Abstract
Aims:
To investigate high-risk sociodemographic and environmental determinants of health (SEDH) potentially associated with adult obesity in counties in the United States using machine-learning techniques.
Materials and Methods:
We performed a cross-sectional analysis of county-level adult obesity prevalence (body mass index ≥30 kg/m2) in the United States using data from the Diabetes Surveillance System 2017. We harvested 49 county-level SEDH factors that were used in a classification and regression trees (CART) model to identify county-level clusters. The CART model was validated using a ‘hold-out’ set of counties and variable importance was evaluated using Random Forest.
Results:
Overall, we analysed 2752 counties in the United States, identifying a national median (interquartile range) obesity prevalence of 34.1% (30.2%, 37.7%). The CART method identified 11 clusters with a 60.8% relative increase in prevalence across the spectrum. Additionally, seven key SEDH variables were identified by CART to guide the categorization of clusters, including Physically Inactive (%), Diabetes (%), Severe Housing Problems (%), Food Insecurity (%), Uninsured (%), Population over 65 years (%) and Non-Hispanic Black (%).
Conclusion:
There is significant county-level geographical variation in obesity prevalence in the United States, which can in part be explained by complex SEDH factors. The use of machine-learning techniques to analyse these factors can provide valuable insights into the importance of these upstream determinants of obesity and, therefore, aid in the development of geo-specific strategic interventions and optimize resource allocation to help battle the obesity pandemic.
Keywords: machine learning, obesity prevalence, public health
1 |. INTRODUCTION
Although obesity prevalence has increased in most countries for the past several decades, it varies widely by country, from <5% in Japan to >30% in the United States.1 Since 1975, the global prevalence of obesity has increased substantially from 3% and 6% to 11% and 15% in 2016 among men and women, respectively.2 Obesity therefore represents a global public health issue, but the United States is one of the global leaders in the obesity pandemic.1 It has been projected that, by 2030, almost one in two people in the United States will have obesity (body mass index [BMI] ≥ 30 kg/m2), and close to one in four will have severe obesity (BMI ≥35 kg/m2).3
Ultimately, obesity is a major risk factor for a plethora of medical conditions such as metabolic disorders (i.e., type 2 diabetes mellitus), cardiovascular diseases, musculoskeletal disease, Alzheimer’s disease, depression, and even some cancer subtypes, making it one of the most important contemporary public health issues.1 Numerous complex and interconnected mechanisms play a role in these complications of obesity. Adipokines and inflammation released by adipose tissue can increase insulin-mediated glucose uptake, resulting in metabolic disorders such as type 2 diabetes.4 In turn, higher circulating levels of free fatty acids can contribute to hypertension, while inflammation, dyslipidaemia and hyperglycaemia place metabolic stress on the heart and lead to myocardial deterioration.5,6 Musculoskeletal complications can arise from excess body weight placing greater mechanical stress on the lower body.5 In the brain, inflammatory mediators reduce neurogenesis and synaptic plasticity. Additionally, impaired insulin and leptin signalling due to altered blood–brain barrier permeability contribute to accumulation of amyloid-β and misfolded or hyperphosphorylated tau protein in Alzheimer’s disease.7,8 Insulin and leptin are implicated in enhanced mood, hence reduced signalling of these hormones can perpetuate the negative emotional states seen in depression.9 Other factors in depression may revolve around behavioural, cognitive and social mechanisms including eating disorders, body image dissatisfaction and stigma, respectively.10 Lastly, the contributions of obesity to cancer pathogenesis are multi-fold. Examples of potential mechanisms include: insulin-like growth factor abnormalities that stimulate cell proliferation; dysregulated sex hormone synthesis brought about by insulin and adipose tissue; and high levels of insulin receptor expression within tumours, which upregulates cellular metabolism and precipitates oxidative stress and further DNA damage.11,12
Although, at its core, obesity is caused by a calorie intake/consumption imbalance, it is crucial to understand its upstream determinants.13 While initial focus was drawn to individual-level ‘obesogenic’ behaviours such as sedentarism and overconsumption of high-caloric food, more recently, greater attention has been given to the overarching population-level factors that help shape the downstream effects and influence individual-level obesogenic behaviours.13,14
These upstream determinants include various sociodemographic and environmental determinants of health (SEDH) that could help us understand the substantial county-, state- and region-level obesity prevalence variability.3,15 Examples of sociodemographic factors include socioeconomic status (SES), location and ethnicity. Lower SES and education levels are associated with higher BMI. Studies have shown that the Southern and Midwest states tend to have the highest levels of obesity in the United States and rural areas, compared to urban areas, are associated with higher odds of obesity.16,17 Ethnic differences play a role, particularly among women, with non-Hispanic Black women having the highest prevalence of obesity.18 Environmental factors include, but are not limited to food availability, fast food culture, transportation, work environment, and technology. Food deserts and a fast-food culture of large portion sizes, speed eating, junk food, and sweet drinks have been positively associated with obesity. In terms of transportation, neighbourhood walkability has been associated with a decreased prevalence of obesity. Jobs and industries have shifted towards requiring less physical activity, and increased screen usage may place individuals at increased risk of obesity.17,19 Of note, these sociodemographic and environmental factors are inter-related and influence each other. For example, differences in obesity prevalence seen in urban and rural areas may be related to discrepancies in the built environment (types of industry, availability of transportation, etc.).
