Abstract
The prognosis of patients with osteosarcoma who experience recurrence or progression (R/P) is extremely poor, and more effective and less toxic therapies are needed. In the current study, the clinical data of osteosarcoma patients who experienced R/P were retrospectively analyzed to verify the reliability of O‐6‐methylguanine‐DNA methyltransferase (MGMT) protein expression or MGMT promoter methylation for predicting the response to off‐label temozolomide (TMZ)‐containing chemotherapy. Of the 30 evaluable patients, 9 (30%) showed no/low MGMT protein expression, whereas all 16 evaluable patients had unmethylated MGMT promoter irrespective of MGMT protein expression levels. Twenty‐three patients received TMZ‐containing chemotherapy for measurable lesions (n = 14) or as adjuvant therapy following resection of recurrent lesions (n = 9). Among 14 patients with radiologically measurable lesions, the objective response rate was higher in the MGMT no/low‐expression group (50.0%) than in the MGMT intermediate/high‐expression group with borderline significance (0%, p = 0.066). The 6‐month progression‐free survival (PFS) rate in patients with radiologically measurable lesions was significantly higher in the MGMT no/low‐expression group (50.0%) than in the MGMT intermediate/high‐expression group (0%, p = 0.036). In the multivariate analysis of the 23 patients receiving TMZ‐containing chemotherapy, MGMT expression and disease status before TMZ‐containing chemotherapy were significantly associated with PFS. No severe adverse effects were observed during TMZ‐containing chemotherapy. MGMT protein expression, but not MGMT promoter methylation, could predict a favorable outcome in patients receiving TMZ‐containing chemotherapy.
Keywords: biomarker, O‐6‐methylguanine DNA methyltransferase, osteosarcoma, recurrence, temozolomide
Temozolomide (TMZ)‐containing chemotherapy exerts powerful and long‐lasting antitumor effects in a certain percentage of patients with osteosarcoma experiencing recurrence or progression. O‐6‐methylguanine‐DNA methyltransferase (MGMT) protein expression, but not MGMT promoter methylation, is a reliable predictor of the effect of TMZ‐containing chemotherapy.
1. INTRODUCTION
Recently developed advances in multidrug chemotherapy agents such as high‐dose methotrexate, adriamycin, and cisplatin, in combination with orthopedic surgery, have improved the clinical outcomes of patients with osteosarcoma, resulting in long‐term survival rates greater than 60%. 1 By contrast, the prognosis of patients with osteosarcoma who experience recurrence or progression (R/P) remains poor, with long‐term survival rates of <20%. 2 , 3 , 4 Salvage chemotherapies, such as ifosfamide (IFM) + etoposide (ETP) and gemcitabine (GEM) + docetaxel, or molecular‐targeted therapies, such as sorafenib, regorafenib, and cabozantinib, have shown limited efficacy. 1
Temozolomide (TMZ) is an alkylator that has shown excellent antitumor activity in patients with high‐grade glioma, 5 , 6 , 7 as well as in a subgroup of extracranial solid tumors including osteosarcoma. 8 , 9 The DNA repair enzyme O‐6‐methylguanine‐DNA methyltransferase (MGMT) inhibits the antitumor activity of alkylators. 5 Because low levels or loss of functional MGMT protein expression increase the susceptibility to alkylators, MGMT promoter methylation or no/low MGMT protein expression predicts a favorable clinical outcome in high‐grade glioma. 5 , 6 , 7 , 10 , 11 However, the clinical utility of MGMT as a marker in patients with extracranial solid tumors remains unclear.
Here, we examined the effect of MGMT protein expression and MGMT promoter methylation on the clinical outcomes of patients with osteosarcoma who experience R/P, particularly on the response to off‐label TMZ‐containing chemotherapy, in a retrospective multicenter analysis.
2. MATERIALS AND METHODS
2.1. Study design and data collection
Clinical information and formalin‐fixed paraffin‐embedded (FFPE) samples collected at R/P were obtained from 30 patients diagnosed with high‐grade osteosarcoma between 2000 and 2020 who experienced R/P at one of eight institutions. Nine patients were reported previously. 8 , 9 The radiological response to chemotherapy was evaluated according to the RECIST guidelines (version 1.1). 12 The use of unapproved drugs was approved by the ethical committee or patient safety unit of each institution, and written informed consent was obtained from each patient and their family. Treatment‐related adverse events were assessed according to the NCI's Common Terminology Criteria for Adverse Events, version 5 (http://evs.nci.nih.gov/ftp1/CTCAE).
2.2. Immunohistochemistry analysis
MGMT protein expression in tumor tissues was analyzed by immunohistochemistry (IHC) using an anti‐MGMT mAb (clone MT3.1; Merck Millipore), as reported previously. 10 The slides were reviewed by a pathologist blinded to the clinical information. MGMT immunopositivity was semiquantitatively evaluated using the H‐score, as described previously. 13 MGMT immunostaining intensity was scored using a scale ranging from 0 to 3: 0, no staining; 1+, weak staining; 2+, intermediate (int) staining; 3+, strong staining. The H‐score was calculated as the percentage of positive cells multiplied by the immunostaining intensity score and defined as a continuous variable ranging from 0 to 300. According to the value of the 25th percentile (20) and 75th percentile (175), H‐scores ≤30, >30 and <175, and ≥175 were defined as no or low, int, and high MGMT protein expression, respectively.
