Abstract
The increasing incidence of hepatocellular carcinoma (HCC) is of great concern not only in the United States but throughout the world because of two major reasons: firstly, HCC is one of the most lethal form of malignancies with less than 10% survival rate and secondly, a lack of prudent diagnostics makes early detection of HCC nearly impossible. The poor prognosis of HCC accentuates the need to develop new diagnostic markers and therapeutic approaches. In this review we discuss recent advances made in the discovery of molecular biomarkers and their significance in the detection of HCC. We focus on three major classes of biomarkers: serological, tumor, peri-tumoral tissue and cancer stem cell markers. Considerable progress has been made recently in our understanding of HCC at the molecular level increasing the potential of molecular targeted therapy. A number of molecular targets have been identified that have been showing promising results. Of particular interest is Sorafenib, a multi-tyrosine kinase inhibitor that has been approved for the HCC treatment. Inhibitors of other molecular targets such as VEGF, EGFR, mTOR etc. are emerging as plausible therapeutic agents for the treatment of HCC and are discussed in this review.
Keywords: Hepatocellular carcinoma (HCC), multi-kinase inhibitor
BACKGROUND
Hepatocellular carcinoma (HCC) is one of the deadliest form of primary cancers with a 5-year survival rate of 10% or less [1]. HCC is the fifth most common cancer worldwide and ranks third among all primary cancer-related mortalities [2]. Development of hepatocellular carcinoma is generally a multi-step process that results from an acute or chronic hepatic injury caused by viral infection, toxic accumulation, impaired metabolism or congenital predisposition [3]. Liver damage progression over decades leads to fibrosis and cirrhosis, the primary indication of HCC-development. Although more than 80% of the HCC cases occur in Eastern Asia or sub-Saharan Africa due to higher propensity to viral infection, a sharp increase of HCC associated mortality has been documented in the West in the last half century [3].
In Asia and sub-Saharan Africa, Hepatitis B and Hepatitis C infections dominantly contribute to acute and chronic liver insult. Poor vaccination practice in these areas is reflected by endemic hepatitis and high malignancy rate due to HCC. Heavy alcohol consumption for prolonged periods is often suggested to be responsible for HCC as it is associated with liver cirrhosis. However, a direct carcinogenic role of alcohol is yet to be investigated [2]. Aflatoxin, which causes DNA damage is known to be a strong hepato-carcinogen and often thought to accelerate the development of malignancy in patients with infectious hepatitis [2].
Though HCC rarely develops without liver cirrhosis or pre-existing viral infection, several studies indicated that obesity can lead to fatty acid associated liver toxicity and lipid deposition in hepatocytes causing steatosis which can give rise to NASH (nonalcoholic steatohepatits) and NAFLD (nonalcoholic fatty liver disease), depending on the severity of the disease [4]. NASH and NAFLD are essentially characterized by inflammation and apoptosis of hepatocytes [5]. Some studies suggest continuous apoptosis leads to hepatocytic mitogenesis and pressure of incessant cell turnover may give rise to tumorigenesis [4,5].
Early stages of HCC are generally asymptomatic and the tumor grows rapidly [6]. Surgical cytoreduction followed by chemotherapy and liver transplant are the options available only at early and locally confined stages of HCC. Limited treatment option for late diagnosis explains the low survival rate [7,8]. Only early diagnosis can improve this statistics and therefore, detailed study of the molecular markers involved in HCC can be of profound importance.
Cancer is known to be an accumulation of molecular aberrations and is characterized by altered genetic structures and expressions; resulting in modified protein structures and expression of particular proteins. Traditionally tumor biopsy and in more recent days analysis of body fluids help to detect these anomalies and these identifications can be used as molecular markers. Studies during last two decades revealed specific biomarkers associated with particular types of cancers. Discovery of molecular markers significantly improved the survival rate in some cancers as early diagnosis helped to monitor the tumor load, assess the tumor metastasis, tumor advancement and evaluate the therapeutic strategy [9].
Ultrasonography (US) is most commonly used to identify hepatic lesions. But specificity of ultrasonography often depends on individual skill of analysis. In addition, ultrasonography is not very sensitive technique to identify small hepatic lesions [10]. Combined approaches, such as ultrasonography along with serum biomarkers have been in practice for last three decades. In this review, we discuss the most frequently used and considered to be potential molecular markers for the diagnosis of HCC.
Currently available therapeutic options for HCC mostly depend on surgical resection, liver transplantation, radiofrequency ablation, chemoembolization and radioembolization [11,12]. Patients with portal invasion, which indicates poor prognosis, primarily undergo aggressive hepatectomy followed by chemotherapy regime. Patients with hepatitis infection-associated HCC are often treated with interferon therapy (to tackle viral load) and with nucleotide analogs [13,14]. Chemotherapy drugs such as Cisplatin, Oxaplatin, Doxorubicin, and Gemcitabine are also commonly used to treat HCC patients. However, in recent years molecular targeted therapies have shown promising results during clinical trials of several cancers including HCC. A number of signaling pathways in hepatocellular carcinoma are altered and aberrant due to epigenetic changes or mutational changes in structural changes. Hepatocellular carcinoma is highly vascularized with dense micro-vessel population in tumors. One of the most commonly used drugs to target angiogenesis related signaling pathways is Sorafenib, a small molecule tyrosine kinase inhibitor [15]. Small molecule kinase inhibitors and monoclonal antibodies have shown tremendous potential in HCC treatment when used in combination with some conventional chemotherapeutic drugs. In this review, we provide a precise account of currently available or emerging molecular targeted therapies.
A. MOLECULAR BIOMARKERS AND THEIR IMPORTANCE IN DIAGNOSIS AND THERAPY
Molecular markers used for HCC-diagnosis can be categorized into three major types based on the biological samples used for this purpose and are summarized in Fig. (1). In this review, we discuss briefly these three classes of molecular biomarkers:
Fig. (1).
