Abstract
Objective
To investigate how individual social determinants of health (SDOH) and cumulative social disadvantage (CSD) affect survival and receipt of liver transplant (LT) in patients with hepatocellular carcinoma (HCC).
Methods
We enrolled 139 adult patients from two Indianapolis hospital systems between June 2019 and April 2022. Structured questionnaires collected SDOH and social risk factor data. We compared SDOH and CSD by race, gender and disease aetiology, assigning one point per adverse SDOH. Multivariable competing risk survival analysis assessed associations between SDOH, CSD, survival and LT receipt.
Results
Black patients experienced higher CSD than white patients in the cohort (5.4±2.5 vs 3.2±2.1, p<0.001). Black patients were significantly more likely to have household incomes <US$15 000 per year (52.6% vs 18.3%, p=0.003), to be insured by Medicaid (57.9% vs 33.0%, p=0.04), and to live in high Social Deprivation Index areas (68.4% vs 17.5%, p<0.001) than white patients. Patients with hepatitis C virus and alcohol-related liver disease had more adverse SDOH than those with metabolic dysfunction-associated steatotic liver disease, while there were no significant differences by gender. On multivariable analysis, a higher health literacy score was a significant predictor of survival (HR 2.54, 95% CI 1.19 to 5.43 CI, p=0.02) and higher CSD was associated with a lower probability of receipt of LT (HR 0.80, 95% CI 0.68 to 0.95, p=0.01).
Conclusions
There are significant racial and aetiology-related differences in SDOH burden. Low health literacy and high CSD are linked to worse outcomes in HCC patients. Health literacy screening and targeted interventions for those with high CSD could improve LT access and survival rates.
Keywords: LIVER TRANSPLANTATION, LIVER, HEPATOCELLULAR CARCINOMA, ECONOMIC EVALUATION
WHAT IS ALREADY KNOWN ON THIS TOPIC
Racial and socioeconomic disparities in hepatocellular carcinoma (HCC) survival and curative therapy receipt are linked to social determinants of health (SDOH) and social risk factors. However, prospective cohort data on social adversity are missing and it is unclear if cumulative adversity or individual SDOH impact outcomes more significantly.
WHAT THIS STUDY ADDS
This study finds that SDOH, social needs and social risk factors differ by race and disease aetiology and that health literacy and cumulative social disadvantage are linked to key outcomes in HCC patients.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
Health literacy screening should be considered for HCC patients, and interventions should target those with cumulative disadvantages to improve the receipt of liver transplant.
Introduction
Hepatocellular carcinoma (HCC) is one of the fastest-rising causes of cancer-related death in the USA.1 However, morbidity and mortality from HCC are borne disproportionately by racial and ethnic minorities, those with low socioeconomic status and those from deprived neighbourhoods.2 3 Reasons for survival disparities are complex but are hypothesised in part to be related to the inability of populations experiencing health disparities to progress through the complex HCC care continuum to access curative therapies.4 Accessing health services is a complicated endeavour driven by social, health system and patient-level factors.5 However, because of the unequal distribution of the social determinants of health (SDOH), the greatest challenges are often experienced by marginalised groups. SDOH are the social and economic conditions that influence individual and group differences in health.6 Structural inequities contribute to deficits in social networks, high burdens of social risk factors and unhealthy behaviours.6
SDOH have been associated with a decreased utilisation of relevant treatments and poor outcomes in other malignancies.7 8 However, the current understanding of SDOH in HCC remains limited, primarily focusing on data extracted from cancer registries and medical records, often overlooking individual-level SDOH that likely impact access to curative therapies and survival. This results in a lack of detailed insight into how SDOH intersect with factors such as race, gender and disease aetiology, hindering tailored interventions. Moreover, while many currently validated SDOH and social need screeners address multiple domains, underscoring the need for a comprehensive understanding of a patient’s experience, the association between SDOH and liver health outcomes has largely been based on single assessments of SDOH. It is not clear that this realistically reflects the experience of most patients. Furthermore, cumulative social disadvantage (CSD) has been shown to be associated with all-cause mortality and accessing health services; we hypothesise that similar associations will be seen in survival and accessing liver transplant (LT) given the complexities of this care cascade.9 10
There are currently no prospective data on SDOH, social needs and social risk factors in patients with HCC. A better understanding of CSD across an exhaustive range of SDOH domains may afford a provider or health system nuanced information to better identify at-risk individuals to target for interventions. Hence, our study aims are to (1) investigate the extent to which individual SDOH versus CSD differ by race, gender and disease aetiology in patients with HCC and (2) to examine how both CSD and individual SDOH are associated with survival and the receipt of LT in patients with HCC.
Methods
Study population and setting
We conducted a prospective cohort study of patients with HCC. Patients were enrolled from two hospital systems in the Indianapolis Metropolitan area (Indiana University Health/University and Methodists Hospitals and Eskenazi Health System (safety-net hospital)) from June 2019 to April 2022. Patients were recruited by trained study personnel from hepatology, interventional radiology and oncology outpatient clinics. A total of 164 patients were approached of whom 139 provided written consent to participate in the study (online supplemental figure 2). Strengthening the Reporting of Observational Studies in Epidemiology guidelines were followed for reporting outcomes of this study. All authors had access to study data and reviewed and approved the final manuscript.
Patients were included in the study if they were diagnosed with HCC confirmed by imaging or biopsy and were at least 18 years of age. Patients were excluded if they were post-transplant for any solid organ.
Exposures
SDOH data collection and conceptual framework
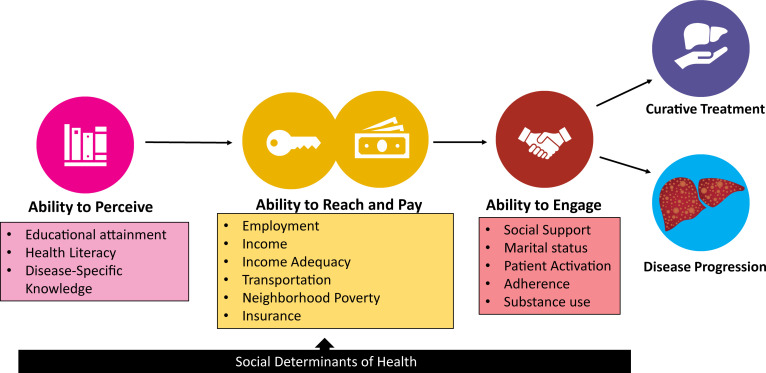
Data were collected through structured in-person questionnaires administered by trained personnel. Responses were recorded electronically. For Spanish-speaking patients, the survey and consent form were translated and administered by a Spanish-speaking coordinator. The assessment of SDOH, social risk factors and health behaviours was guided by Levesque’s framework and the Alcaraz et al model.11 12 We developed a conceptual framework detailing the relationship between SDOH, social risk factors, health behaviours and HCC outcomes, exploring three access-to-care domains: (1) perception, (2) payment and reach and (3) engagement (figure 1).
