Abstract
Background
Gastric cancer (GC) is the fourth leading cause of cancer mortality worldwide. Peritoneal metastasis (PM) is a significant cause of death in patients with GC, and presents a major challenge in clinical diagnosis and treatment. Predicting the occurrence of PM in high-risk patients, and diagnosing and treating PM in advance to improve patient survival, remains an unsolved problem in clinical practice. Given the low positive rate of cytology and difficulty in diagnosing occult PM, new molecular markers and detection technologies for early diagnosis require urgent validation. The primary objective of this study is to observe and evaluate the predictive effect of intraoperative peritoneal lavage fluid (PLF) circulating tumour cells (CTC) and circulating tumour DNA (ctDNA) levels in patients with pT4NxM0/pT1-3N+M0 GC on metachronous PM after R0 resection.
Methods and analysis
This prospective single-centre clinical study is conducted at Renji Hospital, Shanghai Jiao Tong University School of Medicine. In this study, 200 cases of patients with pT4NxM0/pT1-3N+M0 gastric adenocarcinoma older than 18 years will be screened. Participants will undergo intraoperative PLF CTC and ctDNA testing and will be followed up for 2 years, with imaging assessments performed every 3–6 months until PM occurrs. The primary outcome is the incidence of PM 1 year after surgery, which will be estimated using Clopper-Pearson method, with 95% CIs calculated and compared between groups. Secondary outcome include the incidence of PM 2 years after surgery, overall survival and disease progression. Data will be analysed using the Kaplan-Meier method and the log-rank test.
Ethics and communication
Informed consent has been obtained from all subjects. This protocol has been approved by the Ethics Committee of Renji Hospital, Shanghai Jiao Tong University School of Medicine (LY2023-142-B). The findings will be disseminated through peer-reviewed manuscripts, reports and presentations.
Trial registration number
ChiCTR2300074910.
Keywords: gastroenterology, gastroduodenal disease, gastrointestinal tumours
STRENGTHS AND LIMITATIONS OF THIS STUDY.
A prospective study on the detection of circulating tumour cells and circulating tumour DNA in peritoneal lavage fluid for predicting peritoneal metastasis in patients with high-risk gastric cancer.
A 2-year follow-up period with regular assessments ensures thorough monitoring of the patients.
This detection method and protocol can be extended to other stages of gastric cancer populations.
The necessity to enrol a large number of patients may extend the study period significantly, potentially delaying the availability of results and their translation into clinical practice.
The monocentric design of this study is a limitation.
Background
In the 2020 global cancer data released by WHO’s International Agency for Research on Cancer, the number of new cases of gastric cancer (GC) was 1.08 million, ranking fifth in the world in incidence and fourth in mortality.1 Metastasis of GC, particularly peritoneal metastases (PM), is a significant cause of death in patients with GC. About 10%–30% of patients with GC are found to have PM at the time of initial diagnosis, and more than half of patients with stage II–III GC develop PM within 5 years after radical surgery.2 Moreover, with the expansion of the distribution of PM, the survival rate of patients decrases. In a study on the prognosis of patients with PM from GC, the 2-year survival rates of patients with peritoneal spread confined to the transverse colon and several nodules distal to the peritoneum below the transverse colon were 21% and 8%, respectively, while the 2-year survival rate was only 4% for patients with extensive metastases found in the distal peritoneum.3
According to the ‘seed-soil’ hypothesis, PM is a multistep process involving the isolation of cancer cells from the primary tumour, their entry into the abdominal microenvironment, adhesion to peritoneal mesothelial cells and subsequnt infiltration and growth.4 The primary focus of GC penetrates the serous layer into the abdominal cavity to form peritoneal-free cancer cells, which constitute the pathological basis for PM. Therefore, indentifying detached and free abdominal cancer cells in patients with GC has become a core issue in clinical decisions for the prevention and treatment of PM. The Japanese GC Association classifies stage IV GC when cancer cells are detected in ascites or peritoneal lavage fluid cytology.5 The American Joint Committee on Cancer eighth edition tumour, node, metastases classification of GC classifies the presence of ascites and the detection of cancer cells in postparacentesis cytology as M1. In fact, as the gold standard for diagnosing intraperitoneal-free tumour cells, the positive rate of cytology of peritoneal lavage fluid is influenced by numerous factors, including the volume of intraoperative lavage fluid retained, the timeliness of sample submission for testing and the abnormal morphology of exfoliated cells. In a meta-analysis of 19 studies, the frequency of detection of cell-free cancer cells in peritoneal lavage fluid in patients with GC ranged from 6.25% to 54.4% and was influenced by the depth of tumour invasion, the degree of differentiation and the use of chemotherapy.6
Currently, conventional cytological examinations exhibit limited sensitivity in detecting occult PM, while the advancement of liquid biopsy holds the potential to enable early detection of PM.