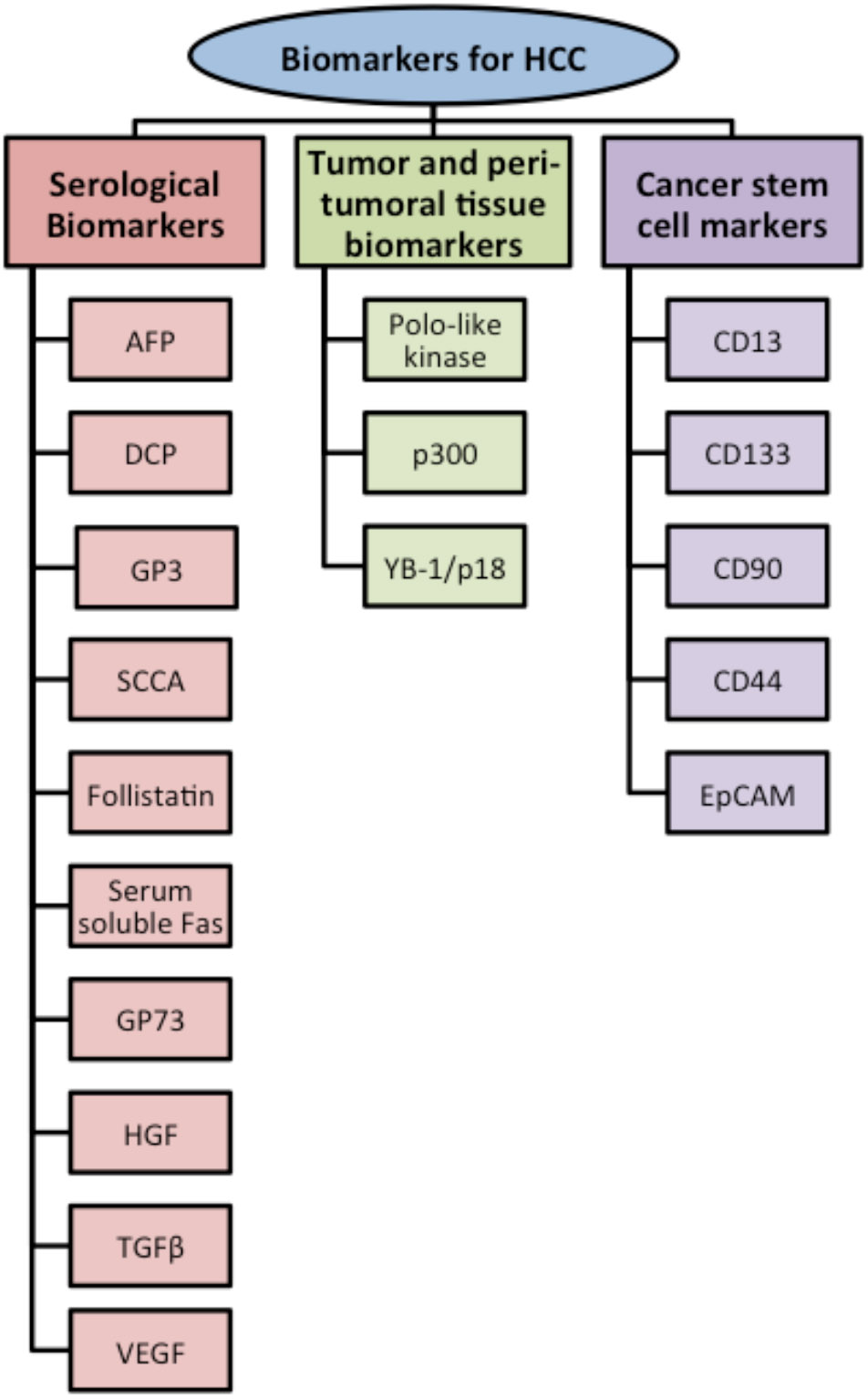
Classification of biomarkers used to diagnose Hepatocellular carcinoma (HCC).
Serological markers,
Tumor and peri-tumoral tissue markers, and
Cancer stem cell markers.
I. Serological Biomarkers
α-Fetoprotein (AFP)
Alpha-fetoprotein, a 70 kD glycoprotein is the most widely used serum marker for HCC diagnosis. AFP is generally synthesized by embryonic yolk sack, fetal liver and intestinal tract. The expression of AFP, which is functionally uncharacterized, become repressed after birth and is documented to be expressed only in pregnant women. From the 1960s, AFP has been associated with several pathological conditions of liver- acute and chronic liver diseases, with high degree of hepatocyte regeneration, hepatitis B infection and liver cirrhosis. AFP is also used as a molecular marker for embryonic cancer, gastric and lung cancer [16,17].
Though AFP is the most commonly used HCC biomarker, its sensitivity and predictive accuracy often depends on the AFP cut-off value used for the tests. Some studies suggest values greater than 400 ng/ml should be considered to be the cut-off value for HCC diagnosis [10]. However, only 30% of the patients ever show serum AFP level higher than 100 ng/ml [10,16]. It has been suggested that a progressive build-up of AFP should be taken as an alarming sign and follow-up with routine diagnostic work is recommended. Other studies were conducted with an AFP cut-off value of 20 ng/ml to make the tests more sensitive. But even with lower AFP cut-off levels it was highly difficult to identify patients with small (< 3 cm) tumors. Sensitivity of HCC diagnosis is shown to be 60-80% with a 20 ng/ml AFP cut-off value, however, the sensitivity decreases to 20-40% for the detection of small tumors [16,17]. On the other hand, as several chronic liver conditions and hepatitis infections are characterized by high serum-AFP level, low AFP cut-off levels often produce false positive results. AFP is also not a very useful diagnostic tool for recurrent HCC cases [8].
Des-Gamma Carboxyprothrombin (DCP)/ Protein Induced by Vitamin K Absence (PIVKAII)
DCP is a commonly used HCC biomarker in eastern Asia (especially Japan) and North America. DCP is an abnormal prothrombin protein found in the serum of HCC patients [18].
Hepatocytes synthesize prothrombins and the process involves γ-carboxylation and vitamin K- dependent γ-glutamyl carboxylase. A deficiency in vitamin K or reduced levels of γ-glutamyl carboxylase results in the secretion of immature prothrombin, which lacks essential γ-carboxyglutamic acid residues in blood [18]. This abnormal, immature prothrombin is also known as Desgamma carboxypothrombin or DCP [19]. Although the association between HCC and DCP is not fully characterized, DCP can be a useful tool to diagnose portal vein invasion by hepatic tumor and aggressive tumor progression. DCP has been a widely used HCC biomarker since 1984 [20], however, it fails to identify individuals with small tumors.
Several studies suggest that better diagnostic results can be achieved if AFP and DCP are measured simultaneously as HCC biomarkers. The diagnostic cut-off value for DCP is 60 mAU/ml and 80 mAU/ml when measured alone and along with AFP, respectively. Higher serum DCP levels generally corresponds to higher proliferation activity of tumor, poor prognosis and intrahepatic spreading [21]. Even though some studies indicate that DCP analysis shows lower sensitivity, it is considered to have higher specificity than AFP with respect to HCC diagnosis [22].
Glypican-3 (GP3)
Glypican-3, an oncofetal gene, is known to be largely expressed in nonalcoholic steatohepatitis (NASH)-associated HCC [6]. GP3 is a member of heat-shock protein (HSP) family and plays a critical role in cell growth, differentiation and migration [6]. GP3 is believed to promote HCC growth by stimulating canonical Wnt signaling pathways [6,23]. Initially, only GP3 mRNA was thought to be elevated in HCC and was used as a biomarker [23], however, a study in 2003 by Capurro et al., showed immunoblotting and ELISA can be used to measure GP3 in patients’ serum [23]. Serological sensitivity and specificity of GP3 as HCC biomarker is 53% and 95%, respectively. Combined use of AFP and GP3 has been suggested to be more useful as HCC biomarkers [6,23].
Squamous Cell Carcinoma Antigen (SCCA)
SCCA-1 and SCCA-2 are both approximately 45 kD proteins, members of serine proteinase inhibitor (sepin) family. SCCA is detected in high concentrations in serum of individuals suffering from certain types of squamous and epithelial malignancy. Although, SCCA is present in the cytoplasm of squamous epithelium, it is believed to be secreted to the circulatory system only by certain cancers [24]. HCC show higher concentration of SCCA than the peritumoral tissues. Also, serum concentration of SCCA is significantly higher in HCC than in the patients with non-malignant liver cirrhosis. But level of serum SCCA does not help to make any inference regarding tumor size, progression and prognosis [24,25].
