Abstract
Objective
To evaluate the effectiveness of 2 interventions for caregivers of patients with acquired brain injury (ABI) transitioning home after inpatient rehabilitation, to prepare them for the role of caregiving and reduce stress and depression.
Design
Controlled trial with participants randomly assigned to (1) usual care (UC), (2) clinician-delivered Problem-Solving Training (PST), or (3) peer-led Building Better Caregivers (BBC) training; both experimental interventions initiated during the inpatient rehabilitation stay, delivered virtually, of similar intensity (six 60-minute sessions), and focused on managing stress and building skills related to caregiving.
Setting
Nonprofit rehabilitation hospital specializing in care of persons with acquired brain and spinal cord injuries.
Participants
Caregivers (n=169) of patients with ABI (54 stroke; 115 other ABI) admitted for rehabilitation whose discharge location was home with care provided by family members (caregivers: 83% women, 62% White, age [mean ± SD]: 51±11.5 y). Participants were recruited from February 2021 to November 2022, when COVID-19 restrictions were in place.
Interventions
Noted above.
Main Outcome Measures
Caregiver-reported stress, depressive symptoms, and caregiving self-efficacy; patient unplanned hospital readmissions and emergency department visits 30 days post discharge.
Results
Only 61% of participants in the 2 intervention groups completed 3 or more of 6 intervention sessions and only 53% completed all data collection surveys. Statistically significant improvements between UC and PST groups were noted for caregiver stress (p=.039). Positive differences in caregiver self-efficacy found between UC and the BBC intervention groups approached significance at 30 days after discharge (p=.054). Patient unplanned hospital readmissions and days hospitalized were also higher, albeit not statistically significant, for UC participants than both intervention groups.
Conclusions
Although positive findings were noted, results were negatively affected by study limitations including low enrollment and limited engagement (intervention completion and follow-up outcomes assessment). These limitations resulted, in part, from restrictions put into place during the COVID-19 pandemic, which limited contact with study participants and required alterations to the BBC intervention likely influencing its effectiveness. Despite limitations noted, the encouraging findings suggest the need for further research.
Keywords: Acquired brain injury, Caregiver stress and burden, Family caregivers, Inpatient rehabilitation, Rehabilitation, Transition support services
Acquired brain injury (ABI) is a significant cause of long-term disability, affecting more than 6 million Americans.1, 2, 3 More than 80% of ABI survivors who require hospitalization are discharged home4, many with significant care and supervision needs resulting from physical and cognitive impairments.5 Family members often must take on responsibility of long-term caregiving, resulting in high levels of caregiver stress and burden, and increased likelihood of unplanned hospital readmissions for patients.6, 7, 8, 9, 10, 11
The problem of caregiver stress and lack of preparation for caregiving is widely acknowledged.7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23 The long-term, adverse effect of caregiver stress/burden affects both patient and caregiver.15,16 The family's ability to cope with stress can influence the quality of support they provide and, consequently, the extent of recovery after ABI.19, 20, 21, 22, 23 Effective problem-solving can improve self-efficacy, and decrease anxiety and depression for caregivers.19 Caregivers with greater self-efficacy in managing their loved one's care needs report being better equipped to cope with the demands of caregiving.20
Most inpatient rehabilitation programs provide support to family members of patients with ABI in preparation for discharge, including training in care routines needed by patients (eg, bowel and bladder management, tube feeding, medication management), counseling to help cope with patients’ behavioral and emotional changes, and discharge planning (eg, assistance with locating needed resources locally). However, it is often difficult to engage families in intervention efforts during the inpatient stay because of the overwhelming consequences of their loved one's injury. Family members don't yet understand or accept the long-term and potentially permanent nature of the patient's care needs, the complexity of continuing medical care, the changing family dynamics, and the financial effect of ongoing medical costs and lost productivity of family members.
As a result of this lack of understanding, family members do not appreciate the importance of interventions to help them manage stress and improve caregiving self-efficacy. Because the patient has not yet returned home, many of the care and supervision needs have not become evident. Families still “wish for the best” outcome for their loved ones and are reluctant to prepare for the role of caregiving. Once home, the opportunity to deliver a preventive transition-support intervention is often lost due to funding limitations.
Several structured interventions have been developed for family caregivers and some have shown efficacy in reducing caregiver stress and improving self-efficacy.24, 25, 26, 27, 28, 29, 30 However, these interventions were not originally developed for delivery during the acute stage of recovery - before the patient is discharged home with family caregivers. The interventions also vary in ways that have been identified as important by caregivers, such as group vs. individualized delivery, facilitation by a clinician vs. peer mentor, and in-person vs. online participation. The purpose of this research was to evaluate 2 promising caregiver interventions with established efficacy, initiated during the inpatient stay, on caregiver outcomes of stress, depression, and self-efficacy; and secondarily on patient health outcomes.
We sought to answer the following research questions: (1) Will caregivers engage during the acute injury stage in the interventions; (2) will the interventions be effective in reducing stress and depression, and improving self-efficacy; and (3) will intervention effects impact health outcomes for patients?
