Abstract
Background
Advanced cancer patients commonly suffer from a fatigue-sleep disturbance symptom cluster. Baduanjin is considered a promising mind-body exercise for relieving the fatigue-sleep disturbance symptom cluster. However, few studies have investigated a tailored Baduanjin for advanced cancer patients. The proposed study will create an optimized Baduanjin exercise program to adapt to advanced cancer patients and evaluate the effect of a Simple Sitting Baduanjin (SSBDJ) mind-body exercise on the fatigue-sleep disturbance symptom cluster among advanced cancer patients.
Methods
The study will be a prospective, assessor-blinded, two-arm, randomized controlled trial, involving a 12-week intervention and 4-week follow-up. A total of 108 advanced cancer patients experiencing the fatigue-sleep disturbance symptom cluster will be recruited from two tertiary general hospitals in China. Participants will be randomized to an experimental group (n = 54) or a control group (n = 54). The experimental group will receive a 12-week SSBDJ intervention plus the usual care, and the control group will receive only the usual care. Outcomes including fatigue-sleep disturbance symptom cluster, fatigue, sleep disturbance, and quality of life will be measured before the intervention, at the 4th, 8th, and 12th weeks of the intervention, and 4 weeks after the intervention. The intention-to-treat principle and a generalized estimating equation will be used to analyze data.
Discussion
This study may produce a new Baduanjin exercise prescription that is user-friendly, simple to execute, more targeted, and adaptable. If proven effective, this approach may be integrated into routine cancer care to manage the fatigue-sleep disturbance symptom cluster and improve QOL in advanced cancer patients.
Trial registration
Chinese Clinical Trial Registry, ChiCTR-2,300,072,331. Registered on 9 June 2023.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12906-024-04652-6.
Keywords: Advanced cancer, Baduanjin, Sleep, Fatigue, Quality of life, Symptom cluster
Background
Cancer is a life-threatening disease. According to the latest estimates of the global cancer burden, China accounts for 24.2% and 26.5% of cancer incidence and mortality worldwide, respectively [1]. Moreover, 52.8% of Chinese patients with lung cancer, gastric cancer, esophageal cancer, colorectal cancer, and breast cancer are diagnosed at an advanced stage [2]. The prevalence of fatigue is up to 80.0% and that of sleep disturbance is 70.8% in advanced cancer patients [3, 4]. They have significant physiological and behavioral associations in advanced cancer patients, forming the fatigue-sleep disturbance symptom cluster, which is one of the most commonly reported symptoms of patients with advanced cancer [5–9]. It negatively affects the patient’s psychological, physical, and social life, leading to poor quality of life (QOL) [10].
Exercise therapy is energy-saving, cost-effective, and easy to implement. There is considerable evidence of the benefits of exercise in advanced cancer patients, with reductions in CRF and improvements in sleep quality and QOL [11, 12]. However, previous studies also have revealed that most patients cannot tolerate universal exercise prescriptions due to treatment-related side effects, compromised mobility, and debility [13, 14]. It is therefore important to make tailored adjustments to exercise prescriptions based on a patient’s conditions [14, 15]. Unlike those physical exercises focused on stretching, strengthening, contracting, and relaxing muscles and joints, Baduanjin (BDJ) Qigong is a mind-body exercise characterized by slow and fluid movements, mental focus, and controlled breathing [16, 17]. It is especially suitable for those who are frail or not inclined towards endurance or vigorous activity. Traditional BDJ consists of eight movements aiming at enhancing inner energy and blood flow, unblocking meridians, regulating internal organs and somatic systems, calming the mind, and relaxing the body [18, 19]. A recent meta-analysis has indicated that BDJ has positive effects on the management of symptoms, such as fatigue and sleep disturbance in cancer patients [20]. However, BDJ is typically performed in a standing position, which may not be suitable for frail patients with advanced cancer.
Sitting Baduanjin (SBDJ) is a redesigned mode of the traditional BDJ, which can be practiced in a chair or bed. It has been used in frail patients, including patients with disability, patients receiving mechanical ventilation, and postoperative patients with myocardial infarction [21–24]. Previous studies have indicated that SBDJ is effective and free of adverse effects, but its application in cancer patients has not yet been investigated. Given the physical capabilities of patients with advanced cancer, it is worth developing a tailored SBDJ to address their fatigue-sleep disturbance symptom cluster [25]. Therefore, based on the traditional Chinese medicine pathogenesis of CRF with sleep disturbance and the principle of Chinese medicinal Qigong theory, our research team previously developed a four-movement Simple Sitting Baduanjin (SSBDJ) program for advanced cancer patients. In this proposed study, we will test the effectiveness of SSBDJ for the fatigue-sleep disturbance symptom cluster, fatigue, sleep disturbance, and QOL in advanced cancer patients. We hypothesize that advanced cancer patients engaging in SSBDJ will have significant reductions in the fatigue-sleep disturbance symptom cluster, fatigue, sleep disturbance, and an improvement in QOL.