Follistatin
Follistatin is a secreted glycoprotein and interact closely with TGFβ superfamily proteins and activin. In liver activin A acts as negative regulator of hepatocyte proliferation and induces apoptosis. Follistatin binds to activin A with high affinity and inhibits receptor binding of activin A [26]. Follistatin abolishes activin A regulated signaling pathways leading to apoptosis and promotes growth and proliferation of hepatocytes. Thus, activin A and follistatin can be used as biomarkers for HCC. However, activin A and follistatin are deregulated in several chronic hepatic disorders including inflammation, fibrosis and liver failure, and using activin A or follistatin alone can lead to false positive results for HCC. Follistatin cannot be used as a biomarker for alcoholic or nonalcoholic liver diseases for the same reasons [26,27].
Serum Soluble Fas
Death-inducing Fas and Fas-ligand (FasL) play unique role in hepatic tumor growth and metastasis. Fas/FasL system, which plays a major role in development, maturation and functioning of B- and T- lymphocytes, is known to be up-regulated in chronic liver diseases and accelerates the progression of the disease. But like many other solid tumor system, HCC expresses low levels of Fas and FasL in order to invade the killing lymphocytes [28,29]. Interestingly, several independent studies reported the presence of elevated level of soluble Fas (sFas) in the serum of patients suffering from HCC.
Patients with lupus, leukemia and hepatoma often express several isoforms of soluble Fas (sFas), generated by alternative splicing or in-frame deletion of exon. Despite the fact that the characterization of soluble splice variant of Fas is incomplete, sFas is believed to act as a decoy receptor, which prevents Fas/FasL binding and inhibits Fas-mediated apoptosis [29,30]. Several independent studies showed that serum sFas is significantly higher in HCC patients when compared with patients suffering from chronic hepatitis C or liver cirrhosis. These studies also suggested that the level of sFas could be used to categorize the progression of liver diseases as sFas levels reflects the degree of hepatic damage [29,31]. According to El Bassiouny et al, evaluation of hepatic carcinogenesis should be done by taking both Fas and sFas into account; as hepatic Fas level is significantly low and sFas is significantly higher in hepatic carcinoma when compared with serum samples collected from chronic hepatitis C and liver cirrhosis [31].
Golgi Protein 73 (GP73)/ Golph2
GP73 is a Golgi-specific 73 kD trans-membrane glycoprotein, normally localized in the membranes of cis-Golgi complex. GP73 is expressed by biliary epithelial cells in normal liver and serum level of GP73 is reported to be elevated in a number of chronic and acute, benign and malignant liver diseases. Several independent studies suggested GP73 as a potential biomarker for HCC diagnosis and post-operative monitoring [16,32,33]. Even though serum GP73 level is found to be increased in chronic hepatitis infections, liver cirrhosis and hepatocellular carcinoma, a study by Mao et al. demonstrated the utility of GP73 in monitoring the disease progression from hepatitis B to liver cirrhosis and then finally into HCC [34]. The study with 4217 serum samples taken from healthy individuals and patients suffering from benign and malignant liver diseases showed serum GP73 elevation level is modest in hepatitis B virus carriers, moderate in liver cirrhosis patients and dramatically increased in HCC patients [34]. GP73 is also shown to be useful to distinguish between benign and malignant tumors and also between liver and non-liver malignancies due to its significantly high serum level in HCC patients [34,35]. Independent studies demonstrated a drop in GP73 level after surgical cytoreduction of HCC, however, post-surgery increase of GP73 level in serum correlates with tumor reappearance. According to Hann et al, post-operative GP73 build up takes place even with the appearance of small tumor lesions, which are undetectable by ultrasonography [36]. It has been suggested that the sensitivity of GP73 as a diagnostic tool for HCC is better than AFP [16,34]. GP73 has a great potential to be used as a serum marker for diagnosis, surveillance, recurrence and post-operative management of HCC.
HGF
Hepatocyte growth factor (HGF), which plays a crucial role in liver regeneration in response to hepatic injury, is considered to be a useful biomarker for several tumors including HCC. Fibroblasts and adipose tissues are main storage compartments of HGF and it is known to be secreted by Kupffer cells and sinusoidal endothelial cells [37]. Recent studies indicated that cancer cells within solid tumors secrete HGF and store it within the tumor [38,39].
HGF binds to the proto-oncogene tyrosine kinase receptor c-Met, present on hepatocyte surface and activates a number of transcription factors, pro-metastatic and pro-angiogenic proteins. Several studies revealed HGF plays a pivotal role in aggressive metastasis of HCC by stimulating the expression or secretion of matrix metalloproteinases (MMPs), vascular endothelial growth factor (VEGF). Despite several theories suggesting that HGF accelerate early stages of tumor development, evidence implicates its strong role in advanced metastasis and late tumor growth [38,40].
Patients with HCC show a much higher level of serum HGF than observed in patients with chronic hepatitis or liver cirrhosis. Yamagamim et al observed that all patients with HCC had serum HGF level higher than 0.31 ng/ml [41]. Patients with serum HGF level higher than 0.6 ng/ ml showed poor prognosis, even when serum AFP and DCP level remained normal. Although, elevated level of HGF can be falsely associated with several chronic liver diseases, recent clinical studies suggest that HGF has a high potential as biomarker for HCC [32,41].
TGFβ
The transforming growth factor β (TGFβ) family of cytokines regulates growth and differentiation of both normal and transformed hepatocytes and plays a pivotal role in liver regeneration. Active, dimer TGFβ family of cytokines is known to phosphorylate and activate SMAD family of transcription factors and regulate growth inhibition and apoptosis of normal cells [38,42]. But recent studies suggest that this multi-functional cytokine acts as a tumor suppressor during early stages of tumor development and as a proto-oncogene during the late phase of carcinogenesis. Proto-oncogenic functions of late TGFβ are associated with increased invasiveness and tumor metastasis by overexpression of HIF 1A, VEGF, MMP and VIM and positive cell-cycle regulation by over-activation of cyclin and cyclin-dependent kinases [43].
Independent clinical studies showed elevated TGFβ1 mRNA and protein levels in HCC tissues and patient serum. Higher serum TGFβ levels in patients, where chronic hepatitis B lead to HCC, generally indicate poor prognosis [43,44]. Song et al suggested sensitivity of TGFβ as HCC biomarker is much higher than AFP, especially to detect HCC at the early stage [42]. In a test group of 234 patients, TGFβ was used as a successful serologic marker with a sensitivity of 68% with a cut-off value of 800 pg/ ml [42]. Although TGFβ appears to be a potential candidate for serologic marker in HCC diagnosis, its overexpression in liver cirrhosis, due to reduced hepatic clearance, might lead to some false positive results [32].
VEGF
Vascular endothelial growth factor (VEGF) is the most well-characterized angiogenic factor closely associated with tumor progression and prognosis in several carcinogenesis. Recent studies reveal that this endothelial cell mitogen is a potential indirect biomarker of HCC progression [45]. In hepatocellular carcinoma VEGF is also associated with venous invasion and metastasis and serum of patients with remotely metastasized tumor showed much higher level of serum VEGF when compared with serum sample collected from HCC patients without metastasis. Moreover, as elevated serum VEGF level is closely related with microscopic venous invasion and intrahepatic metastasis, VEGF can be of immense importance as a biomarker to screen patients suitable for liver transplant [46]. Higher serum VEGF level in patients with small HCC (< 5 cm) indicates poor prognosis and poor 3-year tumor-free survival chance after liver transplantation surgery [16,47].
