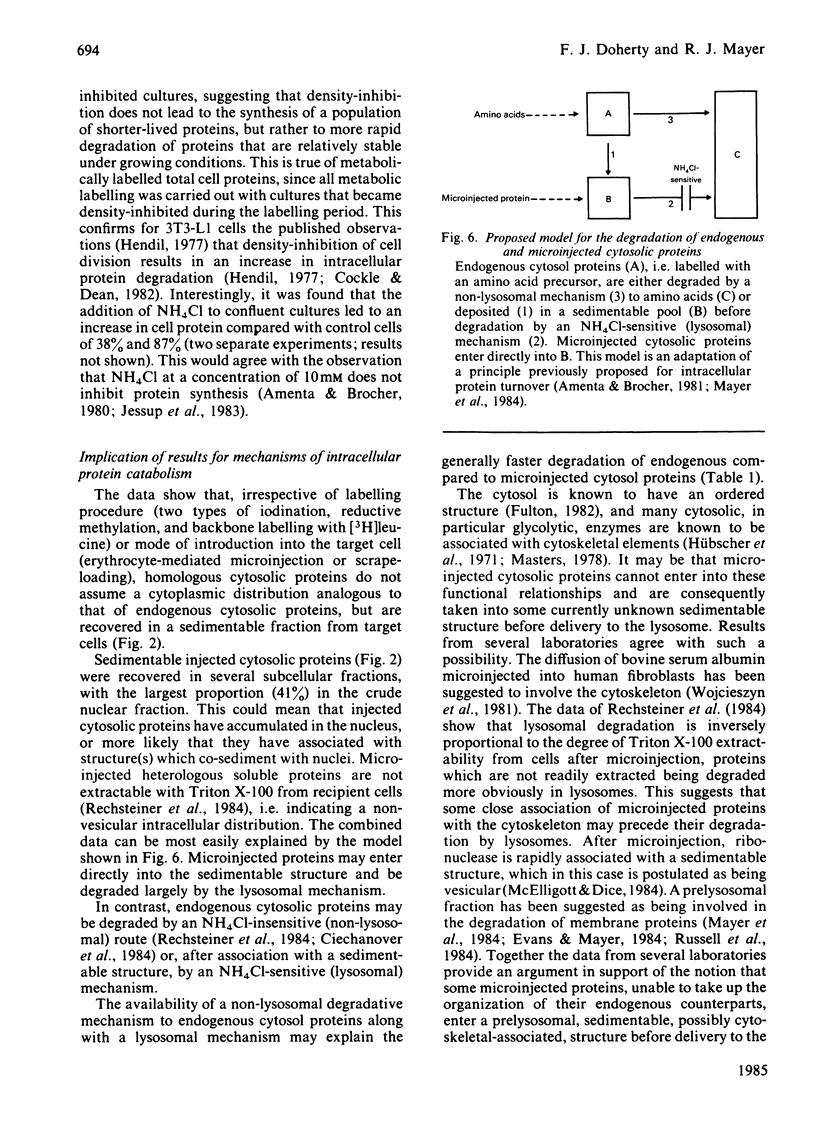
Abstract
Homologous cytosol was introduced into 3T3-L1 cells by two different methods. Erythrocytes loaded with radiolabelled cytosolic proteins extracted from 3T3-L1 cells were fused with the aid of Sendai virus to 3T3-L1 cells, which were then seeded to confluent and non-confluent cultures. Cytosolic proteins were also introduced into cells by the technique of scrape-loading. In confluent cells, injected cytosolic proteins were recovered largely (54-93%) in a sedimentable (6 X 10(6) gav.-min) fraction from recipient cells irrespective of the method of introduction or of radiolabelling of the injected proteins [( 125I]iodination, reductive methylation with NaB3H4 and backbone labelling with L-[4,5-3H]leucine). The degradation of microinjected cytosolic proteins was in all cases inhibited by the lysosomotropic agent NH4Cl to a greater extent (32-75%) than that observed for endogenous cytosolic (less than or equal to 19%) proteins (labelled with L-[4,5-3H]leucine). In growing cells both endogenous total cell proteins and microinjected proteins were degraded at a slower rate than in confluent cell monolayers. The inhibition by NH4Cl of the degradation of both the endogenous and microinjected proteins is decreased compared with the inhibition observed in confluent monolayers. The results are discussed in terms of the cytoplasmic capacity to segregate microinjected homologous proteins before protein degradation can take place.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amenta J. S., Brocher S. C. Lack of effect of NH4Cl on protein synthesis in cultured fibroblasts. Exp Cell Res. 1980 Dec;130(2):305–311. doi: 10.1016/0014-4827(80)90007-5. [DOI] [PubMed] [Google Scholar]
- Amenta J. S., Brocher S. C. Mechanisms of protein turnover in cultured cells. Life Sci. 1981 Mar 16;28(11):1195–1208. doi: 10.1016/0024-3205(81)90444-6. [DOI] [PubMed] [Google Scholar]
- Arias I. M., Doyle D., Schimke R. T. Studies on the synthesis and degradation of proteins of the endoplasmic reticulum of rat liver. J Biol Chem. 1969 Jun 25;244(12):3303–3315. [PubMed] [Google Scholar]
- Bigelow S., Hough R., Rechsteiner M. The selective degradation of injected proteins occurs principally in the cytosol rather than in lysosomes. Cell. 1981 Jul;25(1):83–93. doi: 10.1016/0092-8674(81)90233-6. [DOI] [PubMed] [Google Scholar]
- Capecchi M. R., Capecchi N. E., Hughes S. H., Wahl G. M. Selective degradation of abnormal proteins in mammalian tissue culture cells. Proc Natl Acad Sci U S A. 1974 Dec;71(12):4732–4736. doi: 10.1073/pnas.71.12.4732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciechanover A., Finley D., Varshavsky A. The ubiquitin-mediated proteolytic pathway and mechanisms of energy-dependent intracellular protein degradation. J Cell Biochem. 1984;24(1):27–53. doi: 10.1002/jcb.240240104. [DOI] [PubMed] [Google Scholar]
