Summary
Background:
The effect of the increasing lifetime burden of non-major cardiovascular conditions on risk for a subsequent major adverse cardiovascular event among survivors of childhood cancer has not been assessed. We aimed to characterize the prevalence of major adverse cardiovascular event and their association with the cumulative burden of non-major adverse cardiovascular events in childhood cancer survivors.
Methods:
This is a longitudinal cohort study with participant data obtained from an ongoing cohort study at St Jude Children’s Research Hospital: the St Jude Lifetime Cohort Study (SJLIFE). Prospective clinical follow-up was of 5-year survivors of childhood cancer who were diagnosed when aged younger than 25 years from 1962 to 2012. Age-frequency, sex-frequency, and race-frequency matched community-control participants completed a similar one-time clinical assessment. 22 cardiovascular events were graded using a St Jude Children’s Research Hospital-modified version of the National Cancer Institute Common Terminology Criteria for Adverse Events (version 4.03). Cumulative incidence and burden of the primary outcome of major adverse cardiovascular events (cardiomyopathy, myocardial infarction, stroke, and other cardiovascular-related mortality) were estimated. Rate ratios (RR) of the association of major adverse cardiovascular events with 22 non-major adverse cardiovascular events were estimated using multivariable piecewise-exponential regression adjusting for attained age, age at diagnosis, sex, race and ethnicity, treatment era, diagnosis of diabetes, and exposure to cardiotoxic cancer therapies. The St Jude Lifetime Cohort study is registered with ClinicalTrials.gov, NCT00760656, and is ongoing.
Findings:
9602 5-year survivors of childhood cancer, and 737 community controls were included in the longitudinal follow-up (from Sept 13, 2007, to Dec 17, 2021). The median follow-up was 20·3 years (IQR 12·0–31·4) from the date of primary cancer diagnosis (4311 [44.9%] were females). By the age of 50 years (analysis stopped at age 50 years due to the low number of participants older than that age), the cumulative incidence of major adverse cardiovascular events among survivors was 17·7% (95% CI 15·9–19·5) compared with 0·9% (0·0–2·1) in the community controls. The cumulative burden of major adverse cardiovascular events in survivors was 0·26 (95% CI 0·23–0·29) events per survivor compared with 0·009 (0·000–0·021) events per community control participant. Increasing cumulative burden of grade 1–4 non-major adverse cardiovascular events was associated with an increased future risk of major adverse cardiovascular events (one condition: RR 4·3, 95% CI 3·1–6·0; p<0·0001; two conditions: 6·6, 4·6–9·5; p<0·0001; and three conditions: 7·7, 5·1–11·4; p<0·0001). Increased risk for major adverse cardiovascular events was observed with specific subclinical conditions (eg, grade 1 arrhythmias [RR 1·5, 95% CI 1·2–2·0; p=0·0017]), grade 2 left ventricular systolic dysfunction (2·2, 1·6–3·1; p<0·0001), grade 2 valvular disorders (2·2, 1·2–4·0; p=0·013), but not grade 1 hypercholesterolaemia, grade 1–2 hypertriglyceridaemia, or grade 1–2 vascular stenosis.
Interpretation:
Among an ageing cohort of survivors of childhood cancer, the accumulation of non-major adverse cardiovascular events, including subclinical conditions, increased the risk of major adverse cardiovascular events and should be the focus of interventions for early detection and prevention of major adverse cardiovascular events.
Funding:
The US National Cancer Institute and the American Lebanese Syrian Associated Charities.
Introduction
Advances in the treatment of childhood cancer and supportive care since 1975 have resulted in a remarkable increase in 5-year survival rates, which now exceed 85%.1 Yet, compared with the general population, survivors have a four-times increased risk of health-related mortality accelerating beyond 40 years from their diagnosis, with cardiovascular death being the most common non-cancer cause. 2 Additionally, decades after exposure, chest radiation and anthracyclines continue to be associated with cardiac-related morbidity and mortality in a dose-dependent manner. 2–4 Traditional cardiovascular risk factors (hypertension, obesity, dyslipidaemia, and diabetes) were previously found to potentiate the therapy-associated risk of developing cardiovascular conditions in a near-multiplicative fashion, providing modifiable targets for intervention. 5 However, the longitudinal progression of subclinical cardiovascular conditions, the effect of the cumulative burden of cardiovascular risk factors as survivors age, and their associations with risk for major adverse cardiovascular events is not known. 6
Major adverse cardiovascular events are a common composite endpoint of the leading causes of cardiovascular morbidity and mortality, and are defined by heart failure, myocardial infarction, stroke, and other cardiovascular-related mortality. 7 Studies conducted in adults in the general population reported a higher risk of major adverse cardiovascular events for individuals with subclinical cardiac conditions, such as asymptomatic left ventricular systolic dysfunction and pre-hypertension (blood pressure 120–139/80–89 mm Hg), than those without such conditions. 8,9 For survivors of childhood cancer, international consensus guidelines recommend screening for the early detection of specific cardiovascular conditions including cardiomyopathy and traditional cardiovascular risk factors among survivors at high risk. 10–12 Yet, it remains unknown how these subclinical non-major cardiovascular conditions detected with early screening progress from non-major adverse cardiovascular events to major adverse cardiovascular events in this population.
To address this knowledge gap, we leveraged the large, clinically assessed, longitudinal population of childhood cancer survivors participating in the St. Jude Lifetime Cohort (SJLIFE) study and performed detailed evaluations for 22 non-major adverse cardiovascular events. This study aimed to describe the cumulative burden of both major adverse cardiovascular events and non-major adverse cardiovascular events among survivors of various primary childhood cancers and a community controls sample. We then evaluated the associations between the cumulative burden of non-major adverse cardiovascular events and the subsequent occurrence of major adverse cardiovascular events in survivors.
Methods
Study design and participants
We used data from SJLIFE, which is a longitudinal cohort with prospective clinical follow-up of survivors of paediatric malignancies diagnosed in individuals who were aged younger than 25 years from 1962 to 2012, who were treated at St Jude Children’s Research Hospital, and who survived at least 5 years from the initial cancer diagnosis. This study included survivors from study inception (Sept 13, 2007) until the last contact date on Dec 17, 2021. Survivors in SJLIFE were followed from entry at 5-years survivorship until death, or if they requested to be removed from the study. Additionally, from study inception (Sep 13, 2007) SJLIFE is ongoingly recruiting age-frequency, sex-frequency, and race-frequency matched community control participants aged at least 5 years with no history of cancer diagnosis, with 737 participants as of Dec 17, 2021. These participants completed a similar one-time clinical assessment. Additional details on study design and methods have been previously described.13,14 Participation included comprehensive clinical, laboratory, and survey-based assessments. As part of the SJLIFE study,14 participants were invited for a longitudinal follow-up at 5-year intervals with almost half of the participants having completed one or more subsequent follow-up assessments. SJLIFE had ethics approval by the St Jude Children’s Research Hospital institutional review board. Written informed consent was obtained from all study participants.