Figure 1. Modified Levesque’s conceptual framework for social determinants of health, social needs and health behaviours associated with accessing liver transplant and survival in complex care cascade requiring both cancer and transplant care as in hepatocellular carcinoma.
Ability to perceive: definition and measures
Within the ability to perceive health information domain, we measured health literacy, disease-specific knowledge and level of educational attainment. Measuring health literacy is complex and there is no one agreed on instrument. We used the Brief Health Literacy Screen (BHLS), a three-item questionnaire with response options based on a 5-point Likert scale.13 14 To measure disease-specific knowledge, patients described the cause of their liver disease in their own words. This was compared with the known cause from their medical records, and the per cent concordance between patient and specialist diagnoses was calculated. Finally, participants reported their highest level of education.
Ability to pay and reach: definition and measures
In the ability to pay and reach domain, we collected data on insurance type, income, income adequacy, employment, transportation type, health-related transportation adequacy and neighbourhood deprivation. Insurance types were classified as private, Medicaid (including Medicare with Medicaid supplement) and Medicare. Income was categorised into six brackets, from less than US$15 000 to more than US$100 000 annually. Income adequacy was assessed with a single-item question about financial situations, rated on a scale from having enough money for special things to having difficulty paying bills.15 Employment was categorised as employed full time, part time, unemployed, retired or on disability. Two questions were asked regarding health-related transportation: (1) Do you often have trouble getting to doctors’ appointments? and (2) How do you get to your doctors’ appointments?
To measure census-tract level disadvantage, we calculated the Social Deprivation Index (SDI) using variables from the American Community Survey. These measures quantify social inequities across small geographical areas to evaluate their association with health outcomes.16 17 Additionally, we collected data on census-tract income below the federal poverty level for increased specificity.
Ability to engage: definition and measures
The ability to engage and access care was measured by assessing social support, marital status, patient adherence and substance use history. Instrumental social support was measured using the National Institutes of Health Patient-Reported Outcomes Measurement Information System (PROMIS) form 6a-version 2.018. Marital status was self-reported as divorced, married, separated, widowed and never married. Patient adherence rate was measured using three questions from the Medical Outcomes Study Specific Adherence Scale of Patient Adherence questionnaire.19 Substance use was assessed by asking about alcohol use in the past 90 days and any history of illegal non-marijuana substance use.
Demographic and clinical characteristics
Race, ethnicity and gender were self-reported. Race was categorised as black, white, Asian Pacific Islander, Native-American or American Indian, and others. Ethnicity was classified as Hispanic or non-Hispanic, and gender as male, female or transgender. Model for end-stage liver disease sodium (MELD-Na) score, Child Pugh Category and Charlson Comorbidity Index were recorded from the EMR at enrolment. Disease aetiologies were classified as alcohol-related liver disease (ALD), hepatitis C virus (HCV), metabolic dysfunction-associated steatotic liver disease (MASLD) and other. Tumour characteristics, including number, size, Barcelona Clinic Liver Cancer (BCLC) 2018 staging, Milan status and alpha-fetoprotein (AFP) levels (<500 and ≥500), were documented at diagnosis.
Cumulative social adversity
Health centres are starting to use various SDOH/social need screening tools in clinical practice. Our goal was not to create a universally applicable score, as different SDOH may dominate in each setting and health systems use diverse tools. Instead, we tested the hypothesis that CSD, measured by assigning one point per adverse social determinant, varies by race, gender and disease aetiology and is linked to survival and curative therapy receipt in HCC. An additive approach using varied individual-level SDOH data is widely recognised in healthcare settings.9 20 21
To define CSD, 1 point was given for each adverse SDOH within each domain as follows: Ability to perceive: education attainment—less than high school; health Literacy—Q1 BHLS Score; disease-specific knowledge—inaccurate answer. Ability to reach and pay: employment—on SSI, disability, unemployed; income—less than 15K; income adequacy—first two financial situation categories (enough or little spare money and difficulty paying bills or no spare money); transportation—transportation trouble=yes; neighbourhood poverty—Q4 SDI; insurance—Medicaid. Ability to engage: social support—Q1 PROMIS social support score; marital status—not married; adherence—adherence score Q1; substance use—alcohol use in last 90 days. The total maximum cumulative burden score was 13.
Outcomes
This analysis examined the associations between the CSD and its components and (1) race, (2) gender, (3) disease aetiology, (4) survival status and (5) receipt of LT among patients with BCLC 0-B HCC.
Statistical methods
Individual characteristics were summarised using means, SD, medians, quartile ranges for continuous variables, and frequencies and percentages for categorical variables. Categorical variables were compared using χ2 tests or Fisher’s exact test when necessary, and Mantel-Haenszel χ2 tests for ordinal responses. Continuous variables were compared using analysis of variance for normally distributed data and Kruskal-Wallis tests for non-normally distributed data. All tests were two sided with a 0.05 significance level.
We hypothesised that the impact of CSD would be more pronounced in accessing LT than resection, given the rigours of completing LT evaluation. Therefore, in our primary survival analysis, participants were categorised into three outcomes: transplanted, alive without transplant (including patients alive who underwent resection) and deceased without transplant. In a sensitivity analysis, participants were categorised into three outcomes: transplanted or resected, alive without transplant or resection and deceased without transplant or resection.
The outcomes of LT and survival were analysed using the Fine and Gray method, treating death as a competing risk for LT and vice versa.22 Only patients with BCLC stage 0 and with A and B disease were included in the LT models while all patients were included in the survival models. For both outcomes, subjects who were alive without transplant were censored at last known date alive as of December 2022. For the outcome of LT, deceased non-transplanted subjects were censored at time of death. For the outcome of survival, non-deceased transplanted subjects were censored at time of transplant.
