Abstract
Objective
To evaluate the costs, time to surgery, and clinical outcomes associated with implementing a streamlined hypoglossal nerve stimulator (HGNS) implantation pathway.
Study Design
Retrospective cohort study.
Setting
Single tertiary care center in the United States from 2016 to 2023.
Methods
Patients with a lack of complete concentric collapse of the velum during volitional snore on in‐office laryngoscopy qualified for the streamlined HGNS pathway. This pathway consisted of confirmatory drug‐induced sleep endoscopy (DISE) followed immediately by HGNS implantation during the same surgical encounter. Outcomes were compared to patients in the traditional pathway (standalone DISE followed by HGNS implantation on a later date).
Results
A total of 68 patients (13 streamlined, 55 traditional) with obstructive sleep apnea who underwent HGNS implantation were included. Patients were predominately male (70.6%) and White (95.6%) and had a mean (SD) age of 63.5 (10.0) years. The streamlined pathway was associated with a significant reduction in both hospital costs (mean difference $9258, 95% confidence interval [CI]: 3690‐14,825; P = .002) and time to surgery (mean decrease of 3.82 months, 95% CI: 0.83‐6.80 months; P = .013) compared to the traditional pathway. Patients in both groups had reduction in apnea‐hypopnea index and Epworth Sleepiness Scale score, with no significant differences in comparisons between groups.
Conclusion
In select patients, the streamlined HGNS pathway may expedite time to surgery and reduce hospital costs with comparable clinical outcomes to a traditional 2‐stage pathway. Further research is warranted to validate patient selection and better understand longitudinal outcomes.
Keywords: cost‐benefit analysis, electric stimulation therapy, hospital costs, hypoglossal nerve, obstructive sleep apnea, patient‐reported outcome measures, time‐to‐treatment
An estimated 25% of adults in the United States, or 65 million individuals, 1 suffer from obstructive sleep apnea (OSA). Patients with OSA experience not only sleep deprivation, impaired cognitive function, and altered mood but also an increased risk of hypertension, heart disease, stroke, arrhythmias, and motor vehicle accidents. 2 , 3 Additionally, OSA can exacerbate metabolic disorders, contributing to insulin resistance and type 2 diabetes. 4 There is not enough evidence to support that treatment of OSA can reduce the risk of cardiovascular outcomes or all‐cause mortality. 3 , 5 , 6 Although continuous positive airway pressure (CPAP) reduces disease severity in OSA and improves sleep‐related quality of life, 7 it is limited by poor adherence, with an estimated 25% of patients with OSA who do not tolerate CPAP. 8
High‐level evidence supports the efficacy of hypoglossal nerve stimulation in treating OSA that is refractory to positive airway pressure modalities. 9 , 10 , 11 , 12 , 13 Since its approval by the Food and Drug Administration in 2014, over 50,000 patients have been implanted. 14 However, before undergoing hypoglossal nerve stimulator (HGNS) implantation, patients are required to undergo a drug‐induced sleep endoscopy (DISE) to rule out complete concentric collapse (CCC) of the palate, 15 which was associated with inferior HGNS outcomes in early studies. 16 DISE is typically performed as a standalone procedure in an operating room or endoscopy suite as a prerequisite to HGNS implantation. This 2‐stage process can introduce barriers to HGNS implantation due to the associated costs, 17 extra time required away from work or caregiving responsibilities, and delays in implantation due to surgery scheduling. To address these barriers, our team piloted a streamlined DISE‐HGNS protocol, in which select patients could undergo a DISE followed by HGNS implantation during the same surgical encounter if DISE confirmed a lack of CCC of the palate. 18
Our primary aim for this study was to evaluate costs and time to surgery in the streamlined DISE‐HGNS protocol compared to the traditional pathway. A secondary aim was to evaluate differences in OSA outcomes based on polysomnography and Epworth Sleepiness Scale (ESS). We hypothesized that the streamlined DISE‐HGNS protocol would be associated with reduced costs and earlier HGNS implantation, and that there would be no significant difference in postimplantation OSA parameters.
Methods
This study was approved by the University of Michigan Institutional Review Board (IRB#: HUM00189394).
Patient Sample
We retrospectively identified patients 18 years or older who underwent HGNS implantation at our institution through October 2023. Two study authors (P.T.H. and J.J.S.) performed all of the surgeries for this sample. Patients were excluded if cost data were not available.