Procedures
22 individual cardiovascular events were evaluated during the SJLIFE core health visit and were graded using a St Jude Children’s Research Hospital-modified version of the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE; version 4.03) with grade 1 events being mild, grade 2 events being moderate, grade 3 events being severe or disabling, and grade 4 events being life-threatening (table 1; appendix pp 3–8).13 Interim clinical events occurring before the most recent assessment were recorded and validated through abstraction of participants’ medical records. Cardiovascular events were categorised into seven disease groups: coronary artery disease, arrhythmias, ventricular dysfunction, structural defects, vascular disease, hypertension, and dyslipidaemia. To account for recurrency without overestimating multiply assessed conditions, events and grades were pre-determinedly defined as either chronic non-recurrent, chronic recurrent, or single recurrent, as previously described.4 To minimise participation bias, non-major adverse cardiovascular events and major adverse cardiovascular events of eligible non-participants were estimated using a multiple imputation method with known treatment exposures and other known information, as previously described.4,6 All events have been centrally reviewed by the SJLIFE study team. Date and cause of death for survivors were obtained through linkage with the US National Death Index (updated up to December, 2016), and through the St Jude Children’s Research Hospital Cancer Registry updated yearly for all patients treated at St Jude Children’s Research Hospital (for death occurring before the US National Death Index inception or after latest update). Cause of death was recorded using ICD-9 and ICD-10 codes.
Table 1. Grouped categories and individual Common Terminology Criteria of Adverse Events (CTCAE)-graded (major and non-major adverse cardiovascular events).
| Additional condition description | |
|---|---|
| Arrhythmias | |
| Atrioventricular block | 1st degree (PR interval via electrocardiogram > 200 milliseconds), 2nd degree (Mobitz type 1 or 2), 3rd degree (complete), Bundle branch block, QRS interval via ECG >120 milliseconds, Stokes–Adams syndrome. CTCAE grade 1–4 (non-MACE) and CTCAE grade 5 (MACE) |
| Cardiac dysrhythmia | Atrial fibrillation, atrial flutter, supraventricular arrhythmia, supraventricular tachycardia, paroxysmal atrial tachycardia, ventricular arrhythmia, and lethal arrhythmia requiring defibrillation. CTCAE grade 1–4 (non-MACE) and CTCAE grade 5 (MACE). |
| Conduction abnormalities | Sick sinus syndrome, Wolff–Parkinson–White syndrome. CTCAE grade 1–4 (non-MACE) and CTCAE grade 5 (MACE). |
| Prolonged QT corrected interval | CTCAE grade 1–4 (non-MACE). |
| Sinus bradycardia | Heart rate less than 50 beats per min (sustained after resting for 10 min in the Human Performance Laboratory at St Jude Children’s Research Hospital). CTCAE grade 1–4 (non-MACE) and CTCAE grade 5 (MACE). |
| Sinus tachycardia | Heart rate more than 110 beats per min (sustained after resting for 10 minutes in the Human Performance Laboratory at St Jude Children’s Research Hospital). CTCAE grade 1–4 (non-MACE) and CTCAE grade 5 (MACE). |
| Ventricular dysfunction | |
| Left ventricular systolic dysfunction | CTCAE grade 2 (subclinical; non-MACE) and CTCAE grades 3–5 (MACE). |
| Pulmonary hypertension | CTCAE grade 1–4 (non-MACE) and CTCAE grade 5 (MACE). |
| Cor pulmonale | Right heart failure. CTCAE grade 1–4 (non-MACE) and CTCAE grade 5 (MACE). |
| Right ventricular systolic dysfunction | CTCAE grade 1–4 (non-MACE) and CTCAE grade 5 (MACE). |
| Coronary artery disease | |
| Coronary artery disease | CTCAE grade 2 (non-MACE) and CTCAE grades 3–5 (MACE). |
| Dyslipidaemia | |
| Hypertriglyceridaemia | CTCAE grade 1–4 (non-MACE). |
| Hypercholesterolaemia | CTCAE grade 1–4 (non-MACE). |
| Hypertension | |
| Hypertension | CTCAE grade 1–4 (non-MACE) and CTCAE grade 5 (MACE). |
| Structural defects | |
| Valvular disorders | CTCAE grade 1–4 (non-MACE) and CTCAE grade 5 (MACE). |
| Aortic root aneurysm | CTCAE grade 1–4 (non-MACE) and CTCAE grade 5 (MACE). |
| Pericarditis | CTCAE grade 1–4 (non-MACE) and CTCAE grade 5 (MACE). |
| Vascular disease | |
| Cerebrovascular accidents | Lacunes, haemorrhagic stroke , ischemic stroke. CTCAE grade 1 (non-MACE) and CTCAE grades 2–5 (MACE). |
| Stenosis or occlusion of vessel | Stenosis or occlusion of vessel other than coronary or cerebral vessels (eg, carotid and subclavian). CTCAE grade 1–4 (non-MACE) and CTCAE grade 5 (MACE). |
| Thrombus | CTCAE grade 1–4 (non-MACE) and CTCAE grade 5 (MACE). |
| Arteriovenous malformation | CTCAE grade 1–4 (non-MACE) and CTCAE grade 5 (MACE). |
| Raynaud phenomenon | CTCAE grade 1–4 (non-MACE). |
Major adverse cardiovascular event (MACE) defined as cardiomyopathy (CTCAE grade 3–5 left ventricular systolic dysfunction), myocardial infarction (CTCAE grade 3–5 coronary artery disease), stroke (CTCAE grade 2–5 cerebrovascular accident), and other cardiovascular-related mortality (other CTCAE grade 5 cardiac events).
Trained research staff at St Jude Children’s Research Hospital abstracted data on primary cancer diagnosis and treatment exposures from the medical records of individuals eligible for participation in the SJLIFE using a structured protocol. Cumulative anthracycline doses were calculated using doxorubicin equivalent ratios. 12,15 The cumulative dose received for chest radiation and anthracyclines was stratified following the risk groups defined by the International Late Effects of Childhood Cancer Guidelines Harmonization Group. 12
Potential confounders were also abstracted from the medical records and comprised of sex, age at diagnosis, attained age, race and ethnicity, and treatment era. Modifiable covariates included a diagnosis of type 2 diabetes (defined by CTCAE grades 2–4 abnormal glucose metabolism), BMI (categorised as underweight [<18·5 kg/m2], normal [≥18·5 to <25 kg/m2], overweight [≥25 to <30 kg/m2], and obese [≥30 kg/m2]), and physical activity (dichotomised as meeting the Centers for Disease Control and Prevention recommendation for physical activity [yes or no; 150 min per week]).
Outcomes
The primary outcome was the incidence and risk of a composite of major adverse cardiovascular events, defined by cardiomyopathy (CTCAE grade 3–5 left ventricular systolic dysfunction, used to estimate heart failure among survivors in line with previous studies16), myocardial infarction (grade 3–5 coronary artery disease), stroke (grade 2–5 cerebrovascular accident), and other cardiovascular-related mortality (other grade 5 cardiac events; table 1; appendix pp 3–8).
Statistical analysis
Characteristics of survivors of childhood cancer and controls were summarised as median and IQR for continuous variables and as count and percentages for categorical variables. At-risk follow-up time for cardiovascular events began at 5 years after childhood cancer diagnosis and ended upon the date of last contact or death. Analysis stopped at age 50 years due to the low number of participants older than that age. Cumulative burden was estimated for survivors and controls following the method previously employed.6 Briefly, using the method of mean cumulative count,17 this metric estimates the mean count of chronic health conditions that develops per person during a specific timescale (eg, age), accounting for multiple types of recurrent events and competing risk of death (non- cardiovascular cause of death in our case). The 95% CI was estimated by age, sex, race and ethnicity, treatment exposure, and primary cancer diagnosis using the non-parametric bootstrap percentile method with 1000 iterations.
