Abstract
Hepatitis delta virus (HDV) small delta antigen (S-HDAg) plays a critical role in virus replication. We previously demonstrated that the S-HDAg phosphorylation occurs on both serine and threonine residues. However, their biological significance and the exact phosphorylation sites of S-HDAg are still unknown. In this study, phosphorylated S-HDAg was detected only in the intracellular compartment, not in viral particles. In addition, the number of phosphorylated isoforms of S-HDAg significantly increased with the extent of viral replication in transfection system. Site-directed mutagenesis showed that alanine replacement of serine 177, which is conserved among all the known HDV strains, resulted in reduced phosphorylation of S-HDAg, while the mutation of the other two conserved serine residues (2 and 123) had little effect. The S177A mutant dramatically decreased its capability in assisting HDV RNA replication, with a preferential and profound impairment of the antigenomic RNA replication. Furthermore, the viral RNA editing, a step relying upon antigenomic RNA replication, was also abolished by this mutation. These results suggested that phosphorylation of S-HDAg, with serine 177 as a presumable site, plays a critical role in viral RNA replication, especially in augmenting the replication of antigenomic RNA.
Hepatitis delta virus (HDV) is a defective virus and requires its helper virus, hepatitis B virus (HBV), to supply the envelope proteins for viral assembly (36, 37). HDV RNA genome is a 1.7-kb single-stranded circular RNA and is thought to replicate by a double rolling-circle mechanism (13, 26, 48). Both polarities of multimeric RNA intermediates are generated during replication and processed into genomic and antigenomic monomers. Host RNA polymerase II (Pol II) may be the enzyme responsible for HDV RNA replication (14, 24, 25) since the process is inhibited both by a low dose of α-amanitin and by a monoclonal antibody specific for Pol II. However, it is not clear how RNA Pol II recognizes HDV RNA as a template. HDV replication is also dependent on the expression of the small form of hepatitis delta antigen (S-HDAg). S-HDAg is not a replicase itself and its function in the replication process is still unclear. Therefore, one hypothesis proposed that S-HDAg modulates the specificity of RNA Pol II to facilitate the HDV RNA-dependent RNA transcription (20), while another study suggested that an unidentified RNA dependent RNA polymerase may play that role (31).
During the HDV life cycle, a posttranscriptional RNA editing specifically converts the amber stop codon (UAG) of S-HDAg to a Trp codon (UGG). This event leads to the production of large HDAg (L-HDAg) with 19 extra amino acids at the C terminus (4, 23). The L-HDAg functions as both a suppressor for replication and a key factor for virion assembly (9, 22). This editing event occurs on the antigenomic RNA at a late stage in the viral replication cycle (6, 35) and therefore completes the viral life cycle.
L-HDAg and S-HDAg have been identified as phosphoproteins with different phosphorylation patterns. (8, 15, 32, 51). We have established a two-dimensional system (nonequilibrium pH gradient gel electrophoresis [NEPHGE]) to separate phosphorylated isoforms of HDAg's (32). In this system, the S-HDAg has been resolved into two phospho-isoforms, while the L-HDAg has been resolved into only one. However, the role of HDAg phosphorylation in the life cycle of HDV remains unclear. It has been demonstrated that protein kinase C-specific inhibitor H7 decreases the phosphorylation level of S-HDAg and suppresses viral RNA replication (51). Therefore, S-HDAg phosphorylation may influence its function as a replication cofactor. These observations prompted us to investigate the correlation between S-HDAg phosphorylation and HDV replication and to identify the phosphorylation sites involved.
MATERIALS AND METHODS
Plasmid construction.
pCD2G contains a tandem dimer of wild-type HDV cDNA (1.7-kb XbaI fragment, genomic sense) with sequences derived from pSVD3 (19). Under the control of the human cytomegalovirus (CMV) immediate-early promoter (pCMV-2) (47), this construct transcribed HDV genomic RNA (G RNA). pCD2AG, like pCD2G, also contains an HDV cDNA dimer but in a different orientation; therefore, pCD2AG transcribed antigenomic HDV RNA (AG RNA) driven by a CMV promoter. These two constructs were used for the expression of strand-specific HDV RNA in transfected cells. After cotransfecting with a construct expressing HBV surface antigen (HBsAg), pS1X (46), the HDV virions could be packaged and secreted into cultured medium. pCDm2G and pCDm2AG, with a two-base deletion in the HDAg open reading frame (ORF), are derived from pCD2G and pCD2AG, respectively. Under the control of the CMV promoter, pCDm2G transcribes nonreplicating HDV genomic RNA and pCDm2AG transcribes nonreplicating HDV antigenomic RNA. As no HDAg's are produced, both plasmids require a functional S-HDAg in trans for viral replication (47). pCD2G177 and pCD2AG177 are similar to wild-type pCD2G and pCD2AG but with a serine-to-alanine mutation at serine 177 encoded in the HDAg ORF. pCDAg-S and pCDAg-L contained an HDAg ORF and expressed S-HDAg and L-HDAg, respectively (32).
Site-directed mutagenesis of S-HDAg.
Site-directed mutagenesis was performed by the Transformer Site-Directed Mutagenesis Kit (Clontech, Palo Alto, Calif.). It works by simultaneously annealing two primers (one is a universal selection primer and the other is a mutagenic primer [described later]) to one strand of denatured pCDAg-S. The mutagenic primers used in site-directed substitution of serines 2, 123, and 177 are as follows (the mutated sequences are underlined): Ser 2/Ala, 5′-CCGAGATGGCCCGGTCCGAG-3′; Ser 123/Ala, 5′-CAAGAACCTCGCCAAGGAGG-3′; Ser 177/Ala, 5′-GTCCCGGAGGCCCCCTTCTC-3′. After synthesizing the second strand with T4 DNA polymerase, the mixed population of plasmids contained mutated and unmutated plasmids which could be distinguished by digestion with KpnI. This primary selection step greatly reduced the proportion of the unmutated plasmids present in the mixture and enriched the mutated ones when they were transformed into mutS of Escherichia coli. The DNA isolated from the pooled transformants was subjected to a second round of KpnI digestion, and the second transformation was used to amplify and clone the mutated plasmids. Every mutant clone was confirmed by sequence analysis. Three serine-substituted mutants (mutation at position 2, 123, or 177) were designated S2A, S123A, and S177A, respectively.
DNA transfection.
The DNA transfection was performed by calcium phosphate precipitation (49). For kinetic study, 15 μg of pCD2G was cotransfected with 15 μg of HBsAg-expressing plasmid (pS1X) into HuH-7 cells (4 × 106 cells/100-mm-diameter petri dish). In a metabolic labeling experiment, 5 μg of plasmid together with 5 μg of salmon sperm DNA were used to transfect HuH-7 cells (106 cells/60-mm-diameter petri dish). For analysis of replication, 5 μg of pCDAg-S (or S-HDAg mutants) and 5 μg of either pCDm2G or pCDm2AG were applied to HuH-7 cells (106 cells/60-mm-diameter petri dish).
Immunoprecipitation of HDAg's.