Circulating tumour DNA (ctDNA) is a fragment of DNA present in the extracellular environment, secreted following tumour necrosis, apoptosis or phagocytosis by macrophages. It usually consists of strands <145 bp in length, and can be detected in body fluids such as blood, urine, ascites, pleural fluid and saliva.7 Mutations in ctDNA can be used to identify some potentially modifiable driver genes and provide personalised biomarkers for detecting residual disease or monitoring tumour levels during treatment.8 Minimal residual disease (MRD) was originally used for haematological malignancies to describe residual leukaemia cells that cannot be detected by conventional cell morphology.9 MRD can be defined as a small number of tumour cells or tumour-derived molecular abnormalities that persist in patients after treatment, which cannot be detected with current medical imaging techniques, and represents an insidious stage of cancer progression that may eventually lead to disease recurrence.10 In May 2022, the Food and Drug Administration issued the draft guidelines for the drug development of ctDNA for early stage solid tumours, encouraging the use of ctDNA for MRD detection and evaluation. The guidelines support the use of ctDNA as a marker for MRD after surgery and neoadjuvant chemotherapy, and enriching the evaluation and testing of patients at higher risk of disease recurrence or death.
CTC are a general term for various types of tumour cells present in the peripheral blood, which are shed from solid tumour lesions (primary or metastatic) and enter the peripheral blood circulation due to spontaneous or therapeutic procedures.11 Since CTC is derived from tumour tissue and contain the same biological information as tumour tissue, it is highly accurate to use it as the basis for tumour identification and characterisation. Numerous studies have confirmed that CTC is present in advanced tumours and in the blood samples of patients in the early and middle stages. Further research found that the appearance of CTC occurs even earlier than imaging detection. At present, CTC can be used to evaluate the efficacy and prognosis of solid tumours such as breast cancer, prostate cancer, colorectal cancer and liver cancer.12 In a study, Zeng et al13 detected preoperative peripheral folate receptor-positive circulating tumour cells (FR+CTC) in patients with GC. The level of FR+CTC in the GC group was significantly higher than that in the benign disease group (p<0.001). Additionally, the preoperative FR+CTC level in patients with PM was significantly higher than that in patients without PM, indicating that FR+CTC was closely related to peritoneal/liver metastasis of GC. This finding could provide an important basis for the diagnosis of GC and assist in distinguishing between benign and malignant tumours. The sensitivity and specificity of FR+CTC in detecting GC PM were 77.8% and 54.5%, respectively. The combination of FR+CTC with other biomarkers significantly improved the diagnostic performance of PM, with a combined sensitivity of 100% and an area under the receiver operating characteristic curve of 0.86. FR+CTC was significantly correlated with PM (p=0.025), serving as an independent risk factor for PM and could be used as a non-invasive biomarker for predicting PM in GC.
In summary, this study will include patients with GC classified as pT4NxM0/pT1-3N+M0 for intraoperative intraperitoneal lavage CTC and ctDNA detection, and will conduct a 2-year follow-up to explore the predictive effect of peritoneal lavage fluid CTC and ctDNA on metachronous PM after GC.
Methods and analysis
Study design
The study is designed as a prospective, single-centre study and will be conducted from August 2023 to August 2026 at Renji Hospital, Shanghai Jiao Tong University School of Medicine. Data will be collected and analysed to explore the predictive effect of intraoperative peritoneal lavage fluid CTC and ctDNA on postoperative metachronous PM in patients with pT4NxM0/pT1-3N+M0 GC. The study will be conducted in accordance with the applicable regulations and the Declaration of Helsinki. Additionally, this protocol has been approved by the Ethics Committee of Renji Hospital, Shanghai Jiao Tong University School of Medicine (KY2019-191) and registered in the China Clinical Trials Registry (ChiCTR2300074910).
