Abstract
Background
Locally advanced oral cavity squamous cell carcinoma (OCSCC) presents a significant clinical challenge despite being partially responsive to standard treatment modalities. This study investigates the prognostic implications of programmed death-ligand 1 (PD-L1) expression in these tumors, focusing on its association with treatment outcomes and the immune microenvironment.
Methods
We assessed tumor-infiltrating lymphocytes (TILs) in 132 patients with OCSCC to evaluate their impact on survival. Multiplex immunohistochemistry staining for CD3, CD68, CD11c, PD-L1, and P40 was used to explore correlations with clinical outcomes in patients with early-stage (n=22) and locally advanced (n=36) OCSCC. These initial findings were validated through differential gene expression analysis, gene set enrichment, and immune cell deconvolution in a The Cancer Genome Atlas cohort of 163 locally advanced OCSCC tumors. Additionally, single-cell RNA sequencing (scRNA-seq) on a smaller cohort (n=10) further characterized the PD-L1hi or PD-L1lo cancer cells in these tumors.
Results
Elevated PD-L1 expression was associated with poor outcomes in patients with locally advanced OCSCC undergoing standard adjuvant therapy, irrespective of “hot” or “cold” classification based on TILs assessment. PD-L1hi tumors exhibited an active immune response phenotype, enriched with M1 macrophages, CD8+ T cells and T regulatory cells in the tumor microenvironment. Notably, the negative impact of PD-L1 expression on outcomes was primarily attributed to its expression by cancer cells, rather than immune cells. Furthermore, scRNA-seq revealed that immune interactions were not essential for PD-L1 upregulation in cancer cells, instead, complex regulatory networks were involved. Additionally, PD-L1lo locally advanced tumors exhibited more complex pathway enrichment and diverse T-cell populations compared with those in the early-stage.
Conclusion
Our findings underscore the prognostic significance of PD-L1 expression in locally advanced OCSCC, and unveil the complex interplay between PD-L1 expression, immune responses, and molecular pathways in the tumor microenvironment. This study provides insights that may inform future therapeutic strategies, including the possibility of tailored immunotherapeutic approaches for patients with PD-L1hi locally advanced OCSCC.
Keywords: Tumor Microenvironment, Adjuvant, Head and Neck Cancer, Immune Checkpoint Inhibitor, Radiotherapy/Radioimmunotherapy
WHAT IS ALREADY KNOWN ON THIS TOPIC
Surgery followed by adjuvant radiation or chemoradiation therapy is the standard treatment for locally advanced oral cavity squamous cell carcinoma (OCSCC). Unfortunately, the majority of patients have a high risk of recurrence even with multimodality treatment.
Immune checkpoint blockade therapy is approved for the treatment of patients with recurrent or metastatic head and neck cancer, but not in the adjuvant setting.
The tumor immune microenvironment has been investigated to predict response to immune checkpoint blockade and outcome in patients with head and neck cancer.
WHAT THIS STUDY ADDS
Elevated programmed death-ligand 1 (PD-L1) expression indicates unfavorable outcomes in patients undergoing standard treatment for locally advanced OCSCC, irrespective of “hot” or “cold” classification based on tumor-infiltrating lymphocytes assessment.
PD-L1hi tumors display an active immune response phenotype in the tumor microenvironment.
The association between PD-L1 expression and adverse outcomes is particularly notable when expressed on cancer cells, rather than on immune cells.
The complex interplay between PD-L1 expression, immune response, and molecular pathways was observed in OCSCC with PD-L1hi and PD-L1lo cancer cells.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
A comprehensive understanding of the tumor microenvironment in early-stage and locally advanced OCSCC is needed in guiding therapeutic strategies.
Patients with locally advanced OCSCC with PD-L1hi tumor may derive more substantial benefits from adjuvant immune checkpoint blockade, making it a potentially preferable approach over radiation or chemoradiation therapy following surgical resection.
Background
Locally advanced oral cavity squamous cell carcinoma (OCSCC) ranks among the most prevalent malignancies affecting the head and neck region. Locally advanced OCSCC constitutes a diverse group including all stage III/IV oral cavity tumors without distant metastasis.1 The standard therapeutic approach involves surgery followed by adjuvant radiation or chemoradiation therapy,2 yet recurrence remains a formidable risk for a majority of patients.
In 2016, immune checkpoint blockades (ICBs)—nivolumab and pembrolizumab, which target programmed death 1 (PD-1), were approved by the US Food and Drug Administration for the treatment of patients with head and neck squamous cell carcinoma (HNSCC). However, the approval was restricted to patients with recurrent or metastatic diseases3 and durable clinical responses were attained in only 13–18% of these patients.4 5 Notably, the utilization of ICBs in the adjuvant setting remains limited.
Immune checkpoints are a key regulator of the immune response in the tumor immune microenvironment (TME). Components of the TME have been linked to the response to ICBs,6 as well as outcomes7 in patients with HNSCC. For instance, higher tumor-infiltrating CD8 counts have been associated with improved overall survival in patients with HNSCC.8 However, the heterogeneity within the broader category of head and neck cancers, including tumors from diverse anatomical locations such as the oral cavity, hypopharynx, larynx and oropharynx,9 has often been overlooked. Recent research has underscored the distinct immune landscape from different anatomical sites in the head and neck.10
Hence, an in-depth immunological characterization of OCSCC and its association with clinical outcomes is currently lacking. This study aims to bridge this gap by conducting a comprehensive examination of the tumor microenvironment in OCSCC, focusing on those at a locally advanced stage. The objective is to identify predictive biomarkers of patient outcomes, thereby facilitating the customization of therapeutic strategies for this patient cohort.
Materials and methods
Patient cohort and sample collection
At enrollment, clinical information was recorded. A comprehensive review of all available medical records and patient questionnaires was conducted for the cohort. The clinical database for this cohort was last updated in December 2015. The study analyzed 132 patients with OCSCC, who met the inclusion criteria and were treated between 2007 and 2010, referred to as the “VCCC cohort”. Tumor samples were collected during surgical resections and were preserved as formalin-fixed, paraffin-embedded (FFPE) sections for further analysis. A thorough pathological review was undertaken to confirm tumor presence. Samples were classified into early-stage (stage I/II, n=78) and locally advanced stage (stage III/IV without distant metastasis, n=45) based on the eighth edition of the American Joint Committee on Cancer tumor, node, metastases system.11
For validation purposes, this study leveraged publicly available data from The Cancer Genome Atlas (TCGA) HNSCCs cohort.12 From this cohort, tumors originating from the oral cavity and classified as stage III/IV without distant metastasis, were selected for analysis. This subset included 163 samples and was designated as the “TCGA – locally advanced OCSCC cohort” (online supplemental table 1). Access to the data was facilitated through the Genomic Data Commons data portal and included RNA sequencing counts, T-cell receptor (TCR), virus status, and mutations, and the analysis performed using the R package TCGAbiolinks.13 Data was downloaded in March 2020.
Single-cell RNA sequencing (scRNA-seq) data from 18 patients with OCSCC were downloaded in October 2023 from a published study14 via Gene Expression Omnibus (GEO), through accession number GSE103322. This cohort encompassed data not only for stromal cells but also cancer cells, presenting an opportunity to delve into the characteristics of cancer cells expressing varying levels of PD-L1 and their interaction with immune cells.
Tumor-infiltrating lymphocytes assessment
Four µm sections were cut and stained with H&E from each FFPE block in the study. The H&E slides were evaluated by a trained pathologist to define “tumor” and “stromal” regions. Tumor-infiltrating lymphocytes (TILs) were evaluated on H&E-stained slides, applying the criteria set by the International Immuno-Oncology Working Group.15 This standardized approach facilitates the consistent and reproducible assessment of TILs. The criteria encompass both tumorous TILs (tTILs), located within the confines of the tumor nests or cell islands, and stromal TILs (sTILs), situated in the stromal areas surrounding the tumor cells. A trained pathologist scored the percentages of tTILs and sTILs for each sample in the study (online supplemental table 2). The data obtained from the TILs assessment were then correlated with patient survival outcomes.