Streamlined Versus Traditional HGNS Pathways
Potential candidates for HGNS were first evaluated through a multidisciplinary sleep clinic that provided a comprehensive assessment of sleep disorders from both a sleep surgeon and a sleep medicine physician. Patients were considered potential candidates for HGNS if they had moderate‐to‐severe OSA based on a sleep study (and less than 25% central events), inability to tolerate positive airway pressure treatment, and body mass index (BMI) less than 32.0. Other nonsurgical (eg, weight loss, positioning, mandibular advancement devices, etc) and surgical (eg, palate surgery, tonsillectomy, hyoid suspension, maxillomandibular advancement, etc) treatment options for OSA were considered based on patient and clinical factors. The decision to proceed with HGNS implantation was based on a combination of clinical factors and multidisciplinary discussion between the patient and treatment team.
In 2021, our team initiated a streamlined HGNS pathway in which select patients could undergo DISE immediately followed by HGNS implantation during the same surgical encounter. To qualify for this pathway, patients had to demonstrate a lack of CCC of the soft palate during in‐office volitional snore with flexible laryngoscopy, which has a high sensitivity and positive predictive value for predicting DISE outcomes. 18 Patients also had to meet the same criteria for HGNS implantation by the sleep surgeon based on comprehensive clinical evaluation. Patients were counseled preoperatively that if confirmatory DISE showed CCC of the soft palate, the surgeon would not proceed with HGNS implantation. The streamlined pathway was approved by Blue Cross Blue Shield (BCBS) of Michigan; therefore, only patients with BCBS of Michigan were offered this pathway.
Patients enrolled in the traditional HGNS pathway included patients who met criteria for HGNS implantation based on comprehensive clinical evaluation by the sleep surgeon, and who subsequently underwent standalone DISE followed by HGNS implantation on a future date. This group included both patients who presented prior to the implementation of the streamlined HGNS pathway as well as those who were selected for the traditional pathway based on insurance carrier (any insurer other than BCBS of Michigan) or based on patient or surgeon preference.
Exposure and Outcome Variables
We abstracted demographic and clinical variables, including age, sex, self‐identified race and ethnicity, BMI, clinical encounter dates, ESS scores, and polysomnographic data from patient charts.
Primary outcome variables included cost and time to surgery. Cost variables included total insurance payment, total patient costs, and total hospital costs for each surgical encounter. Insurance payments were the dollar amount paid to the hospital by the insurance company after negotiating the final price. Patient costs were the out‐of‐pocket costs paid by the patient, including deductibles, copays, and co‐insurance. Hospital costs were the total amount of spending reported by the hospital for each surgical encounter. Hospital costs were distinct from “hospital charges.” Hospital costs were calculated as the sum of all professional and facility costs for the surgical encounter, including professional costs for both surgeons and anesthesiologists. The facility costs that were incorporated into the total hospital costs included items such as equipment, operating room supplies, and pharmaceuticals. For the traditional pathway, costs for the 2 surgical encounters (DISE and HGNS implantation) were added to obtain the total costs. All financial data was adjusted for the regional wage index and inflation based on 2023 dollars using publicly available data. 19 , 20 Adjustment for inflation used a multiplier based on the relative consumer price index for each year compared to 2023. Time to HGNS implantation was defined as the time from the initial clinic visit with the sleep surgeon to the date of HGNS implantation.
Secondary outcomes included changes in the apnea‐hypopnea index (AHI), Sher20 responder status, and ESS. We abstracted sleep study data from the most recent preoperative sleep study and the most recent sleep study available after HGNS implantation. Postoperative sleep studies included both titration polysomnograms and home sleep apnea tests. AHI calculation was based on the American Academy of Sleep Medicine hypopnea definition of at least a 30% reduction in airflow and a 3% reduction in oxygen saturation or arousal. 21 Responder status was defined based on the Sher20 criteria (at least a 50% reduction in AHI and postoperative AHI < 20 events/h). 22
Statistical Analysis
We summarized patient demographic and clinical characteristics using descriptive statistics. For patients with available preoperative and postoperative sleep studies, we used χ 2 tests, Fisher's exact test, and 2‐sided t tests to compare patient characteristics between the streamlined and traditional pathways. Next, we used 2‐sided t tests to evaluate differences in costs (insurance payments, patient costs, and hospital costs) and time to surgery between the streamlined and traditional pathways.