Study participants
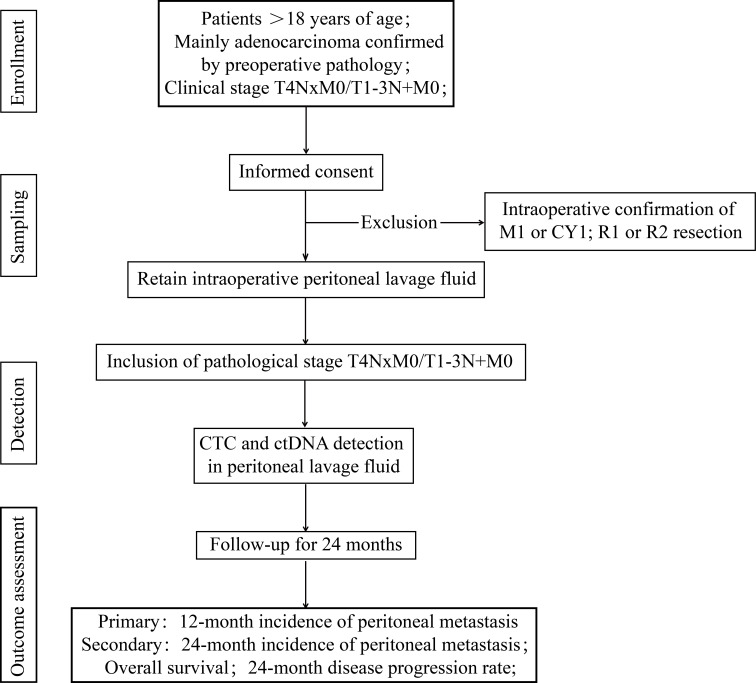
Written informed consent will be obtained from subjects with cT4NxM0/cT1-3N+M0 GC prior to the start of the study to retain their intraoperative intraperitoneal lavage fluid. Within 4 weeks after surgery, based on the judgement of gastrointestinal surgeons and postoperative pathology reports, patients with pT4NxM0/pT1-3N+M0 will be screened out and their peritoneal lavage fluid will be tested. Eligible patients will undergo blood tests and imaging evaluations every 3–6 months until 2 years postoperatively. The end of study is defined as the date of the last follow-up visit for the last enrolled patient. Figure 1 presents a flow chart of the experiment.
Figure 1. Flow chart summarising the study procedure. CTC, circulating tumour cells; ctDNA, circulating tumour DNA.
Complete inclusion/exclusion criteria are detailed in table 1 and table 2.
Table 1. Inclusion criteria.
| 1 | Patients voluntarily join the study and sign an informed consent form. |
| 2 | Aged ≥18 years, both men and women. |
| 3 | There is histologically confirmed, untreated gastric cancer or gastro-oesophageal junction cancer, and histological examination confirms that pT4NxM0/pT1-3N+M0 gastric cancer is mainly adenocarcinoma. |
| 4 | Eastern Cooperative Oncology Group score: 0~1. |
| 5 | Have not received antitumour therapy for gastric cancer before surgery, including chemotherapy, targeted, immune and local radiotherapy. |
Table 2. Exclusion criteria.
