Abstract
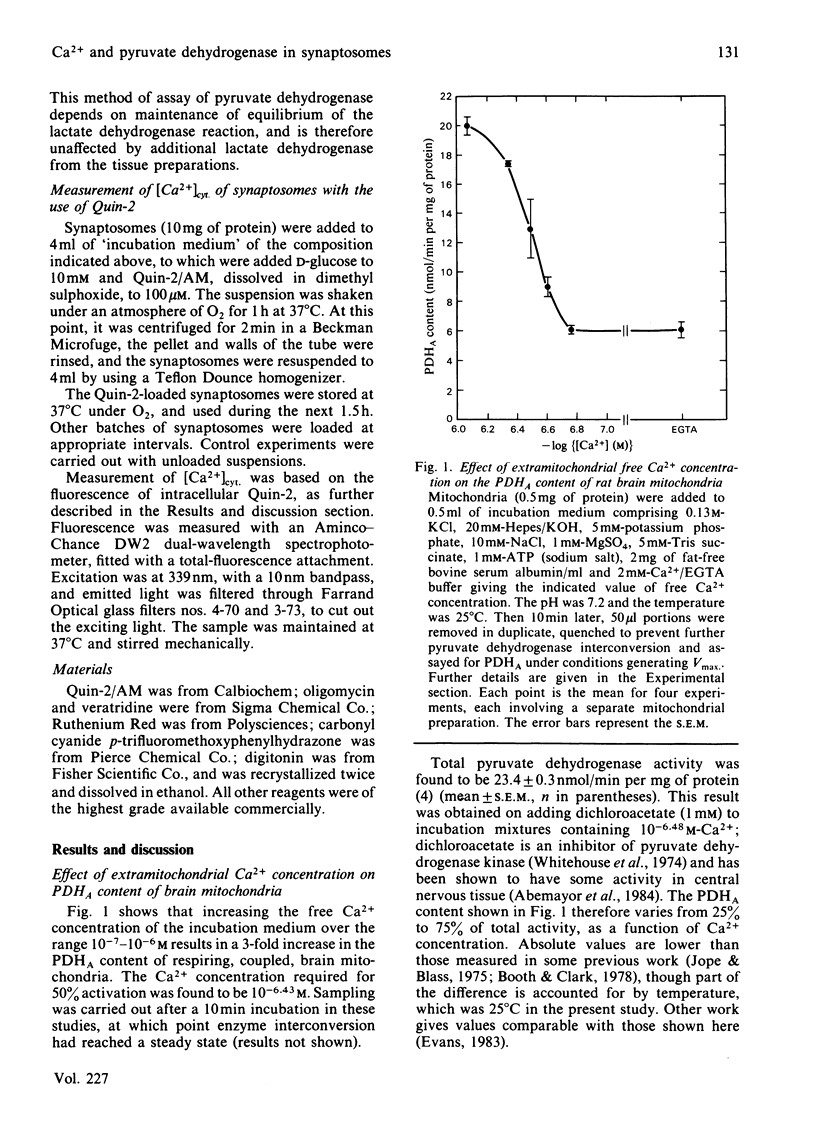
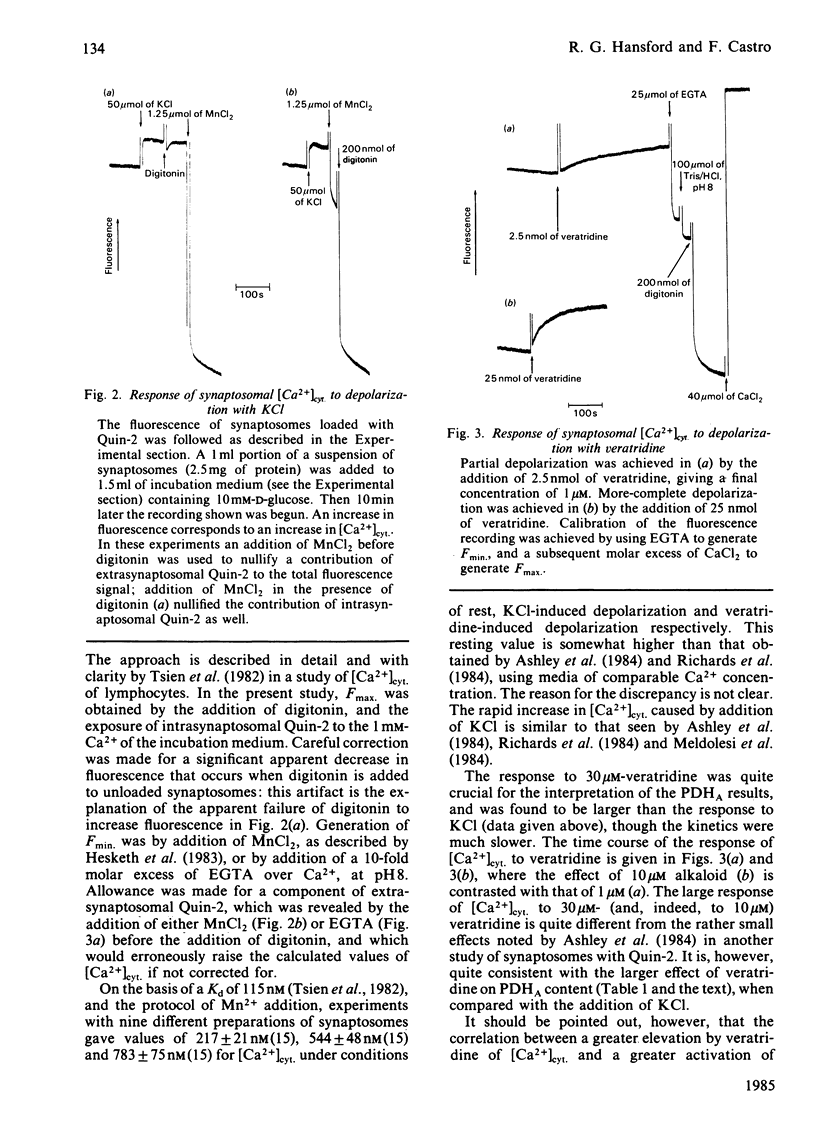
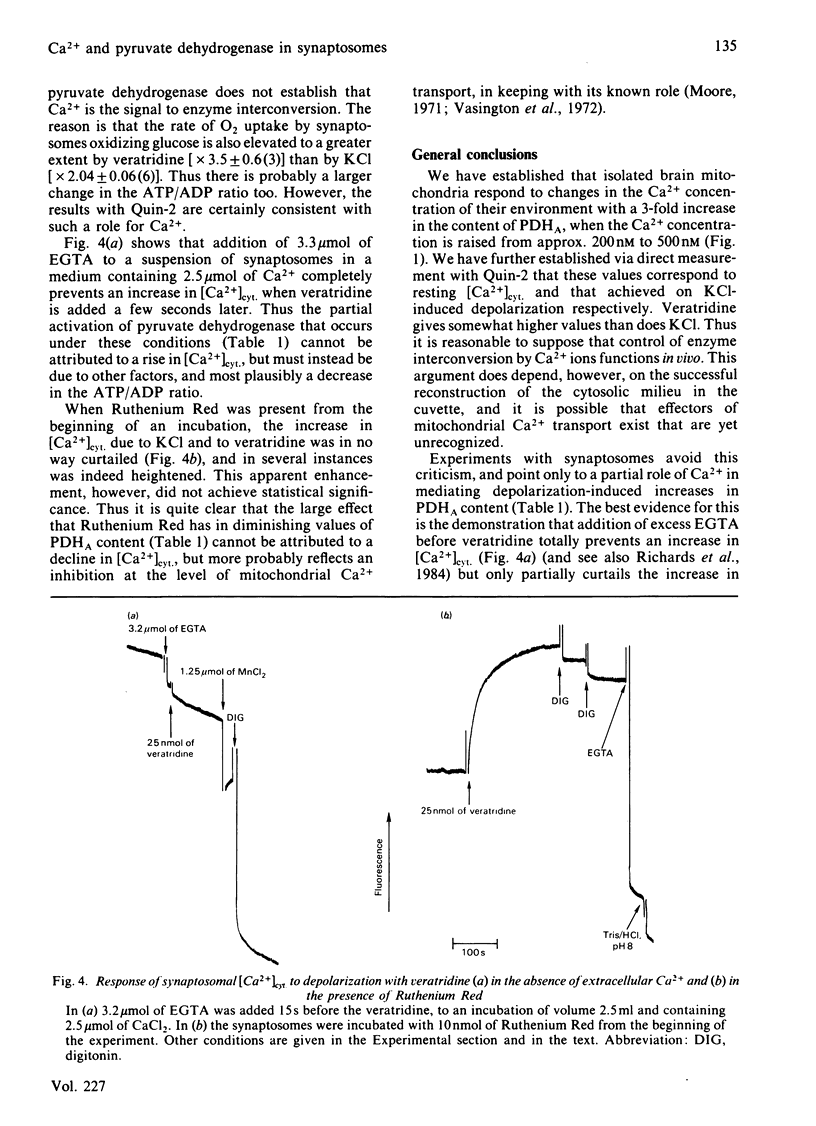
The steady-state content of active (dephospho) pyruvate dehydrogenase (PDHA) of suspensions of coupled rat brain mitochondria oxidizing succinate was found to be markedly increased with increasing free Ca2+ ion concentration of the medium, with a half-maximal effect at 10(-6.43) M Ca2+. Other ions were present in these studies at concentrations appropriate for the cytosol. Depolarization of the plasma membrane of synaptosomes caused an increase in the steady-state content of PDHA, with veratridine giving a larger increase than depolarization by 33 mM-KCl. Values were 68 +/- 1% (n = 13) and 81 +/- 1% (n = 19) of maximal activity, for control incubations and incubations in the presence of 30 microM-veratridine, respectively. Measurements of cytosolic free Ca2+ concentrations ([Ca2+]cyt.) in these suspensions of synaptosomes, with the use of the fluorescent Ca2+-indicator Quin-2, indicated an increase on depolarization, with the change due to 30 microM-veratridine being larger in extent than that due to 33 mM-KCl. Values were 217 +/- 21 nM (n = 15), 544 +/- 48 nM (n = 15) and 783 +/- 75 nM (n = 14) for control, KCl-depolarized and veratridine-depolarized synaptosomes respectively. Experiments in which synaptosomes were treated with Ruthenium Red, an inhibitor of mitochondrial Ca2+ uptake, gave much lower resting contents of PDHA (42 +/- 2% of maximal), but failed to prevent totally an increase on depolarization. Addition of an excess of EGTA to the synaptosomal suspension just before the addition of veratridine resulted in a partial diminution in the response of PDHA content. Parallel studies with Quin-2 indicated no increase in [Ca2+]cyt. on addition of veratridine, under these conditions. Thus an increase in [Ca2+]cyt. forms only a part of the mechanism whereby pyruvate dehydrogenase interconversion responds to depolarization. A decrease in the ATP/ADP ratio may also be important, as inferred from the results of experiments with ouabain, which inhibits the Na+ + K+-dependent ATPase.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abemayor E., Kovachich G. B., Haugaard N. Effects of dichloroacetate on brain pyruvate dehydrogenase. J Neurochem. 1984 Jan;42(1):38–42. doi: 10.1111/j.1471-4159.1984.tb09694.x. [DOI] [PubMed] [Google Scholar]