- Cockle S. M., Dean R. T. Derangement of regulation of protein degradation in transforming fibroblasts. Biosci Rep. 1982 Feb;2(2):107–114. doi: 10.1007/BF01116176. [DOI] [PubMed] [Google Scholar]
- DE DUVE C., PRESSMAN B. C., GIANETTO R., WATTIAUX R., APPELMANS F. Tissue fractionation studies. 6. Intracellular distribution patterns of enzymes in rat-liver tissue. Biochem J. 1955 Aug;60(4):604–617. doi: 10.1042/bj0600604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David G. S., Reisfeld R. A. Protein iodination with solid state lactoperoxidase. Biochemistry. 1974 Feb 26;13(5):1014–1021. doi: 10.1021/bi00702a028. [DOI] [PubMed] [Google Scholar]
- Evans P. J., Mayer R. J. Comparison of the degradative fate of monoamine oxidase in endogenous and transplanted mitochondrial outer membrane in rat hepatocytes. Implications for the cytomorphological basis of protein catabolism. Biochem J. 1984 Apr 1;219(1):61–72. doi: 10.1042/bj2190061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans P. J., Mayer R. J. Degradation of transplanted mitochondrial proteins by hepatocyte monolayers. Biochem J. 1983 Oct 15;216(1):151–161. doi: 10.1042/bj2160151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freikopf-Cassel A., Kulka R. G. Regulation of the degradation of 125 I-labeled glutamine synthetase introduced into cultured hepatoma cells by erythrocyte ghost-mediated injection. FEBS Lett. 1981 Jun 1;128(1):63–66. doi: 10.1016/0014-5793(81)81080-0. [DOI] [PubMed] [Google Scholar]
- Fulton A. B. How crowded is the cytoplasm? Cell. 1982 Sep;30(2):345–347. doi: 10.1016/0092-8674(82)90231-8. [DOI] [PubMed] [Google Scholar]
- Geiger P. J., Bessman S. P. Protein determination by Lowry's method in the presence of sulfhydryl reagents. Anal Biochem. 1972 Oct;49(2):467–473. doi: 10.1016/0003-2697(72)90450-2. [DOI] [PubMed] [Google Scholar]
- HUNTER W. M., GREENWOOD F. C. Preparation of iodine-131 labelled human growth hormone of high specific activity. Nature. 1962 May 5;194:495–496. doi: 10.1038/194495a0. [DOI] [PubMed] [Google Scholar]
- Harris H., Watkins J. F., Ford C. E., Schoefl G. I. Artificial heterokaryons of animal cells from different species. J Cell Sci. 1966 Mar;1(1):1–30. doi: 10.1242/jcs.1.1.1. [DOI] [PubMed] [Google Scholar]
- Hendil K. B. Degradation of abnormal proteins in growing and in quiescent fibroblasts in culture. FEBS Lett. 1981 Jun 29;129(1):77–79. doi: 10.1016/0014-5793(81)80759-4. [DOI] [PubMed] [Google Scholar]
- Hendil K. B. Intracellular degradation of hemoglobin transferred into fibroblasts by fusion with red blood cells. J Cell Physiol. 1980 Dec;105(3):449–460. doi: 10.1002/jcp.1041050309. [DOI] [PubMed] [Google Scholar]
- Hendil K. B. Intracellular protein degradation in growing, in density-inhibited, and in serum-restricted fibroblast cultures. J Cell Physiol. 1977 Sep;92(3):353–364. doi: 10.1002/jcp.1040920304. [DOI] [PubMed] [Google Scholar]