Multiplex immunohistochemistry
A total of 58 samples were selected for multiplex immunohistochemistry (mIHC) staining, comprising 22 early-stage and 36 locally advanced-stage samples (online supplemental table 3). All patients in the early-stage group underwent adjuvant therapy, aligning their treatment with that received by patients in the locally advanced-stage group. mIHC was performed on tumor sections using the OPAL POLARIS 7-color Automation IHC Kit (Akoya BioSciences, California, USA) as per manufacturer’s instructions, to identify CD3 (SP7, Thermo Fisher), CD11c (EP157, Dako), CD68 (514H12, Biocare Medical), PD-L1 (SP263, Roche Diagnostics), P40(BC28, Biocare Medical) and DAPI (Invitrogen) (online supplemental table 4). Automated staining was performed on the sections using Leica Biosystems BOND RX autostainer (Wetzlar, Germany) according to the manufacturer’s instructions. For detailed steps please refer to the method section in Jacquelot et al paper.16 All slides were subsequently imaged on a Vectra Polaris quantitative pathology imaging system (Akoya Biosciences). High-powered images at 20× were selected using Phenochart (Akoya Biosciences) and imported into inForm Software V.2.4 (Akoya Biosciences) for deconvolution. The deconvoluted files from inForm V.2.4 were exported into HALO (Indica Labs, New Mexico, USA) and merged to perform tissue and cell segmentation as well as cell phenotyping using the HighPlex V.3.0.3 module on HALO software. Cell segmentation was assessed based on all cells counter-stained with 4′,6-diamidino-2-phenylindole (DAPI). Manual annotations in HALO segmented the tissue into “tumor” and “stromal” regions guided by pathologist markups of H&E stained sections. Cell phenotyping on inForm was undertaken by selecting at least five representative cells per phenotype, and then performing reiterations until at least 20 representative cells per phenotype were selected. Cell phenotyping on HALO was performed by thresholding the individual markers in the panel for each slide. The density of cell subsets was subsequently calculated per mm2.
Differential gene expression, gene sets enrichment, immune cell deconvolution analyses of TCGA—locally advanced OCSCC cohort
Trimmed mean of M-values normalized counts from bulk RNA-seq data was processed using edgeR.17 FilterByExpr was used to filter out lowly expressed genes and normalized the gene expression to be counts per million (cpm) based on normalized library sizes. High versus low expression of PD-L1 (PD-L1hi vs PD-L1lo) were defined based on the top and bottom quartiles of messenger RNA (mRNA) expression, using cut-off cpm values of 21.365 and 4.5968, respectively. Differential gene expression analyses were performed between PD-L1hi and PD-L1lo samples using edgeR (V.3.40.1). Genes with a false discovery rate <0.05 were determined as differentially expressed. We quantified the pathway enrichment scores of hallmark gene sets collection18 for each sample using gsva function under the parameters of “Gaussain” kcdf and “gsva” method from GSVA R package (V.1.46.0).19 Spearman’s correlation was performed to assess the correlation between individual pathway enrichment scores and PD-L1 mRNA expression across all samples using the cor.test function from stats R package (V.4.2.1).20 The abundance of immune cell types in each sample was estimated using quanTIseq21 and CIBERSORT22 deconvolution algorithms implemented in immunedeconv R package.23
Single-cell RNA sequencing analysis
Published scRNA-seq data14 from the GEO database (Accession Number GSE103322), available on National Centre for Biotechnology Information (NCBI, https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE103322) were used in this study. We excluded all single-cell samples from the lymph node and only used those from the primary tumor. A total of 18 samples were in the scRNA-seq cohort, with 15 samples having cancer cells and 3 samples (samples 8, 9 and 23) with no cancer cells evaluable. We used the TPM (transcript per million) normalized expression values and cell type annotation provided by the authors. Single-cell data was processed using Seurat (V.5.0.2).24,26 All cancer cells were subset from the 15 samples. ScaleData was used to normalize the gene expression across cells and determine the top 2,000 variable genes to perform principal component analysis (PCA). Uniform manifold approximation and projection (UMAP) reductions and cell neighbors were calculated using 25 dimensions from the PCA reduction. Clusters were detected with a resolution of 0.8. Five samples (samples 7, 10, 12, 13 and 24) were dropped for further analyses due to the limited number (cell number less than 10) of cancer cells. The remaining 10 samples were grouped based on their PD-L1 expression on cancer cells and tumor stages. To compare the gene expression profiles between PD-L1hi locally advanced tumors and PD-L1lo locally advanced tumors, FindMarkers was performed to explore the differentially expressed genes (DEGs) using the default parameters. Then gene set enrichment analysis (GSEA) was performed using the GSEA function with minGSSize of 15 and maxGSSize of 500 from clusterProfiler (V.4.10.1)27 28 on the hallmark gene set collection18 to find the significantly enriched pathways based on DEGs. To compare the gene expression profiles between PD-L1lo early-stage tumors and PD-L1lo locally advanced tumors, we performed the same analysis as above. T-cell populations annotation was performed using SingleR (V.2.4.1)29 based on the cell type reference of MonacoImmuneData30 downloaded from celldex (V.1.12.0).29 All non-T cell groups were grouped as “other cells”, and all minor T-cell subtypes with fewer than 10 cells were grouped as “other T cells”. All further analyses and plots were generated in R (V.4.3.3) using tidyverse,31 ggplot2 (V.3.4),32 ComplexHeatmap,33 scCustomize (V.2.1.2)34 and EnhancedVolcano (V.1.20.0).35
Statistical analysis
All statistical analyses were performed using the GraphPad Prism software (V.9.2.0, GraphPad, San Diego, California, USA) unless stated otherwise. Statistical analyses of quantifications were performed with the two-tailed Mann-Whitney U test between two groups as appropriate. For survival analyses, Kaplan-Meier plots were drawn, and statistical differences were evaluated using the log-rank Mantel-Cox test. For the correlation analyses, Pearson correlation was employed to assess relationships between cell densities and PD-L1 expression. Spearman’s correlation was performed to assess the correlations between individual hallmark gene sets and PD-L1 mRNA expression. A p≤0.05 was considered statistically significant.
Results
Elevated PD-L1 expression in locally advanced OCSCC indicates unfavorable outcomes after standard treatment
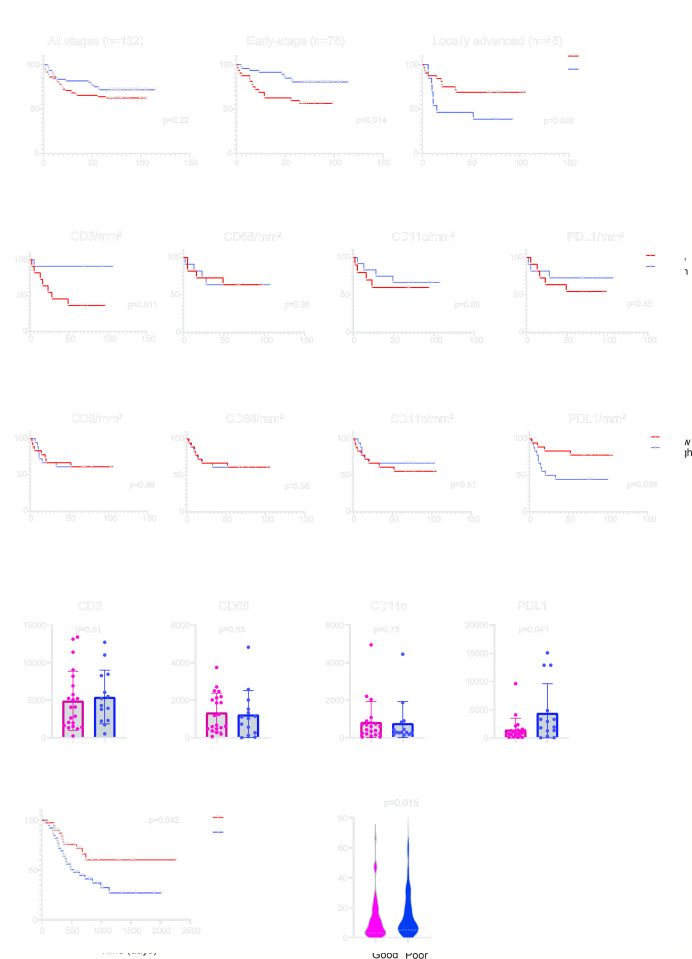
In light of the clinical relevance of TILs in breast cancer,15 we employed the same TILs evaluation method proposed by the International Immuno-Oncology Working Group36 to evaluate TILs in OCSCC. No significant difference in overall survival was observed when assessing patients across all clinical stages (figure 1A left panel). Surprisingly, when the outcomes of early-stage and locally advanced stages were compared, we observed divergent survival outcomes. TILs, more specifically sTILs, were associated with prolonged survival in the early-stage patients (p<0.05, figure 1A middle panel), but with poor outcomes in patients with locally advanced tumors (p<0.05, figure 1A right panel). We hypothesized that the presence of TILs in the tumor microenvironment may reflect distinct immune states that, in turn, bring about varying survival outcomes. Adjuvant radiation or chemoradiation is routinely employed following surgical resection of locally advanced OCSCC, and the improved patient survival noted in our study cohort (online supplemental figure S1A), aligned with previous studies.37 Notably, the prognostic significance of sTILs remained consistent irrespective of the type of adjuvant therapy the patients underwent (online supplemental figure S1A).