Overall, VEGF alone is not useful as a diagnostic marker and is insufficient to predict prognosis of liver cirrhosis to HCC, even though incidence of elevated serum VEGF level correlates with higher AFP and DCP level in serum [48]. However, Poon et al suggested that serologic VEGF levels > 500 pg/ ml can be used as an independent molecular marker to predict portal vein invasion by HCC and degree of metastasis [46].
II. Tumor and Peri-Tumoral Tissue Biomarkers
Polo-Like Kinase
Polo-like kinase (PLK) is a family of cell cycle controlling kinase and is known to be aberrantly up-regulated in several cancers. PLK-1 is a highly conserved serine/threonine kinase and regulates several mitosis related mechanisms [49]. Overexpression of PLK-1 is used as a prognostic marker for glioma, lymphoma, breast, bladder and gastric cancers and independent studies revealed its association with HCC [49]. Immuno-histochemical studies followed by mRNA analysis showed PLK-1 is highly elevated in HCC and this increase is up to 82.22 % when compared to that of surrounding normal tissues and regenerating nodules [49]. Elevated PLK-1 in tumors corresponds to venous invasion, poor prognosis and low 5-year survival rate [50]. PLK-1 is a potential biomarker which can be used for pre-surgery screening of HCC patients [49,50].
p300
p300 is a transcriptional co-activator belonging to histone acetyltransferase family and is known to regulate cell proliferation, cell-cycle, apoptosis, and DNA damage response [51,52]. p300 is up-regulated in various cancers and believed to play an important role in the tumorigenesis of HCC [51]. Immuno-histochemical studies, RT-PCR and immunoblotting revealed elevated level and different distribution of p300 in HCC tissues compared to adjacent non-malignant liver tissues [52]. High p300 level in tumors is closely associated with aggressive migration, intrahepatic metastasis, vascular invasion of tumors and poor prognosis. As high p300 level correlates with significantly shortened survival, it is a potential biomarker for pre-surgery screening and a useful index to design treatment strategy for HCC patients [51,52].
Y-Box Binding Protein-1 (YB-1)/p18
Y-box protein is a member of an evolutionary conserved protein family and has been recently documented to be up-regulated in breast, ovarian, non-small cell lung cancer and hepatocellular carcinoma [53]. Full length and fragmented YB-1 is actively secreted by both transformed and non-transformed cells. The 18 kD fragment of YB-1, known as YB-1/p18 is found in the plasma sample of 80% of the patients suffering from advanced hepatocellular carcinoma. While all the healthy individuals showed the presence of full-length 50 kD YB-1, only plasma samples from patients with advanced HCC have YB-1/p18 which harbors the evolutionary conserved cold-shock domain [54]. YB-1 is believed to up-regulate the transcription of EGFR, PDGF, MMP-2 and other proliferation-associated genes and is often associated with aggressive metastasis, development of drug-resistance and decreased apoptosis [54]. High plasma levels of YB-1/p18 indicate advanced malignancy and poor prognosis of HCC. As overexpression of YB-1/p18 often correlates with early relapse of tumor growth after surgery, it can be used as a molecular marker for pre-operative screening and post-operative maintenance [54]. The expression level of YB-1/p18 is not influenced by the degree of hepatic damage associated with liver cirrhosis or acute liver inflammation and thus makes it a potentially more powerful biomarker than AFP or other conventionally used HCC biomarkers [53,54]. More characterizations are needed before its clinical use.
III. Cancer Stem Cell Markers
For almost 40 years cancer stem cells (CSC) are hypothesized to play a crucial role in the development of chemotherapy and radiation therapy resistance and tumor recurrence. Independent studies during the last decade have identified CSC as a small subset of cells present within the heterogeneous, bulk population of cancer cells and to be responsible for the relapse of malignant tumors after the removal of primary tumors [55-57]. It is theorized that CSC lose the potential to repair tissue due to genetic mutation, but that they retain the some phenotypes of stem cells such as self-renewal and pluripotency to develop different cell types in tumor tissue [58]. CSC are found to be either dormant in the G0 phase of cell cycle or dividing at a much slower rate than the rest of the cancer cell population and thus CSC do not get affected by conventional chemo or radiation therapy, which especially targets highly proliferating cells [59]. A CSC not only sustains the tumor regeneration ability, but also drives the mechanisms of tumor progression and differentiation through its self-renewal property [55-57]. This indispensible sub-population of tumor cells can either recapitulate the original primary tumor or form an extremely heterogeneous cancer cell population and is therefore responsible for high rates of recurrence related mortality among cancer patients [60]. CSC is believed to play pivotal role in the progression of HCC, its recurrence after radical hepatectomy and metastasis of cancer to lungs and bones [59]. Some of the cell surface antigens or markers which help to identify CSC in tumors or cell lines are c-Kit, CD13, CD133, CD44, CD90, EpCAM, OV6 etc [55]. Independent studies suggest comprehensive understanding of CSC markers and their expression pattern is more effective in characterizing CSC harboring HCC than using single CSC marker [61].
CD13
CD13, also known as aminopeptidase N (APN), is a functional marker to identify dormant cancer stem cells in HCC. This cell-membrane antigen is a zinc-dependent metallopeptidase and plays an important role in post-secretory processing of several types of peptides [59]. CD13 is expressed during fetal liver formation and liver regeneration and is believed to play specific role in hepatocyte differentiation. Although RT-PCR did not exhibit an increase in CD13 mRNA, immuno-histochemical study showed elevated CD13 protein level in fibrous capsule of liver, which is very often a site of recurrence for HCC [59,62]. Apart from its usual role in cell proliferation, peptide cleavage, CD13 is uniquely associated with bile canaliculi formation, bile acid secretion in HCC [62]. CD13+ HCC showed reduced ROS-induced DNA damage upon chemo or radiation therapy. In SCID mice model, CD13+ cells are documented to be highly tumorigenic and develop chemotherapy resistance with the elevated expression of some ABC transporters and ROS scavenging pathways [59]. HCC targeted therapy which combines CD13 inhibitor along with conventional chemotherapy drug 5-FU or ROS-inducing drug, is suggested to be more effective [59,63]. Canalicular staining specific for CD13 is a potential diagnostic tool to identify and design therapeutic approaches for HCC patients [62].
CD133
CD133, also known as prominin-1 or AC133, is a 97 kD cell surface glycoprotein [58]. CD133 is a member of prominin family and is a prominent stem cell marker for various normal and cancerous tissues. Seventy percent of the advanced stage HCC harbor CD133+ cells and CD133 harboring tumors are often characterized by elevated cell proliferation, high tumor grade, increased recurrence and poor prognosis when compared with CD133− cells harboring tumors [55]. CD133 is also believed to develop chemo-resistance in HCC by playing a crucial role in the up-regulation of AKT/PKB and Bcl-2 pathways [63]. Immunostaining and RT-PCR are used to detect CD133 in HCC. However, CD133 alone is not a specific marker to determine tumor grade for HCC as it is a prominent circulating endothelial progenitor cell marker and often produce false positive result in liver cirrhosis patients [63,64].
