Abstract
IE62, the major transcriptional regulatory protein encoded by varicella-zoster virus (VZV), is associated with the tegument of gradient-purified virions. Here, we show that most, if not all, of the association requires the expression of open reading frame 66 (ORF66), a protein kinase. The association of IE62 with wild-type VZV virions was confirmed using immunoelectron microscopy with IE62-specific antibodies, which reacted with virions in ultrathin sections of VZV-infected cells. Fractionated purified virions from cells infected with recombinant VZV ROka contained substantial levels of the 175-kDa virion IE62 protein and also contained the ORF66 protein. However, virions from cells infected with recombinant VZV ROka66S, in which ORF66 is disrupted, lacked not only the ORF66 protein but also most of the virion 175-kDa IE62 polypeptide. The virion-associated protein kinase activity was still present in ROka66S virions, although the 175-kDa protein substrate for the virion kinase was absent, implying that the virion protein kinase is encoded by genes other than ORF66. The very low levels of IE62 in ROka66S virions indicate that ORF66 protein mediates the redistribution of IE62 to sites of tegument assembly. IE62 was resolved into several species from VZV-infected cells which showed mobility differences between ROka and ROka66S, and a specific form of IE62 was detected in ROka virions. These results are consistent with a role for the ORF66-mediated phosphorylation of IE62 that results in cytoplasmic distribution of the regulatory protein for tegument inclusion. They support a model in which VZV tegument acquisition occurs in the cytoplasm. As such, two unusual features of VZV IE62, namely, its virion inclusion and its phosphorylation and nuclear exclusion by the ORF66 protein kinase, are functionally linked.
Varicella-zoster virus (VZV) is the ubiquitous human alphaherpesvirus that causes chickenpox upon primary infection and herpes zoster following reactivation from a long period of latency (reviewed in reference 1). In lytically infected cells, VZV gene expression occurs in a sequential cascade (34) and is likely regulated predominantly at the transcriptional level like that seen in cells infected with herpes simplex virus type 1 (HSV-1) (15). Viral genes are subdivided into immediate-early, early, and late, depending upon the requirements for their transcription and the timing of their synthesis. In transfected cells, transcription of VZV promoter-reporter constructs is influenced by a subset of VZV proteins including those encoded by open reading frames (ORFs) 4, 61, 62, 63, 10, and 29, and it is thus likely that these are the predominant regulatory proteins in VZV-infected cells (reviewed in reference 19).
The major transactivator of viral genes is the product of the ORF62 gene, which stimulates transcription from all VZV promoters studied to date, including its own in certain cells (16, 24, 28, 31, 32). In VZV-infected cells, ORF62 is expressed as an immediate-early gene (10) and encodes a 1,310-residue protein designated IE62. IE62 migrates as multiple forms between 170 and 180 kDa, partly as a result of its phosphorylation by both cellular and virus-encoded protein kinases (10, 20, 21, 30). IE62 has close homologs in all alphaherpesviruses identified to date, indicating common functional roles in transcriptional control. Linear comparisons of IE62 to HSV-1 ICP4 and pseudorabies virus IE175 indicate two regions of high homology representing two-thirds of the protein, separated by three regions of low homology (2). IE62 is, to a large extent, functionally conserved with HSV-1 ICP4, as it can complement HSV-1 ICP4 mutants and replace ICP4 in the context of the HSV-1 genome (4, 9). However, IE62 has features which have not been found in the corresponding homologs of other alphaherpesviruses. IE62 is relatively abundant in purified virions, where it is associated with the tegument (18, 21). It has been proposed that virion-associated IE62 may play a role in stimulating immediate-early events upon infection (18, 21, 28). The factors which direct IE62 into the virion tegument have not been resolved. A second unusual property of IE62 is its targeting by both of the VZV-encoded protein kinases. The protein kinase encoded by ORF47 can specifically phosphorylate IE62 in in vitro phosphorylation reactions (30), although the functional consequences of phosphorylation are not clear. Specific phosphorylation of IE62 mediated by the protein kinase encoded by ORF66 results in nuclear exclusion of IE62 in the late stages of infection (20, 22). In cells infected with a VZV recombinant (ROka66S) which does not express the ORF66 protein kinase, IE62 remains completely nuclear at all stages of infection (22). While the functional significance of the nuclear exclusion of IE62 by the ORF66 protein kinase was not clear, it was postulated that ORF66 may downregulate IE62 nuclear functions, such as the transcriptional activation of VZV genes.
Recent evidence has suggested that VZV can mature through the cytoplasmically located trans-Golgi network, where virions acquire tegument (40, 41, 44). As IE62 is an abundant tegument protein (21), we hypothesized that the cytoplasmic forms of IE62 may be a prerequisite for tegument inclusion during virion maturation. To explore this possibility, purified virions obtained from cells infected with ROka and the mutant ROka66S were examined for the presence of IE62. We show here that, indeed, ROka66S virions lack most of the virion form of IE62 protein.
MATERIALS AND METHODS
Cells and virus.
VZV strain Scott (isolate 71004) is a partly characterized wild-type isolate (21) and was used at less than 15 passages beyond its original isolation. Recombinant VZV ROka66S (deficient in the expression of ORF66) was detailed previously (14) and was kindly provided by Jeffrey Cohen, National Institute of Allergy and Infectious Diseases, Bethesda, Md. All VZVs were grown at 35°C on a human melanoma cell line (MeWo cells), as previously described (20), in Eagle's minimal essential medium supplemented with 5% Serum Plus (Hazleton Biologics Inc., Lenexa, Kans.), 5% fetal bovine serum, and an antibiotic mixture of 100 U of penicillin/ml and 0.1 mg of streptomycin/ml.
Antibodies.
