Abstract
Background
Evidence on how to improve daily physical activity (PA) levels following total knee arthroplasty (TKA) or medial uni-compartmental knee arthroplasty (mUKA) by motivational feedback is lacking. Moreover, it is unknown whether a focus on increased PA after discharge from the hospital improves rehabilitation, physical function, and quality of life. The aim of this randomized controlled trial (RCT) nested in a prospective cohort is (a) to investigate whether PA, physical function, and quality of life following knee replacement can be increased using an activity monitoring device including motivational feedback via a patient app in comparison with activity monitoring without feedback (care-as-usual), and (b) to investigate the potential predictive value of PA level prior to knee replacement for the length of stay, return to work, and quality of life.
Methods
The study is designed as a multicenter, parallel-group, superiority RCT with balanced randomization (1:1) and blinded outcome assessments. One hundred and fifty patients scheduled for knee replacement (TKA or mUKA) will be recruited through Odense University Hospital, Denmark, Vejle Hospital, Denmark and Herlev/Gentofte Sygehus, Denmark. Patients will be randomized to either 12 weeks of activity monitoring and motivational feedback via a patient app by gamification or 'care-as-usual,' including activity monitoring without motivational feedback. The primary outcome is the between-group change score from baseline to 12-week follow-up of cumulative daily accelerometer counts, which is a valid proxy for average objectively assessed daily PA.
Discussion
Improving PA through motivational feedback following knee replacement surgery might improve post-surgical function, health-related quality of life, and participation in everyday life.
Trial registration
ClinicalTrials.gov, ID: NCT06005623. Registered on 2023–08-22.
Trial status
Recruiting.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12891-024-07878-0.
Keywords: Knee arthroplasty surgery, Knee osteoarthritis, Motivational feedback, App, RCT
Background
Burden of disease
Musculoskeletal disorders, including knee osteoarthritis (OA), result in decreased physical activity (PA), leading to an increased incidence of chronic diseases [1]. Moreover, knee OA leads to work-related inactivity, a vicious downward spiral of increased symptoms, and a marked decrease in quality of life [2, 3]. The lifetime risk of developing symptomatic knee OA is estimated to be 45% [4].
Total- or uni-compartmental knee arthroplasty and patient satisfaction
Knee replacement surgery by total knee arthroplasty (TKA) and medial uni-compartmental knee arthroplasty (mUKA) are two of the most frequently performed procedures in orthopedic surgery [5], and the lifetime risk of undergoing knee replacement if one suffers from OA is 30% [6]. Worldwide, over a million patients receive knee replacement surgery each year, and the numbers are expected to increase further due to the growing elderly population [7].
Despite successful surgical procedures, knee replacement patients still demonstrate decreased function and PA level, earlier retirement, less income, increased cost for health and home care compared to matched controls [8, 9]. Moreover, nationwide data underline the need for optimized treatment and/or rehabilitation as 20% of patients are dissatisfied one-year post-surgery, and poor postoperative knee function is negatively associated with patient satisfaction [10].
Physical activity
Early mobilization on the day of surgery and a short length of hospital stay (median one day) have been achieved using the so-called fast-track protocols for knee replacement [11, 12]. However, little is known about the actual PA level and return to active daily living of the patients after discharge and in the early rehabilitation phase. Studies suggest that higher levels of PA before surgery can lead to better postoperative outcomes, including faster recovery and improved functional capacity [13, 14]. However, more evidence is needed to fully understand this relationship.
Low levels of PA have consequences such as increased all-cause mortality and chronic comorbidity [15]. This is also evident for knee OA patients [16]. On the contrary, regular PA at moderate or high intensity is associated with substantial health benefits [17]. Thus, evidence on improving PA with the perspective of a better post-knee replacement outcome is needed.
Only a few studies have objectively evaluated PA levels in OA patients, and thus, the influence of optimizing PA following knee replacement still needs to be answered. In a Dutch study, De Groot et al. (2007) observed that PA levels of patients with end-stage OA of the hip and knee were reduced (11%) compared to matched healthy controls [18]. Reduced PA was also found in Swedish patients scheduled for TKA compared to a healthy population [19]. In addition, it was shown that age and body mass index (BMI) were negatively associated with PA levels, indicating that specific interventions to improve PA, especially for heavier and older OA patients, are needed. Recently, a Danish study on fast-track TKA observed that PA levels were significantly reduced three weeks following TKA compared to preoperatively, calling for early stratified physiotherapeutic interventions [9]. The overall post-surgical improvement in physical activity for knee replacement patients has been questioned. Holsgaard-Larsen and Roos (2012) [18] found that PA levels remained low after surgery. Similarly, Kahn and Schwarzkopf (2016) [19] concluded that post-surgery activity levels were not significantly higher than pre-surgery levels. Furthermore, Hodges et al. (2018) [20] concluded that PA levels improved after TKA, but 12 months after surgery, about half of the patients did not meet the World Health Organization's recommendation for activity. This calls for improved and perhaps also individually stratified rehabilitation strategies in patients at risk for reduced postoperative PA and function [21].
