Abstract
Background: Smoking status is known to be an independent and significant predictor of health outcomes related to aging and plays a crucial role in overall mortality rates. This cohort study investigated the relationship between smoking status and survival outcomes over follow-up periods of 9 and 21 years. Methods: The sample consisted of 3526 participants with a mean age of 64 ± 12 years, 44.1% of whom were male. The median follow-up duration was 6315 days, with an interquartile range of 3441 to 7727 days. Smoking status [i.e., Brinkmann index (BI)] was calculated by multiplying the number of years smoked by the number of cigarettes smoked daily. Based on this, participants were categorized into non-smokers, former smokers, and current smokers. The data were analyzed using Cox regression, employing age as the time variable and accounting for various risk factors. Results: A total of 1111 participants (49.2%) were confirmed to have died. Among these, 564 were male (36.2% of all male participants), and 547 were female (27.8% of all female participants). The multivariate-adjusted odds ratio (95% confidence interval) for all-cause mortality compared with never-smokers was 1.51 (1.17-1.96) for former smokers with BI > 800, 1.61 (1.20-2.17) for current smokers with BI of 400-799 and 1.62 (95% CI, 1.24-2.10) with BI of ≥800 (P for trend <0.001). Participants who died within three years of follow-up were excluded to avoid the possibility of reverse causation, but the results were essentially unchanged. Conclusion: We found that the BI is a valid predictor of future mortality risk and that BI 800 for former smokers and BI 400 for current smokers were useful cutoff values. Efforts to control smoking should focus not only on current smokers but also on former smokers to reduce the risk of premature death associated with smoking.
Keywords: Smoking status, all-cause mortality, community-dwelling persons, cohort study
Introduction
Cigarette smoking is the leading cause of premature death worldwide, with more than 7 million of these deaths directly attributable to tobacco consumption. 1 It also represents the primary risk factor for male mortality, accounting for 20.2% of deaths among men 2 and contributing 200 million disability-adjusted life-years. 3 While tobacco use has declined significantly in recent years, 4 smoking remains a leading cause of preventable disease worldwide.
Japan is among the top ten countries with the highest smoking rates worldwide, alongside other Asian nations such as China, India, and Indonesia. 5 It is estimated that tobacco constitutes the highest risk factor driving most death and disability in the country. Smoking continues to be a significant risk factor for men, resulting in an approximate loss of nearly two years of life expectancy by the age of 40. Moreover, it contributes an almost 15% rise in mortality rates among men aged 15 to 60. 6 In Japan, more men than women smoke cigarettes, but the male smoking rate has declined over the past two decades, from 43.3% to 32.1% between 2002 and 2014, while smoking among women has declined slightly from 10.2% to 8.5%. The downward trend observed over the last five decades has recently plateaued, as reported by the Ministry of Health in 2020. 7 Over time, there has been a decrease in daily cigarette consumption among Japanese men, but the percentage of male smokers with a Brinkman index between 20 and 200 still increased from 15.5% in 2003 to 34.0% in 2017, while the proportion of female smokers consuming 10 or fewer cigarettes per day (low-intensity) rose from 43.4% to 53.4%. 8 Meanwhile, an expanding collection of literature indicates that individuals who smoke at low intensities face a heightened risk of mortality.9,10 In a prospective cohort study involving 505,500 nationally representative U.S. adults, daily smokers had a 2.32 times higher mortality risk, while lifelong nondaily smokers had a 1.82 times higher mortality risk compared to never smokers. Significant associations were found for smoking 6 to 10 cigarettes per month, with risks increasing with higher-intensity use. Mortality risks decreased when smokers reduced their smoking from daily to nondaily, although the benefits of complete cessation were much greater. 10 A longitudinal follow-up study of 16,701 Chinese aged 45 years and older showed that smokers with 30 pack-years or more had a higher risk of premature death than non-smokers, but no significant association with all-cause mortality. 11 Thus, understanding regarding the health hazards associated with smoking status remains limited 12 and controversial. Currently, only a few studies exist that have investigated the health effects of low-intensity smoking, specifically in the Japanese population.
This study aimed to achieve three main goals: firstly, to explore the correlation between smoking habits and various risk factors; secondly, to delve into the connection between smoking habits and overall mortality rates; and thirdly, to assess the percentage of premature deaths attributed to smoking across all causes.
