Abstract
Background
Selective laser trabeculoplasty (SLT), a National Institute for Care and Health Excellence recommended first-line treatment for open-angle glaucoma and ocular hypertension, is increasingly delivered by optometrists. This retrospective multicentre observational study evaluates real-world outcomes of SLT comparing optometrist-treated to ophthalmologist-treated eyes.
Methods
Adults aged ≥40 years receiving first SLT treatment at three UK hospital eye units (Aintree, Manchester, Macclesfield) between 1 August 2018 and 1 August 2021 were analysed using anonymised local audit data. Outcomes included intraocular pressure (IOP), visual acuity (VA), drop burden, complications including post-SLT IOP spikes, and composite treatment failures including repeat laser or glaucoma surgery, evaluated at 6-monthly intervals up to 24 months. Groups were compared with parametric and non-parametric tests, accounting for intereye correlation, and Kaplan-Meier survival analysis using composite treatment failure endpoints was conducted.
Results
207 eyes (131 patients) were analysed, 84 (56 patients) optometrist-treated eyes compared with 123 ophthalmologist-treated eyes (75 patients). No statistically significant differences (p>0.05) were found in change in VA, IOP or glaucoma drops from pre-SLT baseline between optometrist and ophthalmologist-treated eyes, at all time points. More cataracts were detected in optometrist-treated eyes, however, this did not affect differences in VA or cataract surgery frequency. More optometrist-treated eyes underwent glaucoma surgery, however, ophthalmologist-treated eyes had higher drop burden and chance of composite treatment failure up to month 18.
Conclusion
Outcomes of SLT treatment by optometrists and ophthalmologists are comparable up to 24 months post-treatment. Ophthalmologist-treated eyes may have had more aggressive eye-drop treatment, preventing the need for surgery.
Keywords: Glaucoma, Treatment Lasers, Intraocular pressure
WHAT IS ALREADY KNOWN ON THIS TOPIC
Selective laser trabeculoplasty (SLT) is a first-line, cost-effective therapy for open-angle glaucoma and ocular hypertension recommended by the National Institute for Health and Care Excellence.
WHAT THIS STUDY ADDS
SLT can be safely and effectively delivered by trained optometrists with comparable efficacy and safety outcomes to ophthalmologists up to 24 months post-treatment.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
This study supports the training of optometrists to deliver SLT as part of newer glaucoma service delivery models, with appropriate training, oversight and support from glaucoma specialist colleagues.
Introduction
Open-angle glaucoma (OAG) and ocular hypertension (OHT) are lifelong conditions estimated to affect up to 4% and 10% of adults, respectively, aged 48 and above in the UK.1 Monitoring and management of elevated intraocular pressure (IOP) is important to prevent individuals with OAG and OHT from losing sight due to progressive damage to the optic nerve, with options including pressure-lowering drugs (usually administered as eye-drops), non-surgical laser procedures and surgery. Shortfalls in the capacity of eye services to diagnose, treat and monitor patients with OAG and OHT have been under scrutiny in recent years,2 and current projected rates of eye specialist training and certification are unlikely to match demands of ageing populations.3 This has led to calls to develop new models of glaucoma care to improve case-finding, referral quality, treatment and monitoring,4 which include using novel technologies and expanding roles of non-physician professionals such as optometrists, specialist nurses, physicians associates and ophthalmic technicians in shared-care models.5 6
Selective laser trabeculoplasty (SLT) is a relatively quick, cost-effective and safe treatment in patients with OHT and OAG recommended as a first-line treatment by the National Institute for Health and Care Excellence in the 2022 updated guidelines.7,9 SLT involves the application of laser energy to the trabecular meshwork (TM), which is theorised to induce TM remodelling leading to improved aqueous humour outflow and reducing IOP, and is conventionally undertaken under direct observation using a gonioscopic lens.10 It is non-invasive, has standardised guidelines available for reference,11 and has distinct advantages compared with eye-drops and surgery in that the benefits of treatment are less dependent on adherence to eye-drops, and the risks of surgical intervention are avoided.
An increasing number of hospital eye services in the UK have been training and commissioning optometrists to deliver SLT treatments,12 which has led to interest in formalising this practice with evidence-based guidelines on training, oversight and implementation.13 14 Given that optometrist-delivered SLT is already being used in the UK National Health Service (NHS), there is scope for a real-world evaluation of outcomes of optometrist-delivered SLT to support changes in glaucoma service models.
The purpose of this study was to evaluate the efficacy and safety of SLT delivered as part of routine clinical care comparing optometrists to ophthalmologists, using routinely collected clinical data.