2.3. Methylation data analysis
Genomic DNA was extracted from the FFPE samples using the QIAamp DNA FFPE Tissue Kit (Qiagen), DNA quality was assessed using Illumina's Infinium HD FFPE QC assay following Illumina's recommendations, and samples with ΔCq value <5 were considered suitable for restoration with the Infinium HD FFPE DNA Restore Kit (Illumina). Selected DNA samples were used for bisulfite conversion with the EZ DNA methylation kit (Zymo Research) according to the manufacturer's instructions. Bisulfite‐converted DNA samples were then used for the DNA restoration process and the methylation assays using Infinium MethylationEPIC BeadChips version 2 (Illumina). MGMT promoter methylation status was predicted with R package MGMT‐STP27 with a default probability cut‐off of 0.358, as reported previously. 14
2.4. Statistical analysis
The characteristics of patients in the two groups were compared using Fisher's exact test for categorical variables. First relapse‐free interval (RFI) was defined as the time from initial diagnosis to the first R/P, and a cut‐off value of 18 months was set as reported previously. 3 The probability of overall survival (OS), defined as the duration of survival between the initiation of TMZ‐containing chemotherapy and either death or the last follow‐up, and progression‐free survival (PFS), defined as the duration of survival between the initiation of TMZ‐containing chemotherapy and either disease progression, death, or the last follow‐up, were estimated using the Kaplan–Meier method. The log‐rank test and Cox proportional hazard model were used for univariate and multivariate analyses, respectively. The factors included in the analyses were patient age group, sex, first RFI, primary tumor site, R/P site, salvage chemotherapy after R/P, local treatment for primary site after R/P, local treatment for metastasis after R/P, disease status before TMZ‐containing chemotherapy, MGMT protein expression, and MGMT promoter methylation. Factors with p < 0.05 in the univariate analysis, and/or MGMT protein expression were included in the multivariate analysis. All statistical analyses were carried out using EZR (version 1.32; Saitama Medical Center, Jichi Medical University), which is a graphical user interface for R (R Foundation for Statistical Computing). 15
3. RESULTS
3.1. MGMT protein expression and MGMT promoter methylation in patients with osteosarcoma who experience R/P
We first assessed MGMT protein expression and MGMT promoter methylation status in 30 patients with osteosarcoma who experienced R/P. Patient characteristics and treatments after initial diagnosis of the 30 patients (17 male and 13 female) included are presented in Table 1. The median age at diagnosis was 14 years (range, 6–60 years). Three patients had distant metastases (two lung alone, one lung and bone). All patients received adjuvant and/or neoadjuvant multidrug chemotherapy. Twenty‐eight patients underwent local treatment (surgery and/or radiotherapy) for primary lesions whereas none of the patients underwent surgery for metastases.
TABLE 1.
Characteristics of 30 patients with osteosarcoma who experienced recurrence or progression.
| Characteristic | All patients (n = 30) | MGMTint/high (n = 21) | MGMTno/low (n = 9) | p value | |||
|---|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | ||
| Age at diagnosis, years; median (range) | 14 (6–60) | 14 (6–47) | 13 (8–60) | 0.666 | |||
| 0–19 | 22 | 73.3 | 16 | 76.2 | 6 | 66.7 | |
| ≥20 | 8 | 26.7 | 5 | 23.8 | 3 | 33.3 | |
| Sex | |||||||
| Male | 17 | 56.7 | 12 | 57.1 | 5 | 55.6 | 1.000 |
| Female | 13 | 43.3 | 9 | 42.9 | 4 | 44.4 | |
| Primary tumor site | |||||||
| Extremity | 26 | 86.7 | 17 | 81.0 | 9 | 89.8 | 0.287 |
| Axial | 4 | 13.3 | 4 | 19.0 | 0 | 10.2 | |
| Metastasis at initial diagnosis | |||||||
| No | 27 | 90.0 | 19 | 90.4 | 8 | 88.9 | 1.000 |
| Yes | 3 | 10.0 | 2 | 9.6 | 1 | 11.1 | |
| Local treatment for primary site during initial treatment | |||||||
| Surgery | 26 | 86.7 | 17 | 81.0 | 9 | 100.0 | 1.000 |
| Radiotherapy | 1 | 3.3 | 1 | 4.8 | 0 | 0.0 | |
| Surgery and radiotherapy | 1 | 3.3 | 1 | 4.8 | 0 | 0.0 | |
| No | 2 | 6.7 | 2 | 9.5 | 0 | 0.0 | |
| Use of other alkylators during initial treatment | |||||||
| No | 3 | 10.0 | 3 | 14.3 | 4 | 44.4 | 1.000 |
| Yes | 27 | 90.0 | 18 | 85.7 | 5 | 55.6 | |
| First RFI, months; median (range) | 18 (2–71) | 19 (2–71) | 16 (4–67) | 1.000 | |||
| <18 | 15 | 50.0 | 10 | 47.6 | 5 | 55.6 | |
| ≥18 | 15 | 50.0 | 11 | 52.4 | 4 | 44.4 | |
Abbreviations: int/high, intermediate/high expression; no/low, no/low expression; MGMT, O‐6‐methylguanine‐DNA methyltransferase; RFI, relapse‐free interval.