Methods
Study Design and Population
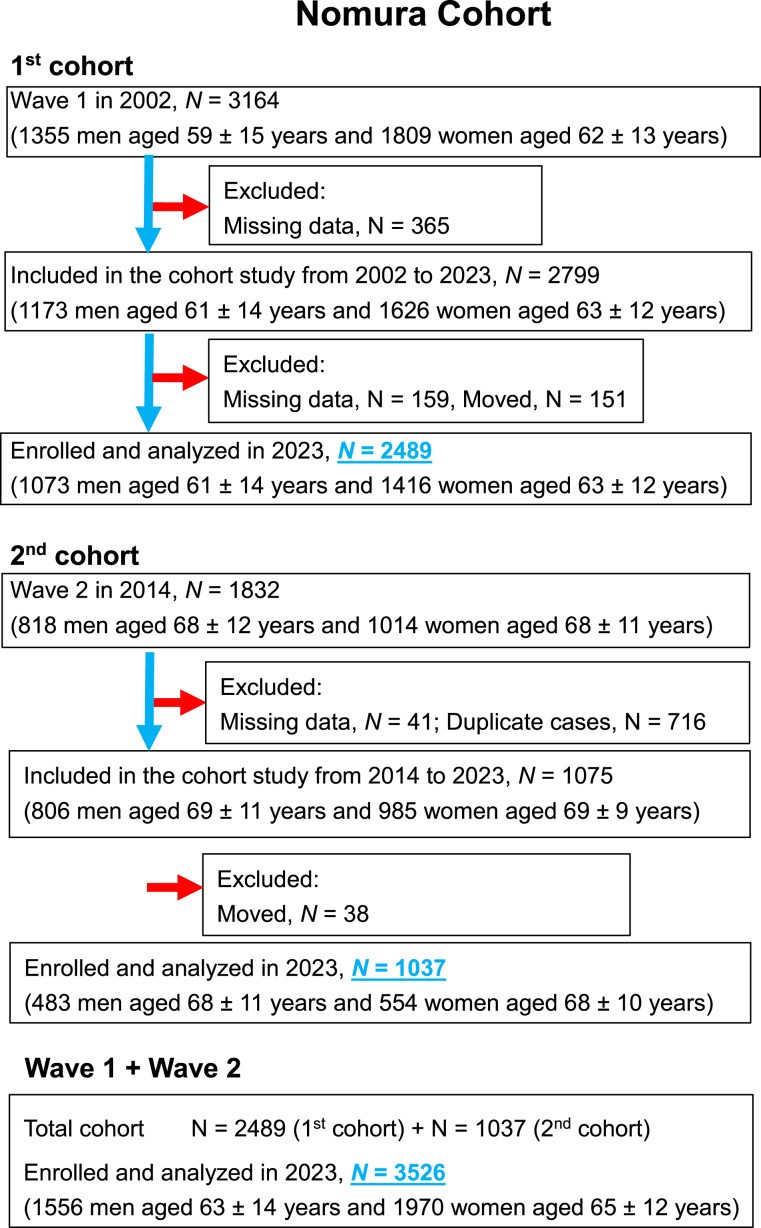
The analysis presented here forms a segment of the Nomura investigations, 13 which commenced in 2002 (for the initial cohort) and 2014 (for the subsequent cohort). The recruitment process focused on individuals living in rural areas of Ehime Prefecture, Japan, particularly those participating in community-centered annual health checkups at the Nomura Health and Welfare Center. These values are generally estimated from previous research found in the literature. To illustrate sample size calculation for cohort studies, we will use data from a study on smoking status and premature death among Japanese persons. The study employed standard confidence and power values (95% and 80%, respectively). The effect of smoking on mortality was calculated from the past literature as a survival rate of 0.88 for nonsmokers, 0.79 for past smokers, and 0.80 for current smokers over a 12-year period, yielding a figure of 269 in the required sample size formula for R2.8-0. 14 The initial cohort consisted of 3164 individuals and the subsequent cohort of 1832 individuals, aged between 22 and 95 years. All actions adhered strictly to the pertinent guidelines and regulations. Data on participants’ medical backgrounds, current health status, and medication usage were acquired through a self-administered questionnaire (Supplemental File). A visual representation of the participant inclusion and exclusion process is depicted in Figure 1. Follow-up assessments were conducted 21 years after the initial cohort and 9 years after the second cohort. The residency status of participants was verified using Japan’s Basic Resident Register. The present investigation analyzed assessment data from both cohorts (N = 3526).
Figure 1.
Flowchart of participants.
Ethics Approval and Consent
The study protocol was approved by the Institutional Review Board (IRB) of Ehime University Hospital (IRB: 1903018), and all participants provided written informed consent.
Evaluation of Risk Factors
Clinical records provided the demographic and risk factor information. Body mass index (BMI) was calculated by dividing weight (in kilograms) by the square of height (in meters). Smoking status [i.e., Brinkmann index (BI)] was calculated by multiplying the number of years smoked by the number of cigarettes smoked per day. Based on this, participants were categorized into non-smokers, former smokers (BI 1-799 and ≥ 800) and current smokers (BI 1-399, 400-799, and ≥ 800). 15 Daily alcohol intake was measured using the Japanese liquor unit (equivalent to 22.9 grams of ethanol). Participants were categorized into four groups based on their alcohol consumption: non-drinkers, occasional drinkers (< 1 unit/day), light daily drinkers (1-2 units/day), and heavy daily drinkers (2-3 units/day), with no participants exceeding 3 units/day. Systolic blood pressure (SBP) and diastolic blood pressure (DBP) were measured using an automated sphygmomanometer. After a minimum rest period of 5 minutes, the participants’ right upper arm was fitted with a properly sized cuff while seated. The mean of two consecutive readings was analyzed. Participants were instructed to fast overnight before measurements were taken for triglycerides (TG), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), serum uric acid (SUA), creatinine (Cr), and blood glucose (BG) levels. The glomerular filtration rate (eGFR) was estimated using the chronic kidney disease epidemiology collaboration (CKD-EPI) equation, modified with a Japanese coefficient. The equation used for males with creatinine (Cr) levels at or below 0.9 mg/dL was 141 × (Cr/0.9)^–0.411 × 0.993^age × 0.813. For those with Cr levels above 0.9 mg/dL, the equation was 141 × (Cr/0.9)^–1.209 × 0.993^age × 0.813. For females with Cr levels at or below 0.7 mg/dL, the equation used was 144 × (Cr/0.7)^–0.329 × 0.993^age × 0.813, and for those with Cr levels above 0.7 mg/dL, the equation was 144 × (Cr/0.7)^–1.209 × 0.993^age × 0.813. 16
Participants were identified as hypertensive if their SBP was 140 mmHg or higher, their DBP was 90 mmHg or higher, or if they were undergoing antihypertensive treatment. They were categorized as having hypertriglyceridemia if their TG levels were 150 mg/dL or higher and hypo-HDL cholesterolemia if their HDL-C levels were below 40 mg/dL. High-LDL cholesterolemia was defined as LDL-C levels of 140 mg/dL or higher or if the participant was using antidyslipidemic medication. Those exhibiting BG levels of 126 mg/dL or above and utilizing antidiabetic medication were designated as diabetic. SUA levels of 7.0 mg/dL or above or usage of an SUA-lowering medication were considered indicative of hyperuricemia. CKD was characterized by an eGFR value ≤ 60 mL/min/1.73 m2. Conditions such as ischemic heart disease, ischemic stroke, and peripheral vascular disease were grouped under cardiovascular disease (CVD).
