Abstract
Rare earth elements (REEs) are becoming increasingly important in the development of modern and green energy technologies with the demand for REEs predicted to grow in the foreseeable future. The importance of REEs lies in their unique physiochemical properties, which cannot be reproduced using other elements. REEs are sourced through mining, with global exploration of additional commercially viable mining sites still ongoing. However, there is a growing need for recycling of REEs due to the current supply of REEs not matching the growing demand, the environmental impact of REE mining and processing (the so-called “balance problem”), and the generation of large volumes of harmful electronic waste (e-waste). Industrial REE processing is mainly carried out by hydrometallurgy processes, particularly solvent extraction (SX) and ion exchange (IX) technologies. However, these methods have a significant environmental impact due to their intensive use of harmful and nonsustainable reagents. This Review highlights the development of approaches involving polymer-based extracting materials for REE manufacturing as more sustainable alternatives to current industrial REE processing methods. These materials include supported liquid membranes (SLMs), solvent impregnated resins (SIRs), macro and micro capsules, polymer inclusion membranes (PIMs), and micro polymer inclusion beads (μPIBs). Polymer-based extracting materials have the advantage of more economical regent usage while applying the same extractants used in commercial SX, enabling applications analogous to the current industrial process. These materials can be fabricated by a variety of methods in a diverse range of physical formats, with the advantages and disadvantages of each material type described and discussed in this Review along with their applications to REE processing, including e-waste recycling and mineral processing.
1. Introduction
1.1. Rare Earth Elements (REEs)
Rare earth elements (REEs), as defined by the International Union of Pure and Applied Chemistry (IUPAC), predominantly consist of the lanthanides (Lns) La, Ce, Pr, Nd, Pm, Sm, Eu, Gd, Tb, Dy, Ho, Er, Tm, Yb, and Lu, but also include Sc and Y, elements commonly found alongside the lanthanides in mineral deposits.1,2 Despite the misnomer that REEs are “rare”, they are not especially so, the term first having been created in the 19th century when very few “rare earth” containing mineral deposits had been discovered.2 Hence, when REEs were first isolated and studied, their abundance was genuinely rare when compared to the quantities of other known elements.1,2 Despite retaining the title “rare”, REEs are almost just as abundant in the earth’s crust as some of the most common industrial metals, namely, Cr, Ni, Cu, Zn, Mo, and Sn.3 This Review briefly outlines the importance and supply of REEs, the growing need for their recycling from end-of-life (EOL) electronic waste (e-waste), followed by a description of current industrial-scale processing methods (section 2), and finally alternative technologies (sections 3 and 4). These technologies involve the use of polymer-based materials incorporating extractant reagents, which are currently being developed and researched. This Review focuses on their applications to the recycling of REEs.
1.2. REEs in Technology
REE applications are set to grow rapidly in the near future due to their significant roles in the development of green technologies for sustainable global economies,1,2,4−6 such as implementation in wind turbines as a means to generate energy, battery technologies to store it, efficient lighting to enable productive energy usage, screen displays, and electric vehicles (EVs).5,7 REEs have also facilitated the miniaturization of technology products such as computers, lasers, and screen displays by enabling the development of smaller and more efficient components.2,4,7 Industrial processes also rely on REEs, which play important roles as catalysts and in polishing powders but also as additives in specialized metal alloys, glasses, and ceramics.2,4 The properties of REEs arise largely from their Ln 4f electron orbitals, which are shielded by external 5s and 5p electron orbitals, enabling the REEs’ unique magnetic (i.e., their unpaired 4f electrons responsible for strong paramagnetism) and optical (i.e., sharp emission and absorbance bands) properties.2,8−10 These unique physiochemical properties cannot be replicated by other elements and have given rise to indispensable REE applications, which global society now heavily relies on.1,2,4−6 Further applications of REEs are predicted to emerge over the coming decades, which will be accompanied by an increasing demand for higher purity and a wider range of REEs.2,4,11,12
1.3. Supply of REEs
Despite their relatively common abundance, finding suitable REE mineral deposits of economic value is still challenging, with extensive global REE mineral exploration still taking place today.4,6,13,14 REEs first became well-known to the general public during the so-called REE crisis in 2010, when China began to restrict REE production and export through the introduction of export tariffs and quotas, enforcing environmental regulations, and no longer providing new mining licenses.1,15,16 Importantly, at this stage, China accounted for over 95% of global REE production, having dominated the market since the mid-1990s.15−17 Hence, global manufacturers no longer had access to the high volume of cheap REEs previously provided by China, causing REE market prices to spike significantly.4,15,16,18
Although China’s export tariffs and quotas were eventually overturned and prices decreased in the following years, the so-called REE crisis prompted the global community to diversify its supply of REEs, with the critical REEs, namely, Y, Nd, Dy, Tb, and Eu, having now been highlighted as valuable and vulnerable resources, specifically for Europe, the U.S., and Japan.1,15,16,18
Current global REE production is considerable, presently measuring at hundreds of thousands of tons of annual rare earth oxide (REO) production and having already exceeded 100 000 t of REOs annually since the early 2000s.19−21 Although there are hundreds of different REE minerals, only a few are of commercial value, specifically the phosphate minerals monazite ((Ce, La, Y, Th) PO4), xenotime (YPO4), and carbonate bastnäsite (La, Ce, Y) F CO3.22 Presently, the large majority of REE mining reserves and operations currently supplying REEs is in China. However, China’s share in this production has decreased from over 95% of global production in 2010 to 58% in 2020,17,20 as shown in Table 1. Other than China, significant global reserves have also been located in the U.S., Canada, Australia, Russia, Brazil, India, Myanmar, Greenland, Madagascar, South Africa, Tanzania, Thailand, and Vietnam.19,20 Since 2012, additional exploration in Australia, Canada, Greenland, and most recently Sweden and the U.S. has demonstrated that many more commercial REE mineral deposits are likely to be found.13,23−25 The U.S., Australia, Myanmar, and Thailand are currently among the top 5 REO producers with a global share in 2020 of 16%, 9%, 13%, and 2%, respectively,19,20 compared to having no REO production taking place in 2010,17 demonstrating that the REE crisis has prompted additional exploration for REE minerals internationally.14,26 The REO production of these countries in 2010 and 2020 is compared in Table 1.
Table 1. U.S. Geological Survey of Global REE Production as REO in 2010 versus 202017,20.
| country | 2010 production (t) | % share | 2020 production (t) | % share |
|---|---|---|---|---|
| China | 130000 | 97 | 140000 | 58 |
| U.S. | 0 | 39000 | 16 | |
| Australia | 0 | 21000 | 9 | |
| Myanmar | 0 | 31000 | 13 | |
| Thailand | 0 | 3600 | 2 | |
| other | 3400 | 3 | 5400 | 2 |
| total | 133400 | 100 | 240000 | 100 |
Thus, although China is still currently the main global producer and holds a majority of currently known REE deposits,19,20 a significant number of undiscovered REE mineral deposits are expected to be present around the globe, with even deep ocean deposits being considered viable.1,4
1.4. Environmental Impacts of REE Mining
There is a growing concern regarding increasing concentrations of REEs in the environment and the resulting implications for ecosystems and human health,27−29 especially when considering the limited understanding of these implications compared to those of other industrial metals.27,30 Currently, the main sources of environmental REE contamination are open cut mining, industrial processing effluents, and mine tailings,2,29,31,32 with some REEs also having been detected leaching from urban and industrial e-waste disposal sites.4 REE ores can also contain radioactive compounds, such as U or Th, which are also released into the environment from REE mining operations.2,4,28 Increases in anthropogenic REE concentrations within areas surrounding mining operations and waterways downstream from these operations have already been detected, along with observed REE bioaccumulation in humans residing around and/or working at REE mining sites.4,28,32,33 This has resulted in a number of studies of the bioaccumulation of REEs in human hair, blood, urine, brain, bone, liver, and lungs.27,28 REE accumulation in humans has been linked to cytogenetic damage to sperm sex cells in humans, oxidative stress induced by redox active REEs, damaged respiratory tracts, and heart disease.4,28,29,32,33 Exposure routes for REEs have been identified including contaminated soil, dust, water, and even food,27,29 with crops like rice being particularly susceptible to REE accumulation.4 The impact of high REE concentrations on plants and ecosystems is also becoming apparent, Thomas et al. having extensively studied the effect of La3+, Ce3+, and Y3+ on plant germination and biomass development, with concerns for plant health upon exposure to high REE concentrations.34 The limited number of studies on environmental indicator species, along with a poor understanding of the toxicity thresholds for REEs, have also been widely highlighted with concern.27−29 Thus, these large knowledge gaps provide a strong incentive to find alternative sources of REEs to reduce global dependence on REE mining and minimize generation of waste containing REEs.
1.5. Alternative REE Sources and the “Balance Problem”
Various industrial waste products have been investigated as potential sources of REEs, including red mud from aluminum processing and phosphor gypsum from phosphorus production.6,32,35 Fly ash from coal fired power stations can also contain commercially viable concentrations of REEs, with some coal deposits already containing high or even commercially viable REE concentrations.4,36 REEs can also be produced as byproducts of U, Fe, and Ti mining operations, where REEs are secondary products after being enriched in the mine tailings or drainage.32,35 Some REE mine tailings still contain commercially viable REO levels, including Mountain Pass (U.S.), which has tailings containing REO concentrations of 3–5%.32 However, these sources alone are not sufficient to satisfy future REE demands, particularly regarding the critical REE supply.
Market pressure and increasing complications in REE supply chains have led researchers into the area of REE recycling from EOL e-waste, which is now a major topic within REE research.12,15,36−38 Moreover, the volume of e-waste produced globally every year is increasing, with spent electronic devices such as mobile phones, computers, and laptops predominantly ending up in landfill, a trend which is predicted to grow significantly in the near future.39,40 Rene et al. stated that in 2019 alone, global e-waste generation was 53.6 million metric tons, and that e-waste generation would increase by 33% over the next decade as the use of electronic devices increases.40
Despite increasing e-waste generation, there has been little improvement in the recycling of REEs from e-waste as reported by Binnemans et al. in 2013 that less than 1% of REEs were recycled.15,38,41 The slow development of REE recycling projects is due to the presence of REEs in electronics at low concentrations and only in specific components, making them difficult to isolate from the bulk e-waste material.12,37,38 Handling and environmental storage protocols are also a contributing factor to the high cost of e-waste recycling in developed nations.40 Hence, until recently, the economic benefit of recycling REEs has been marginal, but rising REE market prices are set to make REE recycling a much more economically viable venture.4,37,38 The cumulative stockpile of viable REE e-waste resources (NiMH batteries, lamp phosphors, and REE permanent magnets (REPMs)) globally in 2020 was estimated by Mudali et al. to be 375 000 metric tons, of which 29 167 metric tons could be available REEs.38 Thus, REE recycling projects in many developed countries are said to be approaching maturity and are set to be applied to industrial scale REE recycling in the near future.38
The development of REE recycling methods would have numerous advantages, the most important being the avoidance of “wasting” REEs, which could be used to service future technological demands, particularly critical REEs, such as Y, Nd, Dy, Tb, and Eu.15,38,39 Second, recycling REEs would help solve the so-called balance problem.15 The balance problem is unique to REEs and describes the disparity between the demand for specific critical REEs and the natural abundance of other REEs with which they occur, leading to the production of other REEs at a surplus.15,42 This is currently most noticeable with Nd, one of the most commonly used REEs,12 which is required for REPMs. However, to mine and process the required Nd, large amounts of La and Ce are produced in excess of global demand,12,42 which could be avoided if Nd were recycled. Third, recycling REEs could have a lower environmental impact than mining and ore processing since REEs are already purified prior to their use in electronic components. As such, their recycling could be less chemically intensive and thus presents less of an environmental hazard. Recycling of e-waste would also reduce the environmental impact of harmful elements like REEs being released into the environment after leaking from waste disposal sites4,39,43 or being released from operational mining sites.2,29,31,32
There has been a significant amount of research into the leaching and digestion of REEs from EOL e-wastes,4,37,38,44−46 focusing on the recovery of REPMs from computer hard drives, which have the highest recyclable REE content (viz., Pr, Nd, and Dy);12,45,47,48 NiMH batteries as a source of La, but also Ce, Nd, Pr, and Y;49,50 and fluorescent light tubes as a source of primarily Eu and Y, but also Tb, Gd, La, and Ce.46,51−54 Other potential REE sources that could be recycled are glass polishing powders, catalysts, some small electrical components, and even some REE-containing glass materials.15 However, less research interest exists in REE recovery from these sources since they are less widely available and can be more difficult to successfully remove REEs from.38
2. Industrial Processing of REEs
2.1. Commercial Industrial Processing of REEs
Currently, the most common and economically viable method for processing REEs is hydrometallurgy, where ores or e-waste are digested in strong acidic or alkaline solutions before processing.37,38 Pyrometallurgy has also been widely applied to e-waste recycling,38 but it is energy intensive37 and emits significant quantities of toxic gases.36 Hydrometallurgy techniques applied to commercial REE separation have varied over time and include selective oxidation, selective reduction, fractional crystallization, fractional precipitation, ion exchange (IX), and solvent extraction (SX), with IX and SX being the predominant separation techniques applied.2,22
SX is the most commonly used method because it can process larger volumes of material and is not very energy intensive,2,37 thus making SX a more cost-effective method for separating REEs, even when the desired purity requires numerous separation stages as can be the case with industrial REE processing.4,22
2.2. Solvent Extraction (SX) of REEs
Due to the diversity and complexity of REE ores, there are numerous commercial extraction methods, which have been developed for separating REEs, requiring a variety of different extractants, shown in Table 2.22,55 The most common and widely used extractants are organophosphorus acid extractants such as di(2-ethylhexyl) phosphoric acid (D2EHPA) and 2-ethylhexylyphosphonic acid mono-2-ethylhexyl ester (EHEHPA), carboxylic acids such as neodecanoic acid (Versatic 10) and naphthenic acid, solvating extractants such as tributyl phosphate (TBP), and quaternary ammonium anion exchange extractants such as Aliquat 336 (A336).2,22,55 For high selectivity and thus greater purification capabilities, the acidic extractants, that is, the organophosphorus acids and carboxylic acids, are commonly used.22,55 These acidic extractants form dimers in nonpolar solvents, and the SX process for the extraction of REEs can be described by eq 1.22,55,56
| 1 |
where REE is the rare earth element, H2A2 represents the organic acid dimer, and (aq) and (org) refer to the aqueous and organic phases, respectively.
