Abstract
This study investigated the role of O6‐methylguanine‐DNA methyltransferase promoter (MGMTp) methylation hierarchy and heterogeneity in grade 2–3 gliomas, focusing on variations in chemotherapy benefits and resection dependency. A cohort of 668 newly diagnosed grade 2–3 gliomas, with comprehensive clinical, radiological, and molecular data, formed the basis of this analysis. The extent of resection was categorized into gross total resection (GTR ≥100%), subtotal resection (STR >90%), and partial resection (PR ≤90%). MGMTp methylation levels were examined using quantitative pyrosequencing. Our findings highlighted the critical role of GTR in improving the prognosis for astrocytomas (IDH1/2‐mutant and 1p/19q non‐codeleted), contrasting with its lesser significance for oligodendrogliomas (IDH1/2 mutation and 1p/19q codeletion). Oligodendrogliomas demonstrated the highest average MGMTp methylation levels (median: 28%), with a predominant percentage of methylated cases (average methylation levels >20%). Astrocytomas were more common in the low‐methylated group (10%–20%), while IDH wild‐type gliomas were mostly unmethylated (<10%). Spatial distribution analysis revealed a decrement in frontal lobe involvement from methylated, low‐methylated to unmethylated cases (72.8%, 59.3%, and 47.8%, respectively). In contrast, low‐methylated and unmethylated cases were more likely to invade the temporal‐insular region (19.7%, 34.3%, and 40.4%, respectively). Astrocytomas with intermediate MGMTp methylation were notably associated with temporal‐insular involvement, potentially indicating a moderate response to temozolomide and underscoring the importance of aggressive resection strategies. In conclusion, our study elucidates the complex interplay of MGMTp methylation hierarchy and heterogeneity among grade 2–3 gliomas, providing insights into why astrocytomas and IDH wild‐type lower‐grade glioma might derive less benefit from chemotherapy.
Keywords: astrocytoma, grade 2–3 gliomas, hierarchy, MGMT promoter, oligodendroglioma
Our findings underscore the pivotal role of gross total resection in improving prognosis for astrocytomas (IDH1/2‐mutant and 1p/19q non‐codeleted astrocytomas, IDHmut‐Noncodel), contrasting with the lessened significance for oligodendrogliomas (IDH1/2 mutation and 1p/19q codeletion, IDHmut‐Codel). Astrocytomas exhibited a notable association between the intermediate MGMTp status and temporal‐insular infringement, which suggests a potential moderate effect from temozolomide and emphasizes the importance of an aggressive resection strategy.
Abbreviations
- EOR
extent of resection
- GBM
glioblastoma
- GTR
gross‐total removal
- IDH
isocitrate dehydrogenase
- IDHmut‐Codel
IDH mutant and 1p/19q codeleted oligodendrogliomas
- IDHmut‐Noncodel
IDH mutant 1p/19q non‐codeleted astrocytomas
- IDHwt
IDH wild‐type LGGs
- LGG
lower‐grade glioma
- MGMT
O6‐methylguanine‐DNA methyltransferase
- OS
overall survival
- PFS
progression‐free survival
- PSQ
pyrosequencing
- STR
sub‐total removal
- WHO
world health organization
1. INTRODUCTION
Grade 2–3 gliomas significantly affect the quality and life expectancy of young and middle‐aged individuals. 1 , 2 Traditionally, histological and molecular analyses served as the gold standard for pathological assessment. Nowadays, advanced imaging techniques, encompassing calcific, diffuse, perfused, and metabolic information, have emerged as valuable tools for precise preoperative diagnosis. These imaging modalities contribute to the formulation of individualized surgical plans and accurate prognosis predictions. 3 , 4 , 5
While surgical resection remains a cornerstone in the primary treatment of gliomas, the extent of resection (EOR) exhibits a nuanced relationship with progression‐free survival (PFS) and overall survival (OS) in subtypes of lower‐grade glioma (LGGs). Notably, gross‐total resection (GTR) confers substantial prognostic benefits for IDH mutant 1p/19q non‐codeleted (IDHmut‐Noncodel) astrocytomas. Even minimal residual tumors significantly affect prognosis in this subtype, unlike the less prominent effect observed in IDH mutant 1p/19q codeleted (IDHmut‐Codel) oligodendrogliomas. 6 , 7 , 8 Prior explanations have attributed this phenomenon to inherent tumor behavior, but our study aims to delve deeper into the molecular alterations underpinning these observations, seeking more robust evidence.
O6‐methylguanine‐DNA methyltransferase promoter (MGMTp) is as a pivotal molecular parameter in predicting chemotherapy effectiveness, particularly with temozolomide. Using pyrosequencing (PSQ), known for its accuracy and quantitative assessment across various CpG sites, we aim to surpass the binary classification (methylated or unmethylated) used in previous studies. Building on recent findings in glioblastoma (GBM), where the intermediate methylation status of MGMTp demonstrated a moderate prognosis, 9 , 10 , 11 our study endeavors to unravel the heterogeneity and hierarchy of MGMTp in grade 2–3 gliomas. We seek to elucidate these distinctions to provide insights into why astrocytomas and IDH wild‐type LGGs might derive less benefit from chemotherapy.
2. METHODS AND MATERIALS
2.1. Patients and inclusion criteria
From 2013 to 2021, we included consecutive adult patients with diffuse grade 2–3 gliomas treated at our institute. Comprehensive molecular data, including IDH 1/2 (via next‐generation sequencing), 1p/19q (via fluorescence in situ hybridization), and TERT promoter (via next‐generation sequencing), were collected for integrated diagnosis. For IDH 1/2 wild‐type gliomas (IDHwts), we additionally tested for BRAF V600E mutation, epidermal growth factor receptor (EGFR) amplification, +7/10−, and H3.3/3.1 K27M mutation, following the 2021 World Health Organization (WHO) guidelines for brain tumor classification. 12 Only radiologically highly suspected diffuse LGG in adults were enrolled in this study (tumors with evident enhancement (punctiform [<1 mm in diameter] or poorly defined faint enhancement were tolerated), necrosis, or hemorrhage were excluded). 13 To differentiate from de novo glioblastoma, we retained the term “IDHwts with GBM features” for this radiologically comparable subgroup. The study received approval from the Institutional Review Board of Capital Medical University, adhering to the principles of the Declaration of Helsinki, and all patients provided informed consent.
