Abstract
Homozygous familial hypercholesterolemia (HoFH), is a rare genetic disorder characterized by dual mutations in the low-density lipoprotein receptor (LDLR) gene, leading to dysfunctional or absent LDLRs, often accompanied by severe premature Atherosclerotic Cardiovascular Disease (ASCVD) and exhibiting refractoriness to aggressive pharmacological interventions. Double filtration plasmapheresis (DFPP), a form of lipoprotein apheresis (LA), has been effectively utilized as an adjunctive treatment modality to reduce serum LDL-C levels in refractory cases of HoFH. Here, we report a case of a 36-year-old female with HoFH who developed xanthomas on her limbs and waist at age 7. Despite maximum-tolerated doses of statins from age 32, combined with ezetimibe and evolocumab, her LDL-C levels remained critically elevated at 12–14 mmol/L. Her genetic testing confirmed a homozygous LDLR mutation. At 35 years old, she experienced exertional chest pain, and percutaneous coronary intervention revealed severe calcific left main stenosis, necessitating stent implantation. Subsequently, she initiated once every 1–2 months DFPP. Pre-DFPP, her LDL-C and total cholesterol (TC) levels were 13.82 ± 3.28 and 15.45 ± 0.78 mmol/L, respectively. Post-DFPP, her LDL-C and TC levels significantly decreased to 2.43 ± 0.33 mmol/L (81.76 ± 4.11% reduction) and 3.59 ± 0.41 mmol/L (76.76 ± 2.75% reduction), respectively. Lipoprotein (a) and triglycerides also decreased by 89.10 ± 1.39% and 42.29 ± 15.68%,respectively. Two years later, there was no progression of coronary artery disease, and her symptoms and xanthomas regressed significantly. Collectively, DFPP effectively reduces LDL-C levels in refractory cases of HoFH and contributes to delaying ASCVD progression, representing an efficacious adjunctive therapeutic modality.
Keywords: Homozygous Familial Hypercholesterolemia, Lipoprotein Apheresis, Double Filtration Plasmapheresis, Proprotein Convertase Subtilisin Kexin 9 Inhibitor
Introduction
Homozygous familial hypercholesterolemia (HoFH) is an exceptionally rare and potentially fatal genetic disorder, characterized by an inherent refractoriness to aggressive pharmacological interventions. The underlying pathogenesis primarily involves pathogenic mutations in genes encoding the low-density lipoprotein receptor (LDLR), apolipoprotein B, proprotein convertase subtilisin/kexin type 9 (PCSK9), and LDL receptor adaptor protein 1 (LDLRAP1), with LDLR mutations accounting for over 80% of cases [1]. Due to the presence of mutations in both homologous alleles, LDLR function is severely impaired or entirely abolished, leading to a significant impediment in the clearance of LDL-C from circulation. Individuals with untreated HoFH exhibit serum LDL-C levels 2–3 times higher than those with heterozygous familial hypercholesterolemia (HeFH), often exceeding the critical threshold of 13.0 mmol/L (500 mg/dL) [2]. The majority of patients manifest extensive atherosclerotic lesions at an early age, during childhood or adolescence. This condition precipitates severe cardiovascular complications, including acute myocardial infarction and sudden cardiac death. Furthermore, it is associated with atherosclerotic manifestations affecting both the valvular and supravalvular regions of the aortic root [3]. Effective management of LDL-C levels is paramount in preventing and slowing the progression of ASCVD in patients with HoFH. Recent advancements in pharmacological developments have yielded promising results. Notably, PCSK9 inhibitors (PCSK9i), which target and increase the number of LDLRs on cellular surfaces, have demonstrated remarkable efficacy in lowering LDL-C levels [4]. Extensive studies have shown that the addition of PCSK9i to intensified statin therapy in patients with cardiovascular disease (CVD) significantly decreases LDL-C levels, stabilizes coronary atherosclerotic plaques, and reduces the risk of cardiovascular events [5, 6]. However, treatment-refractory cases of HoFH present significant therapeutic challenges, exhibiting a lack of response to pharmacological interventions, including maximum tolerated doses of statins, ezetimibe, and PCSK9i. In such instances, lipoprotein apheresis (LA) emerges as an essential therapeutic modality. Accumulating evidence suggests that regular LA can effectively control LDL-C levels, reduce the risk of adverse cardiovascular events, and delay the progression of ASCVD [7, 8].