Immunoprecipitation was performed using a standard method as described in detail elsewhere (32). Briefly, cells were lysed in radioimmunoprecipitation buffer and immunoprecipitated with protein G-agarose-bound mouse monoclonal anti-HDAg antibody (5 μg/ml), D9-3. Proteins were eluted from protein G-agarose by using a urea–NP-40 sample solubilizer for NEPHGE–sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) or using 2× Laemmli's sampling buffer for Western blotting.
Isolation of viral particles.
On day 12 posttransfection, cultured medium was collected from HuH-7 cells which had been cotransfected with pCD2G and pS1X. Then, 30 ml of the cultured medium was clarified by centrifuging at 12,000 rpm and 4°C for 30 min (JA-17 rotor; Beckman). The supernatant was layered over a 20% sucrose cushion (1.5 ml of 20% sucrose in 10 mM phosphate buffer–0.85% NaCl in 10 ml of culture medium) and centrifuged at 25,000 rpm and 4°C overnight (SW 28 rotor; Beckman) (7). The pellet was dissolved in 250 μl of solubilizer, and 50 μl was used for two-dimensional system.
Two-dimensional gel electrophoresis (NEPHGE/SDS-PAGE).
One-dimensional NEPHGE was based on a modified protocol from BASO-DALT (33, 50) and was fine-tuned by us previously (32). The proteins from immunoprecipitation were resuspended in urea–NP-40 sample solubilizer and loaded onto the well of one cylindrical gel (12 cm [length] by 4 mm [diameter]) covered with a 4 M urea overlay solution. One-dimension gel was made with 3.3% acrylamide (30% acrylamide and 1.8% bis-acrylamide) containing 9 M urea, 2% NP-40, 1.6% pharmalyte (pH 3.5 to 10), and 0.4% servalyte (pH 9 to 11). The gel was installed vertically in an electrophoresis apparatus (Desaga GmbH, Heidelberg, Germany) with 20 mM NaOH in the lower chamber and 10 mM H3PO4 in the upper chamber. Lysozyme and trypsinogen (Sigma, St. Louis, Mo.) were used as the references for pI markers and were run in parallel cylindrical gels. The first dimension was electrophoresed at 400 V for 60 min and then at 800 V for 67.5 min. After electrophoresis, the cylindrical gels were extruded from the glass tubes by syringes and each one was soaked in 10 ml of equilibration buffer (10% glycerol, 4.9 mM dithiothreltol, 2% SDS, and 0.125 M Tris, adjusted to pH 6.8) for 10 min.
The second-dimension gel was a standard discontinuous SDS-PAGE employing a 20-by-20-by-0.15-cm gel (12% separation gel and 5% stacking gel) with a beveled plate to assemble the glass-plate sandwich (BRL vertical gel electrophoresis system, model V16-2). This plate provided a wider space to accommodate the thick cylindrical gel from NEPHGE. After equilibration, the extruded tube gel was laid on top of the stacking gel and sealed with 0.5% agarose. Electrophoresis was carried out overnight (70 V, 16 to 18 h), and gels were electrotransferred onto nitrocellulose membrane (Hybond-c super, Amersham Pharmacia Biotech, Little Chalfont, England), followed by Western blotting.
Western blot analysis.
For detection the immunoprecipitated HDAg's, proteins were subjected to electrophoresis in an SDS–12% PAGE, followed by a Western blot procedure (7, 12). HDAg's were detected by an ECL Western blot detection system (Amersham Parmacia Biotech) with a human polyclonal antibody against both forms of HDAg. For detection of protein from whole-cell lysate, 50-μg volumes of proteins were subjected to Western blotting and detected with D9-3, a monoclonal antibody against HDAg (7).
In vivo 32P-orthophosphate labeling.
HDAg's were in vivo labeled as previously described (32). Briefly, transfected cells were starved in 0.8 ml of phosphate-free Dulbecco modified Eagle medium (DMEM) (Gibco BRL/Life Technologies) for 2 h, followed by incubation with 0.5 mCi of 32P-orthophosphate (PBS-13; Amersham Parmacia Biotech)/ml for 4 h. Cells were then immunoprecipitated with D9-3 antibody as described above. The immune complex was resuspended in 2× Laemmli's sampling buffer and boiled for 5 min. Seven-eighths of the eluted proteins were analyzed by electrophoresis in an SDS–12% PAGE gel and visualized by autoradiography. The remaining one-eighth was subjected to a parallel SDS-PAGE and then transferred to a nitrocellulose membrane for Western blot analysis.
Northern blot analysis.
Total RNAs were isolated from transfected HuH-7 cells by using RNAzol B solution, following the procedure described by the manufacturer (Tel-Test, Inc.). Transfected cells were washed with cold phosphate-buffered saline twice and lysed with 1 ml of RNAzol B solution/60-mm-diameter dish. After adding 0.2 ml of chloroform and incubating on ice for 15 min, the lysate was centrifuged at 12,000 rpm for 20 min at 4°C. The RNA was in the aqueous phase and precipitated by adding an equal volume of isopropanol. RNAs were electrophoresed after denaturation with glyoxal and dimethyl sulfoxide (28): 10 μg of total RNA was mixed with 20 μl of glyoxal solution and electrophoresed at 100 V for about 2 h. The gel was then electrotransferred with 0.25× TAE (Tris-acetate-EDTA: 10 mM Tris-acetate, 0.25 mM EDTA) at 90 V for 1.5 h. The membrane (Nytran; Schleicher & Schuell, Dassel, Germany) was then cross-linked and hybridized with a strand-specific riboprobe (synthesized by in vitro transcription) (47).
RNA transfection.
The HDV genomic and antigenomic RNAs were in vitro transcribed from pCDm2AG and pCDm2G, respectively, after linearization by HindIII digestion with T7 MEGAscript (Ambion, Austin, Tex.). The capped mRNA for expressing wild-type and serine 177 mutant S-HDAg were transcribed after linearization by HindIII digestion with T7 mMESSAGE mMACHINE (Ambion) from pCDAg-S and S177A, respectively (30). The RNA transfection was performed by using the lipofectin (Gibco BRL/Life Technologies) method according to the protocol provided by the manufacturer. Briefly, 1 day prior to transfection, HuH-7 cells were seeded onto a 60-mm-diameter dish with 60% confluence in DMEM. To prepare solution A, 21 μl (1 μg/μl) of lipofectin (Gibco BRL/Life Technologies) was diluted in 500 μl of opti-MEM (Gibco BRL/Life Technologies) and stood at room temperature for 30 to 45 min. For solution B, 3 μg of genomic or antigenomic RNA together with 7.5 μg of capped mRNA were diluted in 500 μl of opti-MEM (Gibco BRL/Life Technologies). The two solutions were combined and incubated at room temperature for 10 to 15 min. At the same time, HuH-7 cells were washed twice with opti-MEM (Gibco BRL/Life Technologies), which was followed by adding the RNA-lipofectin mixture. After 4 h, culture medium was replaced with 4 ml of DMEM. During transfection, medium was replaced once with 4 ml of DMEM on day 3 posttransfection. On day 6 post-RNA transfection, cells were harvested for Northern and Western analyses.
Analysis of editing by RT-PCR.