Methods/design
Study design
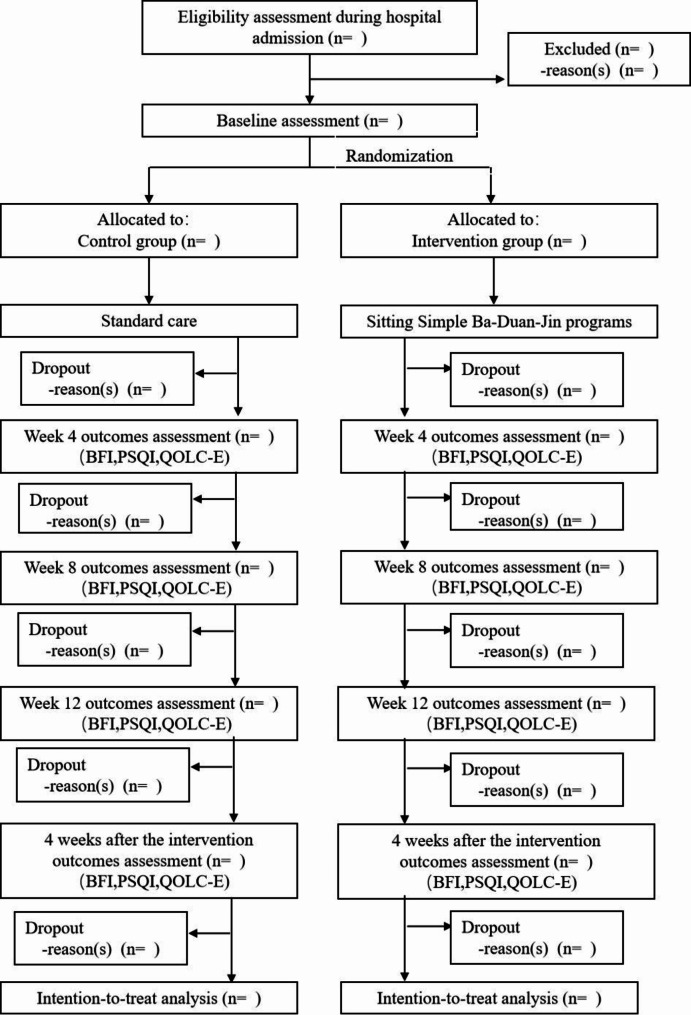
The study is a prospective, assessor-blinded, two-arm randomized controlled trial following the guidelines of the Standard Protocol Items: Recommendations for Intervention Trials (SPIRIT) statement [26]. The participants will be randomly allocated to either the SSBDJ group or the control group. The study period will last for 16 weeks, with a 12-week SSBDJ intervention and a 4-week follow-up. This study will follow the Consolidated Standards of Reporting Trials (CONSORT) flow chart [27] (Fig. 1).
Fig. 1.
CONSORT flow chart for the study
Study setting
The study will be implemented at two general hospitals affiliated with a Fujian medical university, in Southeast of China.
Sample size calculation
The sample size was calculated via the formula  for comparison of two sample means, where N1 and N2 are the sample sizes of the experimental group and the control group respectively, α = 0.05, β = 0.10,
for comparison of two sample means, where N1 and N2 are the sample sizes of the experimental group and the control group respectively, α = 0.05, β = 0.10, = 1.96, and
= 1.96, and 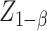 = 1.28. Referring to Lu et al.’s study [28], using CRF, as an outcome,
= 1.28. Referring to Lu et al.’s study [28], using CRF, as an outcome, 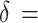 1.4, σ1 = 2.1, and σ2 = 1.9, 43 subjects per group were calculated. Considering a 20% dropping-out rate, 54 subjects per group were needed. Using sleep quality as an outcome,
1.4, σ1 = 2.1, and σ2 = 1.9, 43 subjects per group were calculated. Considering a 20% dropping-out rate, 54 subjects per group were needed. Using sleep quality as an outcome, 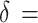 2.8, σ1 = 1.1, and σ2 = 2.0, it was determined that 7 subjects were needed in each group. Considering the 20% dropping-out rate, 9 subjects per group were needed. Accordingly, at least 108 subjects will be required with 54 in the control group and 54 in the SSBDJ group.