Although LA is a well-established therapy for refractory HoFH, expanding our understanding of its indications, techniques, and clinical outcomes remains valuable. In this case report, we present a female patient with refractory HoFH and early-onset ASCVD, who experienced significant clinical benefits from undergoing regular LA sessions over a two-year follow-up period, notably demonstrating delayed progression of ASCVD. Remarkably, despite a lower treatment frequency (12 treatments in 2 years, averaging once every two months, far less than the usually recommended once every two weeks or once a week), the patient still exhibited good clinical stability.
Case report
A 36-year-old woman was admitted to the hospital following a series of recurring episodes characterized by exertional chest tightness and pain that had persisted for more than a year. She had a long-standing history of hypercholesterolemia dating back to the age of 7, at which time she had already developed multiple nodular xanthomas, a clear indication of her underlying lipid disorder. Notably, despite being suspected but not diagnosed with HoFH at the age of 7, she did not initiate statin therapy until the age of 32. During this 32-year period, the patient did not receive any lipid-lowering treatment, suggesting that she may have had a relatively mild phenotype of HoFH.
At the age of 32, in an attempt to manage her persistently elevated LDL-C levels, she initiated the maximum tolerated statin therapy, specifically rosuvastatin at a daily dosage of 20 mg. however, achieving effective control remained an elusive goal. Despite the subsequent addition of ezetimibe and evolocumab to her treatment regimen, her LDL-C levels fluctuated between 12–14 mmol/l, highlighting a refractory response to conventional therapies. Genetic testing revealed a homozygous LDLR mutation at c.1104C > A (p.C368X), providing a molecular explanation. Remarkably, throughout this, she remained asymptomatic.
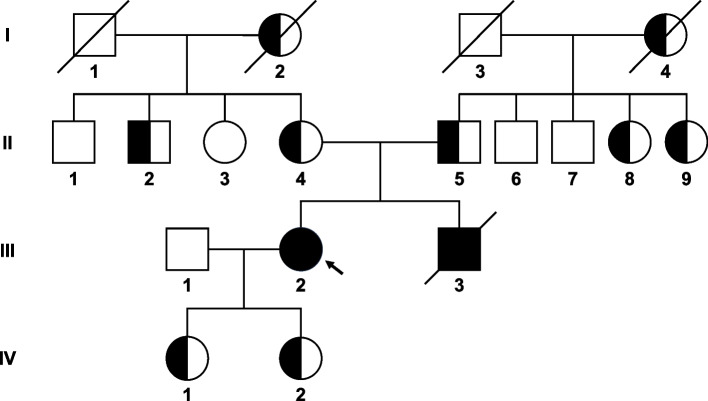
At the age of 35, the patient started to endure recurrent episodes marked by chest tightness and pain, which were triggered by physical exertion or emotional excitement. Each episode persisted for 2–3 min and was alleviated by rest. These distressing occurrences necessitated hospitalization for comprehensive treatment in February 2022. Her family history revealed a consanguineous marriage in her parental lineage, a potential risk factor for the development of her condition. she had a brother who succumbed to sudden death at the age of 25 following an episode of heavy alcohol consumption, also presenting with xanthomas and had markedly elevated LDL-C levels of approximately 20 mmol/L, but his condition remained undiagnosed and untreated. Furthermore, genetic testing confirmed that both her parents, daughters, two aunts, as well as one uncle, were carriers of the LDLR gene mutation at the c.1104C > A (p.C368X) site, indicating they have HeFH (Fig. 1A).
Fig. 1.
Pedigree of the present familial hypercholesterolemia family. The proband is indicated by an arrow. Squares indicate males; circles, females; slashes, deceased individuals; shaded (black) symbols, individuals with homozygous familial hypercholesterolemia; half-shaded (black) symbols, individuals with heterozygous familial hypercholesterolemia. I-2 and I-4 are full sisters
Physical examination revealed multiple large nodular xanthomas on her limbs, waist, and buttocks, further corroborating her clinical presentation (Fig. 1B). Auscultation detected a diastolic murmur at the aortic valve area and a grade 2 soft systolic murmur at the left sternal border between the 3rd and 4th ribs, indicative of potential valvular involvement. Laboratory findings showed markedly elevated LDL-C levels of 13.2 mmol/l and total cholesterol (TC) levels of 15.7 mmol/l.