Health technology
Health technology, such as wearables and motivational feedback using gamification and nudging principles, are new features within health science. Gamification is traditionally defined as using game mechanics in non-gaming contexts to engage audiences [18] while nudging is a subtle and non-coercive method to influence decisions and behaviors for individual or societal benefit [22]. The use of smartphone apps and wearable devices in monitoring patients’ recovery are on the rise. Constantinescu et al. (2022) [23], in a systematic review, emphasized the potential of the technology to improve rehabilitation and increased self-engagement in TKA patients. However, the review concluded that more research is needed to fully understand the long-term effects and maximise the potential of these technologies. This highlights the need for further exploration into how motivational feedback, through nudging and gamification strategies, can be integrated to sustain physical activity and optimize health outcomes. Incorporating wearables and principles of motivational feedback such as gamification and nudging into health science could catalyze an individualized approach focused on increasing patient motivation for PA with expected improved physical functioning, faster and safer return to work, and increased quality of life. Thus, integrating individual rehabilitation goals into wearables may prioritize a more personalized approach and, thereby, potentially improve the effectiveness of rehabilitation following knee replacement surgery. The use of gamification and nudging has been shown to increase PA in overweight and obese adults [24]. Furthermore, a recent RCT study from Van der Walt et al. (2018) [25], on TKA and THA patients has shown that feedback, only on step counts per day, can play a crucial role in motivating patients to increase their daily PA.
SENS motion® (SENS Innovation ApS, Copenhagen, Denmark [26]) is a wireless medical accelerometer for collecting objective data on PA from large cohorts of patients. SENS motion® has developed a patient app with motivational feedback (Appendix 1, Fig. 3; App interface). The app has shown that hospitalized elderly patients with heart and lung diseases were motivated to be physically active for 51 min more each day [27]. The SENS system’s ability to monitor PA has been validated on knee-OA patients [28] and thus, the patient app with motivational feedback may provide a clinically relevant impact on patient self-mobilisation following discharge from knee replacement surgery.
Objectives of the studies
This multi-center randomized controlled trial (RCT) nested in a prospective cohort aims to investigate whether PA following knee replacement surgery (TKA or mUKA) can be optimized using an activity monitoring device, including motivational feedback, compared with activity monitoring without feedback (care-as-usual). Furthermore, a prospective cohort will investigate the predictive value of PA level prior to knee replacement for the post-surgical length of stay, return to work, and quality of life.
Hypotheses
RCT study
Using an activity monitoring device the first 12 weeks after discharge, including visual and motivational feedback on PA, shows a superior effect on increased PA (primary outcome), steps per day, minutes of PA, self-reported PA, self-reported function and pain, quality of life, global perceived effect on patient experience with knee problems, and return to work (secondary outcome measures) compared to no motivational feedback from the activity monitor, defined as 'care as usual', in knee replacement patients.
Prospective cohort
PA prior to knee replacement is a predictive measure for length of hospital stay, post-surgical function, quality of life, and return to work.
Methods/Design
Patient involvement
The current hypotheses have been discussed with our local patient panel, and it has raised various considerations about how the patient app should provide motivational feedback. These considerations include the choice between using a smartphone or a tablet, the delivery of feedback through notifications or on request, and whether the feedback should align with official PA guidelines or be customized to the individual's progress.
Subsequently, qualitative interviews were conducted with patients (n = 10) who had tested the app for one month with the aim of providing input for improvements of a prototype of the patient app. Feedback from these interviews suggested minor improvements that were implemented in an updated patient-specific app version before the inclusion of the first patient.
Study design
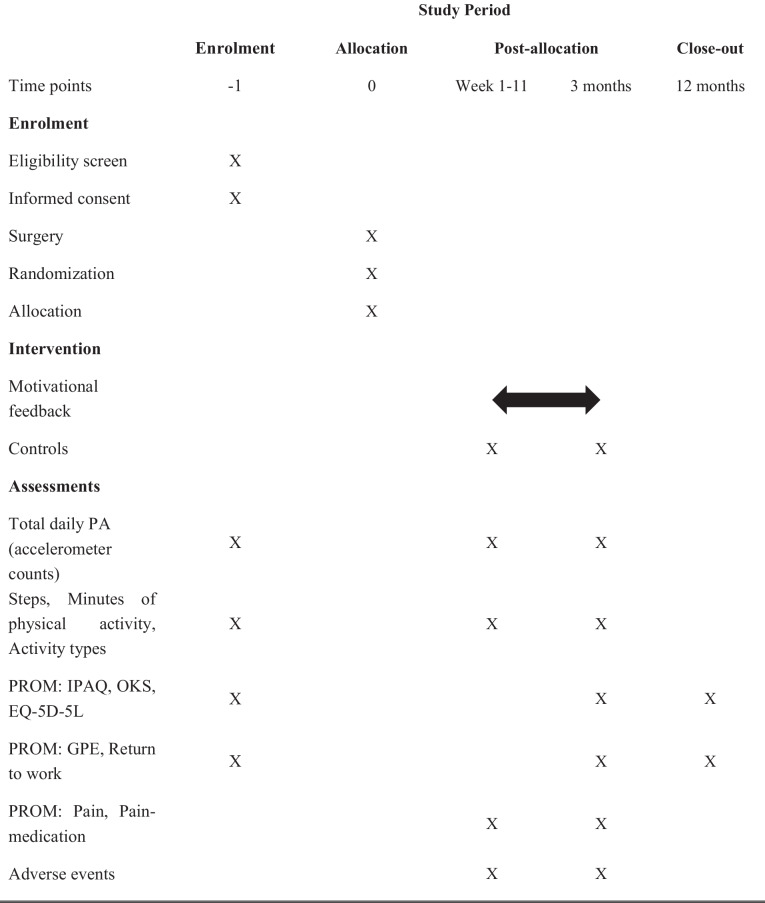
The RCT is a multicenter randomized (1:1) parallel-group superiority trial with a blinded statistical analysis toward group allocation (level of evidence: II) nested in a longitudinal prospective cohort study. The cohort study includes patients unwilling to participate in the RCT study. The study protocol adheres to the SPIRIT Statement (Standard Protocol Items: Recommendations for Interventional Trails) (see Additional file 1 for the SPIRIT Checklist and Fig. 1 for the SPIRIT Figure). The reporting of the RCT study will follow the CONSORT Statement (Consolidated Standards of Reporting Trials) [29], while the cohort study will be reported in accordance with the STROBE statement [30].