Figure 1. Elevated PD-L1 expression in locally advanced OCSCC indicates poor outcome. (A) Survival stratification based on stromal TILs (sTILs) assessment on H&E slides from patients with OCSCC with all stages (n=132), early-stage (n=78) or locally advanced stage (n=45). (B) Survival stratification based on CD3+, CD68+, CD11c+ or PD-L1+ cell densities (per mm2 in early-stage OCSCC (n=22). (C) Survival stratification based on CD3+, CD68+, CD11c+ or PD-L1+ cell densities (per mm2 in locally advanced OCSCC (n=36). (D) Differences of CD3+, CD68+, CD11c+ or PD-L1+ cell densities (per mm2 between patients with good or poor outcomes in locally advanced OCSCC (n=36). (E) Survival differences between PD-L1hi (top 25% n=41) and PD-L1lo (bottom 25% n=41) in the TCGA—locally advanced OCSCC cohort. (F) Differences in PD-L1 messenger RNA expression between patients with good or poor outcomes in the TCGA—locally advanced OCSCC cohort (n=163). Data are expressed as mean±SEM. OCSCC, oral cavity squamous cell carcinoma; PD-L1, programmed death ligand 1; TCGA, The Cancer Genome Atlas; TILs, tumor-infiltrating lymphocytes.
To further investigate the composition of immune cells in the tumor microenvironment, we performed mIHC to identify immune cells as CD3+ (T cells), CD68+ (macrophage), CD11c+ (dendritic cell), PD-L1+ and P40+ (cancer cells). All 36 tumor blocks of locally advanced OCSCC along with 22 early-stage tumors from the “VCCC cohort” were retrieved. All patients in the early-stage group underwent adjuvant therapy, aligning their treatment with that received by patients in the locally advanced-stage group. In the early-stage tumors, increased CD3+ cell density in the tumor was associated with significantly superior patient survival (p<0.05, figure 1B), while no significant differences in survival were observed using CD68+, CD11c+ or PD-L1+ cell densities individually (figure 1B). In contrast, CD3+, CD68+ or CD11c+ cell density did not stratify patients’ survival in the locally advanced OCSCC (figure 1C). Instead, patients with increased PD-L1+ cell infiltration had a poor survival outcome (p<0.05, figure 1C). When we further stratified the locally advanced OCSCC into those with good versus poor outcomes, significantly more PD-L1+ cells were found in the poor outcome group (p<0.05, figure 1D), while no differences were observed in CD3+, CD68+, and CD11c+ cells between the two groups (figure 1D).
To validate the clinical relevance of PD-L1 expression in locally advanced OCSCC, we identified 163 locally advanced OCSCC in the TCGA cohort. Consistent with our findings in mIHC, PD-L1hi patients experienced inferior outcomes compared with PD-L1lo patients (p<0.05, figure 1E). The PD-L1 (CD274) expression in the patients with poor outcomes was significantly higher than in the good outcome group (p<0.05, figure 1F), affirming the clinical relevance of PD-L1 as a potential prognostic marker.
PD-L1hi tumors display an active immune response phenotype within the tumor microenvironment
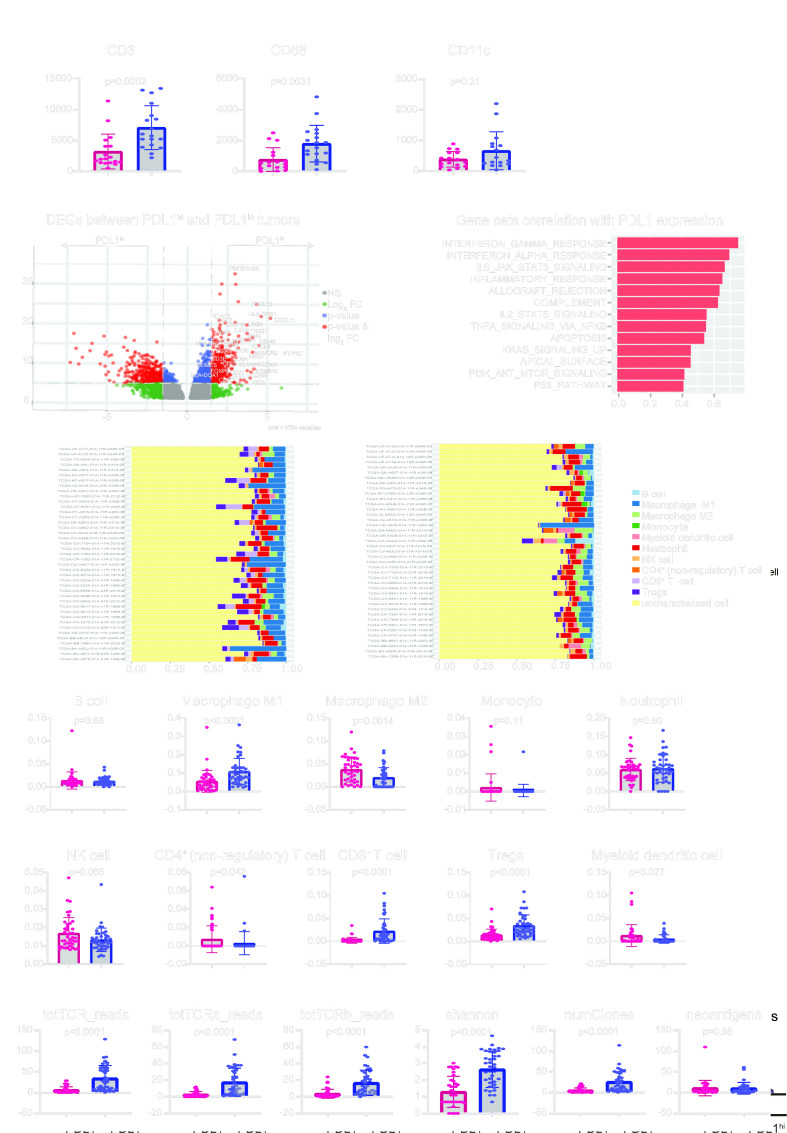
In light of the adverse prognostic value of PD-L1 in locally advanced OCSCC, we further characterized the tumor microenvironment in the “VCCC cohort” and the “TCGA-locally advanced OCSCC cohort”, respectively. In the VCCC cohort, we observed higher infiltration of CD3+ cells (p<0.001) and CD68+ cells (p<0.005), but not CD11c+ cells, in the PD-L1hi group compared with the PD-L1lo group (figure 2A). A positive and significant correlation was observed between CD3+ T cells and PD-L1+ cells, (r=0.49. p<0.05) as well as CD68+ macrophage cells and PD-L1+ cells (r=0.55, p<0.05) (online supplemental figure S1B). These positive correlations between PD-L1 expression and the density of T cells or macrophages in the tumor suggest the PD-L1 expression likely represents a result of dynamic immune cell interactions.
Figure 2. PD-L1hi tumors demonstrate an active immune response in the tumor microenvironment in locally advanced OCSCC. (A) Differences in CD3+, CD68+ and CD11c+ cells densities (per mm2 between PD-L1hi (n=18) and PD-L1lo (n=18) locally advanced OCSCC. (B) Differentially expressed genes between PD-L1hi (n=41) and PD-L1lo (n=41) samples from the TCGA—locally advanced OCSCC cohort. (C) Spearman’s correlation was performed to assess the correlations between individual hallmark gene sets and PD-L1 mRNA expression. Gene sets with rho values above 0.40 were illustrated. (D) Differences in immune cell composition using quanTIseq deconvolution analysis between PD-L1hi (n=41, left) and PD-L1lo (n=41, right) samples. (E) Differences of B cell, M1, M2, monocyte, neutrophil, NK cell, CD4+ non-regulatory cell, CD8+ T cell, Tregs, and myeloid dendritic cell populations between PD-L1hi (n=41) and PD-L1lo (n=41) samples using quanTIseq method. (F) Differences of total TCR, TCR-α, and TCR-β reads, Shannon’s entropy index, number of clones and neoantigens between PD-L1hi (n=41) and PD-L1lo (n=41) samples. Data are expressed as mean±SEM. DEGs, differentially expressed genes; FDR, false discovery rate; NK cell, natural killer cell; OCSCC, oral cavity squamous cell carcinoma; PD-L1, programmed death ligand 1; TCGA, The Cancer Genome Atlas; TCR, T-cell receptor; Tregs, T regulatory cells.