2.8, σ1 = 1.1, and σ2 = 2.0, it was determined that 7 subjects were needed in each group. Considering the 20% dropping-out rate, 9 subjects per group were needed. Accordingly, at least 108 subjects will be required with 54 in the control group and 54 in the SSBDJ group.
Inclusion and exclusion criteria
Adult patients (aged ≥ 18 years) will be recruited for the study if they are (1) diagnosed with advanced cancer (stages IIIB and IV); (2) experiencing both fatigue and sleep disturbance (the score of the 0–10 numerical rating scales was more than one for each symptom over the previous one month); (3) having Karnofsky Performance Scale scores ≥ 60; and (4) able to communicate in Mandarin Chinese.
The exclusion criteria for the study are (1) prescribed psychostimulants, antidepressant medications, or hypnotics for the treatment of fatigue and sleep disturbance symptoms; (2) having contraindications to exercise, including unstable vital signs, acute infection, spondylolisthesis, epilepsy, severe cardiovascular disease, lower extremity venous thrombosis, or acute episodes of disc herniation; (3) suffering from mental illness or confusion; or (4) having regular exercise habits.
Recruitment
An oncological physician from the study setting will refer potential patients to the research assistant. She/he will further identify the potential patients and whether they meet the inclusive criteria but do not breach any exclusion criteria. Once the eligible patients agree to participate in this study, they will be invited to complete a written informed consent form. They will be informed that they have the right to withdraw from the study at any time without facing any negative consequences.
Randomization and allocation concealment
The study will adhere to a rigorous process of randomization. Before randomization, an impartial individual who is not involved with subject recruitment or data collection will prepare two randomization lists via the Research Randomizer website (https://www.randomizer.org), consisting of a unique set of numbers for each of the two participating hospitals. Each sequence will contain 54 numbers; 1 to 54. The numbers will then be carefully placed inside sequentially coded, sealed, opaque envelopes. These envelopes will be entrusted to the custodian of the random numbers, who is responsible for their safekeeping. Once the subjects have been included in the study, the researchers will contact the random numbers custodian, who has played no part in the grouping process. The custodian will then systematically open the envelopes in the order prescribed by their respective envelope numbers, unveiling the allocated groupings. If the random numbers inside the envelopes fall within the range of 1 to 27, the subjects will be assigned to the control group. If the random numbers correspond to the range of 28 to 54, the subjects will be assigned to the experimental group.
Blinding
Due to the visible nature of the SSBDJ intervention, blinding the study intervention facilitator (i.e., the first author) and participants will be impossible. Thus, blinding will only be applied to the outcome assessor (i.e., a research assistant) in this RCT, to avoid potential detection bias during data collection. Two research assistants will be responsible for data collection and will not be involved in the subject recruitment process.
SSBDJ group
The SSBDJ consists of four movements [18, 19]: Two Hands Held Up to Heaven, Wise Owl Gazes Backwards, Sway the Head and Shake the Spine, and Two Hands Hold the Feet. In this study, the first author will act as the SSBDJ instructor. She is a registered nurse and a certified ontological exercise instructor. First, the patient’s exercise risk will be assessed using the Medical Assessment Form before Exercise for Patients with Malignant Tumors and Physical Activity Assessment Form, as recommended by the American College of Sports Medicine [12]. The facilitator will then train the eligible patients to practice SSBDJ using a PPT presentation and on-site demonstration during their stay in the hospital. With the hospital as the starting point of the intervention, the researchers will lead the patients through their practice during their hospitalization, and self-practice at home after discharge.
The four SSBDJ movements are linked together with smooth transitions from one movement to the next. Participants will be required to repeat each movement six times before transitioning to the next. Participants will practice SSBDJ in a stepwise manner [18, 19], for 12 weeks of 15-minute to 20-minute sessions (Table 1). In the first 2 weeks, SSBDJ will be practiced three times a week and the patients will mainly learn the SSBDJ posture. In the next 2 weeks, the frequency of the intervention will be four times per week, and the patients will begin to learn special breathing coordination associated with SSBDJ. From the 5th to 12th weeks the frequency of the intervention will be five times per week, and the participants will further learn mind regulation. Each session time will be appropriately adjusted according to the patient’s target heart rate for termination of exercise (60% of maximum heart rate, maximum heart rate = 207 − 0.7 x age) [10].
Table 1.