Methods
Study design and selection criteria
This is a retrospective, observational, multicentre study of patients treated at three UK regional hospital eye units (Aintree University Hospital, Manchester Royal Eye Hospital and East Cheshire NHS Trust) between 1 August 2018 and 1 August 2021. Patients selected for analysis were adults aged ≥40 years with a diagnosis of OHT or glaucoma, a pre-SLT IOP reading within 30 days, no previous laser trabeculoplasty, cyclodiode laser or glaucoma surgical interventions, receiving first treatment of SLT to one or both eyes and at least 12 months of post-SLT outcome data. Clinical data used in this analysis had been extracted and anonymised from local electronic medical record (EMR) systems as part of local SLT audits or service evaluation studies, and the anonymised data were shared for this multicentre analysis. Authors checked anonymised records against eligibility criteria, and no additional patient data verification was undertaken. The study was conducted in accordance with the REporting of studies Conducted using Observational Routinely-collected health Data (RECORD) statement. No review from an institutional review board or research ethics committee was deemed necessary as no identifiable human data were used, and the anonymised data were collected as part of local SLT audit and service evaluation pathways, in accordance with health research authority guidance.
Patient and public involvement
No patients or members of the public were involved in this research, as this was a retrospective analysis using previously collected, anonymised outcome data and with no prospective recruitment.
Outcome measures
Outcomes were assessed at 6, 12, 18 and 24 months post-SLT. Key clinical outcome data were changes in IOP, visual acuity (VA) and medication burden (number and type of eye-drops), complications and other events such as repeat SLT or surgery. Key safety outcomes were proportion of eyes with loss of ≥0.2 logarithm of the minimum angle of resolution (LogMAR) acuity, sight-threatening diagnoses and immediate post-SLT IOP spikes of ≥30 mm Hg, or ≥10 mm Hg IOP rise compared with prelaser IOP. Proportions of eyes meeting any one composite measure of treatment failure were summarised at each time point, the criteria of which included:
IOP reduction <20% of baseline at two consecutive visits or at last recorded visit.
IOP >21 mm Hg at two consecutive visits or at last recorded visit.
≥1 additional medication required to control IOP.
Repeat SLT.
Glaucoma surgical intervention.
As patients had been seen at various time points, data were taken from the closest visit to the estimated optimal time in months (6, 12, 18 or 24) from baseline, with a window of ±60 days. Non-LogMAR VA data were converted to LogMAR using published conversion methods.15 Outcome data were analysed on eyes with data available at the respective time points.
Statistical analysis
Continuous data were summarised as means, standard deviation (SD) and 95% confidence intervals (CI) if normally distributed, or medians and interquartile range (IQR) if not. Categorical data were summarised as frequencies and percentages. The t-test (paired and unpaired), Mann-Whitney U and χ2 tests were used to compare outcomes between groups for continuous parametric, non-parametric and categorical data, respectively, using two-way analyses for continuous data and a confidence level of 95%. Kaplan-Meier survival analysis was conducted to compare time-to-failure (represented as the failure function) between optometrist and ophthalmologist-treated eyes. To handle intereye correlation for change in continuous outcome measures, intraclass correlation coefficients of change in continuous outcome variables between paired eyes with data available at each time point were calculated according to methods described by Bland and Altman.16 Data showing high correlation (≥0.8) were averaged for both eyes, otherwise one eye was chosen at random for analysis at each time point using STATA random sampling algorithms, following the methods described by Armstrong.15 Sensitivity analysis using data from the fellow (unchosen) eye was undertaken to examine any differences in results. Data were analysed using STATA MP V.17.0 (StataCorp).
Results
Baseline characteristics
207 eyes (131 patients) were analysed, with 84 eyes (56 patients) treated by optometrists compared with 123 eyes (75 patients) treated by ophthalmologists. Baseline characteristics were well matched for age, gender and diagnosis between groups (table 1). Only 17% (14/84) of optometrist-treated and 18% (22/123) of ophthalmologist-treated eyes were on no drops at baseline. There appeared to be more white patients among optometrist-treated eyes, however, due to limited ethnicity data this trend could not be formally verified. All three sites used optometrists to deliver SLT, however, one site (Macclesfield) did not have any ophthalmologists delivering SLT within the period of available data, due to the structure of the local glaucoma service. Most ophthalmologist-treated eyes were treated by glaucoma clinical fellows or ophthalmologists in specialty training. The amount of SLT energy used was similar between optometrists, ophthalmologists in specialty training and fellows, but higher in consultants and associate specialists (senior ophthalmologists).
