Abstract
Background
S100β is a biomarker of astroglial damage, the level of which is significantly increased following brain injury. However, the characteristics of S100β and its association with prognosis in patients with acute ischemic stroke following intravenous thrombolysis (IVT) remain unclear.
Methods
Patients in this multicenter prospective cohort study were prospectively and consecutively recruited from 16 centers. Serum S100β levels were measured 24 h after IVT. National Institutes of Health Stroke Scale (NIHSS) and hemorrhagic transformation (HT) were measured simultaneously. NIHSS at 7 days after stroke, final infarct volume, and modified Rankin Scale (mRS) scores at 90 days were also collected. An mRS score ≥ 2 at 90 days was defined as an unfavorable outcome.
Results
A total of 1072 patients were included in the analysis. The highest S100β levels (> 0.20 ng/mL) correlated independently with HT and higher NIHSS at 24 h, higher NIHSS at 7 days, larger final infarct volume, and unfavorable outcome at 3 months. The patients were divided into two groups based on dominant and non-dominant stroke hemispheres. The highest S100β level was similarly associated with the infarct volume in patients with stroke in either hemisphere (dominant: β 36.853, 95% confidence interval (CI) 22.659–51.048, P < 0.001; non-dominant: β 23.645, 95% CI 10.774–36.516, P = 0.007). However, serum S100β levels at 24 h were more strongly associated with NIHSS scores at 24 h and 3-month unfavorable outcome in patients with dominant hemisphere stroke (NIHSS: β 3.470, 95% CI 2.392–4.548, P < 0.001; 3-month outcome: odds ratio (OR) 5.436, 95% CI 2.936–10.064, P < 0.001) than in those with non-dominant hemisphere stroke (NIHSS: β 0.326, 95% CI − 0.735–1.387, P = 0.547; 3-month outcome: OR 0.882, 95% CI 0.538–1.445, P = 0.619). The association of S100β levels and HT was not significant in either stroke lateralization group.
Conclusions
Serum S100β levels 24 h after IVT were independently associated with HT, infarct volume, and prognosis in patients with IVT, which suggests the application value of serum S100β in judging the degree of disease and predicting prognosis.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12916-024-03517-6.
Keywords: S100β, Intravenous thrombolysis, Acute ischemic stroke, Outcome, Astroglial injury
Background
The global burden of disability caused by stroke has increased in recent years [1]. Intravenous thrombolysis (IVT) has the highest level of evidence for the treatment of acute disabling ischemic stroke [2]; however, approximately half of these patients do not achieve favorable outcomes following IVT [3]. Timely and accurate assessment of patients’ conditions can help neurologists formulate treatment strategies and adjust therapeutic regimes.
S100β is a calcium-binding protein mainly concentrated in astrocytes, and its elevation reflects astrocyte injury [4, 5]. Due to damage to brain tissue and the blood–brain barrier in patients with stroke, S100β is released and enters the bloodstream, resulting in abnormally high levels of S100β in the blood [6]. Previous studies found that serum S100β concentrations were highly correlated with final infarct volume in patients with ischemic stroke [7, 8]. Moreover, Branco et al. demonstrated that S100β levels in peripheral blood at 48 h post-stroke were associated with functional outcome at 3 months [9], which suggests S100β as a prognosis marker for patients with stroke. In patients following IVT, thrombolytic drugs may decrease the final infarct size but increase the breakdown of the blood–brain barrier [10]. Therefore, the characteristics of serum S100β levels in these patients and their relationship with prognosis are unique. Further investigation with a large patient sample size remains warranted.
In the present study, we collected blood samples 24 h after IVT from patients at 16 centers in northeast China to (a) test the serum S100β levels in patients 24 h after IVT; (b) explore the relationship between serum S100β levels and hemorrhagic transformation (HT); and (c) explore the association between serum S100β levels and final infarct volume, National Institutes of Health Stroke Scale (NIHSS), and modified Rankin Scale (mRS) at 90 days in these patients.
Methods
This study was conducted using serum samples from a prospective cohort of patients who underwent IVT. This study was approved by the Ethics Committee of the First Hospital of Jilin University (2015–156). Written informed consent was obtained from all participants, and these patients had the right to withdraw from the study at any point.
Participants
In this prospective study, we enrolled consecutive patients who met the eligibility for IVT and underwent alteplase IVT, and additionally met the following inclusion criteria between September 2016 and April 2023 at the First Hospital of Jilin University and between September 2021 to January 2022 at the other 15 hospitals in northeast China (Fig. 1, Additional file 1: Table S1). The additional inclusion criteria were (a) age > 18 years, (b) underwent standard alteplase treatment (0.9 mg/kg), and (c) had been functioning independently before the stroke (mRS, 0–1; range, 0 (no symptoms) to 6 (death)).
Fig. 1.
Geographical distribution of the hospitals
Clinical parameters
The following baseline clinical variables were collected: demographic features, vascular risk factors, and clinical data. The demographic features included name, age, and sex. Vascular risk factors included cigarette smoking, alcohol consumption, hypertension, diabetes mellitus, dyslipidemia, hyperhomocysteinemia, previous ischemic stroke, and coronary heart disease [11, 12]. Clinical information included blood pressure, heart rate, blood glucose, onset-to-alteplase bolus time, stroke severity, infarct location, stroke subtypes, and bridging therapy. Blood pressure and heart rate were measured on admission. Blood glucose levels were measured the morning following admission after overnight fasting. Stroke severity was assessed using the NIHSS at admission, 24 h, and 7 days after IVT via physical examination [13]. Stoke subtypes were classified according to the Trial of Org 10,172 in the Acute Stroke Treatment (TOAST) classification [14]. In addition, the mRS score at 90 days was recorded via telephone interviews with each patient or next of kin. A mRS score ≤ 1 was defined as a favorable outcome, and an unfavorable outcome was defined as a mRS score > 1 90 days after stroke onset. Any cause of death within 90 days was also recorded. HT was defined as any visible hemorrhage on brain computed tomography 24 h after IVT and classified as hemorrhagic infarction type 1 (HI1), hemorrhagic infarction type 2 (HI2), parenchymal hematoma type 1 (PH1), parenchymal hematoma type 2 (PH2), and remote parenchymal hematoma (rPH) according to the European Collaborative Acute Stroke Study (ECASS) [15]. Infarct volume was calculated on brain diffusion-weighted magnetic resonance imaging obtained 3–7 days after stroke onset, using open-source 3D Slicer software, version 5.3.0 [16]. The mRS and imaging parameters were assessed by examiners blinded to the clinical parameters and serum biomarker levels.