Evaluation of these SEDH variables, however, is often limited by the use of conventional regression-based models, and many are unable to contemplate a broad scope of SEDH factors.20–22 Therefore, in this study we set out to explore the relationship of SEDH and adult obesity prevalence across US counties using machine-learning models.
2 |. METHODS
2.1 |. Socioeconomic and environmental data sources (exposures)
Table S1 outlines the 50 variables analysed in the study, which comprise county-level SEDH variables that were harvested from previously published sources.23,24 The environmental indicators were obtained from the Agency’s Environmental Justice Screening tool (EPA-EJSCREEN) 2020 version (containing data from 2014 to 2020). These indicators, originally in census block group level, were converted into county-level exposures following the method described in the technical documentation guide of EJSCREEN.25 The sociodemographic variables were sourced from the Area Health Resources Files and County Health Rankings and Roadmaps databases. These SEDH indicators encompass a wide array of fields that include access to healthcare, behavioural risk factors, population characteristics, and other health-related variables.
2.2 |. Study endpoints
We analysed the county-level prevalence of obesity in the United States. Our outcome was defined as the percentage of the adult population (aged ≥18 years) reporting a BMI ≥30 kg/m2 (age-adjusted). This metric was obtained from the Diabetes Surveillance System from the Centres for Disease Control, 2017. We adopted 2017 data to harmonize with the other 49 variables included in the study. Counties that lacked data were excluded. Obesity prevalence is reported using the median and interquartile range (IQR).
2.3 |. Machine learning and data analysis
We employed the classification and regression trees (CART) method to define groups of counties that share SEDH characteristics and had similar levels of obesity prevalence (‘clusters’). We then visualized these county clusters on a map to examine their geographic distributions. We additionally applied the Random Forest method to validate the results of the CART analysis and to evaluate the relative importance of SEDH in predicting obesity prevalence.
The CART method uses conditional inferences (‘if-then’ rules) to sequentially divide counties into smaller, homogeneous groups characterized by combinations of the predictors (i.e., SEDH) and their association with the outcome (i.e., obesity prevalence).26 For model performance and interpretation purposes, we limited our trees to a maximum depth of six splits and a minimum of 150 counties in each terminal node (i.e., the final group of counties, or the ‘cluster’). Each split at the tree has passed a Pearson’s correlation test with statistical significance at p < 0.05. We then labelled the terminal nodes, or county clusters, with letters of the alphabet from the lowest to the highest median obesity prevalence. We used an 80% random sample of all counties as the main CART model and evaluated its results using the remaining 20%, the ‘hold-out’ sample.
Random Forest aggregates an ensemble of trees using random variable selection and bootstrap sampling.26 It then produces an average of the outputs from these trees as a prediction, from which we can calculate the relative importance of SEDH in predicting obesity prevalence according to the mean decrease in node impurity, a measure that captures how much each variable contributes to reducing uncertainty or disorder in the prediction.
We used R version 4.3.0 for statistical analyses and QGIS version 3.22.3 for mapping. No institutional review board approval was needed due to the publicly available nature of the data.
3 |. RESULTS
The study examined 2752 counties in the United States with an over-all median (IQR) obesity prevalence of 34.1% (30.2%, 37.7%). Based on a training dataset comprising 80% of the counties (n = 2204), CART determined 11 terminal nodes (or clusters), within which counties shared similar obesity prevalence and SEDH characteristics based on splitting branches (Figure 1). These clusters were labelled in alphabetical order, A to K, according to their increasing median obesity prevalence. A 60.8% relative increase in obesity prevalence was observed across the spectrum (from 25% to 40.2% comparing clusters A and K). To guide county-cluster stratification, CART identified seven key variables (Physically Inactive, Diabetes, Severe Housing Problems, Food Insecurity, Uninsured, Population over 65 years and Non-Hispanic Black) among the 49 potential SEDH to serve as splitting points, each with a statistically significant splitting value. A detailed description of each variable is available in Table S1.
FIGURE 1.
Classification and regression tree analysis to predict county-level obesity prevalence. Each path down to a terminal node represents a sociodemographic and environmental determinants of health cluster of counties. Box plots in the terminal nodes represent the median percentage of the adult population (aged ≥18 years) reporting a body mass index ≥30 (age-adjusted). The minimum number of counties in a terminal node was set to 150. Clusters were labelled alphabetically based on median obesity prevalence, from lowest to highest.
The CART analysis identified Physically Inactive as the first node, splitting the tree into the left and right sides. The left side of the tree (Physically Inactive ≤ 22.9%) comprised three clusters (A, B, D). Physically Inactive (≤ 18.3%) was used again to identify the cluster with the lowest obesity prevalence (Cluster A: 25%, IQR 21.7%, 27.7%) and separate it from Clusters B and D, which had the second and fourth lowest obesity prevalence. The latter differed only with regard to the percentage of diabetes, which was ≤9.1% in Cluster B (28.7%, IQR 25.4%, 31.2%) and >9.1% in Cluster D (31.6%, IQR 29.3%, 34.3%).
