ABSTRACT
Ocular syphilis is a serious complication of Treponema pallidum infection that can occur at any stage of syphilis and affect any eye structure. It remains unknown if certain T. pallidum strains are associated with ocular infections; therefore, we performed genotyping and whole genome sequencing (WGS) to characterize strains from patients with ocular syphilis. Seventy-five ocular or non-ocular specimens from 55 ocular syphilis patients in 14 states within the United States were collected between February 2016 and November 2020. Sufficient T. pallidum DNA was available from nine patients for genotyping and three for WGS. Genotyping was done using the augmented Centers for Disease Control and Prevention typing scheme, and WGS was performed on Illumina platforms. Multilocus sequence typing allelic profiles were predicted from whole genome sequence data. T. pallidum DNA was detected in various specimens from 17 (30.9%) of the 55 patients, and typing was done on samples from 9 patients. Four complete strain types (14d10/g, 14b9/g, 14d9/g, and 14e9/f) and five partial types were identified. WGS was successful on samples from three patients and all three strains belonged to the SS14 clade of T. pallidum. Our data reveal that multiple strain types are associated with ocular manifestations of syphilis. While genotyping and WGS were challenging due to low amounts of T. pallidum DNA in specimens, we successfully performed WGS on cerebrospinal fluid, vitreous fluid, and whole blood.
IMPORTANCE
Syphilis is caused by the spirochete Treponema pallidum. Total syphilis rates have increased significantly over the past two decades in the United States, and the disease remains a public health concern. In addition, ocular syphilis cases has also been on the rise, coinciding with the overall increase in syphilis rates. We conducted a molecular investigation utilizing traditional genotyping and whole genome sequencing over a 5-year period to ascertain if specific T. pallidum strains are associated with ocular syphilis. Genotyping and phylogenetic analysis show that multiple T. pallidum strain types are associated with ocular syphilis in the United States.
KEYWORDS: ocular syphilis, Treponema pallidum subsp. pallidum, PCR, typing, multilocus sequence typing, United States
INTRODUCTION
Syphilis, a sexually transmitted disease, caused by the bacterium Treponema pallidum subsp. pallidum (hereafter referred to as T. pallidum), can progress through varied clinical manifestations and have serious outcomes if untreated. There has been a resurgence of syphilis in the United States (U.S.) over the past decade. Surveillance data collected by the Centers for Disease Control and Prevention (CDC) show that the primary and secondary syphilis (P&S) rate increased from 5.5 cases per 100,000 population in 2013 to 17.7 cases per 100,000 population in 2022 (1). Men who have sex with men (MSM) are disproportionately affected by syphilis, accounting for 45.1% of all male P&S-reported cases in the U.S. in 2022.
Since 2014, there have been various reports of ocular syphilis (OS) in the U.S. that have been documented in other studies (2, 3). OS surveillance data reported by 13 states show an increase in prevalence estimates of syphilis patients presenting with ocular manifestations from 1% (369 cases) in 2019 to 1.4% (640) in 2021 with higher rates among persons with secondary syphilis and syphilis of unknown or late duration and among persons reporting injection drug use (4). OS is an inflammatory eye condition that can affect any eye structure and can occur during any stage of syphilis (5, 6). Uveitis is the most common manifestation with resultant vision loss and blindness reported in some cases (6, 7). However, other clinical manifestations of OS can be relatively non-specific. There has been increased clinical recognition of OS after 12 cases were reported at or around the same time period involving two clusters in Seattle and San Francisco in 2015 (8). More recently, a cluster of OS cases involving five women and a common male sex partner was reported in south west Michigan, suggesting that a common T. pallidum strain might have been associated with increased risk for OS manifestations (9). This is the first reported OS cluster among heterosexuals with epidemiologic linkage.
Molecular typing of ocular and non-ocular specimens from 14 OS cases in the U.S. using the enhanced CDC typing (ECDCT) scheme identified 5 T. pallidum strain types (8d/g, 14d/g, 13_/g, _ _/c, and _ _/f), suggesting a prevalent oculotropic strain was not involved (5). A recently published study that genotyped non-ocular specimens from six OS patients in Massachusetts using ECDCT revealed partial genotypes and two possible strain types (f and g) based on sequencing of the tp0548 gene (10).
Predominant strain types identified worldwide with ECDCT are 14d/f, 14d/g, and 14f/f (11–16). To improve strain discrimination, a fourth typing target based on a variable guanine mononucleotide repeat (MNR) in the ribosomal protein S1 gene (rpsA, tp0279) has been added to ECDCT and the new typing method is referred to as the augmented CDC typing method (ACDCT) (17). A multilocus sequence typing (MLST) method for T. pallidum, based on three genes targets (tp0136, tp0548, and tp0705), was described in 2018 and the common allelic profiles identified with this typing method include 1.1.1, 1.1.2, 1.1.8, 1.3.1, and 9.7.3 (18–22).
Penicillin is the treatment of choice for syphilis and failure of non-treponemal titers to decrease fourfold within 12 months after therapy might be suggestive of treatment failure in some patients; however, clinical evidence of penicillin resistance in T. pallidum has not been reported to date (23–26). Since macrolide treatment failure for syphilis was first reported in San Francisco in 2004, there has been an increasing prevalence of T. pallidum strains harboring either the A2058G or A2059G 23S rRNA mutation associated with azithromycin resistance in many countries (27–29). Although the CDC treatment guidelines do not recommend macrolides for treatment of syphilis, periodic surveillance for macrolide and other resistance markers is necessary considering the benzathine penicillin shortage in the U.S. and use of alternative treatment regimens such as doxycycline and tetracycline (24). Strain type 14d/f has been shown to be associated with neurosyphilis and heterosexuals and macrolide resistance (11, 30). In addition, 14d/f and 14d/g strain types were associated with the A2058G mutation in South Africa (31). MLST allelic profiles 1.3.1 and 1.26.1 have been shown to be associated with the A2058G mutation, 1.1.3 with A2059G, and 1.1.8 with macrolide susceptible strains (22, 32, 33).
Recent advances in next-generation sequencing methods have enabled whole genome sequencing (WGS) of T. pallidum directly from clinical specimens, and global phylogenetic analysis reveals that T. pallidum strains belong to either a SS14-like lineage or a Nichols-like lineage with most strains being SS14 like (34, 35). However, metagenomic sequencing remains challenging, particularly in specimens with low T. pallidum bacterial loads. We recently developed a DNA enrichment method, based on selective whole genome amplification (SWGA), that enables WGS of T. pallidum from lesion swabs with low genomic DNA (36).
The aim of this project was to perform genotyping and WGS of T. pallidum to characterize strains associated with OS. We also screened WGS data for mutations associated with macrolide resistance and non-synonymous single nucleotide polymorphisms (SNPs) in three penicillin binding protein (PBP) genes, pbp1(TPANIC_0500), pbp2 (TPANIC_0760), mrcA (TPANIC_0705), and tp47 (TPANIC_0574), a putative β-lactamase gene that could potentially be associated with penicillin resistance (26, 35). In addition, since doxycycline is being used as an alternative therapy for syphilis, we screened for mutations in the 16S RNA gene, which are known to confer resistance to doxycycline and tetracycline in other bacteria (37–39).