Blood sampling and serum S100β measurement
Venous blood samples were drawn from the cubital vein of each patient 24 h after IVT, centrifuged to obtain serum, transferred to cryovials, and kept frozen at − 80 °C in the Department of Biobank, Division of Clinical Research, the First Hospital of Jilin University until analysis within 2 h following collection. The determination of the level of biomarker S100β was performed at the laboratory of the Stroke Center, the First Hospital of Jilin University, via an automated magnetic particle-based chemiluminescent enzyme immunoassay analyzing system (MS-Fast/Aceso 80A, Sophonix, Beijing, China) as previously reported [17]. The test reagent is individually pre-loaded in a single cartridge. The entire detection procedure is completely automated and requires only 28 min. Briefly, the procedure is as follows: S100β proteins were combined with an alkaline phosphatase-labeled antibody and a biotinylated antibody to form a sandwich structure. An excess of streptavidin-coated magnetic particles was introduced and combined with the biotinylated antibody to form a complex. The complexes were enriched by a magnetic field, thereby enhancing signal sensitivity and allowing them to be captured. After washing, a luminescent substrate was added and cleaved by the enzyme in the complex to form an unstable excited-state intermediate. When the excited-state intermediate returns to its ground state, it emits photons. The light intensity was detected using a photomultiplier tube and converted into the concentration of the test sample. Serum samples were processed and assayed by laboratory technicians blinded to all clinical parameters, and the assay results were not provided to the healthcare providers. The lower limit of quantification was 0.05 ng/mL, while the upper limit was 10 ng/mL. The coefficient of variation was < 8.0%. Concentrations of S100β below the limit of quantification were analyzed as 0.05 ng/mL, whereas those above as 10 ng/mL.
Statistical analysis
Statistical analyses were performed using IBM SPSS Statistics (version 26.0; Statistical Package for the Social Sciences, Armonk, NY, USA). The distribution of data was assessed using a one-sample Kolmogorov–Smirnov test. Continuous variables are expressed as means and standard deviations or medians and interquartile ranges, according to a normal or skewed distribution. Categorical variables are described as frequencies and percentages. Study participants were divided into three groups according to tertiles of S100β levels. Clinical variables and outcome parameters were compared among the three groups using one-way analysis of variance, χ 2 test, Fisher’s exact test, or the Kruskal–Wallis test, based on the measurement level. Comparisons were corrected post hoc using Bonferroni adjustment. S100β values between the two independent groups were compared using Mann–Whitney U test. S100β values between patients with different types of HT were compared using the Kruskal–Wallis test.
To explore the correlation between S100β levels and HT, NIHSS, infarct volume, and 3-month outcome, univariable and multivariable linear regression and binary logistic regression were used. Five models were applied in the sensitivity analysis: Model 1 was unadjusted; Model 2 was adjusted for age and sex; Model 3 was adjusted for age, sex, and vascular risk factors (including cigarette smoking, alcohol consumption, hypertension, diabetes mellitus, dyslipidemia, hyperhomocysteinemia, previous ischemic stroke, and coronary heart disease); Model 4 was adjusted for age, sex, vascular risk factors, and clinical data (including blood pressure, heart rate, blood glucose, admission NIHSS score, onset-to-alteplase bolus time, TOAST, infarct location, and bridging therapy); and Model 5 was adjusted using the confounders of Model 4 except for admission NIHSS score.
Subgroup analyses were reported in unadjusted and adjusted models (adjusted for the same prespecified covariates as in the primary analyses) across different stroke lateralizations, and the heterogeneity of the association of S100β with clinical parameters and outcomes was assessed. A two-sided P < 0.05 was considered statistically significant.
Results
Participant characteristics
Of the 1580 patients who received alteplase IVT, blood samples were collected from 1131; 22 declined to participate in the study during follow-up, 37 were lost to 3-month follow-up, and the remaining 1072 were included in the analysis. The flowchart of the study is shown in Additional file 1: Fig. S1. The characteristics of the patients are listed in Table 1, of whom 478 (44.6%) had favorable outcomes (mRS 0–1) and 52 (4.9%) died due to all causes at 3 months. Computed tomography was available for 1070 patients, of whom 93 (8.7%) had HT 24 h after IVT.
Table 1.
Clinical characteristics and outcomes
| Variables | Total (n = 1072) | T1 (S100β ≤ 0.08 ng/mL, n = 385) | T2 (0.08 < S100β ≤ 0.20 ng/mL, n = 340) | T3 (S100β > 0.20 ng/mL, n = 347) | χ 2/F | P |
|---|---|---|---|---|---|---|
| Demographics | ||||||
| Age (year) | 62.58 ± 11.40 | 61.91 ± 10.77 | 62.58 ± 12.02 | 64.30 ± 11.30 | 5.995 | 0.003 |
| Sex (male, n (%)) | 758 (70.7%) | 217 (70.4%) | 244 (71.8%) | 243 (70.0%) | 0.279 | 0.870 |
| Vascular risk factors | ||||||
| Cigarette smoking, n (%) | 545 (50.8%) | 207 (53.8%) | 185 (54.4%) | 153 (44.1%) | 9.376 | 0.009 |
| Alcohol consumption, n (%) | 428 (39.9%) | 165 (42.9%) | 154 (45.3%) | 109 (31.4%) | 15.951 | < 0.001 |
| Hypertension, n (%) | 542 (50.6%) | 190 (49.4%) | 162 (47.6%) | 190 (54.8%) | 3.822 | 0.148 |
| Diabetes mellitus, n (%) | 216 (20.1%) | 76 (19.7%) | 69 (20.3%) | 71 (20.5%) | 0.065 | 0.968 |