Representative IHC staining images of MGMT no expression (H‐score: 0) and high expression (H‐score: 270) are shown in Figure 1A,B, respectively.
FIGURE 1.
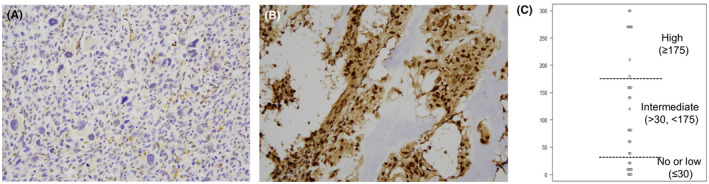
O‐6‐methylguanine‐DNA methyltransferase (MGMT) protein expression in patients with osteosarcoma who experienced recurrence or progression. (A) Patients with osteosarcoma with no MGMT expression (H‐score: 0) and high MGMT expression (H‐score: 270) (magnification, ×200). (C) MGMT protein expression in the 30 patients analyzed.
The H‐score ranged from 0 to 300, and the 25th percentile, 50th percentile (i.e., median), and 75th percentile were 30, 80, and 175, respectively (Figure 1C). MGMT no or low, int, and high MGMT protein expression were detected in 9 (30.0%), 13 (43.3%), and 8 (26.7%) patients. None of the patient characteristics or treatments after initial diagnosis were significantly associated with MGMT expression level (Table 1).
Of 23 patients, 7 were excluded from the MGMT promoter methylation analysis due to insufficient quality of DNA extracted from FFPE tissue sections. The MGMT promoter was unmethylated in all 16 evaluable patients with methylation values ranging from 0.00625 to 0.03387. MGMT protein expression levels were no/low expression in four, int expression in six, and high expression in six patients.
3.2. Impact of MGMT protein expression level on response to TMZ‐containing chemotherapy
Next, we examined the association between MGMT protein expression and clinical outcome in the 23 patients receiving TMZ‐containing chemotherapy. The characteristics of the 23 patients and the treatments received after R/P are listed in Table 2. Adjuvant treatment with TMZ‐containing chemotherapy was given to patients who were in remission (n = 9) or to those with radiologically measurable lesions who were in nonremission (n = 14). The median time of the first RFI was 19 (range, 2–71) months. Seventeen patients had received salvage chemotherapy, such as GEM + DOC (n = 14) and IFO + ETP (n = 6). Four patients had received molecular‐targeted therapy, including pazopanib, regorafenib, sorafenib + everolimus, and palbociclib (n = 1 each). All patients received TMZ + ETP, 8 , 9 except one patient who received TMZ + irinotecan. 16 The median number of treatment cycles of TMZ‐containing chemotherapy was 4 (range, 1–68).
TABLE 2.
Characteristics of 23 patients with osteosarcoma treated with temozolomide (TMZ)‐containing chemotherapy after recurrence or progression (R/P).
| Characteristic | All patients (n = 23) | MGMTint/high (n = 17) | MGMTno/low (n = 6) | p value | |||
|---|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | ||
| Age at TMZ‐containing chemotherapy, years; median (range) | 17 (11–48) | 19 (11–48) | 16 (13–47) | 1.000 | |||
| 0–19 | 17 | 73.9 | 12 | 70.6 | 5 | 83.3 | |
| ≥20 | 6 | 26.1 | 5 | 29.4 | 1 | 16.7 | |
| Sex | |||||||
| Male | 12 | 52.2 | 10 | 58.8 | 2 | 33.3 | 0.371 |
| Female | 11 | 47.8 | 7 | 41.2 | 4 | 66.7 | |
| First RFI, months; median (range) | 19 (2–71) | 19 (2–71) | 22 (4–67) | 1.000 | |||
| <18 | 11 | 47.8 | 8 | 47.1 | 3 | 50.0 | |
| ≥18 | 12 | 52.2 | 9 | 52.9 | 3 | 50.0 | |
| Primary tumor site | |||||||
| Extremity | 20 | 87.0 | 14 | 82.4 | 6 | 100.0 | 0.539 |
| Axial | 3 | 13.0 | 3 | 17.6 | 0 | 0.0 | |
| R/P site | |||||||
| Lung alone | 9 | 39.1 | 7 | 41.2 | 2 | 33.3 | 0.392 |
| Local | 1 | 4.4 | 0 | 0 | 1 | 16.7 | |
| Others | 13 | 56.5 | 10 | 58.8 | 3 | 50.0 | |
| Salvage chemotherapy after R/P | |||||||
| No | 6 | 26.1 | 6 | 35.3 | 0 | 0.0 | 0.144 |
| Yes | 17 | 73.9 | 11 | 64.7 | 6 | 100.0 | |
| Use of other alkylators before TMZ‐containing chemotherapy | |||||||