The right side of the tree (Physically Inactive > 22.9%) contained eight clusters (G, E, C, H, F, I, J, K). Diabetes was used a second time at the threshold of 11.8% to set apart Clusters G, E and C (Diabetes ≤ 11.8%) from Clusters H, F, I, J and K (Diabetes > 11.8%). Severe Housing Problems > 14.5% was used to delineate Cluster C (31.4%, IQR 28.5%, 34.2%) from Clusters G and E. The latter were differentiated by the percentage of Uninsured that was ≤12.1% in Cluster G (34.8%, IQR 32.4%, 37.5%) and greater than >12.1% in Cluster E (33.5%, IQR 30.1%, 36.6%). When analysing the ramifications of Diabetes >11.8% (H, F, I, J, K), Food Insecurity >18% could single out Cluster K as the county cluster with the highest obesity prevalence (40.2%, IQR 36.8%, 43.4%). Next, Physically Inactive was used a third time to separate Clusters H and F (≤ 30.1%) from Clusters I and J (>30.1%). While Cluster H (35.7%, IQR 33.2%, 37.9%) was characterized by a lower Population over 65 years (≤ 20.9%), when compared to Cluster F (33.6%, IQR 30.5%, 36.5%), Cluster I (36.8%, IQR 33.7%, 39.9%) was characterized by a lower percentage of Non-Hispanic Black people (≤7.1%) when compared to Cluster J (38.1%, IQR 35.6%, 40.1%).
We applied the main CART structure to the test set (n = 548) and labelled the clusters (A to K) accordingly. We then compared the median obesity prevalence across the training and testing set, and the results showed no substantial differences across the 11 clusters (Figure S1). Figure 2A,B maps the geographic distribution of county-level obesity prevalence and the CART-identified county Clusters A to K, respectively.
FIGURE 2.
Map of the county-level prevalence of obesity (%) in the United States, divided by natural breaks (A). (B) County clusters identified by classification and regression tree analysis (A–K), labelled according to lowest to highest obesity prevalence (%).
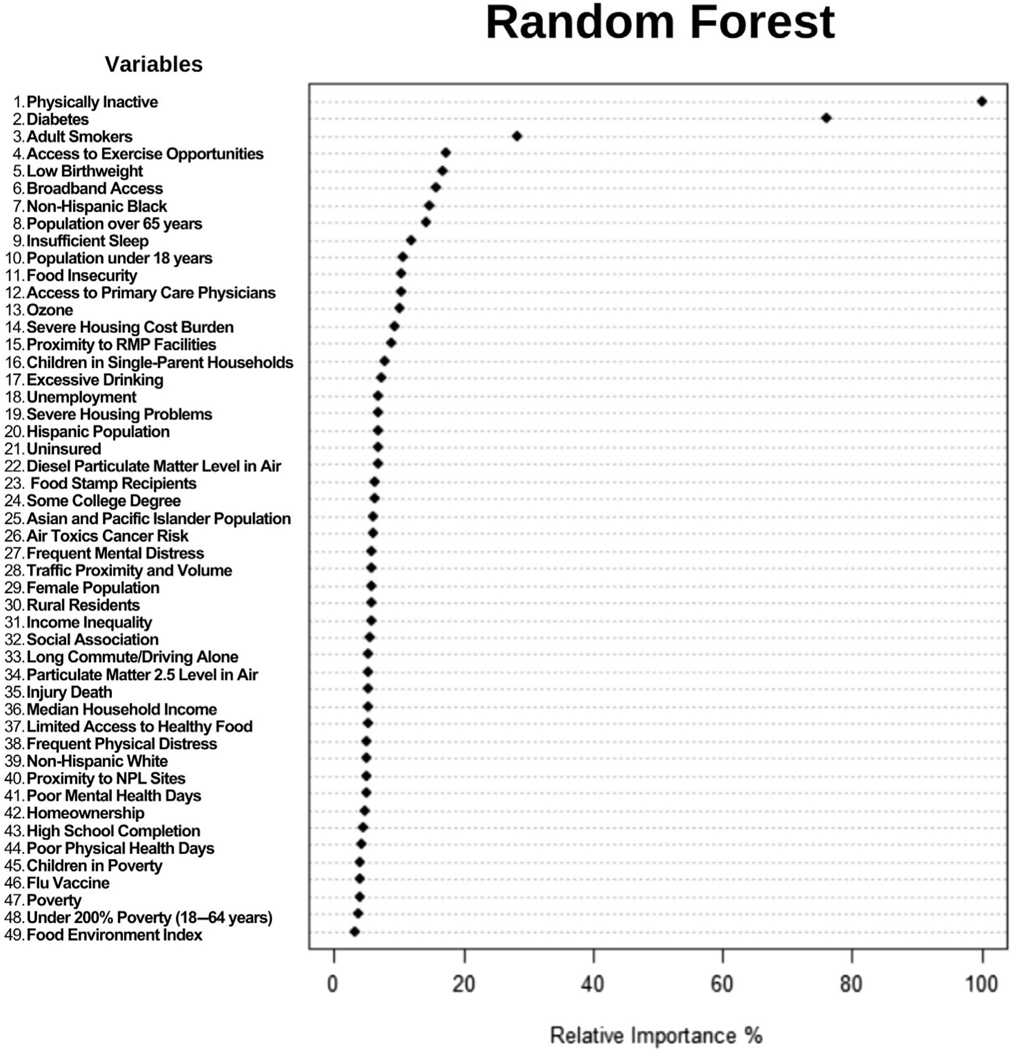
Finally, we employed Random Forest to assess the relative variable importance in respect to the county-level obesity prevalence and the results are displayed in Figure 3. Overall, from the 49 SEDH variables available, the seven variables selected by CART were ranked as 1st (Physically Inactive), 2nd (Diabetes), 7th (Non-Hispanic Black), 8th (Population over 65 years), 11th (Food Insecurity), 19th (Severe Housing Problems) and 21st (Uninsured) in the Random Forest plot.