| Dyslipidemia, n (%) | 727 (67.8%) | 261 (67.8%) | 235 (69.1%) | 231 (66.6%) | 0.511 | 0.775 |
| Hyperhomocysteinemia, n (%) | 479 (44.7%) | 151 (39.2%) | 160 (47.1%) | 168 (48.4%) | 7.379 | 0.025 |
| Previous ischemic stroke, n (%) | 171 (16.0%) | 50 (13.0%) | 56 (16.5%) | 65 (18.7%) | 4.593 | 0.101 |
| Coronary heart disease, n (%) | 233 (21.7%) | 59 (15.3%) | 75 (22.1%) | 99 (28.5%) | 18.740 | < 0.001 |
| Clinical data | ||||||
| SBP (mmHg) | 155.5 (140–174) | 157 (141–180) | 154 (140–171) | 155 (138–171) | 4.915 | 0.086 |
| DBP (mmHg) | 90 (80–86) | 91 (80–100) | 90 (81–100) | 80 (90–100) | 2.741 | 0.254 |
| HR (beats/min) | 78 (68–86) | 76 (68–84) | 78 (69–86) | 78 (68–88) | 1.475 | 0.478 |
| Blood glucose (mmol/L) | 5.88 (5.05–7.19) | 5.50 (4.93–6.85) | 5.83 (5.01–7.15) | 6.38 (5.36–7.94) | 37.234 | < 0.001 |
| Admission NIHSS | 7 (5–11) | 7 (4–10) | 7 (5–11) | 8 (5–13) | 23.191 | < 0.001 |
| NIHSS 24 h | 4 (2–9) | 3 (1–7) | 4 (2–8) | 6 (3–12) | 63.456 | < 0.001 |
| Onset-to-alteplase bolus time (min) | 178 (139–225) | 176 (134.5–221) | 177.5 (144–227) | 180 (140–230) | 2.648 | 0.266 |
| TOAST | 37.411 | < 0.001 | ||||
| LAA | 376 (35.1%) | 111 (28.8%) | 128 (37.6%) | 137 (39.5%) | ||
| SAO | 429 (40.0%) | 187 (48.6%) | 132 (38.8%) | 110 (31.7%) | ||
| CE | 101 (9.4%) | 25 (6.5%) | 25 (7.4%) | 51 (14.7%) | ||
| ODC | 20 (1.9%) | 6 (1.6%) | 9 (2.6%) | 5 (1.4%) | ||
| UE | 146 (13.6%) | 56 (14.5%) | 46 (13.5%) | 44 (12.7%) | ||
| Infarct location | 33.709 | < 0.001 | ||||
| Cortex | 178 (16.6%) | 48 (12.5%) | 57 (16.8%) | 73 (21.0%) | ||
| Subcortex | 487 (45.4%) | 198 (51.4%) | 157 (46.2%) | 132 (38.0%) | ||
| Thalamus | 61 (5.7%) | 26 (6.8%) | 19 (5.6%) | 16 (4.6%) | ||
| Cerebellum | 23 (2.1%) | 5 (1.3%) | 8 (2.4%) | 10 (2.9%) | ||
| Brainstem | 102 (9.5%) | 46 (11.9%) | 32 (9.4%) | 24 (6.9%) | ||
| Multiple | 221 (20.6%) | 62 (16.1%) | 67 (19.7%) | 92 (26.5%) | ||
| Hemisphere | 1.206 | 0.877 | ||||
| Dominant | 493 (46.0%) | 170 (44.2%) | 160 (47.1%) | 163 (47.0%) | ||
| Non-dominant | 488 (45.5%) | 179 (46.5%) | 154 (45.3%) | 155 (44.7%) | ||
| Both | 91 (8.5%) | 36 (9.4%) | 26 (7.6%) | 29 (8.4%) | ||
| Infarct volume (mL)c | 2.37 (0.75–9.17) | 1.94 (0.63–5.58) | 2.02 (0.69–5.82) | 3.84 (0.97–37.76) | 40.188 | < 0.001 |
| Bridging therapy | 44 (4.1%) | 10 (2.6%) | 16 (4.7%) | 18 (5.2%) | 3.568 | 0.168 |
| HTa | 93 (8.7%) | 19 (4.9%) | 20 (5.9%) | 54 (15.6%) | 31.025 | < 0.001 |
| 42.961 | < 0.001 | |||||
| HI1 | 27 (2.5%) | 10 (2.6%) | 5 (1.5%) | 12 (3.5%) | ||
| HI2 | 21 (2.0%) | 0 (0) | 6 (1.8%) | 15 (4.3%) | ||
| PH1 | 20 (1.9%) | 5 (1.3%) | 4 (1.2%) | 11 (3.2%) | ||
| PH2 | 21 (2.0%) | 3 (0.8%) | 3 (0.9%) | 15 (4.3%) | ||
| rPH | 4 (0.4%) | 1 (0.3%) | 2 (0.6%) | 1 (0.3%) | ||
| NIHSS at 7 daysb | 3 (1–7) | 2 (0–6) | 3 (1–6) | 5 (2–10) | 60.433 | < 0.001 |
| Functional long-term prognosis | 28.089 | < 0.001 | ||||
| mRS ≤ 1 (favorable) | 478 (44.6%) | 199 (51.7%) | 164 (48.2%) | 115 (33.1%) | ||
| mRS > 1 (unfavorable) | 594 (55.4%) | 186 (48.3%) | 176 (51.8%) | 232 (66.9%) | ||
| mRS | 70.856 | < 0.001 | ||||
| 0 | 136 (12.7%) | 47 (12.2%) | 51 (15.0%) | 38 (11.0%) | ||
| 1 | 342 (31.9%) | 152 (39.5%) | 113 (33.2%) | 77 (22.2%) | ||
| 2 | 199 (18.6%) | 74 (19.2%) | 65 (19.1%) | 60 (17.3%) | ||
| 3 | 171 (16.0%) | 60 (15.6%) | 55 (16.2%) | 56 (16.1%) | ||
| 4 | 129 (12.0%) | 36 (9.4%) | 37 (10.9%) | 56 (16.1%) | ||
| 5 | 43 (4.0%) | 10 (2.6%) | 8 (2.4%) | 25 (7.2%) | ||
| 6 (death) | 52 (4.9%) | 6 (1.6%) | 11 (3.2%) | 35 (10.1%) | ||
Abbreviations: SBP Systolic blood pressure, DBP Diastolic blood pressure, HR Heart rate, NIHSS National Institutes of Health Stroke Scale, TOAST Trial of Org 10,172 in Acute Stroke Treatment classification, LAA Large artery atherosclerosis, SAO Small artery occlusion, CE Cardioembolism, ODC Other determined cause, UE Undetermined etiology, HI Hemorrhagic infarction, PH Parenchymal hematoma, rPH remote parenchymal hematoma, mRS modified Rankin Scale
aComputed tomography scans at 24 h were available for 1070 patients (T1 = 385; T2 = 339; T3 = 346). Therefore, 1070 patients were included in the analysis of HT
bThe NIHSS scores at 7 days were available for 1056 patients because 16 patients died or discharge within 7 days (T1 = 383; T2 = 338; T3 = 335)
cMagnetic resonance imaging was available for 986 patients (T1 = 345; T2 = 314; T3 = 327). Therefore, 986 patients were included in the analysis of infarct volume
Participants were divided into three groups according to tertiles of S100β levels. A comparison of the clinical variables and outcome parameters is shown in Table 1. A higher percentage of patients in the highest S100β group had HT and unfavorable outcomes, and patients in the highest S100β group had larger infarct volume, and higher 24-h and 7-day NIHSS scores than those with lower S100β levels.
Serum S100β levels and HT
Serum S100β levels were significantly higher in patients with HT 24 h after IVT than in those without HT (0.237 (0.100–0.801) ng/mL vs. 0.118 (0.050–0.240) ng/mL, P < 0.001). The differences in S100β levels between patients with different types of HT (HI1, HI2, PH1, and PH2) and those without HT were significant (H = 35.840, P < 0.001). In binary logistic regression analysis, the highest S100β levels (> 0.20 ng/mL) were significantly correlated with HT, and the correlation was similar in the sensitivity analysis (Additional file 1: Table S2). Further, we explored the heterogeneity in stroke lateralization (dominant, non-dominant, and both hemispheres) of S100β in HT. No significant interaction association was found (P interaction = 0.821). Therefore, further respective analysis in patients with dominant or non-dominant hemisphere stroke was not conducted.
