Abstract
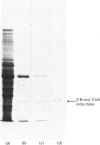
Mitochondrial 2-enoyl-CoA reductase from bovine liver was purified and characterized. A simple three-step purification was developed, involving ion-exchange chromatography to separate the bulk of the NADPH-dependent 2,4-dienoyl-CoA reductase, followed by chromatography on Blue Sepharose and adenosine 2',5'-bisphosphate-Sepharose. Homogeneous enzyme with a subunit Mr of 35 500 is obtained in 35% yield. The Mr of the native enzyme, determined by three different methods, yielded values that suggest that the enzyme is dimeric. NADPH is required as cofactor, and cannot be replaced by NADH. The activity of the purified enzyme towards 2-trans-double bonds in 2-monoene and 2,4-diene structures was investigated. 2-Enoyl-CoA reductase reduced the double bonds in a series of 2-trans-monoenoyl-CoA esters with different chain lengths, but did not exhibit significant activity towards 2-trans-double bonds of 2,4-dienoyl-CoA esters. This result is discussed in the light of analogous observations with enoyl-CoA hydratase.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews P. The gel-filtration behaviour of proteins related to their molecular weights over a wide range. Biochem J. 1965 Sep;96(3):595–606. doi: 10.1042/bj0960595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloch K. Fatty acid synthases from Mycobacterium phlei. Methods Enzymol. 1975;35:84–90. doi: 10.1016/0076-6879(75)35141-0. [DOI] [PubMed] [Google Scholar]
- Bloch K., Vance D. Control mechanisms in the synthesis of saturated fatty acids. Annu Rev Biochem. 1977;46:263–298. doi: 10.1146/annurev.bi.46.070177.001403. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brosnan J. T., Kopec B., Fritz I. B. The localization of carnitine palmitoyltransferase on the inner membrane of bovine liver mitochondria. J Biol Chem. 1973 Jun 10;248(11):4075–4082. [PubMed] [Google Scholar]
- Cuebas D., Schulz H. Evidence for a modified pathway of linoleate degradation. Metabolism of 2,4-decadienoyl coenzyme A. J Biol Chem. 1982 Dec 10;257(23):14140–14144. [PubMed] [Google Scholar]
- Dahlen J. V., Porter J. W. Studies on the synthesis of fatty acids by a beef heart mitochondrial enzyme system. Arch Biochem Biophys. 1968 Sep 20;127(1):207–223. doi: 10.1016/0003-9861(68)90218-x. [DOI] [PubMed] [Google Scholar]
- Dommes P., Dommes V., Kunau W. H. beta-Oxidation in Candida tropicalis. Partial purification and biological function of an inducible 2,4-dienoyl coenzyme A reductase. J Biol Chem. 1983 Sep 25;258(18):10846–10852. [PubMed] [Google Scholar]
- Dommes V., Kunau W. H. 2,4-Dienoyl coenzyme A reductases from bovine liver and Escherichia coli. Comparison of properties. J Biol Chem. 1984 Feb 10;259(3):1781–1788. [PubMed] [Google Scholar]
- Dommes V., Luster W., Cvetanović M., Kunau W. H. Purification by affinity chromatography of 2,4-dienoyl-CoA reductases from bovine liver and Escherichia coli. Eur J Biochem. 1982 Jul;125(2):335–341. doi: 10.1111/j.1432-1033.1982.tb06688.x. [DOI] [PubMed] [Google Scholar]
- Fox J. L., Lynen F. Characterization of the flavoenzyme enoyl reductase of fatty acid synthetase from yeast. Eur J Biochem. 1980 Aug;109(2):417–424. doi: 10.1111/j.1432-1033.1980.tb04810.x. [DOI] [PubMed] [Google Scholar]
- GOLDMAN P., VAGELOS P. R. The specificity of triglyceride synthesis from diglycerides in chicken adipose tissue. J Biol Chem. 1961 Oct;236:2620–2623. [PubMed] [Google Scholar]
- Hiltunen J. K., Davis E. J. Metabolism of pent-4-enoate in rat heart. Reduction of the double bond. Biochem J. 1981 Feb 15;194(2):427–432. doi: 10.1042/bj1940427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinsch W., Klages C., Seubert W. On the mechanism of malonyl-CoA-independent fatty-acid synthesis. Different properties of the mitochondrial chain elongation and enoylCoA reductase in various tissues. Eur J Biochem. 1976 Apr 15;64(1):45–55. doi: 10.1111/j.1432-1033.1976.tb10273.x. [DOI] [PubMed] [Google Scholar]
