Abstract
To date, no therapy has been proven to be efficacious in fully restoring neurological functions after spinal cord injury (SCI). Systemic high-dose methylprednisolone (MP) improves neurological recovery after acute SCI in both animal and human. MP therapy remains controversial due to its modest effect on functional recovery and significant adverse effects. To overcome the limitation of MP therapy, we have developed a N-(2-hydroxypropyl) methacrylamide copolymer-based MP prodrug nanomedicine (Nano-MP) that can selectively deliver MP to the SCI lesion when administered systemically in a rat model of acute SCI. Our in vivo data reveal that Nano-MP is significantly more effective than free MP in attenuating secondary injuries and neuronal apoptosis. Nano-MP is superior to free MP in improving functional recovery after acute SCI in rats. These data support Nano-MP as a promising neurotherapeutic candidate, which may provide potent neuroprotection and accelerate functional recovery with improved safety for patients with acute SCI.
Keywords: Spinal cord injury, Methylprednisolone, Nanomedicine, Neuroprotection, Functional outcomes
Introduction
Spinal cord injury (SCI) affects up to 300,000 people in the United States (US) with about 17,700 new SCI cases each year.1 SCI often results in catastrophic neurological impairments and life-long disability which present significant health, economic and social challenges for the patients, their families and the society.2,3 Total health care costs associated with SCI exceed $10 billion annually in the US, and lifetime per person direct and indirect costs can exceed $3 million.1,4 Multiple promising therapies for SCI have been investigated, including surgical, non-pharmacological interventions (e.g., hypothermia), pharmacological agents (e.g., targeting myelin-associated inhibitors of regeneration), and cellular transplantation therapies (e.g., Schwann cells and human embryonic stem cells).5 Unfortunately, none of these attempted strategies demonstrated sufficiently robust efficacy to be widely accepted by the clinical community.5
To date, methylprednisolone (MP) is the only FDA approved therapy for acute SCI treatment, although it is no longer widely used clinically.6 Damage to the spinal cord results both from the initial trauma and then from secondary processes that occur several hours to days following the initial injury. The secondary injury processes induce oxidative stress, calcium-mediated damage, excitotoxicity, immune reactions that culminate in neuronal apoptosis.7 While the extent of primary injury is determined by the initial trauma, which is not amenable to therapeutic intervention, the relatively slow progression of secondary neurological injury response (hours to days after the initial injury) provides a crucial post-acute SCI window of opportunity to improve functional outcomes. Should spinal cord tissue be spared due to dramatic reductions in secondary injury, one may anticipate significantly improved neurofunctional recovery. It is with this premise, systemic MP therapy was developed as an option to treat acute traumatic SCI.6,8
In 1990, the second National Spinal Cord Injury Study (NASCIS II) trial reported its comparison of MP, naloxone and placebo showed no difference between the groups for functional recovery in patients with acute SCI. However, a subgroup of patients identified on post hoc analysis who were treated within 8 h of injury with MP (30 mg/kg bolus followed by 5.4 mg/kg over 23 h) had better motor scores than the placebo group.9 Based on this tenuous result, MP quickly became established as a standard of care. In 1997 and 1998, based on findings of the NASCIS III trial, it was recommended to initiate a 24-h course of MP if treatment was started within 3 h of injury and a 48-h course if treatment was started within 3–8 h of injury6,10 with both adhering to NASCIS II protocol. The use of systemic high-dose MP in patients with acute SCI remains controversial because of the modest neuroprotective benefit, which should be weighed against its potential serious adverse side effects, including susceptibility to infection and wound complications, gastric bleeding, sepsis, diabetic complications, pneumonia, and acute corticosteroid myopathy.8,9,11–13 Because of these concerns, the Guidelines for the Management of Acute Cervical Spine and Spinal Cord Injuries list MP administration merely as a treatment option to be considered for the treatment of acute SCI.14 Current AOSpine guidelines recommends that its administration be considered within 8 h after acute SCI and administered for 24 h in those without medical contraindications.15,16
While the overarching objective of the current systemic MP therapy is to dampen the secondary injury pathology after SCI, the regimen exposes the entire body to high doses of MP in order to achieve effective local MP concentrations at the injury site, which results in the serious adverse side effects that in turn hinder the utility of higher and more efficacious MP levels at the lesion. Therefore, we hypothesize that targeted MP delivery to the injury site, once achieved, will significantly mitigate the systemic exposure of MP therapy and allow the medication to be delivered at far higher concentrations to the SCI site. As the direct outcome of such an approach, we anticipate markedly improved functional neurological outcome and safety profile. As such, we envision that improved MP therapy may be integrated as part of the standard post SCI care protocol to address the unmet urgent need among SCI patients.
Polymeric glucocorticoid (GC) prodrug nanomedicines have been developed and tested extensively as novel therapeutic agents for inflammatory and autoimmune diseases. When administered systemically, their passive targeting to inflammatory tissues in pathologies such as rheumatoid arthritis (RA),17–19 lupus nephritis (LN),20–22 traumatic brain injury,23 or orthopedic implant loosening24,25 is governed by Extravasation through Leaky Vasculature and Inflammatory cell-mediated Sequestration (ELVIS) effect.26 Upon delivery to the sites of pathology, the prodrugs are activated by pathological environmental cues (e.g., acidosis and/or reduced lysosomal pH), which provides an additional level of specificity of drug for the pathological site. This unique pathophysiology-driven prodrug design has been proven to be very effective to potentiate the anti-inflammatory and immunoregulatory efficacy of GC and drastically reduce their toxicities. To ensure streamlined clinical translation, well-established biocompatible water-soluble polymers such as N-(2-hydroxypropyl) methacrylamide (HPMA) copolymers and polyethylene glycol have been used as the carrier systems in prodrug nanomedicine design. Given the extensive inflammation associated with acute SCI,27 we posit that the polymeric GC prodrug nanomedicine design that has been successfully used in other inflammatory pathologies in AA and LN may also be applied to improve the treatment of acute SCI. Despite many other HPMA-conjugated glucocorticoids, no group has synthesized HPMA-based MP.
Several studies have demonstrated that, local MP nanoparticle formulations significantly reduced lesion volume and improved behavioral outcomes in animals. However, these reported approaches utilized local delivery via surgical procedures, which is not practical for most patients after acute SCI.28–31 The main objective of the present study is to develop a HPMA copolymer-based MP prodrug nanomedicine (Nano-MP) via intravenous injection that selectively delivers MP to the spinal cord lesion. We first performed the biodistribution study to examine the selective uptake of the polymeric prodrug at the injured spinal cord (Study 1) after SCI. Additional acute analysis (Study 2 Part I) was performed at 2 days post injury to quantify specific protein expression indicative of secondary injury. Subacute analysis (Study 3) was performed at 7 days post injury to quantify the degree of the injury-related cellular reactivity responsible for wound-healing as well as neural apoptotic indices. The functional recovery of the animals was assessed pre-operatively and post-operatively over 2 months (Study 4 Part I). In parallel, Study 2 Part II, Study 3 and Study 4 Part II were conducted to assess reductions in side effects with the use of Nano-MP compared to systemic parent MP molecule; the findings of this work will be reported as a separate report in conjunction with this report in this issue of the journal. Because approximately 80 % of persons with SCI are male, these studies were performed using male rats. The design and major endpoints of Study 1–4 are outlined in Fig. 1.
Fig. 1.