| No | 3 | 13.0 | 3 | 17.6 | 0 | 0.0 | 0.539 |
| Yes | 20 | 87.0 | 14 | 82.4 | 6 | 100.0 | |
| Local treatment for primary site after R/P | |||||||
| No | 20 | 87.0 | 14 | 82.4 | 6 | 100.0 | 0.539 |
| Yes | 3 | 13.0 | 3 | 17.6 | 0 | 0.0 | |
| Local treatment for metastasis after R/P | |||||||
| No | 4 | 17.4 | 3 | 17.6 | 1 | 16.7 | 1.000 |
| Yes | 19 | 82.6 | 14 | 82.4 | 5 | 83.3 | |
| Disease status before TMZ‐containing chemotherapy | |||||||
| Nonremission | 14 | 60.9 | 10 | 58.8 | 4 | 66.7 | 1.000 |
| Remission | 9 | 39.1 | 7 | 41.2 | 2 | 33.3 | |
| No. of treatment cycles with TMZ‐containing chemotherapy | 4 (1–68) | 4 (1–17) | 7 (1–68) | ||||
|
Follow‐up after initiation of TMZ‐containing chemotherapy; months, median (range) |
23 (1–87) | 23 (3–68) | 25 (1–87) | ||||
Abbreviations: int/high, intermediate/high expression; MGMT, O‐6‐methylguanine‐DNA methyltransferase; no/low, no/low expression; RFI, relapse‐free interval; TMZ, temozolomide.
The objective response rate (ORR, complete and partial response) was 14.3%, which was observed in 2 of 14 patients with radiologically measurable lesions. The ORR in the MGMTno/low group (50.0%) was higher than that in the MGMTint/high group with borderline significance (0%, p = 0.066; Figure 2).
FIGURE 2.
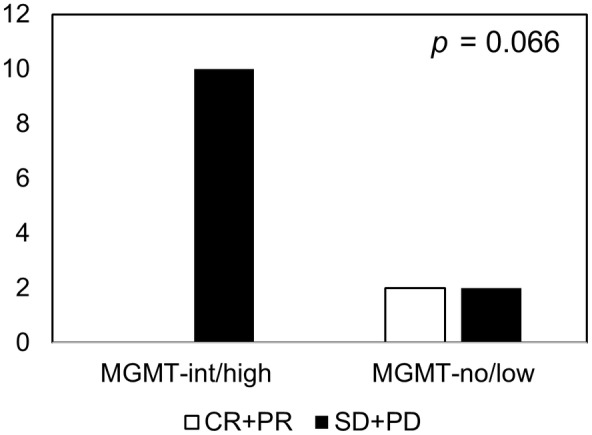
Response to temozolomide‐containing chemotherapy in patients with osteosarcoma who experienced recurrence or progression, grouped by O‐6‐methylguanine‐DNA methyltransferase (MGMT) protein expression. CR, complete response; int/high, intermediate/high expression; no/low, no/low expression; PD, progressive disease; PR, partial response; SD, stable disease.
3.3. Impact of MGMT protein expression on clinical outcome
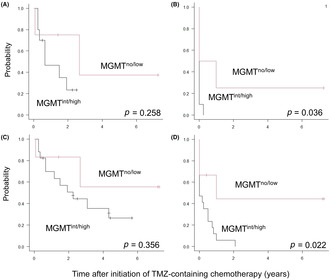
Among the 14 patients with radiologically measurable lesions receiving TMZ‐containing chemotherapy, the 2‐year OS rate was higher in the MGMTno/low group (75.0 [95% confidence interval (CI), 12.8%–96.1%]) than in the MGMTint/high group (23.3% [95% CI, 3.6%–52.9%], p = 0.258; Figure 3A), although the difference was not statistically significant. The 6‐month PFS rate was significantly higher in the MGMTno/low group (50.0% [95% CI, 5.8%–84.5%]) than in the MGMTint/high group (0%, p = 0.036; Figure 3B). Of note, a female patient with low MGMT protein expression achieved partial remission of multiple recurrences to the liver by long‐term TMZ + ETP treatment, and was alive and disease‐free approximately 2 years after curative surgery, as reported previously. 9 Among the nine patients receiving TMZ‐containing chemotherapy in remission, the 2‐year OS and 6‐month PFS rates in the MGMTint/high group (n = 7) were 85.7% (95% CI, 33.4%–97.9% and 57.1% (95% CI, 17.2%–83.7%), respectively, while the remaining two patients in the MGMTno/low group were alive and free from disease for 5 and 86 months, respectively. Collectively, among all 23 patients, the 2‐year OS rate was higher in the MGMTno/low group (83.3% [95% CI. 27.3%–97.5%]) than in the MGMTint/high group (50.7% [95% CI, 25.1%–71.6%], p = 0.356; Figure 3C), although the difference was not statistically significant. Multivariate analysis identified disease status before TMZ‐containing chemotherapy as the sole independent factor associated with OS (Table 3). The 6‐month PFS rate was significantly higher in the MGMTno/low group (66.7% [95% CI, 19.5%–90.4%]) than in the MGMTint/high group (23.5% [95% CI, 7.3%–44.9%], p = 0.022; Figure 3D]. Multivariate analysis indicated that MGMT protein expression and disease status before TMZ‐containing chemotherapy were significantly associated with PFS (Table 3).