The cumulative incidence by age was estimated for all cardiovascular events using the Fine–Gray model, accounting for left truncation.18 The cumulative incidence of major adverse cardiovascular events 10 years after participants’ baseline visit was estimated using the Gray model. Survivors were stratified by treatment-related risk groups (adopted from the International Late Effects of Childhood Cancer Guidelines Harmonization Group cardiomyopathy risk groups; appendix p 11)12 and by the cumulative burden of grades 1–2 non-major adverse cardiovascular events (zero, one, two, three, four, and above), measured at the time of the baseline visit.19
Multivariable regression analysis was restricted to eligible survivors clinically assessed with an on-campus visit. The follow-up period started at 5 years after primary cancer diagnosis and ended at earliest major adverse cardiovascular events, competing event (non-cardiovascular-related mortality), or censoring (last contact date). Piecewise-exponential regression analysis was performed to assess associations between cumulative burden of 22 non-major adverse cardiovascular events, treated as time-dependent variables, and subsequent major adverse cardiovascular events. The time segments in each of which a constant hazard was estimated by the models were single years of attained age. The linearity and proportional hazard assumption were assessed and validated for each covariate by the inclusion of an interaction term between the covariate and attained age (time dependent). Analysis was adjusted for non-modifiable confounders (ie, sex, age at diagnosis, attained age, race and ethnicity, treatment era, treatment exposure [anthracycline, alkylating agents, platinum-containing agents, and neck, chest, and cranial radiation]), and modifiable confounders (ie, diagnosis of diabetes, BMI, and physical activity) treated as time-dependent variables (appendix p 12). The number of participants with missing data on any treatment predictor used in the regression model was 152 (2·9%) of 5229 clinically assessed participants. Consequently, we were able to use the complete-case analysis. The rate ratio (RR) with 95% CI was estimated using the standard large-sample inference methods of generalised linear models. Analytical models considered the total counts of 22 non-major adverse cardiovascular events, the counts by grade, and by individual conditions separately. Sensitivity analysis was performed assessing the risk of non-major adverse cardiovascular events on major adverse cardiovascular events for survivors of leukaemia and central nervous system tumours.
P-values less than 0·05 were considered statistically significant. All analysis was performed using R (version 3.5.2) or SAS (version 9.4) and all statistical tests were two sided. SJLIFE is an ongoing study registered with Clinicaltrials.gov, NCT00760656.
Role of funding source
The funders had no role in the study design, data collection, data analysis, data interpretation, or the writing of the report.
Results
Non-major adverse cardiovascular events and major adverse cardiovascular events were described among 9602 5-year survivors eligible for the SJLIFE Study at the time of this analysis, and 737 SJLIFE community-control participants. 4311 (44·9%) were female, and 2546 (26·5%) were diagnosed with acute lymphoblastic leukaemia, 1731 (18·0%) with central nervous system tumours, and 880 (9·2%) with Hodgkin lymphoma (table 2). The follow-up period extended from study inception (Sept 13, 2007) until Dec 17, 2021, with a median follow-up time of 20·3 years (IQR 12·0–31·4) from the date of primary cancer diagnosis. Association analyses of major adverse cardiovascular events with history of non-major adverse cardiovascular events was limited to the 5229 participants with clinical assessment at baseline on-campus visit (appendix pp 9–10). Clinically assessed SJLIFE participants were older at last contact or death (median 32·1 years [IQR 23·3–41·4] vs non-clinically assessed survivors 25·0 [17·1–36·3]) and had a higher proportion of females (2498 [47·8%] of 5229 participants vs 1813 [41·5%] of 4373) and non-Hispanic White participants (4042 [77·3%] of 5229 vs 3159 [72·2%] of 4373) compared with non-assessed survivors; 2922 (55·9%) were exposed to any dose of anthracyclines and 1100 (21·0%) to chest-directed radiotherapy. Among all eligible survivors, 6419 (66·9%) of 9602 participants had at least one grade 1 to 5 cardiovascular condition (major adverse cardiovascular events or non-major adverse cardiovascular events). Grade 1–2 hypertension was most prevalent (3852 [40·1%] of 9602 participants), and left ventricular systolic dysfunction was the most common cause of cardiovascular death (37 [<1%] of 9602; appendix pp 13–14).
Table 2. Demographic and treatment characteristics of survivors of childhood cancer in St. Jude Lifetime Cohort and community controls.
| Overall (n=9602) |
Clinically assessed survivors (n=5229) |
Non-clinically assessed survivors (n=4373) |
Community controls (n=737) |
|
|---|---|---|---|---|
| Sex | ||||
| Male | 5291 (55.1%) | 2731 (52.2%) | 2560 (58.5%) | 326 (44.2%) |
| Female | 4311 (44.9%) | 2498 (47.8%) | 1813 (41.5%) | 411 (55.8%) |
| Age at diagnosis, years | ||||
| Median (IQR) | 6·4 (2·8–12·6) | 6·4 (2·8–12·7) | 6·4 (2·8–12·7) | ·· |
| Age at censor or death, years | ||||
| Median (IQR) | 29·0 (19·8–39·4) | 32·1 (23·3–41·4) | 25·0 (17·1–36·3) | 35·1 (27·2–43·5) |
| Length of follow-up from diagnosis, years | ||||
| Median (IQR) | 20·3 (12·0–31·4) | 23·4 (15·1–33·5) | 16·2 (9·2–27·5) | ·· |
| Race and ethnicity | ||||
| Non-Hispanic White | 7201 (75.0%) | 4042 (77.3%) | 3159 (72.2%) | 595 (80.7%) |
| Non-Hispanic Black | 1626 (16.9%) | 844 (16.1%) | 782 (17.9%) | 48 (6.5%) |