For patients with available sleep study data, we used the two one‐sided t tests (TOSTs) procedure with an equivalence margin of ±15 events/h to evaluate for differences in change in AHI between the streamlined and traditional pathways. We used TOST with an equivalence margin of ±2 points to evaluate differences in change in ESS between the 2 pathways. For TOST, statistical equivalence can be inferred if the 95% confidence interval (CI) for the mean difference between groups is contained within the equivalence margin and the P value is less than .05. We assessed differences in Sher20 responder status between the 2 groups using Fisher's exact test.
Statistical significance was set at P < .05 for all analyses. We used SAS software version 9.4 for all analyses and GraphPad Prism version 9.1.0 (GraphPad Software) to create the figures.
Results
Baseline Characteristics
A total of 68 patients who underwent HGNS implantation from 2016 to 2023 were included. Patients had a mean (SD) age of 63.5 (10.0) years. Most patients were male (n = 48, 70.6%) and white (n = 65, 95.6%). A total of 55 patients (80.9%) were included in the traditional HGNS pathway, and 13 patients (19.1%) were included in the streamlined HGNS pathway. All patients in the streamlined protocol had BCBS of Michigan insurance. In the traditional pathway, 28 patients (50.9%) had BCBS, 24 (43.6%) had Medicare, 2 (3.6%) had Medicaid, and 1 (1.8%) had Tricare insurance.
Differences in Costs and Time to Surgery
Among all patients, the mean (SD) insurance payment was $52,555 (SD 39,402), the mean (SD) patient cost was $306 (SD 724), and the mean (SD) hospital cost was $41,026 (SD 9496).
When stratified by pathway, the mean (SD) insurance payment was $63,871 (35,205) in the streamlined group and $49,880 (40,159) in the traditional group (mean difference $−13,990, 95% CI: −38,191 to 10,210; P = .253). The mean (SD) patient cost was $446 (1001) in the streamlined group and $273 (651) in the traditional group (mean difference $−172, 95% CI: −620 to 276; P = .446). The mean (SD) hospital cost was $34,039 (2058) in the streamlined group and $43,297 (8967) in the traditional group (mean difference $9258, 95% CI: 3690 to 14,825; P = .002) (Figure 1).
Figure 1.
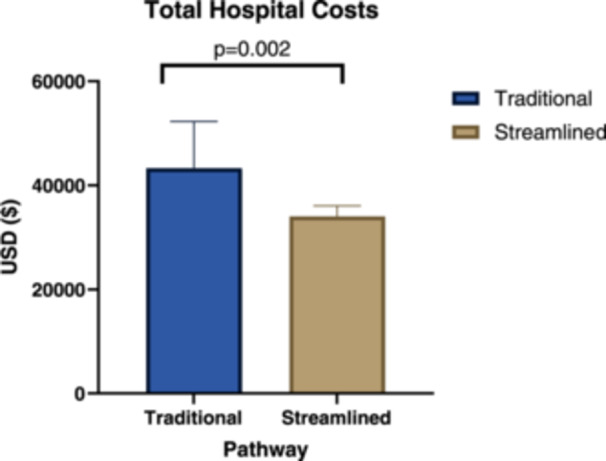
Differences in hospital costs by pathway.
The mean (SD) time from the initial clinic visit with a sleep surgeon to HGNS implantation was 3.62 (2.48) months in the streamlined group and 7.44 (5.21) months in the traditional group (mean difference of 3.82 months, 95% CI: 0.83‐6.80 months; P = .013) (Figure 2).
Figure 2.
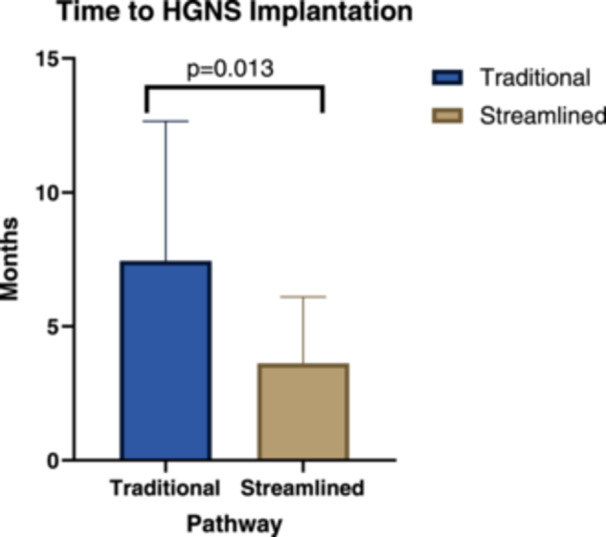
Differences in time to HGNS implantation by pathway. HGNS, hypoglossal nerve stimulator.