- Akerman K. E., Heinonen E. Qualitative measurements of cytosolic calcium ion concentration within isolated guinea pig nerve endings using entrapped arsenazo III. Biochim Biophys Acta. 1983 Jul 13;732(1):117–121. doi: 10.1016/0005-2736(83)90193-1. [DOI] [PubMed] [Google Scholar]
- Akerman K. E., Nicholls D. G. Intrasynaptosomal compartmentation of calcium during depolarization-induced calcium uptake across the plasma membrane. Biochim Biophys Acta. 1981 Jul 6;645(1):41–48. doi: 10.1016/0005-2736(81)90509-5. [DOI] [PubMed] [Google Scholar]
- Ashley R. H., Brammer M. J., Marchbanks R. Measurement of intrasynaptosomal free calcium by using the fluorescent indicator quin-2. Biochem J. 1984 Apr 1;219(1):149–158. doi: 10.1042/bj2190149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baudry M., Fuchs J., Kessler M., Arst D., Lynch G. Entorhinal cortex lesions induce a decreased calcium transport in hippocampal mitochondria. Science. 1982 Apr 23;216(4544):411–413. doi: 10.1126/science.7071588. [DOI] [PubMed] [Google Scholar]
- Blaustein M. P. Effects of potassium, veratridine, and scorpion venom on calcium accumulation and transmitter release by nerve terminals in vitro. J Physiol. 1975 Jun;247(3):617–655. doi: 10.1113/jphysiol.1975.sp010950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaustein M. P., Goldring J. M. Membrane potentials in pinched-off presynaptic nerve ternimals monitored with a fluorescent probe: evidence that synaptosomes have potassium diffusion potentials. J Physiol. 1975 Jun;247(3):589–615. doi: 10.1113/jphysiol.1975.sp010949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth R. F., Clark J. B. The control of pyruvate dehydrogenase in isolated brain mitochondria. J Neurochem. 1978 May;30(5):1003–1008. doi: 10.1111/j.1471-4159.1978.tb12392.x. [DOI] [PubMed] [Google Scholar]
- Browning M., Bennett W. F., Kelly P., Lynch G. Evidence that the 40,000 Mr phosphoprotein influenced by high frequency synaptic stimulation is the alpha subunit of pyruvate dehydrogenase. Brain Res. 1981 Aug 10;218(1-2):255–266. doi: 10.1016/0006-8993(81)91305-6. [DOI] [PubMed] [Google Scholar]