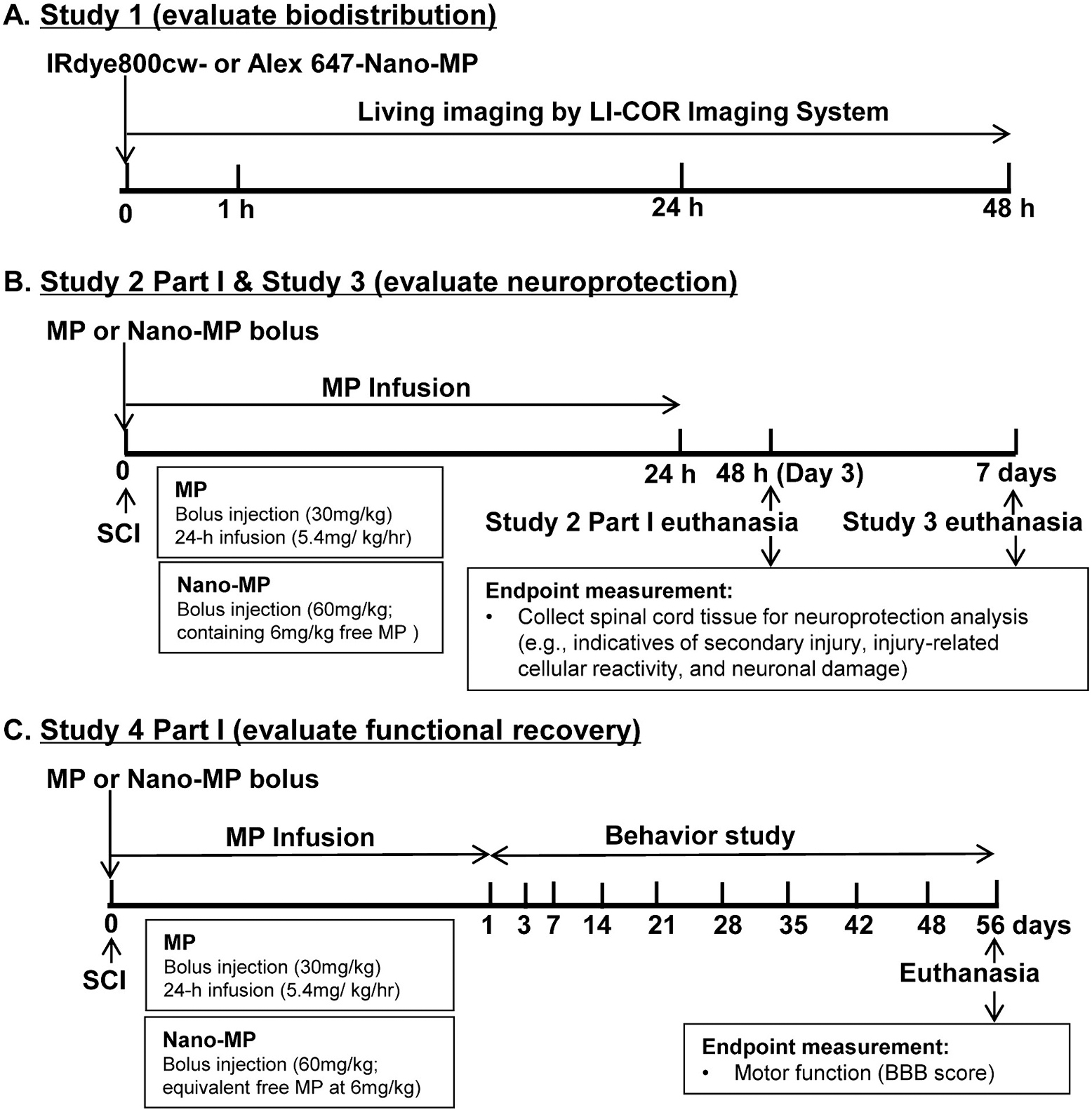
The experimental design and a summary on major endpoints of each study.
Materials and methods
Materials, instruments and synthesis of Nano-MP
Please refer to “Supplemental Materials” for this part in details.
Surgery and in vivo experimental design
All animals were maintained on a 12:12-h light/dark cycle with lights on at 07:00 am in a temperature-controlled (20 ± 2 °C) vivarium, with food and water ad libitum. All procedures were approved by the IACUC at James J. Peters Veterans Affairs Medical Center and conformed to all guidelines and regulations for the protection of the welfare of animals. Complete spinal cord transection model was selected to be used in Study 1, 2 and 3, and was conducted as previously described.32–38 For the function recovery assessment in Study 4, a moderate contusion SCI was produced at the interspace between the 8th and 10th thoracic vertebra using by an Infinite Horizontal (IH) Impactor.39,40 Post SCI induction, urine was manually expressed 3 times/day until automaticity develops, and then as needed. Baytril was administered postoperatively for the first 3–5 days then as needed to manage cloudy/bloody urine or wound infection. Sham-transected/ contusion animals received a laminectomy-only surgery. See Supplemental Materials for details of SCI models’ justification, induction procedures and post-surgical care. In Study 1, three groups of animals were investigated: Healthy control (n = 10), laminectomy-only (Sham, n = 10), and SCI animal (SCI, n = 10). In Studies 2, 3 and 4, four groups of animals were planned: laminectomy-only (Sham, n = 12), SCI animals with vehicle (SCI + veh, n = 12), SCI animals treated with MP (SCI + MP, n = 12), and SCI animals treated with Nano-MP (SCI + Nano-MP, n = 12).
Treatments
An Alzet pump was implanted in a subcutaneous pocket during surgery. Immediately after SCI, a single dose of Nano-MP (60 mg/kg containing ~6 mg/kg of free MP) was administered to the SCI rats via intravenous (i.v.) administration. Unconjugated MP (30 mg/kg) and vehicle (saline) were also subjected to a bolus i.v. administration and followed by 24-h infusion of MP via the Alzet pump at 5.4 mg/kg/h in SCI + MP group, or vehicle in SCI + veh and SCI + Nano-MP groups, respectively. On an mg/kg basis, the dosing protocol for intravenous MP administration is identical to that prescribed by the Bracken protocol.10,41 The dose-equivalent free MP level in the systemic Nano-MP treatment is equal to 3.75 % of the systemic MP dose clinically used. Sustained low-dose local delivery of MP (about 5 % of the systemically administered dose) could provide neuroprotection.28 Sham-transected/contusion animals underwent laminectomy and implantation of the same Alzet pumps, with vehicle infusion instead, as baseline controls.
Assessment of Nano-MP biodistribution
In Study 1, two fluorescently tagged MP prodrugs (Nano-MP–IRDye, 1 mg/rat; Nano-MP-Alexa, 2 mg/rat) were co-administered via tail vein immediately after T4 spinal cord transection. Prior to injection or at 1, 24 and 48 h post administration, the animals were anesthetized through inhalation of isoflurane and were optically imaged to evaluate the distribution of the Nano-MP using LI-COR Odessey Small Animal Imaging System. The animals were euthanized on day 3. At euthanasia, the spinal cords were isolated for ex vivo optical imaging with the same imager. The spinal cords were then sectioned and stained with fluorescence-labeled cell markers for confocal microscopic analyses to assess cell-specific localization of Nano-MP.42
Tissue preparation and histological analysis
At 2 and 7 days after injury, animals (n = 5–6/condition) were perfused intracardially with 4 % paraformaldehyde. The spinal cords were removed and fixed overnight in the same fixative. Longitudinal 10 μm thick tissue sections were cryosectioned. Serial sections were blocked and followed by incubation of primary antibodies including: TNF-α, GFAP (astrocyte marker), nitrotyrosine (oxidative stress marker), μ-Calpain (pro-apoptotic protein), ED1 (macrophages/reactive microglia marker), CSPG (impaired axon regeneration marker), Bax (pro-apoptotic marker), activated Caspase-3 (apoptotic marker), and SM32 (motor neuron marker). After washing and incubation with Alexa Fluor 647-conjugated secondary antibodies, slides were mounted and subjected to fluorescent microscopy. For animals that received Nano-MP-Alexa 647 injection, slides were either immunostained with GFAP followed by Alexa 647-conjugated secondary antibody or directly stained with Cresyl violet. Images were taken using the EVOS FL Auto system. Fluorescence intensity quantifications were performed in the ImageJ software (NIH).