Methods
Design
We conducted a randomized trial to compare effects of 2 interventions to UC on the outcomes reported by family caregivers. The research protocol and study materials, including informed consent form for participants, were approved by the hospital's institutional review board prior to initiating the study.
Setting
This research was undertaken at a private, nonprofit hospital specializing in neurorehabilitation. The hospital admits approximately 350 patients annually for comprehensive inpatient rehabilitation after ABI. About 90% of patients are discharged home after the inpatient stay; >80% of patients are in the care of family members.
Participants
We targeted an enrollment of 180 participants (60 per group) based on sample size calculation and available funding for the study. Sample size was calculated based on a repeated-measures analysis for our primary outcomes (caregiver stress, depression, and self-efficacy). We based effect size on previous research by Lorig et al26 with the Building Better Caregivers (BBC) intervention, which demonstrated small to medium effect sizes ranging from 0.30 to 0.56 for these outcomes. Estimating a small effect size (0.40), statistical power of 0.90, alpha level of 0.05, correlation among repeated-measures of 0.99, and 2-tail distribution, we determined that a sample size of 180 participants (60 per group) would be sufficient to detect statistically significant differences for between-group comparisons.
Eligible participants were family members of patients admitted for inpatient rehabilitation who were expected to be discharged home with family. Because of COVID-19 restrictions in place during much of the enrollment period, participants were recruited via email by the study's research coordinator. The study was explained to prospective participants in a phone or email exchange and those interested in participating were asked to provide written informed consent.
Those who consented were asked to answer questions about their demographics and complete baseline surveys for each outcome measure. Participants who completed and returned the baseline surveys were then randomly assigned to 1 of 3 groups, described below. The sample was stratified on severity of patients’ brain injury and caregiver relationship to the patient. Severity was determined by scoring on the Disability Rating Scale measured at hospital admission (moderate ≤12; severe ≥11). Caregiver relationship was categorized as either parent, spouse or significant other, or other relative (sibling, grandparent, etc.). An online calculatora (www.graphpad.com) was used to generate a randomization assignment spreadsheet to determine group assignments. The spreadsheet was prepopulated with (2 × 3=6) tables reflecting each combination of stratification factors (eg, severe injury and parent caregiver). Each table consisted of a randomized list of group assignments (usual care [UC], Problem-Solving Training [PST], BBC) and participants were assigned sequentially as they enrolled in the study.
Interventions and comparators
Based on input from the Family Caregiver Advisory Council, a group of family members of former patients with ABI formed to help guide this project, we selected PST and BBC as the best available interventions for comparison. Past research has demonstrated the efficacy of both interventions, but only PST has been trialed early after injury.29,30 PST is clinician-led, administered one-on-one via phone calls, assigned readings, and practice assignments between calls. PST teaches caregivers how to address problems and apply a specific problem-solving technique (“ABCDEF”) that includes Assessing, Brainstorming, Consideration, Development, Evaluation, and Flexing to address problems identified by the caregiver. The training aims to teach caregivers to apply the strategy in the present and the future, to whatever problems they choose. The 6 PST sessions last for 30-60 minutes and are usually scheduled once a week over 6 weeks.
BBC was developed for caregivers of patients with dementia and has been adapted for caregivers of patients with ABI.26,27 BBC is a variant of the Stanford Chronic Disease Self-Management Program, developed in the late 1970s and used worldwide by individuals managing chronic health conditions and disability.31,32 Members of our ABI caregiver peer support team were trained and certified in delivery of the BBC. Based on their experience and feedback from the Family Caregiver Advisory Council, adaptations were made to reflect concerns, challenges, and resource needs relevant to families during the acute and early stage of recovery. These changes were approved by the Center for Self-Management Resources.
BBC intervention modifications due to COVID-19 pandemic precautions
The BBC intervention is usually delivered in six 60- to 90-minute group workshops comprised of 8-12 family caregivers and led by certified peer facilitators with experience as family caregivers of patients with ABI. Because of COVID-19 precautions, we were unable to convene in-person workshops, so resorted to the use of the secure Zoomb videoconferencing app. We received very positive feedback from participants on the accessibility and value of the Zoom workshops. However, we also faced challenges scheduling group sessions with caregivers, even on Zoom, because of the intense demands on their time and stresses of their loved ones’ injuries. These challenges forced us to convert the intervention from a group workshop format to one-on-one sessions conducted between individual caregivers and one of 3 peer facilitators. The content of the sessions followed key components of the BBC intervention—problem-solving; making an action plan; managing stress and fatigue, difficult care partner behavior, and difficult thoughts or emotions. Participants were also encouraged, though not required, to participate in ongoing peer support opportunities offered by the ABI program as a supplement to the more limited social support available with the one-to-one intervention.
All participants, including those randomized to UC, received the usual discharge planning and family support services offered by the ABI program. These services include nurse instruction in care routines, case management support for discharge, peer support services, referral to family counseling and community services as indicated, and general information resources about brain injury. All participants also had access to the online peer support communityc created for ABI caregivers (facebook.com/shepherdbi.peers).