SSBDJ schedule
| Session | Practice contents | Frequency |
|---|---|---|
| First and second weeks | Body regulation stage: Practicing SSBDJ movements. | 3 times a week |
| Third and fourth weeks | Respiratory regulation stage: Based on the previous stage of skilled movement, special SSBDJ breathing regulation is added. | 4 times a week |
| Fifth to twelfth weeks | Mind regulation stage: Based on the first two stages of body regulation and breathing regulation, mind regulation is added to achieve the unity of three modulations. | 5 times a week |
It is suggested that SSBDJ will be performed 1 h before or after meals in any indoor or outdoor environment with fresh air [18, 19]. Exercise safety strategies will include: (1) informing participants of exercise precautions, assessing their health status, and screening for exercise risks before SSBDJ; (2) monitoring heart rate and any adverse events, which will be recorded in a self-reported adverse event checklist; (3) getting feedback on the participant’s perceptions of doing SSBDJ; and (4) formulating a plan to deal with the possibility of an adverse event. Intervention fidelity will be determined via scrutiny of the Intervention Fidelity Plan for SSBDJ with regard to design, training, and enactment (Table 2) [29].
Table 2.
Intervention fidelity plan for SSBDJ
| Fidelity focus | Recommendation |
|---|---|
| Design |
SSBDJ intervention development is based on the theory that the content validity of SSBDJ interventions needs to be evaluated by experts in Qigong practice or research. The SSBDJ protocol is created to ensure the intervention and the dose are delivered as specified. Assessors/evaluators will be blinded from the SSBDJ intervention. |
| Training |
SSBDJ educational booklet, SSBDJ movement video, and instructors’ feedback are used to facilitate participants’ comprehension. Instructors take field notes (including any observed problems regarding intervention delivery and adverse events) during sessions, and report to the principal investigator. Direct observation of sessions will be conducted to document participants’ performance of SSBDJ exercise in class and to assess if the active components (body movements, respiratory and mind regulation) are delivered. |
| Enactment |
Participants will be required to complete daily exercise logs to track the frequency of home practice and self-reported feelings after practice. Informal weekly discussions via WeChat or telephone will be performed with participants to understand their home practices, including the ability, challenges, or barriers to practicing breathing exercises, body movements, and meditation. Instructors will have regular monthly meetings with the principle investigator to discuss intervention sessions and any observed problems, to prevent intervention drift. |
Control group
Patients in both the intervention and control groups will be provided with the standard care offered by the study hospitals, including personal and medical attention, health education, and emotional assistance. Participants will be required to abstain from engaging in any other traditional Chinese sports exercises throughout the study, with regular reminders at all assessment intervals. For ethical considerations, after we identified the effectiveness of the SSBDJ, we will provide the SSBDJ free of charge for the control group, if the participants would like to receive it.
Outcome measurements
Demographic and clinical characteristics of the participants
A self-designed demographic and clinical data form will be used to collect participants’ sociodemographic data (age, educational background, employment status, marital status, and household income) and medical history (date of diagnosis, disease diagnosis, metastasis, and treatment) at the baseline.
Primary outcome
Symptom cluster
Fatigue. CRF will be measured using the Brief Fatigue Inventory (BFI). The BFI has nine items, with higher scores corresponding to more severe fatigue [30]. As recommended by Mendoza et al. [30], no (0-<1), mild (1-<4), moderate (4-<7), and severe (7–10) fatigue were defined based on the global fatigue score. The reported Cronbach’s alpha of the Chinese version has ranged from 0.90 to 0.92 [31].
Sleep disturbance. Sleep disturbance will be assessed using the Pittsburgh Sleep Quality Index (PSQI). The questionnaire has seven domains; sleep latency, habitual sleep efficiency, subjective sleep quality, sleep duration, use of sleeping medication, sleep disturbance, and daytime dysfunction [32]. Higher total scores indicate poorer sleep quality, global scores of 0–5, 6–10, and 11–21 were considered to indicate no, some, and severe sleep disturbance, respectively. The Chinese version of the PSQI has been demonstrated to be a reliable and valid scale and is used widely in patients with cancer [33].
The occurrence of the fatigue-disturbance symptom cluster in the present study was defined as the presence of two symptoms concurrently. The fatigue-disturbance symptom cluster will be assessed using the total score of fatigue and sleep disturbance measured by BFI and PSQI.
Secondary outcomes
QOL
The QOL concerns referenced in the End-of-Life Questionnaire (QOLC-E) will be used to evaluate patients’ QOL. The measure was developed by Pang et al. [34], based on a Chinese ethnographic study. It is rated on a 4-point scale ranging from 1 (least desirable) to 4 (most desirable), with higher scores indicating greater satisfaction. Its Cronbach’s α coefficient was measured at 0.87.