In the TCGA—locally advanced OCSCC cohort, we observed a list of 6,784 genes differentially expressed between the PD-L1hi and PD-L1lo groups (online supplemental table 5), with a majority of immune-related genes upregulated in the PD-L1hi tumors (figure 2B). Furthermore, a Spearman’s correlation was performed to assess the correlation between individual hallmark gene sets and PD-L1 mRNA expression. The gene sets related to the immune response were positively correlated with PD-L1 expression (figure 2C), including interferon-gamma response (rho=0.75), interferon alpha response (rho=0.70), IL-6 JAK STAT3 signaling (rho=0.67), inflammatory response (rho=0.65), allograft rejection (rho=0.63), complement (rho=0.62), and IL-2 STAT5 signaling (rho=0.55). Interestingly, gene sets including TNF-α signaling via NF-κB (rho=0.55), apoptosis (rho=0.54), KRAS signaling up (rho=0.46), apical surface (rho=0.45), PI3K AKT mTOR signaling (rho=0.42), and P53 pathways (rho=0.41), also had a weak positive correlation with PD-L1 expression. A heatmap of 50 hallmark gene sets in 163 samples from the TCGA—locally advanced OCSCC cohort based on PD-L1 expression is shown in online supplemental figure S2). To further characterize immune cell composition in the tumor microenvironment in the PD-L1hi and PD-L1lo tumors, we used the immune deconvolution method—quanTIseq21 (online supplemental table 6). This showed that PD-L1hi (figure 2D left) and PD-L1lo (figure 2D right) tumors have distinct immune cell compositions. More specifically, PD-L1hi tumors exhibited an active immune response phenotype with increased M1 macrophages (p<0.0001), CD8+ T cells (p<0.0001) and T regulatory cells (Tregs, p<0.0001), while PD-L1lo tumors showed a suppressive phenotype with more M2 macrophage (p<0.005), CD4+ non-regulatory T cells (p<0.05) and myeloid dendritic cells (p<0.05) (figure 2E). No significant differences in B cell, monocyte, neutrophil, or natural killer cell were observed between the two groups (figure 2E). Similar observations were made using the CIBERSORT method (online supplemental table 7), indicating increased percentages of M1 macrophages (p<0.0001) and CD8+ T cells (p<0.0001) in PD-L1hi tumors (online supplemental figure 3A). Further analysis of TCR profiles (online supplemental table 7) revealed an increase in the number of TCR-α (p<0.0001), TCR-β (p<0.0001), and total TCR (p<0.0001) reads in PD-L1hi tumors (figure 2F). Notably, not only was there an increase in the number of T-cell clones (p<0.0001), but also a greater diversity (p<0.0001) within the T-cell repertoire, as evidenced by Shannon entropy measurements, in PD-L1hi tumors (figure 2F). However, the enhanced complexity of the TCR repertoire in PD-L1hi tumors did not stem from an increase in virus pathogens (online supplemental table 8), including cytomegalovirus (CMV), Epstein-Barr virus (EBV), human herpesvirus (HHV), and human papillomavirus (HPV), or immunogenic mutations or neoantigens (online supplemental table 8 and figure 2F). These findings suggest that alternative mechanisms, other than viral pathogens or genetic mutations, may contribute to the significant increase and diversification of TCRs in PD-L1hi tumors. For instance, cytokines in the tumor can affect TCR diversity and number by altering T-cell survival and differentiation. Taken together, PD-L1hi tumors manifest an active immune response phenotype within the tumor microenvironment. The PD-L1 expression in these tumors likely reflects an ongoing antitumor immune response.
PD-L1 expression on cancer cells, rather than immune cells, is associated with adverse outcomes in the locally advanced OCSCC
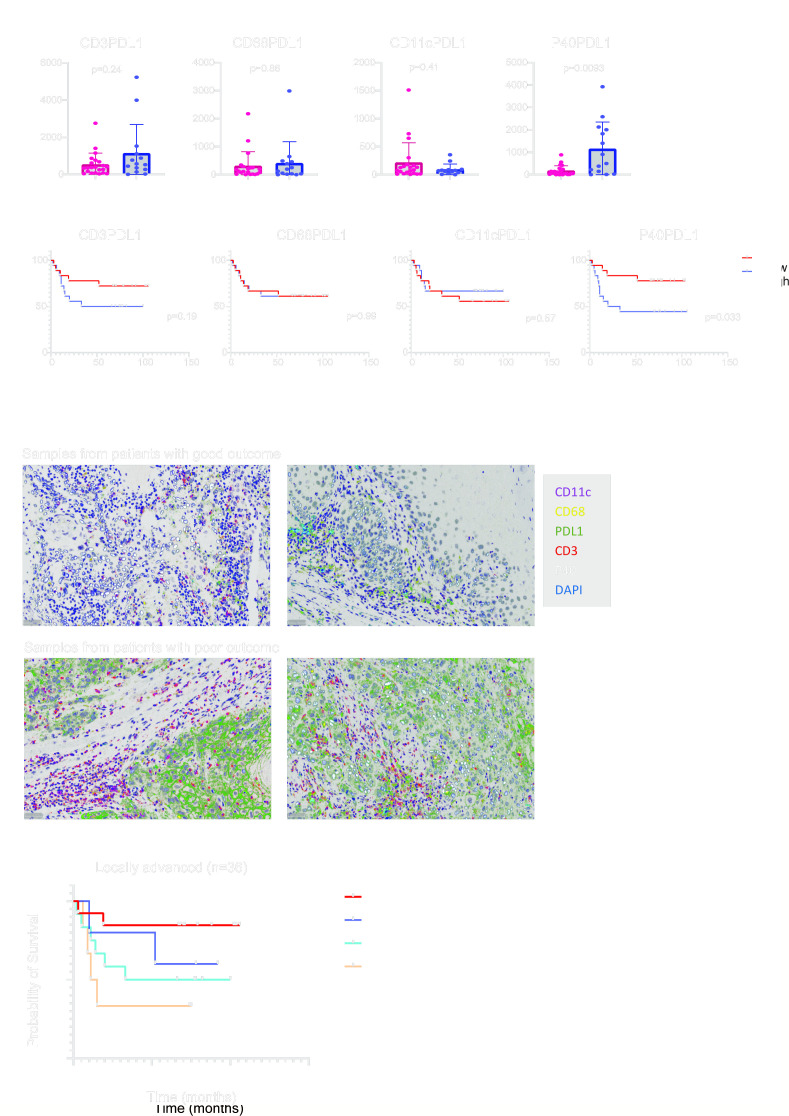
The expression of PD-L1 on cancer cells and non-cancer cells has been shown to impact patient outcomes.38,40 mIHC provided the opportunity to identify which cell type expressed PD-L1 on the cell surface. Interestingly, we observed that PD-L1 on cancer cells (p<0.01), but not immune cells, was significantly elevated in the poor outcome group (figure 3A). In light of this, we next stratified the patients’ survival on the cell types expressing PD-L1. An increased number of PD-L1+ cancer cells (p<0.05) in the tumor was associated with poor outcomes, however, this was not the case for PD-L1+ CD3, CD68 or CD11c cells, (figure 3B). Examples of high-resolution images of mIHC for patients with good and poor outcomes are shown in figure 3C.
Figure 3. PD-L1 expression on the cancer cells, not immune cells, is associated with poor outcomes in the locally advanced OCSCC. (A) Differences in CD3+PD-L1+, CD68+PD-L1+, CD11c+PD-L1+ and P40+PD-L1+ cell densities (per mm2 between patients with good and poor outcomes (n=36). (B) Survival stratification based on CD3+PD-L1+, CD68+PD-L1+, CD11c+PD-L1+ and P40+PD-L1+ cell densities (per mm2 in locally advanced OCSCC (n=36). (C) Examples of multiplex staining for CD11c (magenta), CD68 (yellow), PD-L1 (green), CD3 (red) and P40 (white) in patients with good (n=2) and poor (n=2) outcomes. Scale bar, 50 µm. (D) Survival stratification based on stromal TILs (sTILs) and PD-L1 densities in locally advanced OCSCC tumors (n=36). Data are expressed as mean±SEM. OCSCC, oral cavity squamous cell carcinoma; PD-L1, programmed death ligand 1; TILs, tumor-infiltrating lymphocytes.