Serum S100β levels and infarct volume
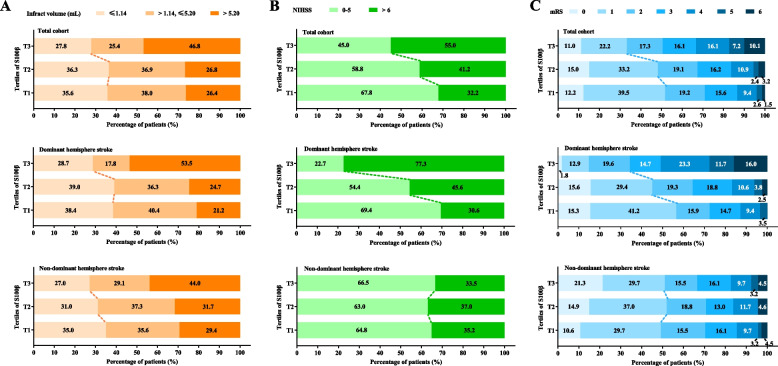
As shown in Additional file 1: Table S2, the highest S100β levels (> 0.20 ng/mL) were independently correlated with larger infarct volume. In the analysis of heterogeneity in stroke lateralization of S100β in infarct volume, the number of patients with bilateral stroke was small (91 patients, 8.5%); therefore, further univariable and multivariable linear regression analyses were conducted in patients with dominant or non-dominant hemisphere stroke. Although the interaction between stroke lateralization and serum S100β levels in predicting infarct volume was significant (P interaction = 0.014), the association between S100β levels and infarct volume was similar between patients with either dominant or non-dominant hemisphere stroke. That is, the highest S100β levels (> 0.20 ng/mL) were independently correlated with larger infarct volume in patients with dominant or non-dominant hemisphere stroke (Table 2 and Additional file 1: Table S3). In addition, the distribution of infarct volume according to S100β levels was similar between patients with dominant and non-dominant hemisphere stroke (Fig. 2A).
Table 2.
The association of tertiles of S100β with clinical parameters and outcome indicators in patients with different lateralization stroke
| Model 1 | Model 2 | Model 3 | Model 4 | |||||
|---|---|---|---|---|---|---|---|---|
| OR/β (95% CI) | P | OR/β (95% CI) | P | OR/β (95% CI) | P | OR/β (95% CI) | P | |
| NIHSS 24 ha (Beta coefficient) | ||||||||
| Dominant hemisphere | ||||||||
| T1 | Reference | Reference | Reference | Reference | ||||
| T2 | 1.447 (0.234–2.661) | 0.020 | 1.442 (0.229–2.654) | 0.020 | 1.357 (0.141–2.572) | 0.029 | 0.575 (− 0.406 to 1.556) | 0.250 |
| T3 | 6.594 (5.386–7.802) | < 0.001 | 6.432 (5.212–7.652) | < 0.001 | 6.447 (5.203–7.691) | < 0.001 | 3.470 (2.392–4.548) | < 0.001 |
| P for trend | < 0.001 | < 0.001 | < 0.001 | < 0.001 | ||||
| Non-dominant hemisphere | ||||||||
| T1 | Reference | Reference | Reference | Reference | ||||
| T2 | − 0.052 (− 1.244 to 1.139) | 0.931 | 0.014 (− 1.172 to 1.201) | 0.981 | − 0.072 (− 1.274 to 1.130) | 0.907 | − 0.009 (− 1.044 to 1.027) | 0.987 |
| T3 | 0.296 (− 0.894 to 1.485) | 0.626 | 0.315 (− 0.869 to 1.498) | 0.602 | 0.472 (− 0.746 to 1.691) | 0.447 | 0.326 (− 0.735 to 1.387) | 0.547 |
| P for trend | 0.636 | 0.608 | 0.462 | 0.559 | ||||
| Infarct volumeb (beta coefficient) | ||||||||
| Dominant hemisphere | ||||||||
| T1 | Reference | Reference | Reference | Reference | ||||
| T2 | − 0.258 (− 13.556 to 13.040) | 0.970 | − 0.010 (− 13.332 to 13.311) | 0.999 | − 0.468 (− 13.850 to 12.913) | 0.945 | − 4.211 (− 17.338 to 8.915) | 0.529 |
| T3 | 46.801 (33.741–59.860) | < 0.001 | 45.939 (32.707–59.170) | < 0.001 | 46.128 (32.671–59.585) | < 0.001 | 36.853 (22.659–51.048) | < 0.001 |
| P for trend | < 0.001 | < 0.001 | < 0.001 | < 0.001 | ||||
| Non-dominant hemisphere | ||||||||
| T1 | Reference | Reference | Reference | Reference | ||||
| T2 | 5.394 (− 6.950 to 17.738) | 0.391 | 5.306 (− 7.044 to 17.656) | 0.399 | 5.890 (− 6.532 to 18.311) | 0.352 | 7.169 (− 5.206 to 19.544) | 0.255 |
| T3 | 20.783 (8.347–33.220) | < 0.001 | 20.661 (8.229–33.093) | < 0.001 | 23.912 (11.114–36.710) | < 0.001 | 23.645 (10.774–36.516) | < 0.001 |
| P for trend | < 0.001 | < 0.001 | < 0.001 | < 0.001 | ||||
| mRS 2–6 c (odds ratio) | ||||||||
| Dominant hemisphere | ||||||||
| T1 | Reference | Reference | Reference | Reference | ||||
| T2 | 1.586 (1.027–2.449) | 0.038 | 1.621 (1.046–2.511) | 0.031 | 1.561 (0.997–2.446) | 0.052 | 1.383 (0.849–2.254) | 0.193 |
| T3 | 7.514 (4.428–12.750) | < 0.001 | 7.160 (4.203–12.199) | < 0.001 | 8.041 (4.602–14.047) | < 0.001 | 5.436 (2.936–10.064) | < 0.001 |
| P for trend | < 0.001 | < 0.001 | < 0.001 | < 0.001 | ||||
| Non-dominant hemisphere | ||||||||
| T1 | Reference | Reference | Reference | Reference | ||||
| T2 | 0.895 (0.581–1.377) | 0.612 | 0.915 (0.592–1.412) | 0.687 | 0.903 (0.581–1.405) | 0.652 | 0.850 (0.528–1.368) | 0.503 |
| T3 | 0.930 (0.605–1.430) | 0.742 | 0.937 (0.607–1.444) | 0.766 | 0.943 (0.602–1.477) | 0.798 | 0.882 (0.538–1.445) | 0.619 |
| P for trend | 0.963 | 0.758 | 0.784 | 0.597 | ||||
The number of patients with both sides of stroke was limited; therefore, the analysis was conducted in patients with dominant or non-dominant hemisphere stroke. The results of Model 5 were the same with those of Models 1–4 and were shown in Additional file 1: Table S3
Model 1 was unadjusted; Model 2 was adjusted for age and sex; Model 3 was adjusted for age, sex and vascular risk factors (including cigarette smoking, alcohol consumption, hypertension, diabetes mellitus, dyslipidemia, hyperhomocysteinemia, previous ischemic stroke and coronary heart disease); Model 4 was adjusted for age, sex, vascular risk factors and clinical data (including SBP, DBP, HR, blood glucose, admission NIHSS, onset-to-alteplase bolus time, TOAST, infarct location, and bridging therapy); Model 5 was adjusted using the confounders of Model 4 except for admission NIHSS score
Abbreviations: OR Odds ratio, CI Confidence interval, SBP Systolic blood pressure, DBP Diastolic blood pressure, HR Heart rate, NIHSS National Institutes of Health Stroke Scale, TOAST Trial of Org 10,172 in Acute Stroke Treatment classification, mRS modified Rankin Scale
aThe NIHSS score at 24 h was determined for all 1072 patients, of which 493 had dominant hemisphere stroke and 488 had non-dominant hemisphere stroke
bMagnetic resonance imaging was available for 986 patients. Therefore, 986 patients were included in the analysis of infarct volume, of whom 454 had dominant hemisphere stroke and 449 had non-dominant hemisphere stroke
cAll 1072 patients were included in the analysis of functional outcomes assessed by mRS, of whom 594 had mRS 2–6 (301 had dominant hemisphere stroke and 241 had non-dominant hemisphere stroke)
Fig. 2.