- Hinsch W., Seubert W. On the mechanism of malonyl-CoA-independent fatty-acid synthesis. Characterization of the mitochondrial chain-elongating system of rat liver and pig-kidney cortex. Eur J Biochem. 1975 May 6;53(2):437–447. doi: 10.1111/j.1432-1033.1975.tb04084.x. [DOI] [PubMed] [Google Scholar]
- Ishidate K., Mizugaki M., Uchiyama M. Biohydrogenation accompanying the beta-oxidation of unsaturated fatty acids by Candida. J Biochem. 1973 Aug;74(2):279–283. [PubMed] [Google Scholar]
- Ishidate K., Mizugaki M., Uchiyama M. Induction of NADPH enoyl-coA reductase in Candida grown in the presence of unsaturated fatty acids. J Biochem. 1974 Nov;76(5):1139–1142. [PubMed] [Google Scholar]
- Kunau W. H., Dommes P. Degradation of unsaturated fatty acids. Identification of intermediates in the degradation of cis-4-decenoly-CoA by extracts of beef-liver mitochondria. Eur J Biochem. 1978 Nov 15;91(2):533–544. doi: 10.1111/j.1432-1033.1978.tb12707.x. [DOI] [PubMed] [Google Scholar]
- Kunau W. H., Dommes V., Dommes P. Degradation of unsaturated fatty acids. 4-Enoyl-CoA reductase: purification, characterization and physiological function. Prog Lipid Res. 1981;20:327–330. doi: 10.1016/0163-7827(81)90065-5. [DOI] [PubMed] [Google Scholar]
- LaBelle E. F., Jr, Hajra A. K. Enzymatic reduction of alkyl and acyl derivatives of dihydroxyacetone phosphate by reduced pyridine nucleotides. J Biol Chem. 1972 Sep 25;247(18):5825–5834. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- MARTIN R. G., AMES B. N. A method for determining the sedimentation behavior of enzymes: application to protein mixtures. J Biol Chem. 1961 May;236:1372–1379. [PubMed] [Google Scholar]
- Mizugaki M., Nishimaki T., Shiraishi T., Yamanaka H. Studies on the metabolism of unsaturated fatty acids. VII. Separation and general properties of reduced nicotinamide adenine dinucleotide phosphate-dependent trans-2-enoyl-coenzyme A reductase from Escherichia coli K-12. Chem Pharm Bull (Tokyo) 1982 Jul;30(7):2503–2511. doi: 10.1248/cpb.30.2503. [DOI] [PubMed] [Google Scholar]
- Mizugaki M., Nishimaki T., Yamamoto H., Nishimura S., Sagi M., Yamanaka H. Studies on the metabolism of unsaturated fatty acids. VIII. Induction of 2,4-dienoyl-CoA reductase in Escherichia coli on the addition of unsaturated fatty acids. J Biochem. 1982 Apr;91(4):1453–1456. doi: 10.1093/oxfordjournals.jbchem.a133836. [DOI] [PubMed] [Google Scholar]
- Mizugaki M., Nishimaki T., Yamamoto H., Sagi M., Yamanaka H. Studies on the metabolism of unsaturated fatty acids. XI. Alterations in the activities of enoyl-CoA hydratase, 3-hydroxyacyl-CoA epimerase and 2,4-dienyl-CoA reductase in rat liver mitochondria and peroxisomes by clofibrate. J Biochem. 1982 Dec;92(6):2051–2054. doi: 10.1093/oxfordjournals.jbchem.a134140. [DOI] [PubMed] [Google Scholar]
- Mizugaki M., Uchiyama M. Possible role of NADPH-dependent enoyl coenzyme A reductase in -oxidation of unsaturated fatty acids. Biochem Biophys Res Commun. 1973 Jan 4;50(1):48–53. doi: 10.1016/0006-291x(73)91061-9. [DOI] [PubMed] [Google Scholar]
- Mizugaki M., Unuma T., Yamanaka H. Studies on the metabolism of unsaturated fatty acids. II. Separation and general properties of reduced nicotinamide adenine dinucleotide phosphate dependent cis-2-enoyl-coenzyme A reductase from Escherichia coli K-12. Chem Pharm Bull (Tokyo) 1979 Oct;27(10):2334–2337. doi: 10.1248/cpb.27.2334. [DOI] [PubMed] [Google Scholar]
- Municio A. M., Odriozola J. M., Pineiro A., Ribera A. In vitro elongation and desaturation of fatty acids during development of insects. Biochim Biophys Acta. 1972 Oct 5;280(2):248–257. doi: 10.1016/0005-2760(72)90091-4. [DOI] [PubMed] [Google Scholar]
- Nugteren D. H. The enzymic chain elongation of fatty acids by rat-liver microsomes. Biochim Biophys Acta. 1965 Oct 4;106(2):280–290. doi: 10.1016/0005-2760(65)90036-6. [DOI] [PubMed] [Google Scholar]
- PASTORE E. J., FRIEDKIN M. The chromatographic separation and recovery of reduced and oxidized pyridine nucleotides. J Biol Chem. 1961 Aug;236:2314–2316. [PubMed] [Google Scholar]
