ABSTRACT
Sporotrichosis, the cutaneous mycosis most commonly reported in Latin America, is caused by the Sporothrix clinical clade species, including Sporothrix brasiliensis and Sporothrix schenckii sensu stricto. Due to its zoonotic transmission in Brazil, S. brasiliensis represents a significant health threat to humans and domestic animals. Itraconazole, terbinafine, and amphotericin B are the most used antifungals for treating sporotrichosis. However, many strains of S. brasiliensis and S. schenckii have shown resistance to these agents, highlighting the importance of finding new therapeutic options. Here, we demonstrate that milteforan, a commercial veterinary product against dog leishmaniasis, whose active principle is miltefosine, is a possible therapeutic alternative for the treatment of sporotrichosis, as observed by its fungicidal activity in vitro against different strains of S. brasiliensis and S. schenckii. Fluorescent miltefosine localizes to the Sporothrix cell membrane and mitochondria and causes cell death through increased permeabilization. Milteforan decreases S. brasiliensis fungal burden in A549 pulmonary cells and bone marrow-derived macrophages and also has an immunomodulatory effect by decreasing TNF-α, IL-6, and IL-10 production. Our results suggest milteforan as a possible alternative to treat feline sporotrichosis.
IMPORTANCE
Sporotrichosis is an endemic disease in Latin America caused by different species of Sporothrix. This fungus can infect domestic animals, mainly cats and eventually dogs, as well as humans. Few drugs are available to treat this disease, such as itraconazole, terbinafine, and amphotericin B, but resistance to these agents has risen in the last few years. Alternative new therapeutic options to treat sporotrichosis are essential. Here, we propose milteforan, a commercial veterinary product against dog leishmaniasis, whose active principle is miltefosine, as a possible therapeutic alternative for treating sporotrichosis. Milteforan decreases S. brasiliensis fungal burden in human and mouse cells and has an immunomodulatory effect by decreasing several cytokine production.
KEYWORDS: Sporothrix brasiliensis, Sporothrix schenckii, sporotrichosis, milteforan, miltefosine, antifungal agent, drug repurposing
INTRODUCTION
Sporotrichosis, a chronic cutaneous and subcutaneous infection, is the most commonly reported mycosis in Latin America and Asia, with a high prevalence in tropical and subtropical areas, including Brazil, Mexico, Argentina, India, Japan, and China (1, 2). Since 1998, Brazil has experienced large outbreaks of sporotrichosis that have been expanding throughout the country, mainly in the southeastern regions, the reason for which Brazil is considered a hyperendemic area (3–5).
Until 2007, Sporothrix schenckii was assumed to be the unique etiological agent for sporotrichosis, but recent molecular analyses have revealed the existence of several sibling species capable of causing infection (6). These species comprise the S. schenckii clinical/pathogenic clade, which includes S. schenckii sensu stricto, S. brasiliensis, Sporothrix globosa, and Sporothrix lurei (7, 8). These species are thermodimorphic fungi, with a mycelial phase that grows in decaying organic matter at 25°C and a yeast phase that develops inside the host during infection (9, 10). The virulence profile varies among the species of the pathogenic clade being S. brasiliensis the most virulent, followed by S. schenckii, both with the capacity to cause severe infection even in immunocompetent individuals, whereas S. globosa and S. luriei are classified as low virulent species (11, 12).
Sporotrichosis can present different clinical manifestations, such as cutaneous (lymphocutaneous and fixed cutaneous), disseminated cutaneous, and extracutaneous (pulmonary, osteoarticular, ocular, meningeal, and visceral) (13). The development of one or other clinical forms depends on different factors, which include the host immune competence, site and depth of inoculation, amount of inoculum, and the etiological agent, all of which should be considered for proper patient management (14).
The transmission of the Sporothrix species is through traumatic implantation with contaminated material, the sapronosis, which is the classical route. However, in hyperendemic zones, such as Brazil, zoonotic infection by S. brasiliensis is highly reported, transmitted mainly by cats through scratching, biting, and even through contact with fluids from infected animals. This zoonotic transmission is considered a severe health problem in Brazil, especially in the area of Rio de Janeiro, due to the rapid spread of S. brasiliensis, which is associated with severe clinical manifestations in both humans and cats (15–18). Besides cats, dogs, albeit to a lesser extent, have also been affected by sporotrichosis, making this infection a significant veterinarian problem. Five thousand one hundred thirteen cases of feline sporotrichosis (from 1988 to 2017) and 244 canine cases (from 1988 to 2014) have been reported by the Evandro Chagas National Institute of Infectious Diseases in Rio de Janeiro, Brazil. However, this number is likely underestimated because sporotrichosis incidence is a mandatory notification only in a few states of Brazil (18).
Identification of the sporotrichosis causative agent is essential for treatment since the Sporothrix species show different antifungal susceptibility profiles (19–21), but this is not always possible, given that the identification of the species requires molecular tools (8). In general, for the treatment of cutaneous forms, itraconazole (ITZ) is considered the gold standard, whereas amphotericin B (AMB) is the first-line antifungal therapy used for disseminated forms (22, 23). However, in the last few years, many S. brasiliensis clinical strains have shown resistance to both azoles and AMB (24–26), complicating sporotrichosis treatment.
Miltefosine (MFS), also known as hexadecyl phosphocholine, is a synthetic glycerol-free phospholipid analog initially used as an antineoplastic drug (27, 28). Nowadays, MFS is the only available oral drug used to treat visceral and cutaneous leishmaniasis in dogs and humans due to its significant antiparasitic activity, in vitro and in vivo, against Leishmania species (29–32). MFS’s action mechanism(s) has yet to be entirely understood. However, it has been demonstrated to act as a multi-target drug associated with the disruption of many vital pathways, such as (i) the inhibition of the biosynthesis of phosphatidylcholine, which causes low levels of this phospholipid (33, 34); (ii) the interference of the cell membrane calcium channels, which induces an increase of intracellular Ca2+ (35, 36); (iii) the inhibition of the sphingomyelin biosynthesis, which increases ceramide concentration (37), resulting in cell apoptosis; and (iv) the immune response, in which its immunomodulatory effects induce the activation of the Th1 response, mainly through the increased production of IFNγ and IL-12, which prevails over the Th2 response driven by Leishmania sp (38).
MFS has also been reported as an antifungal agent in vitro against some of the most clinically significant pathogenic and opportunistic fungi, such as Candida spp., Aspergillus spp., Fusarium spp., and Cryptococcus spp. (39–44). In addition, it was recently shown that MFS has in vitro fungicidal activity against Sporothrix spp., inhibiting the growth of the mycelial phase of S. brasiliensis, S. schenckii, and Sporothrix globosa (45), and the yeast phase of S. brasiliensis strains resistant to (ITZ) and AMB (46). It was also demonstrated that alone or in combination with potassium iodide, MFS inhibits the biofilm formation of S. brasiliensis, S. schenckii, and S. globosa (47, 48). All of this evidence suggests the potential of MFS for treating sporotrichosis. Repurposing orphan drugs, which is the application of existing drugs for different therapeutic purposes than the ones initially marketed for, is a good alternative for treating infections caused by susceptible or resistant microorganisms (49). Such is the case of MFS, which, besides being repurposed for treating leishmaniasis, has been recently designated for treating primary amebic meningoencephalitis and invasive candidiasis (50).
Here, we demonstrate that MFS has fungicidal in vitro activity against both morphologies (hyphae and yeast) of different S. brasiliensis and S. schenckii strains. We also showed that milteforan (ML), a commercial veterinary product against dog leishmaniasis, whose active principle is miltefosine (Virbac), can inhibit and kill Sporothrix species in vitro. ML treatment also increases the killing of S. brasiliensis yeast by the epithelial cells A549 and bone marrow-derived macrophages (BMDMs). Our results suggest ML as a possible veterinary alternative to treat feline sporotrichosis.
RESULTS
ML and MFS have fungicidal activity against Sporothrix spp. in vitro
The in vitro antifungal activity of several drugs against six strains of S. schenckii and S. brasiliensis, three from each species, were assessed according to their MIC and MFC values for the mycelial and yeast phases (Table 1). From these drugs, ITZ has already been reported to show fungistatic activity against Sporothrix spp., whereas terbinafine (TRB), AMB, and MFS are fungicidal drugs (19, 23, 24). On the other hand, voriconazole (VCZ) was reported to show low activity in inhibiting Sporothrix growth, whereas caspofungin (CSP) does not exhibit antifungal activity in vitro (20).
TABLE 1.