- Browning M., Dunwiddie T., Bennett W., Gispen W., Lynch G. Synaptic phosphoproteins: specific changes after repetitive stimulation of the hippocampal slice. Science. 1979 Jan 5;203(4375):60–62. doi: 10.1126/science.214855. [DOI] [PubMed] [Google Scholar]
- Coutinho O. P., Carvalho C. A., Carvalho A. P. Calcium uptake related to K+-depolarization and Na+/Ca2+ exchange in sheep brain synaptosomes. Brain Res. 1984 Jan 9;290(2):261–271. doi: 10.1016/0006-8993(84)90943-0. [DOI] [PubMed] [Google Scholar]
- Crompton M., Moser R., Lüdi H., Carafoli E. The interrelations between the transport of sodium and calcium in mitochondria of various mammalian tissues. Eur J Biochem. 1978 Jan 2;82(1):25–31. doi: 10.1111/j.1432-1033.1978.tb11993.x. [DOI] [PubMed] [Google Scholar]
- Denton R. M., McCormack J. G., Edgell N. J. Role of calcium ions in the regulation of intramitochondrial metabolism. Effects of Na+, Mg2+ and ruthenium red on the Ca2+-stimulated oxidation of oxoglutarate and on pyruvate dehydrogenase activity in intact rat heart mitochondria. Biochem J. 1980 Jul 15;190(1):107–117. doi: 10.1042/bj1900107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denton R. M., Randle P. J., Martin B. R. Stimulation by calcium ions of pyruvate dehydrogenase phosphate phosphatase. Biochem J. 1972 Jun;128(1):161–163. doi: 10.1042/bj1280161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas W. W. Involvement of calcium in exocytosis and the exocytosis--vesiculation sequence. Biochem Soc Symp. 1974;(39):1–28. [PubMed] [Google Scholar]
- Escueta A. V., Appel S. H. Biochemical studies of synapses in vitro. II. Potassium transport. Biochemistry. 1969 Feb;8(2):725–733. doi: 10.1021/bi00830a038. [DOI] [PubMed] [Google Scholar]
- Evans O. B. Effects of dichloroacetate on brain tissue pyruvate dehydrogenase. J Neurochem. 1983 Oct;41(4):1052–1056. doi: 10.1111/j.1471-4159.1983.tb09050.x. [DOI] [PubMed] [Google Scholar]