One microgram of total RNA from transfected HuH-7 cells was first treated with DNase for 15 min at room temperature, and 1 μl of 25 mM EDTA was added for 15 min at 65°C to stop the reaction. Left on ice for 1 min, RNA was ready for reverse transcription (Thermoscript RT-PCR system; BRL). To specifically amplify mRNA, the RNA was annealed with 0.5 μg of oligo(dT) primer/μl for 10 min at 70°C, put on ice for 1 min, and incubated with 2 μl of 10× PCR buffer, 2 μl of 25 mM MgCl2, 1 μl of 10 mM dNTP, and 2 μl of 0.1 M DTT for 5 min, and then reverse transcriptase (200 U) was added. Reverse transcription (RT) was carried at 42°C for 50 min, then at 37°C for 10 min, and finally at 70°C for 15 min to supper enzyme activity. RNase H was added to digest RNA in the DNA-RNA hybrid for 20 min at 37°C, and the RT products were used for the following PCR: 95°C for 1 min, then 55°C for 1 min, and then 72°C 1 min, repeated for 30 cycles (to label the product, dCTP was replaced by [32P]-dCTP). Finally, the RT-PCR products were digested by NcoI and followed by autoradiography to distinguish the proportion of edited and unedited RNA (6). The extent of cleavage was then quantitated by using a Bio-Imaging Analyzer (FUJIX, BAS 1000).
RESULTS
Changes of HDAg phosphorylation patterns during HDV life cycle.
To investigate the biological roles of HDAg phosphorylation, we examined the extent of phosphorylation patterns of HDAg's during the course of HDV RNA replication by the NEPHGE–SDS-PAGE system (32). HuH-7 cells were cotransfected with pCD2G that contains HDV dimer cDNA to provide HDV RNA replication and with pS1X that could express S, M, and L forms of HBsAg for virus packaging. Therefore, in this system, HDV replication could be initiated and viral particles could be secreted into cultured medium. We investigated the kinetics of HDAg phosphorylation on days 2, 3, 5, and 12 posttransfection. Total lysates were immunoprecipitated with HDAg-specific antibody, and the precipitates were resolved by NEPHGE. Isoforms of HDAg's were detected by Western blotting. On day 2 posttransfection, one phospho-isoform of S-HDAg was detected in addition to the unphosphorylated form (Fig. 1A). Two major phospho-isoforms of S-HDAg were observed on day 3 posttransfection (Fig. 1B). Later, there was a significant increase in the amount of phospho-isoforms of S-HDAg on day 5 (Fig. 1C), compared to the amounts on day 2 and 3. More phospho-isoforms made it difficult to clearly differentiate those isoforms. The phosphorylated patterns of S-HDAg on day 12 were similar to those observed on day 5 (Fig. 1D). Because L-HDAg is expressed only late in the replication cycle through RNA editing, we detected L-HDAg on day 12 with the longer exposure time (Fig. 1E versus D). As for the low expression of L-HDAg during genome replication, it is hard to define the number of phospho-isoforms. However, we have previously observed only one phospho-isoform of L-HDAg by overexpression (32). Interestingly, when we studied the phosphorylated isoforms of HDAg's in virions, only an unphosphorylated form of S-HDAg could be found (Fig. 1F). In contrast to the absence of phospho-isoform of S-HDAg in the secreted viral particle, L-HDAg has one phospho-isoform therein. Thus, it appears that S-HDAg phosphorylation occurs only in the intracellular compartment where replication takes place. This observation provided further support to the previous observation that phosphorylation might play some role in controlling HDV RNA replication (51).
FIG. 1.
Kinetic study of HDAg's in replicating cells and in viral particles. pCD2G (HDV dimeric cDNA) cotransfected with pS1X (a construct expressing HBV surface antigen) into HuH-7 cells. After transfection, there was HDV replication, HDAg expression, and HDV viral particle secretion. Proteins were extracted from the replicating cells on days 2 (A), 3 (B), 5 (C), and 12 (D) posttransfection, and a longer exposure time of cells on day 12 is also shown (E). (F) Secreted viral particles were collected from medium on day 12. These materials were subjected to the NEPHGE–SDS-PAGE system and detected by Western blotting. The unphosphorylated form of HDAg is the most basic spot to the right end. The asterisk above each panel indicates the position of trypsinogen with known pI of 9.3, and the star indicates the position of lysozyme with a pI of 10.5 to 11 as pH markers.
Phosphorylation level of S-HDAg was reduced in serine 177 mutant.
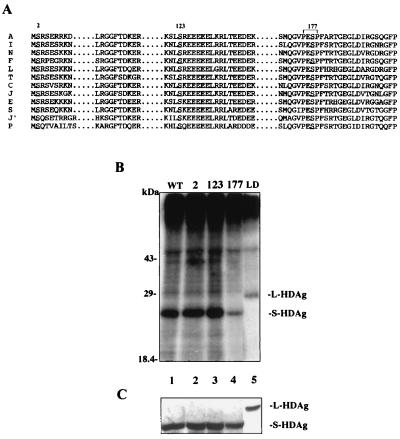
S-HDAg has been previously shown to be phosphorylated at both serine and theronine residues (32). To investigate the effect of phosphorylation on HDV replication, we employed site-directed mutagenesis to replace possible serine and threonine residues. To begin with, we compared currently available HDV sequences (including all three genotypes) and located the conserved serine and threonine residues in the S-HDAg ORF. There are three completely conserved serine residues—serines 2, 123, and 177—in S-HDAg (Fig. 2A). However, no completely conserved threonine residue was found except for a conserved serine/threonine residue (amino acid [aa] 95) in S-HDAg. Therefore, these completely conserved serine residues were first chosen to be substituted into nonphosphorylatable alanine. Three constructs expressing S-HDAg mutant were designated S2A, S123A and S177A.
FIG. 2.
(A) Conserved serine residues among variant HDV strains. On the left are the geographical origins of the HDV isolates: A, American (26); I, Italian (48); N, Nauru (10); F, French (39); L, Lebanon (21); T, Taiwan (11); J, Japan-2 (17); C, Central African (42); J′, Japan-1 (16); P, Peru (5). The numbered residues are the three conserved serine residues among the HDV strains, and PESP encompassing serine 177 is the predicted consensus sequence of the MAP kinase substrate (34). (B) In vivo phosphorylation of HDAg. pCDAg-S (wild type; lane 1), serine-substituted mutants (lanes 2 to 4; the numbers indicate mutated serine residues), and pCDAg-L (L-HDAg, lane 5) were transfected into HuH-7 cells. On day 2 posttransfection, transfected cells were metabolically labeled with [32P]-orthophosphate and immunoprecipitated with monoclonal anti-HDAg antibody. Seven-eighths of the immunoprecipitates were subjected to SDS-PAGE and analyzed by autoradiography. (C) One-eighth of the labeled immunoprecipitates were detected by Western blotting with human polyclonal anti-HDAg antibody. The positions of S-HDAg (24 kDa) and L-HDAg (27 kDa) are indicated.
The phosphorylation level of the three S-HDAg mutants was examined by in vivo phosphate labeling. The wild-type and mutant S-HDAg's were metabolically labeled with 32P-orthophosphate and immunoprecipitated with anti-HDAg monoclonal antibody. No obvious difference in the extent of phosphorylation was observed in S2A and S123A when compared with wild-type S-HDAg (Fig. 2B, lanes 1 to 3). However, in S177A, there was a clear reduction of phosphorylation (Fig. 2B, lane 4). This indicated that serine 177 may be one of the phosphorylation sites in S-HDAg. However, the serine 177 mutant still retained significant phosphorylation, suggesting that additional phosphorylated serine or threonine residue(s) in S-HDAg may be expected.