Fig. 1.
SPIRIT Figure. Template of content for the schedule of enrollment, interventions, and assessments. Abbreviations: PA: Physical activity; PROM: Patient-reported outcome measures; IPAQ: The International Physical Activity Questionnaire; OKS: Oxford Knee Score; EQ-5D-5L: Health-related quality of life; GPE: Global Perceived Effect
Participants, randomization, and blinding
Participants and settings
Patients will be recruited from the Department of Orthopedics and Traumatology, Odense University Hospital, Denmark, and the Department of Orthopedic Surgery, Lillebaelt Hospital, Vejle, Denmark. Prior to inclusion, eligible patients (Table 1; Inclusion and exclusion criteria), will receive verbal and written information about the conditions of the trial and sign a standardized consent form. The longitudinally prospective cohort study includes patients unwilling to participate in the RCT study.
Table 1.
Inclusion and exclusion criteria for patients in the study
| Inclusion | Exclusion |
|---|---|
| Age: 40–85 years | Difficulty adhering to the study protocol |
| Patients scheduled for primary TKA/mUKA | Refusal of standard care |
| Understanding both verbal and written Danish language | Known- or newly diagnosed malignancy or palliative care |
| Participation in an interventional clinical trial during the last 3 months potentially interacts with the aims of the current study | |
| Do not own a smartphone | |
| Undergone surgery using robot-assisted technology (CAS) |
Abbreviations: TKA Total knee arthroplasty, mUKA Medial uni-compartmental knee arthroplasty
Randomization
Randomization is performed internet-based using REDCap Randomize, allocated 1:1. Randomization occurs after baseline measurements on the day of surgery. The randomization is performed in blocks of 2, 4, and 6. No stratifications are applied to the randomization, and the primary investigator is blinded regarding the permuted blocking strategy. A data manager, with no clinical involvement in the trial, prepares the randomization sequence. The allocation is concealed in a password-protected computer file that is only accessible to relevant personnel.
Blinding
The primary investigator (CDS) will be blinded to allocation and will not participate in the randomization of participants. The statistical analysis will only be performed on allocation codes; thus, the data analysts will be blinded concerning intervention allocation. Blinding of patients, surgeons, and nurses (healthcare providers) will not be possible due to the nature of the intervention.
Sample size calculation and statistical procedures
To our knowledge, there are no anchor-based minimum clinically important differences (MCID) specifically for physical activity in knee patients. As a substitute, we use an MCID for steps per day, derived from chronic obstructive pulmonary disease research, corresponding to a change of approximately 17%–35% [31]. Applying a 17% threshold to total accelerometer counts per day, based on a previous study of TKA patients [9], yields an anticipated between-group difference in change score of 50,500 activity counts per day. This expected difference is further supported by findings from Van der Walt et al. (2018) [25], who demonstrated a 17% increase in daily step count in the feedback group compared to the non-feedback group after six months. To achieve 80% statistical power (β = 0.80) and detect statistically significant differences at a two-tailed α level of 0.05, assuming a standard deviation of 101,000 counts per day before and after the intervention [9] a sample size of 62 participants per group is estimated. To account for potential dropouts, we use a rate of 20% based upon evidence from a systematic review showing that dropout rates in RCTs on physical activity are within this percentage [32]. Consequently, we will include a sample of 150 patients in total. When appropriate, all outcome measures will be checked for Gaussian distribution using QQ-plots and parametric statistical and/or non-parametric analyses.
No a priori sample size calculation is made for the cohort study. However, based on a total sample of approximately 1,400 annual knee replacement procedures for OUH and Vejle hospitals, it is reasonable to expect an inclusion of 200 patients, which is acceptable for the current statistical analysis plan on predictive regression models [30].
The main comparative analyses between groups will be performed using an intention-to-treat analysis. Between-group mean differences and 95% confidence intervals will be estimated with a linear regression model. The patient’s baseline score is entered as a covariate and adjusted for potential baseline differences (age, sex, BMI). In addition to the intention-to-treat analysis, a per-protocol analysis will be conducted on patients adhering to the intervention (using the patient app for 5 out of 7 days in a week or > 70% of the available days.
Intervention
Two weeks prior to surgery and 12 weeks following discharge, patients will be equipped with a discrete patch and built-in accelerometer (SENS motion®, Denmark, Copenhagen) attached to the thigh of the leg not undergoing surgery. The patch will be worn 24 h a day, and there will be no need to remove the patch before the end of the study (2 + 12 weeks). Besides raw accelerometer counts (primary outcome measure), the accelerometer also measures daily activity, daily steps, and type of activity. Prior to this protocol, a pilot study evaluated the validity of the SENS accelerometer on a similar patient group, showing that SENS motion® can measure PA and differentiate between different types of activities, such as lying down, sitting, standing, and standing work [33]. Before discharge patients randomized to the intervention group will receive a tablet with the app (“SENS motion”). To simplify usage, the tablet only contains the "SENS motion" app.
