Abstract
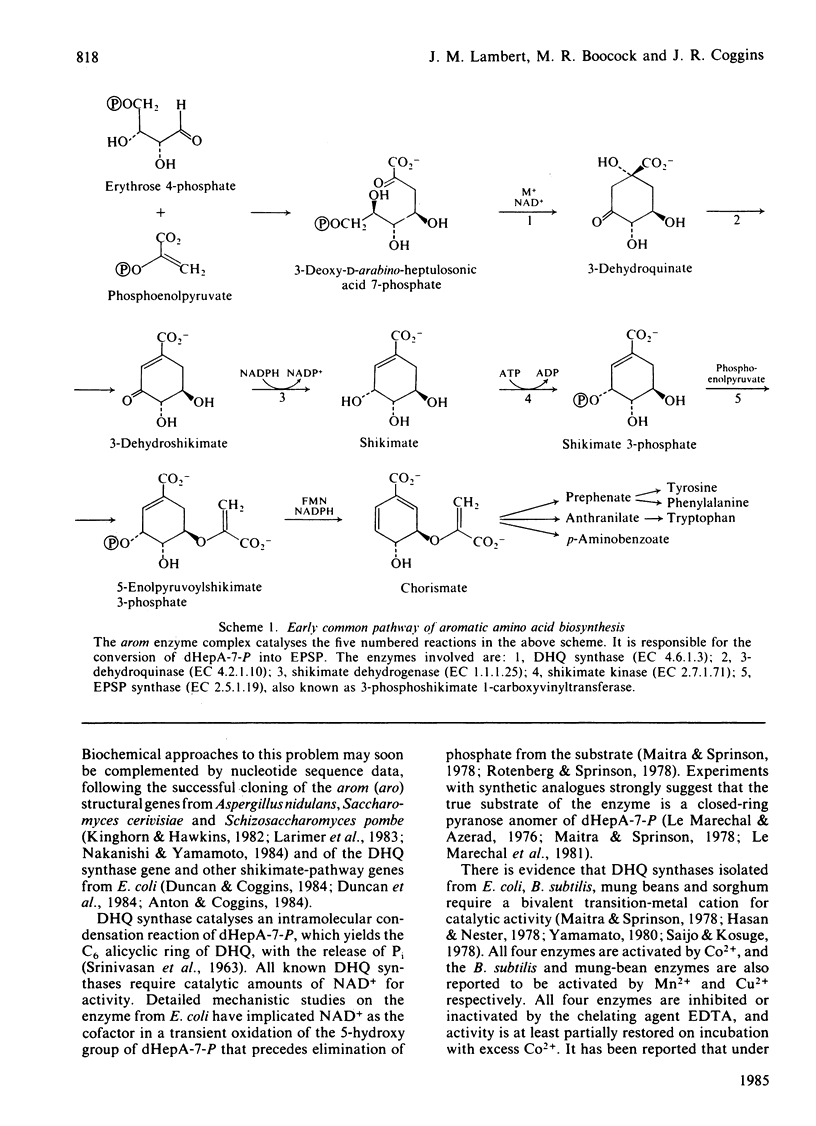
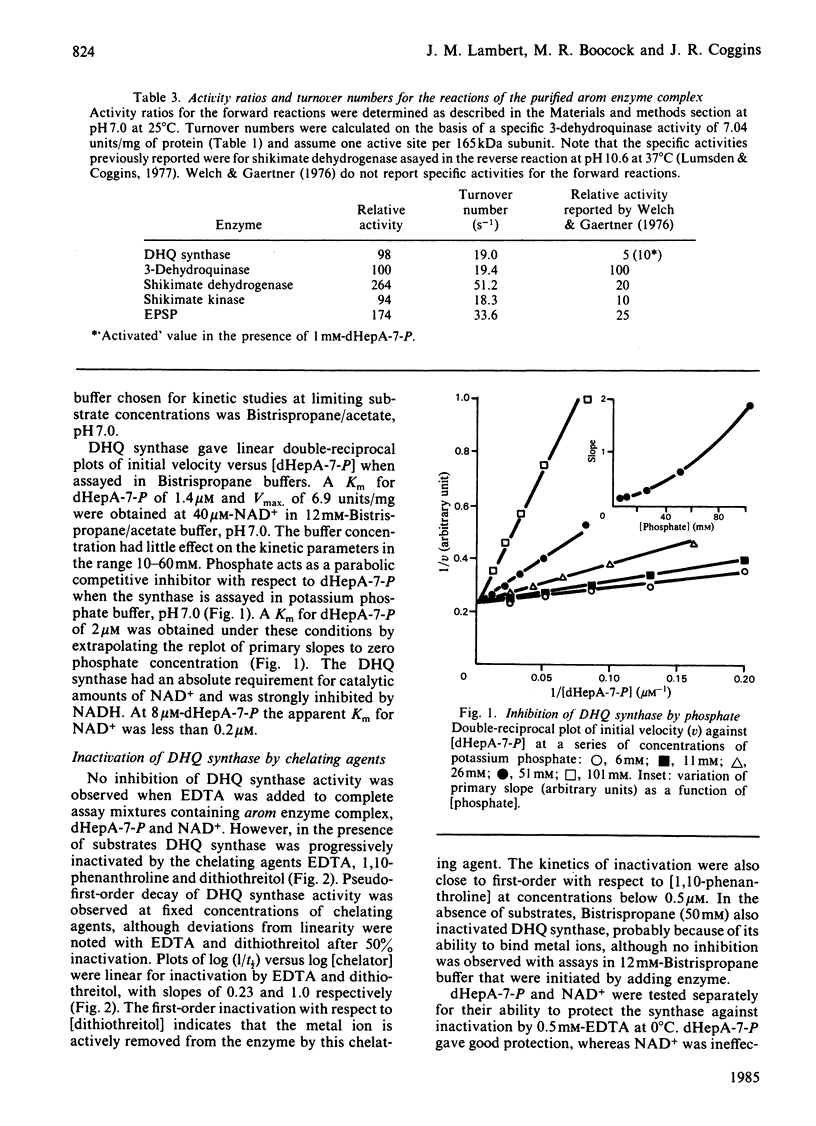
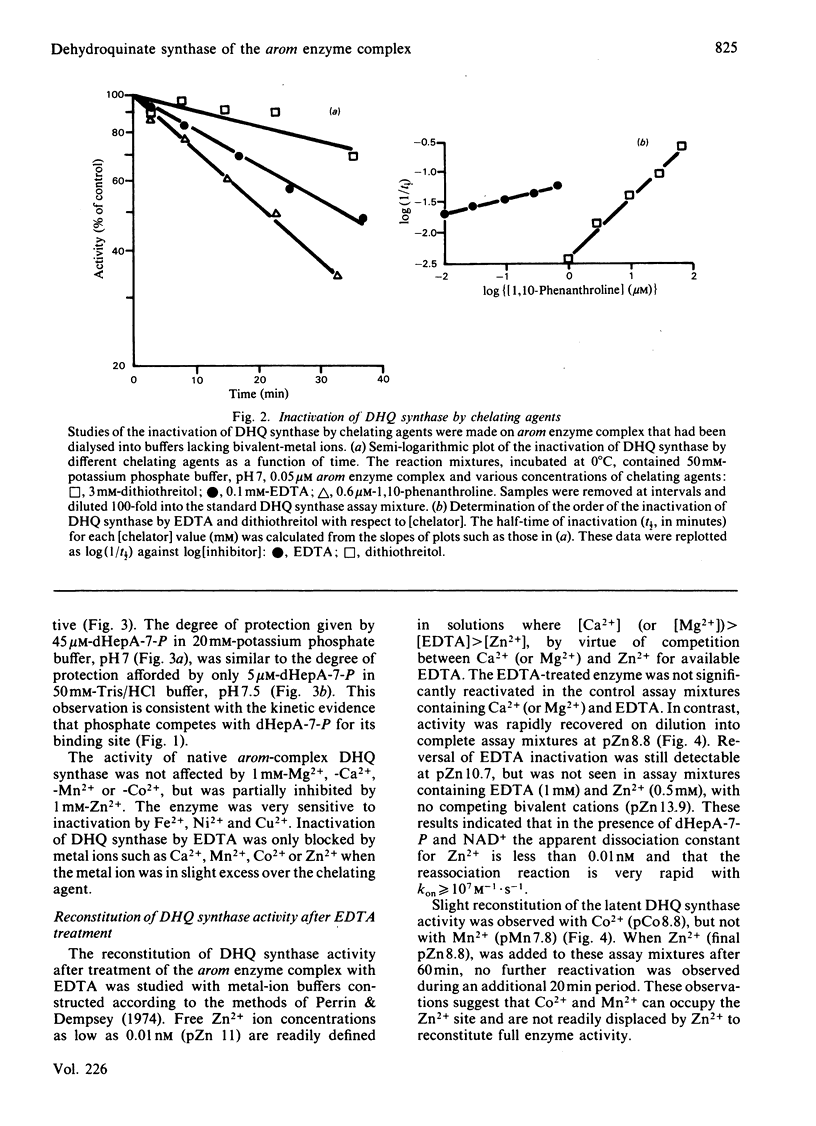
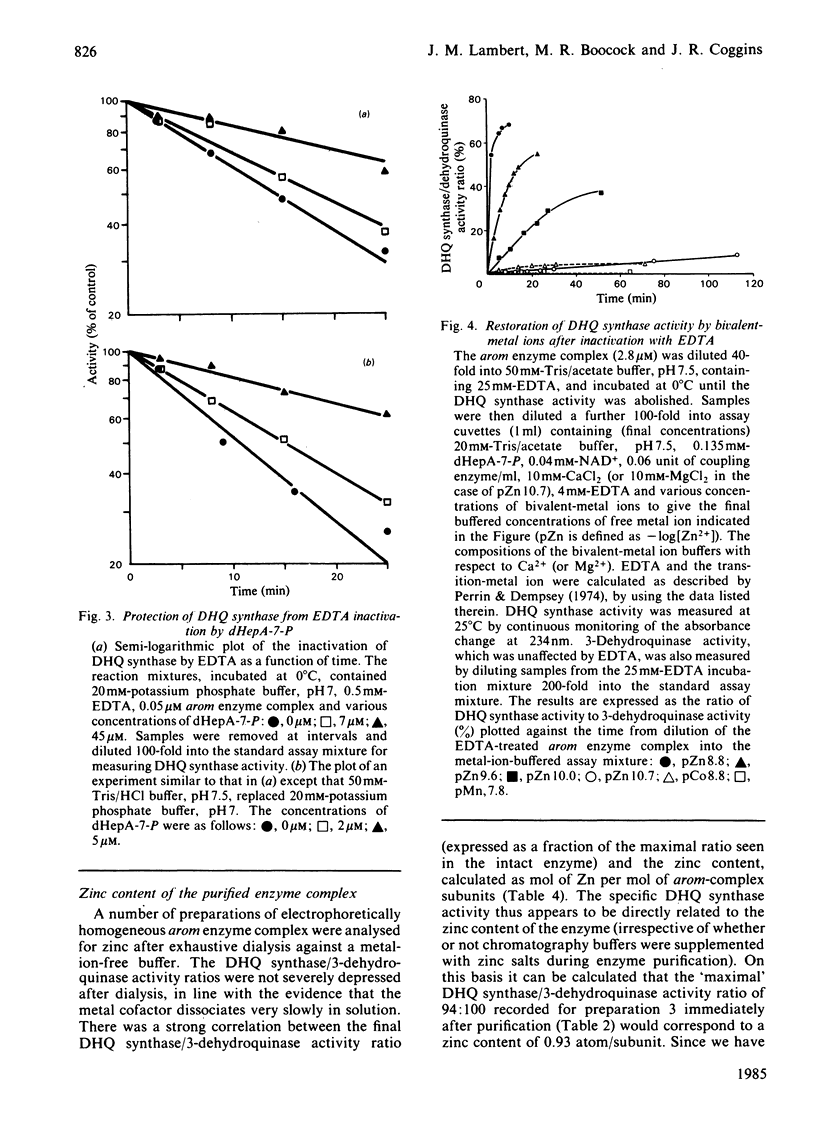
We have demonstrated the co-purification in constant ratio of all five activities of the pentafunctional arom enzyme complex from Neurospora crassa. Progressive inactivation of the 3-dehydroquinate synthase component of the purified enzyme complex by chelating agents was blocked by the substrate, 3-deoxy-D-arabino-heptulosonate 7-phosphate, but not by the cofactor NAD+. Full activity was restored at Zn2+ concentrations as low as 0.05 nM. Atomic absorption data indicated that the intact enzyme complex contained 1 atom per subunit of tightly bound zinc. The arom 3-dehydroquinate synthase had a calculated turnover number of 19s-1, this being within the narrow range of values obtained for the other four activities of the intact multifunctional enzyme. The Km for 3-deoxy-D-arabino-heptulosonate 7-phosphate was 1.4 microM in a phosphate-free buffer; inorganic phosphate was a competitive inhibitor with respect to 3-deoxy-D-arabino-heptulosonate 7-phosphate.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmed S. I., Giles N. H. Organization of enzymes in the common aromatic synthetic pathway: evidence for aggregation in fungi. J Bacteriol. 1969 Jul;99(1):231–237. doi: 10.1128/jb.99.1.231-237.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates D. L., Perham R. N., Coggins J. R. Methods for obtaining peptide maps of proteins on a subnanomole scale. Anal Biochem. 1975 Sep;68(1):175–184. doi: 10.1016/0003-2697(75)90692-2. [DOI] [PubMed] [Google Scholar]