Lipid peroxidation
At 2-day post injury, the animals were sacrificed and sections of spinal cord centered around the site of lesion or laminectomy were dissected out and stored at −80 °C. The injured spinal cords (~ 25 mg) lysed in RIPA buffer and lipid peroxidation was measured using the TBARS Assay Kit following the manufacturer’s instructions. Malondialdehyde (MDA) concentration was determined using the colorimetric method.
Locomotor function testing
Use of the hindlimbs was scored using the Basso, Beattie and Bresnahan (BBB) locomotor score. A score of 0 indicates no hind-limb movement whereas a score of 21 indicates unimpaired locomotion as observed in normal uninjured rats. The test was performed pre-operatively, day 1, 3, and 7 post injury, and then once weekly postoperatively, as described previously43 and in “Supplemental Materials”. Briefly, animals were placed at the center of a square box and observed as they move toward a wall/corner, which was recorded by a video camera. The final BBB scores for each time point were determined by combining the BBB paper record with the results of the video record review by two accessors.
Statistics
Standard power analyses were used to determine the requisite minimum number of animals to ensure sufficient statistical power, as described previously.44 Data are expressed as mean ± SEM; the number of independent samples (n) is provided in the legend of each figure. The statistical significance of differences among means was tested using one-way analysis of variance and Bonferroni’s post hoc test to determine the significance of differences between individual pairs of means using a P value of 0.05 as the cutoff for significance. Statistical calculations were performed using Prism 4.0c (GraphPad Software, CA).
Results
Synthesis of Nano-MP and labeling with fluorescent dyes
Nano-MP was synthesized according to the route shown in Fig. 2. Different from the previous synthesis of HPMA copolymer-based dexamethasone prodrug (P-Dex), in which C3 carbonyl of A-ring was the preferred hydrazone linker formation site, MP preferentially conjugated to the polymeric carrier via C20 carbonyl. Given that HPMA copolymer is not biodegradable, the molecular weight of Nano-MP was maintained at 30 kDa to facilitate its clearance through the kidney glomerular filtration with threshold of 45 kDa for HPMA copolymer.45 The dispersity is narrow with Ð = 1.03. To determine the amount of MP in Nano-MP, it was hydrolyzed in 0.1 N HCl and analyzed with HPLC. The MP content in Nano-MP was determined to be ~10 wt%. Nano-MP–IRDye and Nano-MP-Alexa were synthesized via polymer analogous reaction between primary amine-containing Nano-MP and IRDye 800CW-NHS or Alexa Fluor 647-NHS. The IRDye and Alexa Fluor 647 contents in Nano-MP were determined to be 4.92 × 10−6 mol/g and 5.23 × 10−6 mol/g, respectively.
Fig. 2.

Synthesis of HPMA copolymer-based methylprednisolone prodrug nanomedicine (Nano-MP).
Selective uptake of Nano-MP in the injured spinal cord
To visualize Nano-MP biodistribution in vivo in Study 1, Nano-MP–IRDye and Nano-MP-Alexa were co-administered i.v. immediately after SCI. Blood vessels in the rat ear were visible within 1 h after injection, indicating Nano-MP’s presence in circulation. At day 2 and 3, no fluorescent signal is detectable in the rat ear, suggesting clearance of Nano-MP from the circulation (Fig. 3A). Importantly, the presence of Nano-MP-IRDye in the spinal cord was only detectable in rats with SCI, not in normal or Sham rats with laminectomy (Fig. 3B), confirming the selective targeting and local retention of Nano-MP at the spinal cord injured site. The targeted delivery of Nano-MP to injured spinal cord is further confirmed by the conventional fluorescence microscopy analysis, which demonstrated the presence of Nano-MP-Alexa in the spinal cord section of SCI rats, but in not in the Healthy or Sham controls (Fig. 3C).
Fig. 3.

Selective uptake of Nano-MP in the injured spinal cord. (A) Male rats underwent complete T4 spinal cord transection or laminectomy only. Nano-MP-IRDye (1 mg/rat) was injected via tail veins immediately after SCI. The biodistribution of the Nano-MP in rat ear was examined at different time points post-injection. (B–C) Nano-MP can be detected in the injured spinal cord (B) or spinal cord section (C) at day 3, but not that in normal or laminectomy-only rats. Images were taken using a LI-COR imaging system (X4) for (A) and (B) and fluorescent microscopy for (C) (scale bar = 400 μm), respectively. n = 10/group.
Internalization of Nano-MP by microglia and astrocytes
To further evaluate the subcellular-localization of Nano-MP-Alexa, longitudinal spinal cord sections were examined by fluorescent microscopy. No colocalization between Nano-MP-Alexa 647-fluorescence and Nissl staining (Fig. 4A, B) or SM32 staining (Supplemental Fig. 1) were detectable, suggesting weak internalization by spinal cord motor neurons. Conversely, Nano-MP-Alexa colocalized with CD11+ microglia (Fig. 4C) and GFAP+ astrocytes (Fig. 4D) respectively, suggesting strong sequestrations by these cell phenotypes.
Fig. 4.
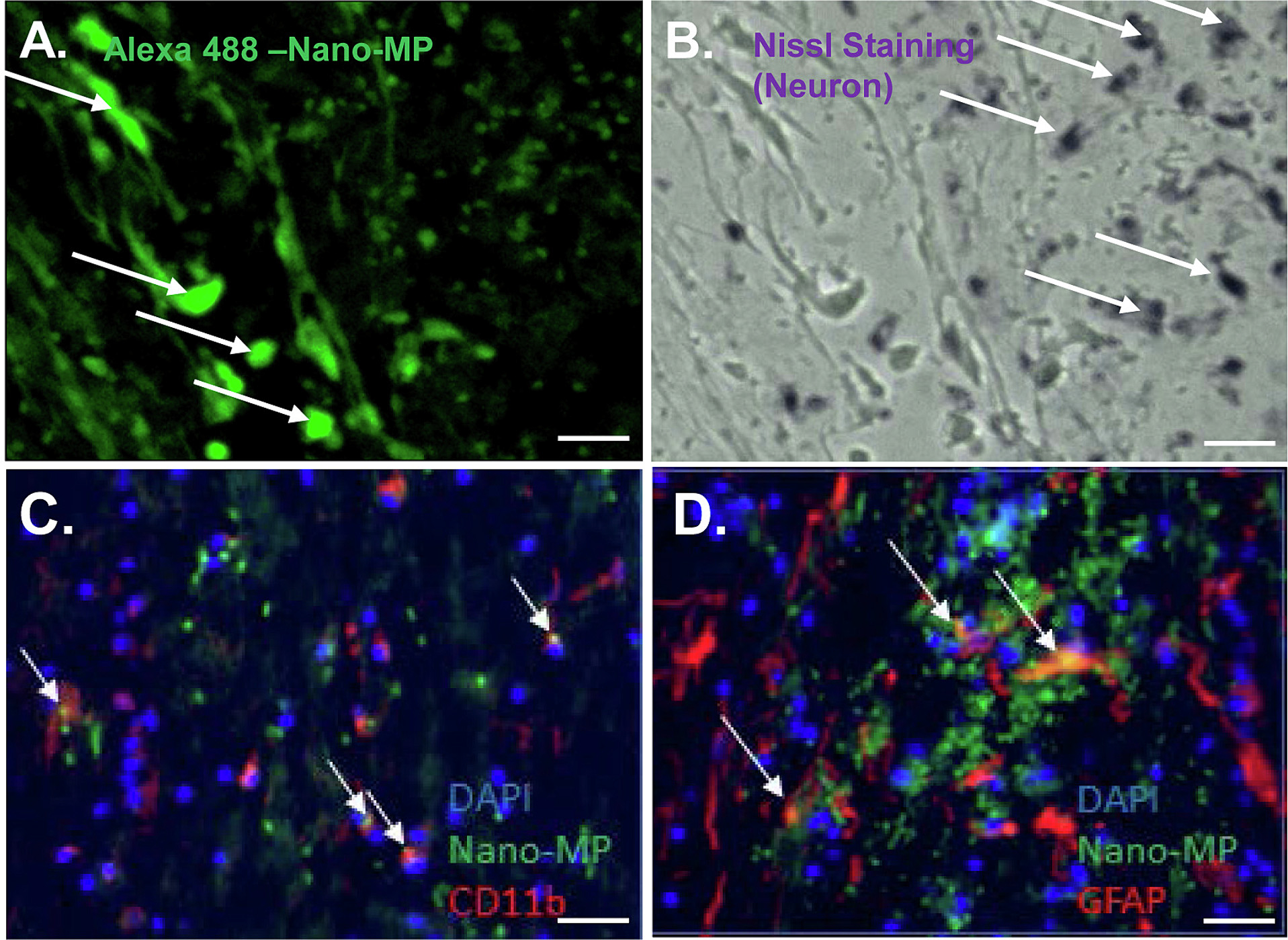
Internalization of Nano-MP by microglia and astrocytes. Nano-MP-Alexa 647 (2 mg/rat) was injected via tail veins immediately after SCI and detected in injured spinal cord at about 1 cm caudal to the lesion epicenter by confocal microscopy. (A and B) Nano-MP (green color) did not accumulate in motor neurons (stained by Nissl Staining; purple). Scale bar = 100 μm. Accumulation of Nano-MP in CD11+ microglia (C) and GFAP+ astrocytes (D) (n = 10/group). Motor neurons were stained by SM32 antibody (red). Nuclei were stained by DAPI (blue). White arrows show double-labeling in intact cells. Scale bar = 20 μm.