| Hispanic | 383 (4.0%) | 204 (3.9%) | 179 (4.1%) | 32 (4.3%) |
| Other | 392 (4.1%) | 139 (2.7%) | 253 (5.8%) | 62 (8.4%) |
| Treatment era | ||||
| Before 1980 | 1421 (14.8%) | 685 (13.1%) | 736 (16.8%) | ·· |
| 1980–94 | 3110 (32.4%) | 1780 (34.0%) | 1330 (30.4%) | ·· |
| 1995 or later | 5071 (52.8%) | 2764 (52.9%) | 2307 (52.8%) | ·· |
| Any death (rate per 10 000 person-year; 95% CI) | 92·3 (87·7–97·1) | 22·9 (20·1–26·0) | 206·6 (195·5–218·2) | ·· |
| Cardiovascular death (rates per 10 000 person-years; 95% CI) | 4·0 (3·1–5·1) | 2·0 (1·3–3·1) | 7·4 (5·4–9·8) | ·· |
| Primary cancer diagnosis | ||||
| Acute lymphoblastic leukaemia | 2546 (26.5%) | 1485 (28.4%) | 1061 (24.3%) | ·· |
| Central nervous system tumour | 1731 (18.0%) | 765 (14.6%) | 966 (22.1%) | ·· |
| Hodgkin lymphoma | 880 (9.2%) | 522 (10.0%) | 358 (8.2%) | ·· |
| Non-Hodgkin lymphoma | 584 (6.1%) | 319 (6.1%) | 265 (6.1%) | ·· |
| Soft-tissue sarcoma | 575 (6.0%) | 316 (6.0%) | 259 (5.9%) | ·· |
| Bone tumour | 563 (5.9%) | 310 (5.9%) | 253 (5.8%) | ·· |
| Wilms tumour | 531 (5.5%) | 317 (6.1%) | 214 (4.9%) | ·· |
| Retinoblastoma | 472 (4.9%) | 308 (5.9%) | 164 (3.7%) | ·· |
| Neuroblastoma | 456 (4.7%) | 249 (4.8%) | 207 (4.7%) | ·· |
| Acute myeloid leukaemia | 428 (4.5%) | 243 (4.7%) | 185 (4.2%) | ·· |
| Germ cell tumour | 200 (2.1%) | 105 (2.0%) | 95 (2.2%) | ·· |
| Other | 636 (6.6%) | 290 (5.5%) | 346 (7.9%) | ·· |
| Chest-directed radiation, Gy * | ||||
| None | 7249/9021 (80.3%) | 4041/5141 (78.6%) | 3208/3880 (82.7%) | ·· |
| 0–14.9 | 313/9021 (3.5%) | 215/5141 (4.2%) | 98/3880 (2.5%) | ·· |
| ≥15–29.9 | 840/9021 (9.3%) | 558/5141 (10.8%) | 282/3880 (7.3%) | ·· |
| ≥30 | 619/9021 (6.9%) | 327/5141 (6.4%) | 292/3880 (7.5%) | ·· |
| Missing | 581/9602 (6.1%) | 88/5229 (1.7%) | 493/4373 (11.3%) | ·· |
| Neck radiation * | ||||
| Yes | 6993/9588 (72.9%) | 4097/5229 (78.3%) | 2896/4359 (66.5%) | ·· |
| No | 2595/9588 (27.1%) | 1132/5229 (21.7%) | 1463/4359 (33.5%) | ·· |
| Missing | 14/9602 (0.1%) | 0 | 14/4373 (0.3%) | ·· |
| Cranial radiation, Gy * | ||||
| None | 6657/8800 (75.6%) | 3784/5138 (73.6%) | 2873/3662 (78.4%) | ·· |
| <50 | 1406/8800 (16.0%) | 869/5138 (16.9%) | 537/3662 (14.7%) | ·· |
| ≥50 | 737/8800 (8.4%) | 485/5138 (9.4%) | 252/3662 (6.9%) | ·· |
| Missing | 802/9602 (8.3%) | 91/5229 (1.7%) | 711/4373 (16.2%) | ·· |
| Anthracycline dose, mg/m2 * | ||||
| None | 4608/9487 (48.6%) | 2291/5213 (43.9%) | 2317/4274 (54.2%) | ·· |
| 0–99.9 | 1628/9487 (17.2%) | 994/5213 (19.1%) | 634/4274 (14.8%) | ·· |
| ≥100–249.9 | 2136/9487 (22.5%) | 1315/5213 (25.2%) | 821/4274 (19.2%) | ·· |
| ≥250 | 1115/9487 (11.7%) | 613/5213 (11.8%) | 502/4274 (11.7%) | ·· |
| Missing | 115/9602 (1.2%) | 16/5229 (0.3%) | 99/4373 (2.3%) | ·· |
| Platinum agent exposure * | ||||
| Yes | 7819/9582 (81.6%) | 4309/5229 (82.4%) | 3510/4353 (80.6%) | ·· |
| No | 1763/9582 (18.4%) | 920/5229 (17.6%) | 843/4353 (19.4%) | ·· |
| Missing | 20/9602 (0.2%) | 0 | 20/4373 (0.5%) | ·· |
| Alkylating agent exposure * | ||||
| Yes | 4106/9581 (42.8%) | 2190/5229 (41.9%) | 1916/4352 (44.0%) | ·· |
| No | 5475/9581 (57.2%) | 3039/5229 (58.1%) | 2436/4352 (56.0%) | ·· |
| Missing | 21/9602 (0.2%) | 0 | 21/4373 (0.5%) | ·· |
Data are n (%) or n/N (%), unless stated otherwise.
Data not available for all patients.
At the age of 50 years, the estimated cumulative incidence of major adverse cardiovascular events among survivors, was 17·7% (95% CI 15·9–19·5) compared with 0·9% (0·0–2·1) in community controls. The cumulative burden of major adverse cardiovascular events in survivors was 0·26 (95% CI 0·23–0·29) major adverse cardiovascular events per survivor compared with 0·009 (0·000–0·021) events per community control participant (appendix pp 15–16; figure 1A). Cardio myopathy was the most frequent major adverse cardiovascular events at the age of 30 years (figures 1B, 2A). The burden of myocardial infarction increased beyond the age of 35 years, surpassing that of cardiomyopathy by the age of 50 years (myocardial infarction: 0·109 (95% CI 0·088–0·130) events per survivor; cardiomyopathy: 0·105 (0·089–0·121) events per survivor at 50 years; figure 1B). The highest burden of major adverse cardiovascular events at the age of 50 years observed in survivors of Hodgkin lymphoma (0·42 [95% CI 0·33–0·51] events per survivor), bone tumours (0·35 [0·15–0·55]), and acute myeloid leukaemia (0·32 [0·15–0·48]; appendix pp 19–20).
Figure 1. Cumulative burden of A) overall major adverse cardiovascular events (MACE) in survivors of childhood cancer compared with community controls, B) specific MACE conditions in survivors, C) overall non-MACE in survivors compared with community controls, and D) specific non-MACE conditions in survivors; N=9,602 for survivors and N=737 for controls.
Shaded areas represent the 95% confidence interval bands for the estimated cumulative burden per individual.
Panels A and C: p-values for survivors vs. controls curves are <0·0001
Figure 2.
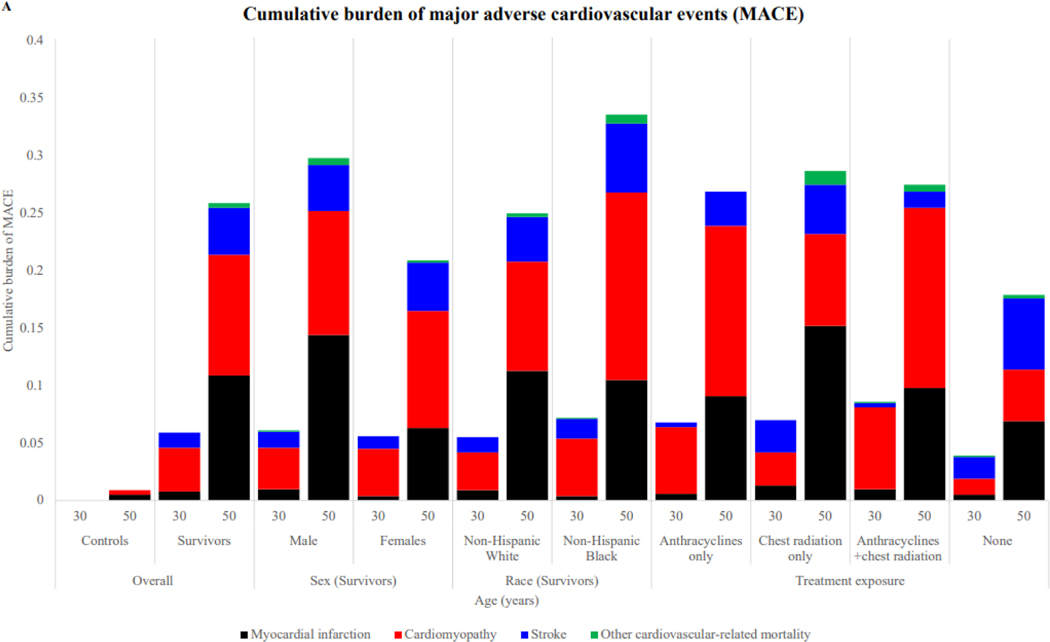
Cumulative burden of cardiovascular conditions at ages 30 and 50 by age, sex, race and cardiotoxic treatment exposure (A) major adverse cardiovascular events (B) non-major cardiovascular events; N=9,602 survivors and N=737 for community controls
The cumulative burden of non-major adverse cardiovascular events at the age of 50 years was 3·3 (95% CI 3·2–3·4) events per survivor compared with 2·4 (2·2–2·6) in community controls (figure 1C; appendix pp 17–18, p 21). Cardiovascular conditions with the highest contribution to the burden of non-major adverse cardiovascular events for survivors at the age of 50 years were dyslipidaemia (1·05 [95% CI 1·01–1·09 events per survivor]), hypertension (0·72 [0·70–0·73]), structural defects (0·65 [0·61–0·68]) and arrhythmias (0·57 [0·54–0·60]; figures 1D, 2B).