Table 2. Reagent Classes and Molecular Formulas of Common Industrial REE Extractants Used in SX Processesa.
| reagent class | molecular formula | extractant |
|---|---|---|
| carboxylic acid | (R1·R2·CH3)·C·COOH | versatic acids: |
| R1 + R2 = C7, Versatic 10; | ||
| R1 + R2 = C6–C8, Versatic 911 | ||
| (R1·R2·R3·R4)·(CH2)nCOOH | naphthenic acids: | |
| R1–R4: varied alkyl groups | ||
| organophosphorus acids | (R1·R2)·POOH | phosphoric acids: |
| R1 = R2 = C4H9CH(C2H5)CH2O–, di-2-ethylhexylphosphoric acid (D2EHPA) | ||
| phosphonic acids: | ||
| R1 = C4H9CH(C2H5)CH2O–, R2 = C4H9CH(C2H5)CH2–, 2-ethylhexylphosphonic acid mono-2-ethylhexyl ester (EHEHPA, HEHEHP, P507, PC88A) | ||
| phosphinic acids: | ||
| R1 = R2 = C4H9CH(C2H5)CH2–, di-2-ethylhexylphosphinic acid (P227) | ||
| R1 = R2 = CH3(CH2)3CH2CH(CH3)CH2–, di-2,4,4-trimethylpentylphosphinic acid (Cyanex 272) | ||
| (R1·R2)·PSOH | monothiophosphinic acids: | |
| R1 = R2 = CH3(CH2)3CH2CH(CH3)CH2–, di-2,4,4-trimethylpentyl-monothiophosphinic acid (Cyanex 302) | ||
| (R1·R2)·PSSH | dithiophosphinic acids: | |
| R1 = R2 = CH3(CH2)3CH2CH(CH3)CH2–, di-2,4,4-trimethylpentyl-dithiophosphinic acid (Cyanex 301) | ||
| chelating exchangers | R1·(CO)·CH2·(CO)·R2 | β-diketones: |
| R1 = R–C6H5, R2 = CH3(CH2)5–, R: alkyl group (LIX 54) | ||
| solvating extractants | (R1·R2·R3)·PO | phosphorus esters: |
| R1 = R2 = R3 = CH2(CH2)2CH2O–, tri-n-butyl-phosphate (TBP) | ||
| R1 = R2 = CH2(CH2)2CH2O–, R3 = CH2(CH2)2CH2–, dibutylbutylphosphonate (DBBP) | ||
| phosphine oxides: | ||
| R1 = R2 = R3 = CH2(CH2)6CH2–, tri-n-octylphosphine oxide (TOPO, Cyanex 921, 923) | ||
| anion exchanger | (R1·R2·R3)·NCH3Cl | primary amines: |
| R = (CH3)3C(CH)2C(CH3)2)4 (Primene JMT, N1923) | ||
| quaternary amines: R1 = R2 = R3 = C8–C10 mixture (Aliquat 336, Adogen 464) |
Reproduced with permission from ref (2). Copyright 2015 Taylor and Francis Group.
Carboxylic acids are commercially applied to separating Y3+ from REEs, as the steric hindrance of the carboxylic acid affects the Y3+ extraction behavior.55,56 For the separation of individual REEs, organophosphorus acids are recommended, namely, D2EHPA and EHEHPA.22,55 In particular, D2EHPA is capable of separating and purifying all REEs,22,55 and it is therefore the most commonly used extractant for SX processing of REE mine ores. The application of D2EHPA in SX for EOL e-waste REE recycling has also been demonstrated to be effective, including for processing REPMs.2,12,37
Anion exchange extractants are also applied industrially, requiring the REEs to form an anionic complex with anions such as SO4–, Cl–, SCN–, or NO3– anions, although NO3– is most commonly used followed by SCN–.22 The extraction of REEs by anion exchange extractants is depicted by eq 2:2,57
| 2 |
where REE is the rare earth element, and A– is the anion of the anion exchanger R4N·A.
Anionic exchange extractants such as A336 usually favor lighter REEs in nitrate media and can be used to separate light REEs from heavy REEs.2,22
Solvating extractants require that the REEs form a neutral complex before extraction, with the most commonly applied system of TBP extraction from NO3– media, described by eq 3.22
| 3 |
This extraction system has been applied to the separation of light REEs from heavy REEs.2,22 The Cl– anion can also be used with TBP but is characterized with distribution coefficients, which are lower than those in NO3– media.22
2.3. Ion Exchange (IX) Separation of REEs
The use of ion exchange (IX) methods for the separation of REEs has a long history dating back to 1893,2 but it was not until the addition of complexing agents, which form REE complexes capable of interacting with IX media, that IX methods had acquired sufficient separation factors for effective REE processing.2 Ethylenediaminetetraacetic acid (EDTA) and hydroxyethylethylene-diaminetriacetic acid (HEDTA) are currently the most commonly used complexing agents in commercial IX separation systems, followed by diethylenetriaminepentaacetic acid (DTPA).2,58 A wide range of commercial resins can be used for IX of REEs, particularly those functionalized with sulfonic acid, amino methyl-phosphonic acid, and quaternary ammonium groups.58 The selectivity of the IX loading step is sufficient to separate REEs from other metals, but not from other REEs, which are later removed by selective elution.58
However, despite achieving high purities, IX methods have low productivity and are costly,58−60 with dilution of the eluent also posing issues for the economics of IX separation processing.2 As such, IX is only suitable for the production of small batches of very high purity REEs when the cost can be justified.22,59 The application of IX is also not suitable for REE recycling purposes, where a high volume processing is required. Moreover, REEs in recyclable waste are already mostly isolated from other REEs, unlike those in ores. Instead, the main industrial method of commercial separation and purification of REEs in hydrometallurgy has been SX, both for mine ore processing and more recently for REE e-waste recycling.22,37,38,40,46
2.4. Environmental Impact of REE Processing
Despite their effective and proven track records in REE separation, mineral processing methods such as SX and IX are recognized as environmentally detrimental processes, intensively applying nonsustainable or toxic reagents, and producing large volumes of acidic, basic, or metal-contaminated wastes.31,32,61 In the case of SX, this is due to the multiple separation stages required to purify REEs, using large volumes of fossil fuel-based organic solvents, which are nonsustainable, toxic to users, and have a significant environmental impact.61,62 Moreover, the separation of REEs using IX produces large volumes of highly acidic waste and is also reagent intensive, requiring the use of expensive complexing agents.59,60 Hence, globally there is significant interest in reducing nonsustainable and environmentally unfriendly reagent usage to minimize the impact of industrial processes on the environment.61,62 This has led to the research and development of alternative and more sustainable technologies and methods for REE separation, which have the potential to replace some existing industrial SX and IX methods.
3. Porous Polymer Supports for REE Separation
3.1. Supported Liquid Membranes
Liquid membrane technologies for the separation of REEs have been of interest to researchers and industry as they are user-friendly and efficient in reagent usage.63,64 The main advantage of liquid membrane systems over conventional SX methods is their ability to simultaneously extract and back-extract the target chemical species involving facilitated transport (Figure 1), which can be done continuously over long periods of time under suitable conditions.41,63−65 Liquid membranes can also utilize the same extraction reagents as industrial SX methods, which enables the membrane separation systems to function analogously to industrial SX systems.63,64
Figure 1.

Schematic representation of facilitated transport across a liquid membrane. The target chemical species (▲) is selectively extracted from the feed solution by the extractant (red ∧), while other chemical species (●) remain in the feed solution. Once transported across the membrane, the target chemical species is back-extracted by a suitable stripping agent (yellow ⧫).
Supported liquid membranes (SLMs) are a type of liquid membrane that has received considerable interest. An SLM consists of an organic phase contained within the pores of an inert porous membrane positioned between aqueous feed and receiving solutions (Figure 2).63,66 The organic phase typically consists of an extractant (also known as carrier), like those applied in industrial SX,63,65 dissolved in an organic solvent (diluent) such as kerosene, hexane, chloroform, toluene, n-decane, or even ionic liquids (ILs).63,65 The supporting membrane is a microporous hydrophobic polymer membrane composed of poly(vinylidene fluoride) (PVDF), polypropylene (PP), or polytetrafluoroethylene (PTFE), and the organic phase is retained within the membrane’s porous structure by capillary forces.63,66
Figure 2.

Schematic representation of a supported liquid membrane (SLM). Reproduced with permission from ref (63). Copyright 2018 Elsevier.
Although SLMs incorporate nonsustainable and toxic organic diluents and extractants similar to those used in SX, the advantage of SLMs is using these same reagents in lower volumes, reducing their environmental impact and thus making separation based on the use of SLMs a greener alternative to conventional SX.63,66 In addition, SLMs can be used as hollow fiber supported liquid membranes (HFSLMs) and hollow fiber renewal liquid membranes (HFRLMs) (Figure 3), thus offering increased surface area for speeding up extraction and back-extraction.63,67
Figure 3.

A schematic representation of an HFRLM setup. Reproduced with permission from ref (67). Copyright 2022 American Chemical Society.
The extraction of REEs by SLMs, HFSLMs, and HFRLMs uses the same extractants applied in industrial SX of REEs,63,65 involving mainly D2EHPA,67−69 Cyanex 272,70,71 EHEHPA,72 and TBP.71,73 Some studies have used less common extractants such as trioctyl phosphine oxide (TOPO),73N,N-dioctyldiglycolamic acid (DODGAA),74 Cyanex 923,75N,N,N′,N′-tetraoctyldiglycolamide (TODGA),76,77 and dinonyl phenyl phosphoric acid (DNPPA).78
SLMs containing TODGA have been applied to the leaching of REEs from phosphate ores,79 and the separation of Nd3+ from monazite leachate using HFSLMs containing DNPPA.78 SLMs have also been applied to REE recycling from e-waste, specifically for Y3+ extraction from digests of fluorescent lamp phosphors using Cyanex 923,75 and for the recovery of Nd3+, Dy3+, Tb3+, and Pr3+ from REPMs using D2EHPA80 and TODGA.76,77 The extraction of Pr3+ and Nd3+ from REPM e-waste using SLMs containing a synergistic extraction system of 1-hexyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide ([C6MIM][NTf2]), TOPO, and TBP has also been investigated.73 Additionally, the recovery of REEs from secondary sources such as coal fly ash using D2EHPA-containing SLMs has been studied.81
The main disadvantage of SLMs, HFSLMs, and HFRLMs is the instability of the membrane’s liquid organic phase.67 Although the liquid phase is contained inside the porous structure of the hydrophobic polymeric membrane support, it is vulnerable to leaching into the adjacent aqueous phases, as it is only retained within the pores by weak capillary forces.65,66 This leads to the performance of the SLM and HFSLM decreasing over time and the leaching reagents contaminating the aqueous phases.65,66 This loss of performance is partially addressed with HFRLMs, but the leaching of the organic phase remains an issue.67
Despite SLMs having demonstrated promise for REE extraction and the ability to separate REEs, the efficiency of membrane-based methods is limited by the comparatively small surface areas available for extraction and back-extraction, which could have significant ramifications when used in large-scale commercial extraction methods.