Table 1. Baseline characteristics of patients/eyes included in analysis disaggregated by treating clinician.
| Optometrist treated | Ophthalmologist treated | |
| Total | ||
| Patients | 56 (26 bilateral) | 75 (48 bilateral) |
| Eyes | 84 | 123 |
| Ophthalmologists by grade (eyes, n %)* | ||
| Consultant | 17 (14) | |
| Fellow | 16 (13) | |
| Associate specialist | 37 (30) | |
| Senior trainee (4 years or more of training) | 11 (9) | |
| Junior trainee (within first 3 years of training) | 42 (34) | |
| Site (eyes, n %) | ||
| Manchester | 32 (38) | 45 (37) |
| Macclesfield | 21 (25) | 0 |
| Aintree | 31 (37) | 78 (63) |
| Data (eyes, n %) at: | ||
| 6 months | 22 (26) | 29 (24) |
| 12 months | 53 (63) | 67 (54) |
| 18 months | 40 (48) | 66 (54) |
| 24 months | 37 (44) | 51 (42) |
| Age (mean, SD) | 69.2 (11.4) | 67.7 (11.6) |
| Sex female, n (%) | 35 (63) | 41 (55) |
| Race, n (%) | ||
| White | 31 (55) | 23 (31) |
| Black | 0 | 2 (3) |
| Asian | 1 (2) | 2 (3) |
| Unknown | 24 (43) | 48 (64) |
| Diagnosis (eyes, n %) at baseline | ||
| OHT | 17 (20) | 32 (26) |
| OAG | 56 (67) | 79 (64) |
| Glaucoma suspect | 7 (8) | 4 (3) |
| NTG | 0 | 4 (3) |
| PDS | 1 (1) | 3 (2) |
| PACG† | 3 (4) | 1 (1) |
| Pseudophakic | 23 (27) | 18 (15) |
| Pre-SLT VA LogMAR, median (IQR) | 0.10 ((0, 0.26) | 0.10 ((0, 0.15)) |
| Pre-SLT IOP, mean (SD) | 25.0 (4.8) | 25.0 (6.1) |
| Pre-SLT proportion of eyes on: | ||
| 0 eye-drops | 14 (17) | 22 (18) |
| 1 eye-drop | 26 (31) | 52 (42) |
| 2 eye-drops | 24 (29) | 31 (25) |
| 3 eye-drops | 20 (24) | 14 (12) |
| 4 eye-drops | 0 | 4 (3) |
| SLT energy mJ, mean (SD) | ||
| Optometrist | 89.6 (23.1) | |
| Consultant | 107.3 (33.2) | |
| Associate specialist | 114 (32.9) | |
| Fellow | 91.8 (39.9) | |
| Trainee | 87.1 (41.4) | |
| Degrees treated, n (%) | ||
| 180 | 4 (5) | 4 (3) |
| 270 | 0 | 1 (1) |
| 360 | 80 (95) | 118 (96) |
Data are n (%), means (SD), or median (IQR).
For clarification and definition of ophthalmologist grades in this analysis: consultants are fully independent glaucoma specialists, fellows are ophthalmologists who have completed ophthalmology specialist training and are undertaking subspecialty training, associate specialists are ophthalmologists with the same clinical experience as consultants but in a non-consultant role, and trainees are doctors undergoing ophthalmology specialist training.
All cases of primary angle closure glaucoma had undergone previous cataract surgery or laser iridotomy to address the closed angle before being treated with SLT.
IOPintraocular pressureIQRinterquartile rangeLogMARlogarithm of the minimum angle of resolutionNTGnormal tension glaucomaOAGopen-angle glaucomaOHTocular hypertensionPACGprimary angle closure glaucomaPDSpigment dispersion syndromeSDstandard deviationSLTselective laser trabeculoplastyVAvisual acuity
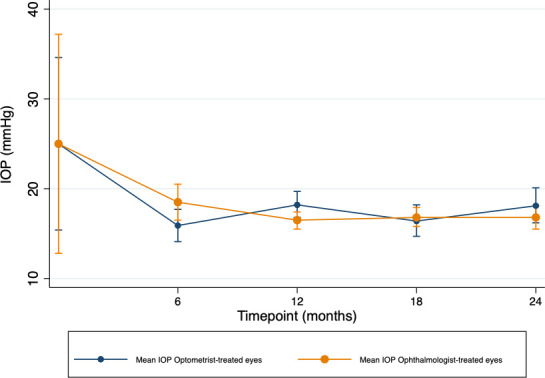
Although there was a small difference in IOP reduction by month 24 favouring ophthalmologist-treated eyes (mean difference optometrist-treated vs ophthalmologist-treated eyes (95% CI) −1.8 mm Hg (−5.1 to 1.5)), none of the differences in IOP reduction at any time point reached statistical significance (p>0.05, table 2). Figure 1 and online supplemental figure S1 show the mean IOP and mean change in IOP over time, respectively. Online supplemental figure S2 shows the median LogMAR VA over time between groups and, accounting for outliers, none of these differences reached statistical significance at any time point (p>0.05).