MIC and MFC values of several antifungals against S. schenckii and S. brasiliensis yeast and mycelial phasesa
| CSP (4–0.06 μg/mL) |
VCZ (4–0.06 μg/mL) |
ITZ (8–0.125 μg/mL) |
MFS (16–0.25 μg/mL) |
ML (16–0.25 μg/mL) |
TRB (4–0.06 μg/mL) |
AMB (8–0.125 μg/mL) |
|||
|---|---|---|---|---|---|---|---|---|---|
| Ss 4820 | Y | MIC | >4 | >4 | 0.25 | 2 | 2 | 1 | 2 |
| MFC | >4 | >4 | 0.5 | 2 | 2 | 1 | 2 | ||
| M | MIC | >4 | >4 | 2 | 2 | 2 | 1 | 2 | |
| MFC | >4 | >4 | >8 | 2 | 2 | 1 | 2 | ||
| Ss 4821 | Y | MIC | >4 | >4 | 0.5 | 2 | 2 | 1 | 2 |
| MFC | >4 | >4 | 2 | 2 | 2 | 1 | 2 | ||
| M | MIC | >4 | >4 | 1 | 2 | 2 | 1 | 2 | |
| MFC | >4 | >4 | >8 | 2 | 2 | 1 | 2 | ||
| Ss 4822 | Y | MIC | >4 | >4 | 0.125 | 2 | 2 | 0.5 | 2 |
| MFC | >4 | >4 | 0.25 | 2 | 2 | 0.5 | 2 | ||
| M | MIC | >4 | >4 | 2 | 2 | 2 | 1 | 2 | |
| MFC | >4 | >4 | >8 | 2 | 2 | 1 | 2 | ||
| Sb 4823 | Y | MIC | >4 | >4 | 2 | 2 | 2 | 0.5 | 2 |
| MFC | >4 | >4 | >8 | 2 | 2 | 0.5 | 2 | ||
| M | MIC | >4 | >4 | 1 | 2 | 2 | 1 | 2 | |
| MFC | >4 | >4 | >8 | 2 | 2 | 1 | 2 | ||
| Sb 4824 | Y | MIC | >4 | >4 | 0.5 | 2 | 2 | 0.5 | >8 |
| MFC | >4 | >4 | 1 | 2 | 2 | 0.5 | >8 | ||
| M | MIC | >4 | >4 | 2 | 2 | 2 | 1 | 2 | |
| MFC | >4 | >4 | >8 | 2 | 2 | 1 | 2 | ||
| Sb 4858 | Y | MIC | >4 | >4 | 0.125 | 2 | 2 | 0.125 | 2 |
| MFC | >4 | >4 | 2 | 2 | 2 | 0.125 | 2 | ||
| M | MIC | >4 | >4 | 1 | 2 | 2 | 1 | 2 | |
| MFC | >4 | >4 | >8 | 2 | 2 | 1 | 2 | ||
Ss: S. schenckii, Sb: S. brasiliensis, Y: yeast phase, M: mycelial phase; CSP: caspofungin, VCZ: voriconazole, ITZ: itraconazole, MFS: miltefosine, ML: milteforan, TRB: terbinafine, AMB: amphotericin B.
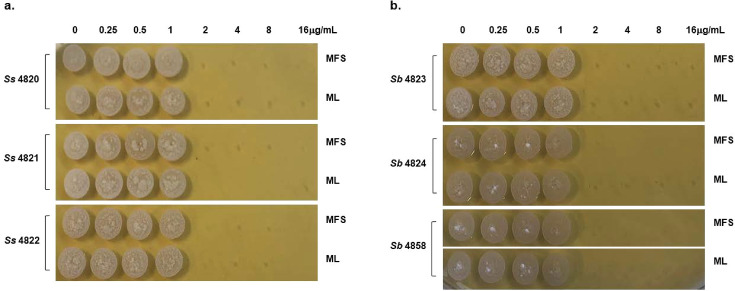
Similar to previous reports, we found that none of the Sporothrix strains, in either yeast or mycelium states, were inhibited by CSP or VCZ. At the same time, both morphologies from all the isolates were sensitive to low concentrations of TRB and AMB (MIC ≤2 µg/mL). For ITZ, all strains’ conidia were highly resistant (MFC >8 µg/mL). At the same time, the yeast phase was more sensitive with MIC and MFC values ≤ 2 µg/mL, except the S. brasiliensis clinical isolate 4823 yeast phase, which shows resistance to the drug (MFC >8 µg/mL), as already reported (51). TRB and AMB present fungicidal activity against Sporothrix species, whereas ITZ is a fungistatic drug (Table 1). MFS and ML also have fungicidal activity in vitro against both morphologies from the S. schenckii and S. brasiliensis strains, with MIC and MFC values ≤ 2 µg/mL (Table 1; Fig. 1).
Fig 1.
In vitro fungicidal activity of miltefosine and milteforan against the yeast morphology of S. schenckii and S. brasiliensis. (a) S. schenckii (strains 4820, 4821, and 4822) and (b) S. brasiliensis (strains 4823, 4824, and 4858) yeast were grown in liquid YDP pH 7.8 at 37°C in the presence of several concentrations of MFS or ML (16, 8, 4, 2, 1, 0.5, and 0.25 µg/mL). After 4 days of incubation, the cells were plated in solid YPD pH 7.8 and incubated for 4 days at 37°C. As a control, yeast cells of each strain were grown without the drugs. The results represent the average of three independent experiments performed by duplicate.
Once we showed the antifungal activity of MFS and ML against Sporothrix spp., we evaluated their ability to interact with some of the drugs already being used for treating sporotrichosis. MIC and MFC values of CSP, VCZ, ITZ, TRB, and AMB in combination with half MIC of MFS or ML were determined for the yeast morphology of each Sporothrix strain (Table 2). No differences in the activity of CSP and VCZ were observed, since neither of these drugs could inhibit S. schenckii or S. brasiliensis growth in the presence of MFS or ML. On the other hand, the interaction of MFS or ML with either ITZ, TRB, or AMB increases the antifungal activity against all of the Sporothrix strains tested, decreasing their MIC and MFC values.
TABLE 2.
MIC and MFC values of MFS and ML combination with several antifungals against S. schenckii and S. brasiliensis yeast phasea
| CSP (16–0.25 μg/mL) |
VCZ (16–0.25 μg/mL) |
ITZ (8–0.125 μg/mL) |
TRB (4–0.06 μg/mL) |
AMB (8–0.125 μg/mL) |
|||
|---|---|---|---|---|---|---|---|
| Ss 4820 | Y | MIC | >16 | >16 | 0.25 | 1 | 2 |
| MFC | >16 | >16 | 0.5 | 1 | 2 | ||
| ML | MIC | >16 | >16 | <0.125 | <0.06 | 1 | |
| MFC | >16 | >16 | 0.5 | <0.06 | 1 | ||
| MFS | MIC | >16 | >16 | <0.125 | <0.06 | 1 | |
| MFC | >16 | >16 | 0.5 | <0.06 | 1 | ||
| Ss 4821 | Y | MIC | >16 | >16 | 0.5 | 0.5 | 2 |
| MFC | >16 | >16 | 2 | 0.5 | 2 | ||
| ML | MIC | >16 | >16 | <0.125 | 0.25 | 0.5 | |
| MFC | >16 | >16 | 0.5 | 0.25 | 0.5 | ||
| MFS | MIC | >16 | >16 | <0.125 | 0.25 | 0.5 | |
| MFC | >16 | >16 | 0.5 | 0.25 | 0.5 | ||
| Ss 4822 | Y | MIC | >16 | >16 | 0.125 | 0.5 | 2 |
| MFC | >16 | >16 | 0.25 | 0.5 | 2 | ||
| ML | MIC | >16 | >16 | <0.125 | 0.25 | 1 | |
| MFC | >16 | >16 | 0.5 | 0.25 | 1 | ||
| MFS | MIC | >16 | >16 | <0.125 | 0.25 | 1 | |
| MFC | >16 | >16 | 0.5 | 0.25 | 1 | ||
| Sb 4823 | Y | MIC | >16 | >16 | 2 | 0.5 | 2 |
| MFC | >16 | >16 | >8 | 0.5 | 2 | ||
| ML | MIC | >16 | >16 | 0.5 | 0.125 | 0.5 | |
| MFC | >16 | >16 | >8 | 0.125 | 0.5 | ||
| MFS | MIC | >16 | >16 | 0.5 | 0.125 | 0.5 | |
| MFC | >16 | >16 | >8 | 0.125 | 0.5 | ||
| Sb 4824 | Y | MIC | >16 | >16 | 0.5 | 0.5 | >8 |
| MFC | >16 | >16 | 1 | 0.5 | >8 | ||
| ML | MIC | >16 | >16 | 0.25 | 0.25 | 8 | |
| MFC | >16 | >16 | 0.25 | 0.25 | 8 | ||
| MFS | MIC | >16 | >16 | 0.25 | 0.25 | 8 | |
| MFC | >16 | >16 | 0.25 | 0.25 | 8 | ||
| Sb 4858 | Y | MIC | >16 | >16 | 0.125 | 0.125 | 2 |
| MFC | >16 | >16 | 2 | 0.125 | 2 | ||
| ML | MIC | >16 | >16 | <0.125 | 0.25 | 0.25 | |
| MFC | >16 | >16 | 0.5 | 0.25 | 0.25 | ||
| MFS | MIC | >16 | >16 | <0.125 | 0.25 | 0.25 | |
| MFC | >16 | >16 | 0.5 | 0.25 | 0.25 | ||
Ss: S. schenckii, Sb: S. brasiliensis; Y: untreated yeasts, ML: yeast treated with milteforan (1 μg/mL), MFS: yeast treated with miltefosine (1μg/mL); CSP: caspofungin, VCZ: voriconazole, ITZ: itraconazole, TRB: terbinafine, AMB: amphotericin B.
Next, in order to determine what kind of interaction MFS has with ITZ, TRB, and AMB, the drug combination responses were analyzed using checkerboard assays and SynergyFinder software (52), which evaluates the potential synergy of two or more drugs. The dose-response data obtained when combining MFS with either TRB, ITZ, or AMB against S. brasiliensis and S. schenckii yeast cells show a likely additive interaction (synergy score from −10 to 10) (Fig. 2). As previously reported for ITZ (46), we found that MFS does not synergize with the drug against S. brasiliensis and S. schenckii.
Fig 2.