CD90
CD90, a 25-37 kD glycoprotein, which is anchored to glycosylphosphatidylinositol and expressed on leukocytes, bone marrow derived mesenchymal stem cells and hepatic progenitor stem cells during embryonic liver development [63,65]. As CD90 is not expressed in adult human liver or in cirrhotic livers, it is considered to be a potential marker for HCC diagnosis and therapeutic approach. CD90+ cells in HCC are believed to represent highly tumorigenic and metastatic tumor cell population and 90% of the blood samples from HCC patients express CD90 harboring CSC [65].
CD44
CD44 is a cell surface adhesion molecule, involved in multiple signaling pathways and is documented as a CSC maker for many malignant tumors including HCC [58]. CD44 binds to extracellular ligands and regulates cell growth, proliferation, differentiation, motility and survival in cancer cells. mRNA analysis and flow-cytometry studies showed CD44 is preferentially expressed in CD133+ and CD90+ HCC population and CD44+CD133+ or CD44+CD90+ cell population in HCC often represent aggressive tumor type. According to several independent studies CD44 can be used as CSC characterizing marker in HCC along with CD133 or CD90 [57,65].
Epithelial Cell Adhesion Molecule (EpCAM)
Epithelial cell adhesion molecule (EpCAM) is expressed during embryonic development and its role in embryogenesis is regulated by Wnt/β-Catenin signaling pathway. Hepatic progenitor cell (HPC) like phenotypes or ‘stemness’ of HCC cells are often associated with Wnt/β-Catenin signaling and EpCAM expression. The EpCAM encoding gene is believed to be a target of Wnt/ β-Catenin signaling. EpCAM harboring HCC cells are often concentrated in the invasive border of the tumors and gene knockdown of EpCAM or β-Catenin reduces tumorigenecity [60,61,66]. Although EpCAM+CD133+ and EpCAM+CD133+ cells are considered to be potential HCC markers, best diagnostic potential is associated with EpCAM and AFP (Alpha-Fetoprotein) expressing HCC cells. EpCAM+AFP+ cells of HCC are characterized with high tumorigenecity, metastasis and portal invasion [60,67]. Microarray analysis and immuno-histochemical studies of HCC indicate EpCAM to be a potential marker and targeted therapy using EpCAM antibody and conventional chemotherapy drug is suggested to be more effective in eradicating CSC and non-CSC harboring HCC [67].
B. MOLECULAR TARGETED THERAPY IN HEPATOCELLULAR CARCINOMA
Unlike many other malignancies, HCC is an accumulation of a number of aberrant oncogenic events. Manifestations of HCC phenotypes are attributable to a number of signaling pathways and concurrent therapy has proved to be more beneficial than sequential targeted. Multi-targeted therapy can be of two types- i) Vertical blockade or inhibition of different points in the same pathway to complete the blockade and stop the feedback loop. ii) Horizontal blockade or targeting more than one signaling pathway [68]. The advantages of synergistic approach are multi-fold: it prevents the activation of redundant signaling pathway that might act as salvage pathway and thus preventing tumors cells from escaping the therapy [37,69]. Table 1 summarizes a brief overview of the current status of molecularly targeted therapies in HCC.
Table 1.
Molecular Targeted Therapies that Inhibit the Signaling Pathways Involved in Hepatocellular Carcinoma (HCC)
| Types of Molecules that Signaling Pathways |
Targeted Signaling Pathways | Names of Drugs | Status of Usage | References |
|---|---|---|---|---|
| Small molecule tyrosine-kinase inhibitors | EGF/EGFR | Sorafenib | Currently used (Nexavar) | 79 |
| Gefitinib | in vivo model | 71 | ||
| Erlotinib | in vivo model | 71 | ||
| Raf/MAPK/ERK | Sorafenib | Currently used | 79 | |
| VEGFR-2,-3 | Sorafenib | Currently used | 79 | |
| VEGF/VEGFR | Brivanib | Phase II clinical trial | 79 | |
| Sunitinib | Discontinued | 79 | ||
| Linifanib | Phase II clinical trial | 79 | ||
| PDGFRβ | Sorafenib | Currently used | 79 | |
| PDGFR | Linifanib | Phase II clinical trial | 79 | |
| FGFR | Brivanib | Phase II clinical trial | 79 | |
| PI3K/mTOR (mTOR-1,-2) | PI-103 | in vitro model | 72 | |
| IGF-1R | BMS-554417, OST-906 | Clinical trial for advanced HCC | 73 | |
| Monoclonal Antibodies | VEGF | Bevacizumab | Clinical trial | 80 |
| EGFR | Cetuximab | Discontinued | 71 | |
| Rapamycin analogs | PI3K/AKT/mTOR | Temsirolumus | Phase I & II clinical trial | 70 |
| Everolimus | Phase I & II clinical trial | 70 |
Small molecule tyrosine kinase inhibitors and monoclonal antibodies are considered to have great potential in the treatment of HCC. These tyrosine kinase inhibitors and monoclonal antibodies target several growth factors and growth factor associated signaling pathways. Some of these signaling pathways which have been thoroughly studied as potential targets of tyrosine kinase inhibitors and monoclonal antibodies are namely EGF/EGFR, VEGF/VEGFR, IGF/IGFR, PDGF, FGF, RAS/RAF/ERK/MAPK, PI3K/AKT/mTOR, Wnt/β-Catenin [37,70].
Epidermal growth factor (EGF) and autocrine signal activator EGFR are associated with several malignancies including HCC. Hepatitis B and Hepatitis C infection are suspected to increase the level of EGF ligands in the liver and overexpression of EGFR is documented in 40-70% of the HCC patients [3,37]. EGFR is known to protect HCC cells from oxidative stress induced apoptosis and also up-regulate tumor angiogenesis by stimulating RAF/ERK/MAPK and mTOR signaling. The mechanism of angiogenesis is very well established in highly vascularized tumors and apart of regulation by EGF, angiogenesis in HCC is also controlled by several growth factors namely VEGF/VEGFR, EGF, FGF, PDGF. Studies showed hepatic injury causes transient up-regulation of growth factors in adult liver, however, in chronically injured liver, expression of growth factors becomes aberrant and initiate the tumor development [69,71,72].
Insulin-like growth factor (IGF) and corresponding receptor system IGFR are also believed to play pivotal role in HCC, though further characterization is needed. IGF-II is overexpressed and tumor suppressor Insulin-like growth factor binding protein (IGFBP-1,-2,-3) is either down-regulated or mutated in 30% of the HCC patients [71]. IGF is essential during fetal development as it regulates growth, cell proliferation, differentiation and apoptosis of hepatocytes [71]. Aberrant activation of IGF-1R and IGF-2R in HCC is associated with reduced apoptosis and overexpression of VEGF respectively and initiation of tumor development [71,73].