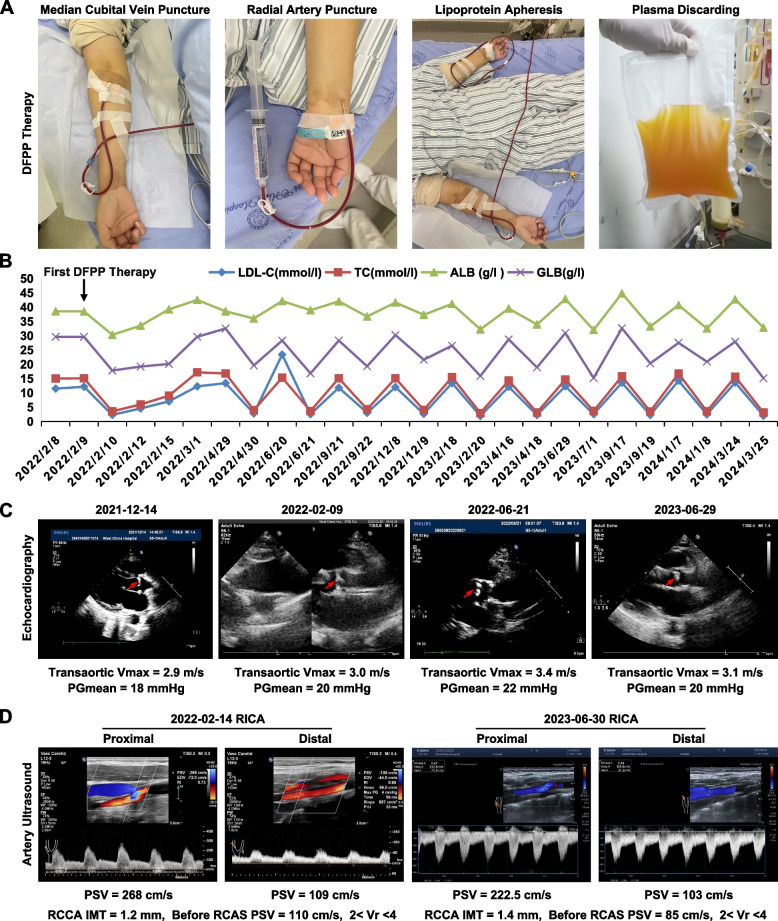
Following treatment initiation, the patient underwent once every 1–2 months LA via peripheral venous access (Fig. 2A). Double filtration plasmapheresis (DFPP) was performed using an OP-08W plasma separator with an EC-50W filter, blood flow 100–130 mL/min, sessions lasting 3–4 h. Over two years, 12 DFPP sessions were conducted, totaling 45 h. The patients tolerated the DFPP treatment well, with no serious adverse reactions observed. Occasional mild dizziness and fatigue were reported during the treatment process, but these did not require interruption of the treatment. The cost of each DFPP treatment session was approximately 7,000 RMB. Pre-DFPP, LDL-C and TC were 13.82 ± 3.28 and 15.45 ± 0.78 mmol/L, respectively. Post-DFPP, LDL-C and TC significantly decreased to 2.43 ± 0.33 and 3.59 ± 0.41 mmol/L with reductions of 81.76 ± 4.11% and 76.76 ± 2.75%. Lipoprotein (a) (Lp(a)) and triglycerides (TG) also significantly decreased by 89.10 ± 1.39% and 42.29 ± 15.68%. Pre-DFPP, albumin and globulin were 41.30 ± 1.92 and 29.36 ± 1.99 g/L. Post-DFPP, they decreased to 34.16 ± 2.67 and 18.31 ± 2.30 g/L (17.14 ± 7.11% and 37.54 ± 7.62% reductions),globulin levels showed a mild decrease (Fig. 2B).
Fig. 2.