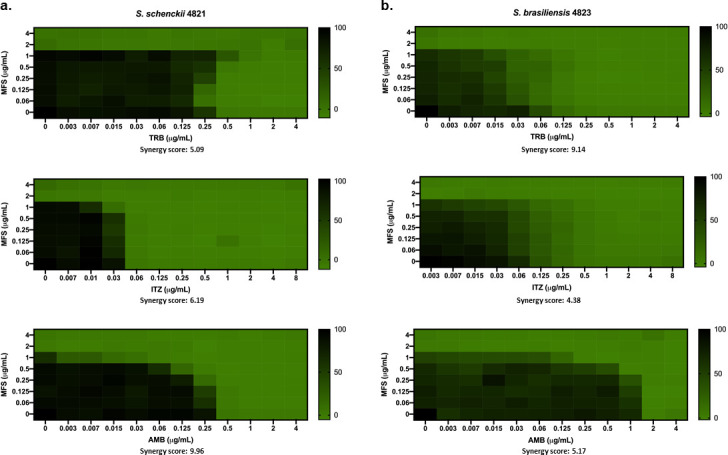
MFS has an additive interaction with ITZ, TRB, and AMB against S. brasiliensis and S. schenckii yeast cells. The synergy score for MFS × TRB, MFS × ITZ, and MFS × AMB against Sporothrix was determined by analyzing the checkerboard assay data with SynergyFinder software. (a) S. schenckii and (b) S. brasiliensis yeasts were grown in liquid YDP pH 7.8 at 37°C in different concentrations of the selected drugs. After 4 days of incubation, the metabolic activity of the cells was assessed by the XTT reduction assay. The results are expressed as the % of metabolic activity and represent the average of three independent experiments.
MFS localizes to the Sporothrix cell membrane and mitochondria and causes cell death
Although the antifungal effect of MFS against Sporothrix has been reported, the localization of the drug in the yeast is still unknown. In Leishmania (53) and A. fumigatus (43), MFS localizes in the cell membrane and the mitochondria, increasing mitochondrial fragmentation and damage. Here, we found that in S. brasiliensis, fluorescent MFS is also localized in the cell membrane and the mitochondria in 47% of the cells investigated (three repetitions of 100 cells each), as shown by MitoTracker colocalization (Fig. 3).
Fig 3.
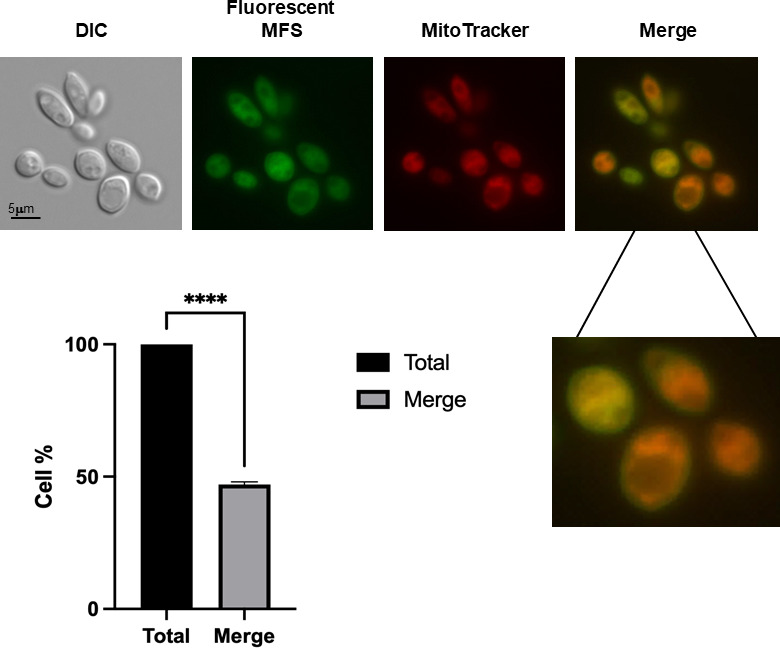
MFS is localized in the mitochondria and cell surface of S. brasiliensis yeast. S. brasiliensis yeast cells were exposed to fluorescent MFS (2 µg/mL) for 1 h and then stained with MitoTracker Deep Red FM. The MFS and MitoTracker signals merged on the mitochondria, whereas the MFS signal was observed on the cell surface. Three independent experiments were performed, and 100 cells were counted for each to calculate a 47.06% ± 1.01% of MFS and MitoTracker colocalization (merge).
Subsequently, to evaluate the viability of the yeast in the presence of MFS, drug-treated cells were stained with propidium iodide (PI) and analyzed by fluorescence microscopy. Since PI only penetrates cells with damaged membranes, PI+ cells are considered to be going through late apoptosis or early necrosis (54). Treatment of S. brasiliensis yeasts with 2, 4, and 8 µg/mL of MFS shows dose-dependent damage of the cells since the PI signal increased with the drug concentration, with a significant difference at 8 µg/mL (Fig. 4), as early as 6 h of exposure, confirming the MFS fungicidal activity against Sporothrix.
Fig 4.
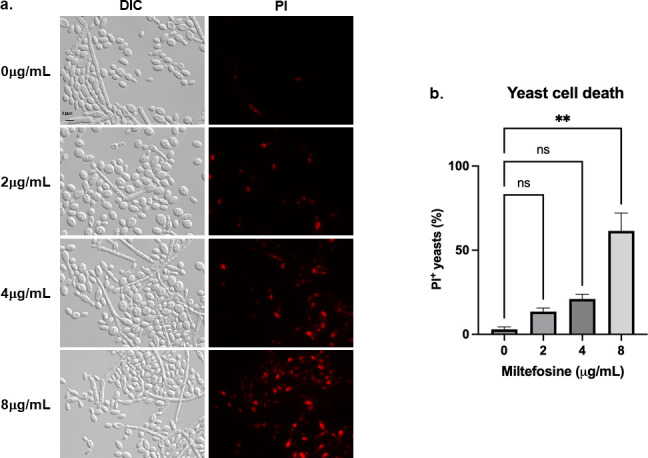
MFS causes dose-dependent death in S. brasiliensis yeast. (a) S. brasiliensis yeasts were exposed to 0, 2, 4, and 8 µg/mL of MFS for 6 h, stained with PI, and analyzed by fluorescence microscopy. (b) Quantification of PI+ yeast exposed to MFS, in which 100 yeast-like cells were counted for each condition. The results represent the average of two independent experiments. **P value < 0.001 when compared with untreated cells; ns: not significant.
ML decreases S. brasiliensis fungal burden in A549 pulmonary cells and bone marrow-derived macrophages (BMDM)
To determine the antifungal activity of ML against S. brasiliensis in the host tissues, two cell lines were used: lung A549 cells and bone marrow-derived macrophages (BMDMs). As shown in Fig. 5a, ML concentrations from 5 to 40 µg/mL did not reduce A549 cell viability compared with the control. A549 cells were challenged with 1:10 and 1:20 ratios (A549-yeast), and we observed a significant reduction of more than 90% in the fungal viability in both ML treatments, which contrasts with TRB treatment that shows about 50% viability (Fig. 5b).
Fig 5.
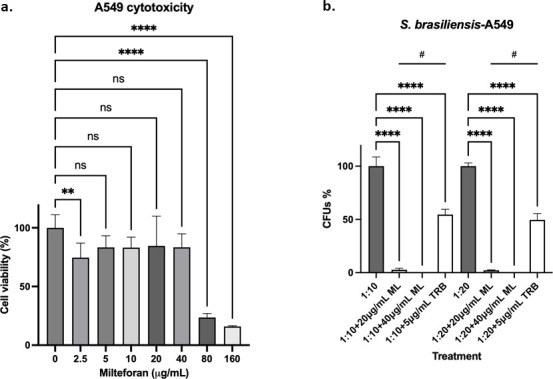
Concentrations from 5 to 40 µg/mL of ML are not toxic to human cells and can significantly decrease S. brasiliensis survival in A549 epithelial cells. (a) A459 epithelial cells were treated with different ML concentrations for 48 h, with a decrease of cell viability only at 80 µg/mL or higher concentrations. (b) A459 cells were challenged with S. brasiliensis yeasts at a proportion of 1:10 and 1:20 and then treated with 20 and 40 µg/mL of ML for 24 h. The fungicidal drug TRB was included as a control. **P-value < 0.01 when compared with untreated cells; ****P-value < 0.0001 when compared with untreated cells; #P-value < 0.0001 when compared with cells treated with TRB.
When we challenged BMDMs with S. brasiliensis at a 1:10 ratio (BMDMs yeast) in the presence of 20 and 40 µg/mL ML, we observed complete clearing of S. brasiliensis compared with TRB that showed about 80% and 40% clearing, respectively, at 24 and 48 h (Fig. 6). Our results strongly indicated that ML can help both A549 and BMDMs to clear S. brasiliensis infection.
Fig 6.
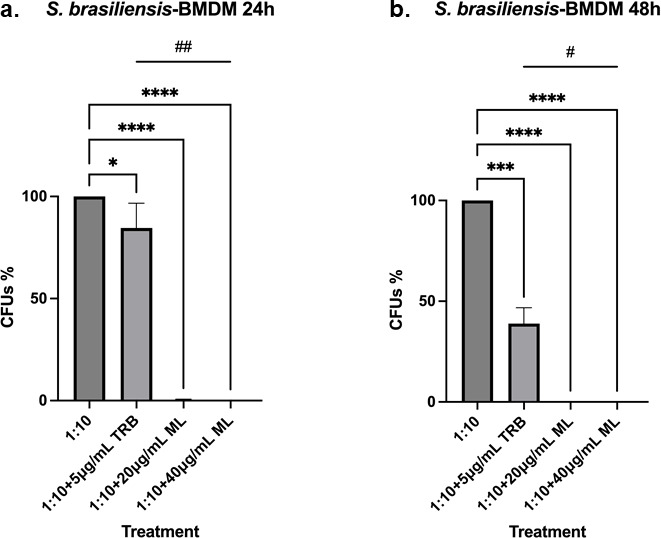
The killing of S. brasiliensis yeasts by BMDM significantly increases in the presence of ML. (a) BMDM cells were infected with S. brasiliensis yeasts and then treated with 20 and 40 µg/mL of ML for 24 h, which decreased the fungal survival by almost 100% when compared with untreated cells. (b) BMDM cells were infected with S. brasiliensis yeasts and were then treated with 20 and 40 µg/mL of ML for 48 h, which decreased the fungal survival to 100% when compared with untreated cells. The fungicidal drug TRB was included as a control. *P-value < 0.05 when compared with untreated cells; ***P-value < 0.0005 when compared with untreated cells; ****P-value < 0.0001 when compared with untreated cells; #P-value < 0.01 when compared with cells treated with TRB; ##P-value < 0.01 when compared with cells treated with TRB.