Antipeptide rabbit antibodies that recognize the product of ORF29 and ORF10 and a multipotent rabbit polyclonal antibody to ORF62 have been described previously (18, 21). New rabbit antibodies to ORF66 were generated against a soluble maltose binding protein-ORF66 fusion antigen expressed in Escherichia coli, in a fashion similar to that detailed elsewhere (18). Cloning of the ORF66 gene for expression in E. coli utilized an EcoRI-BamHI DNA fragment containing the majority (residues 1 to 337) of the ORF derived from the vector pCMV66 (20), which was inserted into corresponding sites in the vector pmalC2 (New England Biolabs Inc., Worcester, Mass.) for generation of the fusion protein. Immunoblot detection of bound antibodies was carried out using a secondary goat antirabbit antibody coupled to horseradish peroxidase, as detailed previously (20), except that bound antibodies were visualized with chemiluminescent substrate. Quantitative analyses were carried out using a Bio-Rad GS 710 calibrated imaging densitometer and Quantity One software (Bio-Rad Inc., Hercules, Calif.).
Immunoelectron microscopy.
Primary antibodies for immunoelectron microscopy were first preabsorbed on uninfected MeWo cells, and the remaining immunoglobulin G was partially purified using ammonium sulfate precipitation. VZV-infected cells for immunoelectron microscopy were grown at 37°C (to minimize syncytium formation) and harvested at 90% or more cytopathic effect by gentle dislodging of infected cells into their own media, followed by washing in phosphate-buffered saline (PBS) at 4°C. Cells were fixed in fresh 5% formaldehyde for 1.5 h; rinsed three times in 1 M ammonium chloride; and successively dehydrated in 50, 75, and 90% dimethyl formamide for 10 min each. Cells were successively infiltrated with Lowacryl-dimethyl formamide concentrations of 30 and 50% for 10 min and twice with 100% Lowacryl for 20 min. Samples were polymerized for 1 h by exposure to UV light, sectioned (80 nm thick) using a microtome, and picked up on Formvar-coated 400-mesh nickel grids. For immunogold labeling, reactions were carried out in 50 μl of solution. Grids were first immersed in 1 M ammonium chloride for 1 h, rinsed, and blocked with 0.1% bovine serum albumin in PBS (PBS-bovine serum albumin) for 2 h. Following washing, grids were incubated with rabbit antibodies diluted in PBS-bovine serum albumin for 12 to 24 h at 4°C and extensively washed in PBS, and bound antibodies were detected with goat antirabbit antibodies conjugated to 10-nm gold particles (Janssen Supplies). Grids were washed extensively in PBS and water and then stained with 4% uranyl acetate and lead citrate solution for 2 min. Grids were again washed, dried, and subsequently examined with a JEOL transmission electron microscope at ×19,000 to ×48,000.
Virion purification and virion protein kinase assay.
Purification of VZV virion particles was achieved essentially as described previously (18). Briefly, VZV-infected MeWo cells grown at 32°C and showing greater than 80% cytopathic effect were harvested, washed in serum-free medium, and subjected to careful Dounce homogenization in serum-free medium to release the cytoplasm but maintain intact nuclei. Virions from the cytoplasmic fractions were combined with the pelleted fraction of cell-released material and were purified by two successive 5 to 15% Ficoll gradients made in PBS. The purity of the virion preparations was monitored by loss of reactivity of antibodies to the nonstructural ORF29 protein, by reactivity with antibodies to ORF10 tegument protein, and by characteristic protein staining following sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and Coomassie blue staining. For comparative immunoblotting studies, virion preparations were first adjusted to give equal levels of the 155-kDa major capsid protein, based upon densitometric analysis of the Coomassie blue-stained band.
Assessment of the protein kinase activity associated with purified virions was detected as detailed previously (21), using approximately 1 μg of virions in a kinase buffer composed of 25 mM HEPES (pH 7.4), 50 mM KCl, 1 mM EDTA, 20 mM MgCl2, 0.1% Nonidet-P40, 5 μM ATP, 50 μCi of [γ-32P]ATP (4,000 Cu/mmol), and a protease inhibitor cocktail (Mini EDTA free; Roche Molecular Chemicals Inc., Indianapolis, Ind.). Incorporation of 32P into phosphoproteins was detected by phosphorimager analysis.
RESULTS
Immunoelectron microscopy of IE62 in virions in VZV-infected cells.