A single dose Nano-MP significantly inhibited oxidative stress in the spinal cord
Oxidative stress is considered a hallmark of SCI or damage to other sites of the central nervous system. MP was reported to have significant antioxidant activities and improve the recovery of patients with SCI in clinical trials, primarily through the inhibition of reactive oxygen induced by lipid peroxidation and inflammation.8 The level of nitrotyrosine, an indirect chemical indicator of toxic nitric oxide and peroxynitric-induced cellular damage, was examined in the spinal cord. SCI-Nano-MP group had significantly lower SCI-induced accumulation of nitrotyrosine at 2 days (−33.9 % vs. SCI + veh; Fig. 5) and 7 days post injury (−34.0 % vs. SCI + veh; Supplemental Fig. 2) whereas the SCI-MP group did not. The TBARS assay was used to assess the levels of MDA as an indication of lipid peroxidation.46 Acute SCI significantly increased the MDA level in the spinal cord as compared to Sham control animals at 2 days post injury (9.44 ± 0.49 μM vs. 5.00 ± 0.62 μM). Notably, while systemic MP treatment had no effect, Nano-MP treatment reduced the MDA level (5.47 ± 0.85 μM), thus protecting the animals from SCI-mediated MDA accumulation and lipid peroxidation in the spinal cord (Fig. 5B).
Fig. 5.

Nano-MP attenuated SCI-induced oxidative stress and lipid peroxidation in the spinal cord. A-a. Representative images showing the nitrotyrosine level in the spinal cord (longitudinally sectioned) and fluorescent intensity quantification (A-b). B. Malondialdehyde (MDA) level was measured using TBARS assay. Data are expressed as Mean ± SEM. n = 5–6/group. *p < 0.05, **p < 0.01, ***p < 0.001 by one-way ANOVA. Scale bar = 40 μm.
A single dose of the Nano-MP reduced inflammation and injury-related cellular markers in the spinal cord
Inflammation responses are a major element of secondary injury and play a pivotal role in regulating the pathogenesis of acute and chronic SCI. From 3 to 24 h post SCI, upregulation of TNF-α could be detected around the injured area.47,48 As expected, acute SCI led to increased TNF-α reactivity in the spinal cord. Interestingly, similar to oxidative stress markers, TNF-α also showed a significant decrease at 2 days after injury (−51.3 % vs. SCI + veh) upon treatment with Nano-MP while MP treatment had no effect (Fig. 6A). At 7 days post injury, both Nano-MP and free MP treatments significantly reduced SCI-induced TNF-α expression to similar levels (−33.9 % and −42.5 % vs. SCI + veh, respectively) (Supplemental Fig. 3). Acute SCI also resulted in increased reactivity of GFAP+ astrocytes (Fig. 7A), ED-1+ macrophages/reactive microglia (Fig. 7B) and CSPG deposition (Supplemental Fig. 4) at 7 days post injury. Both Nano-MP and free MP treatment reduced the GFAP reactivity and ED-1 reactivity (Fig. 7A and B) whereas Nano-MP, but not free MP, inhibited CSPG deposition (indicating greater axon regeneration potential) at 7 days after acute SCI.
Fig. 6.
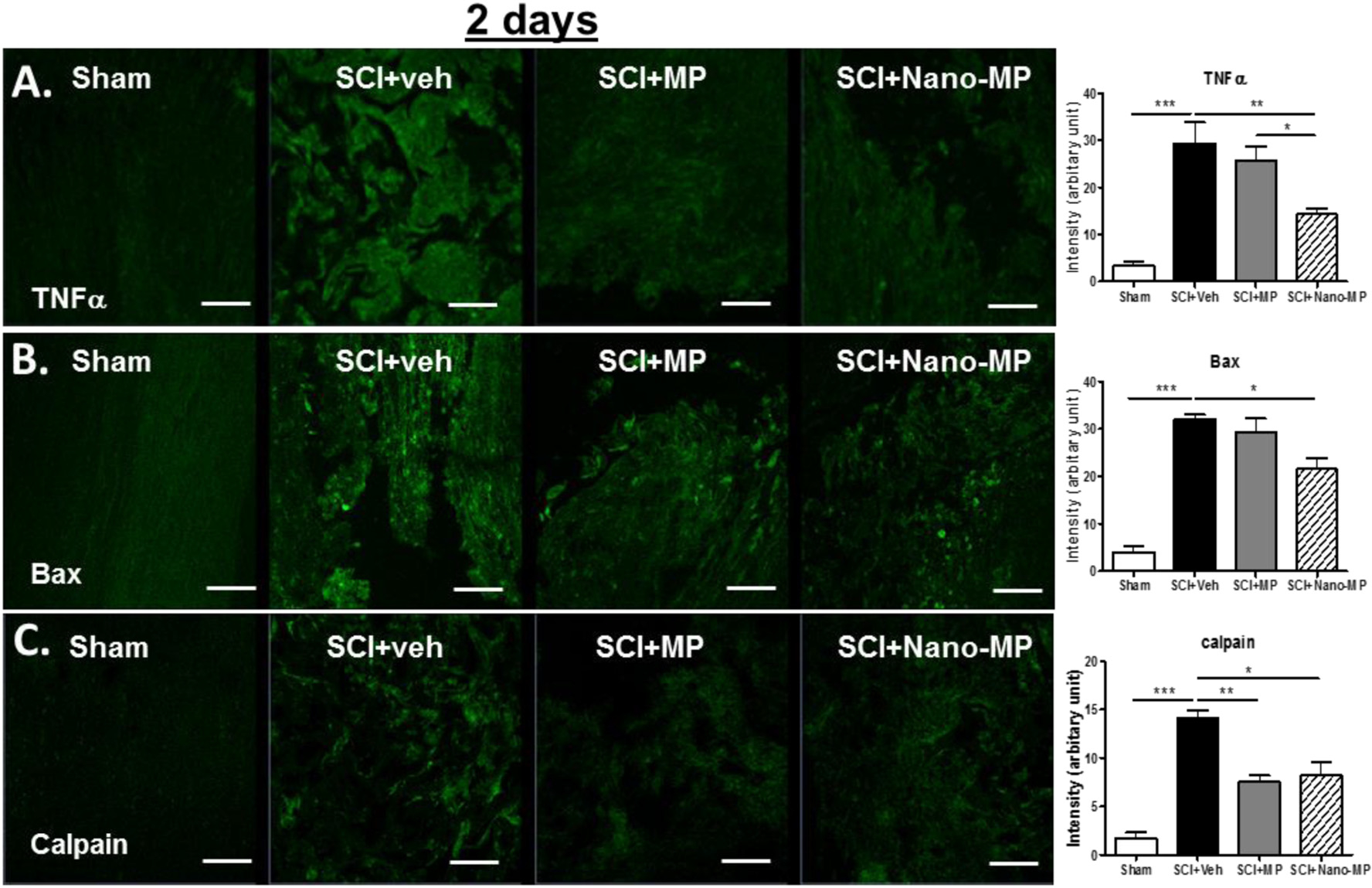
Effects of Nano-MP on inflammation and pro-apoptotic markers after acute SCI. Representative images showing the TNF-α (A), Bax (B) and Calpain (C) in the spinal cord (longitudinally sectioned) and fluorescent intensity quantification (n = 5–6/group). *p < 0.05, **p < 0.01, ***p < 0.001 by one way ANOVA. Scale bar = 40 μm.