- Hübscher G., Mayer R. J., Hansen H. J. Glycolytic enzymes as a multi-enzyme system. J Bioenerg. 1971 May;2(2):115–118. doi: 10.1007/BF01648926. [DOI] [PubMed] [Google Scholar]
- JOHNSON M. K. The intracellular distribution of glycolytic and other enzymes in rat-brain homogenates and mitochondrial preparations. Biochem J. 1960 Dec;77:610–618. doi: 10.1042/bj0770610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessup W., Shirazi M. F., Dean R. T. Inhibition of some spontaneous secretory processes in macrophages and fibroblasts by ammonium chloride. Biochem Pharmacol. 1983 Sep 15;32(18):2703–2710. doi: 10.1016/0006-2952(83)90079-5. [DOI] [PubMed] [Google Scholar]
- Knowles S. E., Ballard F. J. Selective control of the degradation of normal and aberrant proteins in Reuber H35 hepatoma cells. Biochem J. 1976 Jun 15;156(3):609–617. doi: 10.1042/bj1560609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lopez-Saura P., Trouet A., Tulkens P. Analytical fractionation of cultured hepatoma cells (HTC cells). Biochim Biophys Acta. 1978 Nov 1;543(4):430–449. doi: 10.1016/0304-4165(78)90298-2. [DOI] [PubMed] [Google Scholar]
- Mayer R. J., Evans P., Russell S., Amenta J. S. Degradative fate of transplanted proteins. Ciba Found Symp. 1984;103:202–219. doi: 10.1002/9780470720844.ch13. [DOI] [PubMed] [Google Scholar]
- McElligott M. A., Dice J. F. Erythrocyte-mediated microinjection, a technique to study protein degradation in muscle cells. Biochem J. 1983 Dec 15;216(3):559–566. doi: 10.1042/bj2160559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGarry T., Hough R., Rogers S., Rechsteiner M. Intracellular distribution and degradation of immunoglobulin G and immunoglobulin G fragments injected into HeLa cells. J Cell Biol. 1983 Feb;96(2):338–346. doi: 10.1083/jcb.96.2.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeil P. L., Murphy R. F., Lanni F., Taylor D. L. A method for incorporating macromolecules into adherent cells. J Cell Biol. 1984 Apr;98(4):1556–1564. doi: 10.1083/jcb.98.4.1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neff N. T., Bourret L., Miao P., Dice J. F. Degradation of proteins microinjected into IMR-90 human diploid fibroblasts. J Cell Biol. 1981 Oct;91(1):184–194. doi: 10.1083/jcb.91.1.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PENNINGTON R. J. Biochemistry of dystrophic muscle. Mitochondrial succinate-tetrazolium reductase and adenosine triphosphatase. Biochem J. 1961 Sep;80:649–654. doi: 10.1042/bj0800649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rechsteiner M., Chin D., Hough R., McGarry T., Rogers S., Rote K., Wu L. What determines the degradation rate of an injected protein? Ciba Found Symp. 1984;103:181–201. doi: 10.1002/9780470720844.ch12. [DOI] [PubMed] [Google Scholar]
