Abstract
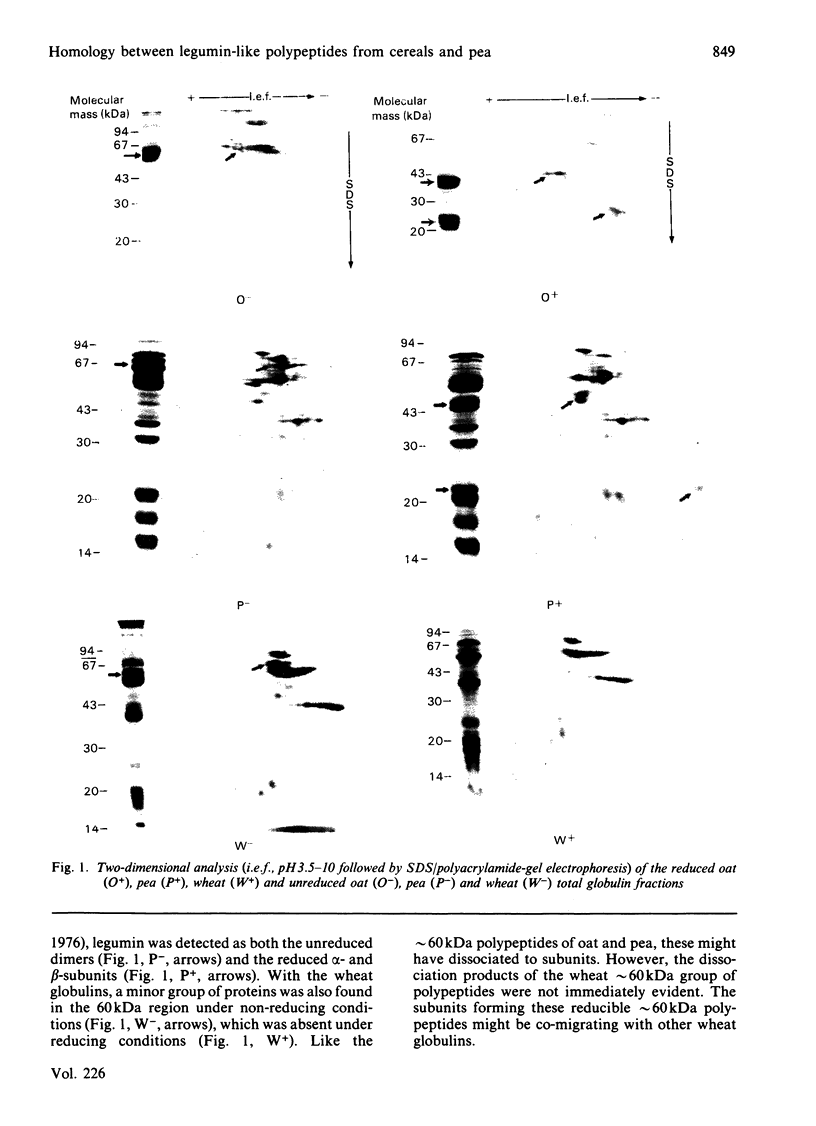
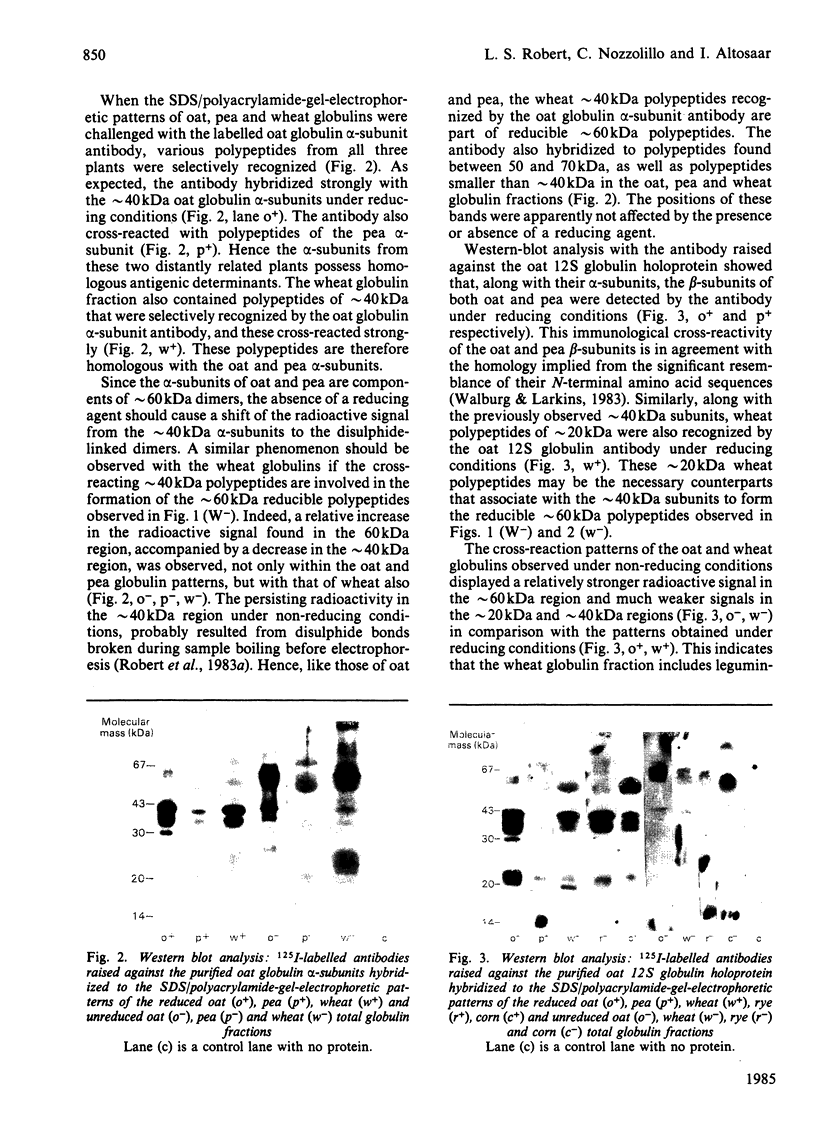
The presence of legumin-like constituents within the globulin fractions of wheat (Triticum aestivum), rye (Secale cereale) and corn (maize, Zea mays) was demonstrated. Two-dimensional analysis of wheat globulins in the presence and absence of a reducing agent revealed the existence of reducible approximately 60 kDa polypeptides. Western-blot analysis with 125I-labelled antibodies raised against the oat (Avena sativa) 12S globulin holoprotein or its alpha-subunits demonstrated, firstly, the immunological homology between the alpha- and beta-subunits of pea (Pisum sativum) legumin and oat 12S globulin, and secondly, the similar occurrence in wheat of antigenically homologous approximately 20kDa and approximately 40 kDa polypeptides that associate via disulphide linkage to form approximately 60 kDa dimers. Western blotting also showed the presence of disulphide-linked approximately 20 kDa and approximately 40 kDa legumin-like subunits within the globulin fractions of rye and corn.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adeli K., Allan-Wojtas P., Altosaar I. Intracellular transport and posttranslational cleavage of oat globulin precursors. Plant Physiol. 1984 Sep;76(1):16–20. doi: 10.1104/pp.76.1.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adeli K., Altosaar I. Characterization of oat vicilin-like polypeptides. Plant Physiol. 1984 May;75(1):225–227. doi: 10.1104/pp.75.1.