MATERIALS AND METHODS
Study population and specimen collection
Clinical specimens from a convenience sampling of OS patients were collected between February 2016 and November 2020. An OS case was defined as a person with clinical symptoms or signs consistent with ocular disease with syphilis of any stage. Pre-antibiotic treatment remnant ocular or non-ocular specimens, collected as part of routine clinical care, including cerebrospinal fluid (CSF), whole blood in ethylenediamine tetra-acetic acid tubes, serum, plasma, vitreous fluid, and a pharyngeal swab [collected for Chlamydia (CT)/Gonorrhea (GC) nucleic acid amplification test (NAAT)], were submitted by medical or ophthalmologic providers through state or local public health laboratories in 14 states (Fig. 1). Specimens were deidentified and frozen after routine diagnostic testing but were not collected and handled using a standardized protocol due to convenience sampling. Specimens were shipped on dry ice to the STD Laboratory Reference & Research Branch at CDC for testing. Participating sites also collected and submitted deidentified data on patients.
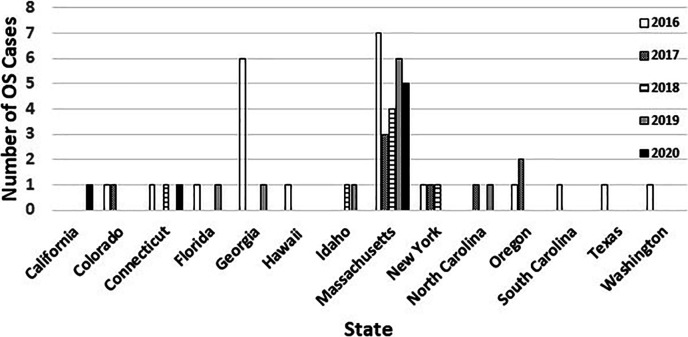
Fig 1.
Distribution of ocular syphilis patients by state and year (n = 55).
PCR detection of T. pallidum
DNA was initially extracted from a 200-µL or 400-µL sample using the QIAamp DNA Mini Kit (Qiagen, Valencia, CA) according to the manufacturer’s instructions and eluted in a final volume of 80 µL. Samples with volumes exceeding 1.2 mL were re-extracted using the QIAamp DNA Blood Midi Kit (Qiagen) and the DNAs eluted in 100 µL of AE Buffer to concentrate DNA for genotyping and WGS. T. pallidum-specific DNA was amplified using a real-time PCR targeting the DNA polymerase I gene (polA) as previously described (36). The sensitivity of T. pallidum PCR was assessed using CSF since it was a well-represented sample type.
Molecular typing and WGS
Strain typing was performed using the ACDCT scheme, which includes four gene targets [determining the number of 60-bp repeats within the arp gene; PCR/restriction fragment length polymorphism analysis of the tpr subfamily II genes (tprE, G, and J); sequencing of an 84-bp variable region within tp0548; and determining the number of MNRs within the rpsA (tp0279) gene as previously described (17, 40)].
WGS was attempted on strains that were either fully or partially typed by ACDCT depending on the availability of DNA. T. pallidum genomic DNA was enriched by SWGA with custom oligonucleotides followed by sequencing on an Illumina NovaSeq 6000 SP and/or MiSeq v2 500 cycle platforms as previously described (36). All bioinformatics analysis including the processing of the post-enrichment raw sequencing reads, removal of the host genome and classification and extraction of T. pallidum sequencing reads, de novo contig assembly, and phylogenetic analysis were performed as previously described (36). MLST allelic profiles of the tp0136, tp0548, and tp0705 genes were predicted by comparing the corresponding gene sequences extracted from the contig assemblies to the PubMLST reference database (last accessed April 2023) and also by Short Read Sequence Typing (SRST2; http://katholt.github.io/srst2/) using T. pallidum short-read sequences as input (41).
23S rRNA point mutations (A2058G, A2059G) associated with macrolide resistance and non-synonymous variants in pbp1, pbp2, mrcA, and tp47 genes were inferred from genomic sequencing data. Mutations at positions 965-967 (AGA965→967TTC) and 1058 (G1058C) in Helicobacter pylori and Propionibacterium spp. 16S rRNA genes, respectively, have been shown to confer genetic resistance to tetracyclines (39). The cognate nucleotides at these positions in T. pallidum are TGA and G, respectively. While resistance to doxycycline or tetracycline has not been reported in T. pallidum, we screened for the above mutations using genomic data. Within-host genetic differences across more than one body site were assessed using inStrain (42).
Statistical methods
Statistical analyses were done using the SPSS (SPSS Inc., Chicago, IL) and R 4.2.2 (R Core Team, 2022). The relative rates were calculated with CSF as reference, and Fisher’s exact test was performed to test if PCR-positive rates were different across different specimen types.
RESULTS
Patient demographic, laboratory, and clinical characteristics
Fifty-five patients diagnosed with OS in 14 states between February 2016 and November 2020 were included in the study (Fig. 1). Twenty-five of the 55 (45.4%) patients were from Massachusetts. Demographic, epidemiologic, and clinical characteristics of patients are shown in Table 1. The majority of cases 48/55 (87.3%) were men with 38.2% identifying as MSM. Twenty-three patients (41.8%) had HIV infection, and 17 (30.9%) patients had secondary syphilis followed by 10 (18.2%) with late latent (>1-year duration) stage. The majority of patients (56.4%) presented with uveitis and some patients presented with multiple symptoms, including blurry vision, vision loss in one or both eyes, and eye pain. Selected demographic characteristics, clinical characteristics, and laboratory results of 17 OS patients with PCR-, genotype-, or WGS-derived data are shown in Table 2.
TABLE 1.
Demographic, epidemiologic, and clinical characteristics of patients with ocular syphilis included in this study (n = 55)a
| Characteristic | Patient (%) |
|---|---|
| Sex | |
| Male | 48 (87.3) |
| Female | 7 (12.7) |
| Age, years | |
| 20 to 29 | 5 (9.1) |
| 30 to 39 | 7 (12.7) |
| 40 to 49 | 12 (21.8) |
| 50 to 59 | 21 (38.2) |
| ≥60 | 7 (12.7) |
| Unknown | 3 (5.5) |
| Sexual orientation | |
| MSM | 21 (38.2) |
| MSM/W | 3 (5.5) |
| MSW | 13 (23.6) |
| WSM | 7 (12.7) |
| Unknown | 11 (20) |
| Race | |
| White | 41 (74.5) |
| Black | 3 (5.5) |
| Asian | 1 (1.8) |
| Unknown | 10 (18.2) |
| HIV positive | |
| MSM | 13 (23.6) |
| MSM/W | 1 (1.8) |
| MSW | 1 (1.8) |
| Unknown | 8 (14.5) |
| HIV negative | |
| MSM | 7 (12.7) |
| MSM/W | 2 (3.6) |
| MSW | 12 (21.8) |
| WSM | 6 (10.9) |
| Unknown | 3 (3.6) |
| Stage of syphilis | |
| Primary | 5 (9.1) |
| Secondary | 17 (30.9) |
| Early latent (<1 year) | 4 (7.3) |
| Late latent (>1 year) | 10 (18.2) |
| Unknown | 19 (34.5) |
| Ocular findingsb | |
| Uveitis | 31 (56.4) |
| Anterior uveitis | 5 (9.1) |
| Posterior uveitis | 6 (10.9) |
| Panuveitis | 17 (30.9) |
| Not specified | 3 (5.5) |
| Chorioretinitis | 7 (12.7) |
| Optic neuritis | 7 (12.7) |
| Vitritis | 3 (5.5) |
| Unknown | 7 (12.7) |
Abbreviations: MSM, men who have sex with men; MSM/W, men who have sex with men and women; MSW, men who have sex with women; WSM, women who have sex with men.