- Berlyn M. B., Giles N. H. Organization of enzymes in the polyaromatic synthetic pathway: separability in bacteria. J Bacteriol. 1969 Jul;99(1):222–230. doi: 10.1128/jb.99.1.222-230.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boocock M. R., Coggins J. R. Kinetics of 5-enolpyruvylshikimate-3-phosphate synthase inhibition by glyphosate. FEBS Lett. 1983 Apr 5;154(1):127–133. doi: 10.1016/0014-5793(83)80888-6. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Burgoyne L., Case M. E., Giles N. H. Purification and properties of the aromatic (arom) synthetic enzyme aggregate of Neurospora crassa. Biochim Biophys Acta. 1969 Nov 4;191(2):452–462. doi: 10.1016/0005-2744(69)90264-2. [DOI] [PubMed] [Google Scholar]
- Case M. E., Giles N. H. Partial enzyme aggregates formed by pleiotropic mutants in the arom gene cluster of Neurospora crassa. Proc Natl Acad Sci U S A. 1971 Jan;68(1):58–62. doi: 10.1073/pnas.68.1.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornell N. W., Crivaro K. E. Stability constant for the zinc-dithiothreitol complex. Anal Biochem. 1972 May;47(1):203–208. doi: 10.1016/0003-2697(72)90293-x. [DOI] [PubMed] [Google Scholar]
- Dixon H. B., Sparkes M. J. Phosphonomethyl analogues of phosphate ester glycolytic intermediates. Biochem J. 1974 Sep;141(3):715–719. doi: 10.1042/bj1410715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floss H. G., Onderka D. K., Carroll M. Stereochemistry of the 3-deoxy-D-arabino-heptulosonate 7-phosphate synthetase reaction and the chorismate synthetase reaction. J Biol Chem. 1972 Feb 10;247(3):736–744. [PubMed] [Google Scholar]
- Frost J. W., Bender J. L., Kadonaga J. T., Knowles J. R. Dehydroquinate synthase from Escherichia coli: purification, cloning, and construction of overproducers of the enzyme. Biochemistry. 1984 Sep 11;23(19):4470–4475. doi: 10.1021/bi00314a036. [DOI] [PubMed] [Google Scholar]