2.2. Pyrosequencing of O6‐methylguanine‐DNA methyltransferase promoter and hierarchy classification
Pyrosequencing (PSQ) was used to determine the methylation levels of CpGs 74–81 following manufacturer instructions. Genomic DNA was extracted from formalin‐fixed paraffin‐embedded (FFPE) tumor sections using QIAamp DNA FFPE Tissue Kits (Qiagen), yielding ≥2 μg of qualified DNA for bisulfite conversion and PCR. Amplification of bisulfite‐treated DNA targeted CpG sites 74–81 in MGMT promoter exon 1 (chromosome 10, 131,265,507–131,265,544). MGMT Methylation Detection Kits (Gene Tech, Shanghai, China) were used, and methylation data were analyzed using a PyroMarker Q96 instrument and software (Qiagen). The analyzed sequences were YGTTTTGYGTTTYGAYGTTYGTAGGTTTTYGYGGTGYGTA. Cases were classified into methylated (>20%), low methylation (10%–20%), and unmethylated (<10%) subgroups. 9 , 10 In this study, DNA methylation sequencing data for LGG and accompanying clinicopathological parameters were downloaded from The Cancer Genome Atlas (TCGA) database (https://portal.gdc.cancer.gov/). Samples with incomplete data were removed, resulting in a final cohort of 502 primary LGG patients. The average methylation level of the MGMT promoter was calculated by averaging the beta values of the identified CpG sites, while the average genome‐wide methylation level was computed by averaging the eta values across all CpG sites. Pearson correlation coefficients were calculated to assess the relationship between MGMT promoter methylation levels and genome‐wide methylation levels.
2.3. Radiological data collection
Preoperative CT (GE Discovery CT750HD, USA) and MRI scans (Siemens Trio Tim or GE Discovery MR750, USA) were collected, including axial T1‐weighted, T2‐weighted fast spin echo, T2 FLAIR, and axial contrast‐enhanced T1‐weighted images. ITK‐SNAP software (version 3.8.0) facilitated tumor region extraction in FLAIR space, co‐registered with T1WI, T2WI, and ADC series. 13 A semi‐automatically segmented region of interest (ROI) was manually corrected by an experienced radiologist (J.C.W, 6 years of radiology experience). All normalized ROI masks were aligned with the Montreal Neurological Institute standard space using Statistical Parametric Mapping (SPM12, www.fil.ion.ucl.ac.uk/spm/software/spm12). A tumor overlapping image was generated with all normalized tumor masks using MRIcroGL software (www.nitrc.org/plugins/mwiki/index.php/mricrogl:MainPage).
2.4. Adjuvant treatment, extent of resection assessment, and follow‐up
Adjuvant therapeutic strategies varied based on factors such as the extent of tumor removal, age, pathology, and patient compliance. Before January 1, 2016, ACNU‐based protocols (ACNU 90 mg/m2, day 1, and VM 60 mg/m2, days 1–3) were predominantly employed due to medical insurance limitations. For WHO grade 3 gliomas and all IDHwts, chemotherapy and radiation therapy (RT) treatment commenced 3–6 weeks post‐surgery. Observation was recommended for low‐risk grade 2 patients, while chemotherapy alone was implemented for oligodendrogliomas. 14
Postoperative MRI at 48–72 h assessed the extent of resection (EOR) based on residual high‐intensity regions on T2/FLAIR images. The degree of removal was calculated as (preoperative tumor volume − postoperative tumor volume)/preoperative tumor volume, categorized as gross total resection (GTR, ≥100%), subtotal resection (STR, >90%), and partial resection (PR, ≤90%).
Standard follow‐up included brain MRI scans (T2WI, FLAIR, and pre‐and post‐contrast T1W sequence) every 6–12 months for at least 5 years, followed by annual or clinically indicated scans. 15 , 16 PFS was defined as the duration from initial surgery to tumor progression, and OS was the duration between initial surgery and death or last follow‐up.
2.5. Statistical analysis
Student's t‐test or the Mann–Whitney U‐test was used for continuous normal distribution or nonparametric data. The χ 2 test or Fisher's exact test was used to compare categorical variables. Survival comparison was performed using Kaplan–Meier plots with log‐rank analysis. GraphPad Prism 9.0.1 (GraphPad Software, USA) and SPSS version 25.0 (IBM Corporation, Armonk, NY, USA) were used for statistical analysis. Probability values were obtained using two‐sided tests with statistical significance defined as p < 0.05.
3. RESULTS
3.1. Demographic description
A total of 668 cases were enrolled in this study, including 320 IDHmut‐Codel oligodendrogliomas, 293 IDHmut‐Noncodel astrocytomas, and 55 IDHwts (Table 1). There were no significant differences in gender distribution or tumor volume among the groups. IDHmut‐Noncodel astrocytomas presented in younger patients (mean ages: IDHmut‐Codel 42.6 years, IDHmut‐Noncodel 39.3 years, IDHwts 42.5 years, p < 0.001) and had a higher proportion of grade 2 tumors (66.6%, 80.5%, and 54.5% for oligodendrogliomas, astrocytomas, and IDHwts, respectively, p < 0.001). Most IDHwts (51/55, 92.7%) harbored TERT promoter mutations, EGFR amplification, and/or +7/10− chromosome alterations, aligning with molecular features characteristic of glioblastoma. A subset of cases (4/55) presented challenges in definitive classification based on our current testing strategy.
TABLE 1.
Clinical and radiological characteristics for grade 2–3 gliomas.
| Characteristics | IDHmut‐Codel | IDHmut‐Noncodel | IDHwt | p |
|---|---|---|---|---|
| Number of cases | 320 | 293 | 55 | |
| Gender (n, %) | ||||
| Female | 146 (45.6) | 135 (46.1) | 29 (52.7) | 0.614 |
| Male | 174 (54.4) | 158 (53.9) | 26 (47.3) | |
| Age (mean, yrs) | 42.6 ± 9.4 | 39.3 ± 9.5 | 42.5 ± 10.1 | <0.001 |
| Grade (n, %) | ||||
| 2 | 213 (66.6) | 236 (80.5) | 30 (54.5) | |
| 3 | 107 (33.4) | 57 (19.5) | 25 (45.5) | <0.001 |
| Volume (median, mL) | 35.3 | 41.6 | 29.4 | 0.414 |
| Tumor location | ||||
| Frontal | 250 (78.1) | 152 (51.9) | 27 (49.1) | |
| Temporal‐insular | 51 (15.9) | 117 (39.9) | 17 (30.9) | <0.001 |
| Parieto‐occipital | 19 (5.9) | 24 (8.2) | 11 (20.0) | |
| CpG sites (median, %) | ||||
| 74 | 30.5 | 16 | 3 | |
| 75 | 34 | 24 | 5 | |
| 76 | 21.5 | 14 | 5 | |
| 77 | 23 | 13 | 3 | |
| 78 | 12 | 9 | 3 | |
| 79 | 29 | 16 | 5 | |
| 80 | 31 | 19 | 3 | |
| 81 | 31 | 16 | 3 | |
| Average | 28 | 18 | 4 | |
| Methylated (average > 12%) | 286 (89.4) | 207 (70.6) | 13 (23.6) | <0.001 |
| MGMTp hierarchy (%) | ||||
| >20% | 234 (73.1) | 116 (39.6) | 10 (18.2) | |
| 11%–20% | 59 (18.4) | 108 (36.9) | 5 (9.1) | <0.001 |
| 0%–10% | 27 (8.4) | 69 (23.5) | 40 (72.7) | |
| EOR (%) | ||||
| GTR | 139 (43.4) | 113 (38.6) | 19 (34.5) | |
| STR | 135 (42.2) | 122 (41.6) | 19 (34.5) | <0.001 |
| PR | 46 (14.4) | 58 (19.8) | 17 (30.9) | |
| Adjuvant treatment (%) | ||||
| Observation | 24 (7.5) | 55 (18.8) | 6 (10.9) | |
| RT | 19 (5.9) | 39 (13.3) | 6 (10.9) | |
| AVM | 23 (7.2) | 15 (5.1) | 1 (1.8) | |
| Temozolomide | 126 (39.4) | 55 (18.8) | 3 (5.5) | |
| RT + AVM | 22 (6.9) | 10 (3.4) | 2 (3.6) | |
| RT + Temozolomide | 106 (33.1) | 119 (40.6) | 37 (67.3) | |
Abbreviations: AVM, ACNU‐based protocols (ACNU 90 mg/m2, day 1, and VM 60 mg/m2, days 1–3); IDHmut‐Codel, IDH mutant and 1p/19q codeleted oligodendrogliomas; IDHmut‐Noncodel, IDH mutant 1p/19q non‐codeleted astrocytomas; IDHwt, IDH wild‐type gliomas; RT, radiation therapy.