3.2. Polymer Particles Supporting and Encapsulating Extractants
The development of effective bead or particle extractant materials is of interest to researchers and industry because of their high specific exposed surface area and the possibility of using separation systems such as fluidized bed reactors and extraction columns. Novel polymer-based particles for the extraction of REEs have been widely investigated, namely, ion imprinted polymer (IIP) particles,82,83 polymer composite nanoparticles,84 solvent impregnated resins (SIRs),85,86 and extractant encapsulating polymer capsules.87 Those which support liquid extractants are deemed by some to have significant advantages by virtue of the large range of extraction reagents, which can be encapsulated and supported inside a range of different base polymers, compared to the limited range of ligands that can be applied to functionalize polymers.85−87 Thus, the encapsulation of a liquid extractant allows for a significant increase in the variety and flexibility of extraction capabilities, greater loading capacities, and simpler and cheaper fabrication.85−87 In most cases, the extractants used are the same as those used in commercial SX, as they are relatively cheap, readily available, and their separation capabilities are well-characterized.85−87
3.2.1. Solvent Impregnated Resins
Solvent impregnated resins (SIRs) were first developed in 1971 by Warshawsky and Grinstead as a means of improving IX methods.86,88 SIRs have the advantage of being very easy to produce because they utilize an already existing polymer framework such as commercially available IX resin beads, which are impregnated with commercially available liquid extractant, loaded up to 50 wt % of the SIR in some cases.85,86 The basis of SIR applications as an alternative to SX is that they have the advantages of being suitable for use in both ion exchange and liquid–liquid extraction methods, possessing the selectivity of the liquid extractants they incorporate, and posing no risk of third phase formation.86,88 SIRs are considered versatile due to the wide range of polymer supports and liquid extractants available, and offer utility by being cheaper to produce for a specific extraction system than it would be to develop new resin materials from scratch, which can be an expensive and time-consuming process.85,86,88
The polymer support usually consists of Amberlite or Lewaitit resin beads, which are composed of styrene (ST)-divinyl benzene (DVB) copolymer or acrylic beads.58,85,86,88,89 A variety of other resin materials are also used, such as Merrifield or Diaion HP2MG (methacrylic ester copolymer) resins,90,91 with custom-synthesized materials having also been reported.92
The polymer supports are washed in solvent, sometimes in combination with acid,90,93 and are then impregnated with an extractant by mixing the beads and liquid extractant in a suitable solvent such as acetone,93 toluene,90 ethanol,91 or methanol,94 which is then evaporated. The resin beads, containing the extractant, are then washed with water.85 Dry methods for preparing SIRs also exist, wherein the extractant is absorbed directly into the beads’ porous structure before the excess extractant is removed by heating.85
The extractants used in SIRs for separation of REEs are those commonly used in industrial SX, namely, D2EHPA,85,86,93−95 EHEHPA,90,93,96,97 Cyanex 272,96,98,99 A336,95 P227,100 Cyanex 923,93 and TODGA.94,101 Recently, Kovalenko et.al. demonstrated that SIRs containing N,N-dioctyl (diphenylphosphoryl) acetamide could be applied to the separation of Nd3+ and Fe3+ in nitric acid leachate of REPMs.102 Extraction using IL impregnated resins has also been investigated, including ILs such as 1-octyl-3-methylimidazolium hexafluorophosphate ([C8MIM][PF6]),91,103 trihexyl(tetradecyl) phosphonium mono-(2-ethylhexyl) 2-ethylhexyl phosphonate ([P66614]-[EHEHP]), and trioctylmethylammonium bis(2,4,4-trimethylpentyl)phosphinate ([N1888][BTMPP]).104 Moreover, new extractants have also been synthesized and applied to SIRs, such as methylcarbamoylmethoxyacetic acid, known as “tripodal broom”,105 (2,3-dimethylbutyl)(2,4,4′-trimethylpentyl)phosphinic acid (INET-3),106 and (2-diphenylphosphoryl)-4-ethylphenoxy)methyl)diphenylphosphine oxide.107
It is well established that extractants leach from SIRs, thus reducing their performance over time and contaminating the adjacent aqueous phases in the same fashion as SLMs do.58,85,86,88 One method for reducing extractant loss is to coat the SIRs with a protective barrier or semipermeable membrane as a means of stabilizing the loaded extractant.85,86,88 This approach has seen limited application with SIRs,88 but has been applied to SIRs fabricated for REE separation, that is, HP2MG beads with EHEHPA applied to the chromatographic separation of La3+ and Ce3+ from EOL car exhaust catalytic converters.90 To stabilize the extractant, the beads were coated with poly(vinyl alcohol) (PVA) and cross-linked by glutaraldehyde or divinyl sulfone (Figure 4), which was found to significantly reduce the leaching of EHEHPA without significantly affecting SIR extraction performance.90 This is a technique that has been used with other SIR systems as a means of stabilizing the impregnated extractant, but this is the first account of it being used in a REE extraction system.88,90
Figure 4.
Schematic representation of the PVA protective coating of SIRs. Reproduced with permission from ref (108). Copyright 2004 Elsevier.
3.2.2. Polymer Capsules
An alternative to using pre-existing polymer supports is to synthesize an inert polymer as part of the fabrication process. Polymer capsules are synthesized porous polymer beads that encapsulate an extractant inside their pores. The main method for fabricating polymer capsules and microcapsules as an extraction medium was first developed in the 1990s by Yoshizawa et al.109−111 using ST and DVB as monomers and 2,2′-azobis-2,4-dimethylvaleronitrile (ADVN) as initiator. For REE extraction and separation, extractants commonly incorporated in polymer capsules are those utilized in industrial SX, for example, polymer capsules containing EHEHPA and in some cases D2EHPA (Table 4).
Table 4. PIMs Applied to REE Extraction and Separationa.
| base polymer | extractant | REs | extraction study | ref |
|---|---|---|---|---|
| CTA | β-diketones | Sc3+, Y3+, La3+, Pr3+, Sm3+, Tb3+, Er3+, Lu3+ | studied the pH effects on transport and flux of selected REEs through the PIM | (139) |
| TODGA | Ce3+ | studied and modeled the extraction and transport of Ce3+ | (140) | |
| Ce3+ | PIMs formed as a hollow fiber and used to transport Ce3+ | (141) | ||
| La3+, Eu3+, Lu3+ | studied the flux and separation factors of selected REEs via transport through the PIM | (142) | ||
| noncyclic carriers | Ce3+ | synthesized noncyclic carriers, which were applied to PIMs used for the transport of Ce3+ | (130) | |
| EHEHPA, Versatic 10 | Sc3+ | studied the separation of Sc3+ from other REEs | (143) | |
| amic carriers | Sc3+ | studied the separation of Sc3+ from other REEs | (144) | |
| Cyphos IL 104 | La3+ | separated Ce3+ via selective transport in the presence of La3+, Cu2+, Co2+, and Ni2+ | (131) | |
| D2EHPA and TBP | La3+, Ce3+ | separated La3+ and Ce3+ via selective transport across the PIM | (145) | |
| PVC | D2EHPA | La3+, Gd3+, Yb3+ | characterized the extraction of selected REEs from sulfuric acid solutions | (129) |
| D2EHPA and Cyanex 272 | Eu3+ | applied a combination of D2EHPA and Cyanex 272 for Eu3+ extraction from nitric acid solutions | (146) | |
| PVDF | [A336][P507] | Lu3+, Yb3+ | PIM applied to the separation of Yb3+ and Lu3+ by membrane transport | (132) |
| Cyphos IL 104 | Lu3+, Yb3+ | PIM applied to the separation of Yb3+ and Lu3+ by membrane transport | (133) | |
| P227 | La3+, Sm3+, Lu3+ | studied and characterized the extraction of selected REEs | (134) | |
| Lu3+ | studied extraction and transport of Lu3+ through the PIM | (135) | ||
| Cyanex 272 | Nd3+, Dy3+, Sm3+ | applied natural deep eutectic solvents to the phase inversion fabrication of PVDF-based PIMs containing Cyanex 272 | (136) | |
| PVDF-EVOH | Cyanex 272 | Lu3+ | fabricated PIMs using new base polymer and characterized extraction of Lu3+ | (137) |
Abbreviations: Cellulose triacetate (CTA), di-2,4,4-trimethylpentylphosphinic acid (Cyanex 272), di-(2-ethylhexyl)phosphoric acid (D2EHPA), di(2-ethylhexyl) phosphinic acid (P227), 2-ethylhexyl hydrogen-2-ethylhexylphoshonate (EHEHPA), N,N,N′,N′-tetraoctyl diglycolamide (TODGA), poly(vinyl chloride) (PVC), poly(vinylidene fluoride) (PVDF), tributyl phosphate (TBP), trialkylmethylammonium di(2-ethylhelxyl) orthophosphinate ([A336][P507]), and trihexyl(tetradecyl)phosphonium bis(2,4,4-trimethylpentyl)phosphinate (Cyphos IL 104).
The polymer capsules can be synthesized using a dispersed phase containing monomers (DVB, ST,112,113 polysulfone (PS),114 or polyethersulfone (PES))115 and initiator ((ADVN) or azobisisobutyronitrile (AIBN)) in a solvent, that is, toluene for St-DVB-based capsules and N-methyl-2-pyrrolidone (NMP) for PS and PES-based capsules.112−115 The dispersed phase can also contain the extractant,116,117 although in some methods the capsules are synthesized prior to loading them with extractant.114 For ST-DVB-based polymer capsules or microcapsules, a reactor containing a continuous phase of deionized water with 2 wt % gum arabic is mixed and heated to 343 K under an inert atmosphere with the dispersed phase added to the reactor in one step.116 For the PS- and PES-based methods, the dispersed phase is added to a room temperature continuous phase of deionized water via droplets (Figure 5) to produce polymer capsules of 1–2 mm diameter.114,115,118
Figure 5.
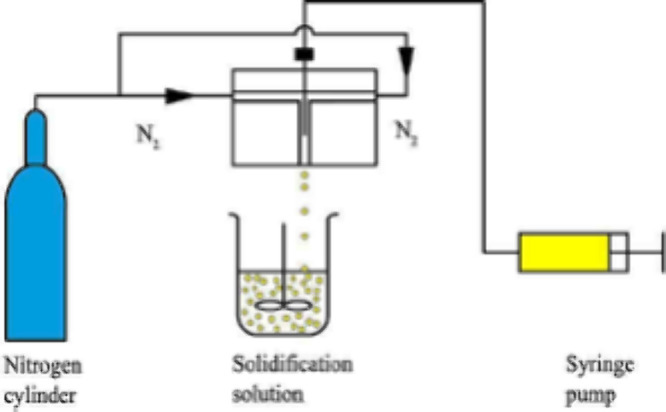
Schematic representation of a microcapsule fabrication apparatus using a coaxial microfluidic method. Reproduced with permission from ref (119). Copyright 2015 Elsevier.
The first report of a polymer capsule fabricated for REE extraction was that by Nishihama et al. in 2002,112 wherein ST-DVB microcapsules containing P227 were fabricated for the separation of Pr3+, Sm3+, Er3+, Nd3+, and Y3+. Since then, several publications have reported this technique and described the development of St-DVB-based microcapsules for the extraction and separation of REEs (Table 4).
Jing et al. and Wang et al. produced polymer capsules using PS as base polymer instead of ST-DVB, which was claimed to exhibit more favorable polymer properties, such as physical robustness, lower toxicity, and high resistance to strong acids, bases, and heated environments.114,118,119 These capsules were developed and characterized not only with EHEHPA as an extractant,118,119 but also with the novel IL trialkylmethylammonium di(2-ethylhelxyl) orthophosphinate ([A336][P507]),114 using La3+, Sm3+, and Er3+ as target REEs.