- Gaertner F. H., Cole K. W. A cluster-gene: evidence for one gene, one polypeptide, five enzymes. Biochem Biophys Res Commun. 1977 Mar 21;75(2):259–264. doi: 10.1016/0006-291x(77)91037-3. [DOI] [PubMed] [Google Scholar]
- Gaertner F. H. Purification of two multienzyme complexes in the aromatic-tryptophan pathway of Neurospora crassa. Arch Biochem Biophys. 1972 Jul;151(1):277–284. doi: 10.1016/0003-9861(72)90498-5. [DOI] [PubMed] [Google Scholar]
- Giles N. H., Case M. E., Partridge C. W., Ahmed S. I. A gene cluster in Nuerospora crassa coding for an aggregate of five aromatic synthetic enzymes. Proc Natl Acad Sci U S A. 1967 Oct;58(4):1453–1460. doi: 10.1073/pnas.58.4.1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gracy R. W., Noltmann E. A. Studies on phosphomannose isomerase. II. Characterization as a zinc metalloenzyme. J Biol Chem. 1968 Aug 10;243(15):4109–4116. [PubMed] [Google Scholar]
- Hankins C. N., Mills S. E. A dimer of a single polypeptide chain catalyzes the terminal four reactions of the L-tryptophan pathway in Euglena gracilis. J Biol Chem. 1977 Jan 10;252(1):235–239. [PubMed] [Google Scholar]
- Hasan N., Nester E. W. Dehydroquinate synthase in Bacillus subtilis. An enzyme associated with chorismate synthase and flavin reductase. J Biol Chem. 1978 Jul 25;253(14):4999–5004. [PubMed] [Google Scholar]
- Jacobson J. W., Hart B. A., Doy C. H., Giles N. H. Purification and stability of the multienzyme complex encoded in the arom gene cluster of Neurospora crassa. Biochim Biophys Acta. 1972 Nov 10;289(1):1–12. doi: 10.1016/0005-2744(72)90101-5. [DOI] [PubMed] [Google Scholar]