Effects of serine 177 mutant on HDV replication by DNA transfection.
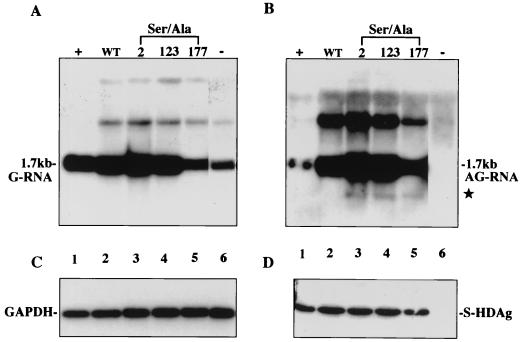
HDV replicates through a two-phase process: the genomic RNA replication followed by antigenomic RNA replication. To study the two phases separately, we adapted a transfection system to detect genomic and antigenomic RNA production. First, an HDV dimeric mutant construct (pCDm2G) which could not self-replicate was used to provide HDV genomic RNA as a starting template (47). It could undergo a replication cycle when supplied S-HDAg in trans. On day 6 posttransfection, intracellular genomic (Fig. 3A) and antigenomic (Fig. 3B) HDV RNAs were analyzed by Northern blotting with strand-specific riboprobes. The antigenomic RNA was produced in the presence of wild-type S-HDAg, indicating that wild-type S-HDAg could help complete the replication cycle (Fig. 3B, lane 2). The antigenomic RNA produced from cotransfection with S2A, S123A, and S177A, respectively, was also detected in an amount similar to that from wild-type S-HDAg (Fig. 3B, lanes 3 to 5). These serine-to-alanine mutations did not significantly affect the ability of S-HDAg to assist antigenomic RNA production.
FIG. 3.
Serine 177 of S-HDAg is dispensable for HDV antigenomic RNA production. pCDm2G was cotransfected with constructs expressing wild-type S-HDAg (lane 2), S-HDAg mutants (lanes 3 to 5; the number above each lanes indicates the location of the mutated residue), or salmon sperm DNA (lane 6, negative control). On day 6 posttransfection, total cellular RNA was extracted and subjected to Northern blotting for detection of genomic RNA (A), antigenomic RNA (B), and GAPDH (glyceraldehyde-3-phosphate dehydrogenase) (C) by using different probes. (B) The star represents mRNA transcribed from S-HDAg-expressing plasmids. The positions of monomeric (1.7-kb) HDV RNA are indicated. (D) The intracellular S-HDAg was detected by Western blotting with anti-HDAg monoclonal antibody. The position of 24-kDa S-HDAg is indicated. Lane 1, a positive control.
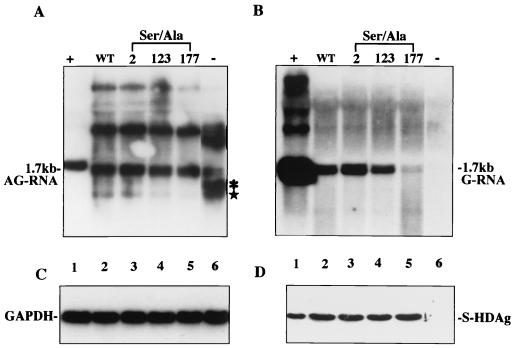
After examining the antigenomic RNA production in Fig. 3, we used the same strategy to examine the effect of these mutations on genomic RNA production. A nonreplicating construct, pCDm2AG, was used as the starting template this time. The genomic RNA was produced when cotransfected with wild-type S-HDAg (Fig. 4B, lane 2), as was the case with the cotransfection of the serine 2 and serine 123 mutants (Fig. 4B, lanes 3 and 4). In contrast, the genomic RNA was hardly detected in the serine 177 cotransfection (Fig. 4B, lane 5). The expression level of each protein was similar (Fig. 4D). Therefore, the serine 177 mutation results in profound impairment (reduced to 3 to 5% of the wild type) of genomic RNA production.
FIG. 4.
Serine 177 of S-HDAg is required for HDV genomic RNA production. Wild-type (lane 2) and mutant (lanes 3 to 5) S-HDAg or salmon sperm DNA (lane 6, negative control) was cotransfected with pCDm2AG. Antigenomic RNA (A), genomic RNA (B), and GAPDH (C) were detected by Northern blotting. (A) The star represents mRNA transcribed from S-HDAg-expressing plasmids, and the asterisk represents primary transcripts from the CMV promoter that were terminated at the poly(A) site for the mRNA. (D) The expression of S-HDAg was analyzed by Western blotting. Samples are organized identically to those in Fig. 3.
Taken together, reduction of protein phosphorylation (Fig. 2B) and impairment of HDV genomic RNA production in S177A (Fig. 4B) were in parallel. These results supported the data from the kinetic study (Fig. 1) that the effect of S-HDAg phosphorylation may correlate with HDV RNA replication. Thus, phosphorylation of serine 177 may strengthen the control of the strand specificity of HDV replication.
Further functional assay of serine 177 mutants by RNA transfection.
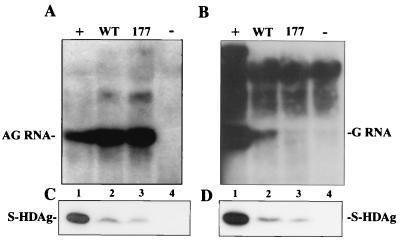
The HDV replication assay presented above was cDNA initiated and was not absolutely specific in genomic and antigenomic RNA transcripts. To study how authentic genomic and antigenomic HDV RNA production was affected by the serine 177 mutant, we employed a recently established cDNA-free RNA transfection system (30). In this RNA transfection, the HDV unit-length genomic and antigenomic RNA templates were in vitro transcribed from nonreplicating constructs: pCDm2AG and pCDm2G, respectively. The capped S-HDAg-encoding mRNA for expressing wild-type and serine 177 mutant S-HDAg were in vitro transcribed from pCDAg-S and S177A.
To detect the antigenomic RNA production, the capped S-HDAg-encoding mRNA was cotransfected with in vitro-transcribed genomic HDV RNA. As shown in Fig. 5A, the production of complementary, unit-length, antigenomic RNA from input in vitro-transcribed genomic HDV RNA template was detected. Consistent with the results in cDNA transfection, the serine 177 mutant still supported the antigenomic RNA production as efficiently as the wild type did (Fig. 5A, lanes 2 and 3). On the other hand, the genomic RNA production was detected when supplied with wild-type S-HDAg and was hardly detected in the presence of the serine 177 mutant (Fig. 5B, lanes 2 and 3) as it was in the cDNA transfection. Both cDNA and RNA transfection systems demonstrated that serine 177 of S-HDAg is involved in modulating HDV replication.
FIG. 5.