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adeli K., Altosaar I. Role of endoplasmic reticulum in biosynthesis of oat globulin precursors. Plant Physiol. 1983 Dec;73(4):949–955. doi: 10.1104/pp.73.4.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinegar A. C., Peterson D. M. Separation and characterization of oat globulin polypeptides. Arch Biochem Biophys. 1982 Nov;219(1):71–79. doi: 10.1016/0003-9861(82)90135-7. [DOI] [PubMed] [Google Scholar]
- Brinegar A. C., Peterson D. M. Synthesis of Oat Globulin Precursors : Analogy to Legume 11S Storage Protein Synthesisa. Plant Physiol. 1982 Dec;70(6):1767–1769. doi: 10.1104/pp.70.6.1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danielsson C. E. Seed globulins of the Gramineae and Leguminosae. Biochem J. 1949;44(4):387–400. doi: 10.1042/bj0440387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREENWOOD F. C., HUNTER W. M., GLOVER J. S. THE PREPARATION OF I-131-LABELLED HUMAN GROWTH HORMONE OF HIGH SPECIFIC RADIOACTIVITY. Biochem J. 1963 Oct;89:114–123. doi: 10.1042/bj0890114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson D. M. Subunit structure and composition of oat seed globulin. Plant Physiol. 1978 Oct;62(4):506–509. doi: 10.1104/pp.62.4.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walburg G., Larkins B. A. Oat seed globulin: subunit characterization and demonstration of its synthesis as a precursor. Plant Physiol. 1983 May;72(1):161–165. doi: 10.1104/pp.72.1.161. [DOI] [PMC free article] [PubMed] [Google Scholar]