Abstract

In this study, some benzofused tricyclic heterocyclic derivatives have been tested as possible new catalysts for anode hydrazine electrooxidation in a direct hydrazine fuel cell (DHFC). Electrochemical studies were carried out in solution media containing 1 M KOH and 1 M KOH + 0.5 M N2H4 using electrochemical techniques such as cyclic voltammetry (CV), electrochemical impedance spectroscopy (EIS), and chronoamperometry (CA). The CV results obtained showed that 5 catalysts promoted the generation of the best current (38.32 mA/cm2), and EIS results confirmed that an electrode modified with the same derivative presented the lowest charge transfer resistance. All these results proved that 5 organic-based catalysts can be used as an anode-efficient catalyst in hydrazine fuel cells.
Introduction
Due to the nonrenewability and environmental impact associated with the use of fossil fuels, the implementation of alternative, affordable, and clean energy sources has become inevitable.1 In this regard, fuel cell-based technologies are destined to play a major role in the near future, as they are able to produce clean energy by converting chemical energy into electrical energy without the need for fossil fuels and without direct emission of carbon dioxide.2
In particular, thanks to their high cell voltage production, direct hydrazine fuel cells (DHFCs) have been attracting increasing attention from researchers, from both academia and industry.3 DHFCs are of particular importance, since they are characterized by a high fuel conversion rate, low cost, and good durability.4 Due to its low electrooxidation potential (−1.21 V) and high hydrogen concentration (12.5% by weight),5,6 hydrazine (N2H4) is the preferred fuel for fuel cells. The following equations could be used to explain the mechanism of the electrooxidation of N2H4 in an alkaline environment:7
| 1 |
| 2 |
| 3 |
Clearly, no CO2 is produced in these reaction, which is very important, as carbon dioxide could poison the electrocatalysts necessary for promoting the electrochemical process.6
A variety of catalysts with high electrocatalytic activity and low cost have been developed as anode catalysts for DHFC in the literature. For instance, Ni–Co,8 Ni3S2@Ni,9 AgNi/MWCNT,10 Cu–Ni,11 nano-CuO/MGCE,12 NiMo/C,13 Ni–Pt/C,14 NPCF/Cu,15 CuNiCo LDH,16 and PtCofiber/Cu4 are reported for N2H4 electrooxidation reactions as anode catalysts. Table 1 displays the maximum current density peak values achieved with these metal-based catalysts.
Table 1. Maximum Current Density and Initial Potential Values for N2H4 Electrooxidation Reported in the Literature.
| Catalyst | Onset Potential (V) | Current Density (mA/cm2) | Preparation | Reference |
|---|---|---|---|---|
| AuPd NCs | - | 5.28 | chemical reduction | (21) |
| PpDP/ZnO | –0.06 | 7.89 | dope | (22) |
| Wash-CNCu | 0.28 | 1.94 | dope | (23) |
| NiFe2O4-rGO | 0.36 | 18.9 | hydrothermal method | (24) |
| NSC | 0.6 | 7.89 | chemical reduction | (25) |
| benzo[b]thiophene | –0.20 | 4.95 | organic synthesis | (20) |
| compound 5 | 0.2 | 38.32 | organic synthesis | this study |
Due to the disadvantages of metals (such as their toxicity, high cost, and non-reusability), organic materials have recently been proposed as anode catalysts in new generation DHFC applications.17 In particular, a metal-free anode catalyst has been recently reported by Sharif and co-workers, and 2-isopropyl-5-methylphenyl-4-oxo-4-(5-(p-tolylethynyl)thiophene-2-yl butanoate organic catalyst exhibited the highest performance with a current density value of 3.66 mA cm–2 (17.24 mA mg–1).18 Other environmentally friendly, inexpensive organic materials have been developed in place of expensive metal catalysts (such as Pt and Au) as anode catalysts in DHFCs.19,20
Polycyclic heterocycles are a particularly important class of heterocyclic systems, as they are present as fundamental cores in natural products and in bioactive principles26 and can also be used for applications in materials sciences.27 In this study, we tested some recently synthesized polycyclic heterocycles as possible anode catalysts with high catalytic activity and long-term stability in hydrazine electrooxidation. In particular, we focused our attention on six derivatives recently reported by our research group,28,29 as shown in Scheme 1.
Scheme 1. Polycyclic Heterocycles Investigated in This Study as Possible Electrocatalysts for Direct Hydrazine Fuel Cell (DHFC) Applications.