The clinical follow-up of the patient. A The direct puncture technique, procedure, and disposal of waste plasma during DFPP therapy. B Changes in low-density lipoprotein cholesterol(LDL-C, mmol/l), total cholesterol(TC, mmol/l), albumin(ALB, g/l), and globulin(GLB, g/l) levels pre and post DFPP therapy. C Echocardiography manifestations: The times of four examinations (2021–12-14 outpatient, 2022–02-09 first DFPP, and two follow-ups on 2022–06-21/2023–06-29) showed irregular thickening and calcification protruding into the lumen at the sinotubular junction of the aortic root, causing localized stenosis. Transaortic maximum velocity (Vmax) and mean paravalvular gradient (PGmean) were recorded for assessment, as transaortic Vmax was 2.9 m/s, PGmean was 18 mmHg (2021–12-14); transaortic Vmax was 3.0 m/s, PGmean was 20 mmHg (2022–02-09); transaortic Vmax was 3.4 m/s, PGmean was 22 mmHg(2022–06-21); transaortic Vmax was 3.1 m/s, PGmean was 20 mmHg(2023–06-29), respectively. D Carotid artery ultrasound manifestations: The times of the two examinations (the first DFPP on 2022–02-14, and a follow-up on 2023–06-30). The results showed moderate to severe stenosis at the origin of the right internal carotid artery (RICA). The peak systolic velocity (PSV) was recorded at the origin and distal end of the RICA, as well as the intima-media thickness (IMT) of the right common carotid artery (RCCA), and the PSV and Velocity Ratio (Vr) changes before the right common carotid artery stenosis (RCAS). The values were: RICA proximal was PSV 268 cm/s, RICA distal PSV was 109 cm/s, RCCA IMT was 1.2 mm, before RCAS PSV was 110 cm/s, 2 < Vr < 4 (2022–02-14) and RICA proximal PSV was 222.5 cm/s, RICA distal PSV was 103 cm/s, RCCA IMT was 1.4 mm, before RCAS PSV was 85 cm/s, 2 < Vr < 4 (2023–06-30)
Simultaneously, Echocardiography at the same period: Calcification of the aortic sinus-ascending aorta junction with associated stenosis, internal diameter narrowed to about 14 mm. The aortic sinus was relatively narrow at about 25 mm. Accelerated antegrade flow at the aortic sinus-ascending aorta junction with the transaortic maximum velocity (Vmax, 3.0 m/s) and the mean transvalvular pressure gradient (PGmean, 20 mmHg). Bicuspid aortic valve thickened and calcified with associated regurgitation, annulus diameter of about 21 mm (Fig. 2C). Bilateral carotid artery ultrasound showed: Bilateral carotid artery intima-media thickening, with a thickness of about 1.2 mm on the right side and 1.2 mm on the left side. The intima-media thickness at the origin of the right subclavian artery was about 1.3 mm. The right internal carotid artery showed stenosis at the origin, with a weak echogenic plaque of 3 mm thickness and increased blood flow velocity, with a peak systolic velocity (PSV) of 268 cm/s. No stenosis was observed in the left carotid artery (Fig. 2D). During the hospitalization, coronary angiography revealed severe calcific ostial stenosis of approximately 95% in the left main coronary artery (LMCA). There was an 80% stenosis in the proximal left anterior descending artery (LAD) and a 70% stenosis in the mid-LAD segment. The right coronary artery (RCA) had a 70% stenosis in the mid-segment and a total occlusion in the distal segment. Consequently, two drug-eluting stents were sequentially implanted from the LMCA to the LAD to restore blood flow. Additionally, three drug-eluting stents were placed in the RCA to revascularize the affected segments and restore coronary perfusion.
During the follow-up period after initiating DFPP treatment, the patient's aortic stenosis and stenosis of the carotid arteries, right subclavian artery, and right internal carotid artery did not worsen significantly. Coronary angiography confirmed the patency of the previously implanted stents, with no evidence of in-stent restenosis.
Furthermore, we summarized the research studies on using LA to treat familial hypercholesterolemia (FH), with a particular emphasis on the pros and cons of LA (Table 1).
Table 1.