- Gibson G. E., Jope R., Blass J. P. Decreased synthesis of acetylcholine accompanying impaired oxidation of pyruvic acid in rat brain minces. Biochem J. 1975 Apr;148(1):17–23. doi: 10.1042/bj1480017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansford R. G., Cohen L. Relative importance of pyruvate dehydrogenase interconversion and feed-back inhibition in the effect of fatty acids on pyruvate oxidation by rat heart mitochondria. Arch Biochem Biophys. 1978 Nov;191(1):65–81. doi: 10.1016/0003-9861(78)90068-1. [DOI] [PubMed] [Google Scholar]
- Hansford R. G. Effect of micromolar concentrations of free Ca2+ ions on pyruvate dehydrogenase interconversion in intact rat heart mitochondria. Biochem J. 1981 Mar 15;194(3):721–732. doi: 10.1042/bj1940721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansford R. G. Studies on the effects of coenzyme A-SH: acetyl coenzyme A, nicotinamide adenine dinucleotide: reduced nicotinamide adenine dinucleotide, and adenosine diphosphate: adenosine triphosphate ratios on the interconversion of active and inactive pyruvate dehydrogenase in isolated rat heart mitochondria. J Biol Chem. 1976 Sep 25;251(18):5483–5489. [PubMed] [Google Scholar]
- Hesketh T. R., Smith G. A., Moore J. P., Taylor M. V., Metcalfe J. C. Free cytoplasmic calcium concentration and the mitogenic stimulation of lymphocytes. J Biol Chem. 1983 Apr 25;258(8):4876–4882. [PubMed] [Google Scholar]
- Jope R., Blass J. P. A comparison of the regulation of pyruvate dehydrogenase in mitochondria from rat brain and liver. Biochem J. 1975 Sep;150(3):397–403. doi: 10.1042/bj1500397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B., Miledi R. The release of acetylcholine from nerve endings by graded electric pulses. Proc R Soc Lond B Biol Sci. 1967 Jan 31;167(1006):23–38. doi: 10.1098/rspb.1967.0011. [DOI] [PubMed] [Google Scholar]
- Knight D. E., Kesteven N. T. Evoked transient intracellular free Ca2+ changes and secretion in isolated bovine adrenal medullary cells. Proc R Soc Lond B Biol Sci. 1983 May 23;218(1211):177–199. doi: 10.1098/rspb.1983.0033. [DOI] [PubMed] [Google Scholar]
- Kovachich G. V., Haugaard N. Pyruvate dehydrogenase activation in rat brain cortical slices by elevated concentrations of external potassium ions. J Neurochem. 1977 May;28(5):923–927. doi: 10.1111/j.1471-4159.1977.tb10651.x. [DOI] [PubMed] [Google Scholar]