Spatial involvement varied significantly, with oligodendrogliomas showing a predilection for frontal lobe involvement (78.1%, 51.9%, and 49.1% for oligodendrogliomas, astrocytomas, and IDHwts, respectively). Astrocytomas were more commonly located in the temporal‐insular area (15.9%, 39.9%, and 30.9% for oligodendrogliomas, astrocytomas, and IDHwts, respectively, p < 0.001, Tables 1 and 2). Treatment protocols are detailed in Table 1.
TABLE 2.
Association between spatial distribution and grade 2–3 gliomas’ molecular status.
| Subgroups (n, %) | Tumor location | ||||
|---|---|---|---|---|---|
| Frontal | Temporal‐insular | Parieto‐occipital | Total | p | |
| All | 429 (64.2) | 185 (27.7) | 54 (8.1) | 668 | |
| IDHmut‐Codel | 250 (78.1) | 51 (15.9) | 19 (5.9) | 320 | |
| IDHmut‐Noncodel | 152 (51.9) | 117 (39.9) | 24 (8.2) | 293 | <0.001 |
| IDHwt | 27 (49.1) | 17 (30.9) | 11 (20.0) | 55 | |
| Methylation classification | |||||
| Methylated | 262 (72.8) | 71 (19.7) | 27 (7.5) | 360 | |
| Low‐methylated | 102 (59.3) | 59 (34.3) | 11 (6.4) | 172 | <0.001 |
| Unmethylated | 65 (47.8) | 55 (40.4) | 16 (11.8) | 136 | |
Abbreviations: IDHmut‐Codel, IDH mutant and 1p/19q codeleted oligodendrogliomas; IDHmut‐Noncodel, IDH mutant 1p/19q non‐codeleted astrocytomas; IDHwt, IDH wild‐type gliomas.
3.2. Prognostic effect of gross total resection in astrocytomas and oligodendrogliomas
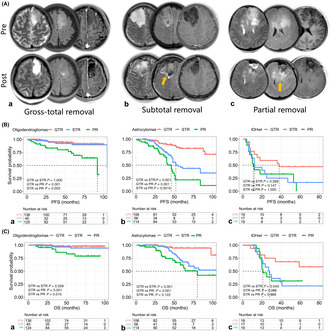
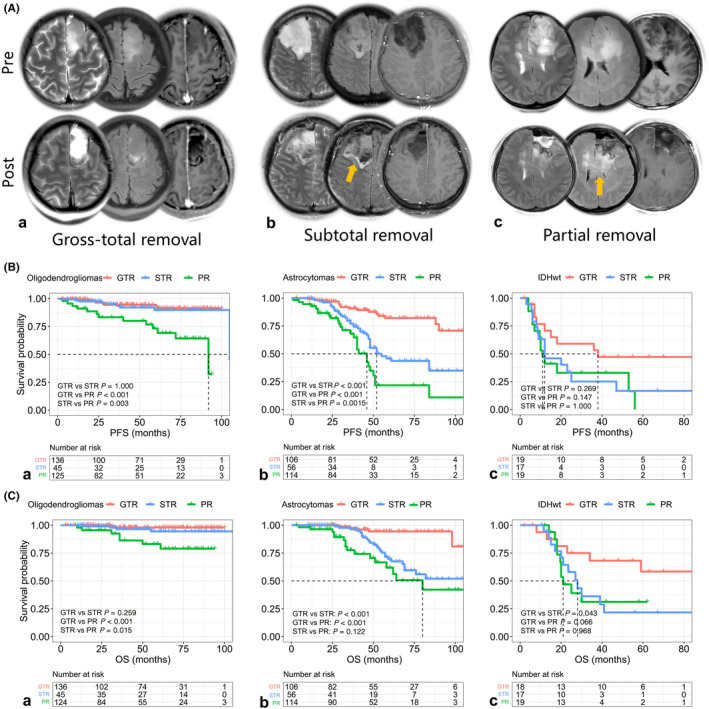
With a median follow‐up of 52.0 months (range: 12–110 months), 22.5% of patients experienced tumor progression, and 13.0% died. After adjusting for age, adjuvant treatment, and MGMT promoter status, oligodendroglioma patients receiving subtotal resection (STR) showed clinical outcomes comparable to those with GTR, both superior to partial resection (PR) patients (median PFS and OS not reached, GTR vs. STR, p > 0.05 for PFS and OS; GTR and STR vs. PR, p < 0.05 for PFS and OS, Figure 1B‐a for PFS and Figure 1C‐a for OS). In contrast, astrocytoma patients benefited significantly from GTR (median PFS for GTR, STR, and PR: unreached, 52.0, and 46.0 months, p < 0.001, Figure 1B‐b; median OS for GTR, STR, and PR: unreached, unreached, and 80.0 months, p < 0.001, Figure 1C‐b). Although limited by sample size, IDHwts also showed a trend toward benefiting from GTR, although this was not statistically significant for PFS and OS (median PFS for GTR, STR, and PR: 38.0, 12.0, and 11.0 months, p = 0.107, Figure 1B‐c; median OS for GTR, STR, and PR: unreached, 28.0, and 21.0 months, p = 0.092, Figure 1C‐c). During the follow‐up period of post‐adjuvant therapy, we observed that oligodendrogliomas might derive substantial benefit from adjuvant chemoradiotherapy and showed tumor regression, whereas astrocytomas, along with IDH wild‐type LGGs, exhibited rapid tumor regrowth and treatment failure (Figure 2). This phenomenon underscored the necessity for further investigation into the underlying mechanisms that drove the distinct residual tumor control effects.