FIGURE 3.
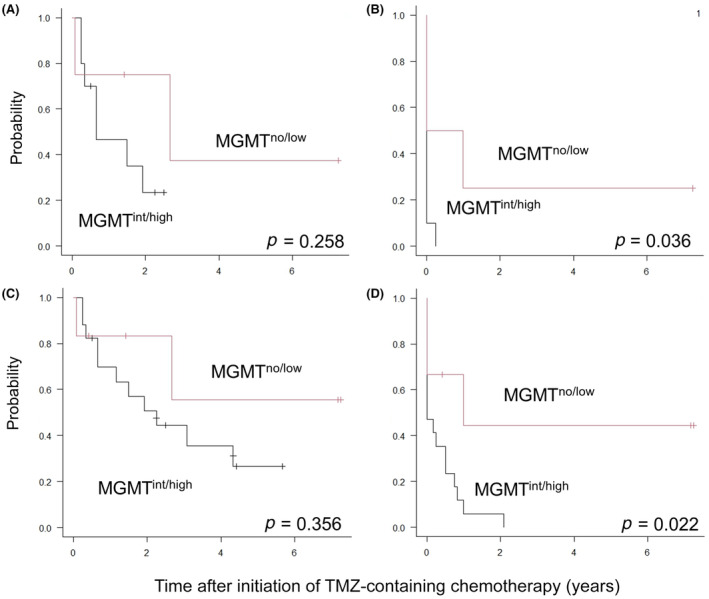
(A, C) Overall survival and (B, D) progression‐free survival rates of (A, B) 14 patients with radiologically measurable lesions and (C, D) all 23 patients receiving temozolomide(TMZ)‐containing chemotherapy grouped by O‐6‐methylguanine‐DNA methyltransferase (MGMT) protein expression. int/high, intermediate/high expression; no/low, no/low expression.
TABLE 3.
Univariate and multivariate analyses of factors affecting overall survival (OS) and progression‐free survival (PFS) in 23 patients receiving temozolomide (TMZ)‐containing chemotherapy for osteosarcoma with recurrence or progression (R/P).
| Variable | Factor (n) | 2‐year OS, % (95% CI) | Univariate analysis | Multivariate analysis | 6‐month PFS, % (95% CI) | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|---|---|---|---|---|
| p value | HR (95% CI) | p value | p value | HR (95% CI) | p value | ||||
| Age group | 0–19 years (17) | 46.9 (20.7–69.5) | 0.283 | N.E. | N.E. | 28.2 (9.6–50.5) | 0.842 | N.E. | N.E. |
| ≥20 years (6) | 83.3 (27.3–97.5) | 50.0 (11.1–80.4) | |||||||
| Sex | Male (12) | 55.6 (23.7–78.7) | 0.672 | N.E. | N.E. | 41.7 (15.2–66.5) | 0.807 | N.E. | N.E. |
| Female (11) | 59.7 (24.1–82.9) | 24.2 (4.4–52.5) | |||||||
| First RFI | <18 months (11) | 24.2 (3.8–54.2) | 0.053 | N.E. | N.E. | 18.2 (2.9–44.2) | 0.194 | N.E. | N.E. |
| ≥18 months (12) | 83.3 (48.2–95.6) | 50.0 (20.8–73.6) | |||||||
| Primary tumor site | Extremity (20) | 61.4 (35.2–79.6) | 0.042 | Reference | 33.8 (14.4–54.4) | 0.709 | N.E. | N.E. | |
| Axial (3) | 33.3 (0.9–77.4) | 3.77 (0.19–76.1) | 0.386 | 33.3 (0.9–77.4) | |||||
| R/P site | Lung alone (9) | 76.2 (33.2–93.5) | 0.438 | N.E. | N.E. | 44.4 (13.6–71.9) | 0.590 | N.E. | N.E. |
| Local (1) | NA (NA–NA) | N.A. (N.A.–N.A.) | |||||||
| Others (13) | 46.2 (19.2–69.6) | 23.1 (5.6–47.5) | |||||||
| Salvage chemotherapy after R/P | No (6) | 33.3 (4.6–67.6) | 0.672 | N.E. | N.E. | 16.7 (0.8–51.7) | 0.108 | N.E. | N.E. |
| Yes (17) | 67.9 (38.8–85.4) | 40.3 (17.6–62.2) | |||||||
| Prior use of other alkylators | No (3) | 33.3 (0.9–77.4) | 0.773 | N.E. | N.E. | N.A. (N.A.–N.A.) | 0.245 | N.E. | N.E. |
| Yes (20) | 61.9 (35.8–79.9) | 39.4 (18.6–59.7) | |||||||
| Local treatment for primary site after R/P | No (20) | 60.9 (34.8–79.3) | 0.017 | Reference | 33.8 (14.4–54.4) | 0.709 | N.E. | N.E. | |
| Yes (3) | 33.3 (0.9–77.4) | 16.7 (0.92–300.5) | 0.057 | 33.3 (0.9–77.4) | |||||
| Local treatment for metastasis after R/P | No (4) | N.A. (N.A.–N.A.) | 0.007 | Reference | N.A. (N.A.–N.A.) | 0.366 | N.E. | N.E. | |
| Yes (19) | 66.2 (39.6–83.3) | 0.11 (0.01–1.76) | 0.119 | 36.8 (16.5–57.5) | |||||
| Disease status before TMZ‐containing chemotherapy | Remission (9) | 87.5 (38.7–98.1) | 0.048 | Reference | 63.5 (23.8–86.6) | 0.043 | Reference | ||
| Nonremission (14) | 37.0 (12.3–62.5) | 5.26 (1.03–26.8) | 0.046 | 14.3 (2.3–36.6) | 5.98 (1.48–24.2) | 0.012 | |||
| MGMT protein expression | No/low (6) | 83.3 (27.3–97.5) | 0.356 | Reference | 66.7 (19.5–90.4) | 0.022 | Reference | ||
| Intermediate/high (17) | 50.7 (25.1–71.6) | 2.96 (0.50–17.5) | 0.232 | 23.5 (7.3–44.9) | 8.69 (1.68–44.8) | 0.010 | |||
Abbreviations: CI, confidence interval; HR, hazard ratio; MGMT, O‐6‐methylguanine‐DNA methyltransferase; N.A., not available; N.E., not evaluated; RFI, relapse‐free interval.