| 1 | Diagnosis of distant metastasis by CT/MR/EUS; intraoperative comfirmation of distant metastasis or CY1; R1 or R2 resection. |
| 2 | Received prior antitumour therapy, including chemotherapy, radiotherapy, targeted or immunotherapy. |
| 3 | Pregnant or lactating women; women of childbearing age are positive at baseline pregnancy test. |
| 4 | Other malignancies (except basal cell or squamous cell carcinoma, superficial bladder cancer, cervical cancer in situ or breast cancer) in the past 5 years. |
| 5 | Uncontrollable pleural effusion, pericardial effusion or ascites. |
| 6 | Severe cardiovascular disease, such as symptomatic coronary heart disease, congestive heart failure of grade ≥II, uncontrolled arrhythmia, myocardial infarction within 12 months prior to enrolment. |
| 7 | Complicated by upper gastrointestinal obstruction/bleeding or digestive dysfunction or malabsorption syndrome. |
| 8 | Complicated by severe uncontrolled concurrent infection or other serious uncontrolled concomitant diseases, moderate or severe kidney injury. |
| 9 | Use of steroids or other systemic immunosuppressive therapy 14 days prior to enrolment. |
| 10 | Patients receiving study medications within 4 weeks prior to enrolment (participation in other clinical trials). |
| 11 | Active autoimmune diseases (including but not limited to uveitis, enteritis, hepatitis, pituitary inflammation, nephritis, vasculitis, hyperthyroidism, hypothyroidism and asthma requiring bronchodilator treatment, etc). Participants with hypothyroidism requiring hormone replacement therapy and skin conditions that do not require systemic therapy (eg, vitiligo, psoriasis or hair loss) were eligible. |
| 12 | History of primary immunodeficiency. |
| 13 | Use of immunosuppressive drugs within 4 weeks prior to the first dose of study treatment, excluding nasal, inhaled or other routes of topical glucocorticoids or physiological doses of systemic corticosteroids (ie, no more than 10 mg/day prednisone or equivalent dose of other glucocorticoids), or use of hormones to prevent contrast allergy. |
| 14 | Receive live attenuated vaccine within 4 weeks prior to the first dose of study treatment or planned for the duration of the study. |
| 15 | Patients with known interstitial pulmonary pneumonia and active pulmonary tuberculosis. |
| 16 | Known history of allogeneic organ transplantation and allogeneic haematopoietic stem cell transplantation. |
| 17 | HIV-positive, active hepatitis B or C (hepatitis B: HBsAg positive and HBV-DNA ≥104 copies/mL; hepatitis C: positive for HCV antibodies and HCV-RNA, requiring concurrent antiviral therapy). |
| 18 | Other factors that may affect participant safety or study adherence as judged by the investigator. Such as serious illness (including mental illness), serious laboratory abnormalities or other family or social factors that require treatment. |
Collection of peritoneal lavage fluid sample
At the beginning of the procedure, the abdominal cavity is rinsed sequentially using >250 mL of warm saline. Direct flushing of the primary lesion should be avoided, and care should be taken to protect the serous surface. The irrigation sequence may include bilateral diaphragmatic regions, supracolic and subhepatic spaces, the omentum, bilateral paracolic sulci and the Douglas fossa. More than 100 mL of lavage fluid is collected from the subdiaphragmatic, subhepatic and the Douglas fossa and sent for detection.
Definition of peritoneal metastasis
In at least one of the CT, MRI or positron emission tomography (PET)-CT (including [18F]-FDG and [68Ga]-FAPI-PET/CT) imaging tests, PM is identified, and the results of multiple imaging tests are consistent. For patients who can afford it or for whom other imaging tests are inconclusive, [68Ga]-FAPI-PET/CT is preferred.
PM is diagnosed when a cytological examination of the ascites obtained from a peritoneal puncture confirms the presence of cancer cells.
For patients who can tolerate general anaesthesia, laparoscopic exploration is performed to confirm the presence of peritoneal lesions, and pathological examination of the biopsy confirms PM.
For patients who cannot tolerate invasive procedures and have negative ascitic fluid examination and/or inconclusive imaging tests, clinicians can diagnose PM based on the patient’s signs, symptoms and laboratory findings.
The earliest date of PM detection by the above examinations is considered the ‘date of PM’.
Outcome measures
Primary outcome
To observe and evaluate the incidence of PM 12 months after surgery in patients with pT4NxM0/pT1-3N+M0 GC.
Secondary outcomes
To observe and evaluate the incidence of PM 24 months after surgery in patients with pT4NxM0/pT1-3N+M0 GC.
To observe and evaluate overall survival (OS) in patients with pT4NxM0/pT1-3N+M0 GC.
To observe and evaluate the disease progression rate 24 months after surgery in patients with pT4NxM0/pT1-3N+M0 GC.