Some patients presented with multiple ocular symptoms.
TABLE 2.
Demographic characteristics, clinical characteristics, and laboratory results of ocular syphilis patients with T. pallidum PCR, genotyping, and WGS data (n = 17)a
| Specimen ID | Location (state) | Sex | Sexual orientation | HIV status | Stage of syphilis | RPR titer | CSF-VDRL titer |
Sample | polA PCR | polA Ct |
23S rRNA mutation | ACDCT strain type | MLST allelic profile | T. pallidum clade | Diagnosis |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OS-1 | Texas | Male | MSM/W | Pos | Unknown | R | 1:2 | CSF | Pos | 44.8 | NA | 14b9/g | NA | NA | Posterior uveitis, blurry vision |
| OS-2 | Georgia | Male | MSM | Pos | Late latent | 1:512 | 1:4 | CSF | Pos | 43.3 | NA | NA | NA | NA | Panuveitis, partial vision loss |
| OS-3 | Hawaii | Male | MSW | Neg | Secondary | 1:256 | R | CSF,blood,plasma,pharyngeal swab | Neg Neg Neg Pos |
NA NA NA 32.4 |
NA NA NA NA |
NA NA NA _b _9/_ |
NA NA NA NA |
NA NA NA NA |
Chorioretinitis, optic neuritis, blurry vision |
| OS-4 | New York | Male | Unknown | Pos | Unknown | 1:512 | R | CSF,blood,serum | Pos Pos Neg |
43.4 36.9 NA |
NA NA NA |
14_10/g 14d10/g NA |
NA X3X NA |
NA SS14 NA |
Posterior uveitis |
| OS-5 | Massachusetts | Male | MSM | Neg | Secondary | Unknown | 1:4 | CSF | Pos | 38 | NA | _ _ _/f | NA | NA | Retinal hypopigmentation |
| OS-6 | Washington | Male | MSM | Neg | Secondary | 1:2,048 | 1:2 | CSF | Pos | 33.5 | NA | 14_ _/_ | NA | NA | Blurry vision |
| OS-8 | Massachusetts | Male | MSM | Pos | Secondary | 1:128 | Unknown | CSFBlood | Pos Neg |
35.7 NA |
NA NA |
NA NA |
NA NA |
NA NA |
Posterior uveitis, blurry vision |
| OS-9 | Massachusetts | Male | MSM | Pos | Secondary | 1:256 | Unknown | CSF | Pos | 37.2 | NA | NA | NA | NA | Panuveitis, partial vision loss, blurry vision |
| OS-10 | North Carolina | Male | MSM | Unknown | Unknown | 1:128 | 1:8 | CSF | Pos | 37.5 | NA | NA | NA | NA | Panuveitis, blurry vision |
| OS-11 | New York | Male | Unknown | Pos | Secondary | 1:4 | R | Vitreous fluid | Pos | 33.9 | NA | NA | NA | NA | Panuveitis, optic neuritis, chorioretinitis |
| OS-12 | Massachusetts | Female | WSM | Neg | Secondary | 1:2 | R | CSF,vitreous fluid | Pos Pos |
31.1 24.7 |
NA A2058G |
14e9/f 14e9/f |
X.1.1 19.1.1 |
SS14 | Panuveitis |
| OS-13 | Massachusetts | Male | MSW | Neg | Secondary | 1:128 | NR | CSF, blood | Pos Pos |
38.3 41.3 |
NA NA |
NA 14d_/_ |
NA NA |
NA NA |
Panuveitis |
| OS-14 | Idaho | Female | WSM | Neg | Late latent | Unknown | R | CSF | Pos | 40.8 | NA | NA | NA | NA | Uveitis, chorioretinitis, loss of vision in both eyes |
| OS-15 | New York | Male | MSM | Pos | Early latent | 1:128 | NR | CSF | Pos | 35.7 | NA | _e_/_ | NA | NA | Posterior uveitis |
| OS-16 | Massachusetts | Male | Unknown | Pos | Late latent | 1:512 | NR | CSF, plasma | Neg Pos |
NA 34.3 |
NA NA |
NA NA |
NA NA |
NA NA |
Optic neuritis, papillitis, blurry vision |
| OS-17 | Idaho | Male | MSM | Pos | Unknown | 1:65,536 | 1:2 | CSF | Pos | 37.1 | NA | NA | NA | NA | Panuveitis, uveitis, partial vision loss, blurry vision |
| OS-18 | Connecticut | Male | MSM | Neg | Secondary | 1:128 | Unknown | Vitreous fluid | Pos | 26.9 | A2058G | 14d9/g | 6c.3.1 | SS14 | Chorioretinitis, posterior uveitis, vitritis |
Abbreviations: ACDCT, augmented CDC typing; CSF, cerebrospinal fluid; Ct, cycle threshold; MLST, multilocus sequence typing; MSM, men who have sex with men; MSW, men who have sex with women; WSM, women who have sex with men; RPR, rapid plasma regain, VDRL, Venereal disease research laboratory; Pos, positive; Neg, negative; NR, non-reactive; R, reactive; NA, not amplifiable; NT, not tested; X, MLST allele not determined.
Denotes ACDCT typing targets that were not amplifiable.
Closest match for tp0136 was to allele 6 with one indel mutation.
PCR detection of T. pallidum
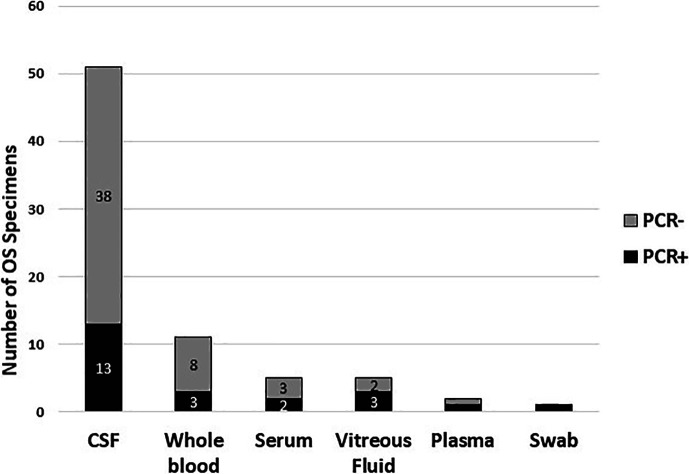
A total of 75 specimens from 55 patients were submitted to CDC for testing, including a pharyngeal swab collected for CT/GC NAAT from a patient who also presented with mucous patches. Overall, 22 of the 75 (29.3%) specimens were T. pallidum PCR positive, including 13 of 51 CSF samples, 3 of 11 blood, 1 of 5 serum, 3 of 5 vitreous fluid, 1 of 2 plasma, and the pharyngeal swab (Fig. 2). The PCR sensitivity rate based on CSF samples was 25.5% (13/51). PCR positivity rates did not appear different across the six specimen types (P value = 0.28, Fisher’s exact test). T. pallidum DNA was detected in at least one specimen from 17 patients (Table 2). Paired specimens that were PCR positive included CSF/blood from two patients and CSF/vitreous fluid and CSF/blood/serum from one patient, respectively. Paired samples that were PCR negative included whole blood and CSF from five patients, CSF and serum from three patients, and CSF and vitreous fluid from one patient (data not shown). PCR cycle threshold (Ct) values ranged from 24.7 to 44.8 with 85% (17/20) of samples having Ct values > 31. Two of the three vitreous fluid samples had Ct values < 27.