Outcome measures
We examined 3 outcomes for caregivers: (1) stress and burden (Kingston Caregiver Stress Scale [KCSS]33); (2) depressive symptoms (Patient Health Questionnaire [PHQ-9]34), and perceived caregiving self-efficacy (Revised Self-Efficacy Scale for Caregivers [RSES]).35 All 3 measures have been used in previous research with caregivers of patients with ABI. Outcome measures were administered at 4 time points: time of enrollment in the study (Time point 1), within 72 hours of discharge from the inpatient rehabilitation program (Time point 2), and approximately 30 and 90 days after discharge (Time points 3 and 4). All 3 instruments were administered at each time point via an email-initiated survey using REDCap.d Participants received a $25 Amazon gift card for initiating outcome surveys at each time point, regardless of whether they completed all 3 instruments.
Data analysis
Missing data were imputed via regression modeling36, 37, 38, specifically growth curve models, to predict all missing data for the 4 time points. For each outcome measure, growth curve models estimated the initial status (intercept) and growth over time (slope) for every participant. These individualized estimates were then used in a simple linear regression formula: Y = b + mX, where b represents intercept estimates, m represents slope estimates, and X represents time from baseline. Each participant's individualized outcome-specific estimates were used in the formula to calculate all their missing data for our 4 time points.
Once missing data were imputed, repeated-measures factorial analysis of variance (ANOVA) was used to evaluate group changes over time (3 groups × 4 time points) for each outcome measure. In cases of significant effects, post hoc analyses were carried out comparing each intervention group separately to UC. We also identified “responders” based on minimal clinically important differences (MCID). Because there are no published MCIDs for the measures used and population studied, a distribution method of 0.5 SDs of the change score from Timepoint 1 (baseline) to Timepoint 4 (3 months after discharge) was used to calculate MCID.
Results
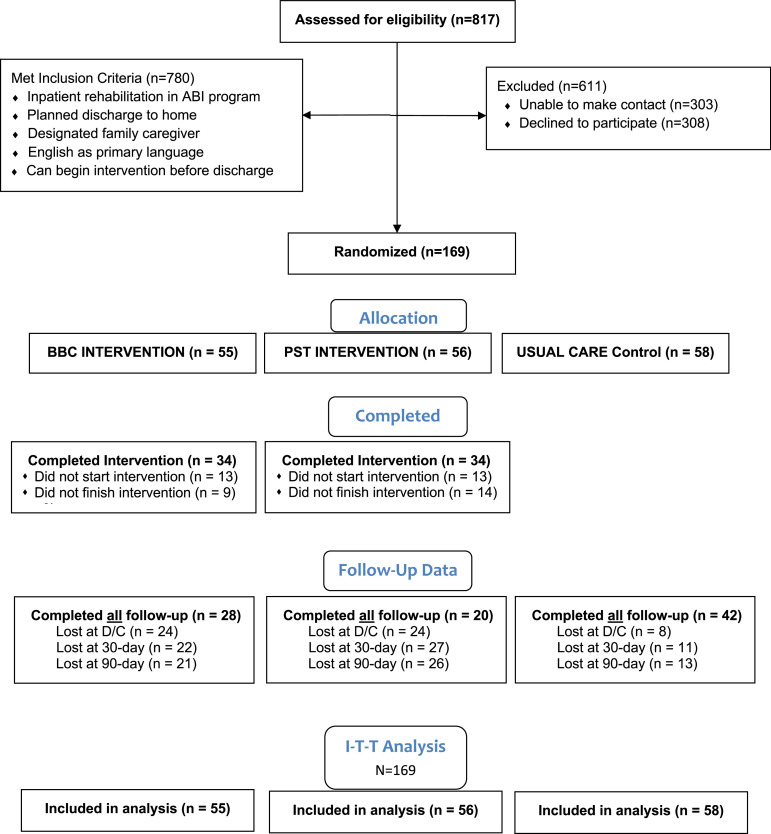
Figure 1 presents the CONSORT diagram outlining study enrollment. Of 817 potentially qualified admissions during the 22-month study enrollment period (February 14, 2021, to November 30, 2022), 780 (95%) met inclusion criteria. Of these eligible candidates, 308 (39%) declined participation and research staff were unable to contact 303 (39%) candidates, in part owing to restricted access in response to COVID-19. As a result, 169 participants were enrolled in the study and randomly assigned to one of the 3 groups. For the intervention groups, 34 of 55 (62%) BBC participants and 34 of 56 (61%) PST participants completed at least 3 intervention sessions; only 52% completed all 6 intervention sessions. An additional 13 participants in each group (23% of the total assigned) failed to initiate any intervention sessions after randomization. Mortality played a role in initiation by caregivers. Six patients passed away during the study: 3 before discharge and 3 after returning home. Four patients had been assigned to 1 of the 2 intervention groups; however, none of their caregivers initiated training. Only 53% of study participants completed outcome measure surveys at all 3 follow-up time points. All 169 participants were included in the “intent-to-treat” data analysis.
Fig 1.
CONSORT flow diagram.