The importance of serine 177 of S-HDAg in supporting HDV genomic RNA production was demonstrated by using a cDNA-free RNA transfection system. (A) Northern blot analysis of antigenomic RNA production from HuH-7 cells cotransfected with in vitro-transcribed genomic RNA and S-HDAg-encoding mRNA (lanes 2 and 3, wild type and serine 177 mutants). (B) Northern blot analysis of genomic RNA production from HuH-7 cells transfected with in vitro-transcribed antigenomic RNA and S-HDAg-encoding mRNA (lanes 2 and 3, wild type and serine 177 mutants). Lanes 1, positive controls which were from cotransfection with pCDm2G and pCDAg-S (A) and cotransfection with pCDm2AG and pCDAg-S (B); lanes 4, negative controls from transfection with only nonreplicating genomic (A) and antigenomic (B) RNA. The S-HDAg expression from genomic RNA transfection (C) or antigenomic RNA transfection (D) was analyzed by Western blotting.
As a consequence of impaired antigenomic RNA replication, serine 177 mutation interrupts L-HDAg production.
During the HDV life cycle, RNA editing plays a central role in modulating viral replication and completing virion package. It has been demonstrated that editing occurs specifically on antigenomic RNA and that the sequence changed is passed to genomic RNA (6). If serine 177 phosphorylation were important for genomic RNA production, a mutation at serine 177 in the HDV genome would very likely interrupt the production of edited genomic RNA and the following production of L-HDAg-encoding mRNA. To further validate the effect of S177A on genomic RNA production, we examined its effects on RNA editing. Two replicating constructs, pCD2G and pCD2AG, which contain a tandem dimer of wild-type HDV cDNA but in opposite orientations, were used to monitor the expression of L-HDAg resulting from editing during the wild-type HDV life cycle. To investigate the effect of serine 177 mutant S-HDAg on the editing event, we constructed the other pair of constructs, pCD2G177 and pCD2AG177. Both contained an HDV mutant dimer with a serine 177 mutation in the ORF but in opposite orientations as well. These dimer constructs were transfected in HuH-7 cells and both genomic and antigenomic RNA were detected on days 6, 9, and 12 posttransfection.
As shown in Fig. 6, wild-type HDV dimer constructs, pCD2G and pCD2AG, could support both antigenomic RNA (Fig. 6C, lanes 1 to 3) and genomic RNA (Fig. 6D, lanes 1 to 3) production. Antigenomic RNA production was also detected in pCD2G177 transfection (Fig. 6C, lanes 4 to 6). However, little genomic RNA was detected when cells were transfected with pCD2AG177 (Fig. 6D, lanes 4 to 6). This corresponded to the results of DNA and RNA cotransfection (Fig. 3, 4, and 5).
FIG. 6.
The expression of L-HDAg, a consequence of RNA editing, was influenced by the serine 177 mutation. The wild-type dimer of HDV, pCD2G and pCD2AG (2Gwt and 2AGwt, respectively), and the Ser 177 dimer mutants, pCD2G177 and pCD2AG177 (2G177 and 2AG177, respectively), were transfected into HuH-7 cells. The HDV RNA and HDAg's were examined on days 6, 9, and 12 posttransfection (D6, D9, and D12). For detection of the antigenomic RNA production from 2Gwt and 2G177, genomic RNA (A) and antigenomic RNA (C) were analyzed by Northern blotting. For detection of the genomic RNA production from 2AGwt and 2AG177, antigenomic RNA (B) and genomic RNA (D) were also analyzed by Northern blotting. The expression of S-HDAg (E) and L-HDAg (F) from these dimer constructs were analyzed by Western blotting and are shown. The positions of L-HDAg and S-HDAg are indicated.
To investigate the expression of L-HDAg as a consequence of editing, HDAg's expressed form these two pairs of constructs were detected by Western blotting. As shown in Fig. 6E and F, both S-HDAg and L-HDAg were expressed from pCD2G and pCD2AG (Fig. 6E and F, lanes 1 to 3). The level of L-HDAg gradually increased from day 6 to day 9 and may have reached a plateau afterward. However, in the transfection of serine 177 dimer mutants, pCD2G177 and pCD2AG177, L-HDAg was barely detected and only S-HDAg was expressed (Fig. 6E and F, lanes 4 to 6). Therefore, the results indicated there might be no L-HDAg expression from the serine 177 dimer mutants. We have noticed that the expression level of serine 177 mutant S-HDAg was lower than that of wild-type S-HDAg in Fig. 6E and F. This result may mislead us to jump to the conclusion that there is no L-HDAg expression in serine 177 mutant transfection. Therefore, we used RT-PCR, a more sensitive experiment, to detect whether edited mRNA (represented by L-HDAg encoding mRNA) was transcribed.
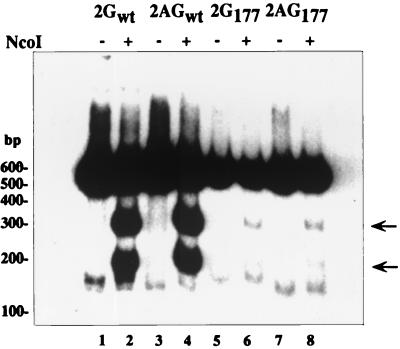
In order to directly demonstrate that the lack of L-HDAg expression from serine 177 dimer mutants was due to the reduction of edited RNA template instead of lower expression of serine 177 mutant HDAg's, we compared the amounts of edited and unedited mRNA in transfected cells. This comparison was established previously by using RT-PCR to amplify HDV RNA and by digesting the RT-PCR products with NcoI to separate edited and unedited sequences (6). We used oligo-(dT) as a primer to specifically amplify polyadenylated mRNA instead of nonpolyadenylated full-length HDV RNA in RT-PCR-based editing. The edited mRNA detected was considered a template for the expression of L-HDAg. This approach can avoid detection of editing in the huge overabundance of full-length, nonpolyadenylated RNA. The total cellular RNA from the transfection of dimer constructs used in Fig. 6 was RT-PCR amplified, and the products were digested by NcoI. As shown in Fig. 7, the products amplified from wild-type dimer constructs (pCD2G and pCD2AG) could be digested into two smaller fragments (Fig. 7, lanes 2 and 4). The cleaved products, reflecting the components of edited mRNA, were found to be around 20 to 25% of total amount of amplified products. However, in the mutant HDV RNA (from transfections of pCD2G177 and pCD2AG177), few NcoI-digested products were detected (less than 1%) (Fig. 7, lanes 6 and 8). A dramatic decrease of edited mRNA from serine 177 mutants supported the conclusion that the failure of yielding edited mRNA in serine 177 mutants may be the consequence of impairment of genomic RNA production.
FIG. 7.
Effect of serine 177 phosphorylation on RNA editing. Total cellular RNA from transfection of wild-type dimers (pCD2G and pCD2AG) and mutant dimers (pCD2G177 and pCD2AG177) as described for Fig. 6 was subjected to an RT-PCR-based editing assay (see Materials and Methods for details) and followed by autoradiography. Arrowheads represent edited RNA.
DISCUSSION
In this study, changes in the phosphorylation pattern of HDAg's during HDV RNA replication were detected by a two-dimensional system (NEPHGE–SDS-PAGE). Furthermore, site-directed mutagenesis studies showed that the reduced phosphorylation level of the serine 177 mutant corresponded to the impairment of its assisting function for HDV antigenomic replication. These results showed a close correlation between phosphorylation and HDV RNA replication and strongly suggested that phosphorylation at serine 177 is a distinguishing feature of S-HDAg in the regulation of HDV RNA replication, particularly strengthening the control of template switch.