Fig 2.
T. pallidum PCR results for ocular syphilis specimens (n = 75).
With respect to the disease stage, T. pallidum DNA was detected in 9/17 (52.9%) secondary cases, 1/4 (25%) early latent, 3/10 (30%) late latent cases, and 4/19 (21%) with an unknown stage (Table 2). There was no difference in PCR positivity by stage of syphilis (P value = 0.18, Fisher’s exact test). The distribution of T. pallidum polA PCR results by RPR and CSF-VDRL titers are shown in Tables S1 and S2, respectively. There was no correlation between PCR positivity and RPR titer (P value = 0.17, Fisher’s exact test), while an association between PCR-negative results and non-reactive CSF-VDRL titer was observed (P value = 0.02, Fisher’s exact test).
Genotyping and WGS analyses
Molecular typing was attempted on all PCR-positive samples, generating complete strain types for 4/17 (23.5%) and partial strain types with amplification of at least one typing target for 5/17 (29.4%) patients (Table 2). ACDCT strain types 14d10/g, 14b9/g, 14d9/g, and 14e9/f were identified in patients from New York, Texas, Connecticut, and Massachusetts, respectively. One patient (OS-12) had the same strain type (14e9/f) in CSF and vitreous fluid samples. Partial types 14d_ /_, 14_ _/_, _ _9/_, _e_/_, and _ _ _/f were identified in five samples, where “ _” indicates targets that were not amplifiable. The partial strain type 14_10/g from the CSF of patient OS-4 matched the complete strain type of 14d10/g in blood, showing no intrapatient genetic heterogeneity in arp, rpsA, and tp0548.
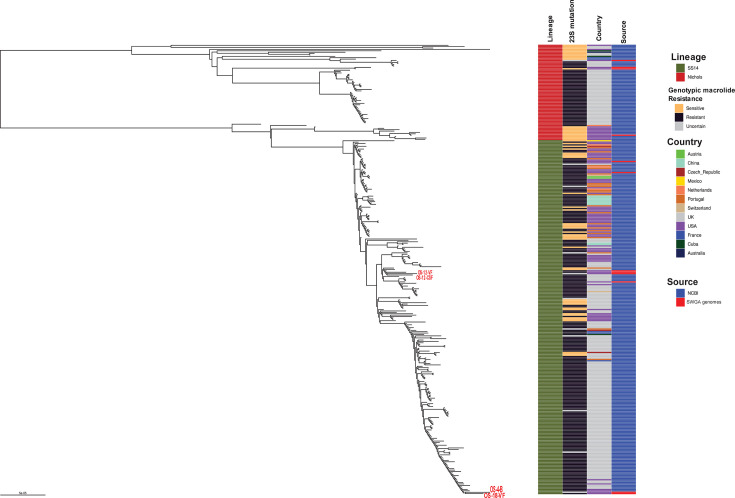
WGS was successful for three patients: blood sample from patient OS-4, CSF and vitreous fluid from OS-12, and vitreous fluid from OS-18, generating 76%–98% genome coverage at 5× read depth (Table S3). Phylogenetic analysis including 323 high-quality publicly available genomes revealed that the three strains belonged to either of two SS14-like subclades of T. pallidum described previously, with the CSF and vitreous fluid sequences from the same patient clustering closely together (Fig. 3; Table 2; Table S4) (34). OS-4 and OS-18 also clustered very closely on the phylogeny. The gene sequences of all the major typing genes used for T. pallidum subtyping of the paired CSF and vitreous fluid samples of OS-12 were compared to determine if intrapatient genetic variability was present. RpsA (tp0279), tp0136, tp0548, and tp0705 were retrievable from both samples with at least 99% coverage of the genes compared with the reference typing genes, while tprG was only recovered from the vitreous fluid (Table S5). In addition, the complete 2,641-bp rpsA gene sequence of OS-12 was retrieved from the WGS data of the CSF specimen, while 76.75% (2,027 bp) of the gene was obtained from the vitreous fluid.
Fig 3.
Maximum likelihood global phylogenetic tree showing four T. pallidum ocular syphilis (red text, this study) and eight additional non-ocular syphilis genomes sequenced by the SWGA/WGS method and 323 genomes from NCBI.
A pairwise comparison of the rpsA gene sequences from CSF and vitreous fluid from the same patient (OS-12) showed a 100% match, suggesting no intrapatient variation within the DNA obtained from the two body sites (Fig. S1). Genome-wide comparison to identify regions of genetic differences between these two samples showed varying SNPs in the tprK gene (TPASS_RS05385), a putative outer membrane protein (Table S6).
The MLST allelic profile of OS-12 was 19.1.1 based on WGS data from the vitreous fluid specimen, but the paired CSF was X.1.1, where the tp0136 profile could not be determined, while the remaining two alleles were identical to the alleles from the paired vitreous fluid specimen (Table 2; Table S5). The allelic profile of OS-18 was 6.3.1, and the tp0136 gene sequence was similar to allele 6 but deferred by one indel. A partial profile (X.3.X) was determined for the blood specimen OS-4. Tpr G and J genes were retrieved from the two vitreous fluid specimens but not from the CSF and blood samples (Table S5).
The A2058G mutation was identified in two of the three strains from which whole genomes were obtained. The complete gene sequences of the 16S rRNA gene were retrieved from the WGS data for all three strains, and comparison to the T. pallidum Nichols reference sequence (NC_021490.2) showed no mutations associated with doxycycline resistance (Fig. S2). Screening of the three PBP and tp47 genes in the three strains did not identify any variants (data not shown).
DISCUSSION
Molecular epidemiological studies can improve our understanding of circulating T. pallidum strains that are driving the syphilis epidemic; determine if certain strain types are associated with clinical manifestations of the disease such as ocular syphilis, neurosyphilis, congenital syphilis, or progression to late-stage disease in untreated individuals. In our study, strain typing and WGS were used to characterize T. pallidum strains during an investigation of OS cases in the U.S. Typing with the ACDCT scheme identified four strain types in four different states, revealing a predominant strain is not responsible for OS in the U.S. In the previous OS investigation by Oliver and colleagues, five strain types were found with ECDCT and 14d/g was present in specimens from four states in addition to being the predominant type among non-ocular strains in Seattle, Washington (5). Two patients with this strain type from neighboring states (New York and Connecticut) were further differentiated into 14d9/g and 14d10/g using ACDCT in our study while phylogenetic analysis showed close relatedness of the strains. The epidemiological relevance of this observation is unclear in the absence of linkage data.
Despite the fact that our data show a predominant strain is not associated with OS, it’s still plausible that certain strains have a propensity to cause OS as suggested previously where the 8d/g type was found in one of two sexual partners and in a third patient with no link to the other two patients (5, 8). In addition, a recent investigation of an ocular syphilis cluster in Michigan involving a heterosexual male partner and five women suggests an oculotropic strain might have been involved; however, molecular typing was not possible due to the lack of T. pallidum DNA (9). Another OS study in Massachusetts reported two partial strain types based on tp0548 sequencing; however, the arp and tpr gene targets could not be amplified in any of the samples, which may have been due to low levels of treponemal DNA (10).