Distribution of A final infarct volume, B NIHSS at 24 h, and C functional outcome assessed by mRS at 90 days according to S100β tertiles in the total cohort and patients with dominant and non-dominant hemisphere stroke. Abbreviations: NIHSS, National Institutes of Health Stroke Scale; mRS, modified Rankin Scale
Serum S100β levels and functional indicators
As shown in Additional file 1: Table S2, in univariable and multivariable linear regression, the highest S100β levels (> 0.20 ng/mL) were independently associated with higher 24-h and 7-day NIHSS scores. We also observed a significant interaction between stroke lateralization (dominant, non-dominant, and both hemispheres) and NIHSS score measured at 24 h after IVT (P interaction < 0.001). In patients with dominant hemisphere stroke, the highest S100β levels (> 0.20 ng/mL) were associated with higher NIHSS score; however, no significant association was found in patients with non-dominant hemisphere stroke (Table 2 and Additional file 1: Table S3). Further, as depicted in Fig. 2B, the association between 24-h NIHSS score and S100β levels was more remarkable in patients with dominant hemisphere stroke than those with non-dominant hemisphere stroke.
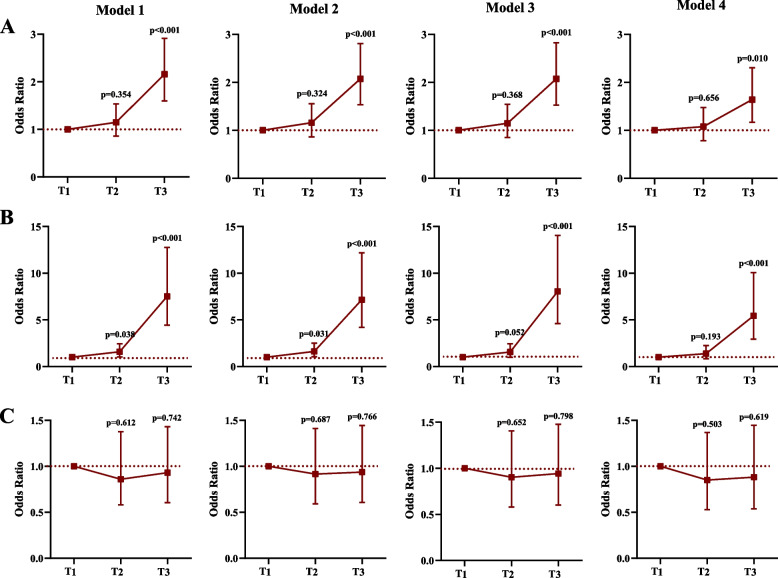
For functional outcomes assessed using the mRS score, S100β levels were significantly increased in patients with unfavorable outcomes compared with those with favorable outcomes (0.140 (0.062–0.393) ng/mL vs. 0.100 (0.050–0.196) ng/mL, P < 0.001). Univariable and multivariable binary logistic regression analyses revealed an independent association between S100β levels and unfavorable outcomes (Additional file 1: Table S2 and Fig. 3A). Moreover, the highest S100β level (> 0.20 ng/mL) was an independent predictive factor for all-cause death within 3 months of IVT (Additional file 1: Table S2).
Fig. 3.
Association between S100β tertiles and unfavorable outcome at 90 days according to different models of A all patients, B patients with dominant hemisphere stroke, and C patients with non-dominant hemisphere stroke. Notes: Model 1 was unadjusted; Model 2 was adjusted for age and sex; Model 3 was adjusted for age, sex and vascular risk factors (including cigarette smoking, alcohol consumption, hypertension, diabetes mellitus, dyslipidemia, hyperhomocysteinemia, previous ischemic stroke and coronary heart disease); Model 4 was adjusted for age, sex, vascular risk factors and clinical data (including SBP, DBP, HR, blood glucose, admission NIHSS, onset-to-alteplase bolus time, TOAST, infarct location, and bridging therapy). Abbreviations: SBP, systolic blood pressure; DBP, diastolic blood pressure; HR, heart rate; NIHSS, National Institutes of Health Stroke Scale; TOAST, Trial of Org 10,172 in Acute Stroke Treatment classification
The heterogeneity in stroke lateralization in the prognostic function (favorable outcome) of S100β was observed (P interaction < 0.001). In patients with dominant hemisphere stroke, the highest S100β levels (> 0.20 ng/mL) were independently associated with unfavorable outcomes at 3 months, and patients with the highest S100β levels (> 0.20 ng/mL) 24 h after IVT presented a 4.4-fold increase in the risk of unfavorable 3-month outcomes compared with those with the lowest S100β levels. However, no significant association was found in patients with non-dominant hemisphere stroke (Table 2, Additional file 1: Table S3 and Fig. 3B, C). The distribution of functional outcomes at 90 days according to S100β tertiles in all patients, patients with dominant hemisphere stroke, and those with non-dominant hemisphere stroke is shown in Fig. 2C.
Discussion
The present study showed that serum S100β levels 24 h after IVT were independently associated with HT, infarct volume, and prognosis in stroke patients who received IVT. Because the detection of S100β is convenient and has guiding significance for the formulation of clinical diagnosis and treatment strategy, it has great potential in clinical application.