Covariates considered for inclusion in the Fine-Gray Cox proportional hazards model included demographic (age, race, ethnicity, gender), clinical characteristics (MELD-NA, Child Pugh Score (CPS), AFP category, Milan status) and all SDOH. Variables were selected for inclusion in the models based on a significance threshold of p<0.05 from univariable analysis. To ensure model stability, as there were only 14 deaths in the reduced cohort, only one covariate was added to the multivariable model, with preference given to the covariate exhibiting the strongest association with the outcomes.23 Models were constructed separately for each outcome: one incorporating the CSD and another incorporating individual SDOH significant at p<0.05. Univariable Fine-Gray Cox proportional hazards models were run for the outcome of LT at each individual number of burdens to compare odds of transplant at various cut points.
Data summaries were produced using R Statistical Software (V.4.1.0). All analytical assumptions were verified, with non-parametric tests being used where necessary. All analyses were performed using SAS V.9.4 (SAS Institute).
Results
There were 139 participants enrolled in the study; 14.4% were black race and 7.9% were Hispanic ethnicity. Most participants were men (73.4%), with an average age of 64.0±8.2 years. The most common cause of liver disease was HCV (46.5%), followed by MASLD (33.9%) and ALD (15.7%). The mean MELD-Na score at enrolment was 10.5 and 61.9% of participants were Child Pugh Category A. Over 75% of the cohort was BCLC stage A or B and 62.6% of the cohort was within Milan criteria at diagnosis (table 1).
Table 1. Demographic and clinical characteristics by survival or liver transplant status.
| Alive without transplant (N=69) | Transplanted (N=41) | Deceased without transplant (N=29) | Total (N=139) | P value | |
| Gender | 0.86* | ||||
| Women | 17 (24.6%) | 12 (29.3%) | 7 (24.1%) | 36 (25.9%) | |
| Men | 51 (73.9%) | 29 (70.7%) | 22 (75.9%) | 102 (73.4%) | |
| Transgender | 1 (1.4%) | 0 (0.0%) | 0 (0.0%) | 1 (0.7%) | |
| Race | 0.01† | ||||
| Asian or Pacific Islander | 1 (1.4%) | 0 (0.0%) | 0 (0.0%) | 1 (0.7%) | |
| Black or African American | 16 (23.2%) | 3 (7.3%) | 1 (3.4%) | 20 (14.4%) | |
| Native American or American Indian | 0 (0.0%) | 1 (2.4%) | 0 (0.0%) | 1 (0.7%) | |
| Other | 0 (0.0%) | 2 (4.9%) | 0 (0.0%) | 2 (1.4%) | |
| White | 52 (75.4%) | 35 (85.4%) | 28 (96.6%) | 115 (82.7%) | |
| Hispanic ethnicity | 0.46 | ||||
| No | 64 (92.8%) | 36 (87.8%) | 28 (96.6%) | 128 (92.1%) | |
| Yes | 5 (7.2%) | 5 (12.2%) | 1 (3.4%) | 11 (7.9%) | |
| Age at diagnosis | 0.96 | ||||
| Mean (SD) | 63.8 (9.1) | 64.1 (6.8) | 64.3 (8.0) | 64.0 (8.2) | |
| Median (Q1, Q3) | 64.8 (59.1, 69.2) | 64.2 (60.2, 69.3) | 64.3 (58.2, 68.2) | 64.4 (59.0, 69.2) | |
| Range | 33.6–80.8 | 51.0–81.1 | 50.2–79.3 | 33.6–81.1 | |
| BMI at the time of HCC diagnosis | 0.09 | ||||
| Mean (SD) | 28.9 (6.2) | 30.1 (5.5) | 32.2 (7.6) | 29.9 (6.4) | |
| Median (Q1, Q3) | 29.0 (25.1, 31.9) | 28.6 (25.9, 34.5) | 32.2 (25.6, 36.5) | 29.3 (25.3, 33.8) | |
| Range | 16.8–48.5 | 22.2–43.2 | 21.2–49.2 | 16.8–49.2 | |
| N-Miss | 4 | 4 | 3 | 11 | |
| CCI | 0.22 | ||||
| Mean (SD) | 7.9 (2.3) | 7.3 (1.4) | 8.4 (2.1) | 7.8 (2.1) | |
| Median (Q1, Q3) | 7.0 (7.0, 9.0) | 7.0 (7.0, 8.0) | 8.0 (7.0, 10.0) | 7.0 (7.0, 8.0) | |
| Range | 5.0–18.0 | 3.0–11.0 | 6.0–15.0 | 3.0–18.0 | |
| MELD-Na Score at diagnosis | 0.23 | ||||
| Mean (SD) | 10.2 (4.2) | 10.5 (4.2) | 11.5 (5.6) | 10.5 (4.5) | |
| Median (Q1, Q3) | 9.3 (7.7, 10.6) | 9.0 (7.7, 10.4) | 10.0 (8.4, 11.2) | 9.4 (7.8, 10.9) | |
| Range | 6.4–29.0 | 6.4–23.0 | 6.9–33.0 | 6.4–33.0 | |
| N-Miss | 3 | 0 | 1 | 4 | |
| Child’s Pugh Score | 0.12 | ||||