We analyzed MLST and ACDCT loci in silico to assess whether WGS data can be used for genotyping strains. A new MLST allelic profile 19.1.1 was identified in one sample while the second sample had the 6.3.1 profile, which has been reported previously (19, 21, 22). We had limited success with in silico typing using ACDCT; one tpr gene sequence was retrieved from two strains; tp0279 sequence was retrieved from one strain, while none of the arp sequences were obtained. The large size of the tpr and arp loci combined with a repeat region in arp and relatively low amount of DNA in extracts may have precluded near-complete genome sequences from being obtained. However, traditional ACDCT or ECDCT of OS specimens with low T. pallidum DNA can be challenging, as shown in our study and in recent OS studies (5, 10).
Phylogenetic analysis of the three strains from which whole genome sequences were obtained showed that they belonged to the T. pallidum SS14 clade, which is one of the two major clades found worldwide including the U.S. (43, 44). All three strains falling within the SS-14 clade may reflect the circulating strains in the U.S.; however, analysis of strains from other jurisdictions is needed to confirm this finding. The CSF and vitreous fluid strains from one case (OS-12) clustered tightly on the phylogenetic tree suggesting minimal genetic heterogeneity between the two body sites. We also compared genome sequences from these paired specimens to determine if there was any variability across the T. pallidum genome. Interestingly, varying SNPs were seen in the tprK gene, which consists of seven variable (V) gene regions (V1-7), and sequence variation is mediated by a gene conversion mechanism (45). Our findings are consistent with previous WGS studies showing intra- and interpatient sequence variabilities of tprK in clinical specimens (46, 47). Antigenic variation in the TprK antigen is hypothesized to enable T. pallidum to evade the host immune system based on experiments in the rabbit model (48). It is possible that antigenic variation in TprK might have enabled the bacterium to evade the host immune system and migrate to the eye and central nervous system, which are immunologically protected sites. Although we identified multiple SNPs in tprK, the T. pallidum genome coverage rates for CSF and vitreous fluid were 91% and 98%, respectively, and therefore do not account for variations in other regions of the genomes.
Penicillin has been the drug of choice for treating syphilis for many decades. Although failure of non-treponemal titers to drop fourfold has been reported in 12.1% of patients worldwide and may represent treatment failure, reinfection, or an undefined immune response, there is no documented clinical evidence of penicillin resistance in T. pallidum (23, 25). Genomic epidemiological studies including strains from various countries have identified 33 SNPs in the three PBPs and a putative β-lactamase gene; however, the relevance of these mutations is unknown (35, 49). None of the strains sequenced in our study had SNPs in any of these genes.
The diagnosis of OS is based on sexual history, reactive syphilis serology, and an ophthalmologic examination in individuals with ocular symptoms. Direct detection of T. pallidum using molecular tests can aid the diagnosis; however, there is limited data on the sensitivity of NAATs for OS. The sensitivity for detecting T. pallidum in CSF was 25.5% in our study. Oliver and colleagues previously reported a CSF PCR sensitivity of 39% (9/23), and a more recent study by Cummings et al. reported a sensitivity of 50% based on six samples (5, 10). The lower sensitivity in our study may have been due to varying times from collection to testing that could have led to DNA degradation since CSF samples are stored without preservatives for routine diagnostics. However, compared with vitreous fluid specimens, non-ocular specimen types (CSF, plasma, and blood) had higher polA PCR Ct values in our study suggesting low numbers of circulating treponemes. Comparison of PCR results based on the disease stage for typeable samples between the Oliver et al. study and ours revealed that the majority of patients (50% vs 35.7%) presented with secondary syphilis, followed by late latent syphilis (28.6% vs 16.7%) and early latent syphilis (21.4% vs 5.6%), and one patient had primary syphilis in the prior study.
Although T. pallidum disseminates throughout the body early in syphilis infection, the low PCR detection rate in non-lesion samples of primary cases presenting with OS is in-keeping with previous studies on non-OS patients (50, 51). In addition, there may have been missed opportunities to detect T. pallidum by PCR in patients who might have had lesions of primary or secondary syphilis as NAATs are rarely performed for syphilis diagnosis in the U.S. due to the lack of an FDA-cleared test. Even though PCR sensitivity was low, our data suggest that NAATs can be used to confirm a diagnosis of OS, especially in difficult clinical cases such as recently reinfected individuals with existing high RPR titers or serofast status. Several studies have shown the potential utility of PCR to detect T. pallidum in oral specimens in the absence of visible lesions. This non-invasive specimen type should be investigated further for improving diagnostic options for OS (52–54).
Our study has several limitations. Due to convenience sampling, patients from Massachusetts were over-represented and the demographics may not be representative of all people who have OS. Remnant specimens were submitted voluntarily after routine diagnostic testing; therefore, we could not ensure specimen adequacy and DNA integrity prior to storage in many cases. This may have contributed to the low PCR positivity rate. Moreover, CSF and blood tend to have lower T. pallidum DNA compared with lesion specimens from primary or secondary syphilis patients (51, 55). A variety of specimen types were collected for testing including a few ocular samples, which limited the interpretation of potential positivity in ocular versus non-ocular specimen types. Since we typed samples only from OS patients, we could not determine if OS genotypes were different from the background non-OS strain types in states where specimens were collected from.
The increase in OS cases appears to coincide with the ongoing syphilis epidemic and may also reflect an increased awareness of ocular manifestations among clinicians. OS can present with a variety of clinical manifestations that are non-specific for syphilis and may be misdiagnosed. It can also lead to serious sequelae including vision loss or blindness. Screening for syphilis should be considered in patients with ocular symptoms. Our findings show that multiple strains are associated with OS; however, further investigation is needed to determine if oculotropic strains are circulating within certain sexual networks.
ACKNOWLEDGMENTS
The authors wish to express their sincere thanks to all the medical providers and public health laboratory personnel for submitting specimens for this project. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
This work was made possible through CDC’s Division of STD Prevention with support from the Advanced Molecular Detection (AMD) program and, in part, by an appointment (K.V., A.D., S.L., and C.M.T.) to the Research Participation Program at CDC, administered by the Oak Ridge Institute for Science and Education through an interagency agreement between the U.S. Department of Energy and CDC.
Study design was done by the following: A.P., S.S.K., C.Y.C., and E.N.K. Data management was done by the following: A.P. and S.J.J. Specimen collection and processing were done by the following: K.V., A.D., S.L., S.S.K., C.M.T., K.P., K.A.W., and R.Y.B. Laboratory experiments and data acquisition were done by the following: K.V., A.D., S.S.K., S.L., C.M.T., D.D., K-.H.C., and A.J. Drafting of the manuscript was done by the following: A.P., S.J.J., and W.C. Bioinformatics and phylogenetics analyses were done by the following: S.J.J. Statistical analyses were done by the following: H.J. Critical review and revision of the manuscript were done by the following: All authors.
Barshak; A. L. Vasquez; A. Achhra; E. Palavecino; D. Green; D. Hata; I. Kasarskis; J. Varkey; J. Gaurner; J. Strymish; K. Wendel; K. Hsu; S. D. Walter; S. Brecher; T. Vindenes; and Z. Wiley are acknowledged as part of the OS Surveillance Working Group.
Findings from this study were presented at the 24th International Society for Sexually Transmitted Diseases Research (ISSTDR) Meeting 2021 in Amsterdam.
This study was determined not to be human subjects research but considered part of routine disease surveillance after project review at CDC; therefore, informed consent was not required.
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Contributor Information
Allan Pillay, Email: apillay@cdc.gov.