FIGURE 1.
The relationship between extent of resection (EOR) and prognosis of grade 2–3 gliomas. (A) Typically illustrative examples of patients who received gross total resection (a, gross total resection [GTR]; b, subtotal resection [STR]; and c, partial resection [PR] (c). Pre and post, preoperative and postoperative T2WI, FLAIR, and T1CE images. Orange arrows represent tumor residuals. (B) Progression‐free survival (PFS) comparison among gliomas: (a) oligodendroglioma patients who received STR were not inferior to those who underwent GTR, but both superior to PR patients; (b) astrocytoma patients might achieve significant tumor control benefits from undergoing GTR (median PFS for GTR, STR, and PR: unreached, 52.0 months and 46.0 months, respectively); and (c) limited by the sample size, we observed a clear trend toward IDH wild‐type gliomas (IDHwts) also benefiting from GTR, although this difference was not statistically significant for PFS (median PFS for GTR, STR, and PR: 38.0, 12.0, and 11.0 months, p = 0.107). (C) Overall survival (OS) comparison among grade 2–3 gliomas: (a) the OS between GTR and STR patients was comparable but both were superior to STR patients in oligodendrogliomas; (b) an evident OS hierarchy was observed for GTR, STR, and PR astrocytoma cases; and (c) GTR might improve the survival of IDHwts (median OS for GTR, STR, and PR: unreached, 28.0 months, and 21.0 months, respectively, p = 0.092).
FIGURE 2.
Some representative outcomes of grade 2–3 gliomas treated with different tumor removal strategies are shown (A, gross total resection [GTR]; B, subtotal resection [STR]; C, partial resection [PR]). (A) GTR can provide significant benefits for gliomas. (B) Oligodendrogliomas with STR exhibit a better response to chemoradiotherapy, and the residual tumor often regresses following adjuvant treatment. However, the small residuals remaining from astrocytomas and IDH wild‐type gliomas (IDHwts) can be a direct source of tumor progression. (C) Patients who undergo PR experience tumor recurrence within a relatively short follow‐up period. Orange arrows represent tumor residuals and red arrows are radiological outcomes. Pre, preoperative; post, postoperative; mon; months after operation.
3.3. Relatively lower O6‐methylguanine‐DNA methyltransferase promoter methylation levels and more low‐methylated cases were observed in astrocytomas
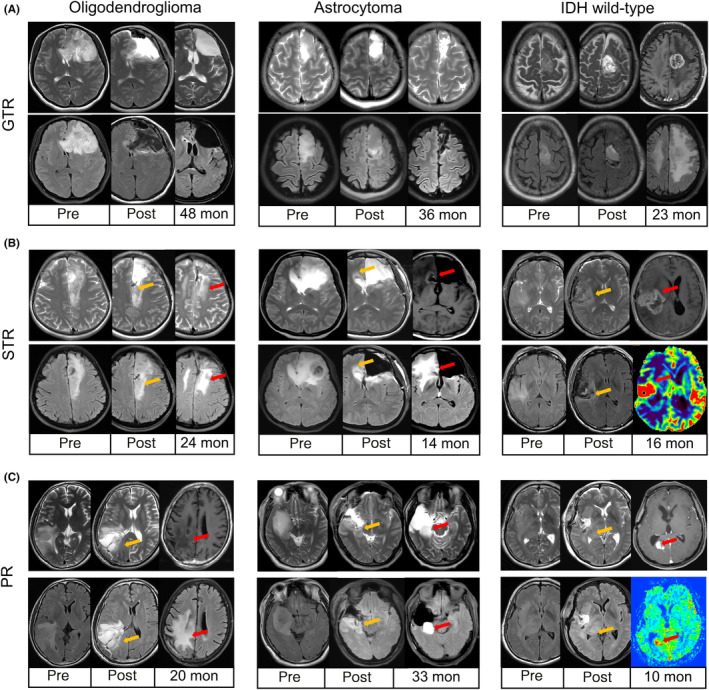
The status of the MGMT promoter is the most critical gene for assessing the effectiveness of chemotherapy. Astrocytomas exhibited relatively lower MGMTp methylation levels, with intermediate levels for each tested CpG site (median for CpGs 74–81: oligodendrogliomas 12%–34%, astrocytomas 9%–18%, IDHwts 3%–5%, all p‐values <0.001, Figure 3A–C). The average CpG results revealed a hierarchical pattern of methylation (median 28%, 18%, and 4% for oligodendrogliomas, astrocytomas, and IDHwts, respectively, p < 0.001, Figure 3C,D).
FIGURE 3.
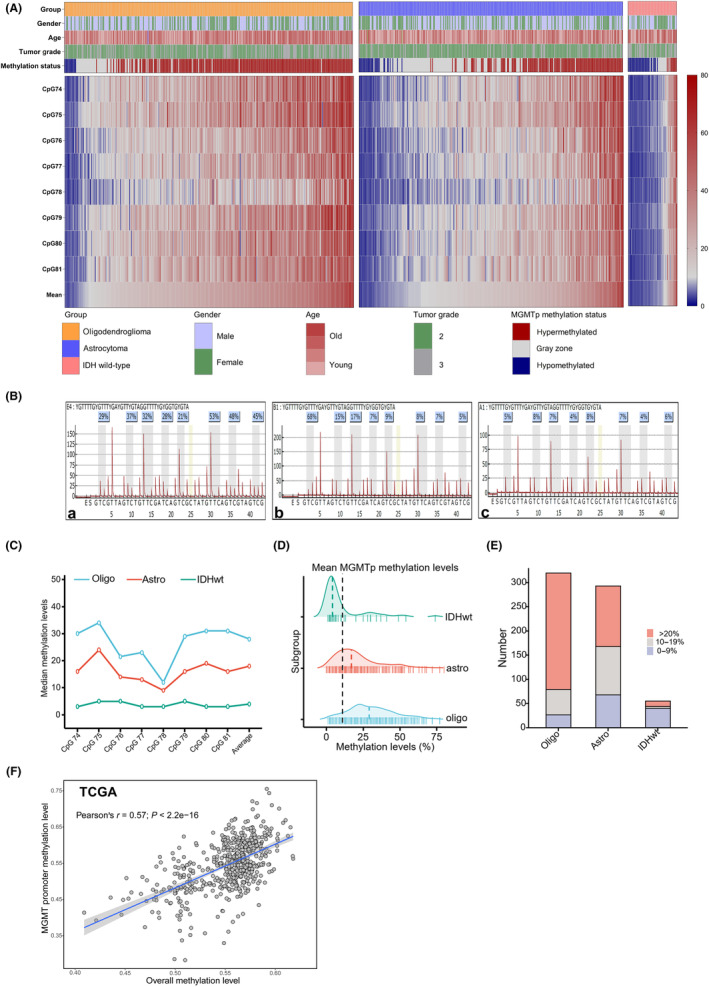
The difference in O6‐methylguanine‐DNA methyltransferase promoter (MGMTp) methylation status among grade 2–3 gliomas. (A) The heatmap of quantitative MGMTp methylation levels for gliomas. The results showed substantial heterogeneity among CpG sites and hierarchy in gliomas. (B) Representative examples for methylated oligodendrogliomas (a), low‐methylated astrocytomas (b), and unmethylated IDHwts (c). (C) The median methylation results of gliomas. Oligodendrogliomas harbored the highest methylation levels for each CpGs tested, followed by astrocytomas and IDH wild‐type gliomas (IDHwts). (D) The distribution of average results of CpGs 74–81 showed hierarchy among gliomas. (E) Tiered average methylation levels revealed that most oligodendrogliomas were methylated (>20%), while low‐methylated (10%–20%) cases increased in astrocytomas and unmethylated (<10%) cases were common in IDHwt subgroups. (F) Correlation between MGMT promoter methylation levels and genome‐wide methylation levels. DNA methylation data were obtained from TCGA database, and Pearson correlation coefficients were calculated to determine the relationship. The analysis included 502 primary LGG patients, and both the MGMT promoter and genome‐wide methylation levels were computed as average beta values of the respective CpG sites. The MGMT promoter methylation levels had a moderate relationship with overall methylation levels in TCGA‐LGGs (r = 0.57, p < 0.001), which implied that MGMTp methylation might only partially serve as a surrogate for G‐CIMP.