We also assessed the ability of the BMDMs to produce cytokines after stimulation by S. brasiliensis and treatment with the drug. It has already been reported that S. brasiliensis yeast stimulates higher production of TNF-α, IL-6, IL-1β, and IL-10 in human monocyte-derived macrophages when compared with S. schenckii, and it is also more phagocytosed (55), which might contribute to the higher virulence of this species.
After infection of BMDMs and treatment during 24 h, we observed a significant decrease in the stimulation of TNF-α and IL-6 when the yeast cells were treated with TRB and 20 and 40 µg/mL of ML, when compared with untreated cells (1:10) (Fig. 7a). However, when compared with TRB treatment, a significant decrease was observed in the stimulation of TNF-α only at 40 µg/mL of ML. In contrast, no difference was observed in the case of IL-6 with both ML concentrations compared with TRB. Finally, for the secretion of IL-10, a significant decrease was only observed when the yeast cells were treated with both ML concentrations. However, no difference was found between the TRB treatment and untreated cells (Fig. 7a).
Fig 7.
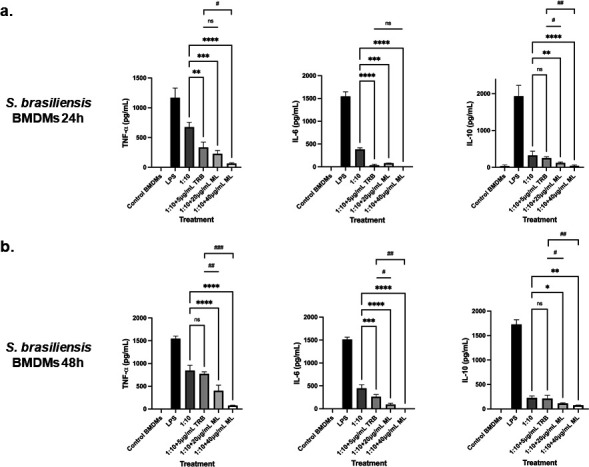
Cytokine secretion by BMDM infected with S. brasiliensis and treated with ML. (a) BMDM cells were infected with S. brasiliensis yeasts and treated with 20 and 40 µg/mL of ML for 24 h. The interaction supernatant was collected and the cytokines TNF-α (ns: not significant; **P-value < 0.005 when compared with untreated cells; ***P-value < 0.0005 when compared with untreated cells; ****P-value < 0.0001 when compared with untreated cells; #P-value < 0.01 when compared with TRB treatment), IL-6 (ns: not significant; ***P-value < 0.0005 when compared with untreated cells; ****P-value < 0.0001 when compared with untreated cells), and IL-10 (ns: not significant; **P-value < 0.005 when compared with untreated cells; ****P-value < 0.0001 when compared with untreated cells; #P-value < 0.0005 when compared with TRB treatment; ##P-value < 0.0001 when compared with TRB treatment) were measured. (b) BMDM cells were infected with S. brasiliensis yeasts and treated with 20 and 40 µg/mL of ML for 48 h. The interaction supernatant was collected and the cytokines TNF-α (ns: not significant; ****P-value < 0.0001 when compared with untreated cells; #P-value < 0.0005 when compared with TRB treatment; ##P-value < 0.0001 when compared with TRB treatment), IL-6 (***P-value < 0.001 when compared with untreated cells; ****P-value < 0.0001 when compared with untreated cells; #P-value < 0.005 when compared with TRB treatment; ##P-value < 0.0001 when compared with TRB treatment), and IL-10 (ns: not significant; *P-value < 0.05 when compared with untreated cells; **P-value < 0.01 when compared with untreated cells; #P-value < 0.05 when compared with TRB treatment; ##P-value < 0.01 when compared with TRB treatment) were measured.
After 48 h of infection, treatment with TRB did not cause a significant decrease in the TNF-α production, whereas both ML concentrations did when compared with untreated cells and TRB treatment (Fig. 7b). In the case of the IL-6 secretion, the same trend as that of 24 h was observed, with the only exception that treatment with 20 and 40 µg/mL of ML results in a significant decrease compared with TRB (Fig. 7b). The secretion of IL-10 did not decrease with the TRB treatment while significantly decreased in macrophages infected and uninfected treated with ML, confirming the participation of this drug in the immune response modulation (Fig. 7b).
DISCUSSION
Although there are several therapeutic options for the treatment of sporotrichosis, fungal resistance and cytotoxicity of the drugs to the host are essential obstacles that hinder the efficient recovery of the patient. ITZ is considered the first-line treatment, an azole known for its fungistatic activity against Sporothrix species (22, 24), which has increased the development of resistance in some isolates, mainly from S. brasiliensis, the most virulent species in the clinical clade (46, 56, 57). TRB, a drug with fungicidal activity against Sporothrix, has been reported to be effective in treating the cutaneous forms but not for the disseminated infections, for which AMB is used. AMB is considered a second-line treatment and is commonly used to treat the invasive and disseminated forms, with the disadvantage that it is very toxic in the doses and time needed to eradicate the infection, in addition to recent reports of isolates resistant to this antifungal agent (22, 46). Additional important variables to take under consideration when trying to establish a protocol for the sporotrichosis treatment, include (i) the etiological agent causing the infection, due to differences in the virulence profiles and the antifungal susceptibilities between the Sporothrix species; (ii) the clinical form affecting the patient; (iii) the immune status of the host, since this mycosis can cause disseminated fatal infections in immunocompromised patients; and (iv) the site of inoculation and the inoculum size (58). Sporotrichosis is a challenging mycosis to treat due to the aforementioned factors, including the difficulty of rapid diagnosis. The emergence of drug resistance and medication toxicity further complicate treatment, especially in endemic areas like Brazil, and these challenges are becoming a growing concern in other countries.
In Brazil, cat-transmitted sporotrichosis due to S. brasiliensis has been a critical health threat since 1998 (5, 8), spreading across the country and affecting both humans and domestic animals such as cats and dogs. This ongoing outbreak underscores the urgent need for new drugs to treat and control this mycosis. One promising approach is drug repurposing, which involves using existing drugs already approved for treating other diseases to treat new infections. This method has been proposed as an excellent alternative for finding new therapies. For instance, commercial MFS, developed initially as an antineoplastic drug (27, 28), is now the only available oral treatment for leishmaniasis in dogs and humans (29–32). Recent studies have shown its effectiveness against Candida species (39, 40). As previously demonstrated (45, 46, 48), we also found that MFS exhibits in vitro fungicidal activity against Sporothrix species by inhibiting the growth of both fungal morphologies. Strains of S. brasiliensis and S. schenckii were sensitive to low concentrations of this drug, with MIC and MFC values of 2 µg/mL for both hyphae and yeast-like cells. Unlike ITZ, no strain resistant to MFS or ML was found in our studies.
We also assessed the ability of MFS to synergize with other drugs used for the treatment of sporotrichosis, including TRB, ITZ, and AMB, and as previously reported for ITZ (45), MFS does not synergize the activity of these antifungals. However, it has an additive effect, suggesting that they do not interact on independent pathways (59).
Although these results in vitro could suggest a positive clinical outcome in the treatment of sporotrichosis with MFS, there is a lack of studies associating the in vitro susceptibility of the Sporothrix species with the in vivo therapeutic response (14). Actually, no association between lower MIC values of AMB and ITR with a higher cure rate was found, which might suggest that other factors, such as the immune status of the patient, the degree of dissemination, the early start of the treatment, and the antifungal absorption in the host, are as important as antifungal susceptibility (60, 61). Therefore, the in vitro findings could differ from the in vivo outcome. An example of this was observed when the effectiveness of MFS was evaluated in cats with refractory sporotrichosis (62). Among 10 cats that had been treated with ITZ or potassium iodide for over a year, one cat received MFS (2 mg/kg orally every 24 h) for 45 days, 6 for 30 days, 1 for 21 days, 1 for 15 days, and 1 for 3 days. Most of the cats treated with MFS showed disease progression, along with hyporexia and weight loss, suggesting MFS treatment failure (62). However, further studies with a larger number of patients and testing different MFS concentrations and treatment duration are needed to assess the MFS efficacy in vivo.
In fungi, the MFS mechanism of action is still poorly understood, but it was found in Saccharomyces cerevisiae that the drug uptake is fast, penetrating the mitochondrial inner membrane and disrupting its potential, which eventually leads to apoptosis. One of the MFS potential targets was identified, COX9, which encodes a subunit of the cytochrome c oxidase complex in the electron transport chain of the mitochondrial membrane (63). An increase in plasma membrane permeability, a decrease in mitochondrial membrane potential, and an increase in ROS production after treatment with MFS were also observed in Cryptococcus neoformans and Scedosporium aurantiacum (41, 64). Interestingly, Scedosporium genera and Sporothrix species are the only medically relevant fungi reported to present rhamnose in their cell walls (65).
In S. brasiliensis yeasts, it was previously found that MFS alters the plasma membrane integrity, decreases the cell wall thickness, increases the microfibrillar layer (peptidorhamnomannan) thickness, and increases the melanin content in the cell wall (24). These results explain the localization of MFS in S. brasiliensis yeasts mitochondria and cell surface that we observed, confirming that MFS mechanism of action is related to the mitochondria and cell membrane integrity and suggesting that this is indeed a multi-target drug.