Besides serine, threonine is also a phosphorylation acceptor in S-HDAg (32). Amino acid 95 is the only completely conserved Ser or Thr residue among all HDV variants (Fig. 2A, aa 95 is threonine in the strain we used). Threonines 134 and 182 are highly but not completely conserved (Fig. 2A, aa 134 was Ala in the S and P isolates, aa 182 was Arg in the S isolate and was His in both the E and L isolates). Other phosphorylation site(s) in S-HDAg might have a greater possibility of occurring on these highly conserved threonine residues (threonines 95, 134, and 182). However, the functional involvement of the less conserved ones (serines 6, 83, 116, 159, 170, and 180 and threonines 37 and 76) could not be completely excluded.
Despite the speculation on the possible phosphorylation sites in S-HDAg, a more solid basis is required. It is necessary to identify the exact amino acid residues to be phosphorylated by biochemical procedures, such as two-dimensional peptide mapping or mass spectrometry (i.e., liquid chromatography-mass spectrometry and matrix-assisted laser desorption ionization–time of flight [mass spectrometry] [MALDI-TOF]). With this information, we can address the biological significance of each phosphorylation of HDAgs in the viral life cycle. Finally, the results also help in searching for the kinases responsible for phosphorylating HDAg's and resolving the biological functions of the HDAg phosphorylation.
As the serine 177 mutation severely affected HDV RNA replication, especially in the antigenomic RNA phase, we speculate that the phosphorylation status of serine 177 in S-HDAg determines or strengthens the control of template specificity of HDV RNA. Furthermore, RNA editing, another important step during the HDV life cycle, has been shown to be dependent upon transcription from edited antigenomic RNA to generate edited genomic RNA (6, 35), also diminishing to an undetected level due to the loss of antigenomic RNA replication (Fig. 4 and 5A). These data indicate that serine 177 phosphorylation is important for the ability of S-HDAg in HDV antigenomic RNA replication to recognize the positive-strand RNA template and dramatically augment their replication. For example, in HDV-infected cells, the amount of viral genomic RNA always exceeds that of antigenomic RNA by 5- to 22-fold (13), suggesting the replication from antigenomic RNA to be more effective than that from genomic RNA. The enhanced transcription from antigenomic RNA is an important step for negative-strand RNA viruses to produce their progeny. This amplification step exists universally in all negative-strand RNA viruses. There are recent experimental data to support that the phase of genomic RNA replication may differ from that of antigenomic RNA in HDV RNA replication. Using a cDNA-free RNA transfection system, one study showed that L-HDAg inhibits genomic but not antigenomic RNA synthesis (29). Using a recombinant S-HDAg to initiate HDV RNA replication by an RNA-protein (RNP) complex transfection assay, another study demonstrated that recombinant S-HDAg could initiate replication from genomic but not antigenomic RNA (40). Both studies indicated that the mechanisms of synthesis of genomic versus antigenomic RNA are different. In addition, the impaired initiation of HDV RNA synthesis from antigenomic RNA by RNP transfection revealed one possibility that posttranslational modification of S-HDAg in mammalian cells regulates the HDV antigenomic RNA replication (40). This provided further support that serine 177 phosphorylation of S-HDAg could be one mechanism in differentiating antigenomic from genomic RNA replication.
It is interesting to note that other negative-strand RNA viruses also encode one phosphoprotein (P protein) in their genome (41, 44). Other than the viral RNA polymerase (the L gene), this P protein is essential for viral replication and requires protein phosphorylation for its function. For the respiratory syncytial virus (RSV) P protein, the phosphorylation of the P protein can modulate its transcription and replication (45). Studies with vesicular stomatitis virus (VSV) P protein indicated that phosphorylation of its amino-terminal domain is required for transcription activity and phosphorylation of carboxyl-terminal domain is required for optimal replication activity. It indicated that phosphorylation at two different domains in the P protein regulates its activity in transcription and replication of the VSV genome. It is conceivable that a differential phosphorylation of the replication factor could help control the two phases of negative-strand RNA viral replication. In fact, for positive-stand RNA viruses, the modulation of positive- versus negative-strand RNA replication also remains unknown. Some viruses also harbor phosphoproteins as replication cofactors, such as HCV NS5a, which has been shown to be a phosphoprotein complex together with viral RNA polymerase (NS5b) (43). Our finding thus bears a general implication for understanding the control of replication template specificity for single-stranded RNA viruses.
In the kinetic study, the low phosphorylation of S-HDAg detected in virions suggested that dephosphorylation may play some role during HDV life cycle. The current study addressed only its role in HDV RNA replication, although there are other possibilities. For example, S-HDAg also functions as a structural protein, the capsid protein, which binds to HDV RNA and forms the nucleocapsid. Since only the unphosphorylated isoform of S-HDAg was detected in viral particles, the transition from the dephosphorylation to the phosphorylation of S-HDAg may also represent a candidate mechanism for the regulation of virus uncoating. It is interesting that several viruses have exploited this mechanism. The core protein of HBV that constitutes the capsids of virus is a phosphoprotein. It has been documented that the core protein packages the pregenomic RNA and viral polymerase in an unphosphorylated manner to form a core particle (18). A cellular protein kinase packaged within the core particle seems to be able to phosphorylate core protein (1, 18). Because phosphorylation can reduce the nucleic acid binding capability of core protein, a consequence of core phosphorylation may be the uncoating of nucleocapsid (52) before genome release. Another example is the capsid protein, CAp 24, of human immunodeficiency virus type 1 (HIV-1). CAp 24 is also a phosphoprotein which is phosphorylated by a virion-associated protein kinase (2). One recent study showed that phosphorylation of CAp 24 is necessary just after entry of the virus in the target cell, and without phosphorylation, the reverse transcription process could not be completely achieved. Therefore, the phosphorylation of CAp 24 revealed a role in the viral uncoating process (3). However, the lack of phosphorylated isoforms of S-HDAg in HDV virion may be due to the fact that the phospho-isoforms are largely unusable for assembly or that the unphosphorylated form were specifically selected for assembly. Those possibilities for HDV assembly will be explored in the future.
Serine 177 of S-HDAg resides in the motif of mitogen-activated protein (MAP) kinase substrate (PESP) (34, 38). Treatment with tetradecanoyl phorbol actetate (TPA) a well-known activator of the cellular MAP kinase pathway, also increased the phosphorylation level of S-HDAg (27, 32). This implied that the MAP kinase or related members carried out serine 177 phosphorylation. However, the identity of this kinase, whether it is erk1 or erk2 or other novel kinases, remained to be clarified. Discovery of such a kinase will advance our understanding of the virus-cell interaction during HDV replication.
ACKNOWLEDGMENTS
This work was supported by grants from National Science Council, Executive Yuan, Taiwan (NSC 89-2320-B-002-240). The work of Pei-Jer Chen was supported in part by an International Research Scholar grant from Howard Hughes Medical Institute.