- Podack E. R., Seubert W. On the mechanism of malonyl-CoA independent fatty acid synthesis. II. Isolation, properties and subcellular location of trans-2,3-hexenoyl-CoA and trans-2,3-decenoyl-CoA reductase. Biochim Biophys Acta. 1972 Oct 5;280(2):235–247. [PubMed] [Google Scholar]
- Prasad M. R., Nagi M. N., Cook L., Cinti D. L. Kinetic evidence for two separate trans-2-enoyl CoA reductases in rat hepatic microsomes: NADPH-specific short chain- and NAD(P)H-dependent long chain-reductase. Biochem Biophys Res Commun. 1983 Jun 15;113(2):659–665. doi: 10.1016/0006-291x(83)91777-1. [DOI] [PubMed] [Google Scholar]
- Pullman M. E. A convenient and versatile method for the purification of CoA thiol esters. Anal Biochem. 1973 Jul;54(1):188–198. doi: 10.1016/0003-2697(73)90262-5. [DOI] [PubMed] [Google Scholar]
- Rüchel R., Gross J. Preparations of continuous gradient gel slabs: a simple technique. Anal Biochem. 1979 Jan 1;92(1):91–98. doi: 10.1016/0003-2697(79)90629-8. [DOI] [PubMed] [Google Scholar]
- STOFFEL W. DER STOFFWECHSEL DER UNGESAETTIGTEN FETTSAEUREN. I. ZUR BIOSYNTHESE HOCHUNGESAETTIGTER FETTSAEUREN. Hoppe Seylers Z Physiol Chem. 1963;333:71–88. doi: 10.1515/bchm2.1963.333.1.71. [DOI] [PubMed] [Google Scholar]
- Sedlmaier H., Tischer W., Rauschenbach P., Simon H. On the mechanism of 2-enoate reductase. Elimination of halogen hydracids from 3-halogeno-2-enoates during reduction with NADH. FEBS Lett. 1979 Apr 1;100(1):129–132. doi: 10.1016/0014-5793(79)81147-3. [DOI] [PubMed] [Google Scholar]
- Seubert W., Lamberts I., Kramer R., Ohly B. On the mechanism of malonyl-CoA-independent fatty acid synthesis. I. The mechanism of elongation of long-chain fatty acids by acetyl-CoA. Biochim Biophys Acta. 1968 Dec 18;164(3):498–517. doi: 10.1016/0005-2760(68)90180-x. [DOI] [PubMed] [Google Scholar]
- Seubert W., Podack E. R. Mechanisms and physiological roles of fatty acid chain elongation in microsomes and mitochondria. Mol Cell Biochem. 1973 May 11;1(1):29–40. doi: 10.1007/BF01659936. [DOI] [PubMed] [Google Scholar]
- Shimakata T., Fujita Y., Kusaka T. Acetyl-CoA-dependent elongation of fatty acids in Mycobacterium smegmatis. J Biochem. 1977 Sep;82(3):725–732. doi: 10.1093/oxfordjournals.jbchem.a131749. [DOI] [PubMed] [Google Scholar]
- Shimakata T., Fujita Y., Kusaka T. Involvement of one of two enoyl-CoA hydratases and enoyl-CoA reductase in the acetyl-CoA-dependent elongation of medium chain fatty acids by Mycobacterium smegmatis. J Biochem. 1980 Oct;88(4):1051–1058. doi: 10.1093/oxfordjournals.jbchem.a133056. [DOI] [PubMed] [Google Scholar]
- Shimakata T., Kusaka T. Purification and characterization of 2-enoyl-CoA reductase of Mycobacterium smegmatis. J Biochem. 1981 Apr;89(4):1075–1080. [PubMed] [Google Scholar]
- Stoffel W., Caesar H., Ditzer R. Der Stoffwechsel der ungesättigten Fettsäuren. IV. Zur beta-Oxydation der Mono- und Polyenfettsäuren Chemische Synthesen von Intermediärprodukten. Hoppe Seylers Z Physiol Chem. 1964;339(1):182–193. [PubMed] [Google Scholar]
- Tischer W., Bader J., Simon H. Purification and some properties of a hitherto-unknown enzyme reducing the carbon-carbon double bond of alpha, beta-unsaturated carboxylate anions. Eur J Biochem. 1979 Jun;97(1):103–112. doi: 10.1111/j.1432-1033.1979.tb13090.x. [DOI] [PubMed] [Google Scholar]
- Vance W. A., Stumpf P. K. Fat metabolism in higher plants. The elongation of saturated and unsaturated acyl-CoAs by a stromal system from isolated spinach chloroplasts. Arch Biochem Biophys. 1978 Sep;190(1):210–220. doi: 10.1016/0003-9861(78)90270-9. [DOI] [PubMed] [Google Scholar]
- Wakil S. J., Stoops J. K., Joshi V. C. Fatty acid synthesis and its regulation. Annu Rev Biochem. 1983;52:537–579. doi: 10.1146/annurev.bi.52.070183.002541. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Wit-Peeters E. M. Synthesis of long-chain fatty acids in mitochondria. Biochim Biophys Acta. 1969 Apr 29;176(3):453–462. doi: 10.1016/0005-2760(69)90212-4. [DOI] [PubMed] [Google Scholar]