Further analysis classified cases into methylated (>20%), low‐methylated (10%–20%), and unmethylated (<10%) subgroups. Oligodendrogliomas had the highest percentage of methylated cases (73.1%), while astrocytomas predominantly fell into the low‐methylated subgroup (36.9%), and unmethylated cases were more common in IDHwts (72.7%, p < 0.001, Table 1 and Figure 3E). A potentially intermediate tumor‐control effect might be observed in astrocytomas due to moderate MGMTp methylation levels. Furthermore, our analysis of DNA methylation sequencing data from TCGA database, comprising 502 primary LGG patients, revealed a moderate correlation between MGMT promoter methylation levels and overall genome‐wide methylation levels (Pearson's r = 0.57, p < 0.001). This finding suggests that MGMT promoter methylation might partially serve as a surrogate marker for whole‐genome epigenetic modification assessment.
3.4. Histological analysis revealed a grade 2 predilection for intermediate methylation cases
Additionally, we observed significant differences in median methylation levels between WHO grade 2 and grade 3 tumors (oligodendrogliomas: 27.0% vs. 36.0%, p < 0.001; astrocytomas: 16.0% vs. 21.0%, p = 0.039; all LGGs: 21.0% vs. 27.0%, p < 0.001, Mann–Whitney U‐test). In the low‐methylation subgroup, 79.7% (137/172) of cases exhibited a lower histological grade (WHO grade 2), significantly higher than in methylated (66.9%, 241/360) and unmethylated tumors (74.3%, 101/136, p = 0.007). Among grade 2 low‐methylated tumors, 67.2% (92/137) were IDHmut‐Noncodel astrocytomas, while oligodendrogliomas (30.7%, 42/137) and IDHwts (2.2%, 3/137) were less prevalent. These findings suggest that low‐methylated tumors might exhibit relatively less aggressive behavior and a predilection for astrocytomas.
3.5. Spatial analysis revealed a close association between low‐methylation cases and temporal‐insular area involvement
We observed that a significant proportion of low‐methylated cases exhibited a preference for temporal‐insular invasion as well. LGGs commonly affected the frontal lobe (64.2%, 429/668, Figure 4A), particularly in oligodendrogliomas (78.1%, 250/320, Figure 4B‐a; astrocytomas, 51.9%, 152/293, Figure 4B‐b; IDHwt, 49.1%, 27/55, Figure 4B‐c). From methylated, low‐methylated to unmethylated cases, there was a decrement in frontal lobe invasion (72.8%, 59.3%, and 47.8%, respectively, Figure 4C) and an increase in temporal‐insular invasion (19.7%, 34.3%, and 40.4%, respectively, Figure 4C). Our results also revealed significant differences in median MGMT promoter methylation levels between tumors located in the frontal and temporal‐insular areas (oligodendrogliomas: 30.0% vs. 23.0%, p = 0.047; astrocytomas: 19.0% vs. 16.0%, p = 0.014; all LGGs: 24.0% vs. 16.0%, p < 0.001). Tumor involvement in the parieto‐occipital lobes was comparable across methylation statuses (methylated, low‐methylated, and unmethylated, 7.5%, 6.4%, and 11.8%, respectively, Figure 4C and Table 2).
FIGURE 4.
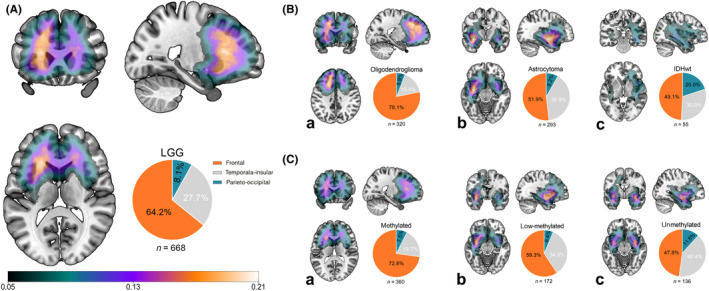
Spatial distribution of grade 2–3 gliomas. (A) Gliomas frequently invaded the frontal lobe and then the temporal‐insular and parieto‐occipital lobes. (B) Oligodendrogliomas (a), astrocytomas (b), and IDH wild‐type gliomas (IDHwts) (c) displayed different spatial preferences. Most of the oligodendrogliomas located in the frontal lobe, and more astrocytomas, showed temporal‐insular involvement. (C) Based on the average results of all CpG sites tested, we simply classified gliomas as (a) methylated (>20%), (b) low methylation (10%–20%), and (c) unmethylated (<10%), and we noticed a decrement of frontal invasion while an inverse trend of temporal‐insular infringement (frontal lobe involvement: 72.8% (262/360) of methylated, 59.3% (102/172) of low‐methylated, and 47.8% (65/136) of unmethylated LGGs, temporal‐insular invasion: 19.7% (71/360) of methylated, 34.3% (59/172) of low‐methylated, and 40.4% (55/136) of unmethylated LGGs, Table 2).