Yadav et al. produced a series of PES-based microporous capsules, made via a fabrication method similar to that used for PS capsules.115,117,120,121 The justification for using PES over other polymers is that PES is a more environmentally friendly polymer with good chemical, mechanical, and thermal stability.117
To date, D2EHPA is the only extractant, which has been used in PES-based capsule fabrication, with these capsules being applied to extraction and separation of Y3+, La3+, Sm3+, and Dy3+.115,117,120,121 One of the key focal points of the research on D2EHPA-based polymer capsules is the effect of additives (e.g., multiwalled carbon nanotubes (MWCNTs), LiCl, and PVA) on the porous structure of the capsules and the subsequent impact on both the extraction performance and the D2EHPA stability. The structural effects were observed using scanning electron microscopy (SEM) and were correlated to extraction results, with capsules fabricated with combining additives PVA and MWCNTs showing the greatest improvement in extraction performance, attributed to the formation of a finer and more uniform porous structure.115,117,120,121
The polymer capsules discussed here have been successfully applied in batch, column, and shallow bed reactor extraction methods, demonstrating the flexibility of the extraction materials in particle form (Table 3). However, it should be noted that like SIRs and SLMs, the extractants in polymer capsules are only retained by weak capillary forces, leading to extractant leaching,115−117 which reduces the long-term reusability and effectiveness of these materials in addition to contaminating the adjacent aqueous phase.
Table 3. Summary of Polymer Capsule Methods for Extraction and Separation of REEsa.
| extractant | polymer | target species | extraction study | ref |
|---|---|---|---|---|
| [A336][P507] | PS | La3+, Sm3+, Er3+ | developed and characterized polymer capsules for extraction and separation of La3+, Sm3+, and Er3+ | (114) |
| D2EHPA | Eu3+ | capsules packed into a column for the extraction and elution of Eu3+ | (87) | |
| EHEHPA | La3+, Sm3+, Er3+ | developed and characterized polymer capsules for the extraction and separation of La3+, Sm3+, and Er3+ | (118) | |
| La3+, Sm3+, Er3+ | developed and characterized polymer capsules for the extraction and separation of La3+, Sm3+, and Er3+ | (119) | ||
| DVB | La3+, Sm3+, Er3+ | modeled the extraction kinetics of selected REEs | (122) | |
| La3+, Ce3+ | characterized polymer capsule extraction of La3+ and Ce3+ in a shallow bed reactor | (123) | ||
| La3+, Ce3+, Pr3+ | shallow bed reactor extraction and separation of La3+, Ce3+, and Pr3+ | (116) | ||
| Sm3+, Eu3+, Gd3+ | characterized the column separation of Sm3+, Eu3+, and Gd3+ | (113) | ||
| EHEHPA/D2EHPA | Y3+, La3+, Ce3+, Pr3+, Sm3+, Eu3+, Gd3+, Ho3+, Er3+ | characterized REE extraction and applied polymer capsules to column separation | (124) | |
| P227 | ST-DVB | Pr3+, Sm3+, Er3+, Nd3+, Y3+ | characterized polymer capsules and their extraction of selected REEs | (112) |
| D2EHPA | PES | Y3+, La3+, Sm3+ | developed and characterized polymer capsule extraction of Y3+, La3+, and Sm3+ | (115) |
| Dy3+ | developed and characterized polymer capsule extraction of Dy3+ | (121) | ||
| Y3+ | developed and characterized polymer capsule extraction of Y3+ | (117) | ||
| Y3+ | developed and characterized polymer capsule extraction of Y3+ | (120) | ||
| dibenzoylmethane | DVB | Gd3+, Dy3+ | batch characterization of Gd3+ and Dy3+ extraction | (125) |
Abbreviations: Di-(2-ethylhexyl)phosphoric acid (D2EHPA), di(2-ethylhexyl) phosphinic acid (P227), divinylbenzene (DVB), 2-ethylhexyl hydrogen-2-ethylhexylphosphonate (EHEHPA), poly(ether sulfone) (PES), polysulfone (PS), styrene (ST), and trialkylmethylammonium di(2-ethylhelxyl) orthophosphinate ([A336][P507]).
4. Nonporous Polymer Supports for REE Separation
4.1. Polymer Inclusion Membranes
Polymer inclusion membranes (PIMs) are solvent-free liquid membranes, which have proven effective as an alternative to SX and SLM systems.64,126,127 PIMs consist of a base polymer, an extractant, and a plasticizer/modifier (if necessary). Base polymers, such as poly(vinyl chloride) (PVC), poly(vinylidene fluoride) (PVDF), poly(vinylidene fluoride-co-hexafluoropropylene) (PVDF-HFP), or cellulose triacetate (CTA), provide the structure and mechanical stability of the PIM.64,126,127 These base polymers are capable of retaining extractants such as those applied in industrial SX systems (Table 3) within the polymer’s internal nanometer-size channels formed between the entangled polymer chains.128 As such, PIMs have demonstrated superior extractant retention over their SLM counterparts.64,128 In some cases, an additional reagent may be added to the membrane liquid phase, that is, a plasticizer, to improve extractant compatibility with the base polymer, thus ensuring that there is no precipitation or exudation of extractant from the PIM, while a modifier may be required to improve the solubility of the extracted adduct in the membrane liquid phase.64,126,127 PIMs are formed mainly by solvent casting of a solution of the base polymer, extractant, and modifier/plasticizer in a volatile solvent, such as tetrahydrofuran (THF) or dichloromethane (DCM) for PVC- and PVDF-HFP-, or CTA-based PIMs, respectively.64,126,127 This is achieved by dissolving the base polymer, extractant, and any required modifier/plasticizer in the solvent and then pouring the resulting PIM solution into a cast (e.g., a glass ring positioned on a glass plate). Once the solvent has evaporated, the PIM can then be simply peeled off and is ready to be used. A successful PIM is transparent, homogeneous, and mechanically durable (Figure 6).64,126,127
Figure 6.

Image of an optically transparent and homogeneous PIM. Reproduced with permission from ref (64). Copyright 2017 John Wiley and Sons.
Most PIMs fabricated for REE separation are fabricated via the solvent casting method, when using PVC or CTA as their chosen base polymer.129−131 However, more recently there has been significant research into the application of PVDF as a base polymer using N,N′-dimethylacetamide (DMAc) as solvent, whereby PIMs are fabricated by phase inversion.132−137 This produces PIMs with highly porous structures, which can be asymmetric.132 To the authors’ knowledge, the effects of these structural differences compared to the solvent-cast PIMs have not yet been studied in more detail.
Although PIMs are solvent-free during application, the small amount of volatile solvent required in the solvent casting fabrication process is nonreusable and fossil fuel-based and thus unsustainable. However, Carner et al. recently demonstrated that PVC- and PVDF-HFP-based PIMs could be successfully fabricated using the greener solvents 2-methyl THF and ethyl acetate, respectively.138 As shown in Table 4, there is a significant number and wide variety of successful PIM compositions that have been applied to REE extraction, transport, and separation. However, as with SLMs, PIMs are limited by their surface area exposed to the feed and receiving solutions, which can impact the effectiveness of large-scale industrial separation processing. Moreover, to the best of the authors’ knowledge, there are no studies applying PIMs to ore processing or the recycling of REEs from EOL e-waste.
4.2. Micro Polymer Inclusion Beads (μPIBs)
Micro polymer inclusion beads (μPIBs) are a new form of polymer inclusion material consisting of the same base polymers, extractants, and modifiers/plasticizers as those used in PIMs, and are fabricated by a recently developed phase inversion microfluidic method.147−149 The apparatus (Figure 7) used for this method is capable of fabricating μPIBs through the formation of droplets containing a THF solution of the base polymer, extractant, and modifier/plasticizer if required (the “μPIB solution”), which then undergo desolvation in an aqueous environment to precipitate the μPIBs.
Figure 7.

Schematic representation of the μPIBs fabrication apparatus. Reproduced with permission from ref (149). Copyright 2022 Elsevier.
A flow of μPIB solution is first merged with an aqueous NaCl “delivery solution” flow (generally 5–15 wt % NaCl, acidified to 0.1 M H2SO4 when applying acidic extractants148,149), causing it to segment before entering the desolvation column. The high salinity of the delivery solution slows the desolvation of THF, enabling the formation of uniform spherical μPIB solution droplets. Complete desolvation of these droplets then occurs under the lower salinity conditions of the solution in the desolvation column, resulting in the formation of μPIBs. The μPIBs can be fabricated to 50–120 μm diameter by manipulating the μPIB solution concentration, and their composition is similar to that of PIMs, although larger extractant mass fractions can be incorporated into μPIBs.149 Like PIMs, μPIBs are a dense and nonporous hydrophobic material. Thus, unlike the previously mentioned polymer capsules and SIRs, which encapsulate their extractant in their polymer porous structure, the extractant is held inside the μPIBs by the polymer matrix itself,64,128 similarly to PIMs. Hence, the extractant is less prone to leaching from μPIBs compared to the other extractant-supporting polymer beads previously mentioned.
The application of μPIBs to the extraction and separation of REEs has been recently demonstrated by Croft et al. in the effective La3+ extraction from pH 2.5 H2SO4 solutions with PVC and PVDF-HFP-based μPIBs containing the extractant D2EHPA at 60 wt %.149,150 PVC was found to be a superior base polymer compared to PVDF-HFP for D2EHPA μPIBs, demonstrating a higher initial mass transfer of La3+.150 PVC-based μPIBs were then applied to the separation of the critical REEs Nd3+ and Dy3+ from digested REPM e-waste, thus demonstrating the utility of this method for the separation of Nd3+ and Dy3+ from Fe3+, Ni2+, Co2+, and boric acid in the digest, which also involved the selective back-extraction of Nd3+ and Dy3+ into 0.3 and 0.5 M H2SO4 stripping solutions, respectively.151
5. Summary of Future Trends and Opportunities
The role of REEs in modern technology is predicted to increase, particularly due to applications in EVs, wind turbines, screen displays, and lighting, among many others. To satisfy this increasing demand, exploration for economically viable mineral deposits and expanding processing of REEs are taking place. However, the mining and processing of REEs are associated with a heavy environmental impact due to the release of REEs into the environment from mining operations and the reagent-intensive nature of REE processing. Hence, recycling of REEs from EOL e-waste is also becoming an area of interest, as the efficient recycling of REEs would solve the so-called “balance problem”, reduce electronic waste generation, and reduce the environmental impact associated with REE mining and processing. Globally there is an increasing push toward developing methods for the recycling of REES from electronic waste, a trend that is likely to increase in the near future. This presents great opportunities for developed economies to begin implementing e-waste recycling and move toward establishing circular economies.
Although current processing techniques, namely, SX and IX, are effective, they are highly reagent intensive, using large volumes of fossil fuel-based solvents and producing large volumes of highly acidic or basic waste. Thus, to reduce the chemically intensive nature of REE processing, researchers have investigated polymer-based extracting materials for greener REE processing.
These materials have been demonstrated to be viable at the laboratory scale, and in many cases, the associated REE separation processes are chemically analogous to their SX counterpart methods, while being less reagent intensive in their application. These materials have been fabricated as membranes, hollow fibers, and beads/particles. The high versatility of liquid supported systems is demonstrated by the large range of commercial and novel extractants that can be applied and the wide range of base-polymers that can be used as a support. The application of SLMs has been most widely investigated for REE processing, including e-waste recycling, the separation of REEs from secondary sources, and mineral processing. SIRs have also shown promising e-waste recycling applications. Polymer capsule extraction materials have demonstrated effective REE extraction and separation performance with synthetic solutions but have not been applied to real-world solutions to the authors’ best knowledge.
A significant challenge that faces porous polymer supported liquid materials is the stability of the supported liquid phase, which can leach from the polymer support over time and has significant implications for both the long-term reusability of these materials and the environmental impact of their use.
Nonporous polymer materials, namely, PIMs and μPIBs, developed with a wide range of extractants and base-polymers, have seen highly successful applications under laboratory conditions in the separation of REEs from REPM e-waste and have demonstrated high degrees of liquid phase stability. While all materials discussed in this Review have shown effective extraction and separation of REEs, current research efforts are focused on developing materials that are reusable, namely, through reducing loss of liquid extractant and maintaining extraction performance for prolonged periods of time. Future research will also need to apply these materials to the pilot-scale industrial processing of REEs to establish their competitiveness with currently used SX methods.