HT is a major complication of IVT treatment, and reliable predictors remain limited. After brain tissue infarction, S100β levels are significantly elevated in the brain and enter the peripheral blood via the damaged blood–brain barrier [6, 18]. Thus, the level of S100β in the blood can partly reflect the extent of damage to the blood–brain barrier [18], suggesting that S100β is associated with HT following IVT. Foerch et al. reported that a S100β value in the highest quintile before thrombolytic therapy was independently associated with any HT [19]. In our study, the highest S100β levels 24 h after IVT were also independently correlated with HT. This suggests that S100β can assist in the assessment of HT both before and after thrombolysis, providing more methods for clinicians to indicate HT.
The prognostic value of functional outcomes based on S100β has been well demonstrated in patients with traumatic brain injury [20–22]. Recently, S100β levels in patients with acute ischemic stroke have been studied. Branco et al. demonstrated that serum S100β levels can potentially predict the 3-month prognosis of stroke patients [9]. However, the association of serum S100β levels with prognosis in stroke patients who received IVT remains unclear. In our multicenter study with a relatively large sample size, we found that the highest S100β level was both independently correlated with short-term (7-day NIHSS score) and long-term (all-cause death at 3 months and 3-month mRS) prognosis. Therefore, serum S100β levels may be used as an early biomarker to predict outcomes in patients with stroke.
In addition, we also found a result worth exploring in this study: S100β level was similarly associated with infarct volume in patients with stroke in dominant and non-dominant hemispheres, but it was only independently associated with 24-h higher NIHSS score and 3-month unfavorable outcomes in patients with dominant hemisphere stroke but the association was not significant in those with non-dominant hemisphere stroke. This may be because S100β level is only related to infarction volume, not infarction location, while the neurological scores (e.g., NIHSS and mRS scores) are affected by location [23–25]. The neurological scores emphasize deficits associated with lesions located in the dominant hemisphere (such as language or dominant hand function), and thus dominant hemisphere stroke is usually associated with higher scale scores [23]. Nevertheless, stroke in the non-dominant hemisphere, which is mainly associated with neglect, cognitive deficit, or apraxia, cannot be adequately reflected by stroke scales and is poorly detected by outcome examiners or patients’ relatives but may significantly influence a patient’s quality of life [24]. Our findings are not isolated. A previous study involving patients after mechanical thrombectomy found similar results: the correlation between infarct volume and functional outcome of patients with infarcts in the right (non-dominant) hemisphere was not as strong as that in patients with left (dominant) hemisphere infarct [25]. Our results, together with previous results, suggest that serum S100β level may be an important marker to assist in determining stroke severity regardless of infarct location. Furthermore, because these scales and S100β each have their own advantages, we speculated that finding different weighted coefficients of scale scores and S100β and combining them to predict a more accurate prognosis for patients after IVT will be a future direction of research.
The S100β detection technique used in this study is also worth popularizing. Traditionally, the quantitative assessment of S100β is performed with an enzyme-linked immunosorbent assay [9, 26, 27], which is manual and requires several hours, limiting the clinical usefulness of S100β as a biomarker. In our study, we used an automated bedside rapid testing technique, a magnetic particle-based chemiluminescent enzyme immunoassay analysis system. Quantitative S100β concentrations can be obtained within 28 min with high sensitivity, specificity, and reproducibility [28]. This convenient and rapid detection method is conducive to the promotion and application of our research results in clinical diagnosis and treatment.
Our study has some limitations. First, the number of patients receiving bridging therapy was limited; therefore, subgroup analysis could not be performed. Further studies remain warranted to explore the association between S100β levels and clinical outcomes in patients undergoing bridging therapy or mechanical thrombectomy. Second, due to the impact of COVID-19, many blood samples could not be collected due to a lack of transportation. Third, as shown in the footnote of Table 1, 86 (8.02%) of the patients did not undergo MRI examination, moreover, the lower the S100β level, the more cases were missing (T1: 10.39%; T2: 7.65%; T3: 5.76%). We considered that this was likely because patients with lower S100β levels may not be willing to undergo further MRI examination for milder disease to avoid higher hospitalization costs. These missing data limited the robustness of our conclusion. Further studies are warranted. Fourth, two parameters, the 24-h NIHSS and HT, were measured at the same time as serum S100β, thus the reported associations of S100β levels and these two parameters cannot be used for causal inference.
Conclusions
Serum S100β levels 24 h after IVT were independently associated with HT, infarct volume, and prognosis in patients with IVT, which suggests the application value of serum S100β in judging the degree of patients’ disease and predicting prognosis.
Supplementary Information
Supplementary Material 1. Fig. S1 and Tables S1-3. Fig. S1. Flowchart of the study; Table S1. Sixteen hospitals in the study; Table S2. The association of tertiles of S100β with clinical parameters and outcome indicators in total cohort; Table S3. The association of tertiles of S100β with clinical parameters and outcome indicators in patients with different lateralization stroke.
Acknowledgements
We thank the Department of Biobank, Division of Clinical Research, the First Hospital of Jilin University, for providing human tissues.
Members of the Biomarkers of Brain Injury Investigator Study Group who participated in data collection: Lijuan Wang (Department of Neurology, Songyuan Central Hospital, Songyuan, China), Yumei Chen (Department of Neurology, Jilin Neuropsychiatric Hospital, Siping, China), Yang Zheng (Stroke Center, Department of Neurology, Siping Central People’s Hospital, Siping, China), Zhi-Mei Yuan (Department of Neurology, Tonghua City Vascular Disease Hospital and Dongchang District People’s Hospital, Tonghua, China), Dongsheng Ju (Department of Neurology, Songyuan Jilin Oilfield Hospital, Songyuan, China), Yun-Fei Ba (Stroke Center, Department of Neurology, Dehuishi People’s Hospital, Changchun, China), Jinhua Chen (Department of Neurology, Affiliated Hospital of Jilin Medical College, Jilin, China), Jiliang Gu (Department of Neurology, Songyuan Hospital of Integrated Traditional Chinese and Western Medicine, Songyuan, China), Anying Wang (Stroke Center, Department of Neurology, Qianguoerros Mongolian Autonomous County Hospital, Songyuan, China), Li-Li Zhao (Department of Neurology, Affiliated Hospital of Jilin Medical College, Jilin, China), Chen-Peng Dong (Department of Neurology, Jilin City Hospital of Chemical Industry, Jilin, China), Li Liu (Department of Neurology, Changchun People’s Hospital, Changchun, China), Zhong-Rui Pei (Department of Neurology, Dongliao First People’s Hospital, Liaoyuan, China), Shuang Yu (Department of Neurology, Jilin People’s Hospital, Jilin, China), Xue Liu (Department of Neurology, Jilin City Hospital of Chemical Industry, Jilin, China), Chun-Li Jiang (Department of Neurology, Jilin City Hospital of Chemical Industry, Jilin, China), Ling He (Stroke Center, Department of Neurology, Jilin Central General Hospital, Jilin, China), and Sun-Juan Zhang (Department of Neurology, Jilin Province People’s Hospital, Changchun, China).