MFS and ML have been reported to be toxic at high doses in mice, with high mortality in concentrations higher than 25 mg/kg (66, 67), with maximum concentrations of the drug in the kidney and liver, probably due to its amphiphilic nature (68, 69). Therefore, we assessed ML cytotoxicity in A549 human pulmonary cells and observed a significant viability reduction at 80 µg/mL. When we tested the ability of ML to decrease the fungal burden in A549 cells and BMDMs, at 24 h, and 24 and 48 h, respectively, we observed that ML could significantly reduce the CFUs more efficiently than the fungicidal drug TRB in both cell types, with an almost complete clearing of the yeast cells as early as 24 h of treatment. These results show that ML is capable of killing the parasitic morphology of S. brasiliensis in the host tissue at a concentration high enough to exert fungicidal activity, yet low enough to avoid toxicity to host cells.
Another proposed mechanism of action for MFS is its immunomodulatory ability, which is essential for treating leishmaniasis. MFS induces the Th1 response and suppresses the Th2 response by increasing the production of proinflammatory cytokines such as IFNγ, TNFα, and IL-12, aiding in the clearance of intracellular pathogens. This immunomodulation is vital to avoid relapses of leishmaniasis, which are associated with an increased Th2 response and higher IL-10 production (32, 38). Here, we observed that ML decreases the fungal burden and the production of TNFα, IL-6, and IL-10 in infected BMDMs. Thus, we propose three non-excluded hypotheses to explain this immune response: (i) the reduction in cytokines might be related to the drug killing the yeasts before they are phagocytosed, as we observed MFS-induced yeast cell death as early as six hours; (ii) MFS, localized to the cell surface, might act as an opsonizing agent, enhancing macrophage recognition and subsequent phagocytosis; and (iii) MFS could bind to essential virulence factors, such as adhesins, or to immunogenic cell wall components like β-glucans. This binding may attenuate S. brasiliensis’ ability to infect and elicit a robust immune response, facilitating fungal clearance. All three mechanisms would reduce the fungal load, tissue damage, and inflammation, thereby decreasing Sporothrix virulence and positioning MFS as a promising alternative treatment for feline sporotrichosis.
As previously mentioned, the in vitro response of an antifungal against Sporothrix may not accurately predict treatment outcomes in an in vivo model, and this has been observed with these pathogenic fungi. Therefore, we only suggest MFS as a potential candidate for treating sporotrichosis in cats, the primary vector for this mycosis transmission. However, the optimal dosage, treatment duration, and specific characteristics of the host need further evaluation to determine the efficacy of MFS for feline sporotrichosis treatment.
MATERIALS AND METHODS
Fungal strains and culture conditions
In this study, three Sporothrix schenckii (ATCC-MYA 4820, ATCC-MYA 4821, and ATCC-MYA 4822) and three S. brasiliensis strains (ATCC-MYA 4823, ATCC-MYA 4824, and ATCC-MYA 4858) were used for the in vitro antifungal susceptibility assays; S. schenckii ATCC-MYA 4821 and S. brasiliensis ATCC- MYA 4823 were used for the checkerboard assays; and S. brasiliensis ATCC-MYA 4823, a highly virulent clinical isolate obtained from feline sporotrichosis (70), was used for the infection assays.
The mycelial phase from Sporothrix spp. was obtained and maintained on solid YPD pH 4.5 [yeast extract 1% (wt/vol), gelatin peptone 2% (wt/vol), and dextrose 3% (wt/vol)] at 28°C for 4 days. In contrast, the yeast morphology was grown in liquid YPD pH 7.8, at 37°C under orbital agitation for 4 days, as previously reported (71). For the experiments in which we needed a single morphology, the cultures were filtered with sterile miracloth (Calbiochem) to avoid contamination with the unwanted fungal morphotype. Each phase was confirmed by observing the cells with light microscopy.
Antifungal drugs
For the in vitro assays, voriconazole (VCZ, Sigma-Aldrich), itraconazole (ITZ, Sigma-Aldrich), amphotericin B (AMB, Sigma-Aldrich), terbinafine (TRB, Sigma-Aldrich), and brilacidin (BRI, supplied by Innovation Pharmaceuticals) were diluted in dimethyl sulfoxide (DMOS); while miltefosine (MFS, Sigma-Aldrich), the milteforan active compound, was diluted in ethanol; and caspofungin (CSP, Sigma-Aldrich) was diluted in distilled water. Milteforan (miltefosine 2%) was purchased from Virbac as an oral solution.
In vitro antifungal susceptibility testing
The minimum inhibitory concentrations (MICs) were determined by the broth microdilution method adapted from protocols published by the Clinical Laboratory Standard Institute for the mycelial and yeast phases (24, 72). Briefly, serial two-fold dilutions of the antifungal drugs were performed in YPD pH 4.5 and 7.8, for mycelial and yeast, respectively, into 96-well microtiter plates to obtain concentrations of 4–0.06 μg/mL for CSP, VCZ, and TRB; 8–0.125 μg/mL for ITZ and AMB; 16–0.25 μg/mL for MFS and ML; and 80–1.25 μM for BRI, with a final concentration of 2 × 103 and 2 × 104 conidia or yeast cells, respectively, in a volume of 100 µL. The plates were incubated at 28°C (for conidia) or 37°C (for yeast) for 4 days, and the MIC was determined by visual inspection and defined as the lowest concentration that inhibits 90-100% of fungal growth about untreated cells. Finally, 5 µL of conidia or yeast cells from each well were grown in drug-free solid YPD pH 4.5 and pH 7.8 at 28°C and 37°C, respectively, for 4 days. The minimum fungicidal concentration (MFC) value was the lowest concentration, showing no fungal growth. Three independent experiments were performed by duplicate.
Checkerboard assays and synergy testing
The drug combination effect was determined through the MIC and MFC values of the yeast phase, as described before. Briefly, serial twofold dilutions of the antifungal drugs were performed in liquid YPD pH 7.8 containing half MIC of MFS or ML (1 µg/mL) in 96-well microtiter plates to obtain concentrations of 16–0.25 μg/mL for CSP and VCZ; 8–0.125 μg/mL for ITZ and AMB; 4–0.06 μg/mL for TRB; and 80–1.25 μM for BRI, with a final concentration of 2 × 104 yeast, in a volume of 100 µL. The plates were incubated at 37°C for 4 days, and the MIC was determined by visual inspection. MIC was defined as the lowest concentration inhibiting 90%–100% of fungal growth in cells treated only with 1 µg/mL of MFS or ML. After MIC determination, 5 µL of yeast from each well were grown in drug-free solid YPD pH 7.8 at 37°C for 4 days. The MFC value was the lowest concentration, which showed no fungal growth.
Checkerboard assays were performed to quantify the interaction (synergistic, additive, or antagonistic) between MFS and ITZ, AMB, or TRB. Briefly, a stock solution of 2 × 105 yeast/mL and each drug (8 µg of MFS and 16 µg/mL of ITZ, 16 µg of AMB, or 8 µg of TRB) were prepared in RMPI-1640. In 96-well microtiter plates, the first antibiotic (MFS) was diluted sequentially along the ordinate. In contrast, the second drug (ITZ, AMB, or TRB) was diluted along the abscissa to obtain a final volume of 100 µL. The plates were incubated at 37°C for 4 days, and the metabolic activity was determined through the XTT reduction assay (47). Briefly, 50 µL of a solution of XTT 1 mg/mL and menadione 1 mM resuspended in water were added to each well, mixed, and incubated in the dark at 37°C for three h. The supernatant of each well was transferred to a new plate and read in a spectrophotometer at 492 nm. Results are expressed as means ± SD of three independent experiments.
The SynergyFinder software (52) was used to determine the type of drug interaction with the following parameters: detect outliners: yes; curve fitting: LL4; method: Bliss; correction: on. The summary synergy scores represent the average excess response due to drug interaction, in which a value less than −10 suggests an antagonistic interaction between two drugs; values from −10 to 10 suggest an additive interaction; and values larger than 10 suggest a synergistic interaction.
Yeast cells death
The effect of ML on the cell membrane potential was assessed by staining with propidium iodide (PI). Yeast cells grown for 4 days in liquid YPD pH 7.8 were treated with 0, 2, 4, and 8 µg/mL of ML for 6 h, stained with PI 20 mM for 30 minutes, and washed with PBS 1 × three times. Fluorescence was analyzed at an excitation wavelength of 572/25 nm and emission of 629/62 nm with the Observer Z1 fluorescence microscope using a 100 × oil immersion lens objective. Differential interference contrast (DIC) and fluorescent images were captured with an AxioCam camera (Carl Zeiss) and processed using AxioVision software (version 4.8). The experiment was performed twice, and at least 100 cells were counted for each treatment. The results were plotted using GraphPad Prism (GraphPad Software, Inc). A P-value < 0.001 was considered significant.
Miltefosine localization
S. brasiliensis yeast cells cultured for 4 days in YPD pH 7.8 were washed three times with PBS 1 × and then treated with the fluorescent MFS analog MT-11 C-BDP (excitation wavelength 450–490nm and emission wavelength 500–550nm) for 6 hours, also in liquid YPD pH 7.8. The cells were washed three times, stained with 250 nM of MitoTracker Deep Red FM (Invitrogen) (wavelength absorbance/emission 644/665 nm), and washed again. The yeast cells were visualized in slides with the Observer Z1 fluorescent microscope using a 100 x oil immersion lens objective. DIC and fluorescent images were captured with an AxioCam camera (Carl Zeiss) and processed using AxioVision software (version 4.8). Two independent experiments were performed, and 100 cells were counted each to calculate the merge %.
Cytotoxicity assay
The cytotoxicity of ML was determined in A549 human lung cancer cells using the XTT reduction assay. A total of 2 × 105 cells/well were seeded in 96-well tissue plates and incubated in Dulbecco’s Modified Eagle Medium (DMEM, Thermo Fischer). After 24 h of incubation with CO2 5%, the cells were treated with different concentrations of ML (0, 2.5, 5, 10, 20, 40, 80, and 160 µg/mL), and after 48 h of incubation, cell viability was assessed using the XTT assay. Briefly, 80 µL of a solution of XTT 1 mg/mL in DMEM, HEPES 1 M, and menadione 8 µg/mL were added to each well, and after 30 min, formazan formation was quantified spectrophotometrically at 450 nm using a microplate reader. Each treatment was performed in triplicate, and the results were plotted using GraphPad Prism (GraphPad Software, Inc). A P-value < 0.0001 was considered significant.