Acknowledgments
This study has been partially financed by the European Union-NextGenerationEU, through the National Recovery and Resilience Plan of the Republic of Bulgaria, project no. BG-RRP-2.004-0008.
Glossary
List of Abbreviations and Acronyms
- μPIBs
micro polymer inclusion beads
- A336
Aliquat 336
- ADVN
2,2′-azobis-2,4-dimethylvaleronitrile
- AIBN
azobisisobutyronitrile
- CTA
cellulose triacetate
- D2EHPA
di(2-ethyl-hexyl)phosphoric acid
- DCM
dichloromethane
- DMAc
N,N′-dimethyl acetamide
- DNPPA
dinonyl phenyl phosphoric acid
- DODGAA
N,N-dioctyldiglycolamic acid
- DTPA
diethylenetriaminepentaacetic acid
- DVB
divinyl benzene
- EDTA
ethylenediaminetetraacetic acid
- EHEHPA
(2-ethylhexyl)phosphonic acid mono-2-ethylhexyl ester
- EOL
end-of-life
- EV
electric vehicle
- e-waste
electronic waste
- HEDTA
hydroxyethylenediaminetetraacetic acid
- HFRLMs
hollow fiber renewal liquid membranes
- HFSLMs
hollow fiber supported liquid membranes
- IUPAC
International Union of Pure and Applied Chemistry
- IX
ion exchange
- NiMH
nickel metal hydride
- PES
polyethersulfone
- PIM
polymer inclusion membrane
- PP
polypropylene
- PS
polysulfone
- PTFE
polytetrafluoroethylene
- PVDF
poly(vinylidene fluoride)
- PVDF-HFP
poly(vinylidene fluoride-co-hexafluoropropylene)
- REE
rare earth element
- REPM
rare earth permanent magnet
- SIR
solvent impregnated resin
- SLM
supported liquid membrane
- ST
styrene
- SX
solvent extraction
- TBP
tributyl phosphate
- TGA
thermogravimetric analysis
- TODGA
N,N,N′,N′-tetraoctyldiglycolamide
- TOPO
trioctyl phosphine oxide
The authors declare no competing financial interest.
References
- Voncken J. H. L.The Rare Earth Elements: An Introduction; Springer: New York, 2016. [Google Scholar]
- Krishnamurthy N.; Gupta C. K.. Extractive Metallurgy of Rare Earths, 2nd ed.; CRC Press: New York, 2015. [Google Scholar]
- Haxel G. B.; Hedrick J. B.; Orris G. J.; Stauffer P. H.; Hendley J. W.. Rare Earth Elements: Critical Resources for High Technology, 2002; http://pubs.er.usgs.gov/publication/fs08702.
- Balaram V. Rare earth elements: A review of applications, occurrence, exploration, analysis, recycling, and environmental impact. Geosci. Front. 2019, 10 (4), 1285–1303. 10.1016/j.gsf.2018.12.005. [DOI] [Google Scholar]
- Li J.; Peng K.; Wang P.; Zhang N.; Feng K.; Guan D.; Meng J.; Wei W.; Yang Q. Critical Rare-Earth Elements Mismatch Global Wind-Power Ambitions. One Earth 2020, 3 (1), 116–125. 10.1016/j.oneear.2020.06.009. [DOI] [Google Scholar]
- Goodenough K. M.; Wall F.; Merriman D. The Rare Earth Elements: Demand, Global Resources, and Challenges for Resourcing Future Generations. Nat. Resour. Res. 2018, 27 (2), 201–216. 10.1007/s11053-017-9336-5. [DOI] [Google Scholar]
- Ault T.; Krahn S.; Croff A. Radiological impacts and regulation of rare earth elements in non-nuclear energy production. Energies 2015, 8 (3), 2066–2081. 10.3390/en8032066. [DOI] [Google Scholar]
- Liu G.Electronic Energy Level Structure. In Spectroscopic Properties of Rare Earths in Optical Materials; Hull R., Parisi J., Osgood R. M., Warlimont H., Liu G., Jacquier B., Eds.; Springer: Berlin Heidelberg, 2005; pp 1–94. [Google Scholar]
- Skomski R.; Sellmyer D. J. Anisotropy of rare-earth magnets. J. Rare Earths 2009, 27 (4), 675–679. 10.1016/S1002-0721(08)60314-2. [DOI] [Google Scholar]
- Tang J.; Zhang P.. A Basis for Lanthanide Single-Molecule Magnets. Lanthanide Single Molecule Magnets; Springer: Berlin Heidelberg, 2015; pp 1–39. [Google Scholar]
- Coey J. M. D. Perspective and Prospects for Rare Earth Permanent Magnets. Engineering 2020, 6 (2), 119–131. 10.1016/j.eng.2018.11.034. [DOI] [Google Scholar]
- Yang Y.; Walton A.; Sheridan R.; Güth K.; Gauß R.; Gutfleisch O.; Buchert M.; Steenari B.-M.; Van Gerven T.; Jones P. T.; Binnemans K. REE Recovery from End-of-Life NdFeB Permanent Magnet Scrap: A Critical Review. J. Sustain. Metall. 2017, 3 (1), 122–149. 10.1007/s40831-016-0090-4. [DOI] [Google Scholar]
- Huleatt M. B.Australian Resource Reviews: Rare Earth Elements 2019. Geoscience Australia, Canberra, 2019; p 130434.
- Van Gosen B. S.; Verplanck P. L.; Seal R. R. II; Long K. R.; Gambogi J.. Rare-Earth Elements; Reston, VA, 2017; http://pubs.er.usgs.gov/publication/pp1802O. [Google Scholar]
- Binnemans K.; Jones P. T.; Blanpain B.; Van Gerven T.; Yang Y.; Walton A.; Buchert M. Recycling of rare earths: a critical review. J. Clean. Prod. 2013, 51, 1–22. 10.1016/j.jclepro.2012.12.037. [DOI] [Google Scholar]
- Chen Y.; Zheng B. What Happens after the Rare Earth Crisis: A Systematic Literature Review. Sustainability 2019, 11 (5), 1288. 10.3390/su11051288. [DOI] [Google Scholar]
- U.S. Geological Survey (USGS) . Mineral Commodity Summaries 2013; Reston, VA, 2013. [Google Scholar]
- Barakos G.; Gutzmer J.; Mischo H. Strategic evaluations and mining process optimization towards a strong global REE supply chain. J. Sustain. Min. 2016, 15 (1), 26–35. 10.1016/j.jsm.2016.05.002. [DOI] [Google Scholar]
- U.S. Geological Survey (USGS) . Mineral Commodity Summaries 2021; Reston, VA, 2021. [Google Scholar]
- U.S. Geological Survey (USGS) . Mineral Commodity Summaries 2022; Reston, VA, 2022. [Google Scholar]
- Dutta T.; Kim K.-H.; Uchimiya M.; Kwon E. E.; Jeon B.-H.; Deep A.; Yun S.-T. Global demand for rare earth resources and strategies for green mining. Environ. Res. 2016, 150, 182–190. 10.1016/j.envres.2016.05.052. [DOI] [PubMed] [Google Scholar]
- Xie F.; Zhang T. A.; Dreisinger D.; Doyle F. A critical review on solvent extraction of rare earths from aqueous solutions. Miner. Eng. 2014, 56, 10–28. 10.1016/j.mineng.2013.10.021. [DOI] [Google Scholar]
- Spandler C.; Slezak P.; Nazari-Dehkordi T. Tectonic significance of Australian rare earth element deposits. Earth-Sci. Rev. 2020, 207, 103219. 10.1016/j.earscirev.2020.103219. [DOI] [Google Scholar]
- Breanna G.; Caroline L.; Kate M.. Outlook for Selected Critical Minerals: Australia 2021; Australian Government, Australia, 2021; https://www.industry.gov.au/sites/default/files/2021-06/outlook_for_selected_critical_minerals_in_australia_2021_report.pdf.
- LKAB. Europe’s largest deposit of rare earth metals is located in the Kiruna area, 2023; https://lkab.com/en/press/europes-largest-deposit-of-rare-earth-metals-is-located-in-the-kiruna-area/ (accessed 14/2/23).
- Paulick H.; Machacek E. The global rare earth element exploration boom: An analysis of resources outside of China and discussion of development perspectives. Resour. Policy 2017, 52, 134–153. 10.1016/j.resourpol.2017.02.002. [DOI] [Google Scholar]
- Ramos S. J.; Dinali G. S.; Oliveira C.; Martins G. C.; Moreira C. G.; Siqueira J. O.; Guilherme L. R. G. Rare Earth Elements in the Soil Environment. Curr. Pollut. Rep. 2016, 2 (1), 28–50. 10.1007/s40726-016-0026-4. [DOI] [Google Scholar]
- Yin X.; Martineau C.; Demers I.; Basiliko N.; Fenton N. J. The potential environmental risks associated with the development of rare earth element production in Canada. Environ. Rev. 2021, 29 (3), 354–377. 10.1139/er-2020-0115. [DOI] [Google Scholar]
- Adeel M.; Lee J. Y.; Zain M.; Rizwan M.; Nawab A.; Ahmad M. A.; Shafiq M.; Yi H.; Jilani G.; Javed R.; et al. Cryptic footprints of rare earth elements on natural resources and living organisms. Environ. Int. 2019, 127, 785–800. 10.1016/j.envint.2019.03.022. [DOI] [PubMed] [Google Scholar]
- Pagano G.; Guida M.; Tommasi F.; Oral R. Health effects and toxicity mechanisms of rare earth elements—Knowledge gaps and research prospects. Ecotoxicol. Environ. Saf. 2015, 115, 40–48. 10.1016/j.ecoenv.2015.01.030. [DOI] [PubMed] [Google Scholar]
- Edahbi M.; Plante B.; Benzaazoua M. Environmental challenges and identification of the knowledge gaps associated with REE mine wastes management. J. Clean. Prod. 2019, 212, 1232–1241. 10.1016/j.jclepro.2018.11.228. [DOI] [Google Scholar]
- Dushyantha N.; Batapola N.; Ilankoon I. M. S. K.; Rohitha S.; Premasiri R.; Abeysinghe B.; Ratnayake N.; Dissanayake K. The story of rare earth elements (REEs): Occurrences, global distribution, genesis, geology, mineralogy and global production. Ore Geol. Rev. 2020, 122, 103521. 10.1016/j.oregeorev.2020.103521. [DOI] [Google Scholar]
- Rim K.-T. Effects of rare earth elements on the environment and human health: A literature review. Toxicology and Environmental Health Sciences 2016, 8 (3), 189–200. 10.1007/s13530-016-0276-y. [DOI] [Google Scholar]
- Thomas P. J.; Carpenter D.; Boutin C.; Allison J. E. Rare earth elements (REEs): Effects on germination and growth of selected crop and native plant species. Chemosphere 2014, 96, 57–66. 10.1016/j.chemosphere.2013.07.020. [DOI] [PubMed] [Google Scholar]
- Costis S.; Mueller K. K.; Coudert L.; Neculita C. M.; Reynier N.; Blais J.-F. Recovery potential of rare earth elements from mining and industrial residues: A review and cases studies. J. Geochem. Explor. 2021, 221, 106699. 10.1016/j.gexplo.2020.106699. [DOI] [Google Scholar]
- Jyothi R. K.; Thenepalli T.; Ahn J. W.; Parhi P. K.; Chung K. W.; Lee J.-Y. Review of rare earth elements recovery from secondary resources for clean energy technologies: Grand opportunities to create wealth from waste. J. Clean. Prod. 2020, 267, 122048. 10.1016/j.jclepro.2020.122048. [DOI] [Google Scholar]
- Sethurajan M.; van Hullebusch E. D.; Fontana D.; Akcil A.; Deveci H.; Batinic B.; Leal J. P.; Gasche T. A.; Ali Kucuker M.; Kuchta K.; et al. Recent advances on hydrometallurgical recovery of critical and precious elements from end of life electronic wastes - a review. Crit. Rev. Environ. Sci. Technol. 2019, 49 (3), 212–275. 10.1080/10643389.2018.1540760. [DOI] [Google Scholar]
- Mudali U. K.; Patil M.; Saravanabhavan R.; Saraswat V. K. Review on E-waste Recycling: Part II—Technologies for Recovery of Rare Earth Metals. Trans. Indian Natl. Acad. Eng. 2021, 6 (3), 613–631. 10.1007/s41403-021-00231-0. [DOI] [Google Scholar]
- Ohene Opare E.; Mirkouei A.. Environmental and Economic Assessment of a Portable E-Waste Recycling and Rare Earth Elements Recovery Process. ASME 2021 International Design Engineering Technical Conferences and Computers and Information in Engineering Conference; 26th Design for Manufacturing and the Life Cycle Conference (DFMLC), 2021; Vol. 5.