Table 2. Change in VA, IOP and eye-drop burden from baseline to months 6, 12, 18 and 24 disaggregated by treating clinician.
| Change in clinical outcomes from baseline | Optometrist-treated eyesN=84 | Ophthalmologist-treated eyesN=123 | Difference in means (95% CI) | Significance of difference in means |
| Month 6 | P>0.05 | |||
| Reduction in IOP, mm Hg (mean, 95% CI) | 7.0 (3.1 to 10.9) | 5.6 (2.9 to 8.4) | 1.4 (–3.1 to 5.8) | |
| Change in LogMAR VA (median, IQR) | 0.08 (0, 0.18) | 0.0 (–0.04, 0.02) | ||
| Change in eye-drop burden (mean, 95% CI) | 0.09 (–0.1 to 0.3) | 0.06 (–0.47 to 0.58) | 0.04 (–0.64 to 0.71) | |
| Month 12 | P>0.05 | |||
| Reduction in IOP, mm Hg (mean, 95% CI) | 7.1 (5.1 to 9.1) | 7.2 (5.2 to 9.3) | −0.12 (–2.9 to 2.7) | |
| Change in LogMAR VA (median, IQR) | 0.18 (–0.08, 0.18) | 0 (–0.08, 0.18) | ||
| Change in eye-drop burden (mean, 95% CI) | −0.18 (–0.40 to 0.04) | −0.31 (–0.60 to –0.02) | 0.13 (–0.24 to 0.50) | |
| Month 18 | P>0.05 | |||
| Reduction in IOP, mm Hg (mean, 95% CI) | 7.4 (4.8 to 10.0) | 7.2 (5.6 to 8.8) | 0.15 (–2.7 to 3.0) | |
| Change in LogMAR VA (median, IQR) | 0 (–0.06, 0.14) | 0 (–0.08, 0.08) | ||
| Change in eye-drop burden (mean, 95% CI) | −0.50 (–1.0 to 0.03) | −0.32 (–0.74 to 0.09) | −0.18 (–0.83 to 0.48) | |
| Month 24 | P>0.05 | |||
| Reduction in IOP, mm Hg (mean, 95% CI) | 6.0 (3.4 to 8.6) | 7.8 (5.6 to 10.0) | −1.8 (–5.1 to 1.5) | |
| Change in LogMAR VA (median, IQR) | 0 (–0.08, 0.06) | 0 (–0.08, 0.08) | ||
| Change in eye-drop burden (mean, 95% CI) | −0.48 (–1.0 to 0.06) | −0.21 (–0.58 to 0.16) | −0.27 (–0.89 to 0.35) |
Data are mean (95% CI) or median (IQR).
95%CI95% confidence intervalIOPintraocular pressureIQRinterquartile rangeLogMARlogarithm of the minimum angle of resolutionVAvisual acuity
Figure 1. Mean intraocular pressure (IOP) of optometrist versus ophthalmologist-treated eyes over time. Mean intraocular pressure between optometrist-treated and ophthalmologist-treated eyes. Error bars show the 95% confidence interval.
Overall, while the proportion of eyes on two eye-drops in optometrist-treated eyes remained constant from pre-SLT to month 24, the proportion of eyes on 3 eye-drops and 1 eye-drop reduced from 24% to 16% and 31% to 16%, respectively, while the proportion of eyes on no drops rose from 16% to 38%. There were no eyes on four eye-drops in the optometrist-treated group. In contrast, the proportion of eyes on 2 eye-drops and four eye-drops in the ophthalmologist-treated group increased from 25% to 33% and 3% to 6%, respectively, from pre-SLT to month 24. The overall proportion of eyes on no eye-drops increased from 18% to 36% from pre-SLT to month 12 but decreased again to 27% by month 24 after the addition of topical drop therapy (online supplemental figure S3).