Summary of the research studies on using Lipoprotein Apheresis (LA) to treat Familial Hypercholesterolemia (FH)
| Author(s) | Year | Study Type | Sample Size | LA Modality | Treatment Duration | TC/LDL-C Reduction | Pros | Cons |
|---|---|---|---|---|---|---|---|---|
| Koga et al. [9] | 1991 | Case Series |
4HeFH 1HoFH |
DFPP LAPP |
2-4 week intervals for 15–62 months | TC 28–52% |
Effectively lowers TC Prevent atherosclerosis progression Improves patient symptoms |
Requires frequent and ongoing sessions, combined with other therapies |
| Koga [10] | 1991 | Case Report | 1 HeFH |
DFPP LAPP |
2-3Week intervals for 17 years | TC 36.5% |
Significant TC reduction, Improved cardiovascular outcomes Resolution of xanthomata |
Required long-term treatment |
| Suzuki et al. [11] | 1996 | Case Series | 5 HeFH |
DFPP LFPP LDL PA |
1–3 years |
LDL-C DFPP 81.4% LFPP 82% LDL PA 74.5% |
Effectively lowers LDL-C, TC, TG Good biocompatibility with less inflammation risk, |
Possible need for albumin supplementation, hypotension risk |
| Yamane et al. [12] | 1998 | Case Series | 4 FH | DFPP | Bi-weekly intervals for 3 years | LDL-C 60–80% |
Long-term LDL-C reduction Prevent atherosclerosis progression |
Required long-term treatment |
| Julius et al. [13] | 2002 | Prospective | 6 FeFH |
DFPP HELP |
Not mentioned |
LDL-C DFPP 61.4 HELP 61.3% |
Effectively lowers LDL-C Selective Removal, |
Reduction in HDL-C |
| Tsai et al. [14] | 2016 | Case Report | 1 HoFH | DFPP | Weekly intervals for 11 years | LDL-C 67 ± 6.1% |
Effectively lowers LDL-C Resolution of xanthomata Prevention of coronary artery disease |
Reduction in HDL-C Requires specialized equipment and technical expertise |
| Gokay et al. [15] | 2016 | Prospective | 10 HoFH |
CF DFPP + CF DFPP |
Bi-weekly intervals for 3–4 years |
LDL-C CF 62.35 ± 7.19% DFPP + CF 63.66 ± 6.63% DFPP 64.79 ± 8.29% |
Effective LDL-C Improved cardiovascular outcomes Resolution or reduction in size of xanthomas |
Reduction in HDL-C Adverse effects like hypotension and nausea Need for calcium and iron supplementation in some patients |
| Albayrak et al. [16] | 2019 | Retrospective | 5 HoFH | DFPP | Bi-weekly intervals for 2 years | LDL-C 69.8% |
Significant Reduction in Lipid Levels Improved cardiovascular outcomes Minimally Invasive |
Reduction in HDL-C Adverse effects like hypotension and nausea |
| Page et al. [17] | 2021 | Case Series |
8 HoFH 8 HeFH |
CF | Biweekly -8weekly intervals for 16 years |
LDL-C HoFH 69% HeFH 72% |
Significant reduction in LDL-C Levels LA well-tolerated Improved Quality of Life Reduced Cardiovascular Events |
High costs Invasive Variable Response to PCSK9i |
| Ozdemir et al. [18] | 2022 | Retrospective |
18 Non-FH 6 HoFH 3 HeFH |
DFPP DALI |
Not mentioned |
LDL-C Non-FH 70.9% HoFH 64.5% HeFH 64.5% |
Significant reduction in Lipid Levels Generally safe with minimal side effects Can be combined with pharmacotherapy for enhanced results |
High cost and limited availability Required long-term treatment |
| Xu et al. [19] | 2022 | Case report | 1 HoFH | DFPP | Bi-weekly intervals for 1 year | LDL-C 57% |
Significant reduction in Lipid Levels Safe and minimal side effects Suitable for therapy-resistant patients |
Inconvenient treatment frequency High cost Time-consuming procedure |
| Zhao et al. [20] | 2023 | Retrospective |
107 HeFH 8 HoFH |
DFPP | Single session | LDL-C 64.2% |
Significant reduction in LDL-C and Lp(a) levels High safety profile with manageable side effects Minimal impact on immunoglobulin levels, particularly IgG and IgA |
High costs Invasive |
HoFH Homozygous Familial Hypercholesterolemia, HeFH Heterozygous Familial Hypercholesterolemia, FH Familial Hypercholesterolemia, DFPP Double Filtration Plasmapheresis, LAPP LDL Adsorbent Plasmapheresis, CF Cascade filtration, DALI Direct Adsorption of Lipoproteins, TC Total Cholesterol, LDL-C Low-Density Lipoprotein Cholesterol, TG Triglycerides, Lp(a) Lipoproteins (a), Ig(G) Immunoglobulin G, Ig(A) Immunoglobulin A
Discussion
This case report highlights the efficacy of lipoprotein apheresis (LA) as an adjunctive therapy for refractory homozygous familial hypercholesterolemia (HoFH) unresponsive to maximal pharmacological intervention. Despite aggressive lipid-lowering therapy with high-intensity statins, ezetimibe, and PCSK9 inhibitors, our patient's LDL-C levels remained critically elevated, necessitating the implementation of LA. The dramatic reductions in LDL-C, total cholesterol, lipoprotein(a), and triglycerides achieved through double filtration plasmapheresis (DFPP) underscore its potential as a life-saving intervention for patients with severe HoFH.