Abbreviations
- CI
Confidence interval
- ECASS
European Collaborative Acute Stroke Study
- HI1
Hemorrhagic infarction type 1
- HI2
Hemorrhagic infarction type 2
- HT
Hemorrhagic transformation
- IVT
Intravenous thrombolysis
- mRS
Modified Rankin Scale
- NIHSS
National Institutes of Health Stroke Scale
- OR
Odds ratio
- PH1
Parenchymal hematoma type 1
- PH2
Parenchymal hematoma type 2
- rPH
Remote parenchymal hematoma
- TOAST
Trial of Org 10172 in the Acute Stroke Treatment
Authors’ contributions
YQ, HJ, RA, TNN, YY, and ZNG conceived the study. YQ, SQ, XKS, PZ, KJZ, and SJW analyzed data. QY, YY, and ZNG developed the model, generated results and figures, and drafted the manuscript. YZ, JHG, XKZ, XDL, CYL, GCL, JW, HJ, YH, LJ, LL, YJ, RHT, YJ, BJZ, ZC, YQ, XL, SL, and XS organized the project and provided medical record material. All authors contributed to the interpretation of results and critical revision of the manuscript, and the authors approved the final version of the manuscript.
Funding
Funding of the trial provided by the National Natural Science Foundation of China (82071291), the Norman Bethune Health Science Center of Jilin University (2022JBGS03), Science and Technology Department of Jilin Province (YDZJ202302CXJD061), Jilin Provincial Key Laboratory (YDZJ202302CXJD017) and Science and Technology Department of Jilin Province (20220303002SF) to Yi Yang, and Talent Reserve Program of the First Hospital of Jilin University (JDYYCB-2023002) to Zhen-Ni Guo.
Availability of data and materials
The data used and/or analyzed during the current study are available in the manuscript and Additional files, except for the values of outcome measures that could be available only from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
This study was approved by the Ethics Committee of the First Hospital of Jilin University (2015–156). Written informed consent was obtained from all participants, and these patients had the right to withdraw from the study at any point.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Yi Yang, Email: yang_yi@jlu.edu.cn.
Zhen-Ni Guo, Email: zhen1ni2@jlu.edu.cn.
on behalf of the Biomarkers of Brain Injury Investigator Study Group:
Lijuan Wang, Yumei Chen, Yang Zheng, Zhi-Mei Yuan, Dongsheng Ju, Yun-Fei Ba, Jinhua Chen, Jiliang Gu, Anying Wang, Li-Li Zhao, Chen-Peng Dong, Li Liu, Zhong-Rui Pei, Shuang Yu, Xue Liu, Chun-Li Jiang, Ling He, and Sun-Juan Zhang
References
- 1.Pandian JD, Gall SL, Kate MP, Silva GS, Akinyemi RO, Ovbiagele BI, Lavados PM, Gandhi DBC, Thrift AG. Prevention of stroke: a global perspective. Lancet. 2018;392(10154):1269–78. 10.1016/S0140-6736(18)31269-8. [DOI] [PubMed] [Google Scholar]
- 2.Berge E, Whiteley W, Audebert H, De Marchis GM, Fonseca AC, Padiglioni C, de la Ossa NP, Strbian D, Tsivgoulis G, Turc G. European Stroke Organisation (ESO) guidelines on intravenous thrombolysis for acute ischaemic stroke. Eur Stroke J. 2021;6(1):I–LXII. 10.1177/2396987321989865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Logallo N, Novotny V, Assmus J, Kvistad CE, Alteheld L, Ronning OM, Thommessen B, Amthor KF, Ihle-Hansen H, Kurz M, et al. Tenecteplase versus alteplase for management of acute ischaemic stroke (NOR-TEST): a phase 3, randomised, open-label, blinded endpoint trial. Lancet Neurol. 2017;16(10):781–8. 10.1016/S1474-4422(17)30253-3. [DOI] [PubMed] [Google Scholar]
- 4.Cao MC, Cawston EE, Chen G, Brooks C, Douwes J, McLean D, Graham ES, Dragunow M, Scotter EL. Serum biomarkers of neuroinflammation and blood-brain barrier leakage in amyotrophic lateral sclerosis. BMC Neurol. 2022;22(1):216. 10.1186/s12883-022-02730-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Michetti F, D’Ambrosi N, Toesca A, Puglisi MA, Serrano A, Marchese E, Corvino V, Geloso MC. The S100B story: from biomarker to active factor in neural injury. J Neurochem. 2019;148(2):168–87. 10.1111/jnc.14574. [DOI] [PubMed] [Google Scholar]
- 6.Herrmann M, Vos P, Wunderlich MT, de Bruijn CH, Lamers KJ. Release of glial tissue-specific proteins after acute stroke: a comparative analysis of serum concentrations of protein S-100B and glial fibrillary acidic protein. Stroke. 2000;31(11):2670–7. 10.1161/01.str.31.11.2670. [DOI] [PubMed] [Google Scholar]
- 7.Buttner T, Weyers S, Postert T, Sprengelmeyer R, Kuhn W. S-100 protein: serum marker of focal brain damage after ischemic territorial MCA infarction. Stroke. 1997;28(10):1961–5. 10.1161/01.str.28.10.1961. [DOI] [PubMed] [Google Scholar]
- 8.Foerch C, Singer OC, Neumann-Haefelin T. du Mesnil de Rochemont R, Steinmetz H, Sitzer M. Evaluation of serum S100B as a surrogate marker for long-term outcome and infarct volume in acute middle cerebral artery infarction. Arch Neurol. 2005;62(7):1130–4. 10.1001/archneur.62.7.1130. [DOI] [PubMed] [Google Scholar]
- 9.Branco JP, Oliveira S, Sargento-Freitas J, Santos Costa J, Cordeiro G, Cunha L, Freire Goncalves A, Pinheiro J. S100beta protein as a predictor of poststroke functional outcome: a prospective study. J Stroke Cerebrovasc Dis. 2018;27(7):1890–6. 10.1016/j.jstrokecerebrovasdis.2018.02.046. [DOI] [PubMed] [Google Scholar]
- 10.Wang R, Zhu Y, Liu Z, Chang L, Bai X, Kang L, Cao Y, Yang X, Yu H, Shi MJ, et al. Neutrophil extracellular traps promote tPA-induced brain hemorrhage via cGAS in mice with stroke. Blood. 2021;138(1):91–103. 10.1182/blood.2020008913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang Y, Cui L, Ji X, Dong Q, Zeng J, Wang Y, Zhou Y, Zhao X, Wang C, Liu L, et al. The China National Stroke Registry for patients with acute cerebrovascular events: design, rationale, and baseline patient characteristics. Int J Stroke. 2011;6(4):355–61. 10.1111/j.1747-4949.2011.00584.x. [DOI] [PubMed] [Google Scholar]