There was a similar proportion of SLT-attributed complications and events in both groups (online supplemental table S1). While there were more secondary diagnoses detected in optometrist-treated eyes compared with ophthalmologist-treated eyes at months 12 and 24, the difference in proportions did not achieve statistical significance. Most of the difference was attributable to cataract (12%, 10/84 optometrist-treated eyes vs 7%, 9/123 ophthalmologist-treated eyes), which did not contribute to any significant differences in LogMAR VA between groups. Two (2%, 2/84) optometrist-treated eyes and five (4%, 5/123) ophthalmologist-treated eyes underwent cataract surgery within the 24-month period. Both optometrist-treated eyes had routine cataract surgery (with no additional glaucoma adjunct devices implanted), whereas in the ophthalmologist-treated eyes, one had routine cataract surgery, three had glaucoma adjunct devices implanted (iStent, Hydrus and Preserflo, respectively), and one had surgery to correct aqueous misdirection syndrome. There were only three reported cases of post-SLT IOP spike overall (one optometrist-treated and two ophthalmologist-treated eyes).
Table 3 shows the eyes that met one of the composite treatment failure criteria, including repeat SLT and surgery. More optometrist-treated eyes (10%, 8/84) underwent penetrating or non-penetrating glaucoma surgery compared with ophthalmologist-treated eyes (5%, 6/123), with an OR of 1.95 (95% CI 0.7 to 5.4, p>0.05). Eyes in both groups that underwent surgery had significantly (p<0.05) higher mean pre-SLT IOP (optometrist-treated mean IOP (95% CI) 27.5 mm Hg (23.9 to 31.1), ophthalmologist-treated mean IOP 29.8 mm Hg (22.2 to 37.5)) compared with those not undergoing surgery (optometrist-treated mean (95% CI) IOP 23.8 mm Hg (22.7 to 24.9), ophthalmologist-treated 24.4 mm Hg (23.2 to 25.5)). Five (63%, 5/8) of optometrist-treated and 4 (36%, 4/11) of ophthalmologist-treated eyes that underwent glaucoma surgery were operated on after month 12. Overall, 54% (45/84) of optometrist-treated eyes met at least one composite failure requirement in 24 months of follow-up, compared with 53% (65/123) of ophthalmologist-treated eyes.
Table 3. Composite treatment failures disaggregated by optometrist-treated versus ophthalmologist-treated eyes.
| Optometrist-treated eyes(n=84) | Ophthalmologist-treated eyes(n=123) | |
| Proportion of eyes requiring repeat SLT, n (%) | 7 (8) | 6 (5) |
| Proportion of eyes undergoing glaucoma surgery, n (%) | 11 (13) | 11 (9) |
| iStent | 2 | 4 |
| Non-penetrating surgery | 4 | 1 |
| Penetrating surgery | 4 | 5 |
| Shunt | 0 | 0 |
| Other | 1 (cyclodiode) | 1 (cyclodiode) |
| Penetrating and non-penetrating glaucoma surgery at specific time points, (n) | ||
| Month 6 | Visco (2) | Visco (1) |
| Month 12 | Trab (1) | Trab (1) |
| Month 18 | Visco (1)+Trab (2)+Preserflo (1) | Trab (2)+Preserflo (1) |
| Month 24 | Visco (1) | Preserflo (1) |
| Overall proportion of eyes achieving composite treatment failure at 24 months, n (%) | 45 (54) | 65 (53) |
| Subgroups of treatment failure* | ||
| IOP reduction <20% baseline at two consecutive visits or last recorded visit | 16 (19) | 32 (26) |
| IOP>21 mm Hg at two consecutive visits or last recorded visit | 13 (15) | 12 (10) |
| ≥1 additional medication | 12 (14) | 19 (15) |
| Repeat SLT required | 7 (8) | 6 (5) |
| Glaucoma surgery | 11 (13) | 8 (7) |
Data are count (%).
Data may be more than proportion of eyes overall that achieved composite treatment failure, as an eye may have met more than one criterion at the respective time point.
IOPintraocular pressureSLTselective laser trabeculoplastyTrabtrabeculectomyViscoviscocanalostomy
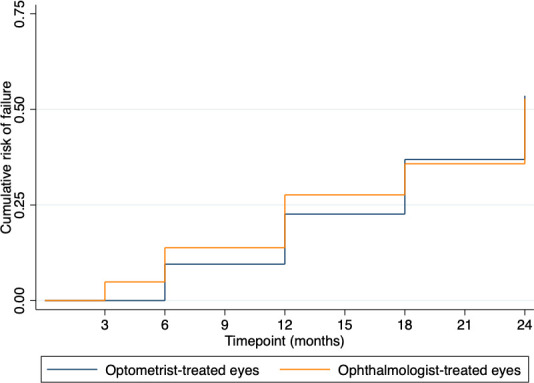
All eyes were included in the survival analysis (figure 2). The estimated probability of failure was slightly higher in ophthalmologist-treated eyes from baseline to month 12, but there was no discernible difference from month 18 onwards.