Recent advances in lipid-lowering therapies, particularly PCSK9 inhibitors, have shown promising results in managing familial hypercholesterolemia. However, their efficacy in HoFH can be limited, especially in patients with null mutations in the LDLR gene [21]. A recent study by Raal et al. demonstrated that evolocumab reduced LDL-C levels by only 21.2% in HoFH patients with null/null mutations compared to 40.8% in those with defective/defective mutations [22]. This highlights the need for alternative therapies in cases of severe, refractory HoFH.
Lipoprotein apheresis has emerged as a crucial therapeutic option for such patients. Our findings align with recent studies demonstrating the significant lipid-lowering effects of LA. An observational study by Schettler et al. reported mean LDL-C reductions of 67.5% with LA techniques [23]. The 81.76% reduction in LDL-C observed in our patient exceeds these averages, possibly due to the use of DFPP, which has shown superior efficacy in some studies [24].
The long-term benefits of LA extend beyond immediate lipid reduction. Recent evidence suggests that regular LA can slow or halt the progression of atherosclerosis and reduce cardiovascular events. A 30-year follow-up study by Stefanutti et al. found that LA significantly reduced the progression of carotid intima-media thickness in FH patients compared to pharmacotherapy alone [25]. Our patient's stable coronary and carotid artery status after two years of LA treatment corroborates these findings. Moreover, the regression of xanthomas observed in our patient aligns with recent research on the extravascular effects of LA. A study by Empen et al. demonstrated significant regression of tendon xanthomas in FH patients undergoing long-term LA, correlating with improved cardiovascular outcomes [26].
Despite its clear benefits, LA is not without limitations. The need for frequent treatments, potential vascular access complications, and the transient nature of lipid reduction necessitates ongoing research into optimizing treatment protocols. Recent studies have explored extended intervals between LA sessions and combination therapies to enhance long-term efficacy [27].
Interestingly, this case patient did not experience the first clinical manifestation of ASCVD until her 30s without treatment, which is relatively late compared to typical HoFH patients. This relatively mild presentation may be related to protective factors or a resilient phenotype. Further research is needed to explore the protective factors that exist in HoFH patients.
Although the frequency of LA treatment in this study was relatively low, good clinical results were still achieved. However, it should be noted that using published equations and rate constants, it is not possible to accurately determine the time-averaged LDL-C concentration of patients during LA treatment intervals. The patient's LDL-C levels may be far above the ideal level for most of the time.
In addition to LA, liver transplantation(LT) is another potential therapeutic option for HoFH. Recent studies have demonstrated that LT can significantly reduce LDL-C levels in patients with HoFH and improve long-term prognosis [28]. However, this approach has inherent limitations, including surgical risks and the necessity for lifelong immunosuppression [29]. Novel drugs such as lomitapide and evinacumab have shown promising potential in the treatment of HoFH. Lomitapide lowers LDL-C by inhibiting microsomal triglyceride transfer protein (MTP), while evinacumab acts by inhibiting angiopoietin-like 3 (ANGPTL3) [30, 31]. These drugs provide new treatment alternatives for patients with refractory HoFH. Other emerging therapies, such as inclisiran (a small interfering RNA targeting PCSK9), also exhibit potential efficacy for refractory HoFH [32]. Nevertheless, the long-term efficacy and safety of these novel drugs in HoFH patients require further investigation. Until these new agents become widely available and proven effective in severe HoFH, LA remains a critical therapeutic option.
Conclusion
In conclusion, for patients with HoFH exhibiting refractoriness to PCSK9i and other lipid-lowering drugs, LA represents an established and indispensable therapeutic modality.Although the frequency of treatment in this case was lower than the standard recommendations, regular LA therapy still promoted the regression of xanthomas and significantly reduced the incidence of coronary events. However, further research is needed to determine the optimal frequency of LA, its long-term efficacy, and its combination with emerging drugs. Additionally, in-depth studies on the protective factors and phenotypic differences present in HoFH patients may provide new insights for the development of individualized treatment strategies.
Acknowledgements
Not applicable.