Table 1 presents the demographic characteristics for all study participants and patients combined and for each comparator group. Only 2 significant differences were noted between comparator groups: (1) patient length of hospital stay was lower for the UC than that for the PST group (p=.05) and a higher proportion of caregivers were women in the UC group (p=.03).
Table 1.
Demographic characteristics of study participants (N=169).
| Characteristic | All | GT | PST | UC | Sign Test | P value |
|---|---|---|---|---|---|---|
| N= | 169 | 55 | 56 | 58 | (t or χ2) | |
| Patient | ||||||
| Severe injury, n (%) | 101 (60) | 33 (60) | 32 (57) | 37 (64) | χ2=0.61 | .74 |
| Disorder of consciousness, n (%) | 21 (12) | 8 (15) | 6 (11) | 7 (12) | χ2=0.38 | .83 |
| Mean length of stay ± SD (d) | 52±28.8 | 53±26.4 | 47±26.6 | t=1.26 | 1.05 | |
| 53±26.4 | 56±32.6 | t=0.54 | .29 | |||
| 56±32.6 | 47±6.6 | t=1.67 | .05* | |||
| Mean age ± SD (y) | 41±16.4 | 40 ± 16.2 | 42±17.6 | t=0.46 | .32 | |
| 40 ± 16.2 | 41±15.5 | t=0.11 | .46 | |||
| 41±15.5 | 42±17.6 | t=0.37 | .34 | |||
| Men, n (%) | 125 (74) | 40 (73) | 38 (68) | 47 (81) | χ2=2.64 | .27 |
| White, n (%) | 111 (66) | 36 (66) | 42 (75) | 33 (57) | χ2=0.12 | .94 |
| Caregiver | ||||||
| Mean age ± SD (y) | 51±11.6 | 50±11.6 | 52±10.6 | t=0.75 | .23 | |
| 50±11.6 | 50±12.4 | t=0.02 | .49 | |||
| 50±12.4 | 52±10.6 | t=0.70 | .24 | |||
| Women, n (%) | 142 (84) | 48 (87) | 41 (73) | 53 (91) | χ2=7.04 | .02* |
| White, n (%) | 103 (61) | 35 (64) | 36 (64) | 32 (55) | χ2=1.58 | .54 |
| Relationship | χ2=4.28 | .37 | ||||
| Parent, n (%) | 68 (40) | 23 (42) | 21 (38) | 24 (41) | ||
| Spouse/partner, n (%) | 84 (50) | 28 (51) | 31 (55) | 25 (43) | ||
| Other, n (%) | 17 (10) | 4 (7) | 4 (7) | 9 (16) | ||
| Payor source | χ2=1.57 | .81 | ||||
| Private insurance, n (%) | 138 (82) | 44 (80) | 48 (86) | 46 (79) | ||
| Medicaid/public, n (%) | 13 (8) | 5 (9) | 4 (7) | 4 (7) | ||
| Other, n (%) | 18 (10) | 6 (11) | 4 (7) | 8 (14) |
P≤.05.
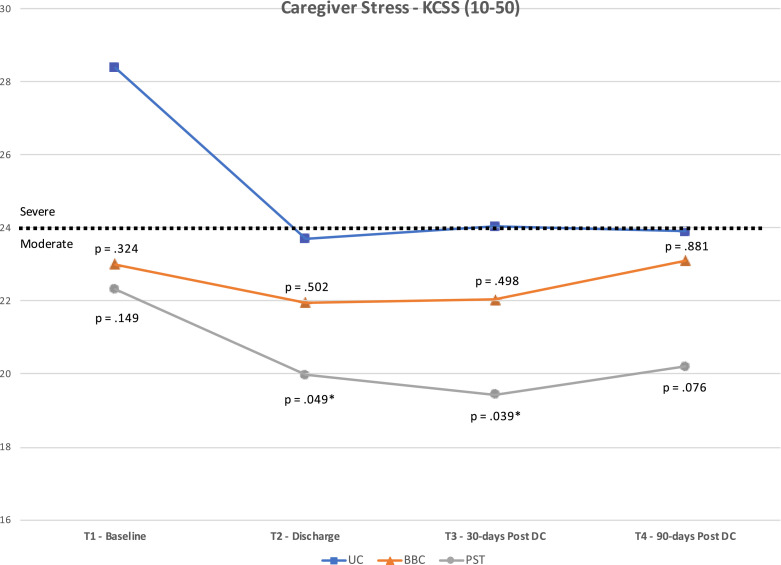
We computed ANOVA for time and group effects for our 3 outcome measures. Results reveal significant (p<.001) time effects for all 3 measures. No statistically significant group × time interaction was observed indicating that changes over time were similar for each group. There was, however, a significant group effect (p=.040) for caregiver stress (KCSS). Post hoc test revealed a significant difference between the PST and UC groups (p=.045). The UC group remained significantly higher in their KCSS score over the course of the study, compared to the PST group (see fig 2). Games-Howell test was used for post hoc analysis as Levine's test of equality of variances was violated.
Fig 2.
Results for Kingston Caregiver Stress Scale.