Figure 2. Kaplan-Meier probability of failure curve. Kaplan-Meier probability of failure curve showing the cumulative risk of failure at respective time points between optometrist-treated compared with ophthalmologist-treated eyes.
Discussion
This study is the first multicentre study evaluating the difference in treatment outcomes between SLT delivered by optometrists compared with ophthalmologists, using time-series data up to 2 years (24 months) and found no statistically significant difference in change in IOP, LogMAR VA or mean reduction in eye-drop burden comparing both groups from pre-SLT baseline up to 2 years.
Although ophthalmologist-treated eyes had (non-significantly) better IOP reduction at 24 months post-SLT (mean difference (95% CI) −1.8 mm Hg (−5.1 to 1.5)) and more stable IOP overall (figure 1 and online supplemental figure S1), they also had a greater proportion of eyes on 2 or more drops (eyes on 2 and 4 drops increased from 25% to 33% and 3% to 6%, respectively), and although both groups had the same proportions of eyes on no drops at month 12 (36% in both groups), by month 24 the proportion of eyes on no drops in the optometrist-treated group was greater than that of ophthalmologist-treated eyes (38% vs 27%). This data suggests that in ophthalmologist-treated eyes there was a tendency for clinicians to increase the number of glaucoma drops through the follow-up period, possibly in response to rising IOP, which may explain the better and more stable IOP control and lower proportion of eyes requiring surgery by 24 months.
Proportionally, there were slightly more new cataract diagnoses in optometrist-treated eyes (12%, 10/84) compared with ophthalmologist-treated eyes (7%, 9/123) at 24 months, despite a greater proportion of eyes in the optometrist group being pseudophakic pre-SLT (27% vs 15%, 23/84 vs 18/123), however, this difference was not statistically significant, did not lead to any significant difference in final LogMAR VA, and the proportion of eyes undergoing cataract surgery was lower in optometrist-treated eyes (2% vs 4%, 2/84 vs 5/123) even though the majority of cataracts were diagnosed in the first 12 months of follow-up. Some of this difference may be attributable to heterogeneity in clinicians reviewing patients, though this could not be verified from available data.
Only one prior UK-based study has evaluated clinical efficacy of SLT delivered by optometrists compared with ophthalmologists. Chadwick et al reported the results of 333 SLT procedures on 325 eyes delivered by 3 non-ophthalmologists (2 optometrists and 1 orthoptist, supervised by a consultant glaucoma specialist)17 and found no significant difference in mean IOP or median IOP reduction at 12 months, and a similar proportion of SLT-attributable complications of 3.9% and 3.8% between groups, both findings of which are comparable to our study. In their cohort, Chadwick et al found 12 eyes (3.7%, 12/325) required filtration surgery within 12 months of SLT, similar to findings in our study in which 3 eyes (3.6%, 3/84, rounded to 4% in the results section) required filtration surgery by month 12. A previous study by Khawaja et al analysing outcomes of ophthalmologist-delivered SLT using EMR data found that 7.7% of eyes (34/439) had required glaucoma filtration surgery by 12–18 months of follow-up.18 In comparison, 10% (8/84) of optometrist-treated eyes, and 5% (6/123) of ophthalmologist-treated eyes in our study underwent penetrating or non-penetrating glaucoma surgery, the difference of which may have been attributable to more aggressive use of eye-drops in the ophthalmologist-treated group.
The total baseline mean (SD) SLT energy of optometrist-treated eyes of 89.6 mJ (23.1) was similar to that of trainee-treated (87.1 mJ (41.4)) and fellow-treated (91.8 mJ (31.9)) eyes, and comparable to total energy administered in the studies by Chadwick et al (82.5 mJ (19.1))17 and the LiGHT trial19 (90.4 mJ (23.5)), but lower than that of consultant (107.3 mJ (33.2)) and associate specialist (114 mJ (32.9)) treated eyes. This may have been due to consultants and associate specialists selecting more challenging or risky cases (necessitating more aggressive treatment to achieve desired IOP reduction), or optometrists, trainees and fellows being more cautious in treatment.