FIGURE 3.
Dot chart of Random Forest analysis displaying the importance of variables for predicting county-level obesity prevalence in the United States. The most important variable is at the top and scaled to 100%. The importance of the remaining variables is shown relative to the top one. NPL, National Priorities List; PM, fine particulate matter; RMP, Risk Management Plan.
4 |. DISCUSSION
Obesity has been an escalating public health issue and point of discussion for several decades, given its increasing prevalence among the US population. According to the Centres for Disease Control, the age-adjusted prevalence of obesity in US adults was 42.4% in the period 2017–2018 and continues to rise despite a temporary plateau in the period 2009–2012.27 By 2030, most adults are projected to be overweight and approximately 50% of adults will have obesity.28 Recognizing that obesity contributes to a growing prevalence of chronic diseases such as cardiovascular disease, diabetes, gastrointestinal disease and arthritis, and some types of cancer, obesity is regarded as one of the greatest threats to public health of the century.29,30 Despite the American Medical Association recognizing obesity as a disease with multiple pathophysiological aspects requiring interventions, little progress has been made in reversing the obesity epidemic.31,32 Implementation of prevention strategies has largely focused on risk factors related to food supply, physical activity, and behaviour changes, which likely reflect the complexity of the disease that continues to challenge healthcare professionals and researchers.33 In this investigation, machine-learning techniques were used to explore the associations between SEDH and adult obesity. CART was subsequently used to provide a clinically applicable visual representation of county-level obesity prevalence. In our assessment, the variables Physically Inactive, Diabetes, Non-Hispanic Black, Population over 65 years, Food Insecurity, Severe Housing Problems and Uninsured were identified as being associated with obesity prevalence. In the CART analysis, these variables were implemented to identify 11 clusters of counties with a 60.8% relative increase in prevalence between the lowest and highest risk county clusters. Additionally, Random Forest—an alternative machine-learning approach—illuminated the ability of the CART method to classify complex SEDH by determining the relative importance of each variable.
The CART analysis identified Physically Inactive as the starting node, which was also the most important variable according to Random Forest. Physically Inactive was utilized twice more as a node further downstream, highlighting the importance of activity as a factor accounting for large variations in obesity prevalence. Studies have shown a positive risk association between physical inactivity/sedentary lifestyle and obesity prevalence. Higher rates of sedentary behaviour and physical inactivity exist among people with obesity.34 Irrespective of BMI, higher physical activity intensity levels and lower sedentary time are associated with lower risk of mortality, and based on these relationships, a combination of lifestyle alterations revolving around being active and reducing time spent sedentary contributes to the prevention of abdominal obesity.35,36
In addition to Physically Inactive, Diabetes was found to be significant in the CART analysis, being utilized twice in the stratification of clusters. This finding was consistent with the Random Forest method, which identified Diabetes as the second most important variable in predicting obesity prevalence among adults. There are significant associations between obesity and diabetes among other chronic conditions including high blood pressure, cardiovascular disease, high cholesterol, asthma, and arthritis.29,37 Likewise, obesity and overweight represent modifiable risk factors for type 2 diabetes and as many as 80% of people are overweight when diagnosed.29
Demographic factors seemed to play an important role in predicting obesity prevalence. Specifically, CART identified Non-Hispanic Black and Population Over 65 Years as two demographic SEDH variables. Although variations have been reported across studies, obesity prevalence in the United States varies by ethnicity. One study demonstrated that non-Hispanic Black individuals were most likely to have obesity (prevalence of 36.1%) and that those living in communities comprising >25% non-Hispanic Black or Hispanic individuals had significantly higher BMI and were more likely to have obesity.38 Meanwhile, in another study, the highest prevalence of relative fat mass-defined obesity was observed in Mexican-American men and women and the highest prevalence of BMI-defined obesity was observed in African-American women.39 Previous studies have also shown age-related disparities across obesity distribution. While it is unclear whether there is a definite positive correlation between older age and obesity, which can vary depending on the surrogate of body fat percentage, age-related trends in obesity are probably explained by age-related changes in body composition.39,40 After the age of approximately 60 years, the proportion of intra-abdominal fat progressively increases and energy intake and total energy expenditure decline, primarily due to decreases in physical activity and basal metabolic rate. Regardless of age, obesity is still associated with disorders that increase functional impairment.40
Like many other public health concerns, SES varies with obesity prevalence. Traditional SES variables identified by CART included Food Insecurity, Severe Housing Problems, and Lack of Insurance. SES affects both the quantity and quality of food consumed. Low-cost foods are highly processed and contain refined grains, sugars, and added fats, which are linked to rising obesity rates. Not only do these food options seem to be of the best value by combining low cost with high energy density, but they may also be the most accessible, particularly in ‘food deserts’. In fact, lower-income households tend to consume more ‘obesogenic’ foods while ‘protective’ foods such as whole grains, lean meats, fish, low-fat dairy products, fresh vegetables, and fruit are more likely to be consumed by groups with higher SES.41–44 High housing costs similarly have the potential to worsen the obesity epidemic. Populations living in areas of high rent burden are more likely to have obesity, which has been demonstrated by modelling investigations in Chicago, Illinois. When households spend >50% of their income on housing, access to healthy foods once again becomes a burden.45,46 Economic status and high housing costs likewise affect housing quality and conditions, which in turn, play a role in sleep patterns—an obesity-related behaviour.47 Beyond food and housing insecurity, lack of health insurance represents an additional risk factor associated with adult obesity. Analyses have revealed disparities in obesity prevalence in disadvantaged neighbourhoods and, conversely, people with obesity constitute one population that is more often uninsured.48–50
Ongoing efforts to improve the obesity pandemic in the United States have largely been unsuccessful. National trends in metabolic phenotypes (as determined by BMI and metabolic health) from 1999 to 2018 demonstrate an increase in metabolically unhealthyobese and metabolically healthy-obese phenotypes, particularly among older adults and populations with lower education levels and income.51 Concurrently, increased incidence of obesity complications, such as cardiovascular disease and poorer outcomes from COVID-19 infection, is a primary concern,52 and the lack of improvement in these trends warrant an investigation of current public health efforts.