At the age of 50 years, participants who were male experienced a higher cumulative burden of both major adverse cardiovascular events and non-major adverse cardiovascular events compared with females, with a respective mean cumulative count of 0·30 (95% CI 0·25–0·35) and 3·46 (95% CI 3·30–3·61) events (vs females 0·21 (0·17–0·24) and 3·16 (3·02–3·30; figure 2). Although the burden of non-major adverse cardiovascular events was comparable between non-Hispanic White (3·40 [95% CI 3·29–3·51]) and non-Hispanic Black (3·13 [2·86–3·40]) survivors, the burden of major adverse cardiovascular events was higher in non-Hispanic Black (0·34 [0·24–0·43]) versus non-Hispanic White (0·25 [0·22–0·28]) survivors.
When survivors were stratified by risk groups based on treatment exposures, the effect of non-major adverse cardiovascular events conditions on the cumulative incidence of major adverse cardiovascular events was greatest for survivors with high-risk treatment exposures (appendix pp 22–24). The 10-year post-baseline visit cumulative incidence of major adverse cardiovascular events increased with increasing cumulative burden of grade 1–2 non-major adverse cardiovascular events detected at the baseline SJLIFE visit.
Compared with survivors without a non-major adverse cardiovascular event, those with one grade 1–4 non-major adverse cardiovascular event had an increased risk of subsequent major adverse cardiovascular events with a RR of 4·3 (95% CI 3·1–6·0; p<0·0001). This association increased with the increasing cumulative burden of non-major adverse cardiovascular events (RR for two conditions 6·6 [95% CI 4·6–9·5]; p<0·0001]; three conditions 7·7 [5·1–11·4]; p<0·0001, and four or more conditions 11·1 [7·5–16·3]; p<0·0001; table 3; appendix pp 25–29).
Table 3. Multivariable analysis of the rates of major adverse cardiovascular events by cumulative burden of grade 1–4 non-major adverse cardiovascular events.
| Major adverse cardiovascular events | Cardiomyopathy | Myocardial infarction | Stroke | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Rate ratio | 95% CI | p value | Rate ratio | 95% CI | p value | Rate ratio | 95% CI | p value | Rate ratio | 95% CI | p value | |
| Anthracycline dose, mg/m2 | ·· | ·· | <0·0001 | ·· | ·· | <0·0001 | ·· | ·· | 0·50 | ·· | ·· | 0·76 |
| None | 1·0 | ·· | ·· | 1·0 | ·· | ·· | 1·0 | ·· | ·· | 1·0 | ·· | ·· |
| 0–100 | 1·0 | 0·7–1·5 | 0·82 | 0·8 | 0·4–1·6 | 0·59 | 1·5 | 0·9–2·7 | 0·13 | 0·8 | 0·4–1·5 | 0·43 |
| ≥100–250 | 1·5 | 1·1–2·0 | 0·019 | 1·9 | 1·2–3·0 | 0·0092 | 1·0 | 0·6–1·7 | 0·88 | 0·9 | 0·4–2·0 | 0·85 |
| ≥250 | 2·6 | 1·9–3·5 | <0·0001 | 4·9 | 3·2–7·4 | <0·0001 | 1·1 | 0·7–1·8 | 0·62 | 0·6 | 0·2–1·9 | 0·42 |
| Chest radiation dose, Gy | ·· | ·· | 0·0001 | ·· | ·· | 0·0046 | ·· | ·· | <0·0001 | ·· | ·· | 0·47 |
| None | 1·0 | ·· | ·· | 1·0 | ·· | ·· | 1·0 | ·· | ·· | 1·0 | ·· | ·· |
| 0–14.9 | 1·5 | 0·8–2·8 | 0·19 | 1·5 | 0·7–3·6 | 0·32 | 3·7 | 1·6–8·3 | 0·0018 | 0·3 | 0·0–1·7 | 0·16 |
| ≥15–29.9 | 1·6 | 1·0–2·6 | 0·050 | 1·7 | 0·9–3·2 | 0·12 | 2·9 | 1·5–5·7 | 0·0019 | 0·4 | 0·1–1·5 | 0·18 |
| ≥30 | 2·5 | 1·7–3·6 | <0·0001 | 2·6 | 1·6–4·4 | 0·0002 | 3·6 | 2·0–6·6 | <0·0001 | 0·8 | 0·3–1·8 | 0·53 |
| Cranial radiation, Gy | ·· | ·· | 0·088 | ·· | ·· | 0·0057 | ·· | ·· | <0·0001 | ·· | ·· | <0·0001 |
| None | 1·0 | ·· | ·· | 1·0 | ·· | ·· | 1·0 | ·· | ·· | 1·0 | ·· | ·· |
| <50 | 0·9 | 0·7–1·2 | 0·45 | 0·5 | 0·3–0·8 | 0·0080 | 0·3 | 0·2–0·6 | <0·0001 | 8·3 | 4·3–16·0 | <0·0001 |
| ≥50 | 1·5 | 1·0–2·2 | 0·039 | 0·5 | 0·3–1·1 | 0·087 | 0·5 | 0·2–1·0 | 0·047 | 19·8 | 9·3–42·1 | <0·0001 |
| Neck radiation exposure | ·· | ·· | 0·0092 | ·· | ·· | 0·11 | ·· | ·· | 0·33 | ·· | ·· | 0·19 |
| No | 1·0 | ·· | ·· | 1·0 | ·· | ·· | 1·0 | ·· | ·· | 1·0 | ·· | ·· |
| 0–29.9 | 0·9 | 0·6–1·5 | 0·76 | 1·0 | 0·5–1·8 | 0·89 | 1·1 | 0·6–2·1 | 0·83 | 1·9 | 0·6–6·5 | 0·29 |
| ≥30 | 1·8 | 1·2–2·7 | 0·0041 | 1·8 | 1·0–3·1 | 0·046 | 1·6 | 0·9–3·1 | 0·14 | 2·1 | 0·9–4·9 | 0·071 |
| Alkylating agents | ·· | ·· | 0·58 | ·· | ·· | 0·0006 | ·· | ·· | 0·91 | ·· | ·· | 0·067 |
| Yes versus No | 1·1 | 0·8–1·3 | 0·58 | 1·9 | 1·3–2·8 | 0·0010 | 1·0 | 0·7–1·4 | 0·91 | 0·7 | 0·4–1·0 | 0·067 |
| Platinum | ·· | ·· | 0·0030 | ·· | ·· | 0·42 | ·· | ·· | 0·0001 | ·· | ·· | 0·012 |
| Yes versus No | 1·6 | 1·2–2·2 | 0·0023 | 1·2 | 0·8–1·8 | 0·42 | 2·9 | 1·8–4·9 | <0·0001 | 2·2 | 1·2–4·2 | 0·012 |
| Cumulative burden of non-MACE | ||||||||||||
| Number of grade 1–4 non-major adverse cardiovascular events | ·· | ·· | <0·0001 | ·· | ·· | <0·0001 | ·· | ·· | <0·0001 | ·· | ·· | <0·0001 |
| None | 1·0 | ·· | ·· | 1·0 | ·· | ·· | 1·0 | ·· | ·· | 1·0 | ·· | ·· |
| 1 | 4·3 | 3·1–6·0 | <0·0001 | 3·9 | 2·5–6·3 | <0·0001 | 8·0 | 4·4–14·7 | <0·0001 | 2·3 | 1·1–4·7 | 0·022 |
| 2 | 6·6 | 4·6–9·5 | <0·0001 | 6·1 | 3·6–10·3 | <0·0001 | 10·3 | 5·4–19·7 | <0·0001 | 4·0 | 1·8–8·6 | 0·0004 |
| 3 | 7·7 | 5·1–11·4 | <0·0001 | 7·8 | 4·4–14·0 | <0·0001 | 12·1 | 6·2–23·8 | <0·0001 | 5·8 | 2·6–12·9 | <0·0001 |
| ≥ | 11·1 | 7·5–16·3 | <0·0001 | 13·8 | 8·0–23·7 | <0·0001 | 12·4 | 6·3–24·4 | <0·0001 | 11·3 | 5·3–24·0 | <0·0001 |
Survivors with missing information on treatment (n=152) were excluded. N=5229 clinically assessed St Jude Lifetime Cohort Study survivors. All variables listed in the table are from the same model; adjusted for attained age as restricted cubic splines, sex, race and ethnicity, age at diagnosis, year of diagnosis, and diagnosis of diabetes. Five knots were used for the restricted cubic splines of attained age, with knots at the age of 10 years, 20 years, 30 years, 40 years, and 50 years. The knots were chosen so the first and last knots were about 5% and 95% of the points and the middle three knots were equally located, and then they were slightly shifted to round numbers.