Sample size calculation
This study aims to investigate the predictive effects of ctDNA and CTC in peritoneal lavage fluid on metachronous PM after radical gastrectomy for pT4NxM0/pT1-3N+M0 GC. According to existing literature (PMID: 34641926), the mutation capsule method predicted PM with 99.0% sensitivity and 91.0% specifity. The prevalence of PM after radical resection of pT4NxM0/pT1-3N+M0 GC was 30.0%. The sensitivity and specificity were estimated to be 90.0% and 80.0%, respectively. Using a confidence level of 1−α=0.95 and a power of 0.9, further statistical analysis can be conducted to evaluate the performance of diagnostic tests or to calculate sample size for future studies. The minimum required sample size is 173 patients, including 52 cases, calculated using PASS V.15.0 software. Considering the annual dropout rate of 10%, 200 people are planned to be included according to the actual situation of this study such as funds, human resources and material resources.
Detection of circulating tumour cell
Most of the leucocytes that may exist in the peritoneal lavage fluid are removed by immunomagnetic bead negative screening. The enriched rare cells are fixed on the surface of the slide after a series of operations such as spinning and fixation. Tumour-related markers on the surface of these rare cells are fluorescently labelled and identified using the classical method of identifying circulating tumour cells. The epithelial markers detected are EpCAM, CK-pan and Claudin18.2, while mesenchymal cell marker include Vimentin and Snail. The epithelial-mesenchymal common marker is folate receptor. The series of markers will be used to identify the residual amount of tumour cells and tumour cell types in the lavage fluid.
Detection of circulating tumour DNA
DNA extraction, nucleic acid concentration determination, library construction, library hybridisation capture and on-machine sequencing are performed on the supernatant of the centrifuged peritoneal lavage solution using tumour-informed ctDNA detection. A double multidimensional noise reduction algorithm is used to correct the single variant signal. The overall significance evaluation is conducted at the sample level based on the significance score of the multivariant signal, ensuring the consistency of specificity criteria for MRD judgement when tracking different numbers of variations.
Statistical analysis
The Clopper-Pearson method is used to estimate the baseline characteristics and PM rate of patients with and without PM, and the 95% CI is calculated and compared between groups. The Kaplan-Meier method is used to estimate OS and progression-free survival, and 95% CIs are calculated and compared between groups. Time to progression (TTP) is described using the mean, SD, median, maximum and minimum values. Solid tumour markers such as Ki67, HER2 levels, microsatellite stability indicators and other biomarker levels in tumour tissue will also be analysed using descriptive statistics. Based on the cut-off values of CTC and ctDNA, the receiver operating characteristic curve of PM is plotted, and the maximum area under the curve is selected.
Data collection and monitoring
Data collection will include patient demographics and disease subclassification based on the Montreal Classification, such as clinical phenotype, disease location and surgical history. The collected patient demographic data will include the identity card number, sex, date of birth, age at diagnosis, disease duration, nationality, height and weight. Laboratory tests (including blood, urine, faecal occult blood test, liver/kidney function, blood glucose, serum electrolytes, serum proteins, clotting tests, thyroid function, tumour markers, etc) should be completed within 14 days prior to the operation. Imaging studies (including chest, abdomen and pelvis CT scans; head CT or MRI; PET-CT and bone scans if clinically necessary) will also be required. Participants and specific follow-up staff will screen for infectious diseases such as HIV, hepatitis B virus, and hepatitis C virus.
Follow-up will be conducted by specific staff for all the participants enrolled in this study. Follow-up visits will be conducted every 3–6 months for 2 years and will be performed annually thereafter. In principle, all the examinations were suggested to be carried out in Renji Hospital. If the patients are re-examined in other hospitals, grade III, first-class hospitals are recommended, and the follow-up staff will track and record the results. The tests include: (1) adverse reactions; (2) physical examination: superficial lymph nodes, abdomen, metastatic signs, etc; (3) peripheral blood routine examination: haemoglobin, red blood cells, white blood cells, lymphocytes, neutrophils, percentage of neutrophils, platelets; (4) blood biochemistry: albumin, prealbumin, total bilirubin, aspartate aminotransferase, alanine aminotransferase, creatinine, urea nitrogen, fasting blood glucose; (5) serum tumour markers: Carcinoembryonic Antigen (CEA), Carbohydrate Antigen 19-9 (CA19-9), Carbohydrate Antigen 72-4 (CA72-4); (6) abdominal and pelvic CT/MRI, or PET-CT imaging tests.