A549 and bone marrow-derived macrophages (BMDMs) killing assays
The cytotoxicity of ML was determined in A549 human lung cancer cells [ATCC, CCL-185, derived from Rio de Janeiro Cell Bank, Brazil (BCRJ-0033) passage (5–10)] using the XTT reduction assay. The cell line A549 and BMDMs were cultured using DMEM supplemented with fetal bovine serum (FBS) 10% and penicillin-streptomycin 1% (Sigma-Aldrich) and seeded at a concentration of 1 × 106 cells/mL in 24-well plates (Corning). The cells were challenged with S. brasiliensis yeasts at a multiplicity of infection of 1:10 and were then treated with ML 20 and 40 µM. We included untreated cells and cells treated with TRB 5 (g/mL) as a control. For the BMDMs, cells treated with LPS were also included as controls. The A549 were incubated for 24 h at 37°C with CO2 5%, while the BMDM were incubated for 24 and 48 h under the same conditions. After incubation, the culture media was removed, each well was washed three times with PBS 1×, and 1 mL of sterile cold water was added to recover and collect the cell monolayer. To assess the number of CFUs, 100 µL of the cell suspensions were plated on YDP pH 4.5 and incubated at 28°C for 4 days. When necessary, the cell suspensions were diluted at 1:100 or 1:1000, and 100 µL were plated. 50 µL of the inoculum adjusted to 1 × 103 cells/mL was also plated to correct the CFU count. Each treatment was performed in triplicate to calculate the CFU %, and the results were plotted using GraphPad Prism (GraphPad Software, Inc). A P-value < 0.0001 was considered significant.
Cytokines quantification
The Elisa-assay kits (R&D Systems) were used to evaluate the concentration of the proinflammatory cytokines TNFα and IL-6, and the anti-inflammatory cytokine IL-10 in the supernatants of the S. brasiliensis and BMDMs interaction for 24 and 48 h, according to the manufacturer’s instruction. The plate’s absorbance was read at 450 nm, and the cytokine concentration (pg/mL) was calculated according to the values obtained in the standard curve of each cytokine. The results were plotted using GraphPad Prism (GraphPad Software, Inc).
Statistical analyses
The GraphPad Prism 10 (GraphPad Software, Inc.) was used for the statistical analyses. The results are reported as the media ± SD from two or three independent experiments performed by duplicate and were analyzed using the Ordinary one-way ANOVA or the Unpaired t-test. The statistical significance was considered with a P-value < 0.05 or lower.
Supplementary Material
ACKNOWLEDGMENTS
We thank the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) grant numbers 2021/04977–5 (GHG) and 2022/08556–7 (LCGC) 2022/08796–8 (CD), 2022/09882–5 (LP), the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Fundação Coordenação de Aperfeiçoamento do Pessoal do Ensino Superior (CAPES) grant number 405934/2022–0 (The National Institute of Science and Technology INCT Funvir), and CNPq 301058/2019–9 from Brazil to GHG., both from Brazil, the National Institutes of Health/National Institute of Allergy and Infectious Diseases from the USA, grant R01AI153356 to GHG. This work was also funded by the Joint Canada-Israel Health Research Program, jointly supported by the Azrieli Foundation, Canada’s International Development Research Centre, Canadian Institutes of Health Research, and the Israel Science Foundation (GHG).
Contributor Information
Gustavo H. Goldman, Email: ggoldman@usp.br.
Alexandre Alanio, Institut Pasteur, Paris, France.
Luana Pereira Borba-Santos, Federal University of Rio de Janeiro, Rio de Janeiro, Brazil.
SUPPLEMENTAL MATERIAL
The following material is available online at https://doi.org/10.1128/spectrum.00474-24.
An accounting of the reviewer comments and feedback.
ASM does not own the copyrights to Supplemental Material that may be linked to, or accessed through, an article. The authors have granted ASM a non-exclusive, world-wide license to publish the Supplemental Material files. Please contact the corresponding author directly for reuse.
REFERENCES
- 1. Rodrigues AM, Della Terra PP, Gremião ID, Pereira SA, Orofino-Costa R, de Camargo ZP. 2020. The threat of emerging and re-emerging pathogenic Sporothrix species. Mycopathologia 185:813–842. doi: 10.1007/s11046-020-00425-0 [DOI] [PubMed] [Google Scholar]
- 2. Chakrabarti A, Bonifaz A, Gutierrez-Galhardo MC, Mochizuki T, Li S. 2015. Global epidemiology of sporotrichosis. Med Mycol 53:3–14. doi: 10.1093/mmy/myu062 [DOI] [PubMed] [Google Scholar]
- 3. Barros MBL, Schubach AO, Schubach TMP, Wanke B, Lambert-Passos SR. 2008. An epidemic of sporotrichosis in Rio de Janeiro, Brazil: epidemiological aspects of a series of cases. Epidemiol Infect 136:1192–1196. doi: 10.1017/S0950268807009727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Falcão EMM, Pires MC de S, Andrade HB, Gonçalves MLC, Almeida-Paes R, do Valle ACF, Bastos FI, Gutierrez-Galhardo MC, Freitas DFS. 2020. Zoonotic sporotrichosis with greater severity in Rio de Janeiro, Brazil: 118 hospitalizations and 11 deaths in the last 2 decades in a reference institution. Med Mycol 58:141–143. doi: 10.1093/mmy/myz024 [DOI] [PubMed] [Google Scholar]
- 5. Gremião IDF, Oliveira MME, Monteiro de Miranda LH, Saraiva Freitas DF, Pereira SA. 2020. Geographic expansion of sporotrichosis, Brazil. Emerg Infect Dis 26:621–624. doi: 10.3201/eid2603.190803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Marimon R, Cano J, Gené J, Sutton DA, Kawasaki M, Guarro J. 2007. Sporothrix brasiliensis, S. globosa, and S. mexicana, three new Sporothrix species of clinical interest. J Clin Microbiol 45:3198–3206. doi: 10.1128/JCM.00808-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. de Beer ZW, Duong TA, Wingfield MJ. 2016. The divorce of Sporothrix and Ophiostoma: solution to a problematic relationship. Stud Mycol 83:165–191. doi: 10.1016/j.simyco.2016.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lopes-Bezerra LM, Mora-Montes HM, Zhang Y, Nino-Vega G, Rodrigues AM, de Camargo ZP, de Hoog S. 2018. Sporotrichosis between 1898 and 2017: the evolution of knowledge on a changeable disease and on emerging etiological agents. Med Mycol 56:126–143. doi: 10.1093/mmy/myx103 [DOI] [PubMed] [Google Scholar]
- 9. de Lima Barros MB, de Almeida Paes R, Schubach AO. 2011. Sporothrix schenckii and Sporotrichosis. Clin Microbiol Rev 24:633–654. doi: 10.1128/CMR.00007-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. de Carolis E, Posteraro B, Sanguinetti M. 2022. Old and new insights into Sporothrix schenckii complex biology and identification. Pathogens 11:297. doi: 10.3390/pathogens11030297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Arrillaga-Moncrieff I, Capilla J, Mayayo E, Marimon R, Mariné M, Gené J, Cano J, Guarro J. 2009. Different virulence levels of the species of Sporothrix in a murine model. Clin Microbiol Infect 15:651–655. doi: 10.1111/j.1469-0691.2009.02824.x [DOI] [PubMed] [Google Scholar]
- 12. Della Terra PP, Rodrigues AM, Fernandes GF, Nishikaku AS, Burger E, de Camargo ZP. 2017. Exploring virulence and immunogenicity in the emerging pathogen Sporothrix brasiliensis. PLoS Negl Trop Dis 11:e0005903. doi: 10.1371/journal.pntd.0005903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gutierrez-Galhardo MC, Freitas DFS, Do Valle ACF. 2015. Clinical forms of human sporotrichosis and host immunocompetence, p 73–82. In Zeppone Carlos I (ed), Sporotrichosis. Springer, Cham. [Google Scholar]