Sensitivity Analysis Among Patients With BCBS Insurance
Among patients with BCBS‐based plans (n = 41), the mean (SD) insurance payment was $63,871 (35,205) in the streamlined group and $69,721 (47,257) in the traditional group (mean difference $5851, 95% CI: −23,953 to 35,654; P = .694). The mean (SD) patient cost was $445 (1001) in the streamlined group and $396 (807) in the traditional group (mean difference $−49.5, 95% CI: −641 to 542; P = .866). The mean (SD) hospital cost was $34,039 (2058) in the streamlined group and $43,252 (5363) in the traditional group (mean difference $9213, 95% CI: 6043 to 12,384; P < .001).
HGNS Outcomes
There were 42 patients (10 streamlined, 32 traditional) with available pre‐ and postoperative sleep study data. There was no significant difference in age, sex, preoperative AHI, preoperative oxygen nadir, preoperative ESS score, type of postoperative sleep study, or time from HGNS implantation to a postoperative sleep study or ESS score between the streamlined and traditional groups (Table 1). Patients in the streamlined group had a lower preoperative BMI compared to patients in the traditional group (mean [SD]: 26.9 kg/m2 [4.2] vs 29.7 kg/m2 [3.3]; P = .037).
Table 1.
Baseline Characteristics in Streamlined and Traditional Groups for Patients With Pre‐ and Postoperative Sleep Studies
| Streamlined (n = 10) | Nonstreamlined (n = 32) | P value | |
|---|---|---|---|
| Age (mean, SD) | 58.4 (10.8) | 64.3 (10.4) | .126 |
| Sex | 1.000 | ||
| Male | 7 (70.0%) | 24 (75.0%) | |
| Female | 3 (30.0%) | 8 (25.0%) | |
| BMI (mean, SD) | 26.9 (4.2) | 29.7 (3.3) | .037 |
| Preoperative AHI (mean, SD) | 37.5 (16.7) | 38.4 (15.8) | .873 |
| Preoperative O2 nadir (mean SD) | 82.0 (7.1) | 78.6 (7.7) | .249 |
| Preoperative ESS (mean, SD) | 8.4 (5.6) | 8.2 (6.0) | .910 |
| Type of most recent sleep study | 1.000 | ||
| Home sleep apnea test | 5 (50%) | 16 (50%) | |
| Titration polysomnogram | 4 (40%) | 12 (37.5%) | |
| Nontitration polysomnogram | 1 (10%) | 4 (12.5%) | |
| Days from HGNS implantation to most recent sleep study (mean, SD) | 264.2 (192.5) | 473.4 (467.0) | .178 |
| Days from HGNS implantation to most recent ESS score (mean, SD) | 382.0 (236.7) | 635.2 (556.1) | .199 |
Abbreviations: AHI, apnea‐hypopnea index; BMI, body mass index; ESS, Epworth Sleepiness Scale; HGNS, hypoglossal nerve stimulator.
The mean (SD) change in AHI was −18.8 (19.1) events/h in the streamlined group and −21.2 (21.6) events/h in the traditional group (mean difference −2.41 events/h, 95% CI: −17.8 to 13.0; TOST P = .053 for an equivalence margin of ±15 events/h). There was no significant difference in responder status based on the Sher20 criteria (60.0% in the streamlined group vs 62.5% in the traditional group; P = 1.000). The mean (SD) change in ESS score was −3.9 (4.7) points in the streamlined group and −3.0 (4.8) points in the traditional group (mean difference 0.89 points, 95% CI: −3.0 to 4.7; TOST P = .280 for an equivalence margin of ±2).
Discussion
Our study highlights the potential for significant hospital cost savings and improved time to surgery using a streamlined HGNS protocol for select patients with OSA. Compared to the traditional pathway, which involves a staged DISE and HGNS on a later date, we found that the streamlined pathway was associated with an average savings of $9258 in hospital costs and 3.8 months improvement in time to surgery from the initial clinic visit. The polysomnogram and ESS outcomes between the traditional and streamlined groups did not differ significantly. Collectively, these findings suggest that the streamlined HGNS pathway is a viable alternative to the traditional pathway and may confer benefits to patients and the health care system.