- 12.Sacco RL, Adams R, Albers G, Alberts MJ, Benavente O, Furie K, Goldstein LB, Gorelick P, Halperin J, Harbaugh R, et al. Guidelines for prevention of stroke in patients with ischemic stroke or transient ischemic attack: a statement for healthcare professionals from the American Heart Association/American Stroke Association Council on Stroke: co-sponsored by the Council on Cardiovascular Radiology and Intervention: the American Academy of Neurology affirms the value of this guideline. Circulation. 2006;113(10):e409-449. [PubMed] [Google Scholar]
- 13.Kwah LK, Diong J. National Institutes of Health Stroke Scale (NIHSS). J Physiother. 2014;60(1):61. 10.1016/j.jphys.2013.12.012. [DOI] [PubMed] [Google Scholar]
- 14.Adams HP Jr, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, Marsh EE 3rd. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993;24(1):35–41. 10.1161/01.str.24.1.35. [DOI] [PubMed] [Google Scholar]
- 15.Hacke W, Kaste M, Fieschi C, Toni D, Lesaffre E, von Kummer R, Boysen G, Bluhmki E, Hoxter G, Mahagne MH, et al. Intravenous thrombolysis with recombinant tissue plasminogen activator for acute hemispheric stroke. The European Cooperative Acute Stroke Study (ECASS). JAMA. 1995;274(13):1017–25. [PubMed] [Google Scholar]
- 16.Fedorov A, Beichel R, Kalpathy-Cramer J, Finet J, Fillion-Robin J-C, Pujol S, Bauer C, Jennings D, Fennessy F, Sonka M, et al. 3D Slicer as an image computing platform for the quantitative imaging network. Magn Reson Imaging. 2012;30(9):1323–41. 10.1016/j.mri.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Qu Y, Zhang P, He QY, Sun YY, Wang MQ, Liu J, Zhang PD, Yang Y, Guo ZN. The impact of serial remote ischemic conditioning on dynamic cerebral autoregulation and brain injury related biomarkers. Front Physiol. 2022;13: 835173. Available from. 10.3389/fphys.2022.835173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kanner AA, Marchi N, Fazio V, Mayberg MR, Koltz MT, Siomin V, Stevens GH, Masaryk T, Aumayr B, Vogelbaum MA, et al. Serum S100beta: a noninvasive marker of blood-brain barrier function and brain lesions. Cancer. 2003;97(11):2806–13. 10.1002/cncr.1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Foerch C, Wunderlich MT, Dvorak F, Humpich M, Kahles T, Goertler M, Alvarez-Sabin J, Wallesch CW, Molina CA, Steinmetz H, et al. Elevated serum S100B levels indicate a higher risk of hemorrhagic transformation after thrombolytic therapy in acute stroke. Stroke. 2007;38(9):2491–5. 10.1161/STROKEAHA.106.480111. [DOI] [PubMed] [Google Scholar]
- 20.Kellermann I, Kleindienst A, Hore N, Buchfelder M, Brandner S. Early CSF and serum S100B concentrations for outcome prediction in traumatic brain injury and subarachnoid hemorrhage. Clin Neurol Neurosurg. 2016;145:79–83. 10.1016/j.clineuro.2016.04.005. [DOI] [PubMed] [Google Scholar]
- 21.Vos PE, Lamers KJ, Hendriks JC, van Haaren M, Beems T, Zimmerman C, van Geel W, de Reus H, Biert J, Verbeek MM. Glial and neuronal proteins in serum predict outcome after severe traumatic brain injury. Neurology. 2004;62(8):1303–10. 10.1212/01.wnl.0000120550.00643.dc. [DOI] [PubMed] [Google Scholar]
- 22.Frankel M, Fan L, Yeatts SD, Jeromin A, Vos PE, Wagner AK, Wolf BJ, Pauls Q, Lunney M, Merck LH, et al. Association of very early serum levels of S100B, glial fibrillary acidic protein, ubiquitin C-terminal hydrolase-L1, and spectrin breakdown product with outcome in ProTECT III. J Neurotrauma. 2019;36(20):2863–71. 10.1089/neu.2018.5809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Foerch C, Misselwitz B, Sitzer M, Berger K, Steinmetz H, Neumann-Haefelin T, Arbeitsgruppe Schlaganfall H. Difference in recognition of right and left hemispheric stroke. Lancet. 2005;366(9483):392–3. 10.1016/S0140-6736(05)67024-9. [DOI] [PubMed] [Google Scholar]
- 24.Fink JN. Underdiagnosis of right-brain stroke. Lancet. 2005;366(9483):349–51. 10.1016/S0140-6736(05)67004-3. [DOI] [PubMed] [Google Scholar]
- 25.Luger S, Koerbel K, Martinez Oeckel A, Schneider H, Maurer CJ, Hintereder G, Wagner M, Hattingen E, Foerch C. Role of S100B serum concentration as a surrogate outcome parameter after mechanical thrombectomy. Neurology. 2021;97(22):e2185–94. 10.1212/WNL.0000000000012918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhou S, Bao J, Wang Y, Pan S. S100beta as a biomarker for differential diagnosis of intracerebral hemorrhage and ischemic stroke. Neurol Res. 2016;38(4):327–32. 10.1080/01616412.2016.1152675. [DOI] [PubMed] [Google Scholar]
- 27.Montaner J, Mendioroz M, Delgado P, Garcia-Berrocoso T, Giralt D, Merino C, Ribo M, Rosell A, Penalba A, Fernandez-Cadenas I, et al. Differentiating ischemic from hemorrhagic stroke using plasma biomarkers: the S100B/RAGE pathway. J Proteomics. 2012;75(15):4758–65. 10.1016/j.jprot.2012.01.033. [DOI] [PubMed] [Google Scholar]
- 28.Jin H, Lin JM, Wang X, Xin TB, Liang SX, Li ZJ, Hu GM. Magnetic particle-based chemiluminescence enzyme immunoassay for free thyroxine in human serum. J Pharm Biomed Anal. 2009;50(5):891–6. 10.1016/j.jpba.2009.06.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material 1. Fig. S1 and Tables S1-3. Fig. S1. Flowchart of the study; Table S1. Sixteen hospitals in the study; Table S2. The association of tertiles of S100β with clinical parameters and outcome indicators in total cohort; Table S3. The association of tertiles of S100β with clinical parameters and outcome indicators in patients with different lateralization stroke.
Data Availability Statement
The data used and/or analyzed during the current study are available in the manuscript and Additional files, except for the values of outcome measures that could be available only from the corresponding author on reasonable request.