Contemporary efforts use a ‘one-size-fits-all’ approach, often addressing lifestyle behaviours as the foundation for weight loss.53 Several nationwide resources target lifestyle modifications based on the premise that ‘regardless of age, race, ethnicity, economic circumstances, or health status’, individuals can benefit from shifting behaviours to support healthier patterns.30 In fact, novel fields of medicine, including lifestyle medicine, have emerged out of these initiatives.54 However, studies have shown that region-specific demographics may drive discrepancies in obesity prevalence. For example, although there is a higher obesity prevalence among individuals with low SES in high-income countries, the reverse seems to be true in low-income countries.47 Moreover, heterogeneity in the distribution of living characteristics, demographics, obesity, and chronic disease prevalence likewise exist at a national level. Several investigations exemplify a correlation between neighbourhood segregation and increased obesity risk among Black women, although these relationships vary among populations of Black men. Interestingly, among Mexican-American women, neighbourhood segregation appears to correlate with lower obesity prevalence.47 Unique, region-specific characteristics likely account for these inconsistencies in obesity prevalence across the United States. Therefore, identifying the impact of SEDH factors on obesity prevalence within specific geographic regions needs to be better understood.
There is a need to change the paradigm of obesity treatment to involve more geo-specific strategies, whether that be through targeted lifestyle modifications or the promotion of underprescribed anti-obesity medications.55 Our study demonstrates that other SEDH-related factors can aid in the identification of areas with higher obesity prevalence, and therefore, deserve more attention in future investigations. Moreover, the use of tree-based machine-learning approaches, such as the CART method, provides a practical representation of the intricate web of SEDH and geographic variation in obesity prevalence.24,26 Analyses such as these can inform the development of obesity prevention and treatment strategies tailored to the unique characteristics of a region, which optimizes the likelihood of local adoption of such strategies and ultimately plays an essential role in reducing obesity-related chronic diseases.
Our study results inspire confidence due to various methodological strengths. First, compared with conventional methods such as linear regression, CART and Random Forest can deal with a greater number of predictors simultaneously without concerns about multicollinearity (i.e., correlations between predictors) thanks to their variable selection and bootstrap sampling strategies. Additionally, our approach incorporates three methods, each contributing to the strengths of our study: CART—identifying county clusters and presenting a ‘pathway’ for each county towards its cluster ‘destination’; geographic information systems—visualizing the CART-identified county clusters on a map, pinpointing the locations where interventions should be targeted; and Random Forest—assessing the relative importance of all predictors. Also, the combined use of CART and Random Forest helps mitigate their respective limitations. CART, being a single-tree model, is sensitive to data changes (such as when data become available in future years). In contrast, Random Forest, an ensemble-of-trees model, provides more stable results. Random Forest, being a black-box model, yields less explainable results regarding how and why a variable becomes less or more important, while CART excels in visualizing the ‘pathway’ of each observation with statistical significance at decision points or splits. This novel combination of tools has been demonstrated successfully in prior studies investigating late-stage breast cancer diagnosis,24 premature cardiovascular mortality,26 and Alzheimer’s disease mortality.56 Our study further demonstrates its utility in understanding obesity prevalence.