Fig. 7.

Effect of Nano-MP on the expressions of injury-related cellular marker and caspase-3 after acute SCI. Representative images showing the GFAP (A), ED-1 (B) and caspase-3 (C) in the spinal cord (longitudinally sectioned) and fluorescent intensity quantification (n = 5–6/group). *p < 0.05, **p < 0.01, ***p < 0.001 by one way ANOVA. Scale bar = 40 μm.
A single dose of the Nano-MP administration significantly enhanced neuroprotection after acute SCI
Apoptosis, as demonstrated by nuclear DNA fragmentation and caspase activation, was a prominent feature in the spinal cord post SCI. After SCI, some cells at the lesion site die by post-traumatic necrosis, whereas others die by apoptosis.49 Pro-apoptotic proteins such as Bax, Caplain (a Ca2+-dependent protease)28,50 and caspase-3 activation play central roles in cell apoptosis. As expected, acute SCI led to upregulation of Bax and Caplain at 2 days post injury. Nano-MP treatment significantly reduced the reactivity to Bax and Caplain at the site of injury when compared to control (veh) treatment (Fig. 6B and C). Nano-MP, but not MP significantly reduced the expression of Bax as compared to SCI control animals at 2 days (−27.0 % and −8.3 % vs. SCI + veh, respectively) post injury (Fig. 6B). Caspase-3 activation was also evaluated. Both Nano-MP and free MP significantly reduced SCI-induced Caspase-3 activation at 7 days post injury (−49.8 % and −30.0 % vs. SCI + veh, respectively), with Nano-MP treatment showing more robust effect than free MP (Fig. 7C).
Further studies were conducted to examine cellular location of caspase-3 and Bax in injured spinal cord tissue sections. As expected, acute SCI led to increased caspase-3 activation and Bax expression. Interestingly, the caspase-3 and Bax immunoreactive signals selectively co-localized with motor neuron-specific immunoreactivity (SM32) as determined by overlapping caspase-3/Bax and SM32 staining (Fig. 8A–b, c and 8B–b,c). Of note, animals in the Nano-MP group displayed significantly reduced immunoreactive signal for caspase-3 activation (Fig. 8A–d) and Bax expression in motor neurons (Fig. 8B–d), suggesting that Nano-MP treatment would lead to a significantly greater reduction in neuronal damage, indicative of enhanced neuroprotection of the spinal cord after acute SCI when compared to MP treatment (Fig. 8A–c and 8B–c). Collectively, these findings support Nano-MP’s potential in providing more significant protection against SCI-induced apoptosis in animals than free MP treatment.
Fig. 8.

Nano-MP decreased SCI-induced motor neuron apoptosis in the spinal cord and promoted functional recovery after acute SCI. Representative images show the caspase-3 (A) or Bax (B) immunofluorescence staining in the spinal cord (longitudinally sectioned, n = 5–6/group). Caspase-3 or Bax stain in green; SMI32 (red) and DAPI (purple) stain motor neuron and nucleus, respectively. Scale bar = 20 μm. Arrow, co-localization. (C) Functional recovery evaluated by the BBB scale after SCI. Vehicle, free MP or Nano-MP was administered immediately after contusion SCI. n = 12/group; *p < 0.05, **p < 0.01 by two-way ANOVA and Bonferroni post tests.
Effects of a single dose of the Nano-MP on functional recovery after acute SCI
The potential impacts of Nano-MP on improving animal functional outcome after acute SCI were assessed (Study 4 Part I). Immediately after a moderate contusion SCI at T9 induced by IH impactor device, rats were administered free MP, Nano-MP, or vehicle. Hindlimb motor function was then assessed pre-operatively, Day 1, 3 and 7 post injury, and then once weekly postoperatively. At Day 1 or 3 after SCI, BBB scores were similar among all groups. At other time points, Nano-MP-treated rats exhibited a more rapid recovery, yielding higher improvement on BBB scores than that of animals given free MP or those in the control groups (Fig. 8C). At 56 days post SCI, Nano-MP-treated rats had an average of ~12 BBB scores and regained the ability to take weight-supported steps without consistent forelimbs and hindlimbs coordination whereas MP-treated rats had an average of ~9 BBB scores and demonstrated weight support in stance only without consistent stepping. This observation demonstrated that Nano-MP is superior to free MP in its ability to improve functional motor recovery after acute SCI in the rodent model employed in this study.
Discussion
SCI is a catastrophic event that causes loss of sensory, motor, and autonomic function due to central nervous system damage. To date, there are no therapeutic approaches that provide neurological functional recovery after acute SCI. MP is an FDA-approved SCI management option to improve neurological functions after acute injury, but it is not routinely used clinically. The use of this drug remains controversial as the modest protective functions on nerve cells are overshadowed by the unfavorable side effects to the rest of the body. To overcome this challenge, we have developed a macromolecular prodrug nanomedicine Nano-MP that can selectively deliver MP to sites of injured and/or inflamed spinal cord in a rat model of acute SCI. Our overarching hypothesis is that such targeted delivery strategy would revitalize MP therapy with improved therapeutic efficacy and safety. In the present study, we demonstrated that a single intravenous injection of Nano-MP is able to preferentially deliver MP to the site of the injured spinal cord where the polymeric prodrug is sequestered and activated predominantly by CD11+ infiltrating inflammatory cells (extrapolated from the action of the parent MP molecule), thereby exerting its therapeutic effects. Nano-MP treatment significantly inhibited lipid peroxidation and inflammation of the injured spinal cord, resulting in reduced neuronal damage and enhanced neuroprotection after acute SCI. Importantly, the administration of Nano-MP provided better functional improvement after acute SCI in rodents compared to the systemic delivery of parent MP. In our companion publication in this issue of the journal, we report that compared to conventional intravenous delivery of the regular MP molecule, Nano-MP that was selectively delivered to the injury site, showed to have far fewer systemic adverse side effects—that is, reduced muscle atrophy, bone loss, and less carbohydrate intolerance. Collectively, this novel targeted-delivery system of MP may be significantly safer than standard MP therapy and can be translated as a highly feasible treatment approach in patients with acute SCI to improve functional recovery.