The strengths of this study include the use of real-world, multicentre, time-series data up to 24 months post-SLT treatment, reflecting practice across multiple eye units, and use of predefined eligibility criteria for inclusion in analysis leading to reasonably comparable groups at baseline. Weaknesses include the lack of race data (a known limitation in existing EMR systems as reflected in the National Ophthalmology Database Audit)20 limiting generalisability of outcomes to different racial groups, and the potential impact of national lockdown commencing 26 March 2020 increasing the risk of missing follow-up data or prioritisation of review to ‘high-risk’ cases only. There were insufficient data on who had initially listed eyes for SLT or reviewed eyes after initial SLT treatment either postoperatively for complications or at subsequent follow-ups, which may have introduced selection bias with respect to allocation of cases to different clinician grades and impacted the likelihood of detecting secondary diagnoses (eg, some studies have shown variable levels of concordance in diagnoses comparing optometrists to ophthalmologists).21 Standard NHS practice usually means patients may be seen by any member of the team, however, decisions on the management of intraoperative and perioperative complications, escalation of treatment or listing for procedures would usually be undertaken by senior ophthalmologists (consultants, fellows or associate specialists). Most ophthalmologist-delivered SLT in this analysis was undertaken by ophthalmology trainees or glaucoma specialist fellows rather than consultants or associate specialists, which may theoretically have led to treatment efficacy bias diminishing the clinical efficacy of SLT, although other real-world UK-based data reported by Khawaja et al disaggregated by clinician grade found that SLT undertaken by ophthalmologists in training was actually more efficacious than treatment by consultants.18 Data on mean deviation or nerve fibre layer metrics were not available within the routinely collected dataset, meaning severity or progression in glaucoma could not be determined in this analysis, which may have contributed to the reason for the higher proportion of filtration surgery required in optometrist-treated eyes, or greater, earlier drop burden in ophthalmologist-treated eyes. Finally, and potentially not a weakness, all three units involved in this study employ experienced optometrists with independent prescribing qualifications (a qualification delivered by the college of optometrists requiring supervised clinical placements and independent exams) and training under direct consultant supervision, including gonioscopic techniques and audited logbooks of cases and outcomes, to deliver SLT. Furthermore, these optometrists work alongside glaucoma specialist ophthalmologists in a shared-care, guideline-driven model, to manage patients with a range of glaucoma complexity, in a collaborative manner. As such our data best reflect units where experienced optometrists work alongside, and share patient care with glaucoma specialist colleagues, and may not be reflective of practices with higher training burdens or outside of the UK NHS context.
As the number of glaucoma patients is expected to increase as the population ages,2 and surveys of the ophthalmic glaucoma workforce have indicated an ongoing shortage of ophthalmologists,22 newer models of service delivery are needed. Expanding the role of non-ophthalmologists to deliver first-line, evidence-based treatments, with appropriate training and oversight, may be a cost-effective and safe model to expand capacity within glaucoma services, ensuring patients are seen and treated in a timely fashion. Formalised training models are currently under development to standardise and accredit this process.13
Our results show that optometrist-delivered SLT may be a viable model of shared-care service delivery, with comparable efficacy and safety outcomes up to 24 months post-SLT treatment, thereby facilitating delivery on the implementation of national guidance.9 We recommend units that employ this model of service delivery ensure rigorous training and audit processes are in place to monitor outcomes, and clear lines of oversight and accountability between those delegated to deliver SLT therapy and glaucoma specialists are in place to ensure patients who experience complications, or who need more intensive care or surgery, are prioritised for consultant review.
supplementary material
Footnotes
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient consent for publication: Not applicable.
Data availability free text: Requests for data sharing should be made to the corresponding author.
Contributor Information
Chan Ning Lee, Email: channing.lee2@nhs.net.
Alexander Delaney, Email: alexander.m.delaney@gmail.com.
Jay A L Richardson, Email: Jay.richardson@nhs.net.
Graham Freeman, Email: graham.freeman@nhs.net.
Patrick J G Gunn, Email: patrick.gunn@mft.nhs.uk.
Stephen Harthan, Email: stephen.harthan@liverpoolft.nhs.uk.
Vincent Dubois, Email: Vincent.dubois@liverpoolft.nhs.uk.
Kenneth Yau, Email: kenneth.yau@mft.nhs.uk.
Christopher Hemmerdinger, Email: christopher.hemmerdinger@nhs.net.
Robert Harper, Email: Robert.Harper@mft.nhs.uk.
Neeru A Vallabh, Email: vallabh@liverpool.ac.uk.
Data availability statement
Data are available on reasonable request.