Grade 3 or higher hematological toxicities included grade 3 neutropenia (n = 4), grade 3 lymphopenia (n = 2), grade 3 anemia (n = 3), and grade 3 thrombopenia (n = 1). None of the patients developed severe neutropenic infection. Grade 3 diarrhea was observed in one patient as nonhematological toxicity.
4. DISCUSSION
The current study showed that TMZ‐containing chemotherapy exerts powerful and long‐lasting antitumor effects in a certain percentage of patients with osteosarcoma who experience R/P. Despite receiving multiple lines of chemotherapy prior to this study, two of the four patients in the MGMTno/low group achieved an objective response in radiologically measurable lesions. Furthermore, TMZ‐containing chemotherapy significantly contributed to increased survival benefit in patients with radiologically measurable lesions and no/low MGMT protein expression. Of note, 6‐month PFS of the patients in the MGMTno/low group was comparable to that in the clinical trials of regorafenib and cabozantinib (approximately 35%). 17 , 18 None of the patients showed severe nonhematological adverse effects or infection during treatment, which supports the incorporation of TMZ to salvage chemotherapy.
Unlike the finding in high‐grade glioma, 5 , 6 , 7 , 10 , 11 MGMT protein expression, but not MGMT promoter methylation, was identified as a reliable predictor of the effect of TMZ‐containing chemotherapy in R/P osteosarcoma. MGMT promoter methylation and no/low MGMT protein expression are observed in 33%–66% and 48%–61% of patients with high‐grade glioma, respectively, showing very high concordance and a significant correlation 5 , 6 , 7 , 10 , 11 and suggesting that MGMT promoter methylation is a primary cause of loss or reduction of MGMT protein expression. By contrast, the current study showed a relatively lower rate (30.0%) of patients with no/low MGMT protein expression in osteosarcoma, as reported previously. 19 , 20 Furthermore, the MGMT promoter was unmethylated in all 16 evaluable patients, which is inconsistent with a previous study in which MGMT promoter methylation was detected in 23.5% of patients. 19 The discordance between MGMT protein expression and MGMT promoter methylation rates might be partially due to differences in the detection method and/or cut‐off level between the two studies. Alternatively, the frequency of MGMT promoter methylation, but not that of no/low MGMT protein expression, might decrease after R/P compared with that at initial diagnosis, as observed in high‐grade glioma. 11 Nonetheless, the discordance between MGMT protein expression and MGMT promoter methylation rates indicates that methylation‐independent mechanisms, such as enhancer activation and microRNAs, might be involved in the repression of MGMT protein expression. 21 , 22
Because TMZ‐containing chemotherapy provides a therapeutic benefit in a minority of patients with osteosarcoma, new therapeutic strategies for decreasing MGMT protein expression need to be developed to maximize the antitumor effect of TMZ. Bromodomain and extraterminal (BET) inhibitor, which downregulates MGMT mRNA expression by reducing BRD4 occupancy at the MGMT promoter in high‐grade glioma, 23 , 24 might be useful to expand the therapeutic targets to patients with MGMT int or high expression.