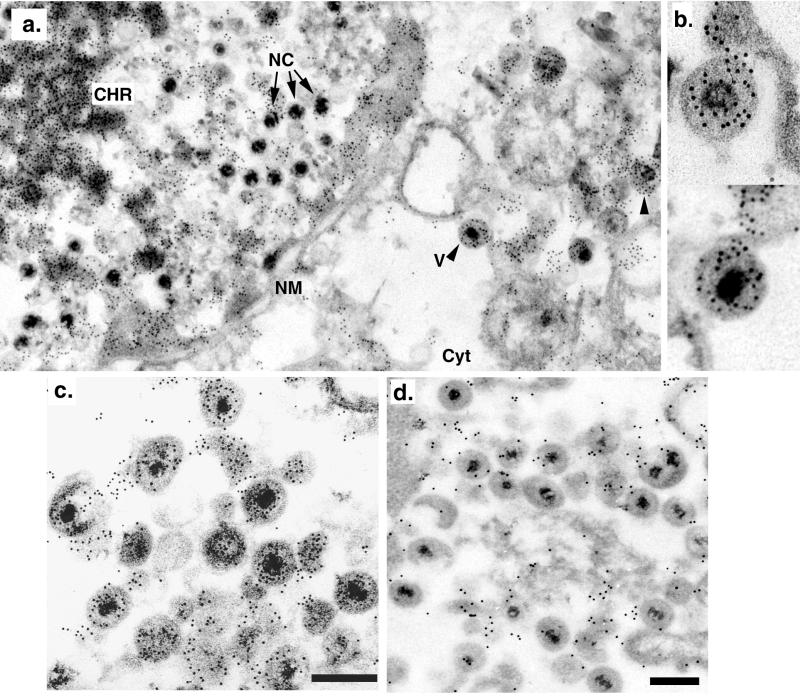
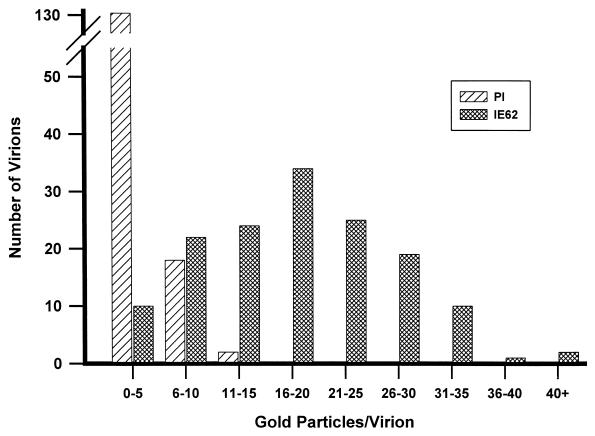
In previous work, the presence of IE62 in virions was demonstrated following purification and their fractionation by Ficoll, sucrose, and potassium tartrate gradients (18, 21). Studies demonstrated that the IE62 protein was a component of the tegument, as virion-associated IE62 protein was protected from trypsin by the envelope and remained associated with nucleocapsids following removal of the envelope but was absent in highly purified nucleocapsids. We first sought to validate the virion association of IE62 using approaches that did not require cellular disruption and gradient purification. The possibility existed that the virion-associated IE62 was due to contaminating infected-cell structures with biophysical properties similar to virions. We analyzed sections of late-stage VZV-infected cells by immunoelectron microscopy using a multipotent and powerful polyclonal antibody to IE62 (20) in conjunction with a secondary antibody covalently linked to 10-nm gold particles. In sections probed with IE62 antibodies, gold particles denoting IE62 presence were heavily distributed over electron-dense stained nuclear chromatin and were also associated with less electron-dense material at the inner border of the nuclear membrane (Fig. 1a). Gold particles were also concentrated in pockets within the cytoplasm which often appeared as vesicle-like structures of 300 to 800 nm. Within infected-cell nuclei, nucleocapsids with characteristic densely staining cores were observed, but these did not appear to be routinely associated with a high distribution of gold particles. However gold particles were concentrated over most tegumented virions in the cytoplasm (Fig. 1b and c). Specificity of the reactivity was demonstrated by probing sections with the specific anti-IE62 preimmune antibody in place of anti-IE62 antibodies (Fig. 1d), and these sections showed some gold particles with no preferential distribution over virions. To quantify the difference between preimmune and immune antibody-probed sections, 150 virions in infected-cell sections on at least five separate grids were evaluated for the number of gold particles directly associated with them (Fig. 2). For these analyses, only virions showing a characteristic densely staining core were scored. In sections of virions probed with preimmune antibody, the majority of virions contained a total mean of 2.6 gold particles per virion. In contrast, virions in sections probed with antibodies to IE62 showed a much higher level of gold particles associated with them, with a mean of 17.9 (±8.7) gold particles per virion. These results provide an additional line of evidence that IE62 is a component of virions and validate subsequent studies in which gradient-purified virions were analyzed.
FIG. 1.
Immunoelectron microscopic detection of IE62 in sections of VZV-infected cells. (a) Section of a VZV-infected cell showing nuclear and cytoplasmic regions (Cyt) separated by the nuclear membrane (NM). Densely staining chromatin structures (CHR), nucleocapsids (NC), and virions (V) are indicated. Arrowheads point to gold-labeled virions in the cytoplasm. (b) Enlargement of two single virions showing gold particles distributed over the virion. The virion shown in the lower part is indicated by “V” in panel a. (c and d) Section of infected-cell cytoplasm showing a concentration of tegumented virions. All sections were probed either with primary antibodies to IE62 (a to c) or with the corresponding preimmune rabbit antibodies at the same dilution (d). All sections were subsequently probed with goat antirabbit antibodies conjugated to 10-nm gold particles. The bars in panels c and d represent 500 nm, and panels c and d are shown at slightly different enlargements. Magnifications for recording the electron microscope images were ×19,000, except for the upper part of panel b, which was ×36,000.
FIG. 2.
Graphic representation of the distribution of gold particles in 150 virions obtained from several sections of VZV-infected cells probed with either IE62-specific antibodies (crosshatched bars) or with preimmune antibodies (hatched bars). Photographic negative images taken at ×19,000 were scanned under high resolution at 1,200 dpi, imaged, and magnified in Adobe Photoshop, and the gold particles over individual virions were counted. Only virions with a darkly staining nucleocapsid, as indicated by “V” in Fig. 1, were evaluated, and only gold particles distributed directly over the electron-dense tegument and nucleocapsid were counted.
Comparison of fractionated virions from ROka- and ROka66S-infected cells.