| Mean (SD) | 6.0 (1.4) | 6.6 (1.9) | 6.6 (1.5) | 6.3 (1.6) | |
| Median (Q1, Q3) | 5.0 (5.0, 7.0) | 6.0 (5.0, 8.0) | 6.0 (5.0, 8.0) | 6.0 (5.0, 7.0) | |
| Range | 5.0–12.0 | 5.0–11.0 | 5.0–10.0 | 5.0–12.0 | |
| N-Miss | 1 | 1 | 1 | 3 | |
| Child Pugh Category | 0.32‡ | ||||
| Child A | 46 (66.7%) | 24 (58.5%) | 16 (55.2%) | 86 (61.9%) | |
| Child B | 20 (29.0%) | 12 (29.3%) | 10 (34.5%) | 42 (30.2%) | |
| Child C | 1 (1.4%) | 4 (9.8%) | 1 (3.4%) | 6 (4.3%) | |
| Unknown/not enough information | 2 (2.9%) | 1 (2.4%) | 2 (6.9%) | 5 (3.6%) | |
| Liver disease aetiology | 0.16§ | ||||
| Alagille | 1 (1.6%) | 0 (0.0%) | 0 (0.0%) | 1 (0.8%) | |
| ALD | 10 (16.4%) | 6 (15.4%) | 4 (14.8%) | 20 (15.7%) | |
| Hepatitis C | 34 (55.7%) | 12 (30.8%) | 13 (48.1%) | 59 (46.5%) | |
| MASLD | 15 (24.6%) | 18 (46.2%) | 10 (37.0%) | 43 (33.9%) | |
| Unknown | 1 (1.6%) | 3 (7.7%) | 0 (0.0%) | 4 (3.1%) | |
| N-Miss | 8 | 2 | 2 | 12 | |
| AFP diagnosis (ng/mL) | 0.04* | ||||
| Mean (SD) | 3379.2 (21382.4) | 320.7 (1590.2) | 3069.6 (7130.2) | 2405.4 (15381.5) | |
| Median (Q1, Q3) | 10.6 (4.0, 93.3) | 5.4 (3.3, 19.7) | 13.7 (5.2, 333.6) | 9.4 (4.0, 65.2) | |
| Range | 0.9–176 144.8 | 1.6–10 120.0 | 1.6–29 513.0 | 0.9–176 144.8 | |
| N-Miss | 1 | 0 | 0 | 1 | |
| AFP category | 0.13 | ||||
| <500 | 55 (80.9%) | 38 (92.7%) | 22 (75.9%) | 115 (83.3%) | |
| ≥500 | 13 (19.1%) | 3 (7.3%) | 7 (24.1%) | 23 (16.7%) | |
| N-Miss | 1 | 0 | 0 | 1 | |
| Milan criteria | 0.004 | ||||
| No | 30 (43.5%) | 7 (17.1%) | 15 (51.7%) | 52 (37.4%) | |
| Yes | 39 (56.5%) | 34 (82.9%) | 14 (48.3%) | 87 (62.6%) | |
| Number of tumours | 0.12 | ||||
| 1 | 43 (63.2%) | 29 (70.7%) | 20 (74.1%) | 92 (67.6%) | |
| 2 | 11 (16.2%) | 10 (24.4%) | 5 (18.5%) | 26 (19.1%) | |
| 3 | 13 (19.1%) | 2 (4.9%) | 1 (3.7%) | 16 (11.8%) | |
| More than 3 | 1 (1.5%) | 0 (0.0%) | 1 (3.7%) | 2 (1.5%) | |
| N-Miss | 1 | 0 | 2 | 3 | |
| Size of largest tumour | 0.10 | ||||
| Mean (SD) | 4.6 (3.5) | 4.4 (4.9) | 4.6 (3.2) | 4.5 (3.9) | |
| Median (Q1, Q3) | 3.6 (2.3, 5.8) | 2.5 (2.0, 4.5) | 3.7 (2.8, 5.5) | 3.2 (2.2, 5.3) | |
| Range | 1.2–18.0 | 1.0–25.0 | 1.1–16.9 | 1.0–25.0 | |
| N-Miss | 1 | 0 | 2 | 3 | |
| BCLC stage | 0.35¶ | ||||
| 0 | 0 (0.0%) | 1 (2.4%) | 0 (0.0%) | 1 (0.7%) | |
| A | 24 (34.8%) | 14 (34.1%) | 7 (25.0%) | 45 (32.6%) | |
| B | 29 (42.0%) | 19 (46.3%) | 12 (42.9%) | 60 (43.5%) | |
| C | 14 (20.3%) | 3 (7.3%) | 7 (25.0%) | 24 (17.4%) | |
| D | 2 (2.9%) | 4 (9.8%) | 2 (7.1%) | 8 (5.8%) | |
| N-Miss | 0 | 0 | 1 | 1 |
*p-value<0.05
Male versus female.
Black versus white.
A versus B versus C.
Hepatitis C versus MASLD versus ALD.
0/A/B vs. C/D
AFPalpha-fetoproteinALDalcohol-related liver diseaseBCLCBarcelona Clinic Liver CancerBMIbody mass index CCICharlson Comorbidity Index HCChepatocellular carcinomMASLDmetabolic dysfunction-associated steatotic liver diseaseMELD-Namodel for end-stage liver disease sodium
Cumulative social adversity by race
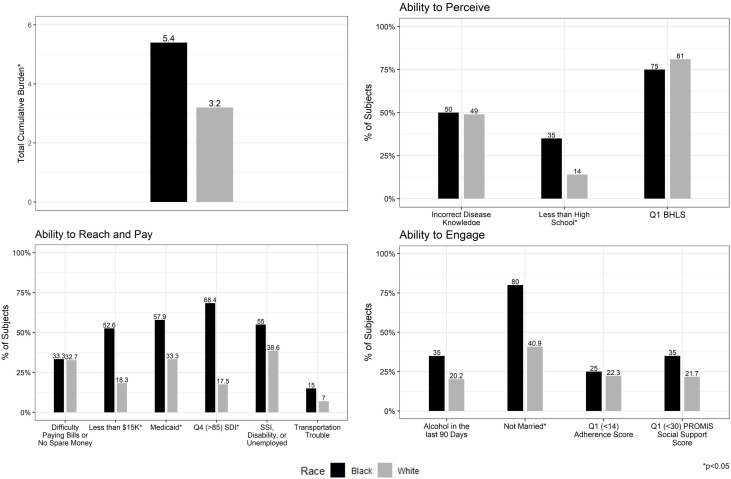
A total of 135 patients were included in this analysis; 20 black and 115 white patients. One patient identified as Asian Pacific Islander, one as Native American and two self-identified as ‘other’. Although their data were not subjected to statistical comparison, the descriptive data are provided in online supplemental table 1A. Black patients had a significantly higher mean CSD than white patients in the cohort (5.4±2.5 vs 3.2±2.1, p<0.001) (figure 2, online supplemental table 1B).
Figure 2. Cumulative social disadvantage and components in black and white patients with hepatocellular carcinoma. PROMIS, Patient-Reported Outcomes Measurement Information System; SDI, Social Deprivation Index; SSI, Social Security Disability Insurance; BHLS, Brief Health Literacy Score.