Compared to metal-based catalyst systems, organic-based anode catalysts can be easily synthesized with different kinds of derivatives, and they can be tested for finding better anode catalysts in DHFC applications. In addition, they do not exhibit any sensitivity to oxygen. Accordingly, the electrocatalytic activity of compounds 1–6 for the electrooxidation of N2H4 has been evaluated with the help of cyclic voltammetry (CV), chronoamperometry (CA), and electrochemical impedance spectroscopy (EIS).
2. Materials and Methods
2.1. Synthesis
Polycyclic heterocycles 1–428 and 5/629 were prepared according to the published procedures.
2.2. Electrochemical Studies
Electrochemical methods including CV, CA, and EIS were performed (1 M KOH + 0.5 M N2H4 solution) using a Gamry-Interface 1010 potentiostat fitted with a 3-electrode system in order to examine the hydrazine electrooxidation (HEO) activity of 1–6 organic catalysts. The working electrode consists of a 3.0 mm diameter glassy carbon disk held in a Teflon cylinder. Before the procedures, electrode surfaces were polished and immediately rinsed with tap water, distilled water, and acetone. In order to create electrodes from catalysts, 5 mg of catalyst was first homogenized in an ultrasonic bath for 10 min with 1 mL of Nafion solution (Nafion 117, Aldrich, comprising 5% Nafion). A volume of 3–5 μL of the resulting catalyst + naphthione mixture was transferred with a micropipet and applied to a 3 mm diameter glassy carbon electrode. The electrodes were then allowed to dry. The stability of the organic catalyst was evaluated using CA measurements over a 1000 s period at potentials of −0.8 V/0.6 V. The EIS was used for the investigation of electrochemical resistance at 316 kHz and 0.046 Hz to 0.005 V amplitude, with varying potentials in the range of −0.8/0.6 V.
3. Results and Discussion
The HEO activity of organic-based catalysts 1–6 was identified by CV analysis. Hydrazine electrooxidation studies on organic-based catalysts were performed in 1 M KOH solution and 1 M KOH + 0.5 M N2H4 solution at a scan rate of 50 mV s–1 and −0.8 and 0.6 V potentials (Figure 1a–c). As it is known, when metal-based catalysts such as Pd, Pt, and Au are used for electrooxidation, significant oxidation peaks are observed in CV. However, these oxidation peaks cannot be observed when organic catalysts are used. The specific activity of compounds 1–6 for HEO obtained from CV results is given in Table 2. As can be seen in Figure 1b and Table 2, organocatalyst 5 exhibited better activity than other catalysts. The specific activity in the total current of N2H4 was calculated as 38.32 mA cm–2. When the electrooxidation performance was carried out in the presence of hydrazine without any organic catalyst, we did not obtain any peaks.
Figure 1.

CV results of organocatalysts 1–6 in (a) 1 M KOH and (b) 1 M KOH + 0.5 M N2H4. (c) CV result of catalyst 5 in 1 M KOH and 1 M KOH + 0.5 M N2H4.
Table 2. HEO Performances of Organic-Based Catalysts.
| Catalyst | Onset Potential (V) | Total Current of KOH (mA/cm–2) | Total Current of Hydrazine(mA/cm–2) | Normal Current, (mA/cm–2) |
|---|---|---|---|---|
| 1 | –0.4 | 0.08 | 0.17 | 0.09 |
| 2 | –0.4 | 0.11 | 0.20 | 0.09 |
| 3 | 0.1 | 0.09 | 27.60 | 27.51 |
| 4 | –0.4 | 0.19 | 0.29 | 0.10 |
| 5 | 0.2 | 0.24 | 38.32 | 38.08 |
| 6 | 0.1 | 0.20 | 22.53 | 22.33 |
Figure 2a,b display the analysis of the hybrid organocatalyst 5, which was obtained at various scan rates (10 and 500 mV s–1) in 1 M KOH + 0.5 M N2H4. Plotting the current value against the square root of the scan rate shows that it increases and changes linearly with the scan rate (see the graph’s inset). This is evidence of a diffusion-controlled reaction for the HEO.
Figure 2.
(a) CV analysis of organocatalyst 5 at different scan rates (10–500 mV s–1). (b) Linear regression of peak currents vs the square root of the scan rates.
The stability of organocatalyst 5 was investigated using CA measurements. The CA curves of organocatalyst 5 at various potentials are shown in Figure 3. CA analysis proved that the best resistance and good stability were obtained at a potential of less than 0.8 V.