Practice-related information
This includes (1) adherence rate, which is the number of SSBDJ sessions conducted divided by the total number of sessions required; (2) participants’ feedback on and satisfaction with the intervention, as determined using a self-designed feedback form; and (3) records of adverse events associated with SSBDJ, which will be obtained from the exercise logs.
Data collection
A trained research assistant who will be blinded to group assignment will conduct all data collection. Instructions will be attached to the homepage of the questionnaire, including the purpose of the research and anonymity and confidentiality requirements. Unified instructions will be used to explain the questionnaire completion method. The principle of diminishing returns and reversibility of exercise training will be applied, which states that an assessment period during the intervention and a follow-up period will assist in determining the expected time course for improvement of outcomes, and what level of exercise must be sustained to produce long-term benefits [35]. Therefore, outcome data will be measured pre-intervention (T0), 4 weeks (T1), 8 weeks (T2), and 12 weeks (T3) after the commencement of SSBDJ, and 4 weeks after completion of the SSBDJ program (T4). This will be accomplished using the BFI, PSQI, and QOLC-E. Practice-related information will be collected throughout the study period.
Data analysis
All data will be analyzed with SPSS software (version 26.0, IBM, Armonk, NY, USA), and statistical testing will be performed at a two-tailed alpha level of 0.05. Participants’ demographic and clinical characteristics will be analyzed using descriptive statistics. Both primary and secondary outcomes will be reported for baseline and follow-up, using standard summary statistics such as means, medians, percentages, and effect sizes. Generalized estimating equations (GEEs) will be used to determine the group, time, and interaction effects on the outcome variables. Type I error will be controlled using the Bonferroni method, which will be adjusted to p < 0.005 (i.e., 0.05 divided by the number of tests, five outcome evaluations will be performed, so the number of tests is 10) [36]. This adjustment aims to ensure the validity and accuracy of the findings for multiple comparisons. An intention-to-treat strategy will be used to analyze the data. Missing data will be imputed with the use of multiple imputations because it is reasonably intuitive, flexible, and could also be used to handle more missing data scenarios [37].
Ethics and dissemination
The study will adhere to ethical standards throughout, and all procedures will be conducted following relevant guidelines and regulations. Ethical approval has been obtained from the Biological and Medical Research Ethics Committee of Fujian University (IRB Ref No. 2023/97), and was registered on the Chinese Clinical Trial Registry Platform (ChiCTR-2300072331). Written informed consent will be obtained from all participants. During participant recruitment, the intervention facilitator will visit potential participants and explain the study’s purpose, procedures, benefits, and potential risks, and the participant’s right to withdraw from the study at any point without negative consequences. Patients will have the opportunity to discuss relevant issues before signing the consent form. The data collected will be stored centrally and kept confidential and anonymous. Data analysis will be conducted by our research team. The data will be used exclusively for this research. Dissemination strategies may include manuscript submission to a peer-reviewed journal and a conference submission.
Discussion
Advanced cancer patients often suffer from the fatigue-sleep disturbance symptom cluster [5]. However, traditional BDJ fails to relieve the symptom cluster of advanced cancer patients due to their limited physical capacity [38]. Therefore, SSBDJ has been developed for advanced cancer patients by our research team, and this study will aim to confirm its effects on the fatigue-sleep disturbance symptom cluster.
The effectiveness of SSBDJ may be attributed to its characteristics. First, SSBDJ is a theory-based intervention tailored to advanced cancer patients with the fatigue-sleep disturbance symptom cluster [12, 18, 19]. Second, it is easily accessible to advanced cancer patients. SSBDJ can be practiced in a sitting position, which matches their physical performance abilities and meets their exercise needs. Third, SSBDJ is a mind-body exercise focused on meditation and deep breathing, which may reduce inflammation, increase melatonin, and affect stress response pathways, thereby reducing the fatigue-sleep disturbance symptom cluster and improving QOL in advanced cancer patients [39, 40]. Fourth, SSBDJ videos and manuals were designed to improve patients exercise compliance, which can also elevate patients’ experiences and outcomes producing a greater effect [41]. The daily exercise logs also will be employed in this study, which contributes to tracking home practice and documenting patients’ self-reported feelings about participating in SSBDJ. Additionally, weekly discussions via WeChat or telephone will be conducted to enhance the exercise self-efficacy [29].