Susan Realegeno, Quest Diagnostics, San Juan Capistrano, California, USA.
DATA AVAILABILITY
All sequencing data associated with this study were submitted to the National Center for Biotechnology Information Sequence Read Archive (SRA) under the BioProject number PRJNA744275.
SUPPLEMENTAL MATERIAL
The following material is available online at https://doi.org/10.1128/spectrum.00581-24.
Pairwise comparison of rpsA gene sequences.
Multiple sequence alignment of the 16S rRNA sequences.
Distribution of T. pallidum polA PCR results by RPR titer.
Distribution of T. pallidum polA PCR results by CSF-VDRL titer.
Whole genome sequencing statistics for OS strains.
List of strains included in the phylogenetic tree.
Results of the pairwise comparison of recovered ACDCT and MLST genes.
Genome-wide comparison of genetic differences between the CSF and vitreous fluid.
ASM does not own the copyrights to Supplemental Material that may be linked to, or accessed through, an article. The authors have granted ASM a non-exclusive, world-wide license to publish the Supplemental Material files. Please contact the corresponding author directly for reuse.
REFERENCES
- 1. Centers for Disease Control and Prevention (CDC) . 2024. Sexually transmitted disease surveillance 2022. US Department of Health and Human Services, Atlanta. [Google Scholar]
- 2. Doris JP, Saha K, Jones NP, Sukthankar A. 2006. Ocular syphilis: the new epidemic. Eye (Lond) 20:703–705. doi: 10.1038/sj.eye.6701954 [DOI] [PubMed] [Google Scholar]
- 3. Tucker JD, Li JZ, Robbins GK, Davis BT, Lobo AM, Kunkel J, Papaliodis GN, Durand ML, Felsenstein D. 2011. Ocular syphilis among HIV-infected patients: a systematic analysis of the literature. Sex Transm Infect 87:4–8. doi: 10.1136/sti.2010.043042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Quilter LAS, Weinstock H, Torrone EA. 2022. Reported ocular manifestations among syphilis cases —13 states, 2019–2021. Sex Transm Dis 49:S58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Oliver S, Sahi SK, Tantalo LC, Godornes C, Neblett Fanfair R, Markowitz LE, Lukehart SA, Marra CM. 2016. Molecular typing of Treponema pallidum in ocular syphilis. Sex Transm Dis 43:524–527. doi: 10.1097/OLQ.0000000000000478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Oliver SE, Aubin M, Atwell L, Matthias J, Cope A, Mobley V, Goode A, Minnerly S, Stoltey J, Bauer HM, Hennessy RR, DiOrio D, Fanfair RN, Peterman TA, Markowitz L. 2016. Ocular syphilis - eight jurisdictions, United States, 2014-2015. MMWR Morb Mortal Wkly Rep 65:1185–1188. doi: 10.15585/mmwr.mm6543a2 [DOI] [PubMed] [Google Scholar]
- 7. Woolston SL, Dhanireddy S, Marrazzo J. 2016. Ocular syphilis: a clinical review. Curr Infect Dis Rep 18:36. doi: 10.1007/s11908-016-0542-9 [DOI] [PubMed] [Google Scholar]
- 8. Woolston S, Cohen SE, Fanfair RN, Lewis SC, Marra CM, Golden MR. 2015. A cluster of ocular syphilis cases - Seattle, Washington, and San Francisco, California, 2014-2015. MMWR Morb Mortal Wkly Rep 64:1150–1151. doi: 10.15585/mmwr.mm6440a6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nettleton WD, Kent JB, Lightheart K, Diesel JC. 2023. A cluster of ocular syphilis cases with a common sex partner - Southwest Michigan, 2022. MMWR Morb Mortal Wkly Rep. Available from: https://www.cdc.gov/mmwr/volumes/72/wr/mm7247a1.htm [DOI] [PMC free article] [PubMed]
- 10. Cummings OW, Durand ML, Barshak MB, Bispo PJM. 2023. Molecular detection and typing of Treponema pallidum in non-ocular samples from patients with ocular syphilis. Ocul Immunol Inflamm:1–5. doi: 10.1080/09273948.2023.2263086 [DOI] [PubMed] [Google Scholar]
- 11. Marra CM, Sahi SK, Tantalo LC, Godornes C, Reid T, Behets F, Rompalo A, Klausner JD, Yin YP, Mulcahy F, Golden MR, Centurion-Lara A, Lukehart SA. 2010. Enhanced molecular typing of Treponema pallidum: geographical distribution of strain types and association with neurosyphilis. J Infect Dis 202:1380–1388. doi: 10.1086/656533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Peng RR, Yin YP, Wei WH, Wang HC, Zhu BY, Liu QZ, Zheng HP, Zhang JP, Huang SJ, Chen XS. 2012. Molecular typing of Treponema pallidum causing early syphilis in China: a cross-sectional study. Sex Transm Dis 39:42–45. doi: 10.1097/OLQ.0b013e318232697d [DOI] [PubMed] [Google Scholar]
- 13. Wu H, Chang SY, Lee NY, Huang WC, Wu BR, Yang CJ, Liang SH, Lee CH, Ko WC, Lin HH, Chen YH, Liu WC, Su YC, Hsieh CY, Wu PY, Hung CC. 2012. Evaluation of macrolide resistance and enhanced molecular typing of Treponema pallidum in patients with syphilis in Taiwan: a prospective multicenter study. J Clin Microbiol 50:2299–2304. doi: 10.1128/JCM.00341-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tipple C, McClure MO, Taylor GP. 2011. High prevalence of macrolide resistant Treponema pallidum strains in a London centre. Sex Transm Infect 87:486–488. doi: 10.1136/sextrans-2011-050082 [DOI] [PubMed] [Google Scholar]
- 15. Read P, Tagg KA, Jeoffreys N, Guy RJ, Gilbert GL, Donovan B. 2016. Treponema pallidum strain types and association with macrolide resistance in Sydney, Australia: new TP0548 gene types identified. J Clin Microbiol 54:2172–2174. doi: 10.1128/JCM.00959-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Flores JA, Vargas SK, Leon SR, Perez DG, Ramos LB, Chow J, Konda KA, Calvo GM, Salvatierra HJ, Klausner JD, Caceres CF. 2016. Treponema pallidum pallidum genotypes and macrolide resistance status in syphilitic lesions among patients at 2 sexually transmitted infection clinics in Lima, Peru. Sex Transm Dis 43:465–466. doi: 10.1097/OLQ.0000000000000465 [DOI] [PubMed] [Google Scholar]
- 17. Pillay A, Lee MK, Slezak T, Katz SS, Sun Y, Chi KH, Morshed M, Philip S, Ballard RC, Chen CY. 2019. Increased discrimination of Treponema pallidum strains by subtyping with a 4-component system incorporating a mononucleotide tandem repeat in rpsA. Sex Transm Dis 46:e42–e45. doi: 10.1097/OLQ.0000000000000935 [DOI] [PubMed] [Google Scholar]