When exploring individual CSD components, black patients were significantly more likely to have less than a high school education (35.0% vs 13.9%, p=0.046); however, disease-specific knowledge (black patients 50.0% vs white patients 49.0%, p=0.94) and health literacy (Q1 health literacy scores in black patients 25.0% vs white patients 19.1%, p=0.55) were similar between the two groups. Black patients were significantly more likely to have household incomes less than US$15 000 per year (52.6% vs 18.3%, p=0.003), to be insured by Medicaid (57.9% vs 33.0%, p=0.04), and to live in neighbourhoods with a high SDI (68.4% vs 17.5%, p<0.001) than white patients. Black and white patients reported similar instrumental social support (Q1 instrumental social support in black patients 35.0% vs white patients 19.6%, p=0.15), however, black patients were significantly more likely to be unmarried (80.0% vs 40.9%, p=0.001). Self-reported adherence was similar between black and white patients (Q1 adherence scores in black patients 25.0% vs white patients 22.3%, p=0.78) (figure 2).
Cumulative social adversity by gender
There was a total of 138 patients included in this analysis, including 102 men and 36 women. There was one patient who identified themselves as transgender. The CSD was similar between men and women in the cohort (3.4±2.4 vs 3.6±2.0, p=0.75) (online supplemental table 2).
There were no significant differences in the individual CSD components by gender. Social determinants with greater than a 10% difference between groups although lacking statistical significance were observed. Women were observed with a higher proportion within the income bracket under US$15 000 (31.2% vs 20.2%, p=0.20), as well as in the category reporting difficulty paying bills compared with men (41.2% vs 28.7%, p=0.18) (online supplemental table 2).
Cumulative burden score by aetiology of liver disease
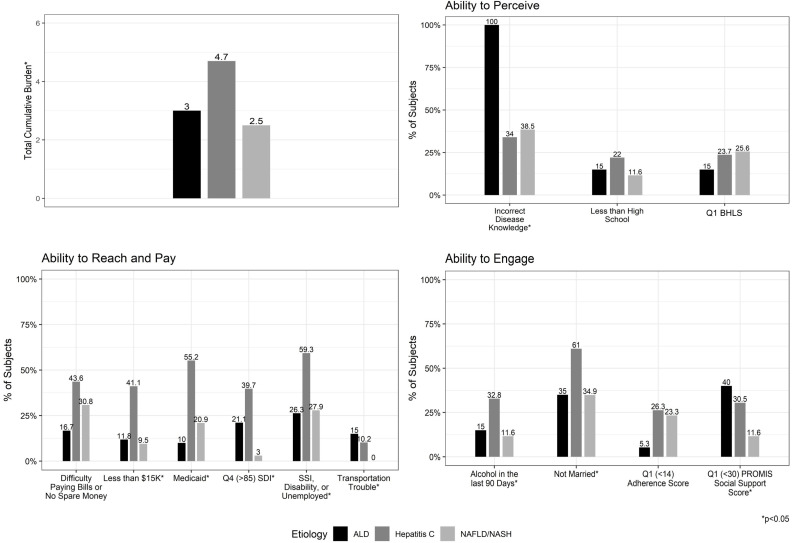
The 122 patients in this analysis had been diagnosed with ALD (n=20), HCV (n=59) and MASLD (n=43). There were 12 patients with unknown or other aetiologies of liver disease; the descriptive data are provided in online supplemental table 3A. The CSD was statistically significantly different by disease aetiology (ALD 2.9±1.7; HCV 4.6±2.4; MASLD 2.4±1.7, p<0.001), with the highest CSD observed in those with HCV (figure 3, online supplemental table 3B).
Figure 3. Cumulative social disadvantage and components by liver disease aetiology. ALD, alcohol-related liver disease; MASLD, metabolic dysfunction-associated steatotic liver disease; PROMIS, Patient-Reported Outcomes Measurement Information System; SDI, Social Deprivation Index, SSI, Social Security Disability Insurance, BHLS Brief Health Literacy Score.
Regarding CSD components, 15 of the 20 ALD patients incorrectly self-reported their aetiology of disease and the other 5 declined to answer yielding a 0% correct disease identification for patients with ALD (ALD 0%, HCV 66.0%, MASLD 61.5%, p<0.001). Patients with underlying ALD were more likely to report issues with transportation (ALD 15.0%, HCV 10.2%, MASLD 0%, p=0.03) than other disease aetiologies. Patients with ALD and HCV were more likely to be in the lowest quartile for instrumental social support (ALD 40.0%, HCV 28.1%, MASLD 9.5%, p=0.02) and also more likely to be unmarried (ALD 35.0%, HCV 61.0%, MASLD 34.9%, p=0.02). 41% of patients with HCV had household incomes less than US$15 000, compared with 11.8% and 9.5% in the ALD and MASLD cohort, respectively (p=0.001). Patients with HCV were also more likely to report unemployment (ALD 26.3%, HCV 59.3%, MASLD 27.9%, p=0.002), to live in neighbourhoods in the highest quartile for SDI (ALD 21.1%, HCV 39.7% and MASLD 7.0%, p=0.001) and to be insured by Medicaid (ALD 10.0%, HCV 55.2%, MASLD 20.9%, p≤0.001) than those with ALD or MASLD. Alcohol use in the past 90 days was highest in the group with HCV, which also included patients with combined HCV-ALD (ALD 15.0%, HCV 32.8%, MASLD 11.6%, p=0.03) (figure 3, online supplemental table 3B).
Association of individual SDOH and CSD with survival and LT
All 139 patients were included in the analysis; there were 41 patients who underwent LT, 29 who died before LT and 69 who were alive without LT at the end of the study period (online supplemental table 4). Participants were followed for a median of 386 days. The group that underwent LT had the lowest CSD (alive 4.1±2.6, LT 2.7±1.9, deceased 3.1±1.7, p=0.01). The group that was alive without LT was most likely to be insured by Medicaid (alive 45.6%, LT 24.4%, deceased 27.6%, p=0.049), unmarried (alive 56.5%, LT 31.7%, deceased 41.4%, p=0.04) and to have used alcohol in the last 90 days (alive 30.4%, LT 9.8%, deceased 17.9%, p=0.03) (online supplemental table 4).
In univariable survival analysis with LT as a competing risk, CSD showed no association with survival (HR 0.93, 95% CI 0.81 to 1.07, p=0.30) (online supplemental table 5). However, BHLS, Charlson score and MELD-Na were found to be significant. On multivariable analysis, quartile 1 BHLS was associated with a significantly higher hazard for death than BHLS quartile 4 (HR 2.54, 95% CI 1.19 to 5.43, p=0.02) (table 2).