In patients who are good candidates for HGNS implantation based on clinical factors and have the probability of favorable DISE findings based on in‐office volitional snore, the streamlined HGNS pathway might expedite their care and save an extra trip to the operating room. Patients undergoing sleep surgery consultation often experience delays in the diagnosis of OSA or referral to a specialist. 23 Untreated OSA is associated with higher health care utilization and costs, 24 , 25 and it confers significant patient morbidity in the form of increased cardiovascular risk, neurocognitive impairment, and diminished quality of life. 3 Expediting definitive surgical treatment of OSA can, therefore, can have important downstream benefits for both the patient and the health care system.
By reducing the number of trips to the operating room, the streamlined HGNS pathway may also improve access for patients with long travel distances, limited transportation, difficulty taking time off work, and caregiving responsibilities. One mixed methods study identified transportation and childcare as key barriers to accessing surgical care, and the prevalence of these barriers was higher in non‐white participants. 26 There is already some indirect evidence to suggest that there are access barriers to HGNS implantation. For example, despite a higher prevalence of OSA among Black and Hispanic individuals compared to White individuals, 27 , 28 a recent study of patients undergoing HGNS implantation in the Adherence and Outcome of Upper Airway Stimulation for OSA International Registry (ADHERE) registry identified only 125 non‐White patients (4.5%) out of 2755 patients in the entire registry. 29 Further research is warranted to better understand upstream access barriers for HGNS implantation and the role of potential interventions.
Our study demonstrated that hospitals saved, on average, $9258 in costs per patient with the streamlined HGNS protocol. This would equate to 1 million dollars in direct health care savings after just 108 surgeries. Although the magnitude of insurance payments and patient out‐of‐pocket costs were higher in the streamlined pathway, these differences were not statistically significant and were largely diminished in the sensitivity analysis limited to BCBS patients. These findings suggest that the type of insurance may be a major driver of the differences in insurance payments and patient costs. In contrast, the clinical care pathway may be the major driver of differences in hospital costs. Other studies have found significant price variation within and between hospitals for the same procedures, 30 , 31 , 32 including HGNS implantation, 33 and one contributor to this is private versus government‐sponsored insurance. 34
Importantly, our findings suggest that streamlined HGNS was not associated with worse surgical outcomes. Although it did not meet the threshold for statistical equivalence, the magnitude of the mean difference in the change in AHI and ESS scores between the 2 groups (−2.41 events/h of 0.89 points, respectively) is small and likely not clinically meaningful. Furthermore, there was no significant difference in Sher20 responder status between the 2 groups (60.0% in the streamlined group vs 62.5% in the traditional group; P = 1.000).
Overall, these findings suggest that the streamlined HGNS pathway can reduce hospital costs and expedite surgery without compromising quality of care. Given the potential benefits of the streamlined HGNS pathway, additional research is warranted to better define patient selection and outcomes. Specifically, more data is needed to validate the test characteristics of in‐office volitional snore in predicting DISE findings and evaluate longitudinal patient‐reported outcomes and objective sleep quality in the streamlined pathway. This could be accomplished via a multi‐institutional trial.
There is a growing body of evidence of factors beyond DISE that can be used to help guide optimal patient selection for HGNS, such as BMI, 35 , 36 Friedman tongue position, 37 supine pharyngeal width, 38 hypopharyngeal cross‐sectional area on tongue protrusion, 35 preoperative AHI, 39 , 40 arousal threshold, 41 and loop gain. 41 In a pilot study of 41 patients, our team found that the pattern of collapse at the level of the soft palate during volitional snore had a high correlation with DISE findings (sensitivity 93.5%, positive predictive value 82.9%). 18 Based on this growing collection of data, stakeholders in sleep medicine could conceivably develop a protocol for HGNS candidacy based on favorable phenotypic criteria (eg, integrating volitional snore, supine pharyngeal width, BMI, and PSG data), and reserving standalone DISE for patients who do not meet these criteria.
Our study is limited by its relatively small sample size, skewed toward patients in the traditional pathway. Due to the limited statistical power, the findings from our study should be interpreted with caution. Furthermore, the sample is from a single institution cohort with an overrepresentation of White patients relative to adult OSA patients in the United States, 3 so it may not be generalizable to our target population. The overrepresentation of White patients in our study is similar to the ADHERE database, 29 and it suggests that there may be upstream access barriers for the surgical treatment of OSA. A potential confounder in our analysis of HGNS outcomes was BMI, which was lower in the streamlined group. Furthermore, although not statistically significant, patients in the traditional pathway had longer times to postoperative sleep studies and ESS scores, which could plausibly affect outcomes. Additional potential unmeasured confounders include socioeconomic variables, patient comorbidities, and changes in populations or practices over time.