- 14. García Carnero LC, Lozoya Pérez NE, González Hernández SE, Martínez Álvarez JA. 2018. Immunity and treatment of sporotrichosis. J Fungi (Basel) 4:3. doi: 10.3390/jof4030100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gremião IDF, Miranda LHM, Reis EG, Rodrigues AM, Pereira SA. 2017. Zoonotic epidemic of sporotrichosis: cat to human transmission. PLoS Pathog 13:e1006077. doi: 10.1371/journal.ppat.1006077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. de Oliveira Bento A, de Sena Costa AS, Lima SL, do Monte Alves M, de Azevedo Melo AS, Rodrigues AM, da Silva-Rocha WP, Milan EP, Chaves GM. 2021. The spread of cat-transmitted sporotrichosis due to Sporothrix brasiliensis in Brazil towards the Northeast region. PLoS Negl Trop Dis 15:e0009693. doi: 10.1371/journal.pntd.0009693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Boechat JS, Oliveira MME, Gremião IDF, Almeida-Paes R, Machado AC de S, Zancopé-Oliveira RM, Oliveira R de VC, Morgado DS, Corrêa ML, Figueiredo ABF, Menezes RC, Pereira SA. 2022. Sporothrix brasiliensis and feline sporotrichosis in the metropolitan region of Rio de Janeiro, Brazil (1998-2018). J Fungi (Basel) 8:749. doi: 10.3390/jof8070749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Morgado DS, Castro R, Ribeiro-Alves M, Corrêa-Moreira D, Castro-Alves J, Pereira SA, Menezes RC, Oliveira MME. 2022. Global distribution of animal sporotrichosis: a systematic review of Sporothrix sp. identified using molecular tools. Curr Res Microb Sci 3:100140. doi: 10.1016/j.crmicr.2022.100140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Marimon R, Serena C, Gené J, Cano J, Guarro J. 2008. In vitro antifungal susceptibilities of five species of Sporothrix. Antimicrob Agents Chemother 52:732–734. doi: 10.1128/AAC.01012-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rodrigues AM, de Hoog GS, de Cássia Pires D, Brihante RSN, Sidrim JJ da C, Gadelha MF, Colombo AL, de Camargo ZP. 2014. Genetic diversity and antifungal susceptibility profiles in causative agents of sporotrichosis. BMC Infect Dis 14:219. doi: 10.1186/1471-2334-14-219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Flórez-Muñoz SV, Alzate JF, Mesa-Arango AC. 2019. Molecular identification and antifungal susceptibility of clinical isolates of Sporothrix schenckii Complex in Medellin, Colombia. Mycopathologia 184:53–63. doi: 10.1007/s11046-018-0310-5 [DOI] [PubMed] [Google Scholar]
- 22. Kauffman CA, Bustamante B, Chapman SW, Pappas PG. 2007. Clinical practice guidelines for the management of sporotrichosis: 2007 update by the infectious diseases society of America. Clin Infect Dis 45:1255–1265. doi: 10.1086/522765 [DOI] [PubMed] [Google Scholar]
- 23. Orofino-Costa R, Freitas DFS, Bernardes-Engemann AR, Rodrigues AM, Talhari C, Ferraz CE, Veasey JV, Quintella L, Sousa MSLA de, Vettorato R, Almeida-Paes R de, de Macedo PM. 2022. Human sporotrichosis: recommendations from the Brazilian society of dermatology for the clinical, diagnostic and therapeutic management. An Bras Dermatol 97:757–777. doi: 10.1016/j.abd.2022.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Borba-Santos LP, Rodrigues AM, Gagini TB, Fernandes GF, Castro R, de Camargo ZP, Nucci M, Lopes-Bezerra LM, Ishida K, Rozental S. 2015. Susceptibility of Sporothrix brasiliensis isolates to amphotericin B, azoles, and terbinafine. Med Mycol 53:178–188. doi: 10.1093/mmy/myu056 [DOI] [PubMed] [Google Scholar]
- 25. Waller SB, Dalla Lana DF, Quatrin PM, Ferreira MRA, Fuentefria AM, Mezzari A. 2021. Antifungal resistance on Sporothrix species: an overview. Braz J Microbiol 52:73–80. doi: 10.1007/s42770-020-00307-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bombassaro A, Spruijtenburg B, Medeiros F, Jacomel Favoreto de Souza Lima B, Ballardin LB, Farias MR de, Vicente VA, de Queiroz-Telles F, Meis JF, de Groot T. 2023. Genotyping and antifungal susceptibility testing of Sporothrix brasiliensis isolates from Southern Brazil. Mycoses 66:585–593. doi: 10.1111/myc.13584 [DOI] [PubMed] [Google Scholar]
- 27. Unger C, Damenz W, Fleer EAM, Kim DJ, Breiser A, Hilgard P, Engel J, Nagel G, Eibl H. 1989. Hexadecylphosphocholine, a new ether lipid analogue studies on the antineoplastic activity in vitro and in vivo. Acta Oncol 28:213–217. doi: 10.3109/02841868909111249 [DOI] [PubMed] [Google Scholar]
- 28. SpruB T, Bernhardt G, Schonenberger H, Engel J. 1993. Antitumour activity of miltefosine alone and after combination with platinum complexes on MXT mouse mammary carcinoma models. J Cancer Res Clin Oncol 119:142–149. doi: 10.1007/BF01229528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Croft SL, Snowdon D, Yardley V. 1996. The activities of four anticancer alkyllysophospholipids against Leishmania donovani, Trypanosoma cruzi and Trypanosoma brucei. J Antimicrob Chemother 38:1041–1047. doi: 10.1093/jac/38.6.1041 [DOI] [PubMed] [Google Scholar]
- 30. Sundar S, Rosenkaimer F, Makharia MK, Goyal AK, Mandal AK, Voss A, Hilgard P, Murray HW. 1998. Trial of oral miltefosine for visceral leishmaniasis. The Lancet 352:1821–1823. doi: 10.1016/S0140-6736(98)04367-0 [DOI] [PubMed] [Google Scholar]
- 31. Croft SL, Engel J. 2006. Miltefosine--discovery of the antileishmanial activity of phospholipid derivatives. Trans R Soc Trop Med Hyg 100 Suppl 1:S4–8. doi: 10.1016/j.trstmh.2006.03.009 [DOI] [PubMed] [Google Scholar]
- 32. Dorlo TPC, Balasegaram M, Beijnen JH, de vries PJ. 2012. Miltefosine: a review of its pharmacology and therapeutic efficacy in the treatment of leishmaniasis. J Antimicrob Chemother 67:2576–2597. doi: 10.1093/jac/dks275 [DOI] [PubMed] [Google Scholar]
- 33. Geilen CC, Wieder T, Reutter W. 1992. Hexadecylphosphocholine inhibits translocation of CTP:choline-phosphate cytidylyltransferase in Madin-Darby canine kidney cells. J Biol Chem 267:6719–6724. doi: 10.1016/S0021-9258(19)50485-9 [DOI] [PubMed] [Google Scholar]
- 34. Cui Z, Houweling M, Chen MH, Record M, Chap H, Vance DE, Tercé F. 1996. A genetic defect in phosphatidylcholine biosynthesis triggers apoptosis in Chinese hamster ovary cells. J Biol Chem 271:14668–14671. doi: 10.1074/jbc.271.25.14668 [DOI] [PubMed] [Google Scholar]
- 35. Paris C, Loiseau PM, Bories C, Bréard J. 2004. Miltefosine induces apoptosis-like death in Leishmania donovani promastigotes. Antimicrob Agents Chemother 48:852–859. doi: 10.1128/AAC.48.3.852-859.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Pinto-Martinez AK, Rodriguez-Durán J, Serrano-Martin X, Hernandez-Rodriguez V, Benaim G. 2018. Mechanism of action of miltefosine on Leishmania donovani involves the impairment of acidocalcisome function and the activation of the sphingosine-dependent plasma membrane Ca2+ channel. Antimicrob Agents Chemother 62:e01614-17. doi: 10.1128/AAC.01614-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wieder T, Orfanos CE, Geilen CC. 1998. Induction of ceramide-mediated apoptosis by the anticancer phospholipid analog, hexadecylphosphocholine. J Biol Chem 273:11025–11031. doi: 10.1074/jbc.273.18.11025 [DOI] [PubMed] [Google Scholar]
- 38. Palić S, Bhairosing P, Beijnen JH, Dorlo TPC. 2019. Systematic review of host-mediated activity of miltefosine in Leishmaniasis through immunomodulation. Antimicrob Agents Chemother 63:e02507-18. doi: 10.1128/AAC.02507-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Machado Vila TV, Sousa Quintanilha N, Rozental S. 2015. Miltefosine is effective against Candida albicans and Fusarium oxysporum nail biofilms in vitro. J Med Microbiol 64:1436–1449. doi: 10.1099/jmm.0.000175 [DOI] [PubMed] [Google Scholar]
- 40. Vila T, Ishida K, Seabra SH, Rozental S. 2016. Miltefosine inhibits Candida albicans and non-albicans Candida spp. biofilms and impairs the dispersion of infectious cells. Int J Antimicrob Agents 48:512–520. doi: 10.1016/j.ijantimicag.2016.07.022 [DOI] [PubMed] [Google Scholar]
- 41. Spadari C de C, Vila T, Rozental S, Ishida K. 2018. Miltefosine has a postantifungal effect and induces apoptosis in Cryptococcus yeasts. Antimicrob Agents Chemother 62:e00312-18. doi: 10.1128/AAC.00312-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Barreto TL, Rossato L, de Freitas ALD, Meis JF, Lopes LB, Colombo AL, Ishida K. 2020. Miltefosine as an alternative strategy in the treatment of the emerging fungus Candida auris. Int J Antimicrob Agents 56:106049. doi: 10.1016/j.ijantimicag.2020.106049 [DOI] [PubMed] [Google Scholar]
- 43. Dos Reis TF, Horta MAC, Colabardini AC, Fernandes CM, Silva LP, Bastos RW, Fonseca MV de L, Wang F, Martins C, Rodrigues ML, Silva Pereira C, Del Poeta M, Wong KH, Goldman GH. 2021. Screening of chemical libraries for new antifungal drugs against Aspergillus fumigatus reveals sphingolipids are involved in the mechanism of action of miltefosine. mBio 12:e0145821. doi: 10.1128/mBio.01458-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Nosratabadi M, Akhtari J, Faeli L, Haghani I, Aghili SR, Shokohi T, Hedayati MT, Zarrinfar H, Mohammadi R, Najafzadeh MJ, Khodavaisy S, Al-Harrasi A, Javan-Nikkhah M, Kachuei R, Salimi M, Fattahi M, Badali H, Al Hatmi AMS, Abastabar M. 2022. In vitro antifungal susceptibility profile of miltefosine against a collection of azole and echinocandins resistant Fusarium strains. J Fungi (Basel) 8:709. doi: 10.3390/jof8070709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Brilhante RSN, Malaquias ADM, Caetano EP, Castelo-Branco D de SCM, Lima RAC de, Marques FJ de F, Silva NF, Alencar LP de, Monteiro AJ, Camargo ZP de, Bandeira T de JPG, Rodrigues AM, Cordeiro R de A, Moreira JLB, Sidrim JJC, Rocha MFG. 2014. In vitro inhibitory effect of miltefosine against strains of Histoplasma capsulatum var. capsulatum and Sporothrix spp. Med Mycol 52:320–325. doi: 10.1093/mmy/myt027 [DOI] [PubMed] [Google Scholar]