Among low‐methylated cases, 34.3% (59/172, Figure 4C‐b) were located in the temporal‐insular area, higher than methylated cases (19.7%, 71/360, Figure 4C‐a) but slightly lower than unmethylated cases (40.4%, 55/136, Figure 4C‐c). Most of the low‐methylated temporal‐insular cases were IDHmut‐Noncodel astrocytomas (76.3%, 45/59), with oligodendrogliomas (22.0%, 13/59) and IDHwts (1.7%, 1/59) being less common. We performed a multivariable logistic regression analysis to identify factors associated with temporal‐insular involvement in gliomas. The variables included in the analysis were molecular diagnosis (categorized as oligodendrogliomas, astrocytomas, and IDH wild‐type LGGs), tumor grade (WHO grade 2 or 3), and quantitative MGMT promoter methylation levels (treated as a continuous variable). The results demonstrated that only quantitative methylation levels were significantly associated with temporal‐insular involvement (β = 0.985, 95% CI: 0.973–0.997, p = 0.015). In contrast, molecular diagnosis (astrocytoma vs. oligodendroglioma: β = 0.549, 95% CI: 0.274–1.098, p = 0.090; IDH wild‐type LGGs vs. oligodendroglioma: β = 1.635, 95% CI: 0.857–3.123, p = 0.136) and tumor grade (WHO grade 3 vs. grade 2: β = 1.279, 95% CI: 0.836–1.956, p = 0.256) were not significantly associated with temporal‐insular location.
4. DISCUSSION
Studies have established that gross total resection (GTR) significantly improves survival for IDHmut‐Noncodel astrocytomas, whereas even small residual tumors can markedly shorten PFS and OS. 6 , 7 , 17 , 18 , 19 , 20 Consequently, an aggressive resection strategy is recommended for eligible IDHmut‐Noncodel cases to maximize prognosis. Notably, the critical prognostic effect of GTR is notably attenuated in IDHmut‐Codel oligodendrogliomas, where subtotal resection (STR) can achieve comparable outcomes following adjuvant chemoradiotherapy. Although the indolent growth pattern of oligodendrogliomas might partly explain this discrepancy, our study systematically explored the key molecular marker, MGMT promoter methylation, across a large cohort of grade 2–3 gliomas, revealing significant differences that influence treatment responses and unequal importance of surgical removal among gliomas in turn.
Previous studies have shown that IDH mutation is strongly associated with methylated MGMTp due to significantly increased overall genomic CpG methylation. 21 IDH 1/2 mutations bring out extensive epigenomic alterations, resulting in the glioma‐CpG island methylator phenotype (G‐CIMP) by remodeling the methylome and transcriptome. 22 , 23 The abnormal accumulation of 2‐hydroxyglutarate (2‐HG) from mutant IDH 1/2 impairs dioxygenases, including histone and DNA demethylases, leading to global hypermethylation status, including the MGMT gene. 22 , 24 , 25 , 26 , 27
Recent studies indicate that MGMTp methylation status varies among IDH mutant gliomas, contrary to traditional stereotypes. MGMTp unmethylation is more common in IDHmut‐Noncodel astrocytomas compared to IDHmut‐Codel oligodendrogliomas. 28 Results from Northwestern Memorial Hospital (NMH) by PSQ assay showed that nearly 20% of IDHmut‐Noncodel astrocytomas (17/97, 17.5%) lacked methylated MGMTp, significantly higher than oligodendrogliomas (3.8%, 2/53). In a large cohort of TCGA cases, 11.7% of IDHmut‐Noncodel astrocytomas (30/257) were MGMTp unmethylated, while only 1/169 (0.6%) of unmethylated cases were observed in IDHmut‐Codel oligodendrogliomas. Kinslow et al. 29 reported that 22.2% (288/1297) of 1p/19q codeleted gliomas showed unmethylated MGMT promoter status, and 52% (370/712) of IDH1/2 mutant astrocytomas were unmethylated. 30 Our study corroborates these findings, demonstrating that oligodendrogliomas are more likely to exhibit hypermethylated MGMT promoter status compared to astrocytomas. Specifically, we observed a moderate and comparable proportion of unmethylated cases (methylation levels <10%) in lower‐grade gliomas: 8.4% (27/320) in oligodendrogliomas and 23.5% (69/293) in astrocytomas. One possible explanation for this hypermethylation in oligodendrogliomas is the gene dosage effect caused by the 1p/19q codeletion in IDH mutant gliomas. Additionally, variations in sample purity, such as the presence of non‐tumor cells, can significantly affect molecular analyses. In the TCGA‐LGG dataset, IDHmut‐Noncodel astrocytomas generally exhibit lower sample purity compared to IDHmut‐Codel oligodendrogliomas, highlighting the complexity of interpreting genomic data within the tumor microenvironment. The moderate correlation between MGMT promoter methylation and overall methylation levels further suggests that these factors play independent roles in determining clinical outcomes.
Various techniques, including methylation‐specific PCR (MSP), quantitative MSP (qMSP), pyrosequencing (PSQ), and immunohistochemistry (IHC), are used to assess MGMTp status. PSQ provides unparalleled quantitative methylation results for individual CpG sites. Recent findings in glioblastomas have shown a hierarchical and heterogeneous pattern of MGMTp methylation levels. Josefine et al. 9 categorized MGMTp status into unmethylated (<10% mean methylation), low‐methylated (10%–20% mean methylation), and highly methylated (>20% mean methylation), with the low‐methylated group demonstrating intermediate prognosis. Chai et al. 11 identified distinct prognostic hierarchies among methylated, unmethylated, and gray‐zone subgroups in malignant gliomas. Our study elaborates on the hierarchy and heterogeneity of MGMTp in grade 2–3 gliomas using a quantitative protocol, suggesting that IDHmut‐Noncodel astrocytomas might have a moderate temozolomide response due to more low‐methylated and unmethylated cases.
Our study also initially revealed the heterogeneous and hierarchical spatial distribution of MGMTp in grade 2–3 gliomas, showing a close relationship between non‐frontal involvement and relatively lower MGMTp methylation levels. Compared to IDHmut‐Codel oligodendrogliomas, more astrocytomas displayed temporal‐insular involvement and intermediate MGMTp status. These findings suggest that different cerebral regions might exhibit distinct mechanisms of tumorigenicity and methylation patterns. More low‐methylated cases in the temporal‐insular zone indicated a relatively compromised temozolomide effect, warranting an aggressive resection strategy.
Controversies persist regarding the prognostic role of MGMTp in WHO grade 2–3 gliomas. We acknowledge the absence of an ideal cutoff value for MGMTp in IDH mutant gliomas and recognize that this parameter might differ within oligodendrogliomas or astrocytomas. Cutoff values from glioblastomas might be unreliable due to distinct molecular backgrounds (e.g. loss copy of chromosome 10). Deriving a reliable cutoff value for prognosis stratification is challenging, as our dataset does not provide a clear threshold for median PFS or OS (22.5% of patients encountered tumor progression, while 13.0% succumbed to the disease, Figure S1). We summarized studies on the prognostic role of MGMTp in grade 2–3 gliomas (Table S1). Although the classification strategy from glioblastomas was used, some results were illuminating. The EORTC study 26,053–22,054 confirmed the survival benefit of temozolomide for IDHmut‐Noncodel astrocytomas, with binary MGMTp classification remaining a prognostic biomarker. 31 Another study (EORTC 22,033–26,033) revealed comparable disease control effects of temozolomide and radiotherapy in oligodendrogliomas but not in astrocytomas, 32 and the RTOG 0424 study confirmed the prognostic significance of MGMTp for LGGs. 33 Notably, the nationwide study by Kinslow et al. suggests survival benefits from chemotherapy in methylated 1p/19q codeleted gliomas, 29 while the research by Kim et al. did not observe prognostic differences in both oligodendrogliomas and astrocytomas. 34 Thus, the prognostic role of MGMTp in grade 2–3 gliomas warrants further validation. 35
Despite limitations such as relatively short follow‐up periods, insufficient molecular testing, the retrospective nature of the study, and its single‐institution focus, our study first revealed the heterogeneity and hierarchy of MGMTp status among grade 2–3 gliomas and pinpointed the spatial distribution of tiered cases based on this gene. These findings are significant for improving our understanding of grade 2–3 gliomas.