One of the well characterized signaling pathways involved in the development and maintenance of HCC is PI3K/Akt/mTOR pathway. Activation of Akt is regulated by EGF or IGF, by constitutive activation of PI3K or by mutation or silencing of tumor suppressor gene PTEN. Akt is known to regulate cell proliferation and migration in HCC. An important mediator of this pathway, the serine-threonine kinase mTOR plays a pivotal role in mitogens and growth factor induced signaling and regulates cell growth, proliferation and monitors the cell cycle progression from G1 phase to S phase. mTOR is also known to initiate and maintain the progression of tumor in HCC. PI3K/Akt/mTOR pathway is aberrantly activated in 30-50% of HCC patient population [71,74,75].
Wnt/β-Catenin signaling, the highly conserved pathway plays crucial role in the maintenance of hepatic health. Wnt/β-Catenin signaling, which is essential for embryonic development regulates cell proliferation, survival, regeneration and self-renewal of hepatocytes [76,77]. Some group of non-invasive tumor is characterized by the over-accumulation of β-Catenin in nucleus [71,76]. Further studies revealed a mutation at exon 3 of this β-Catenin population and its inability to get phosphorylated and degraded by ubiquitination [76]. Wnt/β-Catenin signaling pathway is also associated with the up-regulation of several oncogenes like Cyclin D1, C-Myc, survivin; however, further characterization is necessary to develop targeted therapy for this [77,78].
The major breakthrough in HCC therapy came with the discovery of Sorafenib, the oral multi-kinase inhibitor. This tyrosine-kinase inhibitor competitively inhibits ATP binding to catalytic domains of various kinases. Sorafenib inhibits Raf/ MAPK/ ERK signaling, VEGFR-2, -3, and PDGFR-β. Sorafenib is known to increase apoptosis, and decrease angiogenesis, cell proliferation and overall stalls the tumor cell signaling. Other oral tyrosine-kinase inhibitors sunitinib, linifanib, brivanib block a number of angiogenesis related signaling pathways. Sunitinib is a very promising multi-kinase inhibitor with its potential to block a number of angiogenesis related signaling pathways, linifanib targets VEGFR, PDGFR and brivanib targets VEGFR, FGFR signaling pathways. Unfortunately use of sunitinib was discontinued after adverse effects during phase II and phase III clinical trials. Clinical trials with linifanib and brivanib on advanced, metastatic, unresectable HCC showed promising results in phase II clinical trials. Both of these drugs showed increased efficacy when combined with sorafenib [37,79].
Bevacizumab is the recombinant humanized monoclonal antibody that targets VEGF signaling pathway and block tumor vascularization [80]. Bevacizumab showed promising effects in the treatment of breast and colon cancer and currently being used in clinical trial to treat HCC along with EGFR inhibitor [70,71]. This anti-cancer monoclonal antibody is also being paired with other chemotherapeutic drugs like oxaliplatin or gemcitabine during its clinical trial to treat HCC patients [37].
For last few decades one of the major challenges in anticancer therapy has been to block EGF/EGFR signaling as it plays the central role in many oncogenic molecular pathways. Two tyrosine-kinase inhibitors, gefitinib and erlotinib inhibits EGFR activation by blocking intracellular EGFR domain and after being successfully used for lung and pancreatic cancer treatment showed promising outcome in vivo models. Extracellular EGFR domain is targeted by recombinant chimeric monoclonal antibody cetuximab, however, it did not show much potential during HCC clinical trials [71].
Several other drugs targeting different molecular signaling mechanisms are either in the developmental stage or in clinical trial. Some of them showed real potential when used for sequential target while others worked better in combination with other chemotherapeutic agents or small molecule tyrosine-kinase inhibitors. Three analogs of rapamycin, the natural inhibitor of mTOR, have been developed recently and in vivo studies showed they have better pharmacokinetic properties than rapamycin [70]. Two of these analogs, temsirolumus and everolimus, have been used in phase I and phase II clinical trials of HCC and showed promising outcome in combination with sorafenib, which cannot inhibit PI3K/Akt/mTOR directly [71]. Everolimus arrests cell cycle progression at G1 phase and inhibits cell proliferation. Combination therapy with rapamycin and everolimus on HCC cell lines and in vivo tumor models arrested cell growth, proliferation and reduced tumor growth [70]. PI-103, a small molecule tyrosine-kinase inhibitor, blocks PI3K/mTOR signaling by inhibiting EGF stimulated mTOR-1, -2 and PI3K stimulated AKT activation synergistically [72]. mTOR is also a potential target for many anticancer strategies, which block complementary signaling pathways (Ras and mTOR) to induce apoptosis and inhibit cell proliferation simultaneously. BMS-554417 and OST-906 are potential small molecule tyrosine-kinase inhibitors targeting IGF-1R and currently on different stages of clinical trials for advanced adenocarcinoma and HCC [73]. Data suggests IGF-1R inhibitor shows better clinical outcome in advanced HCC patients when combined with EGFR inhibitor erlotinib or mTOR inhibitor everolimus [71-73,75].
CONCLUSIONS
Hepatocellular carcinoma, which is known for poor prognosis and high 5-year mortality rate requires thorough characterization and novel therapeutic approach to improve the life-expectancy and survival of HCC patients. One of the major steps to improve the diagnostic tool for HCC would be detailed characterization of novel biomarkers. Although there are several biomarkers currently used as predictive, prognostic and post-operative maintenance tools, none of them remotely satisfy the present need for a reliable biomarker, which can be used for precise diagnosis of HCC early enough during hepatitis infection or liver cirrhosis.
Molecular markers are excellent tools to diagnose and follow the course of disease [81]. Some of these serological, tissue and cancer stem cell markers need further characterization before being used as molecular biomarkers. However, these emerging biomarkers have shown potential to improve diagnostic precision, pre-operative screening and post-operative monitoring.
Molecular targeted treatments of HCC mainly depend on cytotoxic chemotherapeutic agents and the multi-kinase inhibitor sorafenib. Novel therapeutic approaches that focus on concurrent inhibition of several signaling pathways rather than a single one, proved to be more effective in HCC treatment. Detailed characterization of molecular profiles associated with HCC is much needed as it would improve the currently available diagnostic tools and would advance the therapeutic practices as well.
ACKNOWLEDGEMENT
This work was supported by NIH’s R01-DK81413 (to SAS) from the National Institute of Diabetes And Digestive and Kidney Diseases. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Diabetes And Digestive and Kidney Diseases or the National Institutes of Health.
Footnotes
CONFLICT OF INTEREST
None declared.