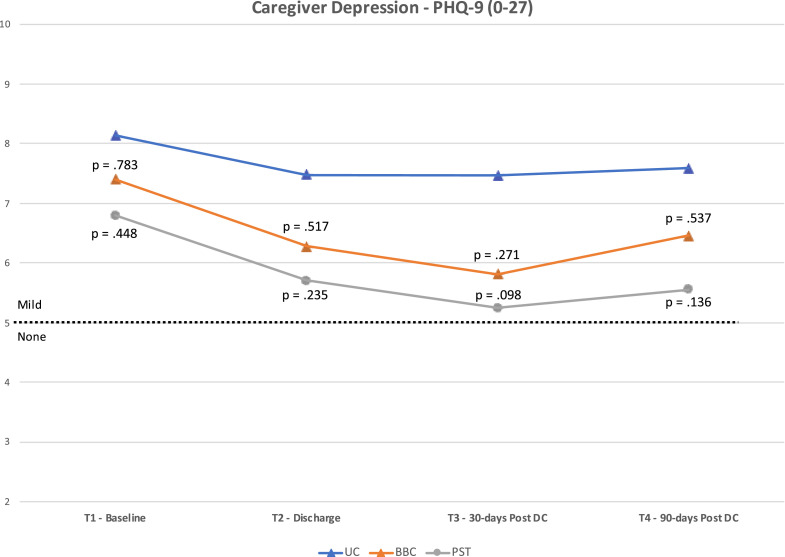
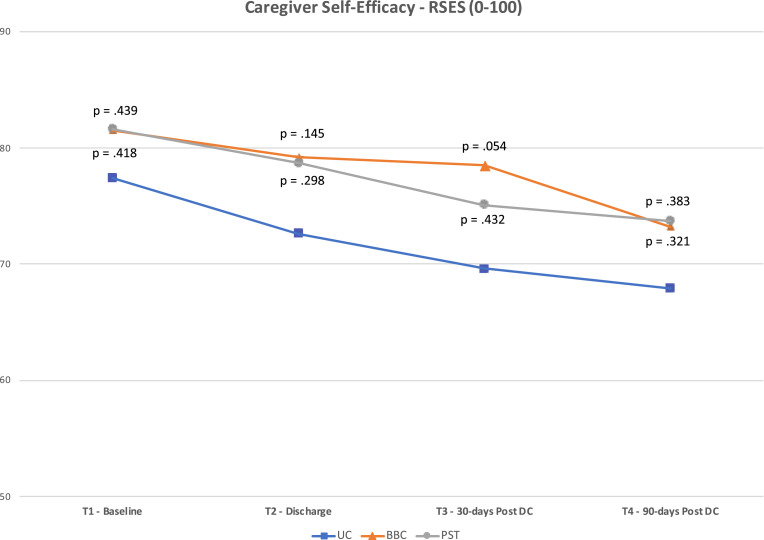
Fig 2, Fig 3, Fig 4 present the mean scores for the 3 caregiver outcome measures (KCSS, PHQ-9, RSES) at each time point. The P values for differences between the means of the UC group and each intervention group are also noted. These P values are based on post hoc, one-way ANOVA using Games-Howell (where homogeneity of variance was violated) or Tukey HSD corrections. The cut-point between “moderate” and “severe” stress on the KCSS and “mild” and “no” depressive symptoms on the PHQ-9 are also noted.
Fig 3.
Results for Patient Health Questionnaire 9.
Fig 4.
Results for Revised Self-Efficacy Scale.
As shown in figure 2, statistically significant differences were found in the KCSS between PST and UC groups at T2 (p=.049; mean difference [MD]=3.7; d=.45) and T3 (p=.039; MD=4.6; d=.46). No significant differences were noted between groups on the PHQ-9 (fig 3) or RSES (fig 4). However, the difference between BBC and UC groups for the RSES approached significance at T3 (p=.054; MD=8.9; d=.44).
Table 2 presents unplanned hospital readmissions and emergency department visits in the first 30 days post discharge for patients of family caregivers enrolled in the study. Overall, unplanned readmissions were quite low (5.3%), which is characteristic of the host hospital across all inpatient rehabilitation programs. There were notable differences between groups in the number of patients with unplanned readmissions and the average number of days rehospitalized. However, the differences were not statistically significant (p=.581)
Table 2.
Health outcomes for patients.
| All | BBC | PST | UC | |
|---|---|---|---|---|
| 30-day unplanned rehospitalizations | ||||
| No. of patients | 9 (5.3%) | 3 (5.5%) | 1 (1.8%) | 5 (8.6%) |
| Mean of days | 5.8 | 4.7 | 2 | 7 |
| ED visits | ||||
| No. of patients | 12 | 5 | 3 | 4 |
Abbreviations: ED, emergency department; No. number.
Discussion
We addressed 3 research questions in this study: (1) would participants engage in interventions to improve caregiving; (2) were the interventions effective; (3) did the interventions affect patient health outcomes?
Would caregivers engage?