The current study had several limitations. First, it was a retrospective study with a small group of patients who experienced R/P. Second, the attending physicians' decision‐making biases could have affected the clinical outcome. Third, the histological response to chemotherapy could not be assessed because of a lack of data in most patients. Finally, the follow‐up period was too short to evaluate the final clinical outcomes, including late adverse effects such as secondary malignancies and infertility. Nonetheless, the present data identify targets for promising salvage therapy in a subgroup of patients with osteosarcoma who experience R/P. Further prospective clinical trials are required to verify the clinical significance of TMZ‐containing salvage chemotherapy according to MGMT protein expression level.
AUTHOR CONTRIBUTIONS
Yoshinori Uchihara: Data curation; formal analysis; investigation; methodology; writing – original draft. Katsutsugu Umeda: Conceptualization; data curation; formal analysis; funding acquisition; investigation; methodology; validation; writing – original draft. Yosuke Yamada: Formal analysis; methodology; visualization. Hiroaki Ito: Data curation; methodology. Keiji Tasaka: Data curation; formal analysis; methodology; software; writing – original draft. Kiyotaka Isobe: Data curation; investigation; writing – original draft. Ryo Akazawa: Data curation; investigation. Naoko Kawabata: Data curation; formal analysis; validation. Satoshi Saida: Data curation; formal analysis; writing – original draft. Itaru Kato: Data curation; formal analysis; writing – original draft. Hidefumi Hiramatsu: Data curation; methodology; supervision. Takashi Noguchi: Data curation; investigation. Akio Sakamoto: Data curation; supervision; writing – original draft. Yoshiki Arakawa: Conceptualization; data curation; supervision. Ayumu Arakawa: Data curation; methodology; writing – original draft. Nobuyuki Yamamoto: Formal analysis; supervision. Yosuke Hosoya: Data curation; investigation; writing – original draft. Suguru Uemura: Data curation; investigation. Ken‐ichiro Watanabe: Conceptualization; data curation; supervision. Hideki Sano: Data curation; formal analysis; supervision. Takashi Taga: Data curation; investigation; supervision. Junko Takita: Conceptualization; funding acquisition; project administration; supervision.
FUNDING INFORMATION
This work was supported by a grant from the Children's Cancer Association of Japan.
CONFLICT OF INTEREST STATEMENT
Junko Takita is an editorial member of Cancer Science. Othe authors have no conflict of interest.
ETHICS STATEMENTS
Approval of the research protocol by institutional review board: This study (G1290) was approved by the institutional ethics committee of Kyoto University Hospital.
Informed consent: Written informed consent was obtained from each patient and their family.
Registry and the registration no. of the study/trial: N/A.
Animal studies: N/A.
5. ACKNOWLEDGEMENTS
We thank all staff for help with histological analysis.
Uchihara Y, Umeda K, Yamada Y, et al. MGMT protein expression is a reliable predictive biomarker for temozolomide‐containing chemotherapy in osteosarcoma. Cancer Sci. 2024;115:3394‐3402. doi: 10.1111/cas.16297
REFERENCES
- 1. Smrke A, Anderson PM, Gulia A, Gennatas S, Huang PH, Jones RL. Future directions in the treatment of osteosarcoma. Cells. 2021;10(1):172. doi: 10.3390/cells10010172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ferrari S, Briccoli A, Mercuri M, et al. Post relapse survival in osteosarcoma of the extremities: prognostic factors for long‐term survival. J Clin Oncol. 2003;21(4):710‐715. doi: 10.1200/JCO.2003.03.141 [DOI] [PubMed] [Google Scholar]
- 3. Bielack SS, Kempf‐Bielack B, Branscheid D, et al. Second and subsequent recurrences of osteosarcoma: presentation, treatment, and outcomes of 249 consecutive cooperative osteosarcoma study group patients. J Clin Oncol. 2009;27(4):557‐565. doi: 10.1200/JCO.2008.16.2305 [DOI] [PubMed] [Google Scholar]
- 4. Leary SE, Wozniak AW, Billups CA, et al. Survival of pediatric patients after relapsed osteosarcoma: the St. Jude Children's Research Hospital Experience. Cancer. 2013;119(14):2645‐2653. doi: 10.2147/CMAR.S261976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hegi ME, Diserens AC, Gorlia T, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005;352(10):997‐1003. doi: 10.1056/NEJMoa043331 [DOI] [PubMed] [Google Scholar]