Based on these results, the researchers assessed and recorded the postoperative survival status of all patients to determine tumour recurrence or metastasis. If patients refuse to be followed up according to our protocol, they will be recorded as missing cases and analysed along with those patients who met the study criteria at the end of the study.
The participants, their family members or local physicians are interviewed by telephone at least every 3 months. Follow-up staff will collect information on survival (date of death and cause of death) and post-treatment until either the end of the study, the subject’s death or if the subject were lost to follow-up, or the study was terminated by the researchers.
Ethics and dissemination
The study has been approved by the Ethics Committee of Renji Hospital, Shanghai Jiaotong University School of Medicine (LY2023-142-B) and registered in the Chinese Clinical Trial Registry (ChiCTR2300074910). The study will be conducted according to the principles of the Declaration of Helsinki and in accordance with Good Clinical Practice standards. Informed consent will be obtained by the coordinators or researchers associated with this protocol. The informed consent document details the course of the study and the associated risks. Written informed consent must be obtained before proceeding with the study. Throughout the course of the clinical study, participants may withdraw their consent at any time. All personal information about the subjects will be kept strictly confidential.
Results will be published in open-access peer-reviewed medical literature and submitted for presentation at national and international meetings.
Discussion
GC is the fourth most common cancer worldwide,14 and PM is a significant cause of death in patients with GC. It is also a major challenge in clinical diagnosis and treatment. Predicting the occurrence of PM in patients with high-risk factors, advancing the diagnosis and treatment threshold forward to the time before PM occurs and improving patient survival benifits are unsolved problems in clinical practice. The limited efficacy of existing imaging and cytological detection methods in early diagnosis of PM renders this issue increasingly challenging.
The detection of CTC and ctDNA for GC in blood has been widely studied, including in early cancer screening, MRD detection and the guidance and detection of advanced disease.15,17 However, ctDNA can also be obtained from non-blood sources, including pleural fluid, ascites, urine and cerebrospinal fluid.18 A large amount of cell-free DNA (cfDNA) with a length of about 140–170 bp from leucocytes in the blood19 can serve as background noise for the detection of ctDNA, whereas the relative lack of clonal haematopoiesis in non-blood samples results in relatively higher concentrations and variant allele frequencies of ctDNA.20 21 Thus, non-blood samples can supplement blood ctDNA test results. As a type of non-blood sample, peritoneal lavage fluid has the advantages of less leucocyte-derived cfDNA interference, higher ctDNA concentration and more importantly, direct contact with the primary lesion or potential PM, making it unique among other body fluid samples in gastrointestinal tumours. In a prospective cohort of 104 patients with GC, Zhao et al22 used a personalised detection method based on mutation analysis technology to detect gene mutations in peritoneal lavage fluid precipitation. The average age of the 104 patients was 61 years, the male-to-female ratio was 3.16 and 59% (61/104) had pT4 GC. After 41 months of follow-up, the technique detected all cases of PM with a sensitivity of 100%, a specificity of 85% and a positive predictive value of 71%. MRD in peritoneal lavage fluid was significantly associated with an increased risk of PM (HR 145.13; 95% CI 20.20 to 18 435.79; p<0.001). In addition, MRD-positive patients were associated with reduced recurrence-free survival and OS.
Based on previous studies, this study will include patients with pT4NxM0/pT1-3N+M0 GC and combine CTC and ctDNA in intraoperative peritoneal lavage fluid, which is not currently covered by clinical study. The main objective of this study was to explore the predictive effect of peritoneal lavage fluid CTC and ctDNA on metachronous PM after GC surgery. We anticipate that this study can create an effective tool for targeting PM in patients with GC. Additionally, considering the current treatment modalities, such as hyperthermic intraperitoneal chemotherapy (HIPEC), intraperitoneal chemotherapy (IP) and neoadjuvant intraperitoneal and systemic chemotherapy, which can improve the prognosis of patients with PM,23 our centre may conduct further research based on this study. This future research will focus on peritoneal chemotherapy guided by peritoneal lavage fluid CTC and ctDNA, as well as patient follow-up studies. We believe that the maturation of this tool can contribute to the prophylactic use of HIPEC/IP for patients with high-risk GC with PM, the monitoring of therapeutic efficacy and outcomes in patients with PM, and serve as a more sensitive diagnostic method for PM during postoperative follow-up of patients with GC.