The proposed study has some potential limitations. First, the program may not be suitable for those advanced cancer patients whose KPS scores are less than 40, which means the illness is worse quickly and their physical capability is very limited. Second, due to the visible nature of the SSBDJ intervention, the blinding of participants and the SSBDJ instructor cannot be accomplished, which may increase the risk of detection bias during the study’s implementation, though outcome assessors will be blinded to intervention allocation. Third, patients will be recruited from general hospitals only, and the findings may not be generalizable to advanced cancer patients in all settings. Future studies with a more rigorous design, with a multi-center, and transregional setting, will be necessary in the future.
If the SSBDJ is proven to be effective, it could be integrated into routine cancer care to manage the fatigue-sleep disturbance symptom cluster and improve QOL in advanced cancer patients. In addition, this study could provide new optimization methods and ideas for the clinical dilemma of advanced cancer patients who are unable to tolerate evidence-based exercise prescriptions.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Not applied.
Abbreviations
- QOL
Quality of life
- CRF
Cancer-related fatigue
- BDJ
Baduanjin
- SBDJ
Sitting Baduanjin
- SSBDJ
Simple Sitting Baduanjin
- KPS
Karnofsky Performance Scale
- BFI
Brief Fatigue Inventory
- PSQI
Pittsburgh Sleep Quality Index
- QOLC-E
The QOL concerns referenced in the End-of-Life Questionnaire
Author contributions
Conceptualization: ZHN. LXY. DTJ. JZJW. XHM; Funding acquisition: XHM; Methodology: ZHN. LXY. DTJ. ZJW. XHM; Project administration: XHM; Supervision: ZJW. XHM. Writing-original draft: ZHN. ZJW; Writing-review & editing: ZHN. ZJW. XHM.
Funding
This study was funded by the Fujian Science and Technology Innovation Joint Fund Project (Grant no. 2021Y9025). The funding sources played no role in the design of the study.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
The study will adhere to ethical standards throughout, and all procedures will be conducted following relevant guidelines and regulations. Ethical approval has been obtained from the Biological and Medical Research Ethics Committee of Fujian University (IRB Ref No. 2023/97), and was registered on the Chinese Clinical Trial Registry Platform (ChiCTR-2300072331). Written informed consent will be obtained from all participants.
Consent for publication
Not applicable.
Trial status
Recruiting participants for the research.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Han B, Zheng R, Zeng H, Wang S, Sun K, Chen R, et al. Cancer incidence and mortality in China, 2022. J Natl Cancer Cent. 2024. 10.1016/j.jncc.2024.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zeng H, Ran X, An L, Zheng R, Zhang S, Ji JS, et al. Disparities in stage at diagnosis for five common cancers in China: a multicentre, hospital-based, observational study. Lancet Public Health. 2021;6:e877–87. 10.1016/S2468-2667(21)00157-2. [DOI] [PubMed] [Google Scholar]
- 3.Chapman EJ, Martino ED, Edwards Z, Black K, Maddocks M, Bennett MI. Practice review: evidence-based and effective management of fatigue in patients with advanced cancer. Palliat Med. 2022;36:7–14. 10.1177/02692163211046754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jakobsen G, Gjeilo KH, Hjermstad MJ, Klepstad P. An update on prevalence, assessment, and risk factors for sleep disturbances in patients with advanced cancer-implications for health care providers and clinical research. Cancers (Basel). 2022;14:3933. 10.3390/cancers14163933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miller AH, Ancoli-Israel S, Bower JE, Capuron L, Irwin MR. Neuroendocrine-immune mechanisms of behavioral comorbidities in patients with cancer. J Clin Oncol. 2008;26:971–82. 10.1200/JCO.2007.10.7805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dun L, Xian-Yi W, Si-Ting H, Xin-yuan Y. Effects of sleep interventions on cancer-related fatigue and quality of life in cancer patients: a systematic review and meta-analysis. Support Care Cancer. 2022;30:3043–55. 10.1007/s00520-021-06563-5. [DOI] [PubMed] [Google Scholar]
- 7.Liu L, Mills PJ, Rissling M, Fiorentino L, Natarajan L, Dimsdale JE, et al. Fatigue and sleep quality are associated with changes in inflammatory markers in breast cancer patients undergoing chemotherapy. Brain Behav Immun. 2012;26:706–13. 10.1016/j.bbi.2012.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu IHC, Balachandran DD, Faiz SA, Bashoura L, Escalante CP, Manzullo EF. Characteristics of cancer-related fatigue and concomitant sleep disturbance in cancer patients. J Pain Symptom Manage. 2022;63:e1–8. 10.1016/j.jpainsymman.2021.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Charalambous A, Berger AM, Matthews E, Balachandran DD, Papastavrou E, Palesh O. Cancer-related fatigue and sleep deficiency in cancer care continuum: concepts, assessment, clusters, and management. Support Care Cancer. 2019;27:2747–53. 10.1007/s00520-019-04746-9. [DOI] [PubMed] [Google Scholar]
- 10.Simão D, Barata PC, Alves M, Papoila AL, Oliveira S, Lawlor P. Symptom clusters in patients with Advanced Cancer: a prospective longitudinal cohort study to Examine their Stability and Prognostic Significance[J]. Oncologist. 2024;29(1):e152–63. 10.1093/oncolo/oyad211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Network NC. version2;. NCCN Clinical Practice Guidelines in Oncology Cancer-Related Fatigue [Cited 2022 February 09]. [Internet]. https://www.nccn.org/ (2022).