Table 2. Multivariable analysis: association of CSD or SDOH with LT or survival.
| Outcome: LT | |||
| Predictor | HR estimate | 95% CI | P value |
| CSD | 0.80 | 0.68 to 0.95 | 0.01 |
| Charlson | 0.79 | 0.64 to 0.97 | 0.02 |
| Outcome: survival | |||
| Predictor | HR estimate | 95% CI | P value |
| BHLS (Q1 vs Q2/3/4) | 2.54 | 1.19 to 5.43 | 0.02 |
| MELD Na | 1.07 | 1.00 to 1.16 | 0.07 |
| Charlson total | 1.19 | 1.05 to 1.34 | 0.01 |
| Milan (yes vs no) | 0.31 | 0.15 to 0.65 | 0.002 |
BHLSBrief Health Literacy ScoreCSDcumulative social disadvantageLTliver transplantMELD-Namodel for end-stage liver disease sodium SDOHsocial determinants of health
In univariable competing risk analysis, CSD and Charlson scores were significantly associated with LT among patients with BCLC0-B HCC; alcohol use in the past 90 days approached significance (online supplemental table 6). In multivariable analysis, a higher CSD remained associated with a lower likelihood for LT (HR 0.80, 95% CI 0.68 to 0.95, p=0.01). On sensitivity analysis exploring both resection and LT together as an outcome among those with BCLC 0-B HCC, CSD was not significantly associated with therapy receipt (HR 0.89, 95% CI 0.74 to 1.06, p=0.189) (table 2).
Discussion
Analysis of administrative datasets and retrospective cohorts has demonstrated an association between the SDOH and both the failure to receive LT and mortality in HCC.6 24 25 However, the capacity to thoroughly examine the influence of SDOH within these datasets is constrained. Moreover, patients, specifically in marginalised groups, often experience multiple adverse SDOH simultaneously, yet our current analysis is limited to exploring each need individually. Our study marks an important effort in prospectively exploring SDOH individually and collectively. Notably, we have identified health literacy to be associated with survival in this cohort. In addition, the data suggest that the cumulative burden of adversity as captured by CSD is a barrier to receipt of LT. Furthermore, we present novel findings on the prevalence of individual-level SDOH in patients with HCC and how those vary by race, gender and disease aetiology. These insights provide important data for tailoring interventions aimed at mitigating disparities in HCC outcomes.
Black patients exhibited significantly higher levels of adverse SDOH in the ability to reach and pay domain while encountering fewer challenges in the ability to perceive or engage domain compared with their white counterparts. Consequently, black patients carried a substantially higher CSD, with an average of two additional burdens compared with white patients in our cohort. Hypotheses regarding racial healthcare disparities have included differences in literacy,26 social support27 and even adherence.28 While these factors may also be playing a role, our preliminary findings suggest that the primary barriers in patients with HCC lie in the realm of accessibility and affordability, with objective measures of literacy, social support and adherence showing comparable levels between the two racial groups. Black race has been associated with a failure to receive curative therapies for HCC.29 30 These data suggest that interventions that help to mitigate the cumulative burden of adversity may improve these outcomes.
Patients with underlying HCV and ALD had additional adverse SDOH than those with HCC and MASLD. Furthermore, it is noteworthy that patients with HCC and ALD demonstrated a 100% failure rate in accurately defining their disease aetiology. This observation may stem from the stigma associated with ALD rather than a genuine lack of understanding among patients. Addressing this stigma is crucial in advancing efforts to improve patient likelihood of transplant candidacy and outcomes.26 Furthermore, patients with underlying ALD and HCV may need tailored support interventions to facilitate access to LT and achieve optimal outcomes.
Knowledge barriers were not limited to patients with ALD, 20% of the cohort had BHLS in the first quartile consistent with inadequate health literacy. Furthermore, health literacy was the only SDOH associated with survival in this cohort. While previous studies have linked low health literacy with reduced odds of being listed for LT, its specific impact on the survival of patients with HCC has not been previously explored.31 32 Health literacy is a complicated construct,33 however, most definitions agree that health knowledge is an important component. Improving health-related knowledge has been associated with behaviour changes and improved cancer-related outcomes.34 The BHLS offers distinct advantages over alternative health literacy screening tools. As a patient-reported outcome measure comprising only three items, the BHLS facilitates quick administration and scoring in outpatient clinical settings.
Despite recommendations for alcohol cessation, 21.7% of patients in our cohort continued to use alcohol, lowering their odds of eligibility for LT. We have previously shown that alcohol use is a barrier to LT for black patients with HCC.29 The need for early screening, diagnosis and referral for treatment of alcohol use disorder within primary care and gastroenterology and hepatology clinics has long been established.35 However, it is not clear how often this is being implemented in practice.
Finally, CSD was associated with the receipt of LT but not with the receipt of all curative therapies. This aligns with the hypothesis that the complexities of the LT care cascade—encompassing numerous system, provider and patient factors—may be exacerbated by SDOH, whereas the path to resection, which involves imaging and surgical consultation, may be more straightforward. Furthermore, the lack of association with any single adverse SDOH, but with CSD, suggests that efforts to understand the full spectrum of social disadvantage experienced by patients are warranted. CSD has also been associated with all-cause mortality and the receipt of treatment for colon cancer.10 36
Next steps
In our cohort, we found that low health literacy was linked to mortality, while CSD was associated with LT receipt. While awaiting larger validation studies, immediate actions are recommended. The National Academy of Science and Engineering outlines a five-step process for integrating, screening and addressing SDOH within the healthcare system: (1) awareness, (2) assistance, (3) adjustment, (4) alignment and (5) advocacy.37 Step 1, awareness, involves screening for SDOH and health literacy using tools like the BHLS, which is efficient, evidenced based and can be completed well before starting a transplant evaluation.35 There are multiple validated social needs screening tools to consider based on languages needed, setting and time available.38 Step 2, assistance, and step 3, adjustment, involve aiding patients who screen positive and adapting practices. The Agency for Healthcare Research and Quality provides a Health Literacy Universal Precaution Toolkit with methods like teach-back and medication reviews to support patients with low health literacy.39 Given limited resources, focusing first on patients with the highest CSD might be considered. Steps 4 and 5, alignment and advocacy, require health systems to invest in community-level social care resources and advocate for policy changes with payers and stakeholders to support these patients.