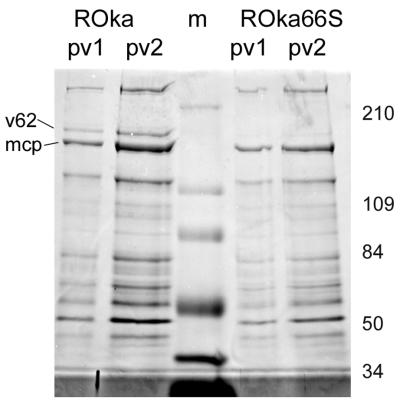
Recent evidence from the analysis of VZV glycoproteins has strongly indicated that VZV virions may acquire at least part of the tegument in the trans-Golgi network within the cytoplasm (11, 12, 41, 44). As ORF66 causes the cytoplasmic accumulation of IE62, we considered the possibility that ORF66 was required to redirect IE62 for cytoplasmic tegument inclusion. Recombinant VZVs lacking either of the two protein kinase genes have been described elsewhere (13, 14). VZVs lacking either kinase grow in tissue culture to similar levels as the wild type, based on assays for the ability to form infectious centers. However, both viruses have been shown elsewhere to be restricted for growth under certain circumstances, and ROka66S (not expressing ORF66) is impaired for growth in human T cells (but not human skin) in the SCID-hu mouse and in cultured T cells (25, 37). To determine if the ORF66 kinase affected virion incorporation of IE62, purified virions were obtained from cells infected with ROka and ROka66S. The proteins from purified virion preparations demonstrated similar Coomassie blue-stained SDS-PAGE-separated polypeptide profiles in two independent preparations of purified virions of each virus (Fig. 3). In ROka virions, the 175-kDa virion polypeptide thought to be IE62 was clearly detected, as expected from previous studies (Fig. 3, “v62”) (18, 21). Surprisingly, ROka66S virions contained only trace levels of the 175-kDa polypeptide, although other virion proteins demonstrated a profile similar to that found in ROka virions. This result suggested that the disruption of expression of the ORF66 protein resulted in the inefficient virion incorporation of the 175-kDa polypeptide.
FIG. 3.
Coomassie blue-stained SDS-PAGE-separated polypeptide profile of two separate preparations of virions obtained from cells infected with either ROka or ROka66S. All preparations were derived following two sequential Ficoll gradient fractionations of infected-cell cytoplasmic extracts. The lane marked “m” represents molecular weight markers used to identify the sizes of the virion polypeptides, which are shown to the right in thousands. The 155-kDa major capsid protein (mcp) and the virion 175-kDa polypeptide (v62) are indicated on the left of the figure.
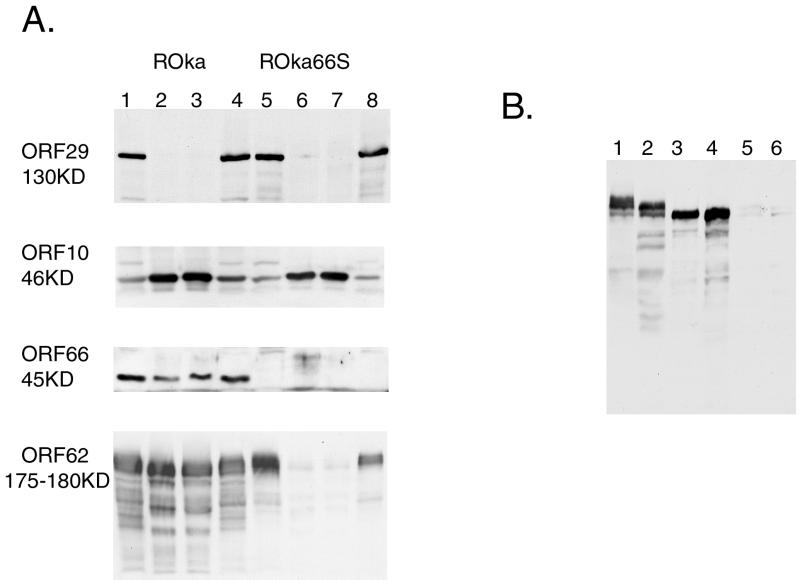
The stained protein profiles of the four virion preparations were normalized for levels of the major capsid protein, and immunoblot analyses of the virions were compared to extracts of infected-cell polypeptides (Fig. 4A). As expected, all virion preparations were virtually devoid of the ORF29 protein, a nonstructural polypeptide (18, 21) involved in DNA replication (Fig. 4A, lanes 2, 3, 6, and 7). In contrast, all virion preparations from both ROka- and ROka66S-infected cells contained high and equivalent levels of the 46-kDa tegument protein from ORF10, which is the VZV homolog of the HSV-1 major tegument protein VP16 and is the VZV transactivator of expression of IE62 (26, 27). Immunoblot analysis with a new specific polyclonal rabbit antibody to ORF66 protein demonstrated low but detectable levels of the ORF66 protein in the virion preparations of ROka, indicating that the ORF66 protein kinase was also a virion protein. As expected, the ORF66-specific antibodies failed to react with the same sized polypeptide in ROka66S virions. In correlation with the very low levels of the 175-kDa polypeptide in the stained polypeptide profile of ROka virions shown in Fig. 3, IE62-specific antibodies detected only trace levels of the virion form of IE62, although it was clearly present in corresponding ROka66S-infected-cell extracts. Quantitative assessment based on the IE62-positive signal in lanes 2, 3, 6, and 7 of Fig. 4A indicated that the normalized levels of IE62 in the ROka66S virions averaged 2.3% of that found in ROka virions. In contrast, the levels of ORF10 protein were similar in ROka and ROka66S virions and were not significantly different. These data indicate that the ORF66 protein kinase is required for most of the abundant association of IE62 with the tegument of virions. As ROka and ROka66S are reported elsewhere to grow at similar levels (14), the data also imply that the abundant levels of IE62 found in virions are not required for the initiation of replication of VZV or its efficient growth in cell culture.
FIG. 4.
(A) Immunoblot analysis of four identical blots of whole-VZV-infected-cell extracts (lanes 1, 4, 5, and 8) and of two separate preparations of VZV-purified virions (lanes 2, 3, 6, and 7) obtained from cells infected with ROka (lanes 1 to 4) or ROka66S (lanes 5 to 8). All virion preparations were normalized for the level of the major capsid protein. Blots were probed with rabbit antibodies to ORF29, -10, -66, and -62, as indicated on the left of each figure. The approximate sizes of the expected polypeptides are shown to the left of each blot. (B) Immunoblot of a higher-resolution SDS-PAGE assay of infected-cell extracts (lanes 1 and 2) and of purified virions (lanes 3 to 6) which has been probed with antibodies to IE62. Extracts and virions are from cells infected with ROka (lanes 2 to 4) and ROka66S (lanes 1, 5, and 6).