- Luft J. H. Ruthenium red and violet. I. Chemistry, purification, methods of use for electron microscopy and mechanism of action. Anat Rec. 1971 Nov;171(3):347–368. doi: 10.1002/ar.1091710302. [DOI] [PubMed] [Google Scholar]
- McCormack J. G., England P. J. Ruthenium Red inhibits the activation of pyruvate dehydrogenase caused by positive inotropic agents in the perfused rat heart. Biochem J. 1983 Aug 15;214(2):581–585. doi: 10.1042/bj2140581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meldolesi J., Huttner W. B., Tsien R. Y., Pozzan T. Free cytoplasmic Ca2+ and neurotransmitter release: studies on PC12 cells and synaptosomes exposed to alpha-latrotoxin. Proc Natl Acad Sci U S A. 1984 Jan;81(2):620–624. doi: 10.1073/pnas.81.2.620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore C. L. Specific inhibition of mitochondrial Ca++ transport by ruthenium red. Biochem Biophys Res Commun. 1971 Jan 22;42(2):298–305. doi: 10.1016/0006-291x(71)90102-1. [DOI] [PubMed] [Google Scholar]
- Nicholls D. G. Calcium transport and porton electrochemical potential gradient in mitochondria from guinea-pig cerebral cortex and rat heart. Biochem J. 1978 Mar 15;170(3):511–522. doi: 10.1042/bj1700511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholls D., Akerman K. Mitochondrial calcium transport. Biochim Biophys Acta. 1982 Sep 1;683(1):57–88. doi: 10.1016/0304-4173(82)90013-1. [DOI] [PubMed] [Google Scholar]
- Ota M., Narahashi T., Keeler R. F. Effects of veratrum alkaloids on membrane potential and conductance of squid and crayfish giant axons. J Pharmacol Exp Ther. 1973 Jan;184(1):143–154. [PubMed] [Google Scholar]
- Pettit F. H., Roche T. E., Reed L. J. Function of calcium ions in pyruvate dehydrogenase phosphatase activity. Biochem Biophys Res Commun. 1972 Oct 17;49(2):563–571. doi: 10.1016/0006-291x(72)90448-2. [DOI] [PubMed] [Google Scholar]
- Reed L. J. Regulation of mammalian pyruvate dehydrogenase complex by a phosphorylation-dephosphorylation cycle. Curr Top Cell Regul. 1981;18:95–106. doi: 10.1016/b978-0-12-152818-8.50012-8. [DOI] [PubMed] [Google Scholar]