The optical imaging results confirmed our hypothesis that Nano-MP can selectively target to the SCI lesion with sustained presence for >3 days, which may be attributed to the compromised blood-spinal cord barrier (BSCB) and the neuroinflammation-induced neovascularization and vascular fenestration. In addition to localizing in the transected spinal cord (Fig. 3), the NIR fluorescent signal associated with Nano-MP was also seen in liver and kidney (data not shown), the two clearance organs, as often observed in other systemic delivery of nanomedicines. Overt extravasation of macromolecules has been reported within the first hour after SCI,51 a period that coincided with marked tissue disruption within the epicenter of the lesion. Abnormal BSCB permeability remains significantly elevated at 24 h after injury. A second peak of abnormal barrier permeability has been observed at 3–7 days postinjury, which coincides with prominent revascularization of the epicenter. BSCB integrity is restored at 21 days post SCI.51 Given this chronological sequence of the peri-spinal cord vasculature remodeling/healing process, Nano-MP was i.v. administered immediately after SCI surgery in our study to take advantage of accessibility to the injured spinal cord. Given this unique pathology-driven passive-targeting of Nano-MP to the injury site, the nanomedicine treatment must be administered as soon as possible post injury, similar to the MP treatment protocol.
Interestingly, as we further investigate the cells that were responsible for Nano-MP’s sustained local retention at SCI pathology, microglia and astrocytes were identified as the main cell phenotypes that sequestered the nanomedicine at sites of SCI pathology. It has been well-established that these two types of cells play pivotal roles in SCI pathogenesis, repair, and healing.1,52–55 As part of the spontaneous repair process, these cells have adopted highly phagocytic phenotypes,56,57 which were responsible for the swift internalization of Nano-MP. Furthermore, activation of microglia and astrocytes results in further acidification of lysosomes,58 where Nano-MP likely resides. The reduced lysosomal pH value would in turn accelerate the cleavage of the hydrazone bond linking MP to the HPMA copolymer carrier and the release of free MP in order to dampen excessive local inflammation and induce resolution and neuroregenerative repair processes. Distinctively different from other attempts at MP formulation improvement,28–31,52,59 the macromolecular prodrug design of Nano-MP is very unique because its simple structure affords a MP therapy with high specificities to SCI pathology on subcellular, cellular and tissue levels. The evidence presented in this report regarding Nano-MP’s capacity to reduce oxidative stress, dampen inflammation, facilitate neuroprotection and regeneration, and most importantly to improve neurofunctional outcome post SCI, validated the principles we adopted in designing Nano-MP. Due to the aforementioned unique features and characteristics of macromolecular prodrugs such as Nano-MP, we may consider the extrapolation of the same design principle to several other drug candidates in order to improve their efficacy and safety. The pharmacological agents for SCI that may be considered include CSPG inhibitors which reduce glia scar, PTEN, Nogo-A and Rho inhibitors, or other neurotrophic factors which promote axon regeneration.
Given the strong evidence presented regarding Nano-MP’s efficacy and safety, we will perform a temporal and/or dose-response curve of Nano-MP and/or other adjunctive therapies on biologic, pathologic and functional outcome measures in the future to facilitate clinical translation of Nano-MP therapy. We will also perform additional experiments to develop in-depth understanding of Nano-MP’s mechanism of action (e.g., inflammatory cascades, glia scar formation, axon regeneration, etc.). Collectively, these studies will guide the design of future treatment regimens by identifying the appropriate time window and the minimum effective dose of Nano-MP or other combinatorial approaches capable of maintaining therapeutic efficacy. As a limitation of the present study, only male animals were investigated. Therefore, it remains of interest and relevance to investigate if the observed beneficial effects of Nano-MP can be reproduced in female animals as well.
In summary, the present work presents the development of a novel macromolecular prodrug nanomedicine Nano-MP, which enabled selective targeting of MP to the injured spinal cord via i.v. systemic administration. The Nano-MP was sequestered in and retained by activated microglia and astrocytes within an SCI lesion via the ELVIS mechanism where it remained bioactive in vivo at least for a few days [verse the intravenous parent MP with a short half-life for 3 h60]. Nano-MP mediated substantially decreases in secondary injury-related inflammation, oxidation, lipid peroxidation, and neuronal death, resulting in considerably enhanced neuroprotection and functional recovery after acute SCI as compared to conventional intravenous delivery of the parent MP molecule. These extraordinary findings and the significantly improved safety profile presented in the companion paper strongly support the potential of Nano-MP for continued efforts toward clinical translation as a highly promising drug candidate for improved clinical management of SCI and its complications.
Supplementary Material
Acknowledgment
This work was supported by the Veterans Health Administration, Rehabilitation Research and Development Service (Merit Review Award I01RX02089-A2 and VA BRAVE funds to WQ and the Center grant B2020–C to WAB and AMS), National Institutes of Health/NINDS (R21 NS111393–01A1 to WQ), National Institutes of Health (R01 AI119090 and R44 DA051278 to DW), and NYS SCIRP DOH01-PART6–2023-00005 to WQ. We thank Dr. Noam Y. Harel for his critical reviewing during the manuscript preparation. We thank Jiangping Pan for her technical assistance.
Abbreviations:
- AA
adjuvant-induced arthritis
- Bax
Bcl-2-associated X protein
- BBB
Basso, Beattie and Bresnahan locomotor scale method
- BSCB
blood-spinal cord barrier
- CMCht/PAMAM
carboxymethyl-chitosan polyamidoamine
- CSPG
chondroitin sulfate proteoglycan
- Dex
dexamethasone
- ELVIS
Extravasation through Leaky Vasculature and subsequent Inflammatory cell-mediated Sequestration
- GC
glucocorticoid
- GFAP
Glial Fibrillary Acidic Protein
- H&E
hematoxylin-eosin
- HPMA
N-(2-hydroxypropyl) methacrylamide
- IH
Infinite Horizons
- LN
lupus nephritis
- MDA
malondialdehyde
- MP
methylprednisolone
- Mw
molecular weight
- Nano-MP
N-(2-hydroxypropyl) methacrylamide copolymer-based methylprednisolone prodrug nanomedicine
- NASCIS
National Acute SCI Study
- NIR
near infrared
- NO
nitric oxide
- NT
nitrotyrosine
- PDI
polydispersity
- RAFT
reversible addition-fragmentation chain transfer
- TBARS
thiobarbituric acid reactive substance assay
- TNFα
tumor necrosis factor alpha
- veh
vehicle
Footnotes
Declaration of competing interest
WZ, ZSJ, YQ, YP, ZC, and CPC have nothing to disclose. WQ, DW, and WAB were coinventors of a patent application (PCT-US20–054559), which covers the development of macromolecular methylprednisolone prodrug nanomedicine (Nano-MP).
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nano.2024.102761.
CRediT authorship contribution statement
Wei Zhao: Writing – review & editing, Visualization, Validation, Methodology, Investigation, Formal analysis, Data curation. Zhenshan Jia: Validation, Methodology, Investigation, Data curation. William A. Bauman: Writing – review & editing, Funding acquisition. Yiwen Qin: Writing – review & editing, Visualization, Investigation, Formal analysis, Data curation, Conceptualization. Yuanzhen Peng: Supervision, Methodology, Investigation, Formal analysis, Data curation. Zihao Chen: Methodology, Formal analysis, Data curation. Christopher P. Cardozo: Writing – review & editing. Dong Wang: Writing – review & editing, Validation, Supervision, Resources, Investigation, Funding acquisition, Formal analysis, Conceptualization. Weiping Qin: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision, Software, Resources, Project administration, Methodology, Investigation, Funding acquisition, Formal analysis, Conceptualization.