Statistical Analysis
The statistical analysis was conducted using IBM SPSS Statistics 28.0 (Statistical Package for Social Science Japan, Inc., Tokyo, Japan) or R 2.8-0. Continuous variables with a normal distribution were presented as means ± standard deviation (SD), whereas median values (interquartile range) were used for non-normally distributed data, such as TG and BG levels. Variables that did not follow a normal distribution were log-transformed, and these transformed values were used in all further analyses. Participants were categorized according to their smoking status. Continuous variables were assessed using Student’s t-test or analysis of variance (ANOVA), while categorical variables were evaluated using the χ2 test to identify differences in means and prevalence across the groups. Subsequently, a Cox proportional hazards regression analysis was performed to estimate hazard ratios (HRs) and 95% confidence intervals (CIs), using age as the primary time variable. The entry time was defined as the participants’ age in days at the time of enrollment, and the exit time was defined as their age at death or at the end of the follow-up period, whichever came first. Adjustments were made for gender, age, BMI, smoking status and drinking behaviors, history of CVD, hypertension, hypertriglyceridemia, hypo-HDL cholesterolemia, high-LDL cholesterolemia, diabetes, CKD, and hyperuricemia. The presence or absence of multicollinearity of the explanatory variables in multivariable analysis was confirmed using the variance inflation factor (VIF). Additionally, we performed subgroup analyses to determine the consistency of the observed association of smoking status with all-cause mortality. The analyses were performed by gender (men and women), age (< 70 years and ≥70 years), BMI (< 25 kg/m2 and ≥25 kg/m2), and drinking habits (never and occasional/light/heavy), and time to death. An interaction test was carried out to assess the effect variable, with adjustments made for all significant confounding variables except the effect variable itself. Statistical significance was assumed at P-values below 0.05.
Results
The sample consisted of 3526 participants, among whom 2526 (71.6%) (male: 39.7%; female: 96.9%) were non-smokers, 481 (13.6%) (male: 29.3%; female: 1.3%) were former smokers, and 519 (14.7%) (male: 31.1%; female: 1.8%) were current smokers. The average age of the participants was 64 ± 12 years, with 44.1% being male. The median follow-up period was 6315 days, with an interquartile range of 3441 to 7727 days. Out of the total participants, 1111 (49.2%) were confirmed to have died. Among these, 564 were men (36.2% of the male participants), and 547 were women (27.8% of the female participants). During follow-up, 774 (30.6%) deaths occurred among the never-smokers, 184 (38.3%) among the former smokers, and 153 (29.5%) among the current smokers from all causes.
Baseline characteristics of the participants according to smoking status are summarized for all males in Table 1. The mean (SD) ages were 65 (12), 66 (12), and 56 (14) for never-smokers, former smokers, and current smokers, respectively. We found that among never-smokers and former smokers, greater BI was associated with higher male ratio, age, alcohol consumption, history of CVD, prevalence of hypertension, diabetes, CKD, and hyperuricemia, while hyper LDL cholesterolemia was lower. Furthermore, among current smokers, greater BI was associated with a higher male ratio, age, alcohol consumption, hypertension, hypo-HDL cholesterolemia, diabetes, CKD, and hyperuricemia.
Table 1.
Baseline characteristics of participants stratified by smoking status.