In conclusion, our findings reveal a significant hierarchy and distinct spatial distribution of MGMT promoter methylation levels among WHO grade 2–3 gliomas. More astrocytomas displayed an intimate association between intermediate MGMTp status and temporal‐insular involvement, which might indicate a moderate effect of temozolomide and warrant an aggressive resection strategy.
AUTHOR CONTRIBUTIONS
Mingxiao Li: Conceptualization; data curation; writing – original draft. Gehong Dong: Investigation; methodology; supervision. Xuzhu Chen: Methodology; visualization; writing – original draft. Xiaohui Ren: Investigation; methodology; supervision; validation; writing – review and editing. Song Lin: Funding acquisition; investigation; writing – review and editing. Yong Cui: Data curation; formal analysis; funding acquisition. Jiang Liu: Conceptualization. Jiancong Weng: Conceptualization. Shaoping Shen: Formal analysis. Haihui Jiang: Formal analysis. Xiaokang Zhang: Formal analysis. Xuzhe Zhao: Formal analysis. Ming Li: Formal analysis. Xijie Wang: Formal analysis. Hongxiang Ren: Formal analysis. Qiang Li: Formal analysis. Yulian Zhang: Formal analysis. Quan Cheng: Project administration. Yanbing Yu: Project administration.
FUNDING INFORMATION
This study was supported by the National High Level Hospital Clinical Research Funding (2023‐NHLHCRF‐BQ‐20, 2022‐NHLHCRF‐YS‐05 and ZRJY2021‐GG05), Elite Medical Professionals Project of China‐Japan Friendship Hospital (ZRJY2024‐QMPY08 & ZRJY2023‐QM25), National Key R&D Program of China (2022YFC2402500), National Natural Science Foundation of China (No.82202983) and Peking University Clinical Scientist Training Program, supported by “the Fundamental Research Funds for the Central Universities”.
CONFLICT OF INTEREST STATEMENT
The authors declare no potential conflicts of interest.
ETHICS STATEMENT
Approval of the research protocol by an Institutional Reviewer Board: The study was approved by the institutional review board of the Capital Medical University.
Informed Consent: The signed consent forms were received from every patient.
Registry and the Registration of the Study: N/A.
Animal Studies: N/A.
Supporting information
Appendix S1.
ACKNOWLEDGMENTS
The authors sincerely thank the patients and their families for their participation in the present study.
Li M, Liu J, Weng J, et al. Unveiling hierarchy and spatial distribution of O6‐methylguanine‐DNA methyltransferase promoter methylation in World Health Organization grade 2–3 gliomas. Cancer Sci. 2024;115:3403‐3414. doi: 10.1111/cas.16268
Mingxiao Li, Jiang Liu and Jiancong Weng contributed equally to this study.
Contributor Information
Quan Cheng, Email: chengquan@csu.edu.cn.
Yanbing Yu, Email: yuyanbing123@126.com.
Song Lin, Email: linsong2005@126.com.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Louis DN, Perry A, Wesseling P, et al. The 2021 WHO classification of tumors of the central nervous system: a summary. Neuro‐Oncology. 2021;23(8):1231‐1251. doi: 10.1093/neuonc/noab106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. van den Bent MJ, Smits M, Kros JM, Chang SM. Diffuse infiltrating oligodendroglioma and astrocytoma. J Clin Oncol. 2017;35(21):2394‐2401. doi: 10.1200/JCO.2017.72.6737 [DOI] [PubMed] [Google Scholar]
- 3. Wu CC, Jain R, Radmanesh A, et al. Predicting genotype and survival in glioma using standard clinical MR imaging apparent diffusion coefficient images: a pilot study from the cancer genome atlas. AJNR Am J Neuroradiol. 2018;39(10):1814‐1820. doi: 10.3174/ajnr.A5794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Smits M, van den Bent MJ. Imaging correlates of adult glioma genotypes. Radiology. 2017;284(2):316‐331. doi: 10.1148/radiol.2017151930 [DOI] [PubMed] [Google Scholar]
- 5. Kickingereder P, Neuberger U, Bonekamp D, et al. Radiomic subtyping improves disease stratification beyond key molecular, clinical, and standard imaging characteristics in patients with glioblastoma. Neuro‐Oncology. 2018;20(6):848‐857. doi: 10.1093/neuonc/nox188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wijnenga MMJ, French PJ, Dubbink HJ, et al. The impact of surgery in molecularly defined low‐grade glioma: an integrated clinical, radiological, and molecular analysis. Neuro‐Oncology. 2018;20(1):103‐112. doi: 10.1093/neuonc/nox176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kawaguchi T, Sonoda Y, Shibahara I, et al. Impact of gross total resection in patients with WHO grade III glioma harboring the IDH 1/2 mutation without the 1p/19q co‐deletion. J Neuro‐Oncol. 2016;129(3):505‐514. doi: 10.1007/s11060-016-2201-2 [DOI] [PubMed] [Google Scholar]
- 8. Ding X, Wang Z, Chen D, et al. The prognostic value of maximal surgical resection is attenuated in oligodendroglioma subgroups of adult diffuse glioma: a multicenter retrospective study. J Neuro‐Oncol. 2018;140(3):591‐603. doi: 10.1007/s11060-018-2985-3 [DOI] [PubMed] [Google Scholar]
- 9. Radke J, Koch A, Pritsch F, et al. Predictive MGMT status in a homogeneous cohort of IDH wildtype glioblastoma patients. Acta Neuropathol Commun. 2019;7(1):89. doi: 10.1186/s40478-019-0745-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Li M, Dong G, Zhang W, et al. Combining MGMT promoter pyrosequencing and protein expression to optimize prognosis stratification in glioblastoma. Cancer Sci. 2021;112(9):3699‐3710. doi: 10.1111/cas.15024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chai RC, Liu YQ, Zhang KN, et al. A novel analytical model of MGMT methylation pyrosequencing offers improved predictive performance in patients with gliomas. Mod Pathol. 2019;32(1):4‐15. doi: 10.1038/s41379-018-0143-2 [DOI] [PubMed] [Google Scholar]
- 12. Li M, Ren X, Dong G, et al. Distinguishing Pseudoprogression from true early progression in isocitrate dehydrogenase wild‐type glioblastoma by interrogating clinical, radiological, and molecular features. Front Oncol. 2021;11:627325. doi: 10.3389/fonc.2021.627325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Li M, Ren X, Chen X, et al. Combining hyperintense FLAIR rim and radiological features in identifying IDH mutant 1p/19q non‐codeleted lower‐grade glioma. Eur Radiol. 2022;32:3869‐3879. doi: 10.1007/s00330-021-08500-w [DOI] [PubMed] [Google Scholar]