These studies suggested mobility differences in IE62 from ROka- and ROka66S-infected-cell extracts, as well as the presence of specific forms of IE62 in ROka virions. Previous studies have not indicated such differences (14, 20, 21). By using extended electrophoretic separation coupled with recirculation of cathode buffers on SDS–7% polyacrylamide gels, at least three forms of IE62 were detected in ROka66S-infected cells (Fig. 4B), and the apparent lowest-mobility form of IE62 in ROka66S extracts either was not present in ROka-infected-cell extracts or migrated as a faster form. Multiple forms and their differential mobilities were also found in infected human foreskin fibroblasts (data not shown). Furthermore, only the fastest-migrating forms of IE62 were found in virions. These results are consistent with previous studies (20) which indicate that the IE62 protein is affected by the ORF66 protein kinase, both at the level of cellular location and at the level of phosphorylation (20, 22). The mobility differences could reflect the specific phosphorylation events of IE62 induced by the ORF66 kinase (22) or could reflect different modification or phosphorylation events resulting from the ORF66-mediated cellular redistribution of IE62. Interestingly, the low levels of IE62 that were detected in ROka66S virions were of a mobility similar to that in ROka virions, and it is possible that the very minor fraction of IE62 found in ROka66S virions represents incorporation in an ORF66-independent fashion.
Virion-associated kinase activity in ROka and ROka66S virions.
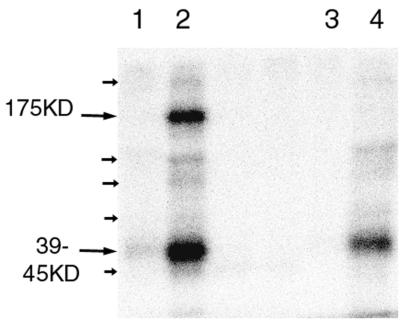
As ORF66 protein kinase is a structural protein, we investigated the virion-associated protein kinase activity of ROka and ROka66S virions. Normalized amounts of the ROka and ROka66S virions were subjected to a virion protein kinase assay as previously described (21). VZV virions contain a protein kinase activity that phosphorylates three predominant polypeptide species of 175, 42, and 37.5 kDa (21). In the absence of MgCl2, all virion preparations showed no significant 32P labeling of virion proteins. However, in the presence of MgCl2, major ROka virion proteins of 175 and 39 to 45 kDa and minor proteins of 300, 85, 70, 60, and 36 kDa were detected (Fig. 5, small arrows). Virion protein kinase activity of ROka66S virions in the presence of MgCl2 resulted in the radiolabeling of the same major and minor species, with the exception that the 175-kDa species was not detected. Taken with the lack of most IE62 in ROka66S virions, these results indicate that (i) the predominant virion protein kinase activity must be encoded by genes other than ORF66 and (ii) the absence of the 175-kDa virion substrate polypeptide in ROka66S virions reflects the absence of the virion form of IE62. There was a noticeable (34%) reduction in the total level of phosphorylation in ROka66S virions, suggesting that ORF66 contributes to virion-associated protein kinase activity.
FIG. 5.
Radiolabeled virion proteins following the incubation of normalized purified virion preparations from ROka (lanes 1 and 2)- and ROka66S (lanes 3 and 4)-infected cells with [γ-32P]ATP in kinase buffer with (lanes 2 and 4) or without (lanes 1 and 3) MgCl2. Major protein species are indicated by large arrows and their molecular masses, and minor species are indicated by small arrows.
DISCUSSION
This work links two unusual features of VZV IE62, features which have not been identified for the corresponding homologous proteins in other alphaherpesviruses to date. Specifically, our data indicate that the abundant association of IE62 with the tegument of VZV virions is functionally interlinked with expression of the ORF66 protein kinase. Taken with the recent demonstration that ORF66 protein kinase induces a cytoplasmic distribution of IE62 at late stages of infection (20, 22), it appears likely that the cytoplasmic accumulation of IE62 is a necessary prior step for virion inclusion. As such, our data support a proposed model for VZV maturation (11, 12, 40) in which at least part of the virion tegument is assembled and acquired outside the nucleus, specifically in the trans-Golgi network.
The immunogold electron microscopy results provided an independent line of evidence for the association of IE62 with the tegument of virions. We considered this alternative, gradient-independent approach important because the abundant levels of IE62 found in gradient-purified virions appear not to be a consistent phenomenon in other alphaherpesviruses, including several alphaherpesviruses closely related to VZV such as equine herpes virus type 1 and pseudorabies virus (17, 42; unpublished data). Previous demonstration of the virion-tegument association of IE62, which relied on virion purification from VZV-infected cytoplasmic extracts (18, 21), was open to the criticism that the apparent virion association of IE62 was due to contaminating vesicles or an IE62-rich VZV-infected-cell structure with biophysical properties very similar to those of virions. The electron microscopy data show that typical densely staining nucleocapsid-containing virions bound IE62-specific antibodies. As expected, nucleocapsids in nuclei did not bind high levels of the gold particles, supporting our previous observation that purified nucleocapsids obtained from nuclei were devoid of IE62 (18). These data validate previous studies and those presented here that use gradient-fractionated virions for the analysis of the IE62 tegument protein.