REFERENCES
- 1.Albin C, Robinson W S. Protein kinase activity in hepatitis B virus. J Virol. 1980;34:297–302. doi: 10.1128/jvi.34.1.297-302.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cartier C, Deckert M, Grangeasse C, Trauger R, Jensen F, Bernard A, Cozzone A, Desgranges C, Boyer V. Association of ERK2 mitogen-activated protein kinase with human immunodeficiency virus particles. J Virol. 1997;71:4832–4837. doi: 10.1128/jvi.71.6.4832-4837.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cartier C, Sivard P, Tranchat C, Decimo D, Desgranges C, Boyer V. Identification of three major phosphorylation sites within HIV-1 capsid. Role of phosphorylation during the early steps of infection. J Biol Chem. 1999;274:19434–19440. doi: 10.1074/jbc.274.27.19434. [DOI] [PubMed] [Google Scholar]
- 4.Casey J L, Bergmann K F, Brown T L, Gerin J L. Structural requirements for RNA editing in hepatitis delta virus: evidence for a uridine-to-cytidine editing mechanism. Proc Natl Acad Sci USA. 1992;89:7149–7153. doi: 10.1073/pnas.89.15.7149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Casey J L, Brown T L, Colan E J, Wignall F S, Gerin J L. A genotype of hepatitis D virus that occurs in northern South America. Proc Natl Acad Sci USA. 1993;90:9016–9020. doi: 10.1073/pnas.90.19.9016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Casey J L, Gerin J L. Hepatitis D virus RNA editing: specific modification of adenosine in the antigenomic RNA. J Virol. 1995;69:7593–7600. doi: 10.1128/jvi.69.12.7593-7600.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chang F L, Chen P J, Tu S J, Wang C J, Chen D S. The large form of hepatitis delta antigen is crucial for assembly of hepatitis delta virus. Proc Natl Acad Sci USA. 1991;88:8490–8494. doi: 10.1073/pnas.88.19.8490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chang M F, Baker S C, Soe L H, Kamahora T, Keck J G, Makino S, Govindarajan S, Lai M M. Human hepatitis delta antigen is a nuclear phosphoprotein with RNA-binding activity. J Virol. 1988;62:2403–2410. doi: 10.1128/jvi.62.7.2403-2410.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chao M, Hsieh S Y, Taylor J. Role of two forms of hepatitis delta virus antigen: evidence for a mechanism of self-limiting genome replication. J Virol. 1990;64:5066–5069. doi: 10.1128/jvi.64.10.5066-5069.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chao Y C, Chang M F, Gust I, Lai M M. Sequence conservation and divergence of hepatitis delta virus RNA. Virology. 1990;178:384–392. doi: 10.1016/0042-6822(90)90335-o. [DOI] [PubMed] [Google Scholar]
- 11.Chao Y C, Lee C M, Tang H S, Govindarajan S, Lai M M. Molecular cloning and characterization of an isolate of hepatitis delta virus from Taiwan. Hepatology. 1991;13:345–352. [PubMed] [Google Scholar]
- 12.Chen P J, Chang F L, Wang C J, Lin C J, Sung S Y, Chen D S. Functional study of hepatitis delta virus large antigen in packaging and replication inhibition: role of the amino-terminal leucine zipper. J Virol. 1992;66:2853–2859. doi: 10.1128/jvi.66.5.2853-2859.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen P J, Kalpana G, Goldberg J, Mason W, Werner B, Gerin J, Taylor J. Structure and replication of the genome of the hepatitis delta virus. Proc Natl Acad Sci USA. 1986;83:8774–8778. doi: 10.1073/pnas.83.22.8774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fu T B, Taylor J. The RNAs of hepatitis delta virus are copied by RNA polymerase II in nuclear homogenates. J Virol. 1993;67:6965–6972. doi: 10.1128/jvi.67.12.6965-6972.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hwang S B, Lee C Z, Lai M M. Hepatitis delta antigen expressed by recombinant baculoviruses: comparison of biochemical properties and post-translational modifications between the large and small forms. Virology. 1992;190:413–422. doi: 10.1016/0042-6822(92)91227-l. [DOI] [PubMed] [Google Scholar]
- 16.Imazeki F, Omata M, Ohto M. Complete nucleotide sequence of hepatitis delta virus RNA in Japan. Nucleic Acids Res. 1991;19:5439. doi: 10.1093/nar/19.19.5439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Imazeki F, Omata M, Ohto M. Heterogeneity and evolution rates of delta virus RNA sequences. J Virol. 1990;64:5594–5599. doi: 10.1128/jvi.64.11.5594-5599.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kann M, Gerlich W H. Effect of core protein phosphorylation by protein kinase C on encapsidation of RNA within core particles of hepatitis B virus. J Virol. 1994;68:7993–8000. doi: 10.1128/jvi.68.12.7993-8000.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuo M Y, Chao M, Taylor J. Initiation of replication of the human hepatitis delta virus genome from cloned DNA: role of delta antigen. J Virol. 1989;63:1945–1950. doi: 10.1128/jvi.63.5.1945-1950.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lai M M. The molecular biology of hepatitis delta virus. Annu Rev Biochem. 1995;64:259–286. doi: 10.1146/annurev.bi.64.070195.001355. [DOI] [PubMed] [Google Scholar]
- 21.Lee C M, Bih F Y, Chao Y C, Govindarajan S, Lai M M. Evolution of hepatitis delta virus RNA during chronic infection. Virology. 1992;188:265–273. doi: 10.1016/0042-6822(92)90756-f. [DOI] [PubMed] [Google Scholar]
- 22.Lee C Z, Chen P J, Chen D S. Large hepatitis delta antigen in packaging and replication inhibition: role of the carboxyl-terminal 19 amino acids and amino-terminal sequences. J Virol. 1995;69:5332–5336. doi: 10.1128/jvi.69.9.5332-5336.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Luo G X, Chao M, Hsieh S Y, Sureau C, Nishikura K, Taylor J. A specific base transition occurs on replicating hepatitis delta virus RNA. J Virol. 1990;64:1021–1027. doi: 10.1128/jvi.64.3.1021-1027.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Macnaughton T B, Gowans E J, Jilbert A R, Burrell C J. Hepatitis delta virus RNA, protein synthesis and associated cytotoxicity in a stably transfected cell line. Virology. 1990;177:692–698. doi: 10.1016/0042-6822(90)90535-y. [DOI] [PubMed] [Google Scholar]
- 25.MacNaughton T B, Gowans E J, McNamara S P, Burrell C J. Hepatitis delta antigen is necessary for access of hepatitis delta virus RNA to the cell transcriptional machinery but is not part of the transcriptional complex. Virology. 1991;184:387–390. doi: 10.1016/0042-6822(91)90855-6. [DOI] [PubMed] [Google Scholar]
- 26.Makino S, Chang M F, Shieh C K, Kamahora T, Vannier D M, Govindarajan S, Lai M M. Molecular cloning and sequencing of a human hepatitis delta (delta) virus RNA. Nature. 1987;329:343–346. doi: 10.1038/329343a0. [DOI] [PubMed] [Google Scholar]
- 27.Marquardt B, Frith D, Stabel S. Signalling from TPA to MAP kinase requires protein kinase C, raf and MEK: reconstitution of the signalling pathway in vitro. Oncogene. 1994;9:3213–3218. [PubMed] [Google Scholar]