This is the first study to prospectively collect individual-level SDOH, social risk and health behaviour data on patients with chronic liver disease or HCC. While our study is the largest to date in this area, a larger sample size may have identified additional SDOH that were significantly associated with the outcomes. Therefore, those SDOH variables that showed modest association, yet did not meet significance, likely warrant further study. While our study sample included patients from both an academic centre and a safety net hospital, both are located in Indiana. It is possible that patients in other parts of the country face different challenges with SDOH like transportation, for example. Furthermore, Indiana is a Medicaid expansion state, therefore, LT may be more widely available than in states where Medicaid was not expanded. Finally, SDOH burdens may not all be equivalent, and therefore, not equally additive as reflected in our CSD.
In this cohort, we observed a significant association between health literacy and survival and the CSD and receipt of LT in patients with HCC. There are existing tools to screen for these barriers and actions we can begin to take to mitigate these barriers for our patients. However, there remains a need for interventions specifically designed to enhance HCC-related knowledge and to address the impact of the cumulative burden of adversity in patients with HCC.
supplementary material
Footnotes
Funding: LN is funded by NMIHD (1K23MD018090-01).
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient consent for publication: Not applicable.
Ethics approval: This study involves human participants and was approved by the Indiana University institutional review board and the Scientific Review Committee of the Simon Comprehensive Cancer Center (IRB #180809754). Participants gave informed consent to participate in the study before taking part.
Data availability free text: All data relevant to the study are included in the article or uploaded as online supplemental information.
Contributor Information
Lauren D. Nephew, Email: lnephew@iu.edu.
Susan M Rawl, Email: srawl@iu.edu.
Allie Carter, Email: alternet@iu.edu.
Nicole Garcia, Email: garcini@iu.edu.
Patrick O Monahan, Email: pmonahan@iu.edu.
John Holden, Email: jhholden@iu.edu.
Marwan Ghabril, Email: mghabril@iu.edu.
Eleazar Montalvan-Sanchez, Email: elmont@iu.edu.
Kavish Patidar, Email: kavish.patidar@bcm.edu.
Archita P Desai, Email: desaiar@iu.edu.
Eric Orman, Email: esorman@iu.edu.
Naga Chalasani, Email: nchalasa@iu.edu.
Data availability statement
All data relevant to the study are included in the article or uploaded as online supplemental information. No additional data are available.
References
- 1.Llovet JM, Kelley RK, Villanueva A, et al. Hepatocellular carcinoma. Nat Rev Dis Primers. 2021;7:6. doi: 10.1038/s41572-020-00240-3. [DOI] [PubMed] [Google Scholar]
- 2.Flores YN, Datta GD, Yang L, et al. Disparities in Hepatocellular Carcinoma Incidence, Stage, and Survival: A Large Population-Based Study. Cancer Epidemiol Biomarkers Prev. 2021;30:1193–9. doi: 10.1158/1055-9965.EPI-20-1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nephew LD, Gupta D, Carter A, et al. Social determinants of health impact mortality from HCC and cholangiocarcinoma: a population-based cohort study. Hepatol Commun. 2023;7:e0058. doi: 10.1097/HC9.0000000000000058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Seif El Dahan K, Reczek A, Daher D, et al. Multidisciplinary care for patients with HCC: a systematic review and meta-analysis. Hepatol Commun. 2023;7:e0143. doi: 10.1097/HC9.0000000000000143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cu A, Meister S, Lefebvre B, et al. Assessing healthcare access using the Levesque’s conceptual framework- a scoping review. Int J Equity Health. 2021;20 doi: 10.1186/s12939-021-01416-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nephew LD, Aitcheson G, Iyengar M. The Impact of Racial Disparities on Liver Disease Access and Outcomes. Curr Treat Options Gastro. 2022;20:279–94. doi: 10.1007/s11938-022-00390-1. [DOI] [Google Scholar]
- 7.Coughlin SS. Social determinants of breast cancer risk, stage, and survival. Breast Cancer Res Treat. 2019;177:537–48. doi: 10.1007/s10549-019-05340-7. [DOI] [PubMed] [Google Scholar]
- 8.Busch EL, Martin C, DeWalt DA, et al. Functional health literacy, chemotherapy decisions, and outcomes among a colorectal cancer cohort. Cancer Control. 2015;22:95–101. doi: 10.1177/107327481502200112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Caleyachetty R, Tehranifar P, Genkinger JM, et al. Cumulative social risk exposure and risk of cancer mortality in adulthood. BMC Cancer. 2015;15:945. doi: 10.1186/s12885-015-1997-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davis RE, Trickey AW, Abrahamse P, et al. Association of Cumulative Social Risk and Social Support With Receipt of Chemotherapy Among Patients With Advanced Colorectal Cancer. JAMA Netw Open. 2021;4:e2113533. doi: 10.1001/jamanetworkopen.2021.13533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Levesque J-F, Harris MF, Russell G. Patient-centred access to health care: conceptualising access at the interface of health systems and populations. Int J Equity Health. 2013;12:18. doi: 10.1186/1475-9276-12-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alcaraz KI, Wiedt TL, Daniels EC, et al. Understanding and addressing social determinants to advance cancer health equity in the United States: A blueprint for practice, research, and policy. CA Cancer J Clin. 2020;70:31–46. doi: 10.3322/caac.21586. [DOI] [PubMed] [Google Scholar]
- 13.Haun J, Luther S, Dodd V, et al. Measurement variation across health literacy assessments: implications for assessment selection in research and practice. J Health Commun. 2012;17 Suppl 3:141–59. doi: 10.1080/10810730.2012.712615. [DOI] [PubMed] [Google Scholar]
- 14.Goodman MS, Griffey RT, Carpenter CR, et al. Do Subjective Measures Improve the Ability to Identify Limited Health Literacy in a Clinical Setting? J Am Board Fam Med. 2015;28:584–94. doi: 10.3122/jabfm.2015.05.150037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Okechukwu CA, El Ayadi AM, Tamers SL, et al. Household food insufficiency, financial strain, work-family spillover, and depressive symptoms in the working class: the Work, Family, and Health Network study. Am J Public Health. 2012;102:126–33. doi: 10.2105/AJPH.2011.300323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Butler DC, Petterson S, Phillips RL, et al. Measures of social deprivation that predict health care access and need within a rational area of primary care service delivery. Health Serv Res. 2013;48:539–59. doi: 10.1111/j.1475-6773.2012.01449.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maroko AR, Doan TM, Arno PS, et al. Integrating Social Determinants of Health With Treatment and Prevention: A New Tool to Assess Local Area Deprivation. Prev Chron Dis. 2016;13 doi: 10.5888/pcd13.160221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.National Institutes of Health Patient-reported outcome measurement information system. 2023. https://commonfund.nih.gov/promis/index Available.