- 6. Spiegl‐Kreinecker S, Pirker C, Fillpits M, et al. O6‐methylguanine DNA methyltransferase protein expression in tumor cells predicts outcome of temozolomide therapy in glioblastoma patients. Neuro‐Oncology. 2010;12(1):28‐36. doi: 10.1093/neuonc/nop003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pandith AA, Qasim I, Zahoor W, et al. Concordant association validates MGMT methylation and protein expression as favorable prognostic factors in glioma patients on alkylating chemotherapy (temozolomide). Sci Rep. 2018;8(1):6704. doi: 10.1038/s41598-018-25169-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Akazawa R, Umeda K, Saida S, et al. Temozolomide and etoposide combination for the treatment of relapsed osteosarcoma. Jpn J Clin Oncol. 2020;50(8):948‐952. doi: 10.1093/jjco/hyaa070 [DOI] [PubMed] [Google Scholar]
- 9. Umeda K, Taura K, Kato I, et al. Intensive multimodal therapy combined with long‐term temozolomide and etoposide treatment for recurrent osteosarcoma to the liver and stomach. J Pediatr Hematol Oncol. 2022;44(4):175‐177. doi: 10.1097/MPH.0000000000002412 [DOI] [PubMed] [Google Scholar]
- 10. Miyazaki M, Nishihara H, Terasaka S, et al. Immunohistochemical evaluation of O6‐methylguanine DNA methyltransferase (MGMT) expression in 117 cases of glioblastoma. Neuropathology. 2014;34(3):268‐276. doi: 10.1111/neup.12091 [DOI] [PubMed] [Google Scholar]
- 11. Park CK, Kim JE, Kim JY, et al. The changes in MGMT promoter methylation status in initial and recurrent glioblastomas. Transl Oncol. 2012;5(5):393‐397. doi: 10.1593/tlo.12253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228‐247. doi: 10.1016/j.ejca.2008.10.026 [DOI] [PubMed] [Google Scholar]
- 13. Rahman WF, Rahman KS, Nafi SN, Fauzi MH, Jaafar H. Overexpression of DNA methyltransferase 1 (DNMT1) protein in astrocytic tumour and its correlation with O6‐methylguanine‐DNA methyltransferase (MGMT) expression. Int J Clin Exp Pathol. 2015;8(6):6095‐6106. [PMC free article] [PubMed] [Google Scholar]
- 14. Baby P, Sciuscio D, Diserens AC, et al. MGMT methylation analysis of glioblastoma on the Infinium methylation BeadChip identifies two distinct CpG regions associated with gene silencing and outcome, yielding a prediction model for comparisons across datasets, tumor grades, and CIMP‐status. Acta Neuropathol. 2012;124(4):547‐560. doi: 10.1007/s00401-012-1016-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kanda Y. Investigation of the freely available easy‐to‐use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013;48(3):452‐458. doi: 10.1038/bmt.2012.244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ju HY, Park M, Lee JA, et al. Vincristine, irinotecan, and temozolomide as a salvage regimen for relapsed or refractory sarcoma in children and young adults. Cancer Res Treat. 2022;54(2):563‐571. doi: 10.4143/crt.2021.178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Duffaud F, Mir O, Boudou‐Rouquette P, et al. Efficacy and safety of regorafenib in adult patients with metastatic osteosarcoma: a non‐comparative, randomized, double‐blind, placebo‐controlled, phase 2 study. Lancet Oncol. 2019;20(1):120‐133. doi: 10.1016/S1470-2045(18)30742-3 [DOI] [PubMed] [Google Scholar]
- 18. Italiano A, Mir O, Mathoulin‐Pelissier S, et al. Cabozantinib in patients with advanced Ewing sarcoma or osteosarcoma (CABONE): a multicentre, single‐arm, phase 2 trial. Lancet Oncol. 2020;21(3):446‐455. doi: 10.1016/S1470-2045(19)30825-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cui Q, Juang W, Guo J, et al. Relationship between hypermethylated MGMT gene and osteosarcoma necrosis rate after chemotherapy. Pathol Oncol Res. 2011;17(3):587‐591. doi: 10.1007/s12253-010-9354-7 [DOI] [PubMed] [Google Scholar]
- 20. Cui Q, Li D, Liu C, et al. The significance of MGMT protein detection in evaluation of osteosarcoma necrosis rate after cisplatin chemotherapy. Bosn J Basic Med Sci. 2011;11(2):80‐83. doi: 10.17305/bjbms.2011.2585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chen X, Zhang M, Gan H, et al. A novel enhancer regulates MGMT expression and promotes temozolomide resistance in glioblastoma. Nat Commun. 2018;9(1):2949. doi: 10.1038/s41467-018-05373-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cabrini G, Fabbri R, Lo Nigro C, et al. Regulation of expression of O6‐methylguanine‐DNA methyltransferase and the treatment of glioblastoma. Int J Oncol. 2015;47(2):417‐428. doi: 10.3892/ijo.2015.3026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bai H, Harmanci AS, Erson‐Omay EZ, et al. Integrated genomic characterization of IDH1‐mutant glioma malignant progression. Nat Genet. 2016;48(1):59‐66. doi: 10.1038/ng.3457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tancredi A, Gusyatiner O, Bady P, et al. BET protein inhibition sensitizes glioblastoma cells to temozolomide treatment by attenuating MGMT expression. Cell Death Dis. 2022;13(12):1037. doi: 10.1038/s41419-022-05497-y [DOI] [PMC free article] [PubMed] [Google Scholar]