Levels of engagement as measured by actual versus targeted enrollment, intervention completion, and loss to follow-up were disappointing but not surprising. We targeted enrollment at 30% of eligible candidates based on a recently completed study at the same hospital, also conducted during the COVID-19 pandemic, that involved delivery of 1-to-1 peer mentoring to family caregivers during the inpatient rehabilitation stay39; we achieved a 33% enrollment rate in the peer mentoring study compared to 22% in the present study. With respect to intervention completion, 84% of participants in the peer mentoring study39 completed the intervention compared to only 61% in the present study. Perhaps more troubling was the sizable percentage of participants (23%) who did not initiate the intervention in the present study compared with only 1 participant in the peer mentoring study. It may be that the interventions were perceived to be too intrusive into the already chaotic lives of caregivers as suggested by many comments from participants that they had difficulty freeing up time to participate in 6 one-hour-long sessions.
Similarly, data collection loss to follow-up was only 27% in the peer mentoring study compared with 47% in the present study. However, a key difference in the method used for follow-up may account for the difference. Telephone interviews between the primary caregiver and a trained interviewer were employed compared to an email-initiated survey in the present study.
Were the interventions effective?
The PST intervention appears to be moderately effective (d=.45) in reducing caregiver stress and the improvements noted remained through the 90-day follow-up time point. The BBC intervention had positive effects, most notably on improving caregiver self-efficacy, but several changes required in intervention delivery (virtual vs in-person, individual vs support group) may have muted overall effectiveness. The changes made were necessitated by the difficulties of delivering a structured, group intervention during the inpatient stay, which may have been the case regardless of COVID-19 restrictions. Delivery must be flexible and a group intervention is likely not practical. Results from the peer mentoring trial34 and findings of positive effects of the PST intervention suggest a one-to-one, peer-delivered version of the PST intervention might be a viable and effective option, assuming caregiver peers are available as interventionists.
Did the interventions affect patient health outcomes?
Both interventions were associated with lower rehospitalizations and hospital days, but the differences were not statistically significant. Given the relatively low incidence rate of unplanned hospitalizations, a much larger sample is likely needed to determine any statistically reliable association. Further, dyadic analyses could identify whether caregiver response (ie, change in outcomes after intervention) was associated with patient health outcomes, providing a more nuanced understanding of the effects of caregiver well-being on patient health.
Study limitations
The most notable limitation of the present study was not achieving the desired sample size owing to restrictions imposed by the COVID-19 pandemic. In addition to not enrolling our desired sample, the percentage of participants in the intervention groups who failed to initiate treatment or complete the optimal “dose” of 6 sessions was disappointingly low, further diminishing the effectiveness of the interventions. In addition, several modifications to the BBC intervention were required in response to COVID-19 restrictions on face-to-face contact. These factors no doubt negatively affected “power” of our statistical analysis.
Rate of dropouts and those lost to follow-up was also much higher than expected, with only half of the enrolled participants completing all outcomes surveys. Many of these losses may be attributed to disruptions brought on by COVID-19. However, the poor follow-up response may also be due to the manner of data collection employed. Rather than telephone interviews, we relied on email-initiated surveys, with 3 follow-up emails to prompt responses for those who did not complete the survey. Although electronic surveys improve efficiency of data collection and could improve compliance by offering participants greater flexibility in responding, they may still require telephone contact to maintain participant engagement and provide reminders and troubleshooting when technical difficulties arise.
Finally, it should be noted as a limitation that study enrollment was initiated before registration of the trial in a national trial registry, as is customary. Participation in the trial was, however, limited to caregivers of patients admitted to the host hospital, not the larger population of caregivers. As such, the generalizability of study findings is limited.
Conclusions
Positive findings were noted despite the several limitations of the present study. The positive findings support continued research into delivery of interventions during the acute phase of ABI to mitigate caregiver stress, improve problem-solving strategies, and instill caregiver self-efficacy. Results suggest that interventions must be flexible in delivery and tailored to individual caregiver needs and concerns. Given the effectiveness of the PST intervention in reducing caregiver stress, and positive if transitory effect of the BBC intervention on caregiver self-efficacy, a peer-led variation of the PST intervention may be a promising next step for research in this area.
Suppliers
-
a.
GraphPad - available at https://www.graphpad.com/quickcalcs/randMenu/
-
b.
Zoom - version 5.17.11 (31580); available at https://zoom.us
-
c.
Facebook - available at https://www.facebook.com/shepherdbi.peers/
-
d.
REDCap - version 13.7.2; available at https://redcap.shepherd.org/index.php
Disclosures
The investigators have no financial or nonfinancial disclosures to make in relation to this project.
Footnotes
The results were previously reported at a symposium presented at the 2023 Annual Meeting of the American Congress of Rehabilitation Medicine, November 2, 2023, Atlanta, GA.
This research was supported by a grant from the National Institute of Disability, Independent Living, and Rehabilitation Research (NIDILRR), US Department of Health and Human Services (award no. 90IFRE0026). Any opinions expressed by the authors are their own and do not represent policies of NIDILRR or the US government.
Clinical Trial Registration No.: NCT06126549.