In separate models, the prevalence of at least one grade 1–2 non-major adverse cardiovascular event was associated with a similar risk for subsequent major adverse cardiovascular events with RRs of 3·5 (95% CI 2·5–4·8; p<0·0001; appendix pp 30–31). Increased risk for major adverse cardiovascular events was observed with subclinical, asymptomatic non-major adverse cardiovascular events, including grade 2 valvular disorders (RR 2·2 [95% CI 1·2–4·0]; p=0·013), grade 2 left ventricular systolic dysfunction (2·2 [1·6–3·1]; p<0·0001)), grade 1 sinus tachycardia (2·6 [1·6–4·0]; p<0·0001), and grade 1 hypertension (1·6 [1·1–2·2; p=0·0057]; figure 3; appendix pp 32–34), not grade 1 hypercholesterolaemia, grade 1–2 hypertriglyceridaemia or grade 1–2 vascular stenosis, among other conditions.
Figure 3. Rate ratios with 95% CIs of associations between selected non-major cardiovascular events and risk for major adverse cardiovascular events.
● Grade 1 ▲ Grade 2 ■ Grade 3–4.
N=5229 clinically assessed St Jude Lifetime Cohort survivors (survivors with missing information on treatment [n=152] on treatment were excluded). Y-axis is on the log scale. Adjusted for attained age as restricted cubic splines, sex, race and ethnicity, age at diagnosis, year of diagnosis, diagnosis of diabetes, anthracycline dose, chest radiation dose, cranial radiation dose, neck radiation dose, and exposure to alkylating or platinum agents. Five knots were used for the restricted cubic splines of attained age, with knots at ages 10 years, 20 years, 30 years, 40 years, and 50 years. The knots were chosen so the first and last knots were about 5% and 95% of the points, and the middle three knots were equally located, and then they were slightly shifted to round numbers.
Discussion
Treatment exposures (anthracyclines and chest, head, and neck radiation) that allowed for both long-term survival and risk for major adverse cardiovascular events in survivors of childhood cancer are immutable.4,20 However, our data suggest that as survivors age, the growing burden of non-major cardiovascular conditions, including subclinical conditions detected only by surveillance screening, are strongly associated with future major adverse cardiovascular events and could be amenable to interventions with the potential to reduce major adverse cardiovascular events risk. We have shown that the presence of a single non-major adverse cardiovascular event of any grade was independently associated with a four-times increased rate of subsequent major adverse cardiovascular event. This rate increases in a step-wise fashion with the increasing number of non-major adverse cardiovascular events, even with low-grade subclinical non-major adverse cardiovascular events and, is particularly noticeable in survivors with moderate and high treatment-related cardiovascular risk. Thus, early discovery, secondary prevention through lifestyle modification, low treatment threshold, and high vigilance for the progression of non-major adverse cardiovascular event conditions should be high-priority targets in survivors.
Treatment-related grades 3–5 cardiomyopathy (ejection fraction <40%) and clinical heart failure are common after anthracycline chemotherapy and chest-directed radiotherapy; progressing to early death among long-term cancer survivors. 20 The updated International Late Effects of Childhood Cancer Guidelines Harmonization Group guidelines recommend screening asymptomatic survivors exposed to at least 100 mg/m2 of anthracycline or at least 15 Gy of chest-directed radiation for cardiomyopathy with echocardiography at a frequency dependent of the exposure dose. 12 However, in the setting of a known decreased ejection fraction (<40%), there are no survivor-specific management guidelines. 12 A previous analysis of a small population of survivors (N=299) suggested that a mildly reduced ejection fraction could predict a future ejection fraction of less than 40%.21 In this analysis using the large SJLIFE population, we have established that a grade 2 left ventricular systolic dysfunction, defined as an asymptomatic mildly reduced ejection fraction (<50–40%), is associated with an increased rate of subsequent major adverse cardiovascular events, independent of cardiotoxic treatments and other cardiovascular conditions. These findings suggest that survivors with asymptomatic mildly reduced ejection fraction could benefit from a lower threshold to initiate treatment compared with individuals from the general population with mildly reduced ejection fraction. Evidence on intervention strategies for asymptomatic mildly reduced ejection fraction is limited. A trial has shown that treatment with low-dose carvedilol, a third-generation beta-blocker, is effective in improving left ventricular end-diastolic diameter and end-systolic wall stress among survivors exposed to anthracyclines with a preserved ejection fraction (>50%).22 However, a single randomised clinical trial in survivors with asymptomatic anthracycline-associated left ventricular systolic dysfunction was inconclusive regarding the benefit of enalapril, an angiotensin converting enzyme inhibitor.23 In the general population, symptomatic mildly reduced ejection fraction is responsive to sodium glucose cotransporter 2 inhibitors, dapagliflozin and empagliflozin, shown by a reduction in progression of heart failure and cardiovascular-related mortality. 24,25 Future trials should investigate the safety and efficacy of these agents among survivors with asymptomatic, treatment-related low-grade left ventricular systolic dysfunction.