- Kinghorn J. R., Hawkins A. R. Cloning and expression in Escherichia coli K-12 of the biosynthetic dehydroquinase function of the arom cluster gene from the eucaryote, Aspergillus nidulans. Mol Gen Genet. 1982;186(1):145–152. doi: 10.1007/BF00422927. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Larimer F. W., Morse C. C., Beck A. K., Cole K. W., Gaertner F. H. Isolation of the ARO1 cluster gene of Saccharomyces cerevisiae. Mol Cell Biol. 1983 Sep;3(9):1609–1614. doi: 10.1128/mcb.3.9.1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Marechal P., Azerad R. The shikimate pathway. IV. 3-dehydroquinate synthetase of E. coli. Substrate analogs and inhibitors. Biochimie. 1976 Nov 13;58(9):1145–1148. doi: 10.1016/s0300-9084(76)80094-6. [DOI] [PubMed] [Google Scholar]
- Lewendon A., Coggins J. R. Purification of 5-enolpyruvylshikimate 3-phosphate synthase from Escherichia coli. Biochem J. 1983 Jul 1;213(1):187–191. doi: 10.1042/bj2130187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumsden J., Coggins J. R. The subunit structure of the arom multienzyme complex of Neurospora crassa. A possible pentafunctional polypeptide chain. Biochem J. 1977 Mar 1;161(3):599–607. doi: 10.1042/bj1610599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumsden J., Coggins J. R. The subunit structure of the arom multienzyme complex of Neurospora crassa. Evidence from peptide 'maps' for the identity of the subunits. Biochem J. 1978 Feb 1;169(2):441–444. doi: 10.1042/bj1690441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MITSUHASHI S., DAVIS B. D. Aromatic biosynthesis. XII. Conversion of 5-dehydroquinic acid to 5-dehydroshikimic acid dy 5-dehydroquinase. Biochim Biophys Acta. 1954 Sep;15(1):54–61. doi: 10.1016/0006-3002(54)90093-1. [DOI] [PubMed] [Google Scholar]