The PD-1/PD-L1 immune checkpoint is a key pathway in the regulation of antitumor immunity.41 This discovery played a pivotal role in the development of the revolutionary ICB.42 Recent studies revealed that cancer cell-intrinsic PD-L1 signaling operates independently of PD-1 engagement or interactions with immune cells.40 This insight elucidates why certain immune-cold tumors exhibit upregulated PD-L1 expression. Taking this into account, we used data on TILs to categorize tumors as either immune “hot” or “cold”. Through the amalgamation of sTILs assessment and PD-L1 expression, we stratified patients into PD-L1hi or PD-L1lo and “hot” or “cold” tumors. Intriguingly, PD-L1hi tumors were associated with inferior outcomes when compared with PD-L1lo tumors, irrespective of their “hot” or “cold” classification based on sTILs assessment (figure 3D). This finding indicates signaling pathways that downregulate PD-L1 expression on cancer cells may confer protective effects on patients undergoing adjuvant radiation or chemoradiation therapy.
Complex regulatory networks other than immune interactions are involved in PD-L1 expression on cancer cells in locally advanced tumors
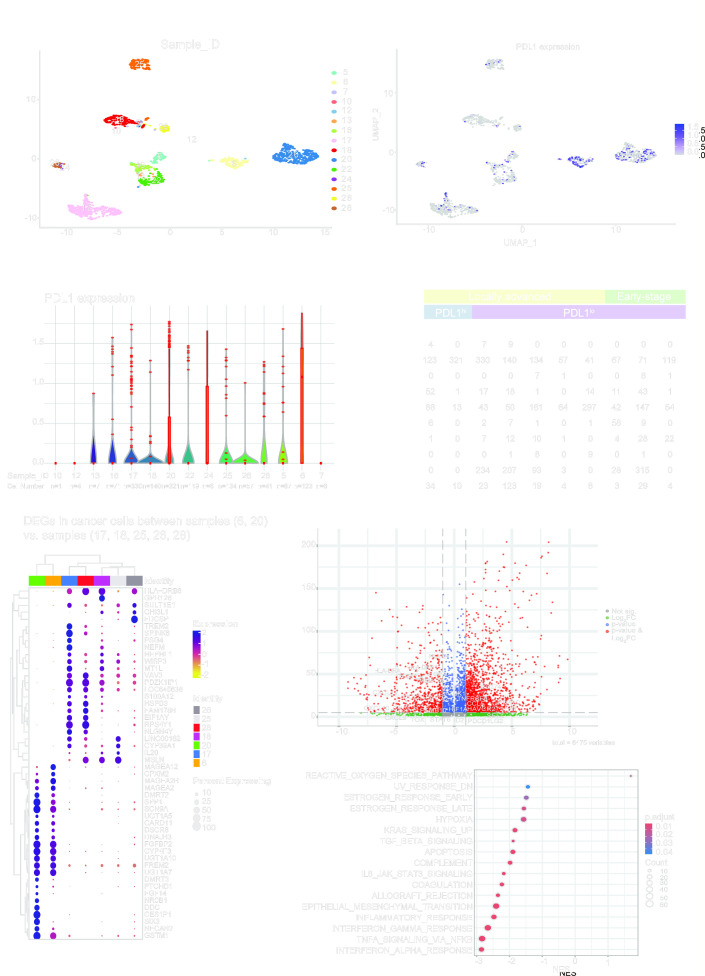
Subsequently, our interest turned to understanding the features of cancer cells that are associated with PD-L1 expression. By using the scRNA-seq cohort from the study conducted by Puram et al,14 we successfully identified OCSCC samples exhibiting either PD-L1hi or PD-L1lo cancer cells. First, cancer cells from 15 samples were clustered based on Sample-IDs (figure 4A), and the expression of PD-L1 on the cancer cells was further determined (figure 4B and C). Notably, 10 of these samples—5, 6, 16, 17, 18, 20, 22, 25, 26, and 28—showed a significant presence of cancer cells (figure 4C). In contrast, samples 7, 10, 12, 13, and 24 had fewer cancer cells (n<10) and were therefore excluded from further analysis. Figure 4D illustrates the remaining 10 OCSCC tumors, detailing their tumor stages, PD-L1 expression levels on cancer cells, and cell compositions. Based on the clinical and pathological information illustrated in online supplemental table 2 from Puram et al,14 and considering that all samples included are without metastasis, sample 5 (T2N1), sample 16 (T2N0), and sample 22 (T1N0) were categorized as early-stage tumors. In contrast, the rest of the samples, such as sample 6 (T4aN2c), sample 17 (T4aN0), sample 18 (T3N1), sample 20 (T4aN2c), sample 25 (T3N1), sample 26 (T4aN2c), and sample 28 (T2N2c), were categorized as locally advanced tumors. Combing the tumor stages and PD-L1 expression levels on cancer cells, we then categorized the samples into three groups: samples 6 and 20 as PD-L1hi locally advanced tumors; samples 17, 18, 25, 26 and 28 as PD-L1lo locally advanced tumors; and samples 5, 16 and 22 as PD-L1lo early-stage tumors.
Figure 4. Complex regulatory networks other than immune interactions are involved in PD-L1 expression on cancer cells in locally advanced tumors. (A) Cancer cell clusters based on sample IDs from the single cell RNA sequencing cohort. (B) PD-L1 expression on individual cancer cells from different tumor samples. (C) Violin plot of PD-L1 expression on individual cancer cells from different tumor samples. (D) Single-cell compositions in the samples (n=10) with evaluable cancer cells (cell number >10). (E) Top 50 differentially expressed genes (DEGs) in cancer cells between samples 6 and 20 (PD-L1hi locally advanced tumors) and samples 17,18, 25, 26 and 28 (PD-L1lo locally advanced tumors). (F) Volcano plot of 6,475 variables from the comparison in panel E with immune-related genes labeled. (G) Enriched hallmark gene set analysis of DEGs from the comparison in panel E. NES, normalized enrichment score; PD-L1, programmed death ligand 1; UMAP, uniform manifold approximation and projection.
Interestingly, samples 6 and 20, despite lacking T cells (n=0), exhibited elevated PD-L1 expression on cancer cells. While this observation is based on a limited number of samples (n=2), it suggests that the PD-L1 expression on cancer cells in these two specimens may be influenced by factors other than the interaction with T cells in the tumor microenvironment. These two samples provided a unique opportunity to elucidate the genes or pathways influencing PD-L1 expression in cancer cells. We then conducted differential gene expression (figure 4E and F and online supplemental table 9) and pathways enrichment analyses (figure 4G and online supplemental table 10) by comparing cancer cells from samples 6 and 20 against samples 17, 18, 25, 26 and 28. Notably, in the PD-L1hi samples (6 and 20), genes from the MAGE family (MAGEA12, MAGEA2B, MAGEA2), the UDP glucuronosyltransferase family (UGT1A5, UGT1A10, UGT1A7), and other genes including CPXM1, DMRT2, SPP1, SCN9A, CARD11, DSCR8, DNAJB3, FGFBP2, CYP4F3, FREM2, and GSTM1 were upregulated. Conversely, genes upregulated in PD-L1lo samples varied, but genes such as HLA-DRB6, SULT1E1, HEPHL1, WISP2, VAV3, PDZK1IP1, and LOC645638 were consistently elevated in all five samples (figure 4E). Interestingly, most immune-related genes including genes associated with antigen presentation (HLA-DRB1, HLADRB6, HLADRA) and T-cell trafficking (CXCL9, CXCL10, CXCL11) were prevalent in the PD-L1lo samples, while genes other than CD274, such as LEF1, PTPRC, SELL, PDCD1LG2 were elevated in the PD-L1hi samples (figure 4F). This may explain the limited T-cell presence observed in samples 6 and 20 (figure 4D).