- 19.Wu J-R, Chung M, Lennie TA, et al. Testing the psychometric properties of the Medication Adherence Scale in patients with heart failure. Heart & Lung. 2008;37:334–43. doi: 10.1016/j.hrtlng.2007.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hagan K, Javed Z, Cainzos-Achirica M, et al. Cumulative social disadvantage and health-related quality of life: national health interview survey 2013-2017. BMC Public Health. 2023;23 doi: 10.1186/s12889-023-16168-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Latham-Mintus K, Weathers T, Irby-Shasanmi A, et al. EXPLORING CUMULATIVE DISADVANTAGE, TELOMERE LENGTH, AND BREAST CANCER AMONG BLACK AND WHITE WOMEN. Innov Aging. 2017;1:1338. doi: 10.1093/geroni/igx004.4910. [DOI] [Google Scholar]
- 22.Fine JP, Gray RJ. A Proportional Hazards Model for the Subdistribution of a Competing Risk. J Am Stat Assoc. 1999;94:496–509. doi: 10.1080/01621459.1999.10474144. [DOI] [Google Scholar]
- 23.Chowdhury MZI, Turin TC. Variable selection strategies and its importance in clinical prediction modelling. Fam Med Community Health. 2020;8:e000262. doi: 10.1136/fmch-2019-000262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wong RJ, Kim D, Ahmed A, et al. Patients with hepatocellular carcinoma from more rural and lower-income households have more advanced tumor stage at diagnosis and significantly higher mortality. Cancer. 2021;127:45–55. doi: 10.1002/cncr.33211. [DOI] [PubMed] [Google Scholar]
- 25.Sobotka LA, Hinton A, Conteh LF. Insurance status impacts treatment for hepatocellular carcinoma. Ann Hepatol. 2019;18:461–5. doi: 10.1016/j.aohep.2018.10.001. [DOI] [PubMed] [Google Scholar]
- 26.Eneanya ND, Winter M, Cabral H, et al. Health Literacy and Education as Mediators of Racial Disparities in Patient Activation Within an Elderly Patient Cohort. J Health Care Poor Underserved. 2016;27:1427–40. doi: 10.1353/hpu.2016.0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wesselman H, Ford CG, Leyva Y, et al. Social Determinants of Health and Race Disparities in Kidney Transplant. Clin J Am Soc Nephrol. 2021;16:262–74. doi: 10.2215/CJN.04860420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xie Z, St Clair P, Goldman DP, et al. Racial and ethnic disparities in medication adherence among privately insured patients in the United States. PLoS ONE. 2019;14:e0212117. doi: 10.1371/journal.pone.0212117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dakhoul L, Gawrieh S, Jones KR, et al. Racial Disparities in Liver Transplantation for Hepatocellular Carcinoma Are Not Explained by Differences in Comorbidities, Liver Disease Severity, or Tumor Burden. Hepatol Commun. 2019;3:52–62. doi: 10.1002/hep4.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wong RJ, Devaki P, Nguyen L, et al. Ethnic disparities and liver transplantation rates in hepatocellular carcinoma patients in the recent era: results from the Surveillance, Epidemiology, and End Results registry. Liver Transpl. 2014;20:528–35. doi: 10.1002/lt.23820. [DOI] [PubMed] [Google Scholar]
- 31.Bittermann T, Dwinnells K, Chadha S, et al. Low Health Literacy Is Associated With Frailty and Reduced Likelihood of Liver Transplant Listing: A Prospective Cohort Study. Liver Transpl. 2020;26:1409–21. doi: 10.1002/lt.25830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gulati R, Nawaz M, Pyrsopoulos NT. Health literacy and liver disease. Clin Liver Dis (Hoboken) 2018;11:48–51. doi: 10.1002/cld.690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baker DW. The meaning and the measure of health literacy. J Gen Intern Med. 2006;21:878–83. doi: 10.1111/j.1525-1497.2006.00540.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Walters R, Leslie SJ, Polson R, et al. Establishing the efficacy of interventions to improve health literacy and health behaviours: a systematic review. BMC Public Health. 2020;20 doi: 10.1186/s12889-020-08991-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Crabb DW, Im GY, Szabo G, et al. Diagnosis and Treatment of Alcohol-Associated Liver Diseases: 2019 Practice Guidance From the American Association for the Study of Liver Diseases. Hepatology. 2020;71:306–33. doi: 10.1002/hep.30866. [DOI] [PubMed] [Google Scholar]
- 36.Javed Z, Valero-Elizondo J, Khan SU, et al. Cumulative Social Disadvantage and All-Cause Mortality in the United States: Findings from a National Study. Popul Health Manag. 2022;25:789–97. doi: 10.1089/pop.2022.0184. [DOI] [PubMed] [Google Scholar]
- 37.National Academies of Sciences E, and Medicine . Health and Medicine Division,Board on Health Care Services; Committee on Integrating Social Needs Care into the Delivery of Health Care to Improve the Nation’s Health. Integrating Social Care into the Delivery of Health Care: Moving Upstream to Improve the Nation’s Health. Washington (DC): National Academies Press (US); 2019. [PubMed] [Google Scholar]
- 38.Kreuter MW, Thompson T, McQueen A, et al. Addressing Social Needs in Health Care Settings: Evidence, Challenges, and Opportunities for Public Health. Annu Rev Public Health. 2021;42:329–44. doi: 10.1146/annurev-publhealth-090419-102204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Quality AfHRa AHRQ health literacy universal precautions toolkit. 2023. https://www.ahrq.gov/health-literacy/improve/index.html Available.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data relevant to the study are included in the article or uploaded as online supplemental information. No additional data are available.