- Rechsteiner M. Red cell-mediated microinjection. Natl Cancer Inst Monogr. 1978 May;(48):57–64. [PubMed] [Google Scholar]
- Rote K. V., Rechsteiner M. Degradation of microinjected proteins: effects of lysosomotropic agents and inhibitors of autophagy. J Cell Physiol. 1983 Jul;116(1):103–110. doi: 10.1002/jcp.1041160116. [DOI] [PubMed] [Google Scholar]
- Russell S. M., Amenta J. S., Mayer R. J. Degradation of proteins in rat liver mitochondrial outer membrane transplanted into different cell types. Evidence for alternative processing. Biochem J. 1984 Jun 1;220(2):489–498. doi: 10.1042/bj2200489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell S. M., Mayer R. J. Degradation of transplanted rat liver mitochondrial-outer-membrane proteins in hepatoma cells. Biochem J. 1983 Oct 15;216(1):163–175. doi: 10.1042/bj2160163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlegel R. A., Rechsteiner M. C. Microinjection of thymidine kinase and bovine serum albumin into mammalian cells by fusion with red blood cells. Cell. 1975 Aug;5(4):371–379. doi: 10.1016/0092-8674(75)90056-2. [DOI] [PubMed] [Google Scholar]
- Schmidt G. W., Mishkind M. L. Rapid degradation of unassembled ribulose 1,5-bisphosphate carboxylase small subunits in chloroplasts. Proc Natl Acad Sci U S A. 1983 May;80(9):2632–2636. doi: 10.1073/pnas.80.9.2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman J. A., Amenta J. S. Sources of error in estimating radioactivity in protein from cell cultures by liquid scintillation counting. Anal Biochem. 1984 Sep;141(2):538–544. doi: 10.1016/0003-2697(84)90082-4. [DOI] [PubMed] [Google Scholar]
- Spiegelman B. M., Green H. Control of specific protein biosynthesis during the adipose conversion of 3T3 cells. J Biol Chem. 1980 Sep 25;255(18):8811–8818. [PubMed] [Google Scholar]
- Tack B. F., Dean J., Eilat D., Lorenz P. E., Schechter A. N. Tritium labeling of proteins to high specific radioactivity by reduction methylation. J Biol Chem. 1980 Sep 25;255(18):8842–8847. [PubMed] [Google Scholar]
- Touster O., Aronson N. N., Jr, Dulaney J. T., Hendrickson H. Isolation of rat liver plasma membranes. Use of nucleotide pyrophosphatase and phosphodiesterase I as marker enzymes. J Cell Biol. 1970 Dec;47(3):604–618. doi: 10.1083/jcb.47.3.604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojcieszyn J. W., Schlegel R. A., Wu E. S., Jacobson K. A. Diffusion of injected macromolecules within the cytoplasm of living cells. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4407–4410. doi: 10.1073/pnas.78.7.4407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zavortink M., Thacher T., Rechsteiner M. Degradation of proteins microinjected into cultured mammalian cells. J Cell Physiol. 1979 Jul;100(1):175–185. doi: 10.1002/jcp.1041000118. [DOI] [PubMed] [Google Scholar]