Figure 3.
CA analysis of organocatalyst 5.
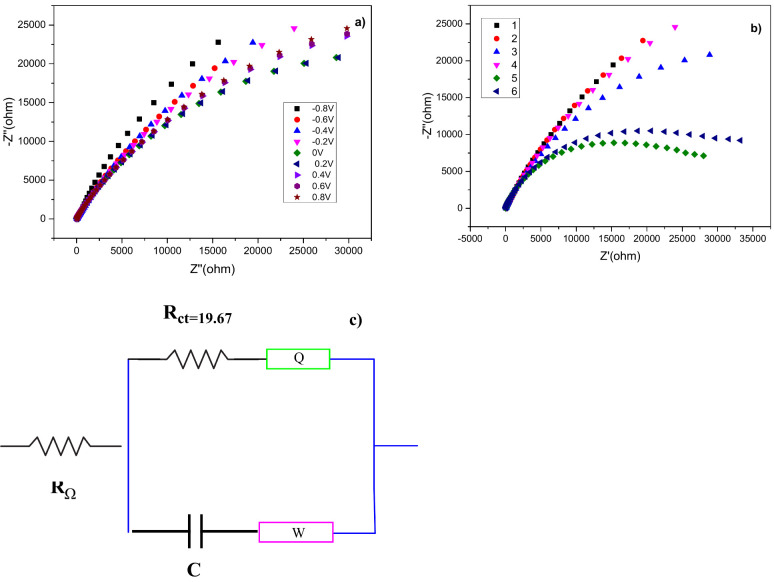
The electrocatalytic resistance of organocatalyst 5 was investigated by using the Nyquist plots from the EIS, as shown in Figure 4a,b. EIS measurements are often used to calculate electrocatalytic resistance, with the diameter of the circle decreasing as resistance increases.30Figure 4a displays the Nyquist plots of the hybrid 5 organic catalyst achieved at different potentials (−0.8, −0.6, −0.4, −0.2, 0.0, 0.2, 0.4, 0.6, and 0.8 V). As seen in Figure 4a, the Nyquist graph showed that the highest electrochemical resistance was obtained when the organic catalyst 5 was taken at 0.2 V. Figure 4b shows the Nyquist plots of organocatalysts 1–6 at a potential of 0.2 V. Organocatalyst 5 gives a smaller Rct value than other catalysts. In DHFC applications, EIS studies also proved that 5 has the best catalytic activity, lowest resistance, and high stability.
Figure 4.
Nyquist plots obtained (a) at different potentials of organocatalyst 5 and (b) compared to all other organocatalysts at 0.2 V in 1 M KOH + 0.5 M N2H4; (c) Rct value.
In Figure 4c, the charge transfer resistance (Rct) values of the 5-based electrode in 1 M KOH + 0.5 M N2H4, −0.8 V (39.89 Ω) > −0.6 V (39.22 Ω) > −0.4 V (38.82 Ω) > −0.2 V (38.75 Ω), > 0 V (38.68 Ω), > 0.2 V (19.67 Ω), > 0.4 V (37.07 Ω), and 0.6 V (36.08 Ω), were found from the different voltage equivalent circuit model. In comparison to other voltages, the electrode of organocatalyst 5 exhibits the maximum carrier transfer performance due to its lowest semicircular shape and Rct of 0.2 V (Table 3).
Table 3. Charge Transfer Resistance (Rct) Values of the C1 Electrode at Different Voltages.
| Electrode | –0.8 V | –0.6 V | –0.4 V | –0.2 V | 0 V | 0.2 V | 0.4 V | 0.6 V |
|---|---|---|---|---|---|---|---|---|
| 5 | 39.89 | 39.22 | 38.82 | 38.75 | 38.68 | 19.67 | 37.07 | 36.08 |
Conclusions
In conclusion, in this study, we tested 6 different benzofused tricyclic heterocyclic derivatives as possible new catalysts for anode hydrazine electrooxidation in a DHFC. The CV results obtained showed that 5 catalysts promoted the generation of the best current (38.32 mA/cm2), and EIS results confirmed that the electrode modified with the same derivative presented the lowest charge transfer resistance. As a result, this study improved the possibility that compound 5 could be used in hydrazine fuel cells as an anode catalyst without needing any metal.
Acknowledgments
The authors would like to acknowledge the networking contribution by the Erasmus+ KA2 Project “Digital Green” 2022-1-TR01-KA220-SCH-000087638.