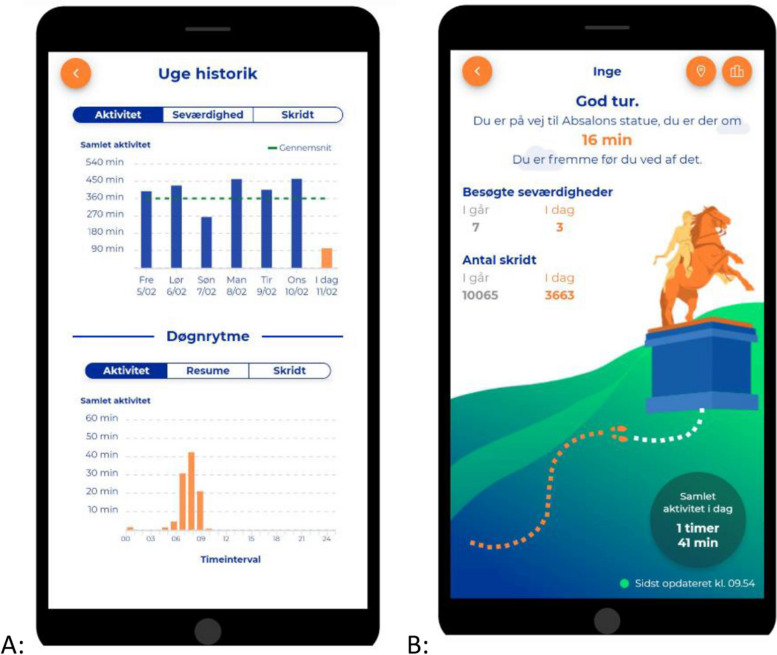
Patients randomized to the intervention group will be motivated to be more physically active through elements of gamification (e.g. achieving step goals) and nudging (supporting notifications). The sensor will measure the PA of the patient and accelerometer counts, irrespectively of the type of activity, will be converted into daily steps, which will be visible to the patients. Through the app, patients will be able to choose between two user interfaces. Interface 1 (Fig. 3B) allows patients to view predefined goals, including locations in a self-selected city whereas Interface 2 (Fig. 3A) provides graphical representations of their daily activity and their history during the period they have worn the accelerometer. The graphical representations include daily steps, physically active minutes, and type of activity. The motivational intervention is reported and described using “The template for intervention description and replication” (TIDieR) [34].
Data from SENS motion sensors are encrypted directly on the sensor itself before being sent to the server. The data is stored pseudonymized with a user ID. The server is located in Germany, and for security reasons, the exact location cannot be disclosed.
Compliance
Both groups will have an app installed on a dedicated tablet (the intervention group) or their smartphone (the control group). For the control group, the app does not provide motivational feedback but is solely used to transfer data from the activity tracker to a central research database. To prevent data loss, weekly SMS messages will be sent to both groups with the message "Have you opened your SENS app this week?”. Furthermore, the researchers can verify proper attachment by following the skin temperature evaluated by the sensor via a web module. If the temperature fluctuates over an extended period, a project manager will call the patient to solve potential issues. A standard for adherence to app interventions has yet to be established. However, based on patient feedback, an adherence rate of 70%, meaning that the intervention group will use the app along with motivational feedback for 5 out of 7 weekdays, seems reasonable.
All patients will receive oral and written information on how to attach the sensor, use the app (without a tablet), and use the tablet with motivational feedback. Moreover, all patients will receive a link to a YouTube video presenting the project and providing instructions on how to use the equipment.
Timing of assessments
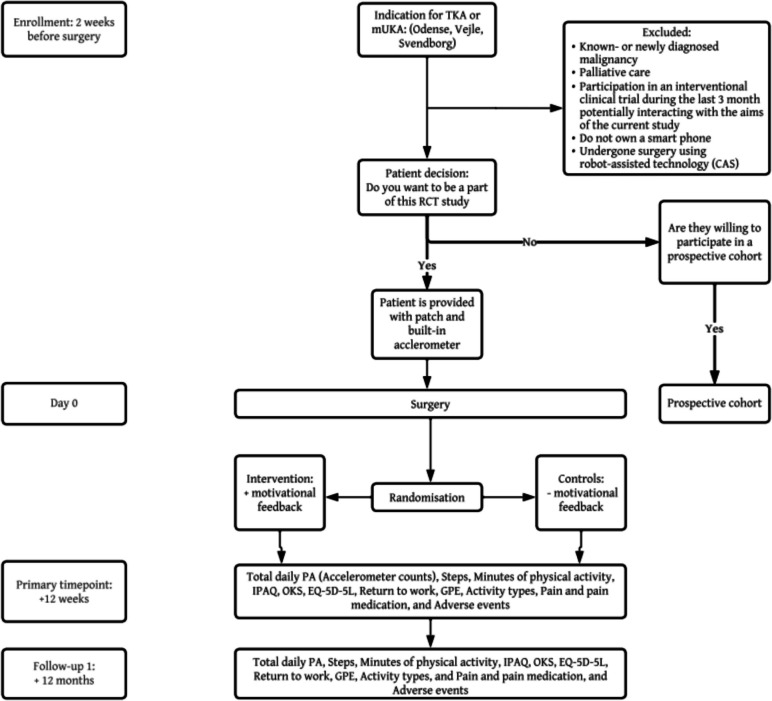
Assessment will be performed at baseline (two weeks prior to surgery and randomization), during the intervention (12 weeks post-surgery) (the primary endpoint), and a long-term follow-up on patient-reported outcomes will be performed at 12 months post-surgery (Fig. 2; Flowchart of the study and Table 2: Outcome measurements).
Fig. 2.
Flowchart of the study. Abbreviations: TKA: Total knee arthroplasty; mUKA: Medial uni-compartmental knee arthroplasty; PA: Physical activity; IPAQ: The International Physical Activity Questionnaire; OKS: Oxford Knee Score; EQ-5D-5L: Health-related quality of life; GPE: Global Perceived Effect
Table 2.