Despite the limitations, this analysis provides data on early outcomes of a novel clinical care pathway. Although the findings from this study are preliminary, they provide a rationale for larger‐scale studies, potentially exploring the streamlined pathway across other payors and populations. Finally, although the streamlined pathway may help reduce barriers to care for patients, deliberative efforts are needed to mitigate upstream access barriers and ensure that it is accessible to all potential candidates.
Conclusion
The streamlined HGNS pathway was associated with reduced hospital costs and expedited surgery for select patients with OSA. More research is warranted to validate patient selection for the streamlined HGNS pathway and assess longitudinal outcomes.
Author Contributions
Nicholas R. Lenze, study design, data collection, statistical analysis, manuscript writing, final approval; Jayden Garcia, study design, data collection, manuscript writing, final approval; Punithavathy Vijayakumar, study design, manuscript review, final approval; Sonja G. Schütz, study design, manuscript review, final approval; Michael J. Brenner, study design, manuscript review, final approval; Jeffrey J. Stanley, study design, manuscript review, final approval; Paul T. Hoff, study design, manuscript review, final approval.
Disclosures
Competing interests
Dr Paul T. Hoff reports involvement on the Inspire Advisory Council, Cryosa safety monitor, and Nyxoah safety monitor. Dr Sonja G. Schütz reports grants or contracts between Huxley Medical Inc, Apple Inc, Oura Health Ltd, and the University of Michigan.
Funding source
Nicholas R. Lenze was supported by the MORE R25 research grant (DC020262).
Acknowledgments
The authors would like to thank Alex Bercu and Brian McPike for retrieving, organizing, and supplying the financial data.
This article was presented at the AAO‐HNSF 2024 Annual Meeting & OTO EXPO; September 28 to October 1, 2024; Miami Beach, Florida.
References
- 1. Blakeslee L, Caplan Z, Meyer J, Rabe M, Roberts A. Age and sex composition: 2020. United States Census Bureau. 2023. Accessed June 12, 2024. https://www2.census.gov/library/publications/decennial/2020/census-briefs/c2020br-06.pdf
- 2. Faria A, Allen AH, Fox N, Ayas N, Laher I. The public health burden of obstructive sleep apnea. Sleep Sci. 2021;14(3):257‐265. 10.5935/1984-0063.20200111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gottlieb DJ, Punjabi NM. Diagnosis and management of obstructive sleep apnea: a review. JAMA. 2020;323(14):1389‐1400. 10.1001/jama.2020.3514 [DOI] [PubMed] [Google Scholar]
- 4. Lee W, Nagubadi S, Kryger MH, Mokhlesi B. Epidemiology of obstructive sleep apnea: a population‐based perspective. Expert Rev Respir Med. 2008;2(3):349‐364. 10.1586/17476348.2.3.349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Balk EM, Adam GP, Cao W, Bhuma MR, D'Ambrosio C, Trikalinos TA. Long‐term effects on clinical event, mental health, and related outcomes of CPAP for obstructive sleep apnea: a systematic review. J Clin Sleep Med. 2024;20(6):895‐909. 10.5664/jcsm.11030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dedhia RC, Bliwise DL, Quyyumi AA, et al. Hypoglossal nerve stimulation and cardiovascular outcomes for patients with obstructive sleep apnea: a randomized clinical trial. JAMA Otolaryngol Head Neck Surg. 2024;150(1):39‐48. 10.1001/jamaoto.2023.3756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Patil SP, Ayappa IA, Caples SM, Kimoff RJ, Patel SR, Harrod CG. Treatment of adult obstructive sleep apnea with positive airway pressure: an American Academy of Sleep Medicine systematic review, meta‐analysis, and GRADE assessment. J Clin Sleep Med. 2019;15(2):301‐334. 10.5664/jcsm.7638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cistulli PA, Armitstead J, Pepin JL, et al. Short‐term CPAP adherence in obstructive sleep apnea: a big data analysis using real world data. Sleep Med. 2019;59:114‐116. 10.1016/j.sleep.2019.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Woodson BT, Strohl KP, Soose RJ, et al. Upper airway stimulation for obstructive sleep apnea: 5‐year outcomes. Otolaryngol Head Neck Surg. 2018;159(1):194‐202. 10.1177/0194599818762383 [DOI] [PubMed] [Google Scholar]