This study has some limitations. Latest research suggests that BMI is not an adequate surrogate for visceral adiposity. BMI does not distinguish between visceral fat and subcutaneous fat, which varies with age, gender and ethnicity. For example, women have more subcutaneous fat than men on average and Asian people have more visceral adiposity than White people and typically have much lower BMIs. BMI is, not surprisingly, less predictive in many populations with higher visceral adiposity including elderly populations. Direct measurement of visceral adiposity could provide much-needed specificity in obesity and will help clarify historic relationships or lack thereof. Another limitation is the cross-sectional characteristic of this study, which is associative by nature. This study focused on county-level risk factors represented by the 49 SEDH variables in the model, and therefore individual-level factors such as genetics, family history, and medications were not incorporated. We excluded counties with no data due to small samples in less populated areas. Future studies should consider consolidating counties using regionalization methods.57,58
In conclusion, the application of machine-learning techniques to the nationwide prevalence data identified Physically Inactive, Diabetes, Non-Hispanic Black, Population over 65 years, Food Insecurity, Severe Housing Problems, and Lack of Insurance as key SEDH variables that delineated clusters of US counties sharing similar rates of obesity prevalence. Comprehensive visualization of these clusters using CART demonstrated significant county-level geographic variation of obesity prevalence in the United States, which provides the potential to advance public health initiatives and policies surrounding obesity.
Supplementary Material
FUNDING INFORMATION
The authors received no funding for this study.
Footnotes
SUPPORTING INFORMATION
Additional supporting information can be found online in the Supporting Information section at the end of this article.
CONFLICT OF INTEREST STATEMENT
None.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1.Blüher M. Obesity: global epidemiology and pathogenesis. Nat Rev Endocrinol. 2019;15(5):288–298. doi: 10.1038/s41574-019-0176-8 [DOI] [PubMed] [Google Scholar]
- 2.Jaacks LM, Vandevijvere S, Pan A, et al. The obesity transition: stages of the global epidemic. Lancet Diabetes Endocrinol. 2019;7(3):231–240. doi: 10.1016/S2213-8587(19)30026-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ward ZJ, Bleich SN, Cradock AL, et al. Projected U.S. state-level prevalence of adult obesity and severe obesity. N Engl J Med. 2019; 381(25):2440–2450. doi: 10.1056/NEJMsa1909301 [DOI] [PubMed] [Google Scholar]
- 4.Pedersen SD. Metabolic complications of obesity. Best Pract Res Clin Endocrinol Metab. 2013;27(2):179–193. doi: 10.1016/j.beem.2013.02.004 [DOI] [PubMed] [Google Scholar]
- 5.Sheehan MT, Jensen MD. Metabolic complications of obesity. Endocrine. 2000;13(2):155–165. [DOI] [PubMed] [Google Scholar]
- 6.Scherer PE, Hill JA. Obesity, Diabetes, and Cardiovascular Diseases: A Compendium. Circ Res. 2016;118(11):1703–1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alford S, Patel D, Perakakis N, Mantzoros CS. Obesity as a risk factor for Alzheimer’s disease: weighing the evidence. Obes Rev. 2018;19(2): 269–280. doi: 10.1111/obr.12629 [DOI] [PubMed] [Google Scholar]
- 8.Picone P, Di Carlo M, Nuzzo D. Obesity and Alzheimer’s disease: molecular bases. Eur J Neurosci. 2020;52(8):3944–3950. doi: 10.1111/ejn.14758 [DOI] [PubMed] [Google Scholar]
- 9.Hryhorczuk C, Sharma S, Fulton SE. Metabolic disturbances connecting obesity and depression. Front Neurosci. 2013;7:7. doi: 10.3389/fnins.2013.00177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Markowitz S, Friedman MA, Arent SM. Understanding the relation between obesity and depression: causal mechanisms and implications for treatment. Clin Psychol Sci Pract. 2008;15(1):1–20. doi: 10.1111/j.1468-2850.2008.00106.x [DOI] [Google Scholar]
- 11.Calle EE, Thun MJ. Obesity and cancer. Oncogene. 2004;23(38):6365–6378. doi: 10.1038/sj.onc.1207751 [DOI] [PubMed] [Google Scholar]
- 12.Avgerinos KI, Spyrou N, Mantzoros CS, Dalamaga M. Obesity and cancer risk: emerging biological mechanisms and perspectives. Metabolism. 2019;92:121–135. doi: 10.1016/j.metabol.2018.11.001 [DOI] [PubMed] [Google Scholar]
- 13.Lakerveld J, Mackenbach J. The upstream determinants of adult obesity. Obes Facts. 2017;10(3):216–222. doi: 10.1159/000471489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Swinburn B, Egger G, Raza F. Dissecting obesogenic environments: the development and application of a framework for identifying and prioritizing environmental interventions for obesity. Prev Med. 1999; 29(6):563–570. doi: 10.1006/pmed.1999.0585 [DOI] [PubMed] [Google Scholar]
- 15.Mills CW, Johnson G, Huang TTK, Balk D, Wyka K. Use of small-area estimates to Describe County-level geographic variation in prevalence of extreme obesity among US adults. JAMA Netw Open. 2020; 3(5):e204289. doi: 10.1001/jamanetworkopen.2020.4289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sung B, Etemadifar A. Multilevel analysis of socio-demographic disparities in adulthood obesity across the United States geographic regions. Osong Public Health Res Perspect. 2019;10(3):137–144. doi: 10.24171/j.phrp.2019.10.3.04 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee A, Cardel M, Donahoo WT. Social and environmental factors influencing obesity. In: Feingold KR, Anawalt B, Blackman MR, et al. , eds. Endotext. MDText.com, Inc; 2000.