| Brinkman Index | Never-Smokers | Former Smokers | Current Smokers | P-Value* | |||
|---|---|---|---|---|---|---|---|
| 0 | 1-799 | ≥800 | 1-399 | 400-799 | ≥800 | ||
| Baseline Characteristics n = 3526 | n = 2526 | n = 335 | n = 146 | n = 124 | n = 205 | n = 190 | |
| Gender (male), n (%) | 617 (24.4) | 311 (92.8) | 145 (99.3) | 99 (79.8) | 196 (95.6) | 188 (98.9) | < 0.001 |
| Age (years) | 65 ± 12 | 64 ± 13 | 71 ± 9 | 45 ± 17 | 56 ± 12 | 63 ± 9 | < 0.001 |
| Body mass index (kg/m2) | 23.2 ± 3.2 | 23.7 ± 3.1 | 23.5 ± 3.0 | 23.2 ± 4.0 | 22.9 ± 3.2 | 23.0 ± 3.0 | 0.032 |
| Drinking habits (never/occasional/light/heavy), n (%) | 67.5/21.2/8.7/2.5 | 9.3/55.2/23.9/11.6 | 13.7/53.4/19.2/13.7 | 14.5/33.1/33.1/19.4 | 12.7/18.5/34.6/34.1 | 8.9/18.9/20.0/52.1 | < 0.001 |
| History of cardiovascular disease, n (%) | 180 (7.1) | 35 (10.4) | 29 (19.9) | 5 (4.0) | 16 (7.8) | 14 (7.4) | < 0.001 |
| Hypertension, n (%) | 1478 (58.5) | 199 (59.4) | 102 (69.9) | 35 (28.2) | 83 (40.5) | 102 (53.7) | < 0.001 |
| Systolic blood pressure (mmHg) | 138 ± 22 | 136 ± 19 | 141 ± 21 | 128 ± 18 | 133 ± 19 | 136 ± 20 | < 0.001 |
| Diastolic blood pressure (mmHg) | 80 ± 11 | 81 ± 11 | 76 ± 12 | 82 ± 11 | 82 ± 11 | 82 ± 11 | < 0.001 |
| Use of antihypertensive medication, n (%) | 825 (32.7) | 125 (37.3) | 71 (48.6) | 16 (12.9) | 22 (10.7) | 40 (21.1) | < 0.001 |
| Hypertriglyceridemia, n (%) | 406 (16.1) | 70 (20.9) | 30 (20.5) | 35 (28.2) | 46 (22.4) | 53 (27.9) | < 0.001 |
| Triglycerides (mg/dL) | 91 (68-126) | 93 (72-140) | 115 (72-136) | 102 (79-154) | 106 (79-143) | 105 (73-161) | < 0.001 |
| Hypo-HDL-cholesterolemia, n (%) | 82 (3.2) | 25 (7.5) | 5 (3.4) | 5 (4.0) | 17 (8.3) | 17 (8.9) | < 0.001 |
| HDL cholesterol (mg/dL) | 64 ± 16 | 61 ± 17 | 60 ± 17 | 60 ± 15 | 60 ± 17 | 56 ± 16 | < 0.001 |
| Hyper-LDL-cholesterolemia, n (%) | 871 (34.5) | 96 (28.7) | 41 (28.1) | 20 (16.1) | 45 (22.0) | 41 (21.6) | < 0.001 |
| LDL cholesterol (mg/dL) | 121 ± 30 | 114 ± 31 | 110 ± 30 | 106 ± 35 | 110 ± 34 | 106 ± 32 | < 0.001 |
| Use of lipid-lowering medication, n (%) | 269 (10.6) | 31 (9.3) | 21 (14.4) | 1 (0.8) | 10 (4.9) | 10 (5.3) | < 0.001 |
| Diabetes, n (%) | 305 (12.1) | 54 (16.1) | 39 (26.7) | 4 (3.2) | 21 (10.2) | 25 (13.2) | < 0.001 |
| Blood glucose (mg/dL) | 100 (91-114) | 105 (94-120) | 110 (95-126) | 94 (88-103) | 98 (89-114) | 101 (89-115) | < 0.001 |
| Use of anti-diabetic medication, n (%) | 206 (8.2) | 40 (11.9) | 29 (19.9) | 4 (3.2) | 16 (7.8) | 18 (9.5) | < 0.001 |
| Chronic kidney disease, n (%) | 246 (9.7) | 53 (15.8) | 26 (17.8) | 4 (3.2) | 10 (4.9) | 17 (8.9) | < 0.001 |
| eGFR (mL/min/1.73 m2) | 77.6 ± 16.2 | 75.8 ± 16.3 | 70.9 ± 16.1 | 89.2 ± 17.5 | 82.8 ± 14.5 | 78.7 ± 14.7 | < 0.001 |
| Hyperuricemia | 205 (8.1) | 99 (29.6) | 49 (33.6) | 31 (25.0) | 48 (23.4) | 53 (27.9) | < 0.001 |
| Serum uric acid (SUA) (mg/dL) | 4.8 ± 1.3 | 5.9 ± 1.4 | 6.1 ± 1.3 | 5.9 ± 1.3 | 5.9 ± 1.5 | 6.0 ± 1.4 | < 0.001 |
| Use of SUA lowering medication, n (%) | 65 (2.6) | 32 (9.6) | 16 (11.0) | 7 (5.6) | 11 (5.4) | 20 (10.5) | < 0.001 |
Data are presented as mean ± standard deviation. Data for triglycerides and blood glucose were skewed and are thus presented as median (interquartile range) values and log-transformed for analysis. * P-values are from ANOVA tests for continuous variables or χ2 tests for categorical variables. Significant values (p < 0.05) are presented in bold.
Kaplan-Meier estimates were plotted to compare survival by BI for never-smokers, former smokers, and current smokers (Figure 2). Regardless of smoking status, a significantly lower survival rate was observed for larger BI. The survival rates of the two groups began to diverge after 800 days, and this pattern persisted throughout the observation period (log-rank test, P < 0.001).
Figure 2.
Analysis of the association between smoking status and all-cause mortality during the follow-up period using a survival function. P-values were obtained through a log-rank test of equality across various strata.