Further Gene Set Enrichment Analysis revealed most immune-related pathways, including complement, IL-6 JAK STAT2 signaling, allograft rejection, inflammatory response, interferon-gamma response, and interferon alpha response were enriched in the PD-L1lo samples, alongside pathways like ultraviolet (UV) response down, estrogen response, hypoxia, Kirsten rat sarcoma virus (KRAS) signaling up, transforming growth factor (TGF)-β signaling, apoptosis, coagulation, epithelial-mesenchymal transition (EMT) and tumor necrosis factor (TNF)-α signaling via nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) (figure 4G and online supplemental table 10). In contrast, the reactive oxygen species (ROS) pathway was the only pathway enriched in the PD-L1hi samples (figure 4G and online supplemental table 10). This emphasizes that immune interactions are not essential for PD-L1 upregulation in cancer cells.
PD-L1lo locally advanced tumors have a more complex pathway enrichment and diverse T-cell infiltrate compared with early-stage tumors
The scRNA-seq cohort offered a valuable opportunity to examine the characteristics of PD-L1lo cancer cells in both early-stage and locally advanced OCSCC tumors. We performed differential gene expression (figure 5A and B and online supplemental table 11) and pathway enrichment analyses (figure 5C and online supplemental table 12) by comparing cancer cells from samples 5, 16, and 22 to those from samples 17, 18, 25, 26, and 28. Interestingly, the number of DEGs between early and advanced stage tumors was significantly lower than those between PD-L1lo and PD-L1hi in locally advanced tumors, with 1,572 and 6,475 DEGs, respectively. Similar patterns were observed in immune-related genes, as depicted in figure 5B. These observations suggest PD-L1 expression might be a more influential factor in representing gene expression changes than the stage of the tumor itself. Further Gene Set Enrichment Analysis identified that inflammatory responses, KRAS signaling downregulation, hypoxia, and TNF-α signaling via NF-κB were predominantly enriched in the locally advanced tumors (samples 17, 18, 25, 26, and 28, shown in figure 5C and online supplemental table 12). These findings indicate these pathways may be involved in modulating the tumor microenvironment when tumor progresses.
Figure 5. More complex pathway enrichment and diverse T cells in PD-L1lo locally advanced tumors compared with early-stage ones. (A) Top 50 differentially expressed genes (DEGs) in cancer cells between samples 5, 16 and 22 (PD-L1 lo early-stage tumors) and samples 17,18, 25, 26 and 28 (PD-L1lo locally advanced tumors). (B) Volcano plot of 1,572 variables from the comparison in panel A with immune-related genes labeled. (C) Enriched hallmark gene set analysis of DEGs from the comparison in panel A. (D) Composition of cell phenotypes of T cells from samples 17, 18 and 25 (PD-L1lo locally advanced tumors) using the Monaco method. (E) Composition of cell phenotypes of T cells from samples 5 and 16 (PD-L1lo early-stage tumors) using the Monaco method. (F) Differences in T-cell composition between PD-L1lo locally advanced tumors (samples 17, 18 and 25) and PD-L1lo early-stage tumors (samples 5 and 16). NES, normalized enrichment score; PD-L1, programmed death ligand 1; Th cells: T helper cells; UMAP, uniform manifold approximation and projection; Vd2 gd T cells: V-delta 2 gamma delta T cell.
Interestingly, samples 6, 20, 26, 28 and 22 exhibited minimal T-cell presence (figure 4D), indicating a “cold” tumor phenotype, while samples 5, 16, 17, 18 and 25 displayed abundant T cells (figure 4D), reflecting a “hot” tumor phenotype. Additionally, all these five “hot” tumors demonstrated low PD-L1 expression on cancer cells (figure 4C). To further understand the composition of these T cells, we next annotated the T cells from PD-L1lo locally advanced tumors (samples 17, 18, and 25) and PD-L1lo early-stage tumors (samples 5 and 16) based on Monaco30 reference data sets (figure 5D and E). The T cells in the PD-L1lo locally advanced tumors were notably more diverse than those in the PD-L1lo early-stage tumors (figure 5F). Specifically, T cells from samples 17, 18, and 25 comprised 11 subsets (figure 5D) whereas only 7 subsets were identified from samples 5 and 16 (figure 5E). Both groups exhibited CD8+ T cells, including central memory and effector memory cells, and CD4+ T cells, including follicular helper cells, Tregs and Th17 cells, although with different percentages(figure 5F). Notably, in PD-L1lo locally advanced tumors, distinct subsets such as terminal effector CD8+ T cells, Th1 and Th2 cells CD4+ T cells, and Vδ−2 γδ T cells were present (figure 5F). The increased diversity of T cells in locally advanced tumors compared with early-stage ones indicates a more complex immune response as the tumor progresses.
Conclusion and discussion
Our study systematically evaluated the prognostic value of PD-L1 in patients with locally advanced OCSCC. The finding that high PD-L1 expression correlates with poorer outcomes after adjuvant therapy challenges existing assumptions and emphasizes the complex role of PD-L1 in cancer progression and treatment response. To understand potential mechanisms to explain this finding, we used multiple cohorts and advanced technologies in this study to unravel the intricate relationships between PD-L1 expression, immune responses, and molecular pathways within the tumor microenvironment.
Our investigations revealed that T cells are indicative of favorable outcomes in early-stage OCSCC, while their prognostic significance diminishes in locally advanced tumors. Conversely, high PD-L1 expression is a strong predictor of poor outcomes in these advanced cases. Both early-stage and locally advanced tumor cohorts assayed with mIHC staining in this study underwent adjuvant therapy, this suggests the influence of radiation on patients’ immune response varies between early-stage and locally advanced tumors. Additionally, scRNA-seq on cancer cells revealed that T-cell populations in early-stage tumors were less diverse than those in advanced tumors. Collectively, these findings led us to hypothesize that in early-stage OCSCC, adjuvant radiation might provoke a more robust immune response, resulting in more diverse immune cell populations and potentially prolonging patient survival. Supporting this, recent studies suggest radiation acts as an “accelerant”, similar to in situ vaccination, by killing tumor cells and eliciting a systemic immune response.43 The most representative example is the abscopal effect: radiation on one site may cause tumor regression at remote and distant non-irradiated sites.44
In contrast, in the locally advanced OCSCC tumors, our study revealed that PD-L1hi tumors exhibit an active immune response phenotype within the tumor microenvironment, as indicated by mIHC and TCGA data. This suggests the preservation of systemic immune response in PD-L1hi locally advanced tumors. However, the benefits from adjuvant radiation or chemoradiation therapy for these patients appear limited. This limitation could be due to the potential suppression of the systemic immune response by these treatments, ultimately contributing to poorer outcomes in this patient cohort. Conversely, patients with PD-L1lo tumors might experience a quicker recovery post-therapy due to the absence of an immunosuppressive environment in the tumor region. Considering the significant PD-L1 expression in these tumors, these patients could derive more substantial benefits from adjuvant ICBs, which could be a more effective alternative to radiation or chemoradiation therapy following surgical resection.
In addition, using scRNA-seq data, we identified complex molecular pathways involved in PD-L1 regulation on cancer cells. We observed that immune interactions are not essential for PD-L1 upregulation in cancer cells. Instead, alternative mechanisms, such as the ROS pathway identified in this study, may independently contribute to PD-L1 upregulation on cancer cells without engaging immune response. The role of ROS on PD-L1 expression is supported by several studies. Roux et al studied how ROS active NF-κB signaling to promote PD-L1 transcription, particularly in tumor-associated macrophages.45 Similarly, Glorieux et al explored the significant role of ROS in mediating K-Ras-induced FGFR1 activation, leading to PD-L1 expression in K-Ras-driven cancers.46 Other mechanisms, such as TP53/mTORC1 signaling,47 JAK/STAT signaling,48 and AKT signaling,49 have been studied in regulating cell-intrinsic PD-L1 expression. Recent discoveries also suggest that cancer cell-intrinsic PD-L1 signals could contribute to tumor growth, stemness, and survival.40
On the other hand, our study also uncovered that activating certain molecular pathways might counteract the effects of immune interactions on PD-L1 expression. For instance, pathways like hypoxia, EMT, estrogen response, KRAS signaling, and TGF-β signaling may inhibit or downregulate PD-L1 expression in cancer cells. Studies demonstrated the hypoxia response in the tumor microenvironment modulates the expression levels for critical molecular targets in immunotherapy, including PD-L1.50 Furthermore, the association between PD-L1 signaling and the EMT program has also been observed in multiple tumor types.51 Additionally, we observed that patients with PD-L1lo locally advanced OCSCC tumors derive a survival benefit from adjuvant radiation or chemoradiation therapy. These findings led us to hypothesize that the adjuvant therapy may influence certain molecular pathways—such as hypoxia, EMT, TGF-β signaling—in cancer cells, making the remaining cancer cells more detectable or vulnerable to immune responses.