- 10. Strollo PJ, Soose RJ, Maurer JT, et al. Upper‐airway stimulation for obstructive sleep apnea. N Engl J Med. 2014;370(2):139‐149. 10.1056/NEJMoa1308659 [DOI] [PubMed] [Google Scholar]
- 11. Certal VF, Zaghi S, Riaz M, et al. Hypoglossal nerve stimulation in the treatment of obstructive sleep apnea: a systematic review and meta‐analysis. Laryngoscope. 2015;125(5):1254‐1264. 10.1002/lary.25032 [DOI] [PubMed] [Google Scholar]
- 12. Freeman CG, Durgham R, Ren E, et al. The impact of hypoglossal nerve stimulation on secondary health outcomes. Ear Nose Throat J. 2024;29:1455613241235538. 10.1177/01455613241235538 [DOI] [PubMed] [Google Scholar]
- 13. Heiser C, Steffen A, Hofauer B, et al. Effect of upper airway stimulation in patients with obstructive sleep apnea (EFFECT): a randomized controlled crossover trial. J Clin Med. 2021;10(13):2880. 10.3390/jcm10132880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.2022 Annual report. Inspire Medical Systems Inc. 2022. Accessed November 10, 2023. https://d18rn0p25nwr6d.cloudfront.net/CIK-0001609550/c301639a-04ba-4ed5-a000-b73f9f75fd52.pdf
- 15. Dedhia RC, Huyett P. A prognostic star was born: drug‐induced sleep endoscopy for hypoglossal nerve stimulation. J Clin Sleep Med. 2020;16(S1):15‐16. 10.5664/jcsm.8882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Vanderveken OM, Maurer JT, Hohenhorst W, et al. Evaluation of drug‐induced sleep endoscopy as a patient selection tool for implanted upper airway stimulation for obstructive sleep apnea. J Clin Sleep Med. 2013;9(5):433‐438. 10.5664/jcsm.2658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ghazizadeh S, Moore K, Kiai K, Mendelsohn AH. Drug‐induced sleep endoscopy performed in the endoscopy suite: a resource utilization. Otolaryngol Head Neck Surg. 2020;162(3):386‐391. 10.1177/0194599820901516 [DOI] [PubMed] [Google Scholar]
- 18. Yalamanchi P, Mott N, Ali SA, et al. Evaluation of in‐office volitional snore as a screening tool for candidacy for hypoglossal nerve stimulation. Otolaryngol Head Neck Surg. 2022;166(3):595‐597. 10.1177/01945998211023733 [DOI] [PubMed] [Google Scholar]
- 19.Wage index files. US Centers for Medicare & Medicaid Services. 2024. Accessed May 24, 2024. https://www.cms.gov/medicare/payment/prospective-payment-systems/acute-inpatient-pps/wage-index-files
- 20.Consumer price index. US Bureau of Labor Statistics. 2024. Accessed May 24, 2024. https://www.bls.gov/cpi/data.htm
- 21. Berry RB, Abreu AR, Krishnan V, Quan SF, Strollo PJ, Malhotra RK. A transition to the American Academy of Sleep Medicine‐recommended hypopnea definition in adults: initiatives of the Hypopnea Scoring Rule Task Force. J Clin Sleep Med. 2022;18(5):1419‐1425. 10.5664/jcsm.9952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sher AE, Schechtman KB, Piccirillo JF. The efficacy of surgical modifications of the upper airway in adults with obstructive sleep apnea syndrome. Sleep. 1996;19(2):156‐177. 10.1093/sleep/19.2.156 [DOI] [PubMed] [Google Scholar]
- 23. Ikeda AK, McShay C, Marsh R, et al. Barriers and communication behaviors impacting referral to sleep surgery: qualitative patient perspectives. J Clin Sleep Med. 2023;19(1):111‐117. 10.5664/jcsm.10260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wickwire EM, Tom SE, Vadlamani A, et al. Older adult US Medicare beneficiaries with untreated obstructive sleep apnea are heavier users of health care than matched control patients. J Clin Sleep Med. 2020;16(1):81‐89. 10.5664/jcsm.8128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ding Q, Kryger M. Greater health care utilization and cost associated with untreated sleep apnea. J Clin Sleep Med. 2020;16(1):5‐6. 