REFERENCES
- [1].He G, and Karin M (2011) Cell Research 21, 159–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].El-Serang HB, and Rudolph KL (2007) Gastroenterology 132, 2557–2576. [DOI] [PubMed] [Google Scholar]
- [3].Newell P, Villanueva A, Friedman SL, Koike K, and Llovet JM (2008) J Hepatol 48, 858–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Caldwell SH, Crespo DM, Kang HS, and Al-Osaimi AM (2004) Gastroenterology 127, 97–103. [DOI] [PubMed] [Google Scholar]
- [5].Marchesini G, Moscatiello S, Di Domizio S, and Forlani G (2008) J Clin Endocrinol Metab 93, 74–80. [DOI] [PubMed] [Google Scholar]
- [6].Ismail MH (2010) Gastroenterol. Res 3, 223–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Altekruse SF, McGlynn KA, and Reichman ME (2009) J. Clin. Oncol 27, 1485–1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Chen L, Ho DW, Lee NP, Sun S, Lam B, Wong KF, Yi X, Lau GK, Ng EW, Poon TC, Lai PB, Cai Z, Peng J, Leng X, Poon RT, and Luk JM (2010) Ann Surg Oncol 17, 2518–2525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Sidransky D. (2002) Nat Rev Cancer. 2, 210–219. [DOI] [PubMed] [Google Scholar]
- [10].Daniele B, Bencivenga A, Megna AS, and Tinessa V (2004) Gastroenterology 127, 108–112. [DOI] [PubMed] [Google Scholar]
- [11].Gish RG, Marrero JA, and Benson AB (2010) Gastroenterol Hepatol (N Y). 6, 1–16. [PMC free article] [PubMed] [Google Scholar]
- [12].Liu JG, Wang YJ, and Du Z (2010) World J Gastroenterol. 16, 3450–3456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Ota H, Nagano H, Sakon M, Eguchi H, Kondo M, Yamamoto T, Nakamura M, Damdinsuren B, and Monden M (2005) Br J Cancer. 93, 557–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Yu LH, Li N, and Cheng SQ (2011) Int J Hepatol 2011:416459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Hsu CH, Yang TS, Hsu C, Toh HC, Epstein RJ, Hsiao LT, Chen PJ, Lin ZZ, Chao TY, and Cheng AL (2010) Br J Cancer. 102, 981–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Gomaa AI, Khan SA, Leen EL, Waked I, and Taylor-Robinson SD (2009) World J Gastroenterol 15, 1301–1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Hsieh CB, Chen TW, Chu CM, Chu HC, Yu CP, and Chung KP (2010) World J Gastroenterol 16, 3049–3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Lok AS, Sterling RK, Everhart JE, Wright EC, Hoefs JC, Di Bisceglie AM, Morgan TR, Kim HY, Lee WM, Bonkovsky HL, and Dienstag JL (2010) Gastroenterology 138, 493–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Ishii M, Gama H, Chida N, Ueno Y, Shinzawa H, Takagi T, Toyota T, and Takahashi TK, R. (2000) Am J Gastroenterol. 95, 1036–1040. [DOI] [PubMed] [Google Scholar]
- [20].Beale G, Chattopadhyay D, Gray J, Stewart S, Hudson M, Day C, Trerotoli P, Giannelli G, Manas D, and Reeves H (2008) BMC Cancer. 18, 200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Nomura F, Ishijima M, Kuwa K, Tanaka N, Nakai T, and Ohnishi K (1999) Am J Gastroenterol 94, 650–654. [DOI] [PubMed] [Google Scholar]
- [22].Suehiro T, Sugimachi K, Matsumata T, Itasaka H, Taketomi A, and Maeda T (1994) Cancer 73, 2464–2471. [DOI] [PubMed] [Google Scholar]
- [23].Capurro M, Wanless IR, Sherman M, Deboer G, Shi W, Miyoshi E, and Filmus J (2003) Gastroenterology 125, 89–97. [DOI] [PubMed] [Google Scholar]
- [24].Uemura Y, Pak SC, Luke C, Cataltepe S, Tsu C, Schick C, Kamachi Y, Pomeroy SL, Perlmutter DH, and Silverman GA (2000) Int J Cancer 89, 368–377. [DOI] [PubMed] [Google Scholar]
- [25].Giannelli G, Marinosci F, Sgarra C, Lupo L, Dentico P, and Antonaci S (2005) Int J Cancer 116, 579–583. [DOI] [PubMed] [Google Scholar]
- [26].Deli A, Kreidl E, Santifaller S, Trotter B, Seir K, Berger W, Schulte-Hermann R, Rodgarkia-Dara C, and Grusch M (2008) World J Gastroenterol. 14, 1699–1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Kreidl E, Oztürk D, Metzner T, Berger W, and Grusch M (2009) World J Hepatol 1, 17–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Nagao M, Nakajima Y, Hisanaga M, Kayagaki N, Kanehiro H, Aomatsu Y, Ko S, Yagita H, Yamada T, Okumura K, and Nakano H (1999) Hepatology 30, 413–421. [DOI] [PubMed] [Google Scholar]
- [29].Jodo S, Kobayashi S, Nakajima Y, Matsunaga T, Nakayama N, Ogura N, Kayagaki N, Okumura K, and Koike T (1998) Clin Exp Immunol 112, 166–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Ozaslan E, Kiliçarslan A, Simşek H, Tatar G, and Kirazli S (2003) J Int Med Res. 31, 384–391. [DOI] [PubMed] [Google Scholar]
- [31].El Bassiouny AE, El-Bassiouni NE, Nosseir MM, Zoheiry MM, El-Ahwany EG, Salah F, Omran ZS, and Ibrahim RA (2008) Medscape J Med. 10, 130. [PMC free article] [PubMed] [Google Scholar]
- [32].Wright LM, Kreikemeier JT, and Fimmel CJ (2007) Cancer Detect Prev 31, 35–44 [DOI] [PubMed] [Google Scholar]
- [33].Bachert C, Fimmel C, and Linstedt AD (2007) Traffic. 8, 1415–1423. [DOI] [PubMed] [Google Scholar]
- [34].Mao Y, Yang H, Xu H, Lu X, Sang X, Du S, Zhao H, Chen W, Xu Y, Chi T, Yang Z, Cai J, Li H, Chen J, Zhong S, Mohanti SR, Lopez-Soler R, Millis JM, Huang J and Zhang H (2010) Gastroenterology 59, 1687–1693. [DOI] [PubMed] [Google Scholar]
- [35].Riener MO, Stenner F, Liewen H, Soll C, Breitenstein S, Pestalozzi BC, Samaras P, Probst-Hensch N, Hellerbrand C, Müllhaupt B, Clavien PA, Bahra M, Neuhaus P, Wild P, Fritzsche F, Moch H, Jochum W and Kristiansen G (2009) Hepatology 49, 1602–1609. [DOI] [PubMed] [Google Scholar]
- [36].Hann HW, Wang M, Hafner J, Long RE, Kim SH, Ahn M, Park S, Comunale MA, Block TM, and Mehta A (2010) Cancer Biomark 7, 269–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Chau GY, W.Y. L, Chi CW, Chau YP, Li AF, Kao HL, and Wu CW (2008) Eur J Surg Oncol. 34, 333–338. [DOI] [PubMed] [Google Scholar]
- [38].Sánchez A, and Fabregat I (2010) World J Gastroenterol 16, 5148–5161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Jiang WG, Martin TA, Parr C, Davies G, Matsumoto K, and Nakamura T (2005) Crit Rev Oncol Hematol 53, 35–69. [DOI] [PubMed] [Google Scholar]
- [40].Vejchapipat P, Tangkijvanich P, Theamboonlers A, Chongsrisawat V, Chittmittrapap S, and Poovorawan Y (2004) J Gastroenterol. 39, 1182–1188. [DOI] [PubMed] [Google Scholar]