The surprisingly low levels of the virion IE62 175-kDa virion protein in ROka66S virions suggest that the ORF66 protein is required for most, if not all, of the virion association of IE62. There are several possible mechanisms by which ORF66 might facilitate IE62 virion tegument incorporation. As the ORF66 protein kinase also appears to be structural, it may facilitate tegument inclusion of IE62 through protein-protein interactions. We have not yet found evidence of such interactions, but IE62 physically interacts with the ORF47 protein kinase (30). Many such physical interactions between tegument proteins must likely occur for tegument assembly, and interactions have been found among the HSV-1 tegument proteins VP16, the virion host shutoff tegument protein (36), and the VP22 tegument protein (7). The VZV tegument protein from ORF10 interacts with glycoproteins gE and gI in the trans-Golgi network (41). A second possible mechanism is that the ORF66 protein kinase facilitates associations leading to tegument inclusion of IE62. While no targets for the protein kinase other than IE62 have been identified, phosphorylation is a well-known mechanism to reversibly activate (or inactivate) protein-protein interactions. Interestingly, phosphorylation by virion and cellular kinases in HSV-1 virions has been shown elsewhere to induce the opposite effect, causing the dissociation of the tegument (29). The third and most likely possibility is that the virion incorporation of IE62 is a consequence of its redirection to the cytoplasm (or cytoplasmic compartments) as a result of phosphorylation events mediated by the ORF66 protein kinase. In the presence of ORF66, IE62 is specifically phosphorylated near its nuclear import signal (20). Current work indicates that the phosphorylation affects the local charge near the IE62 nuclear import signal and inhibits import through the prevention of the binding of the nuclear importins (unpublished data). The cytoplasmic accumulation of IE62 in VZV-infected cells occurs predominantly at late stages of infection but not at immediate-early times when ORF66, a predicted early gene, is not expected to be expressed. ROka66S-infected cells do not accumulate cytoplasmic forms of IE62 at any stage of infection (20).
How, then, does cytoplasmic redirection result in virion association for a tegument protein? Two models for tegument assembly have been proposed which are not necessarily mutually exclusive. Electron microscopy studies have long implied that tegument forms at the inner side of the nuclear membrane and that virions mature as they envelop through the inner nuclear membrane (33). The second model proposes an envelopment-deenvelopment process which releases naked nucleocapsids into the cytoplasm (11, 12, 38–41). For VZV, recent evidence has suggested that virions mature following trafficking to the trans-Golgi network, where preformed tegument envelops nucleocapsids (11, 12, 40, 41, 44). The ORF10 tegument protein preferentially accumulates at the trans-Golgi network in infected cells (41). Several other herpesvirus tegument proteins have shown predominantly cytoplasmic distribution. The HSV-1 VP22 tegument protein is exclusively cytoplasmic and traffics through a Golgi network-mediated secretory pathway during virion maturation (8). HSV-1 with deletion of the tegument protein HSV-1 UL36 results in the distribution of DNA containing nontegumented capsids in the cytoplasm of infected cells (3). For human cytomegalovirus, several tegument proteins accumulate late in the cytoplasm in specific compartments which become associated with the endoplasmic reticulum–Golgi-intermediate compartment (35). Pseudorabies virus may also undergo a two-step nuclear envelopment-deenvelopment process (23). However, not all tegument proteins are cytoplasmic, and the predominantly nuclear distribution of the tegument protein VP13/14 (5, 6) suggests that some tegument acquisition may occur prior to exit from the nucleus. If VZV tegument is a predominantly cytoplasmic phenomenon, then the powerful IE62 nuclear import signal must be overridden, and ORF66 clearly can mediate this (20, 22). We favor a model in which IE62 is phosphorylated in the cytoplasm by early to late expression of ORF66 that subsequently allows redirection of IE62 to the cytoplasmic site of virion tegument assembly (e.g., the trans-Golgi network) rather than to the nucleus for transcriptional control.
Interestingly, VZV IE62 represents one of several tegument proteins which have nuclear roles early in infection that must subsequently associate with the tegument late in infection. These include ORF10, which activates transcription of IE62 in the nucleus by binding to the promoter in conjunction with cell factors (26, 27) but is cytoplasmic late in infection (41). In HSV-1, VP16 and the VP16 accessory proteins encoded by HSV-1 UL46 and -47 may also be bifunctional proteins with nuclear transcriptional and tegument assembly roles (43). It is possible that the nuclear-cytoplasmic distribution of some of these proteins may be controlled by phosphorylation events mediated by the viral protein kinases.
VZV ROka66S has been shown elsewhere to grow to a similar extent as the wild-type recombinant VZV in cell culture (14), suggesting that the abundant virion inclusion of IE62 is not needed at any stage of VZV growth in cultured cells, including the initiation of infection and virion maturation. However, ROka66S is attenuated for growth in T-cell implants in the SCID-hu mouse (25) and in primary cultures of T cells (37). We speculate that one possible reason is that the virion form of IE62 induced by ORF66 may have an auxiliary role for the growth of VZV in T cells. This may indicate that virion IE62 has an important cell-type-dependent role which may be important when certain cell factors required for the initiation of infection are naturally limited.
ACKNOWLEDGMENTS
This work was supported by Public Health Service grant EY09397, CORE Grant for Vision Research EY08098, The Eye & Ear Foundation, and Research to Prevent Blindness, Inc.