After adjusting for confounding factors, the multivariable analysis identified a significant association between smoking status and an increased risk of all-cause mortality (Table 2). The multivariate-adjusted OR (95% CI) for all-cause mortality compared with never smokers was 1.51 (1.17-1.96) for former smokers with BI of ≥800, 1.61 (1.20-2.17) for current smokers with BI of 400-799 and 1.62 (1.24-2.10) for BI of ≥800 (P for trend = 0.001).
Table 2.
Hazard ratios and 95% confidence intervals of baseline smoking status for all-cause mortality.
| Brinkman Index | Never-Smokers | Former Smokers | Current Smokers | P for Trend | |||
|---|---|---|---|---|---|---|---|
| 0 | 1-799 | ≥800 | 1-399 | 400-799 | ≥800 | ||
| Baseline Characteristics n = 3526 | n = 2526 | n = 335 | n = 146 | n = 124 | n = 205 | n = 190 | |
| Prevalence of death, n (%) | 774 (30.6) | 107 (31.9) | 77 (52.7) | 18 (14.5) | 56 (27.3) | 79 (41.6) | < 0.001 |
| Person-years of follow-up | 39,335 | 4681 | 2102 | 1702 | 3397 | 2739 | < 0.001 |
| Model 1 | Reference | 1.25 (1.02-1.53) | 2.86 (2.26-3.62) | 0.37 (0.23-0.59) | 0.81 (0.61-1.06) | 1.54 (1.22-1.94) | < 0.001 |
| Model 2 | Reference | 1.04 (0.83-1.31) | 1.58 (1.22-2.04) | 0.82 (0.51-1.32) | 1.60 (1.20-2.15) | 1.62 (1.26-2.09) | < 0.001 |
| Model 3 | Reference | 1.03 (0.82-1.29) | 1.54 (1.19-1.99) | 0.78 (0.49-1.25) | 1.52 (1.13-2.04) | 1.52 (1.18-1.97) | < 0.001 |
| Model 4 | Reference | 1.05 (0.84-1.32) | 1.51 (1.17-1.96) | 0.79 (0.49-1.26) | 1.61 (1.20-2.17) | 1.62 (1.24-2.10) | < 0.001 |
Model 1 was non-adjusted; model 2 was adjusted for age and gender; model 3 was adjusted for body mass index, drinking habits, and history of cardiovascular disease in addition to covariates in model 1; model 4 was adjusted for hypertriglyceridemia, hypo-HDL-cholesterolemia, hyper-LDL-cholesterolemia, diabetes, chronic kidney disease, hyperuricemia in addition to covariates in model 2.
Significant values (P < 0.05) are presented in bold.
Table 3 shows HRs and 95% CIs of baseline smoking status for all-cause mortality of the participants stratified by gender, age, BMI, and time until death. Consistent with our previous findings, smoking status was associated with a higher risk of all-cause mortality in those with a time until death of ≥1095 days, regardless of gender, age, and BMI. In females, even lower levels of smoking were linked to increased mortality compared to males (P = 0.021 for interaction), while advancing age remained a significant predictor of all-cause mortality even beyond the age of 70 (P = 0.017 for interaction).
Table 3.
Hazard ratios and 95% confidence intervals of baseline smoking status for all-cause mortality by sub-analysis.
| Brinkman Index | Never-Smokers | Former Smokers | Current Smokers | P for Trend | P for Interaction | |||
|---|---|---|---|---|---|---|---|---|
| 0 | 1-799 | ≥800 | 1-399 | 400-799 | ≥800 | |||
| Baseline Characteristics n = 3526 | n = 2526 | n = 335 | n = 146 | n = 124 | n = 205 | n = 190 | ||
| Gender | ||||||||
| Men (n = 1556) | Reference | 0.97 (0.77-1.22) | 1.48 (1.14-1.92) | 0.51 (0.27-0.96) | 1.34 (0.98-1.83) | 1.47 (1.13-1.92) | < 0.001 | 0.021 |
| Women (n = 1970) | Reference | 2.97 (1.21-7.32) | ------- | 2.07 (1.02-4.22) | 6.53 (2.36-18.0) | ------ | 0.001 | |
| Age | ||||||||
| <70 years (n = 2231) | Reference | 0.90 (0.60-1.34) | 1.42 (0.84-2.39) | 1.45 (0.78-2.71) | 1.50 (0.98-2.30) | 1.68 (1.15-2.46) | 0.033 | 0.017 |
| ≥70 years (n = 1295) | Reference | 1.11 (0.84-1.46) | 1.60 (1.18-2.16) | 0.45 (0.21-0.96) | 1.66 (1.09-2.54) | 1.52 (1.04-2.22) | 0.001 | |
| Body mass index | ||||||||
| <25.0 kg/m2 (n = 2575) | Reference | 1.11 (0.85-1.45) | 1.57 (1.16-2.12) | 1.00 (0.60-1.66) | 1.54 (1.09-2.17) | 1.58 (1.17-2.15) | 0.005 | 0.716 |
| ≥25.0 kg/m2 (n = 951) | Reference | 0.81 (0.52-1.26) | 1.25 (0.73-2.15) | 0.24 (0.06-0.99) | 1.57 (0.84-2.92) | 1.71 (1.01-2.91) | 0.024 | |
| Drinking habits | ||||||||
| Never (n = 1818) | Reference | 1.43 (0.87-2.36) | 2.79 (1.66-4.67) | 0.55 (0.22-1.37) | 1.34 (0.60-2.96) | 1.48 (0.75-2.95) | 0.002 | 0.066 |
| Occasional/light/heavy (n = 1708) | Reference | 0.97 (0.74-1.25) | 1.29 (0.95-1.75) | 0.98 (0.56-1.73) | 1.59 (1.15-2.19) | 1.62 (1.22-2.15) | 0.002 | |
| Time to death | ||||||||
| <1095 days (n = 75) | Reference | ------- | ------- | ------- | ------- | ------- | ------- | |
| ≥1095 days (n = 3451) | Reference | 1.04 (0.82-1.31) | 1.55 (1.18-2.03) | 0.81 (0.51-1.31) | 1.64 (1.21-2.23) | 1.56 (1.19-2.06) | < 0.001 | |
The model was adjusted for all covariates in model 4 of Table 2.