- [41].Yamagamim H, Moriyama M, Matsumura H, Aoki H, Shimizu T, Saito T, Kaneko M, Shioda A, Tanaka N, and Arakawa Y (2002) Cancer 95, 824–834. [DOI] [PubMed] [Google Scholar]
- [42].Song BC, Chung YH, Kim JA, Choi WB, Suh DD, Pyo SI, Shin JW, Lee HC, Lee YS, and Suh DJ (2002) Cancer 94, 175–180. [DOI] [PubMed] [Google Scholar]
- [43].Coulouarn C, Factor VM, and Thorgeirsson SS (2008) Hepatology 47, 2059–2067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Fabregat I. (2009) World J Gastroenterol. 15, 513–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Schoenleber SJ, Kurtz DM, Talwalkar JA, Roberts LR, and Gores GJ (2009) Br J Cancer 100, 1385–1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Poon RT, Ng IO, Lau C, Zhu LX, Yu WC, Lo CM, Fan ST, and Wong J (2001) Ann Surg. 233, 227–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Poon RT, Ho JW, Tong CS, Lau C, Ng IO, and Fan ST (2004) Br J Surg 91, 1354–1360. [DOI] [PubMed] [Google Scholar]
- [48].Amaoka N, Osada S, Kanematsu M, Imai H, Tomita H, Tokuyama Y, Sakashita F, Nonaka K, Goshima S, Kondo H, and Adachi Y (2007) J Gastroenterol Hepatol. 22, 2202–2207. [DOI] [PubMed] [Google Scholar]
- [49].He ZL, Zheng H, Lin H, Miao XY, and Zhong DW (2009) World J Gastroenterol. 15, 4177–4182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Pellegrino R, Calvisi DF, Ladu S, Ehemann V, Staniscia T, Evert M, Dombrowski F, Schirmacher P, and Longerich T (2010) Hepatology. 51, 857–868. [DOI] [PubMed] [Google Scholar]
- [51].Yokomizo C, Yamaguchi K, Itoh Y, Nishimura T, Umemura A, Minami M, Yasui K, Mitsuyoshi H, Fujii H, Tochiki N, Nakajima T, Okanoue T, and Yoshikawa T (2011) Cancer Lett 310, 140–147. [DOI] [PubMed] [Google Scholar]
- [52].Li M, Luo RZ, Chen JW, Cao Y, Lu JB, He JH, Wu QL, and Cai MY (2011) J Transl Med. 9, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Yasen M, Kajino K, Kano S, Tobita H, Yamamoto J, Uchiumi T, Kon S, Maeda M, Obulhasim G, Arii S, and Hino O (2005) Clin Cancer Res. 11, 7354–7361. [DOI] [PubMed] [Google Scholar]
- [54].Tacke F, Kanig N, En-Nia A, Kaehne T, Eberhardt CS, Shpacovitch V, Trautwein C, and Mertens PR (2011) BMC Cancer. 11, 185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Oishi N, and Wang XW (2011) Int J Biol Sci 7, 517–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Ma S, Chan KW, Hu L, Lee TK, Wo JY, Ng IO, Zheng BJ, and Guan XY (2007) Gastroenterology. 132, 2542–2556. [DOI] [PubMed] [Google Scholar]
- [57].Zhu Z, Hao X, Yan M, Yao M, Ge C, Gu J, and Li J (2010) Int J Cancer 126, 2067–2078. [DOI] [PubMed] [Google Scholar]
- [58].Lingala S, Cui YY, Chen X, Ruebner BH, Qian XF, Zern MA, and Wu J (2010) Exp Mol Pathol 81, 27–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Haraguchi N, Ishii H, Mimori K, Tanaka F, Ohkuma M, Kim HM, Akita H, Takiuchi D, Hatano H, Nagano H, Barnard GF, Doki Y, and M. M (2010) J Clin Invest. 120, 3326–3339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Terris B, Cavard C, and Perret C (2010) J Hepatol. 52, 280–281. [DOI] [PubMed] [Google Scholar]
- [61].Yamashita T, Forgues M, Wang W, Kim JW, Ye Q, Jia H, Budhu A, and Wang W (2008) Cancer Res. 68, 1451–1461. [DOI] [PubMed] [Google Scholar]
- [62].Röcken C, Licht J, Roessner A, and Carl-McGrath S (2005) J Clin Pathol 58, 1069–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Yang ZF, Ngai P, Ho DW, Yu WC, Ng MN, Lau CK, Li ML, Tam KH, Lam CT, Poon RT, and Fan ST (2008) Hepatology. 47, 919–928. [DOI] [PubMed] [Google Scholar]
- [64].Yoshikawa S, Zen Y, Fujii T, Sato Y, Ohta T, Aoyagi Y, and Nakanuma Y (2009) World J Gastroenterol. 15, 4896–4906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Yang ZF, Ho DW, Ng MN, Lau CK, Yu WC, Ngai P, Chu PW, Lam CT, Poon RT, and Fan ST (2008) Cancer Cell. 13, 153–166. [DOI] [PubMed] [Google Scholar]
- [66].Kimura O, Takahashi T, Ishii N, Inoue Y, Ueno Y, Kogure T, Fukushima K, and Sugamura K (2010) Cancer Sci. 101, 2145–2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Yamashita T, Ji J, Budhu A, Forgues M, Yang W, Wang HY, Jia H, Ye Q, Qin LX, Wauthier E, Reid LM, Minato H, Honda M, Kaneko S, Tang ZY, and Wang XW (2009) Gastroenterology 136, 1012–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].El-Serag HB, and Rudolph KL (2007) Gastroenterology 132, 2557–2576. [DOI] [PubMed] [Google Scholar]
- [69].Yau T, Chan P, Epstein R, and Poon RT (2008) World J Gastroenterol. 14, 6437–6441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Höpfner M, Schuppan D, and Scherübl H (2008) World J Gastroenterol. 14, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Llovet JM, and Bruix J (2008) Hepatology. 48, 1312–1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Gedaly R, Angulo P, Hundley J, Daily MF, Chen C, Koch A, and Evers BM (2010) Anticancer Res. 30, 4951–4958. [PMC free article] [PubMed] [Google Scholar]
- [73].Wu J, and Zhu AX (2011) J Hematol Oncol 4, 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Advani SH (2010) Indian J Med Paediatr Oncol. 31, 132–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Villanueva A, Chiang DY, Newell P, Peix J, Thung S, Alsinet C, Tovar V, Roayaie S, Minguez B, Sole M, Battiston C, Van Laarhoven S, Fiel MI, Di Feo A, Hoshida Y, Yea S, Toffanin S, and Llovet JM (2008) Gastroenterology 135, 1972–1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Fatima S, Lee NP, and Luk JM (2011) World J Clin Oncol. 2, 311–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Noda T, Nagano H, Takemasa I, Yoshioka S, Murakami M, Wada H, Kobayashi S, Marubashi S, Takeda Y, and Monden M (2009) Br J Cancer. 100, 1647–1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Wei W, Chua MS, Grepper S, and So SK (2009) Mol Cancer. 8, 76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Villanueva A, and Llovet JM (2011) Gastroenterology. 140, 1410–1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Hsu CH, Yang TS, Hsu C, Toh HC, Epstein RJ, Hsiao LT, Chen PJ, Lin ZZ, Chao TY, and Cheng AL (2010) Br J Cancer. 102, 981–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Pleguezuelo M, Lopez-Sanchez LM, Rodriguez-Ariza A, Montero JL, Briceno J, Ciria R, Muntane J, and Mata M (2010) World J Hepatol. 2, 127–135. [DOI] [PMC free article] [PubMed] [Google Scholar]