References
- 1.Thurman D, Alverson C, Dunn K, Guerrero J, Sniezek J. Traumatic brain injury in the United States: a public health perspective. J Head Trauma Rehabil. 1999;14:602–615. doi: 10.1097/00001199-199912000-00009. [DOI] [PubMed] [Google Scholar]
- 2.Brain Injury Association of America. Brain injury facts. Available at: http://www.biausa.org/bia-media-center.html
- 3.Centers for Disease Control and Prevention (CDC) Prevalence and most common causes of disability among adults—United States, 2005. MMWR. 2009;58:421–426. [PubMed] [Google Scholar]
- 4.Kim H, Colantonio A, Deber R, Vernich L. Discharge destination from acute care after traumatic brain injury. Can J Neurol Sci. 2006;33:48–52. doi: 10.1017/s0317167100004686. [DOI] [PubMed] [Google Scholar]
- 5.Seel R, Macciocchi S, Velozo C, et al. The safety assessment measure for persons with traumatic brain injury: item pool development and content validity. NeuroRehabilitation. 2016;39:371–387. doi: 10.3233/NRE-161369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marwitz JH, Cifu DX, Englander J, High WM., Jr. A multi-center analysis of rehospitalizations five years after brain injury. J Head Trauma Rehabil. 2001;16:307–317. doi: 10.1097/00001199-200108000-00002. [DOI] [PubMed] [Google Scholar]
- 7.Dillahunt-Aspillaga C, Jorgensen-Smith T, Ehlke S, Sosinski M, Monroe D, Thor J. Traumatic brain injury: unmet support needs of caregivers and families in Florida. PLoS One. 2013;8:e82896. doi: 10.1371/journal.pone.0082896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Douglas JM, Spellacy FJ. Indicators of long-term family functioning following severe traumatic brain injury in adults. Brain Inj. 1996;10:819–839. doi: 10.1080/026990596123936. [DOI] [PubMed] [Google Scholar]
- 9.Knight RG, Devereux R, Godfrey HPD. Caring for a family member with a traumatic brain injury. Brain Inj. 1998;12:467–481. doi: 10.1080/026990598122430. [DOI] [PubMed] [Google Scholar]
- 10.Wallace CA, Bogner J, Corrigan J, et al. Primary caregivers of persons with brain injury: life change 1 year after injury. Brain Inj. 1998;12:483–493. [PubMed] [Google Scholar]
- 11.Leathem J, Heath E, Woolley C. Relatives’ perceptions of role change, social support and stress after traumatic brain injury. Brain Inj. 1996;10:27–38. doi: 10.1080/026990596124692. [DOI] [PubMed] [Google Scholar]
- 12.Kreutzer JS, Gervasio AH, Camplair PS. Primary caregiver's psychological status and family functioning after brain injury. Brain Inj. 1994;8:197–210. doi: 10.3109/02699059409150973. [DOI] [PubMed] [Google Scholar]
- 13.Hassan STS, Wan-Fei K, Raman RA, Jamaludin H, Riji HM. Review on family caregiving and rehabilitation of traumatic brain injury (TBI) Health Med. 2012;6:2423–2434. [Google Scholar]
- 14.Kreutzer JS, Marwitz JH, Kepler K. Traumatic brain injury: family response and outcome. Arch Phys Med Rehabil. 1992;73:771–778. [PubMed] [Google Scholar]
- 15.Kolakowsky-Hayner SA, Miner K, Kreutzer J. Long-term life quality and family needs after traumatic brain injury. J Head Trauma Rehabil. 2001;16:374–385. doi: 10.1097/00001199-200108000-00007. [DOI] [PubMed] [Google Scholar]
- 16.Vangel S, Rapport L, Hanks R. Effects of family and caregiver psychosocial functioning on outcomes in persons with traumatic brain injury. J Head Trauma Rehabil. 2011;26:20–29. doi: 10.1097/HTR.0b013e318204a70d. [DOI] [PubMed] [Google Scholar]
- 17.Kreutzer JS, Rapport LJ, Marwitz JH, Harrison-Felix C, Hart T, Glenn M, Hammond F. Caregivers’ well-being after traumatic brain injury: a multicenter prospective investigation. Arch Phys Med Rehabil. 2009;90:939–946. doi: 10.1016/j.apmr.2009.01.010. [DOI] [PubMed] [Google Scholar]
- 18.Marsh NV, Kersel DA, Havill JH, Sleigh JW. Caregiver burden at 1 year following severe traumatic brain injury. Brain Inj. 1998;12:1045–1059. doi: 10.1080/026990598121954. [DOI] [PubMed] [Google Scholar]
- 19.Elliott TR, Shewchuk RM, Richards JS. Family caregiver social problem-solving abilities and adjustment during the initial year of the caregiving role. J Couns Psychol. 2001;48:223–232. [Google Scholar]
- 20.Man DWK. The empowering of Hong Kong Chinese families with a brain damaged member: its investigation and measurement. Brain Inj. 1998;12:245–254. doi: 10.1080/026990598122728. [DOI] [PubMed] [Google Scholar]
- 21.Leach LR, Frank RG, Bouman DE, Farmer J. Family functioning, social support and depression after traumatic brain injury. Brain Inj. 1994;8:599–606. doi: 10.3109/02699059409151012. [DOI] [PubMed] [Google Scholar]