Collectively, our findings contribute significantly to the understanding of prognostic markers in OCSCC tumors. We demonstrated the utility of TILs as a prognostic marker in early-stage tumors, where the interactions within the tumor microenvironment remain relatively simple and more predictable. This simplicity allows for TILs to serve as a straightforward indicator of immune response and potential tumor reactivity. As the tumor progresses to more advanced stages, however, the complexity of molecular pathways on cancer cells, the diversity of immune responses as well as other factors in the tumor microenvironment increase substantially. Additionally, the impact of standard adjuvant therapy on these factors varies, complicating the prediction of treatment responses and patient survival. Several potential mechanisms were proposed here. Adjuvant therapy may enhance patient survival by triggering robust immune responses, possibly through the abscopal effect or direct killing of cancer cells. Simultaneously, adjuvant therapy can modulate molecular pathways like hypoxia, EMT, and TGF-β signaling, which may unlock the suppression of immune responses, further contributing to its therapeutic efficacy. Conversely, our findings also suggest that adjuvant therapy might have adverse effects, such as dampening systemic immune responses or enhancing cancer cell survival and growth through certain molecular pathways. This dual nature underscores the complexity of predicting adjuvant therapy outcomes using solely immune response factors. Our study has demonstrated PD-L1 expression, especially on cancer cells, could serve as a more reliable indicator of patient survival in locally advanced tumors. This is likely because PD-L1 expression encapsulates the complex interactions between immune cells and cancer cells within the tumor microenvironment, influenced by both intrinsic and extrinsic pathways.
However, it is crucial to acknowledge that the exploratory nature of this analysis necessitates caution in drawing definitive conclusions. The small sample size, particularly within the scRNA-seq cohort, may affect the robustness and generalizability of our findings. An expanded data set that includes a larger number of samples, especially those with high PD-L1 expression and abundant T cells, could unveil additional pathways or mechanisms. This would enhance our understanding of the intricate dynamics between PD-L1 expression and molecular pathways in OCSCC. Furthermore, increasing the sample size would also aid in more accurately validating the prognostic value of PD-L1 in patients with locally advanced disease.
In conclusion, our study contributes significantly to our understanding of the prognostic implications of PD-L1 expression in locally advanced OCSCC tumors following standard adjuvant treatment, and reveals the intricate relationships between PD-L1 expression, immune responses, molecular pathways, and treatment outcomes. They not only highlight the multifaceted nature of adjuvant therapies, and complex interplay in the tumor microenvironment, but also reinforce the potential of PD-L1 as biomarkers for tailoring treatment strategies. Moving forward, it will be essential to further delineate the specific molecular mechanisms by which adjuvant therapies affect tumor and immune dynamics. Continued research into the modulation of cancer cells, immune responses, and tumor microenvironment interactions will be crucial for optimizing treatment outcomes. Ultimately, a deeper insight into these processes will enable the development of more personalized, effective cancer treatments, improving survival and quality of life for patients with OCSCC.
supplementary material
Acknowledgements
We thank the staff from the imaging facilities at the Walter and Eliza Hall Institute of Medical Research and Peter MacCallum Cancer Centre for technical assistance and helpful discussions. We appreciate oncologist Dr Annie Wong’s comments. We are also grateful to Sidharth Puram, Itay Tirosh, and Bradley Bernstein for their technical help with single-cell RNA sequencing analysis.
Footnotes
Funding: This study is funded by the Victoria Comprehensive Cancer Centre research strategic plan, and receives support from an Investigator Grant (Level 3) from the National Health and Medical Research Council (NHMRC) of Australia (1145470, JAT), fellowships from Victorian Cancer Agency (ECRF22016, MW; MCRF17005, DG).
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient consent for publication: Not applicable.
Ethics approval: This study received approval from the Institutional Human Ethics Research Committee (reference number 20/139) at the Victoria Comprehensive Cancer Centre (VCCC), Melbourne, Australia. Participants gave informed consent to participate in the study before taking part.
Data availability free text: The TCGA-HNSC data set is accessible via the National Cancer Institute Genomic Data Commons Data Portal (https://portal.gdc.cancer.gov/). The single-cell RNA sequencing data set can be accessed via Gene Expression Omnibus (GEO) with the accession number GSE103322 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE103322). Data of the VCCC cohort are available upon reasonable request to Paul.Neeson@petermac.org.
Contributor Information
Minyu Wang, Email: Minyu.Wang@petermac.org.
Lei Qin, Email: qinlq@student.unimelb.edu.au.
Kevin Thia, Email: kevin.thia@petermac.org.
Thu Nguyen, Email: ThuNgoc.Nguyen@petermac.org.
Sean MacDonald, Email: Sean.Macdonald@petermac.org.
Simone Belobrov, Email: simonebelobrov@gmail.com.
Sevastjan Kranz, Email: seve.kranz@gmail.com.
David Goode, Email: David.Goode@petermac.org.
Joseph A Trapani, Email: joe.trapani@petermac.org.
David Wiesenfeld, Email: david.wiesenfeld@unimelb.edu.au.
Paul Joseph Neeson, Email: paul.neeson@petermac.org.
Data availability statement
Data are available in a public, open access repository. Data are available upon reasonable request.
References
- 1.Dhar H, Vaish R, D’Cruz AK. Management of locally advanced oral cancers. Oral Oncol. 2020;105:104662. doi: 10.1016/j.oraloncology.2020.104662. [DOI] [PubMed] [Google Scholar]
- 2.Kim D, Li R. Contemporary treatment of locally advanced oral cancer. Curr Treat Options Oncol. 2019;20:32. doi: 10.1007/s11864-019-0631-8. [DOI] [PubMed] [Google Scholar]
- 3.Cohen EEW, Bell RB, Bifulco CB, et al. The society for immunotherapy of cancer consensus statement on immunotherapy for the treatment of squamous cell carcinoma of the head and neck (HNSCC) J Immunother Cancer. 2019;7:184. doi: 10.1186/s40425-019-0662-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ferris RL, Blumenschein G, Jr, Fayette J, et al. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N Engl J Med. 2016;375:1856–67. doi: 10.1056/NEJMoa1602252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mehra R, Seiwert TY, Gupta S, et al. Efficacy and safety of pembrolizumab in recurrent/metastatic head and neck squamous cell carcinoma: pooled analyses after long-term follow-up in KEYNOTE-012. Br J Cancer. 2018;119:153–9. doi: 10.1038/s41416-018-0131-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hanna GJ, Lizotte P, Cavanaugh M, et al. Frameshift events predict anti-PD-1/L1 response in head and neck cancer. JCI Insight. 2018;3:e98811. doi: 10.1172/jci.insight.98811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Spector ME, Bellile E, Amlani L, et al. Prognostic value of tumor-infiltrating lymphocytes in head and neck squamous cell carcinoma. JAMA Otolaryngol Head Neck Surg. 2019;145:1012–9. doi: 10.1001/jamaoto.2019.2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Feng Z, Bethmann D, Kappler M, et al. Multiparametric immune profiling in HPV- oral squamous cell cancer. JCI Insight. 2017;2:e93652. doi: 10.1172/jci.insight.93652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cramer JD, Burtness B, Le QT, et al. The changing therapeutic landscape of head and neck cancer. Nat Rev Clin Oncol. 2019;16:669–83. doi: 10.1038/s41571-019-0227-z. [DOI] [PubMed] [Google Scholar]
- 10.Muijlwijk T, Nijenhuis DNLM, Ganzevles SH, et al. Comparative analysis of immune infiltrates in head and neck cancers across anatomical sites. J Immunother Cancer. 2024;12:e007573. doi: 10.1136/jitc-2023-007573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Amin MB, Edge SB, Greene FL, et al. AJCC Cancer Staging Manual: Springer. 2017. [Google Scholar]