- Richards C. D., Metcalfe J. C., Smith G. A., Hesketh T. R. Changes in free-calcium levels and pH in synaptosomes during transmitter release. Biochim Biophys Acta. 1984 Apr 16;803(4):215–220. doi: 10.1016/0167-4889(84)90110-1. [DOI] [PubMed] [Google Scholar]
- Rubin R. P. The role of calcium in the release of neurotransmitter substances and hormones. Pharmacol Rev. 1970 Sep;22(3):389–428. [PubMed] [Google Scholar]
- Schaffer W. T., Olson M. S. The regulation of pyruvate oxidation during membrane depolarization of rat brain synaptosomes. Biochem J. 1980 Nov 15;192(2):741–751. doi: 10.1042/bj1920741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott I. D., Nicholls D. G. Energy transduction in intact synaptosomes. Influence of plasma-membrane depolarization on the respiration and membrane potential of internal mitochondria determined in situ. Biochem J. 1980 Jan 15;186(1):21–33. doi: 10.1042/bj1860021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsien R. Y. A non-disruptive technique for loading calcium buffers and indicators into cells. Nature. 1981 Apr 9;290(5806):527–528. doi: 10.1038/290527a0. [DOI] [PubMed] [Google Scholar]
- Tsien R. Y. New calcium indicators and buffers with high selectivity against magnesium and protons: design, synthesis, and properties of prototype structures. Biochemistry. 1980 May 27;19(11):2396–2404. doi: 10.1021/bi00552a018. [DOI] [PubMed] [Google Scholar]
- Tsien R. Y., Pozzan T., Rink T. J. Calcium homeostasis in intact lymphocytes: cytoplasmic free calcium monitored with a new, intracellularly trapped fluorescent indicator. J Cell Biol. 1982 Aug;94(2):325–334. doi: 10.1083/jcb.94.2.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasington F. D., Gazzotti P., Tiozzo R., Carafoli E. The effect of ruthenium red on Ca 2+ transport and respiration in rat liver mitochondria. Biochim Biophys Acta. 1972 Jan 21;256(1):43–54. doi: 10.1016/0005-2728(72)90161-2. [DOI] [PubMed] [Google Scholar]
- Whitehouse S., Cooper R. H., Randle P. J. Mechanism of activation of pyruvate dehydrogenase by dichloroacetate and other halogenated carboxylic acids. Biochem J. 1974 Sep;141(3):761–774. doi: 10.1042/bj1410761. [DOI] [PMC free article] [PubMed] [Google Scholar]