- 46. Borba-Santos LP, Gagini T, Ishida K, de Souza W, Rozental S. 2015. Miltefosine is active against Sporothrix brasiliensis isolates with in vitro low susceptibility to amphotericin B or itraconazole. J Med Microbiol 64:415–422. doi: 10.1099/jmm.0.000041 [DOI] [PubMed] [Google Scholar]
- 47. Brilhante RSN, Silva M da, Pereira VS, de Oliveira JS, Maciel JM, Silva I da, Garcia LGS, Guedes G de M, Cordeiro R de A, Pereira-Neto W de A, de Camargo ZP, Rodrigues AM, Sidrim JJC, Castelo-Branco D de S, Rocha MFG. 2019. Potassium iodide and miltefosine inhibit biofilms of Sporothrix schenckii species complex in yeast and filamentous forms. Med Mycol 57:764–772. doi: 10.1093/mmy/myy119 [DOI] [PubMed] [Google Scholar]
- 48. dos Santos GMP, Borba-Santos LP, Vila T, Ferreira Gremião ID, Pereira SA, De Souza W, Rozental S. 2022. Sporothrix spp. biofilms impact in the zoonotic transmission route: feline claws associated biofilms, itraconazole tolerance, and potential repurposing for miltefosine. Pathogens 11:206. doi: 10.3390/pathogens11020206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Peyclit L, Yousfi H, Rolain JM, Bittar F. 2021. Drug repurposing in medical mycology: identification of compounds as potential antifungals to overcome the emergence of multidrug-resistant fungi. Pharmaceuticals (Basel) 14:488. doi: 10.3390/ph14050488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Profounda Pharmaceuticals . PROFOUNDA, INC. Available from: https://www.profounda.com. Retrieved 1313 AugAugust 2023. Accessed , 1313 AugAugust 2023
- 51. Ishida K, Castro RA, Torrado JJ, Serrano DR, Borba-Santos LP, Quintella LP, de Souza W, Rozental S, Lopes-Bezerra LM. 2018. Efficacy of a poly-aggregated formulation of amphotericin B in treating systemic sporotrichosis caused by Sporothrix brasiliensis. Med Mycol 56:288–296. doi: 10.1093/mmy/myx040 [DOI] [PubMed] [Google Scholar]
- 52. Ianevski A, Giri AK, Aittokallio T. 2022. SynergyFinder 3.0: an interactive analysis and consensus interpretation of multi-drug synergies across multiple samples. Nucleic Acids Res 50:W739–W743. doi: 10.1093/nar/gkac382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Santa-Rita RM, Henriques-Pons A, Barbosa HS, de Castro SL. 2004. Effect of the lysophospholipid analogues edelfosine, ilmofosine and miltefosine against Leishmania amazonensis. J Antimicrob Chemother 54:704–710. doi: 10.1093/jac/dkh380 [DOI] [PubMed] [Google Scholar]
- 54. Davey HM, Hexley P. 2011. Red but not dead? Membranes of stressed Saccharomyces cerevisiae are permeable to propidium iodide. Environ Microbiol 13:163–171. doi: 10.1111/j.1462-2920.2010.02317.x [DOI] [PubMed] [Google Scholar]
- 55. Gómez-Gaviria M, Martínez-Duncker I, García-Carnero LC, Mora-Montes HM. 2023. Differential recognition of Sporothrix schenckii, Sporothrix brasiliensis, and Sporothrix globosa by human monocyte-derived macrophages and dendritic cells. Infect Drug Resist 16:4817–4834. doi: 10.2147/IDR.S419629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kohler LM, Soares BM, de Assis Santos D, Da Silva Barros ME, Hamdan JS. 2006. In vitro susceptibility of isolates of Sporothrix schenckii to amphotericin B, itraconazole, and terbinafine: comparison of yeast and mycelial forms. Can J Microbiol 52:843–847. doi: 10.1139/w06-040 [DOI] [PubMed] [Google Scholar]
- 57. Alvarado-Ramírez E, Torres-Rodríguez JM. 2007. In vitro susceptibility of Sporothrix schenckii to six antifungal agents determined using three different methods. Antimicrob Agents Chemother 51:2420–2423. doi: 10.1128/AAC.01176-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. García-Carnero LC, Pérez-García LA, Martínez-Álvarez JA, Reyes-Martínez JE, Mora-Montes HM. 2018. Current trends to control fungal pathogens: exploiting our knowledge in the host-pathogen interaction. Infect Drug Resist 11:903–913. doi: 10.2147/IDR.S170337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Roell KR, Reif DM, Motsinger-Reif AA. 2017. An introduction to terminology and methodology of chemical synergy-perspectives from across disciplines. Front Pharmacol 8:158. doi: 10.3389/fphar.2017.00158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Espinel-Ingroff A, Abreu DPB, Almeida-Paes R, Brilhante RSN, Chakrabarti A, Chowdhary A, Hagen F, Córdoba S, Gonzalez GM, Govender NP, et al. 2017. Multicenter, international study of MIC/MEC distributions for definition of epidemiological cutoff values for Sporothrix species identified by molecular methods. Antimicrob Agents Chemother 61:e01057–17. doi: 10.1128/AAC.01057-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Fichman V, Almeida-Silva F, Francis Saraiva Freitas D, Zancopé-Oliveira RM, Gutierrez-Galhardo MC, Almeida-Paes R. 2022. Severe sporotrichosis caused by Sporothrix brasiliensis: antifungal susceptibility and clinical outcomes. J Fungi (Basel) 9:49. doi: 10.3390/jof9010049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Silva FDS, Cunha S dos S, Baptista A de S, Baptista VDS, Silva K da, Coêlho TFQ, Ferreira AMR. 2018. Miltefosine administration in cats with refractory sporotrichosis. Acta Scient Vet 46:7. doi: 10.22456/1679-9216.83639 [DOI] [Google Scholar]
- 63. Zuo X, Djordjevic JT, Bijosono Oei J, Desmarini D, Schibeci SD, Jolliffe KA, Sorrell TC. 2011. Miltefosine induces apoptosis-like cell death in yeast via Cox9p in cytochrome c oxidase. Mol Pharmacol 80:476–485. doi: 10.1124/mol.111.072322 [DOI] [PubMed] [Google Scholar]
- 64. Rollin-Pinheiro R, Almeida Y de C, Rochetti VP, Xisto M da S, Borba-Santos LP, Rozental S, Barreto-Bergter E. 2021. Miltefosine against Scedosporium and Lomentospora species: antifungal activity and its effects on fungal cells. Front Cell Infect Microbiol 11:698662. doi: 10.3389/fcimb.2021.698662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Mora-Montes HM, García-Gutiérrez K, García-Carnero LC, Lozoya-Pérez NE, Ramirez-Prado JH. 2022. The search for cryptic L-rhamnosyltransferases on the Sporothrix schenckii genome. JoF 8:529. doi: 10.3390/jof8050529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Godinho JLP, Simas-Rodrigues C, Silva R, Ürmenyi TP, de Souza W, Rodrigues JCF. 2012. Efficacy of miltefosine treatment in Leishmania amazonensis-infected BALB/c mice. Int J Antimicrob Agents 39:326–331. doi: 10.1016/j.ijantimicag.2011.11.008 [DOI] [PubMed] [Google Scholar]
- 67. García Bustos MF, Barrio A, Prieto GG, de Raspi EM, Cimino RO, Cardozo RM, Parada LA, Yeo M, Soto J, Uncos DA, Parodi C, Basombrío MA. 2014. In vivo antileishmanial efficacy of miltefosine against Leishmania (Leishmania) amazonensis. J Parasitol 100:840–847. doi: 10.1645/13-376.1 [DOI] [PubMed] [Google Scholar]
- 68. Kötting J, Berger MR, Unger C, Eibl H. 1992. Alkylphosphocholines: influence of structural variation on biodistribution at antineoplastically active concentrations. Cancer Chemother Pharmacol 30:105–112. doi: 10.1007/BF00686401 [DOI] [PubMed] [Google Scholar]
- 69. Jiménez-Antón MD, García-Calvo E, Gutiérrez C, Escribano MD, Kayali N, Luque-García JL, Olías-Molero AI, Corral MJ, Costi MP, Torrado JJ, Alunda JM. 2018. Pharmacokinetics and disposition of miltefosine in healthy mice and hamsters experimentally infected with Leishmania infantum. Eur J Pharm Sci 121:281–286. doi: 10.1016/j.ejps.2018.06.002 [DOI] [PubMed] [Google Scholar]
- 70. Castro RA, Kubitschek-Barreira PH, Teixeira PAC, Sanches GF, Teixeira MM, Quintella LP, Almeida SR, Costa RO, Camargo ZP, Felipe MSS, de Souza W, Lopes-Bezerra LM. 2013. Differences in cell morphometry, cell wall topography and Gp70 expression correlate with the virulence of Sporothrix brasiliensis clinical isolates. PLoS ONE 8:e75656. doi: 10.1371/journal.pone.0075656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Martínez-Álvarez JA, Pérez-García LA, Mellado-Mojica E, López MG, Martínez-Duncker I, Lópes-Bezerra LM, Mora-Montes HM. 2017. Sporothrix schenckii sensu stricto and Sporothrix brasiliensis are differentially recognized by human peripheral blood mononuclear cells. Front Microbiol 8:843. doi: 10.3389/fmicb.2017.00843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Wayne PA. 2017. Reference method for broth dilution antifungal susceptibility testing of yeasts. In CLSI standard M27, 4th ed. Clinical and Laboratory Standard Institute. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
An accounting of the reviewer comments and feedback.