However, there are some methodological limitations to this study. Enrolling a large number of patients is expected to be time-consuming and resource-intensive, which may extend the study period significantly and require substantial financial support. Additionally, there are limited studies on the detection of CTC and ctDNA in peritoneal lavage fluid, which means that the techniques used in this study may have unknown detection errors. The monocentric design of this study further limits the generalisability of the findings to other populations or clinical settings. Furthermore, slight variations in the peritoneal lavage techniques used by different surgeons could become confounding factors in data analysis. This issue can be minimised through standardised training.
The final review paper will be submitted to a peer-reviewed journal for publication and presented at relevant conferences.
Clinical study progress
The recruitment of patients for this study commenced in October 2023, and is currently ongoing with continuous enrollment and collection of follow-up data. Commencement of first subject enrollment: October 2023. Anticipated enrollment of last subject: December 2024. Projected study completion: October 2025.
The funding agency had no influence on the design of the study, nor the collection, analysis and interpretation of data, the writing of the manuscript or the presentation the study results.
Footnotes
Funding: The study was sponsored by The National Natural Science Foundation of China (grant numbers: 81972206 and 82173215).
Prepublication history for this paper is available online. To view these files, please visit the journal online (https://doi.org/10.1136/bmjopen-2023-083659).
Patient consent for publication: Not applicable.
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Contributor Information
Long Bai, Email: bailong001001@163.com.
Yujing Guan, Email: gyji0113@163.com.
Yeqian Zhang, Email: zhangyeqian@renji.com.
Jiayi Gu, Email: gujiayi@renji.com.
Bo Ni, Email: nibo9465@163.com.
Hao-yu Zhang, Email: zhy18018969572@163.com.
Muerzhate Aimaiti, Email: 13279885465@163.com.
Shuchang Wang, Email: sdxjwshch@163.com.
Ben Yue, Email: yueben@163.com.
Xiang Xia, Email: xiangmoumou@163.com.
Zizhen Zhang, Email: zhangzizhen@renji.com.
References
- 1.Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209–49. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Lei Z, Wang J, Li Z, et al. Hyperthermic intraperitoneal chemotherapy for gastric cancer with peritoneal metastasis: A multicenter propensity score-matched cohort study. Chin J Cancer Res. 2020;32:794–803. doi: 10.21147/j.issn.1000-9604.2020.06.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yonemura Y, Bandou E, Kawamura T, et al. Quantitative prognostic indicators of peritoneal dissemination of gastric cancer. Eur J Surg Oncol. 2006;32:602–6. doi: 10.1016/j.ejso.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 4.Kanda M, Kodera Y. Molecular mechanisms of peritoneal dissemination in gastric cancer. World J Gastroenterol. 2016;22:6829–40. doi: 10.3748/wjg.v22.i30.6829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Japanese Gastric Cancer Association Japanese gastric cancer treatment guidelines 2014 (ver. 4) Gastric Cancer. 2014;20:1–19. doi: 10.1007/s10120-016-0622-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kołomańska MM, Głuszek S. Free cancer cells in gastric cancer - methods of detection, clinical and prognostic importance (meta-analysis) Contemp Oncol (Pozn) 2020;24:67–74. doi: 10.5114/wo.2020.94724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thierry AR, El Messaoudi S, Gahan PB, et al. Origins, structures, and functions of circulating DNA in oncology. Cancer Metastasis Rev. 2016;35:347–76. doi: 10.1007/s10555-016-9629-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haber DA, Velculescu VE. Blood-based analyses of cancer: circulating tumor cells and circulating tumor DNA. Cancer Discov. 2014;4:650–61. doi: 10.1158/2159-8290.CD-13-1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gomez-Arteaga A, Guzman ML. Minimal Residual Disease in Acute Myeloid Leukemia. Adv Exp Med Biol. 2018;1100:111–25. doi: 10.1007/978-3-319-97746-1_7. [DOI] [PubMed] [Google Scholar]