Arrhythmias can both be the cause of or exacerbate treatment-related cardiomyopathy. Independent of cardiotoxic treatments, our results suggest that low-grade arrhythmias, particularly atrioventricular blockade, conduction abnormalities, and tachycardia, are associated with an increased risk of major adverse cardiovascular events. The Children’s Oncology Group Long-Term Follow-Up Guidelines recommend a one-time screening electrocardiogram for survivors exposed to anthracyclines and heart-directed radiation (chest, abdomen, spine, total body) at the start of survivorship period, to be repeated as clinically indicated. 10 Our results motivate future opportunities to enhance early detection of subclinical arrhythmias through long-term electrocardiogram using wearable devices and remote monitoring. Additionally, detection of arrhythmias could merit deeper investigation and treatment of underlying causes. Possible drivers include cardiac remodelling and scar tissue. Moreover, the autonomic nervous system modulates cardiac electrophysiology and arrhythmogenesis. 26 Among survivors of childhood cancer, cardiac autonomic dysfunction is common and has been associated with increased mortality. 27,28 Symptoms of exercise intolerance or abnormal post exercise heart rate recovery may indicate autonomic dysfunction. 28 Although not yet investigated among survivors, early intervention on autonomic dysfunction may include supervised graded exercise or autonomic nervous system modulators (e.g. device-based vagal modulation). 26,29
In a previous SJLIFE study, one-third of survivors of childhood cancer were found to have a valvular abnormality. 20 In the general population, advanced valvular disease is associated with cardiac remodelling leading to heart failure and increasing the risk of cardiac-related mortality. A 2022 study investigated the long-term outcomes of valvular disease in a cohort of non-Hispanic Black individuals in their late 50s from the general population and highlighted an association between mild valvular lesions and risk of cardiovascular morbidities and mortality. 30 In this analysis, adult survivors of childhood cancer in their early 30s with a grade 2 valvular disorder, an asymptomatic condition not requiring medical intervention, had an increased rate of major adverse cardiovascular events. This finding suggests that for this high-risk population, early intervention might be necessary (eg, with aggressive blood pressure control and early statin initiation), and most certainly survivors with a grade 2 valve disorder should be referred to a cardiologist for evaluation and more frequent monitoring. Future studies are needed to determine how intervening on specific low-grade valvular disease could improve long-term cardiovascular health in survivors.
Lastly, it is widely accepted that most cardiovascular diseases can be prevented by a healthy lifestyle (eg, physical activity, maintaining healthy weight and eating habits, and non-smoking). Yet, little is known about whether healthy habits could moderate treatment-related cardiovascular risk in survivors of childhood cancer. Moreover, survivor-specific behavioural interventions for lifestyle change are limited.2 The findings call for evaluations of the moderator effect of lifestyle habits on the risk of non-major adverse cardiovascular events on subsequent major adverse cardiovascular events and investigations of the optimal delivery methods to promote healthy habits among survivors.
This study leverages a large cohort of clinically assessed survivors of childhood cancer. However, results should be interpreted with consideration of some limitations. First, unmeasured factors (e.g., smoking status) that can influence cardiovascular disease risk may have confounded our findings. Our assessment of major adverse cardiovascular events started 5 years after primary cancer diagnosis; however, data on behavioural risk factors at that time were unavailable, which prevented adjustments for those covariates in this analysis. Additionally, we did not assess emotional stress or negative social health determinants, which can affect risk for both non-major adverse cardiovascular events and major adverse cardiovascular events. Prospective evaluation of these variables in relation to non-major adverse cardiovascular events and major adverse cardiovascular events could inform the development of intervention strategies. Second, given some minor differences in the demographics of participants with and without clinical assessments, results might not be generalisable to all survivors of childhood cancer. To minimise potential participation bias, we used multiple imputation to describe the growing burden of major adverse cardiovascular events and non-major adverse cardiovascular events for all survivors. Third, some of the treatments used in this cohort do not reflect contemporary standard of care. Additionally, this analysis did not capture the long-term outcomes of newer targeted therapies (eg, immune checkpoint inhibitors and tyrosine kinase inhibitors). Nevertheless, anthracyclines, important cardiotoxic agents, which we did capture, remain extensively used to treat multiple childhood malignancies20.
In summary, we identified that as survivors age, non- major adverse cardiovascular events, including subclinical conditions, will progress and increase the subsequent risk of major adverse cardiovascular events with very high effect sizes, particularly among patients with previous moderate and high-risk cardiotoxic treatment exposures. Unfortunately, high-quality evidence informing survivor-specific therapeutic interventions are limited. Consequently, vulnerable survivors are unlikely to be treated when potentially amenable to intervention, which highlights the need for survivor-specific interventional guidelines and trials for the treatment of subclinical cardiovascular disease.
Supplementary Material
Research in context.
Evidence before this study
Cardiovascular diseases are the primary non-malignant cause of late-effect disease burden and premature mortality among survivors of childhood cancer. Major adverse cardiovascular events (cardiomyopathy, myocardial infarction, stroke, and other cardiovascular-related mortality) are a composite endpoint of the leading causes of cardiovascular morbidity and mortality. We searched PubMed on July 10, 2023, using the search terms “childhood cancer OR paediatric cancer,” “survivor,” and “long-term effects OR late effects OR cardiovascular outcomes”. We searched for studies published between database inception and June 1, 2023, with no language restrictions, to identify publications that investigated risk factors for major adverse cardiovascular events among 5-year survivors of childhood cancer. Previous publications described the risks associated with treatment exposure, demographic factors, germline genetics, and traditional cardiovascular risk factors (hypertension, obesity, dyslipidaemia, and diabetes). To our knowledge, not one study investigated the added contributions of the growing burden of non-major cardiovascular conditions over time, including asymptomatic subclinical conditions, to the subsequent risk of major adverse cardiovascular events.
Added value of this study
In this prospective, longitudinal cohort study with systematic in-person clinical assessment (St Jude Lifetime Cohort, NCT00760656), we identified that the presence of a single non-major adverse cardiovascular event condition of any grade independently increased the rate of major adverse cardiovascular events on longitudinal follow-up. This risk increased in a dose-dependent manner with the growing burden of non-major adverse cardiovascular events. Importantly, leveraging the richness of the St Jude Lifetime Cohort allowed us to simultaneously explore the longitudinal associations of individual cardiovascular conditions and grades with subsequent risk of major adverse cardiovascular events for the first time in an ageing cohort of childhood cancer survivors. We observed that several subclinical non-major adverse cardiovascular events, including asymptomatic valvular disorders, left ventricular systolic dysfunction, and some arrhythmias, independently increased the risk of future major adverse cardiovascular events.
Implications of all available evidence
The incidence and accumulation of non-major adverse cardiovascular events, including sub clinical conditions discovered by screening, increases the risk of subsequent major adverse cardiovascular events. This later finding emphasises the importance of early, pro-active screening for timely detection of non-major adverse cardiovascular events. In addition, as some non-major adverse cardiovascular events could be amenable to interventional efforts, our results suggest the importance of early intervention using lifestyle modifications and medical treatment, with the aim of reducing major treatment-related cardiovascular morbidities and mortality among childhood cancer survivors.
Acknowledgments
This study was supported by a Cancer Center Support grant (P30 CA021765) to St Jude Children’s Research Hospital (grant numbers U01 CA195547 [multiple principle investigators Melissa M Hudson and Kirsten K Ness], R01 CA216354 [multiple principle investigators Yutaka Yasui and Jinghui Zhang]), and the American Lebanese Syrian Associated Charities, Memphis, TN, USA.
Footnotes
Declaration of interest: All authors declare no conflicts of interest.