Significant values (P < 0.05) are presented in bold.
Discussion
A significant finding of this cohort study is that smoking status emerges as a notable and independent predictor of all-cause mortality among community-dwelling adults. Following adjustments for various confounding factors, a notable increase in multivariable HR was noted, beginning from BI 800 for former smokers and from BI 400 for current smokers, compared to those who never smoked. To mitigate the possibility of reverse causality, participants who passed away within three years of follow-up were excluded, with the results remaining largely consistent. Additionally, it was observed that the threshold for smoking-related mortality was lower in women. To our knowledge, there are limited studies illustrating the association between low-intensity smoking status and all-cause mortality among community-dwelling individuals in Japan.
The prevalence of current smokers in Japan decreased from 45.9% in 2002 to 30.3% in 2014 among males, and from 9.9% to 8% among females, as reported by the National Nutrition Survey in Japan. 7 In our survey, we observed that the percentage of current smokers was lower that these figures among both men and women. It’s worth noting that the participants in our study were undergoing annual health check-ups, indicating a potentially higher level of health consciousness compared to those surveyed in the National Nutrition Survey.
Our study found that after adjusting for various confounders, smokers had an approximately 1.6 times higher risk of all-cause mortality. This result aligns with previous research conducted on both Asian and Western populations. In a meta-analysis, the hazard ratio (HR) for all-cause mortality was 1.26 (95% CI: 1.17-1.37) for individuals born before 1920, 1.47 (95% CI: 1.35-1.61) for those born in the 1920s, and 1.70 (95% CI: 1.57-1.84) for those born in 1930 or later. 17 In a recent study by Yang et al. 14 that individual participant data (n = 70,9151) from 16 prospective cohorts conducted in China, Japan, Korea/Singapore, and India/Bangladesh, separately by cohorts, there were 108,287 deaths over a mean follow-up of 12.0 years from tobacco smoking cessation. Their results showed a higher risk of all-cause mortality among former smokers (HR, 1.25; 95% CI, 1.13–1.37) compared with never-smokers, consistent with the findings of our study. Similarly, research by Halipah et al. 18 involving 3353 participants aged 40 and older from the Indonesian Family Life Survey found a smoking prevalence of 40.3%. Current smokers had a higher risk of all-cause mortality compared to non-smokers (HR, 1.48; 95% CI, 1.11-1.98). Akter et al. 19 conducted research in Japan, focusing on employed individuals enrolled in the Japan Epidemiology Collaboration on Occupational Health Study. The study encompassed 79,114 Japanese workers aged between 20 and 85 years. Over six years, 252 deaths were recorded. The findings indicated an adjusted HR for overall mortality of 1.49 (95% CI, 1.10 to 2.01).
An important study from a Western context utilizing data from the US National Health and Nutrition Examination Survey (NHANES) from 1999 to 2014, found a notable increase in all-cause mortality risk among occasional smokers (HR, 1.50; 95% CI, 1.08-2.08) compared to never-smokers. For daily smokers, the adjusted HRs for all-cause mortality were as follows: 1.54 (95% CI, 1.24-1.90) for those smoking fewer than 20 cigarettes per day, 2.09 (95% CI, 1.65-2.63) for those smoking 20-40 cigarettes per day, and 2.78 (95% CI, 1.75-4.43) for those smoking 40 or more cigarettes per day. 20 The CHANCES project, a collaboration of 22 population-based cohort studies involving older adults from Europe and the United States, found that across its combined analysis, there were 99,298 deaths among the 489,056 participants. Current smokers demonstrated a 2-fold increase in the risk of all-cause mortality compared to never-smokers. The elevated HR observed in the CHANCES study might be attributable to the older age of the participants (over 60 years) and the extended observation period, similar to our study. 21 Furthermore, in a meta-analysis identifying 17 studies from 7 countries, a dose-response relationship between amount of smoking (e.g., average number of cigarette smoked per day or pack-years of smoking) and premature mortality was observed, and former smokers were similarly associated with increased mortality and the benefits of smoking cessation were evident in all age groups, including those over 80 years of age. 22 Our study also found a dose-response relationship between Brinkman index and all-cause mortality.