- 18.Wang Y, Beydoun MA. The obesity epidemic in the United States—gender, age, socioeconomic, racial/ethnic, and geographic characteristics: a systematic review and meta-regression analysis. Epidemiol Rev. 2007;29:6–28. doi: 10.1093/epirev/mxm007 [DOI] [PubMed] [Google Scholar]
- 19.Nicolaidis S. Environment and obesity. Metabolism. 2019;100S: 153942. doi: 10.1016/j.metabol.2019.07.006 [DOI] [PubMed] [Google Scholar]
- 20.Ludwig J, Sanbonmatsu L, Gennetian L, et al. Neighborhoods, obesity, and diabetes—a randomized social experiment. N Engl J Med. 2011; 365(16):1509–1519. doi: 10.1056/NEJMsa1103216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tyrrell J, Jones SE, Beaumont R, et al. Height, body mass index, and socioeconomic status: mendelian randomisation study in UK biobank. BMJ. 2016;352:i582. doi: 10.1136/bmj.i582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Min J, Goodale H, Xue H, Brey R, Wang Y. Racial-Ethnic Disparities in Obesity and Biological, Behavioral, and Sociocultural Influences in the United States: A Systematic Review. Adv Nutr. 2021;12(4):1137–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dong W, Bensken WP, Kim U, et al. Variation in and factors associated with US County-level cancer mortality, 2008–2019. JAMA Netw Open. 2022;5(9):e2230925. doi: 10.1001/jamanetworkopen.2022.30925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dong W, Bensken WP, Kim U, Rose J, Berger NA, Koroukian SM Phenotype discovery and geographic disparities of late-stage breast cancer diagnosis across U.S. counties: a machine learning approach. Cancer Epidemiol Biomarkers Prev. 2022;31(1):66–76. doi: 10.1158/1055-9965.EPI-21-0838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.US EPA O. EJScreen: Environmental Justice Screening and Mapping Tool. 2014. https://www.epa.gov/ejscreen (accessed April 2023).
- 26.Dong W, Motairek I, Nasir K, et al. Risk factors and geographic disparities in premature cardiovascular mortality in US counties: a machine learning approach. Sci Rep. 2023;13:2978. doi: 10.1038/s41598-023-30188-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Products—Data Briefs—Number 360. https://www.cdc.gov/nchs/products/databriefs/db360.htm (accessed August 2023).
- 28.Wang Y, Beydoun MA, Min J, Xue H, Kaminsky LA, Cheskin LJ. Has the prevalence of overweight, obesity and central obesity levelled off in the United States? Trends, patterns, disparities, and future projections for the obesity epidemic. Int J Epidemiol. 2020;49(3):810–823. doi: 10.1093/ije/dyz273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smyth S, Heron A. Diabetes and obesity: the twin epidemics. Nat Med. 2006;12(1):75–80. doi: 10.1038/nm0106-75 [DOI] [PubMed] [Google Scholar]
- 30.Dietary Guidelines for Americans, 2020–2025. [Google Scholar]
- 31.Pollack A. Recognizes obesity as a disease. The New York Times. https://www.nytimes.com/2013/06/19/business/ama-recognizes-obesity-as-a-disease.html (accessed August 2023). [Google Scholar]
- 32.Roberto CA, Swinburn B, Hawkes C, et al. Patchy progress on obesity prevention: emerging examples, entrenched barriers, and new thinking. Lancet. 2015;385(9985):2400–2409. doi: 10.1016/S0140-6736(14)61744-X [DOI] [PubMed] [Google Scholar]
- 33.Kyle TK, Dhurandhar EJ, Allison DB. Regarding obesity as a disease: evolving policies and their implications. Endocrinol Metab Clin North Am. 2016;45(3):511–520. doi: 10.1016/j.ecl.2016.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Silveira EA, Mendonça CR, Delpino FM, et al. Sedentary behavior, physical inactivity, abdominal obesity and obesity in adults and older adults: a systematic review and meta-analysis. Clin Nutr ESPEN. 2022; 50:63–73. doi: 10.1016/j.clnesp.2022.06.001 [DOI] [PubMed] [Google Scholar]
- 35.Tarp J, Rossen J, Ekelund U, Dohrn IM. Joint associations of physical activity and sedentary time with body mass index: a prospective study of mortality risk. Scand J Med Sci Sports. 2023;33(5):693–700. doi: 10.1111/sms.14297 [DOI] [PubMed] [Google Scholar]
- 36.de Araújo MDESC, da Conceição Chagas de Almeida M, Matos SMA, de Jesus Mendes da Fonseca M, Pitanga CPS, Pitanga FJG Combined effect of leisure-time physical activity and sedentary behavior on abdominal obesity in ELSA-Brasil participants. Int J Environ Res Public Health. 2023;20(15):6501. doi: 10.3390/ijerph20156501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mokdad AH, Ford ES, Bowman BA, et al. Prevalence of obesity, diabetes, and obesity-related health risk factors, 2001. JAMA. 2003; 289(1):76–79. doi: 10.1001/jama.289.1.76 [DOI] [PubMed] [Google Scholar]