Outcome measurement
| Data Collection instrument | Collection time | ||||
|---|---|---|---|---|---|
| Baseline (2 weeks prior to surgery) | Post surgery (week 1–11) | Post surgery (3 months) | Post surgery (12 months) | ||
| Primary Outcomes | |||||
| Total daily PA (Accelerometer counts) | Accelerometer | X | X | X | |
| Secondary outcomes | |||||
| Steps | Accelerometer | X | X | X | |
| Minutes of physical activity | Accelerometer | X | X | X | |
| IPAQ | PROM | X | X | X | |
| OKS | PROM | X | X | X | |
| EQ-5D-5L | PROM | X | X | X | |
| Return to work | PROM | X | X | X | |
| GPE | PROM | X | X | X | |
| Tertiary outcomes | |||||
| Activity types | Accelerometer | X | X | X | |
| Pain and pain medication | PROM, SMS-survey | X | X | ||
| Adverse events | PROM | X | X | ||
Patient characteristics (Age, gender, BMI, work type and surgery leg) will be obtained at baseline
Abbreviations: PA Physical activit, IPAQ The International Physical Activity Questionnaire, PROM Patient-reported outcome measures, OKS Oxford Knee Score, EQ-5D-5L Health-related quality of life, GPE Global Perceived Effect
Outcome measures
Patient characteristics
At baseline prior to randomization, height and weight will be measured, and gender, age, work type, and surgery leg will be recorded. After randomization (post-surgery), the type of knee arthroplasty (TKA/mUKA) and length of hospital stay will be obtained from patient journals.
Primary outcome measure
PA will be evaluated by SENS motion® as described above. The primary outcome measure is the between-group change score of total daily PA (accelerometer counts) from baseline to 12 weeks following surgery. Accelerometer counts per day is a cumulative and validated variable based on raw accelerometer data in 3 planes and is a proxy for total daily PA [35].
Secondary outcome measures
Steps
Minutes of physical activity
Minutes of physical activity per day measured with SENS motion®.
Self-reported physical activity
Patient-reported PA will be measured by The International Physical Activity Questionnaire Short Form (IPAQ-SF), which is a 7-item questionnaire consisting of open-ended questions surrounding the patient's last seven days' recall of PA [36, 37]. The short and long version of the IPAQ was translated into Danish, and the long version was validated in 2014 [38].
The Oxford Knee Score
The Oxford Knee Score (OKS) is a validated 12-item patient-reported questionnaire designed and developed to assess function and pain following TKA [39]. It was translated and validated into Danish in 2009 [40]. The questionnaire generates scores ranging from 0–48, with a score of 48 representing the best outcome post-TKA. Each item response is graded between 0–4.
General Health
The EQ-5D-5L is a validated patient-reported questionnaire assessing health-related quality of life and consists of the following dimensions: mobility, self-care, usual activities, pain/discomfort, and anxiety/depression. Each dimension uses a 5-point Likert scale [41]. The EQ-5D-5L has been validated in knee OA patients referred for knee arthroplasty surgery [42].
Return to work
At 12 weeks and 12 months follow up, all patients will receive tree questions about changes in work status (retried/sick leave/job-seeking), work type (non-physically demanding/physically demanding), and working time (full-/part-time).
Global Perceived Effect (GPE)
At 12 weeks and 12 months follow-up, two GPE questions will be sent to the patient. A 7-point Likert scale using the following answers “Worse," "Slightly worse," “Very slight worse", "The same, "Minimal improvement," "Slightly better," and "Better” will be used to answer the following question: “How do you experience your knee problems now, compared to before the surgery?”.
Tertiary outcome measures
Activity types
Activity types evaluated by SENS motion®, i.e. time spent sleeping/resting, standing, walking, and cycling, will be considered.
Pain and pain medication
The patients receive weekly text messages (SMS) with a question about their knee pain the previous week. They will be asked to rate their pain on an NRS scale from 0–10, where 0 corresponds to no pain and 10 corresponds to the worst imaginable pain. Furthermore, patients will receive an SMS with a question about their use of pain medication in the last week. The SMS has four options: i) No use of pain medication the past week, ii) use of non-prescription medicine (e.g., Panodil, Pamol, Ibumetin) at least once during the past week, iii) use of strong painkillers (e.g., Morphine, Tramadol) at least once the past week, and iv) use of both non-prescription medicine and strong painkillers at least once the past week.
Adverse events
Reporting of adverse events will be elicited through self-reported questionnaires 30 and 90 days after the operation. All events will be coded according to the Medical Dictionary for Regulatory Activities, as currently required by all regulatory authorities, including the US Food and Drug Administration and the European Agency for the Evaluation of Medicinal Products. Additionally, a project staff member will examine the patient's medical record for more severe adverse events 7 and 90 days after TKA/mUKA (e.g. deep vein thrombosis (DVT), lung emboli (LE), death, and re-operation). The motivational intervention for increased PA is non-invasive, and only minor discomfort associated with the procedure is anticipated. In cases of unacceptable pain (NRS > 5) for consecutive days, the project manager can be directly contacted. Patients are informed to follow standard procedures for PA after knee replacement in which it is specified that patients should be aware of signs such as fatigue and pain, which indicate the need to take a break from exercise.