In terms of the impact of cigarettes on mortality, individuals with low baseline risk, including those who have recently started smoking or who smoke infrequently, generally exhibit lower mortality rates. Conversely, among those who have quit smoking or have never smoked, there exists a possibility that they abstain due to underlying health conditions. In such instances, the mortality rate might be higher for non-smokers due to these pre-existing illnesses. 23 Also, our study showed a significant decrease in HR among men with a BI of 1-399 compared to never smokers. While the HR for mortality was not significant for past smoking, with a BI of 1-799 in men, it was significant in women, as was even the case for current smoking with a BI of 1-399. It can thus be suggested that women should exercise caution even if only a history of low-intensity smoking is present.
Tobacco smoke harbors numerous chemicals detrimental to the health of both smokers and non-smokers. 24 Even minimal exposure to tobacco smoke can pose risks. 25 Of the over 7000 chemicals present in tobacco smoke, at least 250 have been identified as harmful; these comprise compounds like hydrogen cyanide, carbon monoxide, and ammonia. 25 At least 69 are recognized carcinogens. 26 In addition, exposure to tobacco smoke quickly damages blood vessels throughout the body and increases blood clotting, which can lead to the occurrence of atherosclerosis, the narrowing of the arterial vascular cavity, and cause a variety of cardiovascular and cerebrovascular diseases.27,28
This study benefited from a relatively long follow-up period, which enabled more accurate HR estimates. Additionally, the results of our sensitivity analysis, which excluded the first three years of mortality data, were consistent with the main findings, further confirming the validity of the results. However, several caveats should be considered when interpreting the outcomes of this study. Firstly, the participants were predominantly middle-aged and older adults residing in a Japanese rural community (with a mean age of 64 ± 12 years), which may limit the findings’ generalizability to the broader population. Second, participants’ questionnaires were self-administered and may be subject to recall bias and social desirability bias because the questionnaires were not validated. Third, the study specifically targeted individuals listed as deceased in the basic resident register, excluding those who relocated during the research period. Fourth, it is crucial to acknowledge the potential influences of medications, underlying health conditions, and lifestyle changes (for example, smokers quitting and non-smokers becoming smokers) both at the study’s outset and throughout its duration on the observed outcomes, which were not accounted for in the analysis. Lastly, while the study did account for the duration of smoking, it did not explore the impact of the length of time since smoking cessation.
Conclusion
In summary, the present investigation encompassing a significant cohort of Japanese residents uncovered a link between smoking and elevated risks of all-cause mortality, with the degree of risk varying based on smoking intensity. Among females, even light cigarette smokers faced higher all-cause mortality risks compared to non-smokers. Interestingly, individuals who had quit smoking displayed a higher mortality risk compared to lifelong non-smokers. However, for former smokers with a BI of less than 800, their risk decreased to levels comparable to those who never smoked. Our findings offer compelling evidence that both male and female cigarette smokers face heightened mortality risks compared to never-smokers.
Supplemental Material
Supplemental Material for Smoking Status and Premature Death Among Japanese Rural Community-Dwelling Persons by Ryuichi Kawamoto, Asuka Kikuchi, Daisuke Ninomiya, Masanori Abe and Teru Kumagi in Tobacco Use Insights.
Ethical Statement
Ethical Approval and Consent to Participate
The study was approved by the ethics committee of the Ehime University Graduate School of Medicine (IRB: no. 1903018), and informed consent was obtained from all participants. The study was conducted in accordance with ethical standards.
Acknowledgements
We thank Uni-edit (https://uni-edit.net/) for their valuable assistance in editing and proofreading this manuscript.
Author Contributions: RK and AK contributed to the study design, conducted the statistical analysis, and drafted the manuscript. RK, AK, DN, MA, and TK contributed to data acquisition and interpretation. RK and AK contributed to the conception and design of the statistical analysis. RK conceived the study, participated in its design and coordination, and helped draft the manuscript. All authors reviewed and approved the final manuscript.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research received partial support from a Grant-in-Aid for Scientific Research from the Foundation for Development of Community (2024). No additional external funding was received. The funders had no involvement in the study design, data collection and analysis, decision to publish, or manuscript preparation.
Supplemental Material: Supplemental material for this article is available online.
ORCID iD
Ryuichi Kawamoto https://orcid.org/0000-0003-4674-0787
Data Availability Statement
The data set analyzed for this study is not publicly available due to restrictions in the IRB consent but may be available from the corresponding author based on reasonable request.