- 28.McMaster G K, Carmichael G G. Analysis of single- and double-stranded nucleic acids on polyacrylamide and agarose gels by using glyoxal and acridine orange. Proc Natl Acad Sci USA. 1997;74:4835–4838. doi: 10.1073/pnas.74.11.4835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Modahl L E, Lai M M. The large delta antigen of hepatitis delta virus potently inhibits genomic but not antigenomic RNA synthesis: a mechanism enabling initiation of viral eplication. J Virol. 2000;74:7375–7380. doi: 10.1128/jvi.74.16.7375-7380.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Modahl L E, Lai M M. Transcription of hepatitis delta antigen mRNA continues throughout hepatitis delta virus (HDV) replication: a new model of HDV RNA transcription and replication. J Virol. 1998;72:5449–5456. doi: 10.1128/jvi.72.7.5449-5456.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Modahl L E, Macnaughton T B, Zhu N, Johnson D L, Lai M M. RNA-dependent replication and transcription of hepatitis delta virus RNA involve distinct cellular RNA polymerases. Mol Cell Biol. 2000;20:6030–6039. doi: 10.1128/mcb.20.16.6030-6039.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mu J J, Wu H L, Chiang B L, Chang R P, Chen D S, Chen P J. Characterization of the phosphorylated forms and the phosphorylated residues of hepatitis delta virus delta antigens. J Virol. 1999;73:10540–10545. doi: 10.1128/jvi.73.12.10540-10545.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.O'Farrell P Z, Goodman H M, O'Farrell P H. High resolution two-dimensional electrophoresis of basic as well as acidic proteins. Cell. 1977;12:1133–1141. doi: 10.1016/0092-8674(77)90176-3. [DOI] [PubMed] [Google Scholar]
- 34.Pinna L A, Ruzzene M. How do protein kinases recognize their substrates? Biochim Biophys Acta. 1996;1314:191–225. doi: 10.1016/s0167-4889(96)00083-3. [DOI] [PubMed] [Google Scholar]
- 35.Polson A G, Bass B L, Casey J L. RNA editing of hepatitis delta virus antigenome by dsRNA-adenosine deaminase. Nature. 1996;380:454–456. doi: 10.1038/380454a0. . (Erratum, 381:346, 1996.) [DOI] [PubMed] [Google Scholar]
- 36.Rizzetto M. The delta agent. Hepatology. 1983;3:729–737. doi: 10.1002/hep.1840030518. [DOI] [PubMed] [Google Scholar]
- 37.Rizzetto M, Hoyer B, Canese M G, Shih J W, Purcell R H, Gerin J L. delta Agent: association of delta antigen with hepatitis B surface antigen and RNA in serum of delta-infected chimpanzees. Proc Natl Acad Sci USA. 1980;77:6124–6128. doi: 10.1073/pnas.77.10.6124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rothmann K, Schnolzer M, Radziwill G, Hildt E, Moelling K, Schaller H. Host cell-virus cross talk: phosphorylation of a hepatitis B virus envelope protein mediates intracellular signaling. J Virol. 1998;72:10138–10147. doi: 10.1128/jvi.72.12.10138-10147.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Saldanha J A, Thomas H C, Monjardino J P. Cloning and sequencing of RNA of hepatitis delta virus isolated from human serum. J Gen Virol. 1990;71:1603–1606. doi: 10.1099/0022-1317-71-7-1603. [DOI] [PubMed] [Google Scholar]
- 40.Sheu G T, Lai M M. Recombinant hepatitis delta antigen from E. coli promotes hepatitis delta virus RNA replication only from the genomic strand but not the antigenomic strand. Virology. 2000;278:578–586. doi: 10.1006/viro.2000.0680. [DOI] [PubMed] [Google Scholar]
- 41.Takacs A M, Barik S, Das T, Banerjee A K. Phosphorylation of specific serine residues within the acidic domain of the phosphoprotein of vesicular stomatitis virus regulates transcription in vitro. J Virol. 1992;66:5842–5848. doi: 10.1128/jvi.66.10.5842-5848.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tang J R, Hantz O, Vitvitski L, Lamelin J P, Parana R, Cova L, Lesbordes J L, Trepo C. Discovery of a novel point mutation changing the HDAg expression of a hepatitis delta virus isolate from Central African Republic. J Gen Virol. 1993;74:1827–1835. doi: 10.1099/0022-1317-74-9-1827. [DOI] [PubMed] [Google Scholar]
- 43.Tanji Y, Kaneko T, Satoh S, Shimotohno K. Phosphorylation of hepatitis C virus-encoded nonstructural protein NS5A. J Virol. 1995;69:3980–3986. doi: 10.1128/jvi.69.7.3980-3986.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tuffereau C, Fischer S, Flamand A. Phosphorylation of the N and M1 proteins of rabies virus. J Gen Virol. 1985;66:2285–2289. doi: 10.1099/0022-1317-66-10-2285. [DOI] [PubMed] [Google Scholar]
- 45.Villanueva N, Hardy R, Asenjo A, Yu Q, Wertz G. The bulk of the phosphorylation of human respiratory syncytial virus phosphoprotein is not essential but modulates viral RNA transcription and replication. J Gen Virol. 2000;81:129–133. doi: 10.1099/0022-1317-81-1-129. [DOI] [PubMed] [Google Scholar]
- 46.Wang C J, Chen P J, Wu J C, Patel D, Chen D S. Small-form hepatitis B surface antigen is sufficient to help in the assembly of hepatitis delta virus-like particles. J Virol. 1991;65:6630–6636. doi: 10.1128/jvi.65.12.6630-6636.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang H W, Chen P J, Lee C Z, Wu H L, Chen D S. Packaging of hepatitis delta virus RNA via the RNA-binding domain of hepatitis delta antigens: different roles for the small and large delta antigens. J Virol. 1994;68:6363–6371. doi: 10.1128/jvi.68.10.6363-6371.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang K S, Choo Q L, Weiner A J, Ou J H, Najarian R C, Thayer R M, Mullenbach G T, Denniston K J, Gerin J L, Houghton M. Structure, sequence and expression of the hepatitis delta (delta) viral genome. Nature. 1986;323:508–514. doi: 10.1038/323508a0. . (Erratum, 328:456, 1987.) [DOI] [PubMed] [Google Scholar]
- 49.Wigler M, Pellicer A, Silverstein S, Axel R. Biochemical transfer of single-copy eucaryotic genes using total cellular DNA as donor. Cell. 1978;14:725–731. doi: 10.1016/0092-8674(78)90254-4. [DOI] [PubMed] [Google Scholar]
- 50.Willard K E, Giometti C S, Anderson N L, O'Connor T E, Anderson N G. Analytical techniques for cell fractions. XXVI. A two-dimensional electrophoretic analysis of basic proteins using phosphatidyl choline/urea solubilization. Anal Biochem. 1979;100:289–298. doi: 10.1016/0003-2697(79)90232-x. [DOI] [PubMed] [Google Scholar]
- 51.Yeh T S, Lo S J, Chen P J, Lee Y H. Casein kinase II and protein kinase C modulate hepatitis delta virus RNA replication but not empty viral particle assembly. J Virol. 1996;70:6190–6198. doi: 10.1128/jvi.70.9.6190-6198.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yu M, Summers J. Phosphorylation of the duck hepatitis B virus capsid protein associated with conformational changes in the C terminus. J Virol. 1994;68:2965–2969. doi: 10.1128/jvi.68.5.2965-2969.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]