Author Contributions
Raffaella Mancuso: Visualization, Investigation. Bartolo Gabriele: Visualization, Investigation. Omruye Ozok Arici: Visualization, Investigation, Data curation, Writing–original draft. Hilal Kivrak: Conceptualization, Methodology, Supervision, Visualization. Arif Kivrak: Methodology, Supervision.
The authors declare no competing financial interest.
References
- Kumar K. S. A.; Vardhan A. S. S.; Vardhan A. S. S.; Kumar S.; Saket R. K.; Rajendran R.; Eslamian S. Microbial fuel cells for a soil-based green energy conversion system. International Journal of Hydrology Science and Technology 2021, 11 (4), 439–460. 10.1504/IJHST.2021.115491. [DOI] [Google Scholar]
- i Kivrak H.; Atbas D.; Alal O.; Cogenli M. S.; Bayrakceken A.; Mert S. O.; Sahin O. A complementary study on novel PdAuCo catalysts: Synthesis, characterization, direct formic acid fuel cell application, and exergy analysis. Int. J. Hydrogen Energy 2018, 43 (48), 21886–21898. 10.1016/j.ijhydene.2018.09.135. [DOI] [Google Scholar]; ii Caglar A.; Sahan T.; Cogenli M. S.; Yurtcan A. B.; Aktas N.; Kivrak H. A novel Central Composite Design based response surface methodology optimization study for the synthesis of Pd/CNT direct formic acid fuel cell anode catalyst. Int. J. Hydrogen Energy 2018, 43 (24), 11002–11011. 10.1016/j.ijhydene.2018.04.208. [DOI] [Google Scholar]
- Serov A.; Kwak C. Direct hydrazine fuel cells: A review. Appl. Catal., B 2010, 98 (1–2), 1–9. 10.1016/j.apcatb.2010.05.005. [DOI] [Google Scholar]
- Zabielaite A.; Balciunaite A.; Simkunaite D.; Lichusina S.; Stalnioniene I.; Simkunaite-Stanyniene B.; Naruskevicius L.; Tamasauskaite-Tamasiunaite L.; Norkus E.; Selskis A.; et al. High Performance Direct N2H4-H2O2 Fuel Cell Using Fiber-Shaped Co Decorated with Pt Crystallites as Anode Electrocatalysts. J. Electrochem. Soc. 2020, 167 ( (1), ), 054502. 10.1149/2.0052005JES. [DOI] [Google Scholar]
- Abdolmaleki M.; Ahadzadeh I.; Goudarziafshar H. Direct hydrazine-hydrogen peroxide fuel cell using carbon supported Co@Au core-shell nanocatalyst. Int. J. Hydrogen Energy 2017, 42 (23), 15623–15631. 10.1016/j.ijhydene.2017.05.059. [DOI] [Google Scholar]
- Wang Y. C.; Wang Q.; Wan L. Y.; Han Y.; Hong Y. H.; Huang L.; Yang X. D.; Wang Y. S.; Zaghib K.; Zhou Z. Y. KOH-doped polybenzimidazole membrane for direct hydrazine fuel cell. J. Colloid Interface Sci. 2020, 563, 27–32. 10.1016/j.jcis.2019.12.046. [DOI] [PubMed] [Google Scholar]
- Uhm S.; Hong S.; Lee J. Effective Electrode Structure for the Stability of Alkaline Hydrazine Fuel Cells. Applied Chemistry for Engineering 2019, 30 (6), 652–658. 10.14478/ace.2019.1091. [DOI] [Google Scholar]
- Liang B.; Wang Y.; Liu X.; Tan T.; Zhang L.; Wang W.. Nickel-cobalt alloy doping phosphorus as advanced electrocatalyst for hydrazine oxidation. Journal of Alloys Compounds 2019, 807, 151648. 10.1016/j.jallcom.2019.151648. [DOI] [Google Scholar]
- Liu X.; Li Y.; Chen N.; Deng D.; Xing X.; Wang Y. Ni3S2@Ni foam 3D electrode prepared via chemical corrosion by sodium sulfide and using in hydrazine electro-oxidation. Electrochim. Acta 2016, 213, 730–739. 10.1016/j.electacta.2016.08.009. [DOI] [Google Scholar]