- Maitra U. S., Sprinson D. B. 5-Dehydro-3-deoxy-D-arabino-heptulosonic acid 7-phosphate. An intermediate in the 3-dehydroquinate synthase reaction. J Biol Chem. 1978 Aug 10;253(15):5426–5430. [PubMed] [Google Scholar]
- McClure W. R. A kinetic analysis of coupled enzyme assays. Biochemistry. 1969 Jul;8(7):2782–2786. doi: 10.1021/bi00835a014. [DOI] [PubMed] [Google Scholar]
- Nakanishi N., Yamamoto M. Analysis of the structure and transcription of the aro3 cluster gene in Schizosaccharomyces pombe. Mol Gen Genet. 1984;195(1-2):164–169. doi: 10.1007/BF00332740. [DOI] [PubMed] [Google Scholar]
- Nimmo G. A., Coggins J. R. Some kinetic properties of the tryptophan-sensitive 3-deoxy-D-arabino-heptulosonate 7-phosphate synthase from Neurospora crassa. Biochem J. 1981 Dec 1;199(3):657–665. doi: 10.1042/bj1990657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel V. B., Giles N. H. Purification of the arom multienzyme aggregate from Euglena gracilis. Biochim Biophys Acta. 1979 Mar 16;567(1):24–34. doi: 10.1016/0005-2744(79)90168-2. [DOI] [PubMed] [Google Scholar]
- Pittard J., Wallace B. J. Distribution and function of genes concerned with aromatic biosynthesis in Escherichia coli. J Bacteriol. 1966 Apr;91(4):1494–1508. doi: 10.1128/jb.91.4.1494-1508.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rines H. W., Case M. E., Giles N. H. Mutants in the arom gene cluster of Neurospora crassa specific for biosynthetic dehydroquinase. Genetics. 1969 Apr;61(4):789–800. doi: 10.1093/genetics/61.4.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotenberg S. L., Sprinson D. B. Isotope effects in 3-dehydroquinate synthase and dehydratase. Mechanistic implications. J Biol Chem. 1978 Apr 10;253(7):2210–2215. [PubMed] [Google Scholar]