Authors’ contributions
MJG and HW wrote the main manuscript text, conducted the review of literature, and submitted the manuscript. FW, SCL, and LL revised the manuscript for intellectual content and provided data from outside laboratories. BW and LZ revised the manuscript for intellectual content and is the corresponding author and guarantor.
Funding
Not applicable.
Availability of data and materials
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
This patient and family has provided verbal informed consent for this publication.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Mingjing Guan and Hao Wang contributed equally to this work.
Contributor Information
Bo Wang, Email: kingwave@scu.edu.cn.
Ling Zhang, Email: zhangling_crrt@163.com.
References
- 1.Khera AV, Won HH, Peloso GM, et al. Diagnostic yield and clinical utility of sequencing familial hypercholesterolemia genes in patients with severe hypercholesterolemia. J Am Coll Cardiol. 2016;67:2578–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cuchel M, Bruckert E, Ginsberg HN, et al. Homozygous familial hypercholesterolaemia: new insights and guidance for clinicians to improve detection and clinical management. A position paper from the Consensus Panel on Familial Hypercholesterolaemia of the European Atherosclerosis Society. Eur Heart J. 2014;35:2146–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sprecher DL, Schaefer EJ, Kent KM, et al. Cardiovascular features of homozygous familial hypercholesterolemia: analysis of 16 patients. Am J Cardiol. 1984;54:20–30. [DOI] [PubMed] [Google Scholar]
- 4.Qamar A, et al. Interindividual variation in low-density lipoprotein cholesterol level reduction with evolocumab: An analysis of FOURIER trial data. JAMA Cardiol. 2019;4:59–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.O’Donoghue ML, Giugliano RP, Wiviott SD, et al. Long-term evolocumab in patients with established atherosclerotic cardiovascular disease. Circulation. 2022;146:1109. [DOI] [PubMed] [Google Scholar]
- 6.Räber L, Ueki Y, Otsuka T, et al. Effect of alirocumab added to high-intensity statin therapy on coronary atherosclerosis in patients with acute myocardial infarction: the PACMAN-AMI randomized clinical trial. JAMA. 2022;327(18):1771–81. [DOI] [PMC free article] [PubMed]
- 7.Safarova MS, Moriarty PM. Lipoprotein apheresis: current recommendations for treating familial hypercholesterolemia and elevated lipoprotein(a). Curr Atheroscler Rep. 2023;25(7):391–404. [DOI] [PubMed]
- 8.Thompson GR, HEART-UK LDL Apheresis Working Group. Recommendations for the use of LDL apheresis. Atherosclerosis. 2008;198:247–55. [DOI] [PubMed] [Google Scholar]
- 9.Koga N, et al. LDL-apheresis and improvement in the coronary atherosclerosis of familial hypercholesterolemia - correlation of computerized quantitative coronary angiography with autopsy findings. Artif Cells Blood Substit Immobil Biotechnol. 1991;19:37–52. [DOI] [PubMed] [Google Scholar]
- 10.Koga N. Pathological and angiographic regression of coronary atherosclerosis by LDL-apheresis in a patient with familial hypercholesterolemia. Atherosclerosis. 1991;90:245–51. [DOI] [PubMed] [Google Scholar]
- 11.Suzuki M, Yamane S, Matsugane T, et al. Evaluation of double filtration plasmapheresis, thermofiltration, and low-density lipoprotein adsorptive methods by crossover test in treatment of familial hypercholesterolemia patients. Artif Organs. 1996;20:407–12. [DOI] [PubMed] [Google Scholar]
- 12.Yamane S, Matsugane T, Motohashi K, et al. Double filtration plasmapheresis maintains normal adhesion molecule levels. Ther Apher Dial. 1998;2:260–5. [DOI] [PubMed] [Google Scholar]
- 13.Julius U, Metzler W, Pietzsch J, et al. Intraindividual comparison of two extracorporeal LDL apheresis methods: lipidfiltration and HELP. Int J Artif Organs. 2002;25:1180–8. [DOI] [PubMed] [Google Scholar]
- 14.Tsai JL, Chang WT, Chang TC, et al. Long-term follow-up of a homozygous familial hypercholesterolemic patient receiving regular double filtration plasmapheresis - case report and literature review. Blood Purif. 2016;41:264–71. [DOI] [PubMed] [Google Scholar]