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- 1.National Spinal Cord Injury Statistical Center. Spinal Cord Injury Facts and Figures at a Glance. 2018. [Google Scholar]
- 2.Dvorak MF, Noonan VK, Fallah N, Fisher CG, Rivers CS, Ahn H, et al. Minimizing errors in acute traumatic spinal cord injury trials by acknowledging the heterogeneity of spinal cord anatomy and injury severity: an observational Canadian cohort analysis. J Neurotrauma. 2014;31(18):1540–1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Noonan VK, Fallah N, Park SE, Dumont FS, Leblond J, Cobb J, et al. Health care utilization in persons with traumatic spinal cord injury: the importance of multimorbidity and the impact on patient outcomes. Top Spinal Cord Inj Rehabil. 2014;20(4):289–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Krueger H, Noonan VK, Trenaman LM, Joshi P, Rivers CS. The economic burden of traumatic spinal cord injury in Canada. Chronic Dis Inj Can. 2013;33(3):113–122. [PubMed] [Google Scholar]
- 5.Hawryluk GW, Rowland J, Kwon BK, Fehlings MG. Protection and repair of the injured spinal cord: a review of completed, ongoing, and planned clinical trials for acute spinal cord injury. Neurosurg Focus. 2008;25(5):E14. [DOI] [PubMed] [Google Scholar]
- 6.Bracken MB, Shepard MJ, Holford TR, Leo-Summers L, Aldrich EF, Fazl M, et al. Methylprednisolone or tirilazad mesylate administration after acute spinal cord injury: 1-year follow up. Results of the third National Acute Spinal Cord Injury randomized controlled trial. J Neurosurg. 1998;89(5):699–706. [DOI] [PubMed] [Google Scholar]
- 7.Sekhon LH, Fehlings MG. Epidemiology, demographics, and pathophysiology of acute spinal cord injury. Spine (Phila Pa 1976). 2001;26(24 Suppl):S2–12. [DOI] [PubMed] [Google Scholar]
- 8.Hall ED, Springer JE. Neuroprotection and acute spinal cord injury: a reappraisal. NeuroRx. 2004;1(1):80–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bracken MB, Shepard MJ, Collins WF Jr, Holford TR, Baskin DS, Eisenberg HM, et al. Methylprednisolone or naloxone treatment after acute spinal cord injury: 1-year follow-up data. Results of the second National Acute Spinal Cord Injury Study. J Neurosurg. 1992;76(1):23–31. [DOI] [PubMed] [Google Scholar]
- 10.Bracken MB, Shepard MJ, Holford TR, Leo-Summers L, Aldrich EF, Fazl M, et al. Administration of methylprednisolone for 24 or 48 hours or tirilazad mesylate for 48 hours in the treatment of acute spinal cord injury. Results of the third National Acute Spinal Cord Injury Randomized Controlled Trial. National Acute Spinal Cord Injury Study. JAMA. 1997;277(20):1597–1604. [PubMed] [Google Scholar]
- 11.Gerndt SJ, Rodriguez JL, Pawlik JW, Taheri PA, Wahl WL, Micheals AJ, et al. Consequences of high-dose steroid therapy for acute spinal cord injury. J Trauma. 1997;42(2):279–284. [DOI] [PubMed] [Google Scholar]
- 12.Legos JJ, Gritman KR, Tuma RF, Young WF. Coadministration of methylprednisolone with hypertonic saline solution improves overall neurological function and survival rates in a chronic model of spinal cord injury. Neurosurgery. 2001;49(6):1427–1433. [DOI] [PubMed] [Google Scholar]
- 13.Qian T, Guo X, Levi AD, Vanni S, Shebert RT, Sipski ML. High-dose methylprednisolone may cause myopathy in acute spinal cord injury patients. Spinal Cord. 2005;43(4):199–203. [DOI] [PubMed] [Google Scholar]
- 14.Walters BC, Hadley MN, Hurlbert RJ, Aarabi B, Dhall SS, Gelb DE, et al. Guidelines for the management of acute cervical spine and spinal cord injuries: 2013 update. Neurosurgery. 2013;60(suppl 1):82–91. [DOI] [PubMed] [Google Scholar]
- 15.Fehlings MG, Tetreault LA, Wilson JR, Kwon BK, Burns AS, Martin AR, et al. A clinical practice guideline for the Management of Acute Spinal Cord Injury: Introduction, rationale, and scope. Global Spine J. 2017;7(3 Suppl):84S–94S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fehlings MG, Wilson JR, Tetreault LA, Aarabi B, Anderson P, Arnold PM, et al. A clinical practice guideline for the Management of Patients with Acute Spinal Cord Injury: recommendations on the use of methylprednisolone sodium succinate. Global Spine J. 2017;7(3 Suppl):203S–211S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang D, Miller SC, Liu XM, Anderson B, Wang XS, Goldring SR. Novel dexamethasone-HPMA copolymer conjugate and its potential application in treatment of rheumatoid arthritis. Arthritis Res Ther. 2007;9(1):R2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Quan L, Zhang Y, Dusad A, Ren K, Purdue PE, Goldring SR, et al. The evaluation of the therapeutic efficacy and side effects of a macromolecular dexamethasone prodrug in the collagen-induced arthritis mouse model. Pharm Res. 2016;33(1):186–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu XM, Quan LD, Tian J, Alnouti Y, Fu K, Thiele GM, et al. Synthesis and evaluation of a well-defined HPMA copolymer-dexamethasone conjugate for effective treatment of rheumatoid arthritis. Pharm Res. 2008;25(12):2910–2919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yuan F, Nelson RK, Tabor DE, Zhang Y, Akhter MP, Gould KA, et al. Dexamethasone prodrug treatment prevents nephritis in lupus-prone (NZB x NZW)F1 mice without causing systemic side effects. Arthritis Rheum. 2012;64(12):4029–4039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wei X, Zhao G, Wang X, Gautam N, Jia Z, Zhao Z, et al. Head-to-head comparative pharmacokinetic and biodistribution (PK/BD) study of two dexamethasone prodrug nanomedicines on lupus-prone NZB/WF1 mice. Nanomedicine. 2020;29, 102266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhao Z, Jia Z, Foster KW, Wei X, Qiao F, Jiang H, et al. Dexamethasone prodrug nanomedicine (ZSJ-0228) treatment significantly reduces lupus nephritis in mice without measurable side effects - a 5-month study. Nanomedicine. 2021;31, 102302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wei X, Zhao G, Jia Z, Zhao Z, Chen N, Sun Y, et al. Macromolecular dexamethasone prodrug ameliorates Neuroinflammation and prevents bone loss associated with traumatic brain injury. Mol Pharm. 2022;19(11):4000–4009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wei X, Li F, Zhao G, Chhonker YS, Averill C, Galdamez J, et al. Pharmacokinetic and biodistribution studies of HPMA copolymer conjugates in an aseptic implant loosening mouse model. Mol Pharm. 2017;14(5):1418–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ren K, Dusad A, Yuan F, Yuan H, Purdue PE, Fehringer EV, et al. Macromolecular prodrug of dexamethasone prevents particle-induced peri-implant osteolysis with reduced systemic side effects. J Control Release. 2014;175:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yuan F, Quan LD, Cui L, Goldring SR, Wang D. Development of macromolecular prodrug for rheumatoid arthritis. Adv Drug Deliv Rev. 2012;64(12):1205–1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.David S, Zarruk JG, Ghasemlou N. Inflammatory pathways in spinal cord injury. Int Rev Neurobiol. 2012;106:127–152. [DOI] [PubMed] [Google Scholar]