- Rene E. R.; Sethurajan M.; Kumar Ponnusamy V.; Kumar G.; Bao Dung T. N.; Brindhadevi K.; Pugazhendhi A. Electronic waste generation, recycling and resource recovery: Technological perspectives and trends. J. Hazard. Mater. 2021, 416, 125664. 10.1016/j.jhazmat.2021.125664. [DOI] [PubMed] [Google Scholar]
- Akcil A.; Ibrahim Y. A.; Meshram P.; Panda S.; Abhilash Hydrometallurgical recycling strategies for recovery of rare earth elements from consumer electronic scraps: a review. J. Chem. Technol. Biotechnol. 2021, 96 (7), 1785–1797. 10.1002/jctb.6739. [DOI] [Google Scholar]
- Binnemans K.; Jones P. T.; Van Acker K.; Blanpain B.; Mishra B.; Apelian D. Rare-Earth Economics: The Balance Problem. JOM 2013, 65 (7), 846–848. 10.1007/s11837-013-0639-7. [DOI] [Google Scholar]
- Islam M. T.; Huda N. Assessing the recycling potential of “unregulated” e-waste in Australia. Resour. Conserv. Recycl. 2020, 152, 104526. 10.1016/j.resconrec.2019.104526. [DOI] [Google Scholar]
- Jowitt S. M.; Werner T. T.; Weng Z.; Mudd G. M. Recycling of the rare earth elements. Curr. Opin. Green Sustain. Chem. 2018, 13, 1–7. 10.1016/j.cogsc.2018.02.008. [DOI] [Google Scholar]
- Omodara L.; Pitkäaho S.; Turpeinen E.-M.; Saavalainen P.; Oravisjärvi K.; Keiski R. L. Recycling and substitution of light rare earth elements, cerium, lanthanum, neodymium, and praseodymium from end-of-life applications - A review. J. Clean. Prod. 2019, 236, 117573. 10.1016/j.jclepro.2019.07.048. [DOI] [Google Scholar]
- Innocenzi V.; De Michelis I.; Kopacek B.; Vegliò F. Yttrium recovery from primary and secondary sources: A review of main hydrometallurgical processes. Waste Manag. Res. 2014, 34 (7), 1237–1250. 10.1016/j.wasman.2014.02.010. [DOI] [PubMed] [Google Scholar]
- Sprecher B.; Kleijn R.; Kramer G. J. Recycling Potential of Neodymium: The Case of Computer Hard Disk Drives. Environ. Sci. Technol. 2014, 48 (16), 9506–9513. 10.1021/es501572z. [DOI] [PubMed] [Google Scholar]
- Lee C.-H.; Chen Y.-J.; Liao C.-H.; Popuri S. R.; Tsai S.-L.; Hung C.-E. Selective Leaching Process for Neodymium Recovery from Scrap Nd-Fe-B Magnet. Metall. Mater. Trans. A 2013, 44 (13), 5825–5833. 10.1007/s11661-013-1924-3. [DOI] [Google Scholar]
- Innocenzi V.; Vegliò F. Recovery of rare earths and base metals from spent nickel-metal hydride batteries by sequential sulphuric acid leaching and selective precipitations. J. Power Sources 2012, 211, 184–191. 10.1016/j.jpowsour.2012.03.064. [DOI] [Google Scholar]
- Porvali A.; Wilson B. P.; Lundström M. Lanthanide-alkali double sulfate precipitation from strong sulfuric acid NiMH battery waste leachate. Waste Manag. Res. 2018, 71, 381–389. 10.1016/j.wasman.2017.10.031. [DOI] [PubMed] [Google Scholar]
- Yurramendi L.; Gijsemans L.; Forte F.; Aldana J. L.; del Río C.; Binnemans K. Enhancing rare-earth recovery from lamp phosphor waste. Hydrometallurgy 2019, 187, 38–44. 10.1016/j.hydromet.2019.04.030. [DOI] [Google Scholar]
- Yang F.; Kubota F.; Baba Y.; Kamiya N.; Goto M. Selective extraction and recovery of rare earth metals from phosphor powders in waste fluorescent lamps using an ionic liquid system. J. Hazard. Mater. 2013, 254–255, 79–88. 10.1016/j.jhazmat.2013.03.026. [DOI] [PubMed] [Google Scholar]
- Innocenzi V.; De Michelis I.; Vegliò F. Design and construction of an industrial mobile plant for WEEE treatment: Investigation on the treatment of fluorescent powders and economic evaluation compared to other e-wastes. J. Taiwan Inst. Chem. Eng. 2017, 80, 769–778. 10.1016/j.jtice.2017.09.019. [DOI] [Google Scholar]
- Tan Q.; Li J.; Zeng X. Rare Earth Elements Recovery from Waste Fluorescent Lamps: A Review. Crit. Rev. Environ. Sci. Technol. 2015, 45 (7), 749–776. 10.1080/10643389.2014.900240. [DOI] [Google Scholar]
- Qi D.Chapter 2 - Extractants Used in Solvent Extraction-Separation of Rare Earths: Extraction Mechanism, Properties, and Features. In Hydrometallurgy of Rare Earths; Qi D., Ed.; Elsevier: New York, 2018; pp 187–389. [Google Scholar]
- Preez A. C. d.; S Preston J. The solvent extraction of rare-earth metals by carboxylic acids. Solvent Extr. Ion Exch. 1992, 10 (2), 207–230. 10.1080/07366299208918101. [DOI] [Google Scholar]
- Jha M. K.; Kumari A.; Panda R.; Rajesh Kumar J.; Yoo K.; Lee J. Y. Review on hydrometallurgical recovery of rare earth metals. Hydrometallurgy 2016, 165, 2–26. 10.1016/j.hydromet.2016.01.035. [DOI] [Google Scholar]
- El Ouardi Y.; Virolainen S.; Massima Mouele E. S.; Laatikainen M.; Repo E.; Laatikainen K. The recent progress of ion exchange for the separation of rare earths from secondary resources - A review. Hydrometallurgy 2023, 218, 106047. 10.1016/j.hydromet.2023.106047. [DOI] [Google Scholar]
- Kaur H.; Agrawal Y. K. Functionalization of XAD-4 resin for the separation of lanthanides using chelation ion exchange liquid chromatography. React. Funct. Polym. 2005, 65 (3), 277–283. 10.1016/j.reactfunctpolym.2005.06.010. [DOI] [Google Scholar]
- Krättli M.; Müller-Späth T.; Ulmer N.; Ströhlein G.; Morbidelli M. Separation of Lanthanides by Continuous Chromatography. Ind. Eng. Chem. Res. 2013, 52 (26), 8880–8886. 10.1021/ie3031482. [DOI] [Google Scholar]
- Vahidi E.; Zhao F. Environmental life cycle assessment on the separation of rare earth oxides through solvent extraction. J. Environ. Manag. 2017, 203, 255–263. 10.1016/j.jenvman.2017.07.076. [DOI] [PubMed] [Google Scholar]
- Li Z.; Smith K. H.; Stevens G. W. The use of environmentally sustainable bio-derived solvents in solvent extraction applications—A review. Chin. J. Chem. Eng. 2016, 24 (2), 215–220. 10.1016/j.cjche.2015.07.021. [DOI] [Google Scholar]
- Chen L.; Wu Y.; Dong H.; Meng M.; Li C.; Yan Y.; Chen J. An overview on membrane strategies for rare earths extraction and separation. Sep. Purif. Technol. 2018, 197, 70–85. 10.1016/j.seppur.2017.12.053. [DOI] [Google Scholar]
- Kolev S. D.; Almeida M. I. G. S.; Cattrall R. W.. Polymer inclusion membranes. In Handbook of Smart Materials in Analytical Chemistry; Guardia M. d. l., Esteve-Turrillas F. A., Eds.; John Wiley & Sons Ltd.: New York, 2019; pp 439–461. [Google Scholar]
- Amini M.; Rahbar-Kelishami A.; Alipour M.; Vahidi O. Supported Liquid Membrane in Metal Ion Separation: An Overview. J. Membr. Sci. Res. 2018, 4, 121–135. [Google Scholar]
- Martínez J.; Rodríguez Varela R.; Forsberg K.; Rasmuson Å. Factors influencing separation selectivity of rare earth elements in flat sheet supported liquid membranes. Chem. Eng. Sci. 2018, 191, 134–155. 10.1016/j.ces.2018.06.018. [DOI] [Google Scholar]
- Alemrajabi M.; Ricknell J.; Samak S.; Rodriguez Varela R.; Martinez J.; Hedman F.; Forsberg K.; Rasmuson Å. C. Separation of Rare-Earth Elements Using Supported Liquid Membrane Extraction in Pilot Scale. Ind. Eng. Chem. Res. 2022, 61 (50), 18475–18491. 10.1021/acs.iecr.2c03268. [DOI] [Google Scholar]
- Middleton A.; Hsu-Kim H. Separation of Rare-Earth Elements by Supported Liquid Membranes: Impacts of Soluble Iron, Aluminum, and pH in Low-Grade Feedstocks. ACS ES&T Eng. 2023, 3 (8), 1197–1204. 10.1021/acsestengg.3c00060. [DOI] [Google Scholar]
- Pei L.; Wang L.; Yu G. Study on a novel flat renewal supported liquid membrane with D2EHPA and hydrogen nitrate for neodymium extraction. J. Rare Earths 2012, 30 (1), 63–68. 10.1016/S1002-0721(10)60640-0. [DOI] [Google Scholar]
- Wannachod P.; Chaturabul S.; Pancharoen U.; Lothongkum A. W.; Patthaveekongka W. The effective recovery of praseodymium from mixed rare earths via a hollow fiber supported liquid membrane and its mass transfer related. J. Alloys Compd. 2011, 509 (2), 354–361. 10.1016/j.jallcom.2010.09.025. [DOI] [Google Scholar]
- Ramakul P.; Supajaroon T.; Prapasawat T.; Pancharoen U.; Lothongkum A. W. Synergistic separation of yttrium ions in lanthanide series from rare earths mixture via hollow fiber supported liquid membrane. J. Ind. Eng. Chem. 2009, 15 (2), 224–228. 10.1016/j.jiec.2008.09.011. [DOI] [Google Scholar]
- Wannachod T.; Leepipatpiboon N.; Pancharoen U.; Nootong K. Separation and mass transport of Nd(III) from mixed rare earths via hollow fiber supported liquid membrane: Experiment and modeling. Chem. Eng. J. 2014, 248, 158–167. 10.1016/j.cej.2014.03.024. [DOI] [Google Scholar]
- Asadollahzadeh M.; Torkaman R.; Torab-Mostaedi M.; Hemmati A.; Ghaemi A. Efficient recovery of neodymium and praseodymium from NdFeB magnet-leaching phase with and without ionic liquid as a carrier in the supported liquid membrane. Chem. pap. 2020, 74 (12), 4193–4201. 10.1007/s11696-020-01240-z. [DOI] [Google Scholar]
- Baba Y.; Kubota F.; Kamiya N.; Goto M. Selective Recovery of Dysprosium and Neodymium Ions by a Supported Liquid Membrane Based on Ionic Liquids. Solvent Extr. Res. Dev., Jpn. 2011, 18, 193–198. 10.15261/serdj.18.193. [DOI] [Google Scholar]
- Pavón S.; Fortuny A.; Coll M. T.; Sastre A. M. Improved rare earth elements recovery from fluorescent lamp wastes applying supported liquid membranes to the leaching solutions. Sep. Purif. Technol. 2019, 224, 332–339. 10.1016/j.seppur.2019.05.015. [DOI] [Google Scholar]
- Deshmane V. G.; Islam S. Z.; Bhave R. R. Selective Recovery of Rare Earth Elements from a Wide Range of E-Waste and Process Scalability of Membrane Solvent Extraction. Environ. Sci. Technol. 2020, 54 (1), 550–558. 10.1021/acs.est.9b05695. [DOI] [PubMed] [Google Scholar]
- Kim D.; Powell L.; Delmau L. H.; Peterson E. S.; Herchenroeder J.; Bhave R. R. A supported liquid membrane system for the selective recovery of rare earth elements from neodymium-based permanent magnets. Sep. Sci. Technol. 2016, 51 (10), 1716–1726. 10.1080/01496395.2016.1171782. [DOI] [Google Scholar]