Discussion
This randomized controlled superiority multi-center trial will evaluate the effect of activity monitoring and motivational feedback following knee arthroplasty surgery. The RCT study is expected to provide high-level evidence of the potential clinical and functional benefits of activity monitoring and motivational feedback after a TKA/mUKA. This trial is a multicenter RCT, which results in increased external validity and generalizability of the results. While the literature demonstrates consistent results on decreased levels of post-surgical PA, there is limited understanding of how to improve post-surgical PA levels and the return to active daily living following knee replacement surgery. Existing studies indicate that many knee patients do not achieve significant improvements after surgery and do not meet World Health Organization recommendations for activity [20]. Consequently, there is a need for interventions that help patients enhance post-surgical physical activity.
Outcome variables
The primary outcome variable, the between-group change score of total daily PA (accelerometer counts) after 12 weeks, is chosen to examine if short-term PA can be increased with motivational feedback. Furthermore, patient-reported outcome variables are evaluated at 12-month follow-up to evaluate potential long-term effects of the intervention on knee function and quality of life. In addition to the objective PA measurements, patients will respond to a questionnaire regarding their subjective PA. Both approaches are combined to provide a more nuanced understanding of the patients’ PA.
The 12-week follow-up was chosen to identify possible improvements at the end of the acute rehabilitation period, where patients are expected to have returned to an active lifestyle and/or have ended their sick leave. 12 weeks is also a reasonably long timeframe for clinical improvements to occur in patients who have undergone knee-arthroplasty surgery, yet short enough to assume that patients would be able to recall their baseline condition. The 12-month follow-up was selected to capture any potential changes beyond the short-term rehabilitation as an expression of a long-term effect.
Study design
Prior to this RCT, a qualitative study was conducted including 10 TKA/mUKA-patients. Integrating qualitative research before the RCT strengthens the study design by understanding our population and their perspectives. Furthermore, the qualitative study helped optimize the app for the population and their specific needs. This approach facilitates a more comprehensive and well-informed methodology for conducting rigorous and impactful research.
Only a few exclusion criteria will be employed to improve external validity and generalizability. However, the study may potentially be affected by selection bias. An exclusion criterion of “do not own a smartphone” will be used since the use of a smartphone is necessary for the intervention. Furthermore, the trial is based on patients volunteering for a physical intervention, which can also be a cause of selection bias. The accelerometer used in this trial has a maximum memory capacity of 7–14 days. To prevent data loss resulting from a full memory, it is required to establish a weekly data transfer connection to a smartphone using the dedicated application. Therefore, we decided to send a weekly SMS reminder to patients in the intervention and control groups. This approach may introduce attention bias. Moreover, all patients, whether in the intervention or control group, will be aware of wearing the accelerometer, which also may introduce attention bias. However, using their own smartphones to transfer data should minimize the risk of bias in the control group, resulting in more accurate PA levels for comparison.
Public access to the current protocol paper and prior registration on ClinicalTrials.gov will guarantee transparency regarding the implemented methods and definitions of outcome measures.
Perspective
If proven effective, the use of gamification tools and accelerometers introduced in this study can potentially enhance the current rehabilitation process for patients undergoing knee replacement. Furthermore, the use of gamification tools and accelerometers is extensive, and if proven effective, findings might be extrapolated to other conditions where a rehabilitation process plays a crucial role in the patient's return to an active life.
Supplementary Information
Additional file 1: SPIRIT Checklist. Spirit 2013 Checklist: Recommended items to address in clinical trial protocol and related documents (DOC 71,5 KB).
Additional file 2: “The template for intervention description and replication” (TiDieR) Checklist (DOC: 65,0 KB).
Acknowledgements
Study data will be collected and managed using REDCap electronic data capture tools hosted at Open Patient Data Explorative Network (OPEN), Odense University Hospital, Region of Southern Denmark.
Access to data
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Trial status
Protocol version 4.
Date of which recruitment was initiated for qualitative study: 12. February 2023.
Date of which recruitment for the RCT study: 15. September 2023.
Abbreviations
- OA
Osteoarthritis
- PA
Physical Activity
- TKA
Total knee arthroplasty
- mUKA
Medial uni-compartmental knee arthroplasty
- BMI
Body Mass Index
- RCT
Randomized controlled trial
- INT
Intervention group
- CON
Control group
- IPAQ
The International Physical Activity Questionnaires
- OKS
Oxford Knee Score
- EQ-5D-5L
Health-related quality of life
- GPE
Global perceived effect
- ADL
Activities of Daily Living
- QOL
Quality of life
- NRS
Numeric Rating Scale
- DVT
Deep vein thrombosis
- LE
Lung emboli
- OUH
Odense University Hospital
- SDU
University of Southern Denmark
Appendix
Fig. 3.
A Example of the graphical feedback screen (In Danish). B Example of the visual feedback screen. Pictures: Healthcare – SENS Innovation ApS, used with consent
Authors’ contributions
AHL conceived the project. CDS is leading the coordination of the trial and is the primary investigator. AHL and CDS wrote the protocol manual, and MLL, CV, HS, and CMJ assisted with the study design and protocol prepara-tion. AHL and CDS procured the project funding. AHL performed the sample size calculation and designed the statistical analysis plan. CDS will be the blinded analysts on the project. All authors provided feedback on drafts of this article and read and approved the final manuscript.
Funding
Open access funding provided by University of Southern Denmark This project was funded by: The Faculty of Health Sciences, Ph.D fund, Syddansk University, Odense. The Region of Southern Denmark Ph.D-fund; Dept. of Orthopedic Surgery and Traumatology; The Region of Southern Denmark research fund; GigtForeningen.
None of the funders has any role in the study besides providing funding. This protocol has undergone peer review at Syddansk University, Odense.