- 10.Pantel K, Alix-Panabières C. Liquid biopsy and minimal residual disease - latest advances and implications for cure. Nat Rev Clin Oncol. 2019;16:409–24. doi: 10.1038/s41571-019-0187-3. [DOI] [PubMed] [Google Scholar]
- 11.Lin D, Shen L, Luo M, et al. Circulating tumor cells: biology and clinical significance. Signal Transduct Target Ther. 2021;6:404. doi: 10.1038/s41392-021-00817-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lawrence R, Watters M, Davies CR, et al. Circulating tumour cells for early detection of clinically relevant cancer. Nat Rev Clin Oncol. 2023;20:487–500. doi: 10.1038/s41571-023-00781-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zeng CDD, Jin CC, Gao C, et al. Preoperative Folate Receptor-Positive Circulating Tumor Cells Are Associated With Occult Peritoneal Metastasis and Early Recurrence in Gastric Cancer Patients: A Prospective Cohort Study. Front Oncol. 2022;12:769203. doi: 10.3389/fonc.2022.769203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dong Z, Zhang Y, Geng H, et al. Development and validation of two nomograms for predicting overall survival and cancer-specific survival in gastric cancer patients with liver metastases: A retrospective cohort study from SEER database. Transl Oncol. 2022;24:101480. doi: 10.1016/j.tranon.2022.101480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mencel J, Slater S, Cartwright E, et al. The Role of ctDNA in Gastric Cancer. Cancers (Basel) 2022;14:5105. doi: 10.3390/cancers14205105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ning D, Cui K, Liu M, et al. Comparison of CellSearch and Circulating Tumor Cells (CTC)-Biopsy Systems in Detecting Peripheral Blood Circulating Tumor Cells in Patients with Gastric Cancer. Med Sci Monit. 2021;27:e926565. doi: 10.12659/MSM.926565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang Z, Wu H, Chong W, et al. Liquid biopsy in gastric cancer: predictive and prognostic biomarkers. Cell Death Dis. 2022;13:903. doi: 10.1038/s41419-022-05350-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tivey A, Church M, Rothwell D, et al. Circulating tumour DNA - looking beyond the blood. Nat Rev Clin Oncol. 2022;19:600–12. doi: 10.1038/s41571-022-00660-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lo YMD, Chan KCA, Sun H, et al. Maternal plasma DNA sequencing reveals the genome-wide genetic and mutational profile of the fetus. Sci Transl Med. 2010;2:61ra91. doi: 10.1126/scitranslmed.3001720. [DOI] [PubMed] [Google Scholar]
- 20.Tong L, Ding N, Tong X, et al. Tumor-derived DNA from pleural effusion supernatant as a promising alternative to tumor tissue in genomic profiling of advanced lung cancer. Theranostics. 2019;9:5532–41. doi: 10.7150/thno.34070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tu H-Y, Li Y-S, Bai X-Y, et al. Genetic Profiling of Cell-Free DNA From Pleural Effusion in Advanced Lung Cancer as a Surrogate for Tumor Tissue and Revealed Additional Clinical Actionable Targets. Clin Lung Cancer. 2022;23:135–42. doi: 10.1016/j.cllc.2021.09.002. [DOI] [PubMed] [Google Scholar]
- 22.Zhao D, Yue P, Wang T, et al. Personalized analysis of minimal residual cancer cells in peritoneal lavage fluid predicts peritoneal dissemination of gastric cancer. J Hematol Oncol. 2021;14:164. doi: 10.1186/s13045-021-01175-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rau B, Lang H, Koenigsrainer A, et al. Effect of Hyperthermic Intraperitoneal Chemotherapy on Cytoreductive Surgery in Gastric Cancer With Synchronous Peritoneal Metastases: The Phase III GASTRIPEC-I Trial. J Clin Oncol. 2024;42:146–56. doi: 10.1200/JCO.22.02867. [DOI] [PMC free article] [PubMed] [Google Scholar]