- Wannachod T.; Mohdee V.; Suren S.; Ramakul P.; Pancharoen U.; Nootong K. The separation of Nd(III) from mixed rare earth via hollow fiber supported liquid membrane and mass transfer analysis. J. Ind. Eng. Chem. 2015, 26, 214–217. 10.1016/j.jiec.2014.11.032. [DOI] [Google Scholar]
- Xu D.; Shah Z.; Sun G.; Peng X.; Cui Y. Recovery of rare earths from phosphate ores through supported liquid membrane using N,N,N′,N′-tetraoctyl diglycol amide. Miner. Eng. 2019, 139, 105861. 10.1016/j.mineng.2019.105861. [DOI] [Google Scholar]
- Ni’am A. C.; Wang Y.-F.; Chen S.-W.; Chang G.-M.; You S.-J. Simultaneous recovery of rare earth elements from waste permanent magnets (WPMs) leach liquor by solvent extraction and hollow fiber supported liquid membrane. Chem. Eng. Process.: Process Intensif. 2020, 148, 107831. 10.1016/j.cep.2020.107831. [DOI] [Google Scholar]
- Smith R. C.; Taggart R. K.; Hower J. C.; Wiesner M. R.; Hsu-Kim H. Selective Recovery of Rare Earth Elements from Coal Fly Ash Leachates Using Liquid Membrane Processes. Environ. Sci. Technol. 2019, 53 (8), 4490–4499. 10.1021/acs.est.9b00539. [DOI] [PubMed] [Google Scholar]
- Pyrzynska K.; Kubiak A.; Wysocka I. Application of solid phase extraction procedures for rare earth elements determination in environmental samples. Talanta 2016, 154, 15–22. 10.1016/j.talanta.2016.03.022. [DOI] [PubMed] [Google Scholar]
- Fu J.; Chen L.; Li J.; Zhang Z. Current status and challenges of ion imprinting. J. Mater. Chem. A 2015, 3 (26), 13598–13627. 10.1039/C5TA02421H. [DOI] [Google Scholar]
- Mohammadnezhad G.; Keikavousi Behbahan A. Polymer matrix nanocomposites for heavy metal adsorption: a review. J. Iran. Chem. Soc. 2020, 17 (6), 1259–1281. 10.1007/s13738-020-01864-8. [DOI] [Google Scholar]
- Bao S.; Tang Y.; Zhang Y.; Liang L. Recovery and Separation of Metal Ions from Aqueous Solutions by Solvent-Impregnated Resins. Chem. Eng. Technol. 2016, 39 (8), 1377–1392. 10.1002/ceat.201500324. [DOI] [Google Scholar]
- Kabay N.; Cortina J. L.; Trochimczuk A.; Streat M. Solvent-impregnated resins (SIRs) - Methods of preparation and their applications. React. Funct. Polym. 2010, 70 (8), 484–496. 10.1016/j.reactfunctpolym.2010.01.005. [DOI] [Google Scholar]
- Momen M. A.; Dietz M. L. High-capacity extraction chromatographic materials based on polysulfone microcapsules for the separation and preconcentration of lanthanides from aqueous solution. Talanta 2019, 197, 612–621. 10.1016/j.talanta.2019.01.026. [DOI] [PubMed] [Google Scholar]
- Tamang A. M.; Singh N.; Chandraker S. K.; Ghosh M. K. Solvent impregnated resin a potential alternative material for separation dyes, metal and phenolic compounds: A review. Curr. Res. Green Sustain. Chem. 2022, 5, 100232. 10.1016/j.crgsc.2021.100232. [DOI] [Google Scholar]
- Hermassi M.; Granados M.; Valderrama C.; Ayora C.; Cortina J. L. Recovery of Rare Earth Elements from acidic mine waters by integration of a selective chelating ion-exchanger and a solvent impregnated resin. J. Environ. Chem. Eng. 2021, 9 (5), 105906. 10.1016/j.jece.2021.105906. [DOI] [Google Scholar]
- Nishihama S.; Kohata K.; Yoshizuka K. Separation of lanthanum and cerium using a coated solvent-impregnated resin. Sep. Purif. Technol. 2013, 118, 511–518. 10.1016/j.seppur.2013.07.047. [DOI] [Google Scholar]
- Zhu L.; Liu Y.; Chen J.; Liu W. Extraction of scandium (III) using ionic liquids functionalized solvent impregnated resins. J. Appl. Polym. Sci. 2011, 120 (6), 3284–3290. 10.1002/app.33501. [DOI] [Google Scholar]
- Zhang W.; Ye G.; Chen J. TRPO Impregnated Levextrel Resin: Synthesis and Extraction Behavior of Zr (IV) and Nd (III) Ions. Sep. Sci. Technol. 2012, 48 (2), 263–271. 10.1080/01496395.2012.675002. [DOI] [Google Scholar]
- Lee G. S.; Uchikoshi M.; Mimura K.; Isshiki M. Distribution coefficients of La, Ce, Pr, Nd, and Sm on Cyanex 923-, D2EHPA-, and PC88A-impregnated resins. Sep. Purif. Technol. 2009, 67 (1), 79–85. 10.1016/j.seppur.2009.03.033. [DOI] [Google Scholar]
- Horwitz E. P.; McAlister D. R.; Bond A. H.; Barrans R. E. Novel Extraction of Chromatographic Resins Based on Tetraalkyldiglycolamides: Characterization and Potential Applications. Solvent Extr. Ion Exch. 2005, 23 (3), 319–344. 10.1081/SEI-200049898. [DOI] [Google Scholar]
- El-Sofany E. A. Removal of lanthanum and gadolinium from nitrate medium using Aliquat-336 impregnated onto Amberlite XAD-4. J. Hazard. Mater. 2008, 153 (3), 948–954. 10.1016/j.jhazmat.2007.09.046. [DOI] [PubMed] [Google Scholar]
- Liao C.-f.; Jiao Y.-f.; Liang Y.; Jiang P.-g.; Nie H.-p. Adsorption-extraction mechanism of heavy rare earth by Cyanex272-P507 impregnated resin. Trans. Nonferrous Met. Soc. China 2010, 20 (8), 1511–1516. 10.1016/S1003-6326(09)60330-7. [DOI] [Google Scholar]
- Wakui Y.; Matsunaga H.; Suzuki T. M. Distribution of Rare Earth Elements between (2-Ethylhexyl Hydrogen 2-Ethylhexylphosphonate)-Impregnated Resin and Acid Aqueous Solution. Anal. Sci. 1988, 4 (3), 325–327. 10.2116/analsci.4.325. [DOI] [Google Scholar]
- İnan S.; Tel H.; Sert Ş.; Çetinkaya B.; Sengül S.; Özkan B.; Altaş Y. Extraction and separation studies of rare earth elements using Cyanex 272 impregnated Amberlite XAD-7 resin. Hydrometallurgy 2018, 181, 156–163. 10.1016/j.hydromet.2018.09.005. [DOI] [Google Scholar]
- Wang Z.; Ma G.; Li D. Extraction and separation of heavy rare earth(III) with extraction resin containing di(2,4,4-trimethyl pentyl) phosphinic acid (cyanex 272). Solvent Extr. Ion Exch. 1998, 16 (3), 813–828. 10.1080/07366299808934554. [DOI] [Google Scholar]
- Yang B.; Wu S.-Z.; Liu X.-Y.; Yan Z.-X.; Liu Y.-X.; Li Q.-S.; Yu F.-S.; Wang J.-L. Solid-phase extraction and separation of heavy rare earths from chloride media using P227-impregnated resins. Rare Metals 2021, 40 (9), 2633–2644. 10.1007/s12598-020-01549-4. [DOI] [Google Scholar]
- Yadav A. G.; Gujar R. B.; Valsala T. P.; Sathe D. B.; Bhatt R. B.; Mohapatra P. K. A comparative study on the uptake of lanthanides from acidic feeds using extraction chromatography resins containing N,N,N’,N’-tetra-n-alkyl diglycolamides with varying alkyl chain lengths in an ionic liquid. J. Chromatogr. A 2023, 1687, 463683. 10.1016/j.chroma.2022.463683. [DOI] [PubMed] [Google Scholar]
- Kovalenko O. V.; Baulin V. E.; Shulga Y. M.; Baulin D. V.; Gutsev G. L.; Tsivadze A. Y. Composite Resins Impregnated by Phosphorus Organic Extractants for Separation of Rare Earth Elements from Nitrate-Based Leachate of Permanent Magnets. Materials 2023, 16 (19), 6614. 10.3390/ma16196614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X.; Peng B.; Ji Y.; Chen J.; Li D. The solid-liquid extraction of yttrium from rare earths by solvent (ionic liquid) impreganated resin coupled with complexing method. Sep. Purif. Technol. 2008, 63 (1), 61–68. 10.1016/j.seppur.2008.03.038. [DOI] [Google Scholar]
- Zhao Z.; Sun X.; Dong Y.; Wang Y. Synergistic Effect of Acid-Base Coupling Bifunctional Ionic Liquids in Impregnated Resin for Rare Earth Adsorption. ACS Sustain. Chem. Eng. 2016, 4 (2), 616–624. 10.1021/acssuschemeng.5b01253. [DOI] [Google Scholar]
- Nakamura T.; Ohto K.; Inoue K.; Oshima T. Adsorption of rare earth metal ions on novel tripodal broom methylcarbamoylmethoxyacetic acid impregnated resin. Ars Separatoria Acta 2006, 27–36. [Google Scholar]
- Wang J.; Xie M.; Ma J.; Wang H.; Xu S. Extractant (2,3-dimethylbutyl)(2,4,4′-trimethylpentyl)phosphinic acid (INET-3) impregnated onto XAD-16 and its extraction and separation performance for heavy rare earths from chloride media. J. Rare Earths 2017, 35 (12), 1239–1247. 10.1016/j.jre.2017.07.003. [DOI] [Google Scholar]
- Kovalenko O.; Baulin V.; Baulin D.; Tsivadze A. Solvent-Impregnated Resins Based on the Mixture of (2-Diphenylphosphoryl)-4-ethylphenoxy)methyl) diphenylphosphine Oxide and Ionic Liquid for Nd(III) Recovery from Nitric Acid Media. Molecules 2021, 26 (9), 2440. 10.3390/molecules26092440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trochimczuk A. W.; Kabay N.; Arda M.; Streat M. Stabilization of solvent impregnated resins (SIRs) by coating with water soluble polymers and chemical crosslinking. React. Funct. Polym. 2004, 59 (1), 1–7. 10.1016/j.reactfunctpolym.2003.12.011. [DOI] [Google Scholar]
- Yoshizawa H.; Fujikubo K.; Uemura Y.; Kawano Y.; Kondo K.; Hatate Y. Preparation of Divinylbenzene Homopolymeric Microcapsules with Highly Porous Membranes by in situ Polymerization with Solvent Evaporation. J. Chem. Eng. Jpn. 1995, 28 (1), 78–84. 10.1252/jcej.28.78. [DOI] [Google Scholar]
- Yoshizawa H.; Uemura Y.; Kawano Y.; Hatate Y. Preparation and Extraction Properties of Microcapsules Containing Tri-n-Octyl Amine as Core Material. J. Chem. Eng. Jpn. 1993, 26 (2), 198–204. 10.1252/jcej.26.198. [DOI] [Google Scholar]
- Yoshizawa H.; Uemura Y.; Kawano Y.; Hatate Y. Regeneration of Styrene-Divinylbenzene Copolymer Microcapsules Containing Tri-n-Octyl Amine. J. Chem. Eng. Jpn. 1993, 26 (6), 692–697. 10.1252/jcej.26.692. [DOI] [Google Scholar]
- Nishihama S.; Sakaguchi N.; Hirai T.; Komasawa I. Extraction and separation of rare earth metals using microcapsules containing bis(2-ethylhexyl)phosphinic acid. Hydrometallurgy 2002, 64 (1), 35–42. 10.1016/S0304-386X(02)00011-7. [DOI] [Google Scholar]
- Kondo K.; Oguri M.; Matsumoto M. Novel separation of samarium, europium and gadolinium using a column packed with microcapsules containing 2-ethylhexylphosphonic acid mono-2-ethylhexyl ester. Chem. Eng. Trans. 2013, 32, 919–924. [Google Scholar]
- Wang Y.; Wang Y.; Jing Y.; Chen J.; Liu Y. Microcapsules containing ionic liquid [A336][P507] for La3+/Sm3+/Er3+ recovery from dilute aqueous solution. J. Rare Earths 2016, 34 (12), 1260–1268. 10.1016/S1002-0721(16)60162-X. [DOI] [Google Scholar]