- 15.Gokay S, Kendirci M, Kaynar L, et al. Long-term efficacy of lipoprotein apheresis in the management of familial hypercholesterolaemia: Application of two different apheresis techniques in childhood. Transfus Apher Sci. 2016;54:185–93. [DOI] [PubMed] [Google Scholar]
- 16.Albayrak Y, Ateş BA, Aylı M, et al. The efficacy of double filtration plasmapheresis in the treatment of homozygous familial hypercholesterolemia. Transfus Apher Sci. 2019;58:62–5. [DOI] [PubMed] [Google Scholar]
- 17.Page MM, Ekinci EI, Burnett JR, et al. Lipoprotein apheresis and PCSK9 inhibitors for severe familial hypercholesterolaemia: Experience from Australia and New Zealand. J Clin Apher. 2021;36(1):48–58. [DOI] [PubMed] [Google Scholar]
- 18.Özdemir N, Şahin İ, Yıldırım N, et al. Lipoprotein apheresis efficacy and challenges: single center experience. Hematol Transfus Cell Ther. 2022;44:42–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu F, Chu X, Hu Y, et al. Difficult journey to find the best treatment for homozygous familial hypercholesterolemia: Case report. Int Med Case Rep J. 2022;15:97–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mansoorian M, Kazemi K, Nikeghbalian S, et al. Liver transplantation as a definitive treatment for familial hypercholesterolemia: A series of 36 cases. Pediatr Transplant. 2015;19(6):605–11. [DOI] [PubMed]
- 21.Raal FJ, Honarpour N, Blom DJ, et al. Inhibition of PCSK9 with evolocumab in homozygous familial hypercholesterolaemia (TESLA Part B): a randomised, double-blind, placebo-controlled trial. Lancet. 2020;395(10224):435–45. [DOI] [PubMed]
- 22.Zhao L, Gao Y, Liu G, et al. Lipoprotein apheresis in patients with familial hypercholesterolemia: a single center research. Zhonghua Xin Xue Guan Bing Za Zhi. 2023;55:441–9. [DOI] [PubMed]
- 23.Schettler VJJ, Neumann CL, Peter C, et al. The German Lipoprotein Apheresis Registry (GLAR) - almost 5 years on. Clin Res Cardiol Suppl. 2017;12(Suppl 1):44–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stefanutti C, Julius U. Lipoprotein apheresis: state of the art and novelties. Atheroscler Suppl. 2019;40:1–9. [DOI] [PubMed]
- 25.Stefanutti C, Pang J, Di Giacomo S, et al. A cross-national investigation of cardiovascular survival in homozygous familial hypercholesterolemia: the Sino-Roman Study. J Clin Lipidol. 2019;13(4):608–17. [DOI] [PubMed] [Google Scholar]
- 26.Empen K, Otto C, Brodl UC, Parhofer KG. The effects of lipoprotein apheresis in patients with familial hypercholesterolemia: A systematic review and meta-analysis. J Clin Lipidol. 2018;12(2):474–83. [Google Scholar]
- 27.Moriarty PM, Parhofer KG, Babirak SP, et al. Alirocumab in patients with heterozygous familial hypercholesterolemia undergoing lipoprotein apheresis: the ODYSSEY ESCAPE trial. Eur Heart J. 2016;37(48):3588–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cuchel M, Hegele RA, Bruckert E, et al. 2023 Update on European Atherosclerosis Society Consensus Statement on Homozygous Familial Hypercholesterolaemia: new treatments and clinical guidance. Eur Heart J. 2023;44(22):1949–63. [DOI] [PMC free article] [PubMed]
- 29.Page MM, Alex G, Srinivasan S, et al. Long-term outcomes of liver transplantation for homozygous familial hypercholesterolaemia in Australia and New Zealand. Atherosclerosis. 2023:1–10. [DOI] [PubMed]
- 30.Cuchel M, Bloedon LT, Szapary PO, et al. Inhibition of microsomal triglyceride transfer protein in familial hypercholesterolemia. N Engl J Med. 2007;2:356. [DOI] [PubMed] [Google Scholar]
- 31.Rosenson RS, Burgess LJ, Ebenbichler CF, et al. Evinacumab in Patients with Refractory Hypercholesterolemia. N Engl J Med. 2020;383(24):2307–19. [DOI] [PubMed] [Google Scholar]
- 32.Ray KK, Wright RS, Kallend D, et al. Two Phase 3 Trials of Inclisiran in Patients with Elevated LDL Cholesterol. N Engl J Med. 2020;382(16):1507–19. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
No datasets were generated or analysed during the current study.