- 28.Kim YT, Caldwell JM, Bellamkonda RV. Nanoparticle-mediated local delivery of methylprednisolone after spinal cord injury. Biomaterials. 2009;30(13):2582–2590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chvatal SA, Kim YT, Bratt-Leal AM, Lee H, Bellamkonda RV. Spatial distribution and acute anti-inflammatory effects of methylprednisolone after sustained local delivery to the contused spinal cord. Biomaterials. 2008;29(12):1967–1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Karabey-Akyurek Y, Gurcay AG, Gurcan O, Turkoglu OF, Yabanoglu-Ciftci S, Eroglu H, et al. Localized delivery of methylprednisolone sodium succinate with polymeric nanoparticles in experimental injured spinal cord model. Pharm Dev Technol. 2017;22(8):972–981. [DOI] [PubMed] [Google Scholar]
- 31.Lin Y, Li C, Li J, Deng R, Huang J, Zhang Q, et al. NEP1–40-modified human serum albumin nanoparticles enhance the therapeutic effect of methylprednisolone against spinal cord injury. J Nanobiotechnology. 2019;17(1):12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Qin W, Li X, Peng Y, Harlow LM, Ren Y, Wu Y, et al. Sclerostin antibody preserves the morphology and structure of osteocytes and blocks the severe skeletal deterioration after motor-complete spinal cord injury in rats. J Bone Miner Res. 2015;30(11):1994–2004. [DOI] [PubMed] [Google Scholar]
- 33.Qin W, Sun L, Cao J, Peng Y, Collier L, Wu Y, et al. The central nervous system (CNS)-independent anti-bone-resorptive activity of muscle contraction and the underlying molecular and cellular signatures. J Biol Chem. 2013;288(19):13511–13521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Qin W, Zhao W, Li X, Peng Y, Harlow LM, Li J, et al. Mice with sclerostin gene deletion are resistant to the severe sublesional bone loss induced by spinal cord injury. Osteoporos Int. 2016;27(12):3627–3636. [DOI] [PubMed] [Google Scholar]
- 35.Zhao W, Li X, Peng Y, Qin Y, Pan J, Li J, et al. Sclerostin antibody reverses the severe Sublesional bone loss in rats after chronic spinal cord injury. Calcif Tissue Int. 2018;103(4):443–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhao W, Peng Y, Hu Y, Guo XE, Li J, Cao J, et al. Electrical stimulation of hindlimb skeletal muscle has beneficial effects on sublesional bone in a rat model of spinal cord injury. Bone. 2021;144, 115825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Peng Y, Zhao W, Hu Y, Guo XE, Wang J, Hao K, et al. Administration of High-Dose Methylprednisolone Worsens Bone Loss after acute spinal cord injury in rats. Neurotrauma Rep. 2021;2(1):592–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Peng Y, Zhao W, Hu Y, Li F, Guo XE, Wang D, et al. Rapid bone loss occurs as early as 2 days after complete spinal cord transection in young adult rats. Spinal Cord. 2020;58(3):309–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Toro CA, Hansen J, Siddiq MM, Johnson K, Zhao W, Azulai D, et al. The human ApoE4 variant reduces functional recovery and neuronal sprouting after incomplete spinal cord injury in male mice. Front Cell Neurosci. 2021;15, 626192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bramlett HM, Dietrich WD, Marcillo A, Mawhinney LJ, Furones-Alonso O, Bregy A, et al. Effects of low intensity vibration on bone and muscle in rats with spinal cord injury. Osteoporos Int. 2014;25(9):2209–2219. [DOI] [PubMed] [Google Scholar]
- 41.Wu Y, Hou J, Collier L, Pan J, Hou L, Qin W, et al. The administration of high-dose methylprednisolone for 24 h reduced muscle size and increased atrophy-related gene expression in spinal cord-injured rats. Spinal Cord. 2011;49(8):867–873. [DOI] [PubMed] [Google Scholar]
- 42.Van Broeckhoven J, Sommer D, Dooley D, Hendrix S, Franssen A. Macrophage phagocytosis after spinal cord injury: when friends become foes. Brain. 2021;144(10):2933–2945. [DOI] [PubMed] [Google Scholar]
- 43.Basso DM, Beattie MS, Bresnahan JC. A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma. 1995;12(1):1–21. [DOI] [PubMed] [Google Scholar]
- 44.Lenth RV. Java Applets for Power and Sample Size [Computer software] (2006–9). [Available from: http://www.stat.uiowa.edu/~rlenth/Power.
- 45.Seymour LW, Duncan R, Strohalm J, Kopecek J. Effect of molecular weight (mw) of N-(2-hydroxypropyl)methacrylamide copolymers on body distribution and rate of excretion after subcutaneous, intraperitoneal, and intravenous administration to rats. J Biomed Mater Res. 1987;21(11):1341–1358. [DOI] [PubMed] [Google Scholar]
- 46.Hall ED, Bosken JM. Measurement of oxygen radicals and lipid peroxidation in neural tissues. Curr Protoc Neurosci. 2009:1–51 Chapter 7:Unit 7 17. [DOI] [PubMed] [Google Scholar]
- 47.Bethea JR, Nagashima H, Acosta MC, Briceno C, Gomez F, Marcillo AE, et al. Systemically administered interleukin-10 reduces tumor necrosis factor-alpha production and significantly improves functional recovery following traumatic spinal cord injury in rats. J Neurotrauma. 1999;16(10):851–863. [DOI] [PubMed] [Google Scholar]
- 48.Pineau I, Lacroix S. Proinflammatory cytokine synthesis in the injured mouse spinal cord: multiphasic expression pattern and identification of the cell types involved. J Comp Neurol. 2007;500(2):267–285. [DOI] [PubMed] [Google Scholar]
- 49.Byrnes KR, Stoica BA, Fricke S, Di Giovanni S, Faden AI. Cell cycle activation contributes to post-mitotic cell death and secondary damage after spinal cord injury. Brain. 2007;130(Pt 11):2977–2992. [DOI] [PubMed] [Google Scholar]
- 50.Momeni HR. Role of calpain in apoptosis. Cell J. 2011;13(2):65–72. [PMC free article] [PubMed] [Google Scholar]
- 51.Whetstone WD, Hsu JY, Eisenberg M, Werb Z, Noble-Haeusslein LJ. Blood-spinal cord barrier after spinal cord injury: relation to revascularization and wound healing. J Neurosci Res. 2003;74(2):227–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cerqueira SR, Oliveira JM, Silva NA, Leite-Almeida H, Ribeiro-Samy S, Almeida A, et al. Microglia response and in vivo therapeutic potential of methylprednisolone-loaded dendrimer nanoparticles in spinal cord injury. Small. 2013;9(5):738–749. [DOI] [PubMed] [Google Scholar]
- 53.Jha MK, Jo M, Kim JH, Suk K. Microglia-astrocyte crosstalk: an intimate molecular conversation. Neuroscientist. 2019;25(3):227–240. [DOI] [PubMed] [Google Scholar]
- 54.Brennan FH, Li Y, Wang C, Ma A, Guo Q, Li Y, et al. Microglia coordinate cellular interactions during spinal cord repair in mice. Nat Commun. 2022;13(1):4096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Xu L, Wang J, Ding Y, Wang L, Zhu YJ. Current knowledge of microglia in traumatic spinal cord injury. Front Neurol. 2021;12, 796704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liddelow SA, Guttenplan KA, Clarke LE, Bennett FC, Bohlen CJ, Schirmer L, et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature. 2017;541 (7638):481–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sofroniew MV, Vinters HV. Astrocytes: biology and pathology. Acta Neuropathol. 2010;119(1):7–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kreher C, Favret J, Maulik M, Shin D. Lysosomal functions in glia associated with neurodegeneration. Biomolecules. 2021;11(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Qi L, Jiang H, Cui X, Liang G, Gao M, Huang Z, et al. Synthesis of methylprednisolone loaded ibuprofen modified dextran based nanoparticles and their application for drug delivery in acute spinal cord injury. Oncotarget. 2017;8(59):99666–99680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Antal EJ, Wright CE 3rd, Gillespie WR, Albert KS. Influence of route of administration on the pharmacokinetics of methylprednisolone. J Pharmacokinet Biopharm. 1983;11(6):561–576. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