- 14. Li M, Ren X, Jiang H, et al. Supratentorial high‐grade astrocytoma with leptomeningeal spread to the fourth ventricle: a lethal dissemination with dismal prognosis. J Neuro‐Oncol. 2019;142(2):253‐261. doi: 10.1007/s11060-018-03086-8 [DOI] [PubMed] [Google Scholar]
- 15. van den Bent MJ, Wefel JS, Schiff D, et al. Response assessment in neuro‐oncology (a report of the RANO group): assessment of outcome in trials of diffuse low‐grade gliomas. Lancet Oncol. 2011;12(6):583‐593. doi: 10.1016/S1470-2045(11)70057-2 [DOI] [PubMed] [Google Scholar]
- 16. Wen PY, Macdonald DR, Reardon DA, et al. Updated response assessment criteria for high‐grade gliomas: response assessment in neuro‐oncology working group. J Clin Oncol. 2010;28(11):1963‐1972. doi: 10.1200/jco.2009.26.3541 [DOI] [PubMed] [Google Scholar]
- 17. Rossi M, Ambrogi F, Gay L, et al. Is supratotal resection achievable in low‐grade gliomas? Feasibility, putative factors, safety, and functional outcome. J Neurosurg. 2019;132(6):1692‐1705. doi: 10.3171/2019.2.JNS183408 [DOI] [PubMed] [Google Scholar]
- 18. Rossi M, Gay L, Ambrogi F, et al. Association of supratotal resection with progression‐free survival, malignant transformation, and overall survival in lower‐grade gliomas. Neuro‐Oncology. 2021;23(5):812‐826. doi: 10.1093/neuonc/noaa225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Patel SH, Bansal AG, Young EB, et al. Extent of surgical resection in lower‐grade gliomas: differential impact based on molecular subtype. AJNR Am J Neuroradiol. 2019;40(7):1149‐1155. doi: 10.3174/ajnr.A6102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. de Leeuw CN, Vogelbaum MA. Supratotal resection in glioma: a systematic review. Neuro‐Oncology. 2019;21(2):179‐188. doi: 10.1093/neuonc/noy166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Turcan S, Rohle D, Goenka A, et al. IDH1 mutation is sufficient to establish the glioma hypermethylator phenotype. Nature. 2012;483(7390):479‐483. doi: 10.1038/nature10866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lu C, Ward PS, Kapoor GS, et al. IDH mutation impairs histone demethylation and results in a block to cell differentiation. Nature. 2012;483(7390):474‐478. doi: 10.1038/nature10860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Malta TM, de Souza CF, Sabedot TS, et al. Glioma CpG Island methylator phenotype (G‐CIMP): biological and clinical implications. Neuro‐Oncology. 2018;20(5):608‐620. doi: 10.1093/neuonc/nox183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yan H, Parsons DW, Jin G, et al. IDH1 and IDH2 mutations in gliomas. N Engl J Med. 2009;360(8):765‐773. doi: 10.1056/NEJMoa0808710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dang L, White DW, Gross S, et al. Cancer‐associated IDH1 mutations produce 2‐hydroxyglutarate. Nature. 2009;462(7274):739‐744. doi: 10.1038/nature08617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ward PS, Patel J, Wise DR, et al. The common feature of leukemia‐associated IDH1 and IDH2 mutations is a neomorphic enzyme activity converting alpha‐ketoglutarate to 2‐hydroxyglutarate. Cancer Cell. 2010;17(3):225‐234. doi: 10.1016/j.ccr.2010.01.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tateishi K, Wakimoto H, Iafrate AJ, et al. Extreme vulnerability of IDH1 mutant cancers to NAD+ depletion. Cancer Cell. 2015;28(6):773‐784. doi: 10.1016/j.ccell.2015.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Horbinski C, McCortney K, Stupp R. MGMT promoter methylation is associated with patient age and 1p/19q status in IDH‐mutant gliomas. Neuro‐Oncology. 2021;23(5):858‐860. doi: 10.1093/neuonc/noab039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kinslow CJ, Rae AI, Taparra K, et al. MGMT promoter methylation predicts overall survival after chemotherapy for 1p/19q‐Codeleted gliomas. Clin Cancer Res. 2023;29(21):4399‐4407. doi: 10.1158/1078-0432.Ccr-23-1295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kinslow CJ, Siegelin MD, Iwamoto FM, et al. MGMT promoter methylation in 1p19q‐intact gliomas. J Neuro‐Oncol. 2024;166(1):73‐78. doi: 10.1007/s11060-023-04515-z [DOI] [PubMed] [Google Scholar]
- 31. van den Bent MJ, Tesileanu CMS, Wick W, et al. Adjuvant and concurrent temozolomide for 1p/19q non‐co‐deleted anaplastic glioma (CATNON; EORTC study 26053‐22054): second interim analysis of a randomised, open‐label, phase 3 study. Lancet Oncol. 2021;22(6):813‐823. doi: 10.1016/s1470-2045(21)00090-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Baumert BG, Hegi ME, van den Bent MJ, et al. Temozolomide chemotherapy versus radiotherapy in high‐risk low‐grade glioma (EORTC 22033‐26033): a randomised, open‐label, phase 3 intergroup study. Lancet Oncol. 2016;17(11):1521‐1532. doi: 10.1016/s1470-2045(16)30313-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bell EH, Zhang P, Fisher BJ, et al. Association of MGMT promoter methylation status with survival outcomes in patients with high‐risk glioma treated with radiotherapy and temozolomide: an analysis from the NRG oncology/RTOG 0424 trial. JAMA Oncologia. 2018;4(10):1405‐1409. doi: 10.1001/jamaoncol.2018.1977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kim M, Kim S, Park YW, et al. Sex as a prognostic factor in adult‐type diffuse gliomas: an integrated clinical and molecular analysis according to the 2021 WHO classification. J Neuro‐Oncol. 2022;159(3):695‐703. doi: 10.1007/s11060-022-04114-4 [DOI] [PubMed] [Google Scholar]
- 35. Kinslow CJ, Mercurio A, Kumar P, et al. Association of MGMT promoter methylation with survival in low‐grade and anaplastic gliomas after alkylating chemotherapy. JAMA Oncologia. 2023;9(7):919‐927. doi: 10.1001/jamaoncol.2023.0990 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