- 38.Kirby JB, Liang L, Chen HJ, Wang Y. Race, place, and obesity: the complex relationships among community racial/ethnic composition, individual race/ethnicity, and obesity in the United States. Am J Public Health. 2012;102(8):1572–1578. doi: 10.2105/AJPH.2011.300452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Woolcott OO, Seuring T. Temporal trends in obesity defined by the relative fat mass (RFM) index among adults in the United States from 1999 to 2020: a population-based study. BMJ Open. 2023;13(8): e071295. doi: 10.1136/bmjopen-2022-071295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Elia M. Obesity in the elderly. Obes Res. 2001;9(S11):244S–248S. doi: 10.1038/oby.2001.126 [DOI] [PubMed] [Google Scholar]
- 41.Schwartz MW, Seeley RJ, Zeltser LM, et al. Obesity pathogenesis: an Endocrine Society scientific statement. Endocr Rev. 2017;38(4):267–296. doi: 10.1210/er.2017-00111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Darmon N, Drewnowski A. Does social class predict diet quality? Am J Clin Nutr. 2008;87(5):1107–1117. doi: 10.1093/ajcn/87.5.1107 [DOI] [PubMed] [Google Scholar]
- 43.Hanson KL, Connor LM. Food insecurity and dietary quality in US adults and children: a systematic review123. Am J Clin Nutr. 2014; 100(2):684–692. doi: 10.3945/ajcn.114.084525 [DOI] [PubMed] [Google Scholar]
- 44.Crawford PB, Webb KL. Unraveling the paradox of concurrent food insecurity and obesity. Am J Prev Med. 2011;40(2):274–275. doi: 10.1016/j.amepre.2010.11.003 [DOI] [PubMed] [Google Scholar]
- 45.Hohl A, Lotfata A. Modeling spatiotemporal associations of obesity prevalence with biking, housing cost and green spaces in Chicago, IL, USA, 2015–2017. J Transp Health. 2022;26:101412. doi: 10.1016/j.jth.2022.101412 [DOI] [Google Scholar]
- 46.Lotfata A, Tomal M. Exploring housing determinants of obesity prevalence using multiscale geographically weighted regression in Chicago, Illinois. Prof Geogr. 2023;75(3):335–344. doi: 10.1080/00330124.2022.2111692 [DOI] [Google Scholar]
- 47.Baez AS, Ortiz-Whittingham LR, Tarfa H, et al. Social determinants of health, health disparities, and adiposity. Prog Cardiovasc Dis. 2023;78: 17–26. doi: 10.1016/j.pcad.2023.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brakefield WS, Olusanya OA, Shaban-Nejad A. Association between neighborhood factors and adult obesity in Shelby County, Tennessee: geospatial machine learning approach. JMIR Public Health Surveill. 2022;8(8):e37039. doi: 10.2196/37039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Scheinker D, Valencia A, Rodriguez F. Identification of factors associated with variation in US County-level obesity prevalence rates using epidemiologic vs machine learning models. JAMA Netw Open. 2019; 2(4):e192884. doi: 10.1001/jamanetworkopen.2019.2884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ayanian JZ, Weissman JS, Schneider EC, Ginsburg JA, Zaslavsky AM. Unmet health needs of uninsured adults in the United States. JAMA. 2000;284(16):2061–2069. doi: 10.1001/jama.284.16.2061 [DOI] [PubMed] [Google Scholar]
- 51.Liu J, Zhang Y, Lavie CJ, Moran AE. Trends in metabolic phenotypes according to body mass index among US adults, 1999–2018. Mayo Clin Proc. 2022;97(9):1664–1679. doi: 10.1016/j.mayocp.2022.02.013 [DOI] [PubMed] [Google Scholar]
- 52.Arena R, Pronk NP, Woodard C. Physical inactivity and obesity in the United States: At the intersection of politics, socioeconomics, race, and culture. Curr Probl Cardiol. 2023;48(12):102007. doi: 10.1016/j.cpcardiol.2023.102007 [DOI] [PubMed] [Google Scholar]
- 53.Arena R, Laddu D, Pronk NP, Woodard C. The geographic distribution of unhealthy living characteristics according to the American nations model: cultural factors warranting attention. Prog Cardiovasc Dis. 2023;79:100–106. doi: 10.1016/j.pcad.2023.07.002 [DOI] [PubMed] [Google Scholar]
- 54.Rozanski A, Blumenthal JA, Hinderliter AL, Cole S, Lavie CJ. Cardiology and lifestyle medicine. Prog Cardiovasc Dis. 2023;77:4–13. doi: 10.1016/j.pcad.2023.04.004 [DOI] [PubMed] [Google Scholar]
- 55.Claridy MD, Czepiel KS, Bajaj SS, Stanford FC. Treatment of obesity: pharmacotherapy trends of office-based visits in the United States from 2011 to 2016. Mayo Clin Proc. 2021;96(12):2991–3000. doi: 10.1016/j.mayocp.2021.07.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Salerno PR, Dong W, Motairek I, et al. Alzheimer’s disease mortality in the United States: cross-sectional analysis of county-level socio-environmental factors. Arch Gerontol Geriatr. 2023;115:105121. doi: 10.1016/j.archger.2023.105121 [DOI] [PubMed] [Google Scholar]
- 57.Dong W, Kim U, Rose J, et al. Geographic variation and risk factor Association of Early Versus Late Onset Colorectal Cancer. Cancer. 2023;15(4):1006. doi: 10.3390/cancers15041006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Duque JC, Anselin L, Rey SJ. The max-p-regions problem. J Regional Sci. 2012;52(3):397–419. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.