- 22.Testa JA, Malec JF, Moessner AM, Brown AW. Predicting family functioning after TBI: impact of neurobehavioral factors. J Head Trauma Rehabil. 2006;21:236–247. doi: 10.1097/00001199-200605000-00004. [DOI] [PubMed] [Google Scholar]
- 23.Manskow US, Sigurdardottir S, Røe C, et al. Factors affecting caregiver burden 1 year after severe traumatic brain injury: a prospective nationwide multicenter study. J Head Trauma Rehabil. 2015;30:411–423. doi: 10.1097/HTR.0000000000000085. [DOI] [PubMed] [Google Scholar]
- 24.Rivera PA, Elliott TR, Berry JW, Grant JS. Problem-solving training for family caregivers of persons with traumatic brain injuries: a randomized controlled trial. Arch Phys Med Rehabil. 2008;89:931–941. doi: 10.1016/j.apmr.2007.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kreutzer JS, Stejskal TM, Ketchum JM, Marwitz JH, Taylor LA, Menzel JC. A preliminary investigation of the Brain Injury Family Intervention: impact on family members. Brain Inj. 2009;23:535–547. doi: 10.1080/02699050902926291. [DOI] [PubMed] [Google Scholar]
- 26.Lorig K, Thompson-Gallagher D, Traylor L, et al. Building Better Caregivers: a pilot online support workshop for family caregivers of cognitively impaired adults. J Appl Gerontol. 2012;31:423–437. [Google Scholar]
- 27.Powell JM, Fraser R, Brockway JA, Temkin N, Bell KR. A telehealth approach to caregiver self-management following traumatic brain injury: a randomized controlled trial. J Head Trauma Rehabil. 2016;31:180–190. doi: 10.1097/HTR.0000000000000167. [DOI] [PubMed] [Google Scholar]
- 28.Lorig K, Ritter PL, Laurent DD, Yank V. Building better caregivers: a pragmatic 12-month trial of a community-based workshop for caregivers of cognitively impaired adults. J Appl Gerontol. 2019;38:1228–1252. doi: 10.1177/0733464817741682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Juengst SB, Osborne CL, Holavanahalli R, et al. Feasibility study of problem-solving training for care partners of adults with traumatic brain injury, spinal cord injury, burn injury, or stroke during the inpatient hospital stay. Arch Rehabil Res Clin Transl. 2019;1 doi: 10.1016/j.arrct.2019.100009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Juengst SB, Wright B, Driver S, et al. Multisite randomized feasibility study of Problem-Solving Training for care partners of adults with traumatic brain injury during inpatient rehabilitation. NeuroRehabilitation. 2023;52:109–122. doi: 10.3233/NRE-220129. [DOI] [PubMed] [Google Scholar]
- 31.Lorig KR, Sobel DS, Stewart AL, et al. Evidence suggesting that a chronic disease self-management program can improve health status while reducing hospitalization: a randomized trial. Med Care. 1999;37:5–14. doi: 10.1097/00005650-199901000-00003. [DOI] [PubMed] [Google Scholar]
- 32.Lorig KR, Ritter P, Stewart AL, et al. Chronic disease self-management program: 2-year health status and health care utilization outcomes. Med Care. 2001;39:1217–1223. doi: 10.1097/00005650-200111000-00008. [DOI] [PubMed] [Google Scholar]
- 33.Sadak T, Korpak A, Wright JD, et al. Psychometric evaluation of the Kingston Caregiver Stress Scale. Clin Gerontol. 2017;40:268–280. doi: 10.1080/07317115.2017.1313349. [DOI] [PubMed] [Google Scholar]
- 34.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Steffen AM, McKibbin C, Zeiss AM, Gallagher-Thompson D, Bandura A. The revised scale for caregiving self-efficacy: reliability and validity studies. J Gerontol B Psychol Sci Soc Sci. 2002;57:P74–P86. doi: 10.1093/geronb/57.1.p74. [DOI] [PubMed] [Google Scholar]
- 36.Ferguson KK, Yu Y, Cantonwine DE, McElrath TF, Meeker JD, Mukherjee B. Foetal ultrasound measurement imputations based on growth curves versus multiple imputation chained equation (MICE) Paediatr Perinat Epidemiol. 2018;32:469–473. doi: 10.1111/ppe.12486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Andridge RR. Quantifying the impact of fixed effects modeling of clusters in multiple imputation for cluster randomized trials. Biom J. 2011;53:57–74. doi: 10.1002/bimj.201000140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Curran PJ, Obeidat K, Losardo D. Twelve frequently asked questions about growth curve modeling. J Cogn Dev. 2010;11:121–136. doi: 10.1080/15248371003699969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jones M, Holley C, Jacobs M, Batchelor R, Mangin A. Effects of peer mentoring for caregivers of patients with acquired brain injury: a preliminary investigation of efficacy. Arch Rehabil Res Clin Transl. 2021;3 doi: 10.1016/j.arrct.2021.100149. [DOI] [PMC free article] [PubMed] [Google Scholar]