- 12.American College of Sports Medicine. ACSM’ guidelines for exercise testing and prescription. 11th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2022. [Google Scholar]
- 13.Heywood R, McCarthy AL, Skinner TL. Safety and feasibility of exercise interventions in patients with advanced cancer: a systematic review. Support Care Cancer. 2017;25:3031–50. 10.1007/s00520-017-3827-0. [DOI] [PubMed] [Google Scholar]
- 14.Cancer Nutrition Committee. China anticancer association, state key laboratory for market regulation of cancer special medical foods, oncology palliative care committee, Beijing Cancer society. Chinese expert consensus on exercise therapy for cancer patients. Electron J Metab Nutr Cancer. 2022;9:298–311. 10.16689/j.cnki.cn11-9349/r.2022.03.006. [Google Scholar]
- 15.Winters-Stone KM, Medysky ME, Savin MA, Winters-Stone KM, Medysky ME, savin MA. Patient-reported and objectively measured physical function in older breast cancer survivors and cancer-free controls. J Geriatr Oncol. 2019;10:311–6. 10.1016/j.jgo.2018.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yuen M, Ouyang HX, Miller T, Pang MYC. Baduanjin qigong improves balance, leg strength, and mobility in individuals with chronic stroke: a randomized controlled study. Neurorehabil Neural Repair. 2021;35:444–56. 10.1177/15459683211005020. [DOI] [PubMed] [Google Scholar]
- 17.Fang J, Zhang L, Wu F, Ye J, Cai S, Lian X. The safety of Baduanjin exercise: a systematic review. Evid Based Complement Alternat Med. 2021;2021:8867098. 10.1155/2021/8867098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu T, Zhang W. Qigong of traditional Chinese medicine. 10th ed. Beijing, China Traditional Chinese Medicine; 2016.
- 19.Health qigong management center of general administration of sport of China. Health qigong Baduanjin, Beijing; 2018.
- 20.Kuo CC, Wang CC, Chang WL, Liao TC, Chen PE, Tung TH. Clinical effects of Baduanjin qigong exercise on cancer patients: a systematic review and meta-analysis on randomized controlled trials. Evid Based Complement Alternat Med. 2021;2021:6651238. 10.1155/2021/6651238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen G, Hong C, Lin S, Lin P, Tang R, Cai J. A pilot study of the effects of sitting Baduanjin on daily blood pressure fluctuations in the disabled residents of nursing home. Taipei J TCM. 2021;24(3):29–42. 10.6516/TJTCM.202109_24(3).0002. [Google Scholar]
- 22.Chen M. Clinical study of sitting Baduanjin on early rehabilitation of septic patients with mechanical ventilation. Dissertation, Guangzhou: Guangzhou University of Traditional Chinese Medicine; 2019.