References
- 1.Chan MPY, Broadway DC, Khawaja AP, et al. Glaucoma and intraocular pressure in EPIC-Norfolk Eye Study: cross sectional study. BMJ. 2017;358:j3889. doi: 10.1136/bmj.j3889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.The Royal College of Ophthalmologists The way forward: glaucoma. 2017
- 3.The Royal College of Ophthalmologists The royal college of ophthalmologists workforce census 2018. 2018
- 4.Ratnarajan G, Newsom W, Vernon SA, et al. The effectiveness of schemes that refine referrals between primary and secondary care--the UK experience with glaucoma referrals: the Health Innovation & Education Cluster (HIEC) Glaucoma Pathways Project. BMJ Open. 2013;3:e002715. doi: 10.1136/bmjopen-2013-002715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Simons A-S, Vercauteren J, Barbosa-Breda J, et al. Shared Care and Virtual Clinics for Glaucoma in a Hospital Setting. J Clin Med. 2021;10:4785. doi: 10.3390/jcm10204785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hamid S, Desai P, Hysi P, et al. Population screening for glaucoma in UK: current recommendations and future directions. Eye (Lond) 2022;36:504–9. doi: 10.1038/s41433-021-01687-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.National Institute for Health and Care Excellence [NICE] NICE Guidel; 2017. Glaucoma: diagnosis and management nice guideline; pp. 1–41. [PubMed] [Google Scholar]
- 8.Gazzard G, Konstantakopoulou E, Garway-Heath D, et al. Selective laser trabeculoplasty versus drops for newly diagnosed ocular hypertension and glaucoma: the LiGHT RCT. Health Technol Assess. 2019;23:1–102. doi: 10.3310/hta23310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.National Institute for Health and Care Excellence [NICE] Resource impact report: glaucoma: diagnosis and management-partial update (NG81) 2022 [PubMed]
- 10.Kagan DB, Gorfinkel NS, Hutnik CML. Mechanisms of selective laser trabeculoplasty: a review. Clin Exp Ophthalmol. 2014;42:675–81. doi: 10.1111/ceo.12281. [DOI] [PubMed] [Google Scholar]
- 11.European Glaucoma Society Terminology and Guidelines for Glaucoma, 5th Edition. Br J Ophthalmol. 2021;105:1–169. doi: 10.1136/bjophthalmol-2021-egsguidelines. [DOI] [PubMed] [Google Scholar]
- 12.Gunn PJG, Creer RC, Bowen M, et al. Scope of practice of optometrists working in the UK Hospital Eye Service: Second national survey. Ophthalmic Physiol Opt. 2022;42:428–39. doi: 10.1111/opo.12952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Konstantakopoulou E, Varia J, Parmar J, et al. Optometrist-delivered selective laser trabeculoplasty in the HES - a training protocol and early service evaluation. Eye (Lond) 2024;38:2589–95. doi: 10.1038/s41433-024-03086-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Konstantakopoulou E, Jones L, Nathwani N, et al. Selective laser trabeculoplasty (SLT) performed by optometrists-enablers and barriers to a shift in service delivery. Eye (Lond) 2022;36:2006–12. doi: 10.1038/s41433-021-01746-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Armstrong RA. Statistical guidelines for the analysis of data obtained from one or both eyes. Ophthalmic Physiol Opt. 2013;33:7–14. doi: 10.1111/opo.12009. [DOI] [PubMed] [Google Scholar]
- 16.Bland JM, Altman DG. Measurement error and correlation coefficients. BMJ. 1996;313:41–2. doi: 10.1136/bmj.313.7048.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chadwick O, Chia SN, Rotchford A. Establishing an allied health professional delivered selective laser trabeculoplasty service in Scotland. Ophthalmic Physiologic Optic . 2019;39:216–23. doi: 10.1111/opo.12611. [DOI] [PubMed] [Google Scholar]
- 18.Khawaja AP, Campbell JH, Kirby N, et al. Real-World Outcomes of Selective Laser Trabeculoplasty in the United Kingdom. Ophthalmology. 2020;127:748–57. doi: 10.1016/j.ophtha.2019.11.017. [DOI] [PubMed] [Google Scholar]
- 19.Garg A, Vickerstaff V, Nathwani N, et al. Efficacy of Repeat Selective Laser Trabeculoplasty in Medication-Naive Open-Angle Glaucoma and Ocular Hypertension during the LiGHT Trial. Ophthalmology. 2020;127:467–76. doi: 10.1016/j.ophtha.2019.10.023. [DOI] [PubMed] [Google Scholar]
- 20.Henry P, Donachie J, Buchan JC. Year 7 annual report-the sixth prospective report of the national ophthalmology database audit national cataract audit national ophthalmology database audituk 2 NOD audit seventh annual report-sixth prospective audit year report. 2023
- 21.Fung M, Myers P, Wasala P, et al. A review of 1000 referrals to Walsall’s hospital eye service. J Public Health. 2016;38:599–606. doi: 10.1093/pubmed/fdv081. [DOI] [PubMed] [Google Scholar]
- 22.The Royal College of Ophthalmologists Facing workforce shortages and backlogs in the aftermath of COVID-19: The 2022 census of the ophthalmology consultant, trainee and SAS workforce Census Report. 2023
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available on reasonable request.