- Yi Q.; Chu H.; Tang M.; Zhang Y.; Liu X.; Zhou Z.; Nie H. A Novel Membraneless Direct Hydrazine/Air Fuel Cell. Fuel Cells 2014, 14 (6), 827–833. 10.1002/fuce.201400098. [DOI] [Google Scholar]
- Filanovsky B.; Granot E.; Presman I.; Kuras I.; Patolsky F. Long-term room-temperature hydrazine/air fuel cells based on low-cost nanotextured Cu-Ni catalysts. J. Power Sources 2014, 246, 423–429. 10.1016/j.jpowsour.2013.07.084. [DOI] [Google Scholar]
- Wang G.; Gu A.; Wang W.; Wei Y.; Wu J.; Wang G.; Zhang X.; Fang B. Copper oxide nanoarray based on the substrate of Cu applied for the chemical sensor of hydrazine detection. Electrochem. Commun. 2009, 11, 631–634. 10.1016/j.elecom.2008.12.061. [DOI] [Google Scholar]
- Asset T.; Roy A.; Sakamoto T.; Padilla M.; Matanovic I.; Artyushkova K.; Serov A.; Maillard F.; Chatenet M.; Asazawa K.; et al. Highly active and selective nickel molybdenum catalysts for direct hydrazine fuel cell. Electrochim. Acta 2016, 215, 420–426. 10.1016/j.electacta.2016.08.106. [DOI] [Google Scholar]
- Lao S. J.; Qin H. Y.; Ye L. Q.; Liu B. H.; Li Z. P. A development of direct hydrazine/hydrogen peroxide fuel cell. J. Power Sources 2010, 195 (13), 4135–4138. 10.1016/j.jpowsour.2010.01.059. [DOI] [Google Scholar]
- Jia F.; Zhao J.; Yu X. Nanoporous Cu film/Cu plate with superior catalytic performance toward electro-oxidation of hydrazine. J. Power Sources 2013, 222, 135–139. 10.1016/j.jpowsour.2012.08.076. [DOI] [Google Scholar]
- Liu W.; Xie J.; Guo Y.; Lou S.; Gao L.; Tang B. Sulfurization-induced edge amorphization in copper-nickel-cobalt layered double hydroxide nanosheets promoting hydrazine electro-oxidation. Journal Of Materials Chemistry A 2019, 7, 24437–24444. 10.1039/C9TA07857F. [DOI] [Google Scholar]
- Deng J.; Li X.; Imhanria S.; Chen K.; Deng X.; Wang W.. Molybdenum carbide-nitrogen doped carbon composites as effective non-precious electrocatalyst for direct hydrazine fuel cell. Electrochim. Acta 2021, 384, 138417. 10.1016/j.electacta.2021.138417. [DOI] [Google Scholar]
- Sharif K. H.; Kivrak H.; Ozok-Arici O.; Caglar A.; Kivrak A.. Catalytic electro-oxidation of hydrazine by thymol based-modified glassy carbon electrode. Fuel 2022, 330, 125597. 10.1016/j.fuel.2022.125597. [DOI] [Google Scholar]
- i Ozok-Arici O.; Kaya S.; Caglar A.; Kivrak H.; Kivrak A. Glucose Electrooxidation Study on 3-iodo-2-(aryl/alkyl)benzo b thiophene Organic Catalyst. J. Electron. Mater. 2022, 51 (4), 1653–1662. 10.1007/s11664-022-09432-x. [DOI] [Google Scholar]; ii Hamad A. R.; Calis H.; Caglar A.; Kivrak H.; Kivrak A.. Indole-based novel organic anode catalyst for glucose electrooxidation. International Journal of Energy Research. 2022, 46, 1659. 10.1002/er.7282. [DOI] [Google Scholar]
- Er O. F.; Ulas B.; Ozok O.; Kivrak A.; Kivrak H.. Design of 2-(4-(2-pentyllbenzo b thiophen-3-yl)benzylidene)malononitrile based remarkable organic catalyst towards hydrazine electrooxidation. J. Electroanal. Chem. 2021, 888, 115218. 10.1016/j.jelechem.2021.115218. [DOI] [Google Scholar]
- Liang Y.; Zhou Y.; Ma J.; Zhao J. Y.; Chen Y.; Tang Y. W.; Lu T. H. Preparation of highly dispersed and ultrafine Pd/C catalyst and its electrocatalytic performance for hydrazine electrooxidation. Appl. Catal., B 2011, 103 (3–4), 388–396. 10.1016/j.apcatb.2011.02.001. [DOI] [Google Scholar]