Data Sharing: The St. Jude Lifetime Cohort Study (SJLIFE) is funded by the US National Cancer Institute (U01 CA195547). SJLIFE data are publicly available on the St. Jude Survivorship Portal within the St. Jude Cloud at https://survivorship.stjude.cloud/.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- 1.Ehrhardt MJ, Krull KR, Bhakta N, et al. Improving quality and quantity of life for childhood cancer survivors globally in the twenty-first century. Nat Rev Clin Oncol 2023; 20(10): 678–96. [DOI] [PubMed] [Google Scholar]
- 2.Dixon SB, Liu Q, Chow EJ, et al. Specific causes of excess late mortality and association with modifiable risk factors among survivors of childhood cancer: a report from the Childhood Cancer Survivor Study cohort. Lancet 2023; 401(10386): 1447–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Faber J, Wingerter A, Neu MA, et al. Burden of cardiovascular risk factors and cardiovascular disease in childhood cancer survivors: data from the German CVSS-study. European Heart Journal 2018; 39(17): 1555–62. [DOI] [PubMed] [Google Scholar]
- 4.Bhakta N, Liu Q, Yeo F, et al. Cumulative burden of cardiovascular morbidity in paediatric, adolescent, and young adult survivors of Hodgkin’s lymphoma: an analysis from the St Jude Lifetime Cohort Study. Lancet Oncol 2016; 17(9): 1325–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Armstrong GT, Oeffinger KC, Chen Y, et al. Modifiable risk factors and major cardiac events among adult survivors of childhood cancer. Journal of Clinical Oncology 2013; 31(29): 3673–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bhakta N, Liu Q, Ness KK, et al. The cumulative burden of surviving childhood cancer: an initial report from the St Jude Lifetime Cohort Study (SJLIFE). Lancet 2017; 390(10112): 2569–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bosco E, Hsueh L, McConeghy KW, Gravenstein S, Saade E. Major adverse cardiovascular event definitions used in observational analysis of administrative databases: a systematic review. BMC Med Res Methodol 2021; 21(1): 241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Echouffo-Tcheugui JB, Erqou S, Butler J, Yancy CW, Fonarow GC. Assessing the Risk of Progression From Asymptomatic Left Ventricular Dysfunction to Overt Heart Failure: A Systematic Overview and Meta-Analysis. JACC Heart Fail 2016; 4(4): 237–48. [DOI] [PubMed] [Google Scholar]
- 9.Huang Y, Cai X, Liu C, et al. Prehypertension and the risk of coronary heart disease in Asian and Western populations: a meta-analysis. J Am Heart Assoc 2015; 4(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Children’s Oncology Group. Long-term follow-up guidelines for survivors of childhood, adolescent and young adult cancers, version 6·0 (October 2023). 2024. www.survivorshipguidelines.org (accessed Dec 20, 2023).
- 11.van Dalen EC, Mulder RL, Suh E, et al. Coronary artery disease surveillance among childhood, adolescent and young adult cancer survivors: A systematic review and recommendations from the International Late Effects of Childhood Cancer Guideline Harmonization Group. Eur J Cancer 2021; 156: 127–37. [DOI] [PubMed] [Google Scholar]
- 12.Ehrhardt MJ, Leerink JM, Mulder RL, et al. Systematic review and updated recommendations for cardiomyopathy surveillance for survivors of childhood, adolescent, and young adult cancer from the International Late Effects of Childhood Cancer Guideline Harmonization Group. Lancet Oncol 2023; 24(3): e108–e20. [DOI] [PubMed] [Google Scholar]
- 13.Hudson MM, Ehrhardt MJ, Bhakta N, et al. Approach for Classification and Severity Grading of Long-term and Late-Onset Health Events among Childhood Cancer Survivors in the St. Jude Lifetime Cohort. Cancer Epidemiol Biomarkers Prev 2017; 26(5): 666–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Howell CR, Bjornard KL, Ness KK, et al. Cohort Profile: The St. Jude Lifetime Cohort Study (SJLIFE) for paediatric cancer survivors. Int J Epidemiol 2021; 50(1): 39–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feijen EAM, Leisenring WM, Stratton KL, et al. Derivation of Anthracycline and Anthraquinone Equivalence Ratios to Doxorubicin for Late-Onset Cardiotoxicity. JAMA Oncol 2019; 5(6): 864–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mulrooney DA, Armstrong GT, Huang S, et al. Cardiac Outcomes in Adult Survivors of Childhood Cancer Exposed to Cardiotoxic Therapy: A Cross-sectional Study. Ann Intern Med 2016; 164(2): 93–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dong H, Robison LL, Leisenring WM, Martin LJ, Armstrong GT, Yasui Y. Estimating the burden of recurrent events in the presence of competing risks: the method of mean cumulative count. Am J Epidemiol 2015; 181(7): 532–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Geskus RB. Cause-specific cumulative incidence estimation and the fine and gray model under both left truncation and right censoring. Biometrics 2011; 67(1): 39–49. [DOI] [PubMed] [Google Scholar]
- 19.Gray RJ. A Class of K-Sample Tests for Comparing the Cumulative Incidence of a Competing Risk. Ann Stat 1988; 16(3): 1141–54. [Google Scholar]
- 20.Mulrooney DA, Hyun G, Ness KK, et al. Major cardiac events for adult survivors of childhood cancer diagnosed between 1970 and 1999: report from the Childhood Cancer Survivor Study cohort. BMJ 2020; 368: l6794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leerink JM, van der Pal HJH, Kremer LCM, et al. Refining the 10-Year Prediction of Left Ventricular Systolic Dysfunction in Long-Term Survivors of Childhood Cancer. JACC CardioOncol 2021; 3(1): 62–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Armenian S, Hudson MM, Lindenfeld L, et al. Carvedilol for prevention of heart failure in anthracycline-exposed survivors of childhood cancer: Results from COG ALTE1621. Journal of Clinical Oncology 2023; 41(16_suppl): 10013-. [Google Scholar]
- 23.Silber JH, Cnaan A, Clark BJ, et al. Enalapril to prevent cardiac function decline in long-term survivors of pediatric cancer exposed to anthracyclines. J Clin Oncol 2004; 22(5): 820–8. [DOI] [PubMed] [Google Scholar]
- 24.Solomon SD, McMurray JJV, Claggett B, et al. Dapagliflozin in Heart Failure with Mildly Reduced or Preserved Ejection Fraction. N Engl J Med 2022; 387(12): 1089–98. [DOI] [PubMed] [Google Scholar]
- 25.Anker SD, Butler J, Filippatos G, et al. Empagliflozin in Heart Failure with a Preserved Ejection Fraction. N Engl J Med 2021; 385(16): 1451–61. [DOI] [PubMed] [Google Scholar]
- 26.Florea VG, Cohn JN. The autonomic nervous system and heart failure. Circ Res 2014; 114(11): 1815–26. [DOI] [PubMed] [Google Scholar]
- 27.Groarke JD, Tanguturi VK, Hainer J, et al. Abnormal exercise response in long-term survivors of hodgkin lymphoma treated with thoracic irradiation: evidence of cardiac autonomic dysfunction and impact on outcomes. J Am Coll Cardiol 2015; 65(6): 573–83. [DOI] [PubMed] [Google Scholar]
- 28.Christoffersen L, Gibson TM, Pui CH, et al. Cardiac autonomic dysfunction in survivors of childhood acute lymphoblastic leukemia: The St. Jude Lifetime Cohort Study. Pediatric Blood & Cancer 2020; 67(7): e28388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hsu CY, Hsieh PL, Hsiao SF, Chien MY. Effects of Exercise Training on Autonomic Function in Chronic Heart Failure: Systematic Review. Biomed Research International 2015; 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Matsushita K, Gao Y, Rubin J, et al. Association of Mild Valvular Lesions With Long-term Cardiovascular Outcomes Among Black Adults. JAMA Netw Open 2022; 5(5): e2211946. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.