10.5664/jcsm.8152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Johnson CL, Schwartz H, Greenberg A, et al. Patient perceptions on barriers and facilitators to accessing low‐acuity surgery during COVID‐19 pandemic. J Surg Res. 2021;264:30‐36. 10.1016/j.jss.2021.01.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chen X, Wang R, Zee P, et al. Racial/ethnic differences in sleep disturbances: the multi‐ethnic study of atherosclerosis (MESA). Sleep. 2015;38(6):877‐888. 10.5665/sleep.4732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dudley KA, Patel SR. Disparities and genetic risk factors in obstructive sleep apnea. Sleep Med. 2016;18:96‐102. 10.1016/j.sleep.2015.01.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Khan M, Stone A, Soose RJ, et al. Does race‐ethnicity affect upper airway stimulation adherence and treatment outcome of obstructive sleep apnea. J Clin Sleep Med. 2022;18(9):2167‐2172. 10.5664/jcsm.10068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rochlin DH, Rizk NM, Matros E, Wagner TH, Sheckter CC. Commercial price variation for breast reconstruction in the era of price transparency. JAMA Surg. 2023;158(2):152‐160. 10.1001/jamasurg.2022.6402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bartholomew RA, Rathi VK, Suresh K, Sethi RKV, Lee DJ, Xiao R. Hospital‐negotiated pricing of cochlear implants. Otolaryngol Head Neck Surg. 2023;169(3):734‐737. 10.1002/ohn.280 [DOI] [PubMed] [Google Scholar]
- 32. Miller AL, Xiao R, Rathi VK, et al. Hospital prices for pediatric tympanostomy tube placement and adenotonsillectomy in 2021. Laryngoscope. 2023;133(4):948‐955. 10.1002/lary.30236 [DOI] [PubMed] [Google Scholar]
- 33. Bartholomew RA, Russo MV, Xiao R, Rathi VK, Haleem A, Huyett PA. Payer‐negotiated pricing of the hypoglossal nerve stimulator. J Clin Sleep Med. 2022;18(9):2333‐2334. 10.5664/jcsm.10142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Fahmy JN, Mead M, Chung WT, Ibrahim AM, Chung KC. Reported prices for high volume hand surgery in the era of price transparency: implications for future policy iterations. Plast Reconstr Surg. Published online March 4, 2024. 10.1097/PRS.0000000000011378 [DOI] [PubMed] [Google Scholar]
- 35. Boroosan A, Salapatas AM, Friedman M. Clinical predictors of osa treatment success following implantation of a hypoglossal nerve stimulation device. Otolaryngol Head Neck Surg. 2022;167(5):891‐895. 10.1177/01945998221087594 [DOI] [PubMed] [Google Scholar]
- 36. Suurna MV, Steffen A, Boon M, et al. Impact of body mass index and discomfort on upper airway stimulation: ADHERE registry 2020 update. Laryngoscope. 2021;131(11):2616‐2624. 10.1002/lary.29755 [DOI] [PubMed] [Google Scholar]
- 37. Xiao R, Trask DK, Kominsky AH. Preoperative predictors of response to hypoglossal nerve stimulation for obstructive sleep apnea. Otolaryngol Head Neck Surg. 2020;162(3):400‐407. 10.1177/0194599820901499 [DOI] [PubMed] [Google Scholar]
- 38. Weiner JS, Munhall CC, Kent DT. Supine pharyngeal width is associated with complete concentric palatal collapse during drug‐induced sleep endoscopy and hypoglossal nerve stimulator outcomes. Ear Nose Throat J. 2022;29:014556132211483. 10.1177/01455613221148313 [DOI] [PubMed] [Google Scholar]
- 39. Chao TN, Thaler ER. Predictors of success in hypoglossal nerve stimulator implantation for obstructive sleep apnea. World J Otorhinolaryngol Head Neck Surg. 2021;7(1):40‐44. 10.1016/j.wjorl.2020.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Renslo B, Hobelmann K, Sagalow ES, Ananth A, Boon M, Huntley C. Palatal coupling maneuvers do not predict hypoglossal nerve stimulator treatment efficacy. Laryngoscope. 2023;133(2):431‐436. 10.1002/lary.30397 [DOI] [PubMed] [Google Scholar]
- 41. Op de Beeck S, Wellman A, Dieltjens M, et al. Endotypic mechanisms of successful hypoglossal nerve stimulation for obstructive sleep apnea. Am J Respir Crit Care Med. 2021;203(6):746‐755. 10.1164/rccm.202006-2176OC [DOI] [PMC free article] [PubMed] [Google Scholar]