Declarations
Ethics approval and consent to participate
The study has been submitted to The Regional Committee for Medical Research Ethics, which has assessed the project as "not subject to notification”, Project-ID: S-20222000 – 171. The Danish Medicines Agency has declared no need for further permission to carry out the study. The project will be implemented in accordance with the Helsinki Declaration II. Before patients give their written consent to participate, they will be provided with written and oral information on the experimental procedures and potential risks. All subject data will be treated confidentially under the Danish laws about personal data and health.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Williams A, Kamper SJ, Wiggers JH, O’Brien KM, Lee H, Wolfenden L, et al. Musculoskeletal conditions may increase the risk of chronic disease: a systematic review and meta-analysis of cohort studies. BMC Med. 2018;16(1):167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hunter DJ, Schofield D, Callander E. The individual and socioeconomic impact of osteoarthritis. Nat Rev Rheumatol. 2014;10(7):437–41. [DOI] [PubMed] [Google Scholar]
- 3.Sharif B, Garner R, Hennessy D, Sanmartin C, Flanagan WM, Marshall DA. Productivity costs of work loss associated with osteoarthritis in Canada from 2010 to 2031. Osteoarthritis Cartilage. 2017;25(2):249–58. [DOI] [PubMed] [Google Scholar]
- 4.Murphy L, Schwartz TA, Helmick CG, Renner JB, Tudor G, Koch G, et al. Lifetime risk of symptomatic knee osteoarthritis. Arthritis Rheum. 2008;59(9):1207–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Steiner CA, Barrett ML, Weiss AJ, Andrews RM. Trends and projections in hospital stays for Adults Withe Multiple Chronic Conditions 2003-2014. Helahtcare Cost and Utiliation Project (HCUP). Rockville: Agency for Healthcare Research and Quality (US); 2014. [PubMed]
- 6.Burn E, Murray DW, Hawker GA, Pinedo-Villanueva R, Prieto-Alhambra D. Lifetime risk of knee and hip replacement following a GP diagnosis of osteoarthritis: a real-world cohort study. Osteoarthritis Cartilage. 2019;27(11):1627–35. [DOI] [PubMed] [Google Scholar]
- 7.Bitton R. The economic burden of osteoarthritis. Am J Manag Care. 2009;15(8 Suppl):S230–5. [PubMed] [Google Scholar]
- 8.Kjellberg J, Kehlet H. A nationwide analysis of socioeconomic outcomes after hip and knee replacement. Dan Med J. 2016;63(8):A5257. [PubMed] [Google Scholar]
- 9.Luna IE, Kehlet H, Wede HR, Hoevsgaard SJ, Aasvang EK. Objectively measured early physical activity after total hip or knee arthroplasty. J Clin Monit Comput. 2019;33(3):509–22. [DOI] [PubMed] [Google Scholar]
- 10.Liu J, Yang Y, Wan S, Yao Z, Zhang Y, Zhang Y, et al. A new prediction model for patient satisfaction after total knee arthroplasty and the roles of different scoring systems: a retrospective cohort study. J Orthop Surg Res. 2021;16(1):329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jensen CB, Troelsen A, Foss NB, Nielsen CS, Lindberg-Larsen M, Gromov K. 10-year evolution of day-case hip and knee arthroplasty: a Danish nationwide register study of 166,833 procedures from 2010 to 2020. Acta Orthop. 2023;94:178–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kehlet H. Fast-track hip and knee arthroplasty. Lancet. 2013;381(9878):1600–2. [DOI] [PubMed] [Google Scholar]
- 13.Vasta S, Papalia R, Torre G, Vorini F, Papalia G, Zampogna B, et al. The Influence of Preoperative Physical Activity on Postoperative Outcomes of Knee and Hip Arthroplasty Surgery in the Elderly: A Systematic Review. J Clin Med. 2020;9(4):969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Christensen J, Peters C, Gililland J, Stoddard G, Pelt C. Physical activity, pain interference and comorbidities relate to PROMIS physical function in younger adults following total knee arthroplasty. Disabil Rehabil. 2021;43(26):3741–7. [DOI] [PubMed] [Google Scholar]
- 15.Organization WH. Global action plan on physical activity 2018–2030: More active people for a healthier world. World Health Organization: World Health Organization; 2018. [Google Scholar]
- 16.McKevitt S, Healey E, Jinks C, Rathod-Mistry T, Quicke J. The association between comorbidity and physical activity levels in people with osteoarthritis: Secondary analysis from two randomised controlled trials. Osteoarthr Cartil Open. 2020;2(2):100057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wasfy MM, Baggish AL. Exercise Dose in Clinical Practice. Circulation. 2016;133(23):2297–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Groot IB, Bussmann JB, Stam HJ, Verhaar JAN. Actual everyday physical activity in patients with endstage hip or knee osteoarthritis compared with healthy controls. Osteoarthritis Cartilage. 2008;16(4):436–42. [DOI] [PubMed] [Google Scholar]
- 19.Holsgaard-Larsen A, Roos EM. Objectively measured physical activity in patients with end stage knee or hip osteoarthritis. Eur J Phys Rehabil Med. 2012;48(4):577–85. [PubMed] [Google Scholar]
- 20.Hodges A, Harmer AR, Dennis S, Nairn L, March L, Crawford R, et al. Prevalence and determinants of physical activity and sedentary behaviour before and up to 12 months after total knee replacement: a longitudinal cohort study. Clin Rehabil. 2018;32(9):1271–83. [DOI] [PubMed] [Google Scholar]
- 21.Aasvang EK, Luna IE, Kehlet H. Challenges in postdischarge function and recovery: the case of fast-track hip and knee arthroplasty. Br J Anaesth. 2015;115(6):861–6. [DOI] [PubMed] [Google Scholar]
- 22.Sant’Anna A, Vilhelmsson A, Wolf A. Nudging healthcare professionals in clinical settings: a scoping review of the literature. BMC Health Serv Res. 2021;21(1):543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Constantinescu D, Pavlis W, Rizzo M, Vanden Berge D, Barnhill S, Hernandez VH. The role of commercially available smartphone apps and wearable devices in monitoring patients after total knee arthroplasty: a systematic review. EFORT Open Rev. 2022;7(7):481–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Patel MS, Small DS, Harrison JD, Fortunato MP, Oon AL, Rareshide CAL, et al. Effectiveness of Behaviorally Designed Gamification Interventions With Social Incentives for Increasing Physical Activity Among Overweight and Obese Adults Across the United States: The STEP UP Randomized Clinical Trial. JAMA Intern Med. 2019;179(12):1624–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Van der Walt N, Salmon LJ, Gooden B, Lyons MC, O’Sullivan M, Martina K, et al. Feedback From Activity Trackers Improves Daily Step Count After Knee and Hip Arthroplasty: A Randomized Controlled Trial. J Arthroplasty. 2018;33(11):3422–8. [DOI] [PubMed] [Google Scholar]
- 26.Sens Motion - Diskret Aktivitetssensor til Sundhed og Forskning Homepage. Available from: https://www.sens.dk/da/.