- 23.Zhang X, Chen M, Kong L, Lai H, Wang J, Huang H. Nursing care of sitting Baduanjin exercise for 43 patients with acute myocardial infarction after PCI. J Nurs(China). 2019;26:63–5. 10.16460/j.issn1008-9969.2019.19.063. [Google Scholar]
- 24.Bao X, Qiu QX, Shao YJ, Quiben M, Liu H. Effect of sitting ba-duan-jin exercises on balance and quality of life among older adults: a preliminary study. Rehabil Nurs. 2020;45:271–8. 10.1097/rnj.0000000000000219. [DOI] [PubMed] [Google Scholar]
- 25.Zhou S, Kou S, Zhou M, Ma J, Cai J. Effect of Baduanjin four-style combined with emotional nursing on quality of life in patients with gastrointestinal tumors after chemotherapy. Chongqing Med. 2022;51:806–9. 10.3969/i.issn.1671-8348.2022.05.019. [Google Scholar]
- 26.Butcher NJ, Monsour A, Mew EJ, Chan AW, Moher D, Mayo-Wilson E, et al. Guidelines for reporting outcomes in trial protocols: the SPIRIT-outcomes 2022 extension. JAMA. 2022;328:2345–56. 10.1001/jama.2022.21243. [DOI] [PubMed] [Google Scholar]
- 27.Schulz KF, Altman DG, Moher D, Group CONSORT. CONSORT 2010 statement: updated guidelines for reporting parallel group randomized trials. Ann Intern Med. 2010;152:726–32. 10.7326/0003-4819-152-11-201006010-00232. [DOI] [PubMed] [Google Scholar]
- 28.Lu Y, Qu HQ, Chen FY, Li XT, Cai L, Chen S, et al. Effect of Baduanjin qigong exercise on cancer-related fatigue in patients with colorectal cancer undergoing chemotherapy: a randomized controlled trial. Oncol Res Treat. 2019;42:431–9. 10.1159/000501127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chang PS, Chao AM, Jang M, Lu YYF. Intervention fidelity in qigong randomized controlled trials: a method review. Geriatr Nurs. 2019;40:84–90. 10.1016/j.gerinurse.2018.07.001. [DOI] [PubMed] [Google Scholar]
- 30.Mendoza TR, Wang XS, Cleeland CS, Morrissey M, Johnson BA, Wendt JK, et al. The rapid assessment of fatigue severity in cancer patients: use of the brief fatigue inventory. Cancer. 1999;85:1186–96. 10.1002/(sici)1097-0142(19990301)85:5%3C1186::aid-cncr24%3E3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 31.Wang XS, Hao XS, Wang Y, Guo H, Jiang YQ, Mendoza TR, et al. Validation study of the Chinese version of the brief fatigue inventory (BFI-C). J Pain Symptom Manage. 2004;27:322–32. .008 • PubMed: 15050660 • Google Scholar. [DOI] [PubMed] [Google Scholar]
- 32.Buysse DJ, Reynolds CF 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh sleep quality index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 33.Liu X, Tang M, Hu L, Wang A, Wu H, Zhao G, et al. Reliability and validity of the Pittsburgh sleep quality index. Chin J Psychiatry. 1996;02:103–7. [Google Scholar]
- 34.Pang SM, Chan KS, Chung BP, Lau KS, Leung EM, Leung AW, et al. Assessing quality of life of patients with advanced chronic obstructive pulmonary disease in the end of life. J Palliat Care. 2005;21:180–7. 10.1177/082585970502100311. [PubMed] [Google Scholar]
- 35.Winters-Stone KM, Neil SE, Campbell KL. Attention to principles of exercise training: a review of exercise studies for survivors of cancers other than breast. Br J Sports Med. 2014;48:987–95. 10.1136/bjsports-2012-091732. [DOI] [PubMed] [Google Scholar]
- 36.ICH harmonised tripartite guideline. Statistical principles for clinical trials. International conference on Harmonisation E9 Expert Working Group Statistics in medicine, 18; 1999. pp. 1905–1942. [PubMed]
- 37.Grobler AC, Lee K. Intention-to-treat analyses for randomized controlled trials in hospice/palliative care enhanced by principled methods to handle missing data. J Pain Symptom Manage. 2020;60:e28–9. 10.1016/j.jpainsymman.2020.06.038. [DOI] [PubMed] [Google Scholar]
- 38.Yao LQ, Tan JY, Turner C, Wang T. Development and validation of a Tai Chi intervention protocol for managing the fatigue-sleep disturbance-depression symptom cluster in female breast cancer patients. Complement Ther Med. 2021;56:102634. 10.1016/j.ctim.2020.102634. [DOI] [PubMed] [Google Scholar]
- 39.Osypiuk K, Ligibel J, Giobbie-Hurder A, Vergara-Diaz G, Bonato P, Quinn R, et al. Qigong mind-body exercise as a biopsychosocial therapy for persistent post-surgical pain in breast cancer: a pilot study. Integr Cancer Ther. 2020;19(1475601408):1534735419893766. 10.1177/1534735419893766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lei J, Yang J, Dong L, Xu J, Chen J, Hou X, et al. An exercise prescription for patients with lung cancer improves the quality of life, depression, and anxiety. Front Public Health. 2022;10:1050471. 10.3389/fpubh.2022.1050471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ong JW, Ong QO, Metsävainio T, Vaajoki A, Tian JL, He HG. The effectiveness of mind-body therapies for women with Gynecological Cancer: a systematic review and Meta-analysis. Cancer Nurs Published Online April. 2023;24. 10.1097/NCC.0000000000001231. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No datasets were generated or analysed during the current study.