- 18. Liu D, He S-M, Zhu X-Z, Liu L-L, Lin L-R, Niu J-J, Yang T-C. 2020. Molecular characterization based on MLST and ECDC typing schemes and antibiotic resistance analyses of Treponema pallidum subsp. pallidum in Xiamen, China. Front Cell Infect Microbiol 10:618747. doi: 10.3389/fcimb.2020.618747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sahi SK, Zahlan JM, Tantalo LC, Marra CM. 2021. A comparison of Treponema pallidum subspecies pallidum molecular typing systems: multilocus sequence typing vs. enhanced centers for disease control and prevention typing. Sex Transm Dis 48:670–674. doi: 10.1097/OLQ.0000000000001378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fernández-Naval C, Arando M, Espasa M, Antón A, Fernández-Huerta M, Silgado A, Pinatar C, Zarzuela F, González-López JJ, Serra-Pladevall J, Sulleiro E, Pumarola T, Vall-Mayans M, Esperalba J. 2021. Multilocus sequence typing of Treponema pallidum subsp. pallidum in Barcelona. Future Microbiol 16:967–976. doi: 10.2217/fmb-2021-0037 [DOI] [PubMed] [Google Scholar]
- 21. Sweeney EL, Lowry K, Seel M, Rahimi F, Langton-Lockton J, Bletchly C, Nimmo GR, Whiley DM. 2022. Two Treponema pallidum strains account for the majority of syphilis infections, including among females, in Queensland, Australia. Commun Dis Intell (2018) 46. doi: 10.33321/cdi.2022.46.26 [DOI] [PubMed] [Google Scholar]
- 22. Grillová L, Bawa T, Mikalová L, Gayet-Ageron A, Nieselt K, Strouhal M, Sednaoui P, Ferry T, Cavassini M, Lautenschlager S, Dutly F, Pla-Díaz M, Krützen M, González-Candelas F, Bagheri HC, Šmajs D, Arora N, Bosshard PP. 2018. Molecular characterization of Treponema pallidum subsp. pallidum in Switzerland and France with a new multilocus sequence typing scheme. PLoS One 13:e0200773. doi: 10.1371/journal.pone.0200773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Alsallamin I, Alsallamin A, Greene S, Hammad F, Bawwab A. 2022. A case of neurosyphilis with penicillin failure. Cureus 14:e21456. doi: 10.7759/cureus.21456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Workowski KA, Bachmann LH, Chan PA, Johnston CM, Muzny CA, Park I, Reno H, Zenilman JM, Bolan GA. 2021. Sexually transmitted infections treatment guidelines, 2021. MMWR Recomm Rep 70:1–187. doi: 10.15585/mmwr.rr7004a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Seña AC, Zhang XH, Li T, Zheng HP, Yang B, Yang LG, Salazar JC, Cohen MS, Moody MA, Radolf JD, Tucker JD. 2015. A systematic review of syphilis serological treatment outcomes in HIV-infected and HIV-uninfected persons: rethinking the significance of serological non-responsiveness and the serofast state after therapy. BMC Infect Dis 15:479. doi: 10.1186/s12879-015-1209-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mi H-F, Shen X, Chen X-Q, Zhang X-L, Ke W-J, Xiao Y. 2023. Association between treatment failure in patients with early syphilis and penicillin resistance-related gene mutations of Treponema pallidum: protocol for a multicentre nested case-control study. Front Med (Lausanne) 10:1131921. doi: 10.3389/fmed.2023.1131921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Centers for Disease Control and Prevention (CDC) . 2004. Azithromycin treatment failures in syphilis infections--San Francisco, California, 2002-2003. MMWR Morb Mortal Wkly Rep. Available from: https://www.cdc.gov/mmwr/preview/mmwrhtml/mm5309a4.htm [PubMed]
- 28. Šmajs D, Paštěková L, Grillová L. 2015. Macrolide resistance in the syphilis spirochete, Treponema pallidum ssp. pallidum: can we also expect macrolide-resistant yaws strains? Am J Trop Med Hyg 93:678–683. doi: 10.4269/ajtmh.15-0316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chen C-Y, Chi K-H, Pillay A, Nachamkin E, Su JR, Ballard RC. 2013. Detection of the A2058G and A2059G 23S rRNA gene point mutations associated with azithromycin resistance in Treponema pallidum by use of a TaqMan real-time multiplex PCR assay. J Clin Microbiol 51:908–913. doi: 10.1128/JCM.02770-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kanai M, Arima Y, Nishiki S, Shimuta K, Itoda I, Matsui T, Oishi K, Ohnishi M, Nakayama SI. 2019. Molecular typing and macrolide resistance analyses of Treponema pallidum in heterosexuals and men who have sex with men in Japan, 2017. J Clin Microbiol 57:e01167-18. doi: 10.1128/JCM.01167-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Venter JME, Müller EE, Mahlangu MP, Kularatne RS. 2021. Treponema pallidum macrolide resistance and molecular epidemiology in Southern Africa, 2008 to 2018. J Clin Microbiol 59:e0238520. doi: 10.1128/JCM.02385-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pospíšilová P, Grange PA, Grillová L, Mikalová L, Martinet P, Janier M, Vermersch A, Benhaddou N, Del Giudice P, Alcaraz I, Truchetet F, Dupin N, Šmajs D. 2018. Multi-locus sequence typing of Treponema pallidum subsp. pallidum present in clinical samples from France: infecting treponemes are genetically diverse and belong to 18 allelic profiles. PLoS One 13:e0201068. doi: 10.1371/journal.pone.0201068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Vrbová E, Grillová L, Mikalová L, Pospíšilová P, Strnadel R, Dastychová E, Kojanová M, Kreidlová M, Vaňousová D, Rob F, Procházka P, Krchňáková A, Vašků V, Woznicová V, Dvořáková Heroldová M, Kuklová I, Zákoucká H, Šmajs D. 2019. MLST typing of Treponema pallidum subsp. pallidum in the Czech Republic during 2004-2017: clinical isolates belonged to 25 allelic profiles and harbored 8 novel allelic variants. PLoS One 14:e0217611. doi: 10.1371/journal.pone.0217611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Arora N, Schuenemann VJ, Jäger G, Peltzer A, Seitz A, Herbig A, Strouhal M, Grillová L, Sánchez-Busó L, Kühnert D, et al. 2016. Origin of modern syphilis and emergence of a pandemic Treponema pallidum cluster. Nat Microbiol 2:16245. doi: 10.1038/nmicrobiol.2016.245 [DOI] [PubMed] [Google Scholar]
- 35. Beale MA, Marks M, Sahi SK, Tantalo LC, Nori AV, French P, Lukehart SA, Marra CM, Thomson NR. 2019. Genomic epidemiology of syphilis reveals independent emergence of macrolide resistance across multiple circulating lineages. Nat Commun 10:3255. doi: 10.1038/s41467-019-11216-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Thurlow CM, Joseph SJ, Ganova-Raeva L, Katz SS, Pereira L, Chen C, Debra A, Vilfort K, Workowski K, Cohen SE, Reno H, Sun Y, Burroughs M, Sheth M, Chi KH, Danavall D, Philip SS, Cao W, Kersh EN, Pillay A. 2022. Selective whole-genome amplification as a tool to enrich specimens with low Treponema pallidum genomic DNA copies for whole-genome sequencing. mSphere 7:e0000922. doi: 10.1128/msphere.00009-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Xiao Y, Liu S, Liu Z, Xie Y, Jiang C, Xu M, Zhao F, Zeng T, Yu J, Wu Y. 2016. Molecular subtyping and surveillance of resistance genes in Treponema pallidum DNA from patients with secondary and latent syphilis in Hunan, China. Sex Transm Dis 43:310–316. doi: 10.1097/OLQ.0000000000000445 [DOI] [PubMed] [Google Scholar]