- SRINIVASAN P. R., ROTHSCHILD J., SPRINSON D. B. THE ENZYMIC CONVERSION OF 3-DEOXY-D-ARABINO-HEPTULOSONIC ACID 7-PHOSPHATE TO 5-DEHYDROQUINATE. J Biol Chem. 1963 Oct;238:3176–3182. [PubMed] [Google Scholar]
- Smith D. D., Coggins J. R. Isolation of a bifunctional domain from the pentafunctional arom enzyme complex of Neurospora crassa. Biochem J. 1983 Aug 1;213(2):405–415. doi: 10.1042/bj2130405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- THIERS R. E. Contamination in trace element analysis and its control. Methods Biochem Anal. 1957;5:273–335. doi: 10.1002/9780470110218.ch6. [DOI] [PubMed] [Google Scholar]
- Trigalo F., Level M., Szabó L. Phosphorylated sugars. Part XVII. Synthesis of 3-deoxy-D-arabino [1- minus 14 C]heptulosonic acid 7-(dihydrogen phosphate). J Chem Soc Perkin 1. 1975;(6):600–602. [PubMed] [Google Scholar]
- Welch G. R., Gaertner F. H. Coordinate activation of a multienzyme complex by the first substrate. Evidence for a novel regulatory mechanism in the polyaromatic pathway of Neurospora crassa. Arch Biochem Biophys. 1976 Feb;172(2):476–489. doi: 10.1016/0003-9861(76)90101-6. [DOI] [PubMed] [Google Scholar]
- Welch G. R., Gaertner F. H. Influence of an aggregated multienzyme system on transient time: kinetic evidence for compartmentation by an aromatic-amino-acid synthesizing complex of Neurospora crassa. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4218–4222. doi: 10.1073/pnas.72.11.4218. [DOI] [PMC free article] [PubMed] [Google Scholar]