- i Liu H. Y.; Zheng P. L.; Li J. W.; Sun J. C.; Wang R.; Zhao H. H.; Wu J.; Zheng Y.; Liu Q. Y.. In-situ formation of macromolecule P/N/Si flame retardant for epoxy resin with low smoke toxicity and excellent flame-retardancy. J. Appl. Polym. Sci. 2023, 140 ( (23), ), 10.1002/app.53926. [DOI] [Google Scholar]; ii Liu Q.; Liao X.; Tang Y.; Wang J.; Lv X.; Pan X.; Lu R.; Zhao Y.; Yu X.-Y.; Wu H. B. Low-coordinated cobalt arrays for efficient hydrazine electrooxidation. Energy Environ. Sci. 2022, 15 (8), 3246–3256. 10.1039/D2EE01463G. [DOI] [Google Scholar]
- Cazetta A. L.; Zhang T.; Silva T. L.; Almeida V. C.; Asefa T. Bone char-derived metal-free N- and S-co-doped nanoporous carbon and its efficient electrocatalytic activity for hydrazine oxidation. Appl. Catal., B 2018, 225, 30–39. 10.1016/j.apcatb.2017.11.050. [DOI] [Google Scholar]
- Ma Y. Y.; Wang H.; Key J.; Ji S.; Lv W. Z.; Wang R. F. Control of CuO nanocrystal morphology from ultrathin ″willow-leaf″ to ″flower-shaped″ for increased hydrazine oxidation activity. J. Power Sources 2015, 300, 344–350. 10.1016/j.jpowsour.2015.09.087. [DOI] [Google Scholar]
- Liu R.; Yin J. L.; Cao D. X. Ti Foil Supported Three-Dimensional Porous Silver Film as a High Performance Catalyst for Hydrazine Electrooxidation in KOH Solution. Int. J. Electrochem. Sci. 2017, 12 (12), 11805–11817. 10.20964/2017.12.45. [DOI] [Google Scholar]
- i Zora M.; Kivrak A.; Yazici C. Synthesis of Pyrazoles via Electrophilic Cyclization. J. Org. Chem. 2011, 76 (16), 6726–6742. 10.1021/jo201119e. [DOI] [PubMed] [Google Scholar]; ii Labarrios E.; Jerezano A.; Jimenez F.; Cruz M. D.; Delgado F.; Zepeda L. G.; Tamariz J. Efficient Synthetic Approach to Substituted Benzo b furans and Benzo b thiophenes by Iodine-Promoted Cyclization of Enaminones. Journal of Heterocyclic Chemistry 2014, 51 (4), 954–971. 10.1002/jhet.1686. [DOI] [Google Scholar]; iii Stepien M.; Gonka E.; Zyla M.; Sprutta N. Heterocyclic Nanographenes and Other Polycyclic Heteroaromatic Compounds: Synthetic Routes, Properties, and Applications. Chem. Rev. 2017, 117, 3479–3716. 10.1021/acs.chemrev.6b00076. [DOI] [PubMed] [Google Scholar]
- Kulikova M. V.; Krylova A. Y.; Zhagfarov F. G.; Krysanova K. O.; Lapidus A. L. Plant Biomass as a Raw Material for Producing Basic Organic Sysnthesis Products. Chemistry and Technology of Fuels and Oils 2022, 58 (2), 320–326. 10.1007/s10553-022-01387-3. [DOI] [Google Scholar]
- Ziccarelli I.; Mancuso R.; Santandrea D.; Altomare A.; Olivieri D.; Carfagna C.; Gabriele B.. Synthesis of benzothienofuranones and dihydrobenzothienopyranones by palladium iodide-catalyzed carbonylative double cyclization. Journal of Catalysis 2023, 427, 115101. 10.1016/j.jcat.2023.115101. [DOI] [Google Scholar]
- Mancuso R.; De Salvo A.; Russo P.; Falcicchio A.; Della Ca N.; Munoz L.; Gabriele B. Palladium Iodide-Catalyzed Selective Carbonylative Double Cyclization of 4-(2-Aminophenyl)-3-yn-1-ols to Dihydrofuroquinolinone Derivatives. Chinese Journal Of Chemistry 2023, 41, 2801–2809. 10.1002/cjoc.202300277. [DOI] [Google Scholar]
- Ulas B.; Caglar A.; Sahin O.; Kivrak H. Composition dependent activity of PdAgNi alloy catalysts for formic acid electrooxidation. J. Colloid Interface Sci. 2018, 532, 47–57. 10.1016/j.jcis.2018.07.120. [DOI] [PubMed] [Google Scholar]