- 38. Pringle M, Fellström C, Johansson K-E. 2007. Decreased susceptibility to doxycycline associated with a 16S rRNA gene mutation in Brachyspira hyodysenteriae. Vet Microbiol 123:245–248. doi: 10.1016/j.vetmic.2007.02.019 [DOI] [PubMed] [Google Scholar]
- 39. Nguyen F, Starosta AL, Arenz S, Sohmen D, Dönhöfer A, Wilson DN. 2014. Tetracycline antibiotics and resistance mechanisms. Biol Chem 395:559–575. doi: 10.1515/hsz-2013-0292 [DOI] [PubMed] [Google Scholar]
- 40. Katz KA, Pillay A, Ahrens K, Kohn RP, Hermanstyne K, Bernstein KT, Ballard RC, Klausner JD. 2010. Molecular epidemiology of syphilis--San Francisco, 2004-2007. Sex Transm Dis 37:660–663. doi: 10.1097/OLQ.0b013e3181e1a77a [DOI] [PubMed] [Google Scholar]
- 41. Inouye M, Dashnow H, Raven L-A, Schultz MB, Pope BJ, Tomita T, Zobel J, Holt KE. 2014. SRST2: Rapid genomic surveillance for public health and hospital microbiology labs. Genome Med 6:90. doi: 10.1186/s13073-014-0090-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Olm MR, Crits-Christoph A, Bouma-Gregson K, Firek BA, Morowitz MJ, Banfield JF. 2021. inStrain profiles population microdiversity from metagenomic data and sensitively detects shared microbial strains. Nat Biotechnol 39:727–736. doi: 10.1038/s41587-020-00797-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Beale MA, Marks M, Cole MJ, Lee M-K, Pitt R, Ruis C, Balla E, Crucitti T, Ewens M, Fernández-Naval C, et al. 2021. Global phylogeny of Treponema pallidum lineages reveals recent expansion and spread of contemporary syphilis. Nat Microbiol 6:1549–1560. doi: 10.1038/s41564-021-01000-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lieberman NAP, Lin MJ, Xie H, Shrestha L, Nguyen T, Huang M-L, Haynes AM, Romeis E, Wang Q-Q, Zhang R-L, et al. 2021. Treponema pallidum genome sequencing from six continents reveals variability in vaccine candidate genes and dominance of Nichols clade strains in Madagascar. PLoS Negl Trop Dis 15:e0010063. doi: 10.1371/journal.pntd.0010063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Centurion-Lara A, LaFond RE, Hevner K, Godornes C, Molini BJ, Van Voorhis WC, Lukehart SA. 2004. Gene conversion: a mechanism for generation of heterogeneity in the tprK gene of Treponema pallidum during infection. Mol Microbiol 52:1579–1596. doi: 10.1111/j.1365-2958.2004.04086.x [DOI] [PubMed] [Google Scholar]
- 46. Pinto M, Borges V, Antelo M, Pinheiro M, Nunes A, Azevedo J, Borrego MJ, Mendonça J, Carpinteiro D, Vieira L, Gomes JP. 2016. Genome-scale analysis of the non-cultivable Treponema pallidum reveals extensive within-patient genetic variation. Nat Microbiol 2:16190. doi: 10.1038/nmicrobiol.2016.190 [DOI] [PubMed] [Google Scholar]
- 47. Liu D, Tong ML, Luo X, Liu LL, Lin LR, Zhang HL, Lin Y, Niu JJ, Yang TC. 2019. Profile of the tprK gene in primary syphilis patients based on next-generation sequencing. PLoS Negl Trop Dis 13:e0006855. doi: 10.1371/journal.pntd.0006855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Giacani L, Molini BJ, Kim EY, Godornes BC, Leader BT, Tantalo LC, Centurion-Lara A, Lukehart SA. 2010. Antigenic variation in Treponema pallidum: TprK sequence diversity accumulates in response to immune pressure during experimental syphilis. J Immunol 184:3822–3829. doi: 10.4049/jimmunol.0902788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Taouk ML, Taiaroa G, Pasricha S, Herman S, Chow EPF, Azzatto F, Zhang B, Sia CM, Duchene S, Lee A, Higgins N, Prestedge J, Lee YW, Thomson NR, Graves B, Meumann E, Gunathilake M, Hocking JS, Bradshaw CS, Beale MA, Howden BP, Chen MY, Fairley CK, Ingle DJ, Williamson DA. 2022. Characterisation of Treponema pallidum lineages within the contemporary syphilis outbreak in Australia: a genomic epidemiological analysis. Lancet Microbe 3:e417–e426. doi: 10.1016/S2666-5247(22)00035-0 [DOI] [PubMed] [Google Scholar]
- 50. Tantalo LC, Mendoza H, Katz DA, Sahi SK, Marra CM. 2021. Detection of Treponema pallidum DNA in oropharyngeal swabs and whole blood for syphilis diagnosis. Sex Transm Dis 48:915–918. doi: 10.1097/OLQ.0000000000001476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Gayet-Ageron A, Lautenschlager S, Ninet B, Perneger TV, Combescure C. 2013. Sensitivity, specificity and likelihood ratios of PCR in the diagnosis of syphilis: a systematic review and meta-analysis. Sex Transm Infect 89:251–256. doi: 10.1136/sextrans-2012-050622 [DOI] [PubMed] [Google Scholar]
- 52. Towns JM, Leslie DE, Denham I, Wigan R, Azzato F, Williamson DA, Lee D, Chow EPF, Fairley CK, Graves SR, Zhang L, Chen MY. 2021. Treponema pallidum detection in lesion and non-lesion sites in men who have sex with men with early syphilis: a prospective, cross-sectional study. Lancet Infect Dis 21:1324–1331. doi: 10.1016/S1473-3099(20)30838-0 [DOI] [PubMed] [Google Scholar]
- 53. Yang CJ, Chang SY, Wu BR, Yang SP, Liu WC, Wu PY, Zhang JY, Luo YZ, Hung CC, Chang SC. 2015. Unexpectedly high prevalence of Treponema pallidum infection in the oral cavity of human immunodeficiency virus-infected patients with early syphilis who had engaged in unprotected sex practices. Clin Microbiol Infect 21:787. doi: 10.1016/j.cmi.2015.04.018 [DOI] [PubMed] [Google Scholar]
- 54. Dionne JA, Giacani L, Tamhane A, Workowski K, Lieberman NAP, Greninger AL, Perlowski C, Newman L, Hook EW. 2024. Prevalence and predictors of oral Treponema pallidum detection by quantitative polymerase chain reaction in early syphilis. J Infect Dis 229:1628–1636. doi: 10.1093/infdis/jiad582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Peng RR, Wang AL, Li J, Tucker JD, Yin YP, Chen XS. 2011. Molecular typing of Treponema pallidum: a systematic review and meta-analysis. PLoS Negl Trop Dis 5:e1273. doi: 10.1371/journal.pntd.0001273 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Pairwise comparison of rpsA gene sequences.
Multiple sequence alignment of the 16S rRNA sequences.
Distribution of T. pallidum polA PCR results by RPR titer.
Distribution of T. pallidum polA PCR results by CSF-VDRL titer.
Whole genome sequencing statistics for OS strains.
List of strains included in the phylogenetic tree.
Results of the pairwise comparison of recovered ACDCT and MLST genes.
Genome-wide comparison of genetic differences between the CSF and vitreous fluid.
Data Availability Statement
All sequencing data associated with this study were submitted to the National Center for Biotechnology Information Sequence Read Archive (SRA) under the BioProject number PRJNA744275.