- 12.Cancer Genome Atlas N. Comprehensive genomic characterization of head and neck squamous cell carcinomas. Nat New Biol. 2015;517:576–82. doi: 10.1038/nature14129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Colaprico A, Silva TC, Olsen C, et al. TCGAbiolinks: an R/Bioconductor package for integrative analysis of TCGA data. Nucleic Acids Res. 2016;44:e71. doi: 10.1093/nar/gkv1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Puram SV, Tirosh I, Parikh AS, et al. Single-cell transcriptomic analysis of primary and metastatic tumor ecosystems in head and neck cancer. Cell. 2017;Gene Expression Omnibus:24 e24. doi: 10.1016/j.cell.2017.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dieci MV, Radosevic-Robin N, Fineberg S, et al. Update on tumor-infiltrating lymphocytes (TILs) in breast cancer, including recommendations to assess TILs in residual disease after neoadjuvant therapy and in carcinoma in situ: a report of the International Immuno-Oncology Biomarker Working Group on Breast Cancer. Semin Cancer Biol. 2018;52:16–25. doi: 10.1016/j.semcancer.2017.10.003. [DOI] [PubMed] [Google Scholar]
- 16.Jacquelot N, Seillet C, Wang M, et al. Blockade of the co-inhibitory molecule PD-1 unleashes ILC2-dependent antitumor immunity in melanoma. Nat Immunol. 2021;22:851–64. doi: 10.1038/s41590-021-00943-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Robinson MD, McCarthy DJ, Smyth GK. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics. 2010;26:139–40. doi: 10.1093/bioinformatics/btp616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liberzon A, Birger C, Thorvaldsdóttir H, et al. The Molecular Signatures Database (MSigDB) hallmark gene set collection. Cell Syst. 2015;1:417–25. doi: 10.1016/j.cels.2015.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hänzelmann S, Castelo R, Guinney J. GSVA: gene set variation analysis for microarray and RNA-seq data. BMC Bioinformatics. 2013;14:7. doi: 10.1186/1471-2105-14-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Team RC . A Language and Environment for Statistical Computing. 2013. [Google Scholar]
- 21.Finotello F, Mayer C, Plattner C, et al. Molecular and pharmacological modulators of the tumor immune contexture revealed by deconvolution of RNA-seq data. Genome Med. 2019;11:34. doi: 10.1186/s13073-019-0638-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Newman AM, Liu CL, Green MR, et al. Robust enumeration of cell subsets from tissue expression profiles. Nat Methods. 2015;12:453–7. doi: 10.1038/nmeth.3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sturm G, Finotello F, Petitprez F, et al. Comprehensive evaluation of transcriptome-based cell-type quantification methods for immuno-oncology. Bioinformatics. 2019;35:i436–45. doi: 10.1093/bioinformatics/btz363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Satija R, Farrell JA, Gennert D, et al. Spatial reconstruction of single-cell gene expression data. Nat Biotechnol. 2015;33:495–502. doi: 10.1038/nbt.3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Butler A, Hoffman P, Smibert P, et al. Integrating single-cell transcriptomic data across different conditions, technologies, and species. Nat Biotechnol. 2018;36:411–20. doi: 10.1038/nbt.4096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stuart T, Butler A, Hoffman P, et al. Comprehensive integration of single-cell data. Cell. 2019;177:1888–902. doi: 10.1016/j.cell.2019.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu T, Hu E, Xu S, et al. clusterProfiler 4.0: a universal enrichment tool for interpreting omics data. Innovation (Camb) . 2021;2:100141. doi: 10.1016/j.xinn.2021.100141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yu G, Wang L-G, Han Y, et al. clusterProfiler: an R package for comparing biological themes among gene clusters. OMICS. 2012;16:284–7. doi: 10.1089/omi.2011.0118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aran D, Looney AP, Liu L, et al. Reference-based analysis of lung single-cell sequencing reveals a transitional profibrotic macrophage. Nat Immunol. 2019;20:163–72. doi: 10.1038/s41590-018-0276-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Monaco G, Lee B, Xu W, et al. RNA-Seq signatures normalized by mRNA abundance allow absolute deconvolution of human immune cell types. Cell Rep. 2019;26:1627–40. doi: 10.1016/j.celrep.2019.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wickham H, Averick M, Bryan J, et al. Welcome to the tidyverse. JOSS. 2019;4:1686. doi: 10.21105/joss.01686. [DOI] [Google Scholar]
- 32.Wickham H. Ggplot2: Elegant Graphics for Data Analysis. New York: Springer-Verlag New York; 2016. [Google Scholar]
- 33.Gu Z, Eils R, Schlesner M. Complex heatmaps reveal patterns and correlations in multidimensional genomic data. Bioinformatics. 2016;32:2847–9. doi: 10.1093/bioinformatics/btw313. [DOI] [PubMed] [Google Scholar]
- 34.Marsh SE. scCustomize: custom visualizations & functions for streamlined analyses of single cell sequencing. 2021. [DOI]
- 35.EnhancedVolcano LM. EnhancedVolcano: publication-ready volcano plots with enhanced colouring and labeling. 2023. [DOI]
- 36.Salgado R, Denkert C, Demaria S, et al. The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: recommendations by an International TILs Working Group 2014. Ann Oncol. 2015;26:259–71. doi: 10.1093/annonc/mdu450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Foster CC, Melotek JM, Brisson RJ, et al. Definitive chemoradiation for locally-advanced oral cavity cancer: a 20-year experience. Oral Oncol. 2018;80:16–22. doi: 10.1016/j.oraloncology.2018.03.008. [DOI] [PubMed] [Google Scholar]
- 38.Diskin B, Adam S, Cassini MF, et al. PD-L1 engagement on T cells promotes self-tolerance and suppression of neighboring macrophages and effector T cells in cancer. Nat Immunol. 2020;21:442–54. doi: 10.1038/s41590-020-0620-x. [DOI] [PubMed] [Google Scholar]
- 39.Lucas ED, Schafer JB, Matsuda J, et al. PD-L1 reverse signaling in dermal dendritic cells promotes dendritic cell migration required for skin immunity. Cell Rep. 2020;33:108258. doi: 10.1016/j.celrep.2020.108258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kornepati AVR, Vadlamudi RK, Curiel TJ. Programmed death ligand 1 signals in cancer cells. Nat Rev Cancer. 2022;22:174–89. doi: 10.1038/s41568-021-00431-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Freeman GJ, Long AJ, Iwai Y, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192:1027–34. doi: 10.1084/jem.192.7.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gong J, Chehrazi-Raffle A, Reddi S, et al. Development of PD-1 and PD-L1 inhibitors as a form of cancer immunotherapy: a comprehensive review of registration trials and future considerations. J Immunother Cancer. 2018;6:8. doi: 10.1186/s40425-018-0316-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gong J, Le TQ, Massarelli E, et al. Radiation therapy and PD-1/PD-L1 blockade: the clinical development of an evolving anticancer combination. J Immunother Cancer. 2018;6:46. doi: 10.1186/s40425-018-0361-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ngwa W, Irabor OC, Schoenfeld JD, et al. Using immunotherapy to boost the abscopal effect. Nat Rev Cancer. 2018;18:313–22. doi: 10.1038/nrc.2018.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Roux C, Jafari SM, Shinde R, et al. Reactive oxygen species modulate macrophage immunosuppressive phenotype through the up-regulation of PD-L1. Proc Natl Acad Sci U S A. 2019;116:4326–35. doi: 10.1073/pnas.1819473116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Glorieux C, Xia X, He Y-Q, et al. Regulation of PD-L1 expression in K-ras-driven cancers through ROS-mediated FGFR1 signaling. Redox Biol. 2021;38:101780. doi: 10.1016/j.redox.2020.101780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yu J, Ling S, Hong J, et al. TP53/mTORC1-mediated bidirectional regulation of PD-L1 modulates immune evasion in hepatocellular carcinoma. J Immunother Cancer. 2023;11:e007479. doi: 10.1136/jitc-2023-007479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wu R, Wang C, Li Z, et al. SOX2 promotes resistance of melanoma with PD-L1 high expression to T-cell-mediated cytotoxicity that can be reversed by SAHA. J Immunother Cancer. 2020;8:e001037. doi: 10.1136/jitc-2020-001037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ba H, Dai Z, Zhang Z, et al. Antitumor effect of CAR-T cells targeting transmembrane tumor necrosis factor alpha combined with PD-1 mAb on breast cancers. J Immunother Cancer. 2023;11:e003837. doi: 10.1136/jitc-2021-003837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Labiano S, Palazon A, Melero I. Immune response regulation in the tumor microenvironment by hypoxia. Semin Oncol. 2015;42:378–86. doi: 10.1053/j.seminoncol.2015.02.009. [DOI] [PubMed] [Google Scholar]
- 51.Dong P, Xiong Y, Yue J, et al. Tumor-Intrinsic PD-L1 signaling in cancer initiation, development and treatment: beyond immune evasion. Front Oncol. 2018;8:386. doi: 10.3389/fonc.2018.00386. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available in a public, open access repository. Data are available upon reasonable request.