References
- 1.Organization WH . Tobacco. Geneva, Switzerland: WHO; 2022. [Google Scholar]
- 2.Oberg M, Jaakkola MS, Woodward A, Peruga A, Prüss-Ustün A. Worldwide burden of disease from exposure to second-hand smoke: a retrospective analysis of data from 192 countries. Lancet. 2011;377:139-146. [DOI] [PubMed] [Google Scholar]
- 3.GBD 2019 Tobacco Collaborators . Spatial, temporal, and demographic patterns in prevalence of smoking tobacco use and attributable disease burden in 204 countries and territories, 1990-2019: a systematic analysis from the Global Burden of Disease Study 2019. Lancet. 2021;397:2337-2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Organization WH . WHO Global Report on Trends in Prevalence of Tobacco Smoking 2000-2025. 2nd ed. Geneva, Switzerland: WHO; 2018. [Google Scholar]
- 5.Collaborators GT. Smoking prevalence and attributable disease burden in 195 countries and territories, 1990-2015: a systematic analysis from the Global Burden of Disease Study 2015. Lancet. 2017;389:1885-1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gilmour S, Liao Y, Bilano V, Shibuya K. Burden of disease in Japan: using national and subnational data to inform local health policy. J Prev Med Public Health. 2014;47:136-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ministry of Health, Labor and Welfare . Tobacco or Health: https://www.health-net.or.jp/tobacco/statistics/kokumin_kenkou_eiyou_report.html (15 July 2024, date last accessed). Ministry of Health, Labor and Welfare; 2020. [Google Scholar]
- 8.Service NCCCI. Cancer Statistics—Smoking rate Tokyo, Japan. National Cancer Center, Center for Cancer Control and Information Services; 2020. [Google Scholar]
- 9.Inoue-Choi M, McNeel TS, Hartge P, Caporaso NE, Graubard BI, Freedman ND. Non-daily cigarette smokers: mortality risks in the U.S. Am J Prev Med. 2019;56:27-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Inoue-Choi M, Christensen CH, Rostron BL, et al. Dose-response association of low-intensity and nondaily smoking with mortality in the United States. JAMA Netw Open. 2020;3:e206436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang L, Ma Y, Men K, Li C, Zhang Z, Shi G. Tobacco smoke and all-cause mortality and premature death in China: a cohort study. BMC Publ Health. 2023;23:2486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Inoue-Choi M, Freedman ND, Saito E, et al. Low-intensity cigarette smoking and mortality risks: a pooled analysis of prospective cohort studies in Japan. Int J Epidemiol. 2022;51:1276-1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kawamoto R, Ninomiya D, Kumagi T. Handgrip strength is positively associated with mildly elevated serum bilirubin levels among community-dwelling adults. Tohoku J Exp Med. 2016;240:221-226. [DOI] [PubMed] [Google Scholar]
- 14.Yang JJ, Yu D, Shu XO, et al. Reduction in total and major cause-specific mortality from tobacco smoking cessation: a pooled analysis of 16 population-based cohort studies in Asia. Int J Epidemiol. 2022;50:2070-2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nakayama T, Sakai M, Slingsby BT. Japan's Ethical Guidelines for Epidemiologic Research: a history of their development. J Epidemiol. 2005;15:107-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Horio M, Imai E, Yasuda Y, Watanabe T, Matsuo S. Modification of the CKD epidemiology collaboration (CKD-EPI) equation for Japanese: accuracy and use for population estimates. Am J Kidney Dis. 2010;56:32-38. [DOI] [PubMed] [Google Scholar]
- 17.Yang JJ, Yu D, Wen W, et al. Tobacco smoking and mortality in asia: a pooled meta-analysis. JAMA Netw Open. 2019;2:e191474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Holipah H, Sulistomo HW, Maharani A. Tobacco smoking and risk of all-cause mortality in Indonesia. PLoS One. 2020;15:e0242558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Akter S, Nakagawa T, Honda T, et al. Smoking, smoking cessation, and risk of mortality in a Japanese working population - Japan epidemiology collaboration on occupational health study. Circ J. 2018;82:3005-3012. [DOI] [PubMed] [Google Scholar]
- 20.Zhu D, Zhao G, Wang X. Association of smoking and smoking cessation with overall and cause-specific mortality. Am J Prev Med. 2021;60:504-512. [DOI] [PubMed] [Google Scholar]
- 21.Müezzinler A, Mons U, Gellert C, et al. Smoking and all-cause mortality in older adults: results from the CHANCES consortium. Am J Prev Med. 2015;49:e53-e63. [DOI] [PubMed] [Google Scholar]
- 22.Gellert C, Schöttker B, Brenner H. Smoking and all-cause mortality in older people: systematic Review and meta-analysis. Arch Intern Med. 2012;172:837-844. [DOI] [PubMed] [Google Scholar]
- 23.Kenfield SA, Stampfer MJ, Rosner BA, Colditz GA. Smoking and smoking cessation in relation to mortality in women. JAMA. 2008;299:2037-2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martins-Green M, Adhami N, Frankos M, et al. Cigarette smoke toxins deposited on surfaces: implications for human health. PLoS One. 2014;9:e86391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wahl EA, Schenck TL, Machens HG, Egaña JT. Acute stimulation of mesenchymal stem cells with cigarette smoke extract affects their migration, differentiation, and paracrine potential. Sci Rep. 2016;6:22957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hoffmann D, Hoffmann I, El-Bayoumy K. The less harmful cigarette: a controversial issue. a tribute to Ernst L. Wynder. Chem Res Toxicol. 2001;14:767-790. [DOI] [PubMed] [Google Scholar]
- 27.Benjamin RM. Exposure to tobacco smoke causes immediate damage: a report of the Surgeon General. Publ Health Rep. 2011;126:158-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gallucci G, Tartarone A, Lerose R, Lalinga AV, Capobianco AM. Cardiovascular risk of smoking and benefits of smoking cessation. J Thorac Dis. 2020;12:3866-3876. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material for Smoking Status and Premature Death Among Japanese Rural Community-Dwelling Persons by Ryuichi Kawamoto, Asuka Kikuchi, Daisuke Ninomiya, Masanori Abe and Teru Kumagi in Tobacco Use Insights.
Data Availability Statement
The data set analyzed for this study is not publicly available due to restrictions in the IRB consent but may be available from the corresponding author based on reasonable request.