- 27.Dall CH, Andersen H, Povlsen TM, Henriksen M. Evaluation of a technology assisted physical activity intervention among hospitalised patients: A randomised study. Eur J Intern Med. 2019;69:50–6. [DOI] [PubMed] [Google Scholar]
- 28.Ghaffari A, Rahbek O, Lauritsen REK, Kappel A, Kold S, Rasmussen J. Criterion Validity of Linear Accelerations Measured with Low-Sampling-Frequency Accelerometers during Overground Walking in Elderly Patients with Knee Osteoarthritis. Sensors (Basel). 2022;22(14):5289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lorette G, Maruani A. The CONSORT statement (CONsolidated Standards Of Reporting Trials). Ann Dermatol Venereol. 2013;140(6–7):431–5. [DOI] [PubMed] [Google Scholar]
- 30.von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: guidelines for reporting observational studies. Int J Surg. 2014;12(12):1495–9. [DOI] [PubMed] [Google Scholar]
- 31.Teylan M, Kantorowski A, Homsy D, Kadri R, Richardson C, Moy M. Physical activity in COPD: Minimal clinically important difference for medical events. Chron Respir Dis. 2019;16:1479973118816424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bullard T, Ji M, An R, Trinh L, Mackenzie M, Mullen SP. A systematic review and meta-analysis of adherence to physical activity interventions among three chronic conditions: cancer, cardiovascular disease, and diabetes. BMC Public Health. 2019;19(1):636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pedersen BS, Kristensen MT, Josefsen CO, Lykkegaard KL, Jonsson LR, Pedersen MM. Validation of Two Activity Monitors in Slow and Fast Walking Hospitalized Patients. Rehabil Res Pract. 2022;2022:9230081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hoffmann TC, Glasziou PP, Boutron I, Milne R, Perera R, Moher D, et al. Better Reporting of Interventions: Template for Intervention Description and Replication (TIDieR) Checklist and Guide. Gesundheitswesen. 2016;78(3):e174. [DOI] [PubMed] [Google Scholar]
- 35.Skender S, Ose J, Chang-Claude J, Paskow M, Bruhmann B, Siegel EM, et al. Accelerometry and physical activity questionnaires - a systematic review. BMC Public Health. 2016;16:515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Craig CL, Marshall AL, Sjostrom M, Bauman AE, Booth ML, Ainsworth BE, et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. 2003;35(8):1381–95. [DOI] [PubMed] [Google Scholar]
- 37.Lee PH, Macfarlane DJ, Lam TH, Stewart SM. Validity of the International Physical Activity Questionnaire Short Form (IPAQ-SF): a systematic review. Int J Behav Nutr Phys Act. 2011;8:115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hansen AW, Dahl-Petersen I, Helge JW, Brage S, Gronbaek M, Flensborg-Madsen T. Validation of an Internet-based long version of the International Physical Activity Questionnaire in Danish adults using combined accelerometry and heart rate monitoring. J Phys Act Health. 2014;11(3):654–64. [DOI] [PubMed] [Google Scholar]
- 39.Dawson J, Fitzpatrick R, Murray D, Carr A. Questionnaire on the perceptions of patients about total knee replacement. J Bone Joint Surg Br. 1998;80(1):63–9. [DOI] [PubMed] [Google Scholar]
- 40.Mørup-Petersen A, Krogsgaard M, Nielsen R, Paulsen A, Odgaard A. Translation and classical test theory validation of the Danish version of the Oxfod Knee Score. DOS Kongressen 2019; 2019. p. 177.
- 41.Jensen CE, Sorensen SS, Gudex C, Jensen MB, Pedersen KM, Ehlers LH. The Danish EQ-5D-5L Value Set: A Hybrid Model Using cTTO and DCE Data. Appl Health Econ Health Policy. 2021;19(4):579–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Conner-Spady BL, Marshall DA, Bohm E, Dunbar MJ, Loucks L, Al Khudairy A, et al. Reliability and validity of the EQ-5D-5L compared to the EQ-5D-3L in patients with osteoarthritis referred for hip and knee replacement. Qual Life Res. 2015;24(7):1775–84. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: SPIRIT Checklist. Spirit 2013 Checklist: Recommended items to address in clinical trial protocol and related documents (DOC 71,5 KB).
Additional file 2: “The template for intervention description and replication” (TiDieR) Checklist (DOC: 65,0 KB).