- Yadav K. K.; Singh D. K.; Anitha M.; Varshney L.; Singh H. Studies on separation of rare earths from aqueous media by polyethersulfone beads containing D2EHPA as extractant. Sep. Purif. Technol. 2013, 118, 350–358. 10.1016/j.seppur.2013.07.012. [DOI] [Google Scholar]
- Kondo K.; Matsuo T.; Matsumoto M. Adsorptive separation of La, Ce and Pr using microcapsules containing 2-ethylhexylphosphonic acid mono-2-ethylhexyl ester. Hydrometallurgy 2015, 152, 204–213. 10.1016/j.hydromet.2015.01.004. [DOI] [Google Scholar]
- Yadav K. K.; Dasgupta K.; Singh D. K.; Anitha M.; Varshney L.; Singh H. Solvent impregnated carbon nanotube embedded polymeric composite beads: An environment benign approach for the separation of rare earths. Sep. Purif. Technol. 2015, 143, 115–124. 10.1016/j.seppur.2015.01.032. [DOI] [Google Scholar]
- Jing Y.; Wang Y.; Hou H.; Xu J.; Wang Y. Extraction of lanthanides by polysulfone microcapsules containing EHPNA. I. Piercing method. J. Rare Earths 2015, 33 (6), 655–663. 10.1016/S1002-0721(14)60467-1. [DOI] [Google Scholar]
- Wang Y.; Jing Y.; Hou H.; Xu J.; Wang Y. Extraction of lanthanides by polysulfone microcapsules containing EHPNA. II. Coaxial microfluidic method. J. Rare Earths 2015, 33 (7), 765–775. 10.1016/S1002-0721(14)60483-X. [DOI] [Google Scholar]
- Yadav K. K.; Dasgupta K.; Singh D. K.; Anitha M.; Lenka R. K.; Varshaney L.; Singh H. Sorption behavior of Y(III) from chloride medium with polymer composites containing di-2-ethyl hexyl phosphoric acid and multiwall carbon nanotube. Sep. Sci. Technol. 2015, 50 (3), 463–470. 10.1080/01496395.2014.973518. [DOI] [Google Scholar]
- Yadav K. K.; Dasgupta K.; Singh D. K.; Varshney L.; Singh H. Dysprosium sorption by polymeric composite bead: Robust parametric optimization using Taguchi method. J. Chromatogr. A 2015, 1384, 37–43. 10.1016/j.chroma.2015.01.061. [DOI] [PubMed] [Google Scholar]
- Kamio E.; Fujiwara Y.; Matsumoto M.; Valenzuela F.; Kondo K. Investigation on extraction rate of lanthanides with extractant-impregnated microcapsule. Chem. Eng. J. 2008, 139 (1), 93–105. 10.1016/j.cej.2007.07.072. [DOI] [Google Scholar]
- Kondo K.; Matsuo T.; Matsumoto M. A fundamental study on the extraction of rare earth metals using microcapsules containing an extractant. Solvent Extr. Res. Dev., Jpn. 2011, 18, 103–112. 10.15261/serdj.18.103. [DOI] [Google Scholar]
- Kamio E.; Kondo K. Separation and Concentration of Lanthanoids Using Microcapsules Containing Acidic Organophosphorus Compounds as an Extractant. J. Chem. Eng. Jpn. 2002, 35 (6), 574–581. 10.1252/jcej.35.574. [DOI] [Google Scholar]
- Kondo K.; Umetsu M.; Matsumoto M. Adsorption characteristics of gadolinium and dysprosium with microcapsules containing an extractant. J. Water Process Eng. 2015, 7, 237–243. 10.1016/j.jwpe.2015.06.006. [DOI] [Google Scholar]
- Almeida M. I. G. S.; Cattrall R. W.; Kolev S. D. Recent trends in extraction and transport of metal ions using polymer inclusion membranes (PIMs). J. Membr. Sci. 2012, 415–416, 9–23. 10.1016/j.memsci.2012.06.006. [DOI] [Google Scholar]
- Almeida M. I. G. S.; Cattrall R. W.; Kolev S. D. Polymer inclusion membranes (PIMs) in chemical analysis - A review. Anal. Chim. Acta 2017, 987, 1–14. 10.1016/j.aca.2017.07.032. [DOI] [PubMed] [Google Scholar]
- Nagul E. A.; Croft C. F.; Cattrall R. W.; Kolev S. D. Nanostructural characterisation of polymer inclusion membranes using X-ray scattering. J. Membr. Sci. 2019, 588, 117208. 10.1016/j.memsci.2019.117208. [DOI] [Google Scholar]
- Croft C. F.; Almeida M. I. G. S.; Cattrall R. W.; Kolev S. D. Separation of lanthanum(III), gadolinium(III) and ytterbium(III) from sulfuric acid solutions by using a polymer inclusion membrane. J. Membr. Sci. 2018, 545, 259–265. 10.1016/j.memsci.2017.09.085. [DOI] [Google Scholar]
- Hiratani K.; Kusumocahyo S. P.; Kameta N.; Sugaya K.; Shinbo T.; Kanamori T. Synthesis of noncyclic carriers for cerium ion transport through polymer inclusion membrane. Chem. Lett. 2005, 34 (12), 1636–1637. 10.1246/cl.2005.1636. [DOI] [Google Scholar]
- Makowka A.; Pospiech B. Studies on extraction and permeation of lanthanum(III) and cerium(III) using cyphos IL 104 as extractant and ion carrier. Sep. Sci. Technol. 2020, 55, 1–11. 10.1080/01496395.2019.1584635. [DOI] [Google Scholar]
- Chen L.; Chen J. Asymmetric membrane containing ionic liquid [A336][P507] for the preconcentration and separation of heavy rare earth lutetium. ACS Sustain. Chem. Eng. 2016, 4 (5), 2644–2650. 10.1021/acssuschemeng.6b00141. [DOI] [Google Scholar]
- Wang Y.; Chen L.; Yan Y.; Chen J.; Dai J.; Dai X. Separation of adjacent heavy rare earth Lutetium (III) and Ytterbium (III) by task-specific ionic liquid Cyphos IL 104 embedded polymer inclusion membrane. J. Membr. Sci. 2020, 610, 118263. 10.1016/j.memsci.2020.118263. [DOI] [Google Scholar]
- Huang S.; Chen J.; Zou D. A preliminary study of polymer inclusion membrane for lutetium(III) separation and membrane regeneration. J. Rare Earths 2021, 39, 1256. 10.1016/j.jre.2020.07.024. [DOI] [Google Scholar]
- Huang S.; Chen J.; Chen L.; Zou D.; Liu C. A polymer inclusion membrane functionalized by di(2-ethylhexyl) phosphinic acid with hierarchically ordered porous structure for Lutetium(III) transport. J. Membr. Sci. 2020, 593, 117458. 10.1016/j.memsci.2019.117458. [DOI] [Google Scholar]
- Chen L.; Cui R.; Pan W.; Dai J.; Meng M.; Dai X.; Pan J. Role of natural deep eutectic solvents (NADESs) in coagulation bath for PVDF-based membranes on enhanced permeation and separation of rare earth ions. J. Membr. Sci. 2023, 683, 121836. 10.1016/j.memsci.2023.121836. [DOI] [Google Scholar]
- Chen L.; Dong H.; Pan W.; Dai J.; Dai X.; Pan J. Poly (vinyl alcohol-co-ethylene) (EVOH) modified polymer inclusion membrane in heavy rare earths separation with advanced hydrophilicity and separation property. Chem. Eng. J. 2021, 426, 131305. 10.1016/j.cej.2021.131305. [DOI] [Google Scholar]
- Carner C. A.; Croft C. F.; Kolev S. D.; Almeida M. I. G. S. Green solvents for the fabrication of polymer inclusion membranes (PIMs). Sep. Purif. Technol. 2020, 239, 116486. 10.1016/j.seppur.2019.116486. [DOI] [Google Scholar]
- Sugiura M.; Kikkawa M.; Urita S. Carrier-mediated transport of rare earth ions through cellulose triacetate membranes. J. Membr. Sci. 1989, 42 (1), 47–55. 10.1016/S0376-7388(00)82364-9. [DOI] [Google Scholar]
- Kusumocahyo S. P.; Sumaru K.; Iwatsubo T.; Shinbo T.; Kanamori T.; Matsuyama H.; Teramoto M. Quantitative analysis of transport process of cerium(III) ion through polymer inclusion membrane containing N,N,N’,N’-tetraoctyl-3-oxapentanediamide (TODGA) as carrier. J. Membr. Sci. 2006, 280 (1–2), 73–81. 10.1016/j.memsci.2006.01.007. [DOI] [Google Scholar]
- Kusumocahyo S. P.; Kanamori T.; Iwatsubo T.; Sumaru K.; Shinbo T.; Matsuyama H.; Teramoto M. Modification of preparation method for polymer inclusion membrane (PIM) to produce hollow fiber PIM. J. Appl. Polym. Sci. 2006, 102 (5), 4372–4377. 10.1002/app.24991. [DOI] [Google Scholar]
- Ansari S. A.; Mohapatra P. K.; Manchanda V. K. Cation transport across plasticized polymeric membranes containing N,N,N′,N′-tetraoctyl-3-oxapentanediamide(TODGA) as the carrier. Desalination 2010, 262 (1), 196–201. 10.1016/j.desal.2010.06.011. [DOI] [Google Scholar]
- Sharaf M.; Yoshida W.; Kubota F.; Kolev S. D.; Goto M. A polymer inclusion membrane composed of the binary carrier PC-88A and Versatic 10 for the selective separation and recovery of Sc. RSC Adv. 2018, 8 (16), 8631–8637. 10.1039/C7RA12697B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida W.; Baba Y.; Kubota F.; Kolev S. D.; Goto M. Selective transport of scandium(III) across polymer inclusion membranes with improved stability which contain an amic acid carrier. J. Membr. Sci. 2019, 572, 291–299. 10.1016/j.memsci.2018.11.021. [DOI] [Google Scholar]
- Makowka A.; Pospiech B. Synthesis of polymer inclusion membranes based on cellulose triacetate for recovery of lanthanum(III) from aqueous solutions. Autex Res. J. 2019, 19 (3), 288–292. 10.1515/aut-2018-0056. [DOI] [Google Scholar]
- Zaheri P.; Ghassabzadeh H. Preparation of polymer inclusion membrane including mixture of D2EHPA and Cyanex272 for the extraction of Eu from nitrate media. Chem. pap. 2017, 71 (9), 1623–1631. 10.1007/s11696-017-0155-2. [DOI] [Google Scholar]
- Zhang Y.; Cattrall R. W.; Kolev S. D. Fast and environmentally friendly microfluidic technique for the fabrication of polymer microspheres. Langmuir 2017, 33 (51), 14691–14698. 10.1021/acs.langmuir.7b03574. [DOI] [PubMed] [Google Scholar]
- Zhang Y.; Croft C. F.; Cattrall R. W.; Kolev S. D. Microfluidic Fabrication of Micropolymer Inclusion Beads for the Recovery of Gold from Electronic Scrap. ACS Appl. Mater. Interfaces. 2021, 13 (51), 61661–61668. 10.1021/acsami.1c19506. [DOI] [PubMed] [Google Scholar]
- Croft C. F.; Almeida M. I. G. S.; Kolev S. D. Development of micro polymer inclusion beads (μPIBs) for the extraction of lanthanum. Sep. Purif. Technol. 2022, 285, 120342. 10.1016/j.seppur.2021.120342. [DOI] [Google Scholar]
- Croft C. F.; Almeida M. I. G. S.; Kolev S. D. Characterisation of micro polymer inclusion beads by thermogravimetric analysis. Polymer 2023, 283, 126203. 10.1016/j.polymer.2023.126203. [DOI] [Google Scholar]
- Croft C. F.; Almeida M. I. G. S.; Kolev S. D. Online separation of critical rare earth elements from end-of-life permanent magnets using micro polymer inclusion beads (μPIBs). Miner. Eng. 2024, 214, 108779. 10.1016/j.mineng.2024.108779. [DOI] [Google Scholar]