REFERENCES
- 1.Arvin A M. Varicella-zoster virus. Clin Microbiol Rev. 1996;9:361–381. doi: 10.1128/cmr.9.3.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cheung A K. DNA nucleotide sequence analysis of the immediate-early gene of pseudorabies virus. Nucleic Acids Res. 1989;17:4637–4646. doi: 10.1093/nar/17.12.4637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Desai P J. A null mutation in the UL36 gene of herpes simplex virus type 1 results in accumulation of unenveloped DNA-filled capsids in the cytoplasm of infected cells. J Virol. 2000;74:11608–11618. doi: 10.1128/jvi.74.24.11608-11618.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Disney G H, Everett R D. A herpes simplex virus type 1 recombinant with both copies of the Vmw175 coding sequences replaced by the homologous varicella-zoster virus open reading frame. J Gen Virol. 1990;71:2681–2689. doi: 10.1099/0022-1317-71-11-2681. [DOI] [PubMed] [Google Scholar]
- 5.Donnelly M, Elliot G. Fluorescent tagging of herpes simplex virus tegument protein VP13/14 in virus infection. J Virol. 2001;75:2575–2583. doi: 10.1128/JVI.75.6.2575-2583.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Donnelly M, Elliot G. Nuclear localization and shuttling of herpes simplex virus tegument protein VP13/14. J Virol. 2001;75:2566–2574. doi: 10.1128/JVI.75.6.2566-2574.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Elliott G, Mouzakitis G, O'Hare P. VP16 interacts via its activation domain with VP22, a tegument protein of herpes simplex virus, and is relocated to a novel macromolecular assembly in coexpressing cells. J Virol. 1995;69:7932–7941. doi: 10.1128/jvi.69.12.7932-7941.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Elliott G, O'Hare P. Live-cell analysis of a green fluorescent protein-tagged herpes simplex virus infection. J Virol. 1999;73:4110–4119. doi: 10.1128/jvi.73.5.4110-4119.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Felser J M, Kinchington P R, Inchauspe G, Straus S E, Ostrove J M. Cell lines containing varicella-zoster virus open reading frame 62 and expressing the “IE”175 protein complement ICP4 mutants of herpes simplex virus type 1. J Virol. 1988;62:2076–2082. doi: 10.1128/jvi.62.6.2076-2082.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Forghani B, Mahalingam R, Vafai A, Hurst J W, Dupuis K W. Monoclonal antibody to immediate early protein encoded by varicella-zoster virus gene 62. Virus Res. 1990;16:195–210. doi: 10.1016/0168-1702(90)90023-5. [DOI] [PubMed] [Google Scholar]
- 11.Gershon A A, Sherman D L, Zhu Z, Gabel C A, Ambron R T, Gershon M D. Intracellular transport of newly synthesized varicella-zoster virus: final envelopment in the trans-Golgi network. J Virol. 1994;68:6372–6390. doi: 10.1128/jvi.68.10.6372-6390.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gershon M D, Gershon A A. Role of glycoproteins in varicella-zoster virus infection. Contrib Microbiol. 1999;3:43–60. doi: 10.1159/000060309. [DOI] [PubMed] [Google Scholar]
- 13.Heineman T C, Cohen J I. The varicella-zoster virus (VZV) open reading frame 47 (ORF47) protein kinase is dispensable for viral replication and is not required for phosphorylation of ORF63 protein, the VZV homolog of herpes simplex virus ICP22. J Virol. 1995;69:7367–7370. doi: 10.1128/jvi.69.11.7367-7370.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heineman T C, Seidel K, Cohen J I. The varicella-zoster virus ORF66 protein induces kinase activity and is dispensable for viral replication. J Virol. 1996;70:7312–7317. doi: 10.1128/jvi.70.10.7312-7317.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Honess R W, Roizman B. Regulation of herpesvirus macromolecular synthesis. I. Cascade regulation of the synthesis of three groups of viral proteins. J Virol. 1974;14:8–19. doi: 10.1128/jvi.14.1.8-19.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Inchauspe G, Nagpal S, Ostrove J M. Mapping of two varicella-zoster virus-encoded genes that activate the expression of viral early and late genes. Virology. 1989;173:700–709. doi: 10.1016/0042-6822(89)90583-7. [DOI] [PubMed] [Google Scholar]
- 17.Killington R A, Yeo J, Honess R, Watson D H, Duncan B E, Halliburton I W, Mumford J. Comparative analyses of the proteins and antigens of five herpesviruses. J Gen Virol. 1977;37:297–310. doi: 10.1099/0022-1317-37-2-297. [DOI] [PubMed] [Google Scholar]
- 18.Kinchington P R, Bookey D, Turse S E. The transcriptional regulatory proteins encoded by varicella-zoster virus open reading frames (ORFs) 4 and 63, but not ORF 61, are associated with purified virus particles. J Virol. 1995;69:4274–4282. doi: 10.1128/jvi.69.7.4274-4282.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kinchington P R, Cohen J I. Varicella zoster virus proteins. In: Arvin A M, Gershon A A, editors. Varicella zoster virus. Cambridge, United Kingdom: Cambridge University Press; 2000. pp. 74–104. [Google Scholar]
- 20.Kinchington P R, Fite K, Turse S E. Nuclear accumulation of IE62, the varicella-zoster virus (VZV) major transcriptional regulatory protein, is inhibited by phosphorylation mediated by the VZV open reading frame 66 protein kinase. J Virol. 2000;74:2265–2277. doi: 10.1128/jvi.74.5.2265-2277.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kinchington P R, Hougland J K, Arvin A M, Ruyechan W T, Hay J. The varicella-zoster virus immediate-early protein IE62 is a major component of virus particles. J Virol. 1992;66:359–366. doi: 10.1128/jvi.66.1.359-366.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kinchington P R, Turse S E. Regulated nuclear localization of the varicella zoster virus major regulatory protein IE62. J Infect Dis. 1998;178:S16–S21. doi: 10.1086/514263. [DOI] [PubMed] [Google Scholar]
- 23.Klupp B G, Granzow H, Mettenleiter T C. Primary envelopment of pseudorabies virus at the nuclear membrane requires the UL34 gene product. J Virol. 2000;74:10063–10073. doi: 10.1128/jvi.74.21.10063-10073.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ling P, Kinchington P R, Sadeghi-Zadeh M, Ruyechan W T, Hay J. Transcription from varicella-zoster virus gene 67 (glycoprotein IV) J Virol. 1992;66:3690–3698. doi: 10.1128/jvi.66.6.3690-3698.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moffat J F, Zerboni L, Sommer M H, Heineman T C, Cohen J I, Kaneshima H, Arvin A M. The ORF47 and ORF66 putative protein kinases of varicella-zoster virus determine tropism for human T cells and skin in the SCID-Hu mouse. Proc Natl Acad Sci USA. 1998;95:11969–11974. doi: 10.1073/pnas.95.20.11969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moriuchi H, Moriuchi M, Cohen J I. Proteins and cis-acting elements associated with transactivation of the varicella-zoster virus (VZV) immediate-early gene 62 promoter by VZV open reading frame 10 protein. J Virol. 1995;69:4693–4701. doi: 10.1128/jvi.69.8.4693-4701.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moriuchi H, Moriuchi M, Straus S E, Cohen J I. Varicella-zoster virus open reading frame 10 protein, the herpes simplex virus VP16 homolog, transactivates herpesvirus immediate-early gene promoters. J Virol. 1993;67:2739–2746. doi: 10.1128/jvi.67.5.2739-2746.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moriuchi M, Moriuchi H, Straus S E, Cohen J I. Varicella-zoster virus (VZV) virion-associated transactivator open reading frame 62 protein enhances the infectivity of VZV DNA. Virology. 1994;200:297–300. doi: 10.1006/viro.1994.1190. [DOI] [PubMed] [Google Scholar]
- 29.Morrison E E, Wang Y F, Meredith D M. Phosphorylation of structural components promotes dissociation of the herpes simplex virus type 1 tegument. J Virol. 1998;72:7108–7114. doi: 10.1128/jvi.72.9.7108-7114.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ng T I, Keenan L, Kinchington P R, Grose C. Phosphorylation of varicella-zoster virus open reading frame (ORF) 62 regulatory product by viral ORF 47-associated protein kinase. J Virol. 1994;68:1350–1359. doi: 10.1128/jvi.68.3.1350-1359.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Perera L P, Mosca J D, Ruyechan W T, Hay J. Regulation of varicella-zoster virus gene expression in human T lymphocytes. J Virol. 1992;66:5298–5304. doi: 10.1128/jvi.66.9.5298-5304.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Perera L P, Mosca J D, Sadeghi-Zadeh M, Ruyechan W T, Hay J. The varicella-zoster virus immediate early protein, IE62, can positively regulate its cognate promoter. Virology. 1992;191:346–354. doi: 10.1016/0042-6822(92)90197-w. [DOI] [PubMed] [Google Scholar]
- 33.Roizman B, Furlong D. The replication of herpesviruses. In: Frankel-Conrat H, Wagner R R, editors. Comprehensive virology. New York, N.Y: Plenum Press; 1974. pp. 229–403. [Google Scholar]
- 34.Ruyechan W, Ling P, Kinchington P, Hay J. The correlation between varicella zoster virus transcription and the sequence of the viral genome. In: Wagner E K, editor. Herpesvirus transcription and its regulation. Boca Raton, Fla: CRC Press, Inc.; 1991. pp. 301–318. [Google Scholar]
- 35.Sanchez V, Greis K D, Sztul E, Britt W J. Accumulation of virion tegument and envelope proteins in a stable cytoplasmic compartment during human cytomegalovirus replication: characterization of a potential site of virus assembly. J Virol. 2000;74:975–986. doi: 10.1128/jvi.74.2.975-986.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schmelter J, Knez J, Smiley J R, Capone J P. Identification and characterization of a small modular domain in the herpes simplex virus host shutoff protein sufficient for interaction with VP16. J Virol. 1996;70:2124–2131. doi: 10.1128/jvi.70.4.2124-2131.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Soong W, Schultz J C, Parera A C, Sommer M H, Cohen J I. Infection of human T lymphocytes with varicella-zoster virus: an analysis with viral mutants and clinical isolates. J Virol. 2000;74:1864–1870. doi: 10.1128/jvi.74.4.1864-1870.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stackpole C W. Herpes-type virus of the frog renal adenocarcinoma. I. Virus development in tumor transplants maintained at low temperature. J Virol. 1969;4:75–93. doi: 10.1128/jvi.4.1.75-93.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang Z, Gershon M D, Lungu O, Panagiotidis C A, Zhu Z, Hao Y, Gershon A A. Intracellular transport of varicella-zoster glycoproteins. J Infect Dis. 1998;178(Suppl. 1):S7–S12. doi: 10.1086/514268. [DOI] [PubMed] [Google Scholar]
- 40.Wang Z H, Gershon M D, Lungu O, Zhu Z, Gershon A A. Trafficking of varicella-zoster virus glycoprotein gI: T338-dependent retention in the trans-Golgi network, secretion, and mannose 6-phosphate-inhibitable uptake of the ectodomain. J Virol. 2000;74:6600–6613. doi: 10.1128/jvi.74.14.6600-6613.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang Z H, Gershon M D, Lungu O, Zhu Z, Mallory S, Arvin A M, Gershon A A. Essential role played by the C-terminal domain of glycoprotein I in envelopment of varicella-zoster virus in the trans-Golgi network: interactions of glycoproteins with tegument. J Virol. 2001;75:323–340. doi: 10.1128/JVI.75.1.323-340.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yeo J, Killington R A, Watson D H, Powell K L. Studies on cross-reactive antigens in the herpesviruses. Virology. 1981;108:256–266. doi: 10.1016/0042-6822(81)90434-7. [DOI] [PubMed] [Google Scholar]
- 43.Zhang Y, McKnight J L. Herpes simplex virus type 1 UL46 and UL47 deletion mutants lack VP11 and VP12 or VP13 and VP14, respectively, and exhibit altered viral thymidine kinase expression. J Virol. 1993;67:1482–1492. doi: 10.1128/jvi.67.3.1482-1492.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhu Z, Gershon M D, Hao Y, Ambron R T, Gabel C A, Gershon A A. Envelopment of varicella-zoster virus: targeting of viral glycoproteins to the trans-Golgi network. J Virol. 1995;69:7951–7959. doi: 10.1128/jvi.69.12.7951-7959.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]